Abstract
Tumour necrosis factor (TNF) is a central cytokine in inflammatory reactions, and biologics that neutralize TNF are among the most successful drugs for the treatment of chronic inflammatory and autoimmune pathologies. In recent years, it became clear that TNF drives inflammatory responses not only directly by inducing inflammatory gene expression but also indirectly by inducing cell death, instigating inflammatory immune reactions and disease development. Hence, inhibitors of cell death are being considered as a new therapy for TNF-dependent inflammatory diseases.
Subject terms: Cell death and immune response, Necroptosis, Tumour-necrosis factors, Autoinflammatory syndrome
Tumour necrosis factor (TNF) drives inflammatory responses directly by inducing inflammatory gene expression and also indirectly by inducing cell death. This article reviews the various TNF-induced cell death pathways, their mode of execution and the molecular checkpoints that control them, which is revealing new opportunities for the treatment of TNF-mediated diseases.
Introduction
Cell death is increasingly recognized as a major driver of inflammatory disease. Compared with apoptosis, which is generally considered to be immunologically silent, lytic forms of cell death, such as necroptosis, pyroptosis and apoptosis-driven secondary necrosis, release intracellular factors, known as damage-associated molecular patterns, that activate immune receptors and induce inflammatory responses. In addition, the inflammatory signalling cascade may originate from and/or be amplified by loss of barrier function caused by epithelial cell death and the subsequent sensing of pathogen-associated molecular patterns from microorganisms that have breached the epithelial barrier. Therefore, cell demise, in its multiple modalities, acts as an initiator or amplifier of inflammation. Death is, however, not the default response in cells, and is usually suppressed unless certain cell death checkpoints are overridden. On the one hand, cell death-driven inflammation serves as a backup mechanism in microbial infection to ensure optimal antimicrobial responses when inflammatory gene activation has been hijacked by the pathogen. On the other hand, environmental factors and/or genetic predispositions can alter the tight regulation of the cell death processes, leading to unwanted or exacerbated inflammatory responses that may underlie various inflammatory diseases. Accumulating evidence suggests that blocking cell death can reverse the inflammatory pathology state in various mouse models of acute and chronic inflammatory diseases (reviewed in1). Improving our understanding of the interplay between the various cell death modalities, their mode of execution, the molecular checkpoints that control them, and the physiological and pathological conditions that turn them off is therefore needed to identify new therapeutic targets. Moreover, such knowledge will help to define the disorders in which pharmacological cell death inhibitors may provide therapeutic advantage.
The inflammatory cytokine tumour necrosis factor (TNF) is central in orchestrating the inflammatory immune response. Hence, TNF-neutralizing therapies have been highly successful for the treatment of chronic inflammatory and autoimmune pathologies (Box 1). This Review briefly recalls the history and discovery of TNF as a target for therapy, and then focuses on more recent findings demonstrating that TNF indirectly promotes inflammation by inducing cell death. Consequently, direct inhibition of cell death is now being considered as a new therapeutic strategy for the treatment of TNF-mediated diseases, especially to treat patients who are non-responders or show adverse effects to anti-TNF treatment.
Box 1 Anti-TNF biologics are among the most successful drugs in history.
Following the discovery that tumour necrosis factor (TNF) has strong proinflammatory activities, attention turned to the development of biologics that neutralize TNF’s activity for the treatment of inflammatory diseases. This turned out to be highly successful.
TNF was the first cytokine to be validated as a therapeutic target for rheumatoid arthritis. TNF inhibition using a neutralizing antibody was shown to block the synthesis of several other important proinflammatory cytokines in cell cultures and mice, which led to the pivotal concept that TNF is at the apex of an inflammatory cascade of cytokines in rheumatoid arthritis195–197. Soon after, a small-scale clinical study using anti-TNF antibodies was initiated in patients with rheumatoid arthritis, and demonstrated marked clinical improvement in most patients198. Subsequent clinical trials confirmed high efficacy of TNF neutralization in the treatment of rheumatoid arthritis199–201, which paved the way for testing use of TNF inhibitors in other inflammatory autoimmune diseases.
Five distinct antibody- or receptor-based TNF-neutralizing drugs have been approved over the years for treating rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis, ankylosing spondylitis, psoriasis, Crohn’s disease and ulcerative colitis (reviewed in202). The chimeric antibody infliximab (sold under the brand name Remicade) and the TNF receptor 2 (TNFR2)–Fc fusion protein etanercept (Enbrel) were the first two TNF biologics to be approved, in 1998, followed by the first fully human antibody, adalimumab (Humira), in 2002. Certolizumab pegol (Cimzia), a pegylated Fab fragment, was approved in 2008, and another fully human antibody, golimumab (Simponi), was approved in 2009 (reviewed in203). From 2015 on, several of these TNF inhibitors had lost their market exclusivities, allowing biosimilar alternatives to enter the market. Together, these TNF-neutralizing therapies are among the most successful protein-based drugs in history, with global sales estimated at US $30 billion annually.
A short history of TNF
TNF was identified as a serum factor that could induce the haemorrhagic necrosis of tumours in patients following acute bacterial infections2 (Fig. 1). This anticancer activity had already been exploited nearly a century before by the New York surgeon William Coley, who described the treatment of patients with cancer with bacterial extracts termed ‘Coley’s mixed toxins’3,4. Later, lipopolysaccharide (LPS) was isolated from bacterial extracts and shown to induce some tumour regression in experimental cancer studies in mice5. Carswell et al. later demonstrated that it was in fact not the LPS itself that killed the cancer cells but a necrotizing factor produced by the host macrophages in response to LPS. Hence, the necrotizing factor was named ‘tumour necrosis factor’ or ‘TNF’2. In the years after, the genes encoding the human and mouse TNF and TNF receptors were purified, sequenced and cloned6–13, and experimental studies with recombinant TNF were initiated to validate its antitumour potential for cancer treatment (reviewed in14). However, the hope that TNF would be a powerful anticancer drug soon faded when it became clear that administration of the recombinant cytokine induces severe endotoxic shock. Indeed, and independently of these cancer studies, TNF was found to be identical to a previously identified protein named ‘cachectin’, which was responsible for endotoxin-induced wasting disease (cachexia) in mice15 (Fig. 1). These findings also clearly demonstrated that TNF is a pleiotropic cytokine that must be tightly regulated.
Fig. 1. Timeline of key events in the history of TNF and TNF-induced cell death.
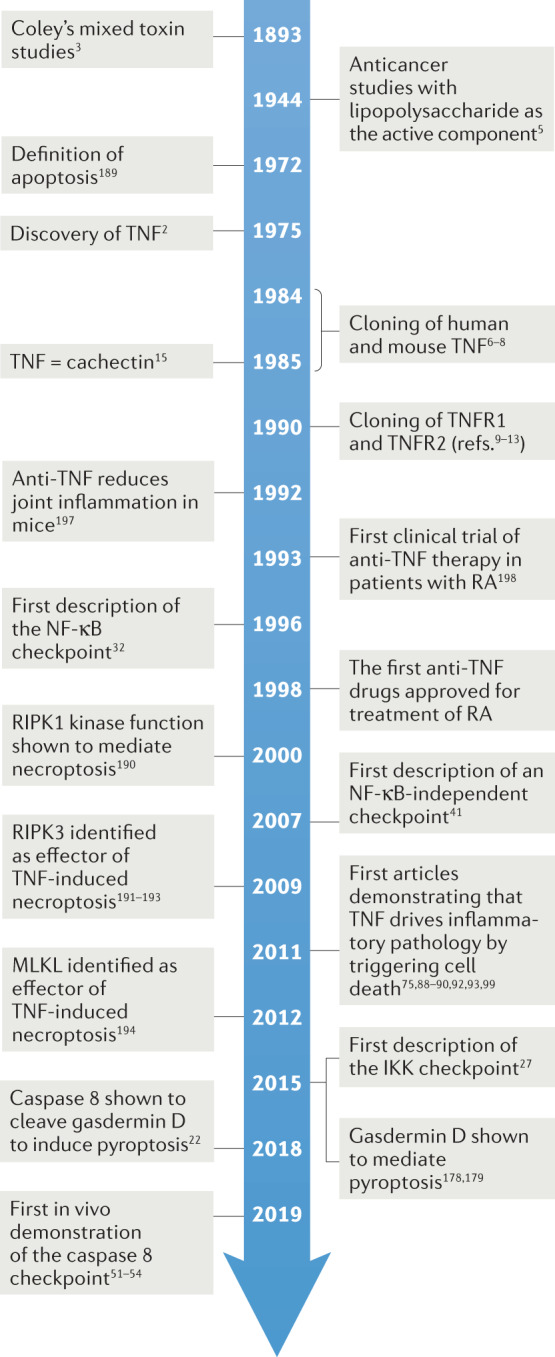
IKK, inhibitor of nuclear factor-κB kinase; MLKL, mixed lineage kinase domain-like protein; NF-κB, nuclear factor-κB; RA, rheumatoid arthritis; RIPK, receptor-interacting serine/threonine protein kinase; TNF, tumour necrosis factor; TNFR, tumour necrosis factor receptor.
Induction of cell death by TNF
The clinical success of anti-TNF biologics in treating inflammatory disorders has been attributed to their effectiveness in blocking TNF from binding to its cognate receptors TNF receptor 1 (TNFR1) and TNFR2. It was long thought that this blockade reduces inflammation by preventing TNFR1 from activating the mitogen-activated protein kinase (MAPK) pathway and the canonical nuclear factor-κB (NF-κB) pathway, which would otherwise collectively lead to the transcriptional upregulation of proinflammatory genes that underlie the inflammatory pathology (Fig. 2). While this initial belief is probably true, it is now clear that binding of TNF to TNFR1 also indirectly promotes and exacerbates inflammation by inducing cell death, in the form of apoptosis, necroptosis or pyroptosis. Indeed, dying cells release intracellular constituents that induce proinflammatory gene expression in neighbouring cells. In addition, epithelial cell death (in the skin or the intestine) may affect barrier integrity, inducing microbial tissue infiltration and inflammation (Fig. 2). Hence, genetic targeting of cell death was shown to reverse the inflammatory phenotype in various mouse models of TNF-induced inflammatory diseases (see later). Consequently, drugs that inhibit cell death, such as inhibitors of receptor-interacting serine/threonine protein kinase 1 (RIPK1), are currently under investigation as alternative therapies for TNF-driven human diseases (reviewed in1,16,17).
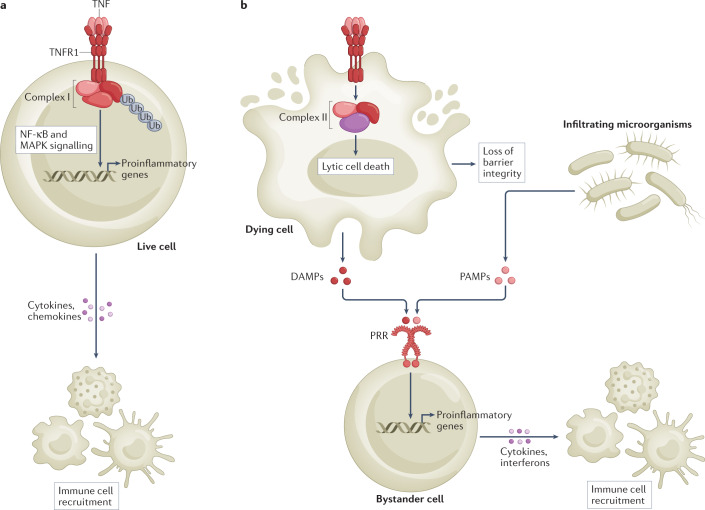
Fig. 2. Inflammatory signalling by TNFR1.
a, Binding of tumour necrosis factor (TNF) to TNF receptor 1 (TNFR1) directly promotes inflammation by activating the nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signalling pathways, which collectively lead to the transcriptional upregulation of genes encoding proinflammatory mediators, such as cytokines and chemokines. b, TNFR1 activation also indirectly promotes inflammation by triggering cell death. Lytic forms of cell death, such as apoptosis-driven secondary necrosis, pyroptosis and necroptosis, release damage-associated molecular patterns (DAMPs) that activate proinflammatory gene expression in bystander cells. In addition, the inflammatory response may originate from and/or be amplified by loss of barrier function caused by epithelial cell death (lytic and non-lytic) and the subsequent sensing of pathogen-associated molecular patterns (PAMPs) from microorganisms that have breached the epithelial barrier. PRR, pattern recognition receptor.
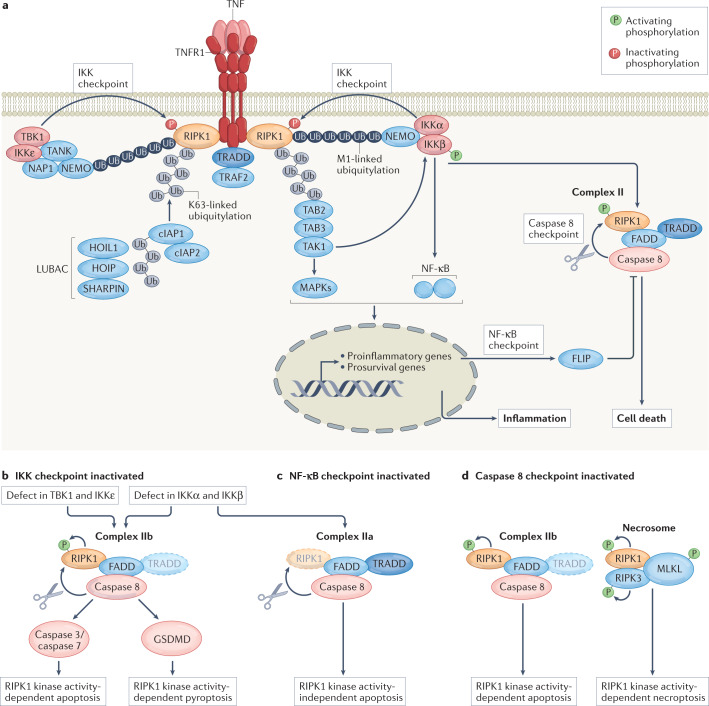
Death is not the default response of cells to TNF. Protectives brakes, or cell death checkpoints, normally actively repress TNF cytotoxicity to protect the organism from its potential detrimental consequences. Thus, while TNFR1 has the ability to trigger cell death, this response proceeds only when one of the cell death checkpoints is inactivated (Fig. 3). The survival versus death outcome of TNFR1 activation depends on the assembly of two distinct, but successive, protein complexes (Fig. 3) (reviewed in18,19). The membrane-bound complex I forms within seconds of TNF sensing, and predominantly leads to inflammatory gene activation. Assembly of complex I starts with the binding of RIPK1 and TNFR1-associated death domain protein (TRADD) to the cytosolic portion of the receptor, allowing the subsequent recruitment of TNFR-associated factor 2 (TRAF2) and of the ubiquitin ligases cellular inhibitor of apoptosis protein 1 (cIAP1), cIAP2 and the linear ubiquitin chain assembly complex (LUBAC; which is composed of HOIL1, HOIP and SHARPIN). Together, these E3 ligases generate a dense network of ubiquitin chains that permits further recruitment of the kinases that activate the MAPK signalling pathway and the canonical NF-κB signalling pathway. More precisely, the K63-linked ubiquitin chains generated by cIAP1 and cIAP2 act as binding stations for the adaptor proteins TGFβ-activated kinase 1-binding protein 2 (TAB2) and TAB3, which recruit the upstream kinase TGFβ-activated kinase 1 (TAK1) for MAPK signalling. In addition, the M1-linked (linear) ubiquitin chains generated by LUBAC are recognized by the adaptor protein NF-κB essential modulator (NEMO), which brings the kinases inhibitor of NF-κB kinase-α (IKKα), IKKβ, TANK-binding kinase 1 (TBK1) and IKKε to the receptor complex. The close proximity of TAK1 and IKKα and IKKβ on the hybrid K63/M1-linked ubiquitin chains then permits activation of IKKα–IKKβ by TAK1, and the subsequent IKKα–IKKβ-dependent activation of the canonical NF-κB pathway (reviewed in18,19) (Fig. 3). The ubiquitin network associated with complex I is negatively regulated by a subset of deubiquitylases, including A20, CYLD and OTULIN, which destabilize the signalling complex and restrict signalling to MAPKs and NF-κB (reviewed in20).
Fig. 3. Signalling by TNFR1 and overview of the three characterized cell death checkpoints in the TNFR1 pathway.
a, Sensing of tumour necrosis factor (TNF) by TNF receptor 1 (TNFR1) leads to the formation of a primary membrane-bound receptor signalling complex (complex I) that activates the mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signalling pathways, leading to proinflammatory gene expression. A secondary, potentially cytotoxic, cytosolic complex (complex II) originates from the dissociation of complex I components from the receptor, and from their association with FAS-associated death domain-containing protein (FADD) and caspase 8. Three cell death checkpoints actively repress TNF cytotoxicity. First, the inhibitor of nuclear factor-κB kinase (IKK) checkpoint consists of the inhibition of receptor-interacting serine/threonine protein kinase 1 (RIPK1) kinase activity through phosphorylation mediated by complexes of IKKα and IKKβ and TANK-binding kinase 1 (TBK1) and IKKε. Second, the NF-κB checkpoint, which consists of the NF-κB-dependent transcriptional upregulation of prosurvival genes (including the gene encoding FLICE-like inhibitory protein (FLIP)). Third, the caspase 8 checkpoint, which consists of RIPK1 inactivation by caspase 8-mediated cleavage. b, Inhibition of the IKK checkpoint leads to activation of RIPK1 in complex I, and the subsequent kinase-dependent assembly of complex IIb, which drives apoptosis or pyroptosis depending on the cellular context. Of note, conditions that affect proper IKKα–IKKβ activation will additionally inactivate the NF-κB checkpoint. c, Conditions leading to inactivation of the NF-κB checkpoint, such as the in vitro use of the translation inhibitor cycloheximide, activate complex IIa and result in RIPK1 kinase activity-independent apoptosis. d, Inhibition of the caspase 8 checkpoint induces RIPK1 cytotoxicity by the kinase-dependent assembly of complex IIb and the necrosome. TNF sensing in caspase 8-inhibited conditions will result only in necroptosis induction. Additional checkpoints may exist. cIAP, cellular inhibitor of apoptosis protein; GSDMD, gasdermin D; LUBAC, linear ubiquitin chain assembly complex; NAP1, NAK-associated protein 1; NEMO, NF-κB essential modulator; TAB, TGFβ-activated kinase 1-binding protein; TAK1, TGFβ-activated kinase 1; TANK, TRAF family member-associated NF-κB activator; TRADD, TNFR1-associated death domain protein; TRAF2, TNFR-associated factor 2; Ub, ubiquitin.
How TNFR1 signalling further evolves to induce cell death is less clear, but it requires the assembly of a secondary cytosolic complex, termed ‘complex II’, which originates from the binding of FAS-associated death domain-containing protein (FADD) to the receptor-dissociated complex I components TRADD and/or RIPK1 (ref.21). Complex II functions as a cytosolic platform for the binding and activation of caspase 8, which can process downstream effector caspases to induce apoptosis, or instead cleave gasdermin D (GSDMD) to trigger pyroptosis, as recently reported22–24. Complex II can further be defined as complex IIa or complex IIb to differentiate the complex that spontaneously assembles upon TNF sensing from the one that additionally forms upon RIPK1 enzymatic activation25,26 (Fig. 3). So far, two cell death checkpoints have been found to inhibit apoptosis induction by these death complexes. The first one (‘IKK checkpoint’) occurs at the level of the receptor, within complex I, and consists of the phosphorylation-dependent inactivation of RIPK1 by IKKα–IKKβ and TBK1–IKKε, thereby preventing complex IIb assembly and subsequent RIPK1 kinase activity-dependent apoptosis induction27–29 (Fig. 3). The fact that single inhibition of IKKα–IKKβ or TBK1–IKKε complexes suffices to switch the TNF response from survival to RIPK1 kinase activity-dependent apoptosis and the observation that the combined inactivation of these kinases further increases RIPK1 cytotoxicity suggest that IKKα–IKKβ and TBK1–IKKε inhibit distinct pools of RIPK1 in TNFR1 complex I. S25 of RIPK1 was identified as a substrate of IKKα–IKKβ, TBK1–IKKε and protein phosphatase 1 regulatory subunit 3G (PPP1R3G)27,28,30, and mimicking phosphorylation on that residue was demonstrated to inhibit RIPK1 activity and cytotoxicity31. However, preventing S25 phosphorylation of RIPK1 is not sufficient to activate RIPK1 by TNF, indicating that IKKα–IKKβ and TBK1–IKKε additionally regulate RIPK1 cytotoxicity independently of S25, possibly by phosphorylating RIPK1 on other residues or, alternatively, by phosphorylating other targets. The second cell death checkpoint (‘NF-κB checkpoint’) occurs downstream in the pathway, in the nucleus, and relies on the NF-κB-dependent transcriptional and translational upregulation of prosurvival proteins, such as FLICE-like inhibitory protein (FLIP; also known as CFLAR), which counteract caspase 8 activation in complex IIa and protect the cells from RIPK1 kinase activity-independent apoptosis25,32. Since IKKα and IKKβ are upstream kinases in the canonical NF-κB pathway, they control two successive checkpoints downstream of TNFR1, which respectively protect the cells from RIPK1 kinase activity-dependent apoptosis (IKK checkpoint) and RIPK1 kinase activity-independent apoptosis (NF-κB checkpoint) (Fig. 3). By contrast, TBK1 and IKKε repress RIPK1 activation only in complex I, and their inactivation consequently only switches the TNF response from survival to RIPK1 kinase activity-dependent cell death, without disturbing NF-κB28.
Activation of the kinases that control the two aforementioned cell death checkpoints is highly dependent on the ubiquitin network associated with complex I. Consequently, conditions that affect ubiquitylation of complex I, such as inhibition of cIAP1, cIAP2 and LUBAC33–40, and also mutations in the RIPK1 ubiquitin acceptor site K377 (K376 in mouse RIPK1)41–43 or deficiencies of A20 and OTULIN44–49, indirectly perturb these cell death checkpoints and activate TNF cytotoxicity. Of note, the inhibitory effect of ubiquitin on RIPK1 death signalling can be dissociated from its role in inducing NF-κB-mediated gene transcription18,41. Interestingly, although the two described cell death checkpoints are in place to restrain caspase 8 processing, a non-lethal pool of caspase 8 is still activated by TNF sensing, and functions as a third checkpoint in the pathway (the ‘caspase 8 checkpoint’), which prevents RIPK1 kinase activity-dependent apoptosis and necroptosis by cleaving RIPK1 (refs.50–54) (Fig. 3). What restrains this pool of activated caspase 8 from inducing cell death is currently unclear, but suggests the existence of additional protective mechanisms. Accordingly, inactivation of caspase 8 switches the TNF response to RIPK1 kinase activity-dependent necroptosis, which additionally requires recruitment of the kinase RIPK3 and of the potential pore-forming pseudo-kinase mixed lineage kinase domain-like protein (MLKL) to complex II, now called the ‘necrosome’. Association between RIPK3 and RIPK1 occurs via their RIP homotypic interaction motifs, and appears to be sufficient to activate RIPK3 within the necrosome. The phosphorylation of MLKL by RIPK3 then induces a conformational change in MLKL resulting in its oligomerization and translocation from the cytosol to the plasma membrane, where it induces cell death via unknown mechanisms. The enzymatic activity of RIPK1 is dispensable for complex I and complex IIa assembly, but is required for the formation of complex IIb and the necrosome. Depending on the cellular context, the catalytic activity of RIPK1 can therefore promote apoptosis, caspase 8-mediated pyroptosis or necroptosis downstream of TNFR1 (refs.19,23) (Fig. 3).
All three cell death checkpoints described so far were shown to be essential to prevent TNF-dependent embryonic lethality or severe inflammatory pathology in mice. Moreover, mutations that affect these checkpoints have been identified as the cause of autoinflammatory diseases in humans, further providing clinical relevance of these cell death checkpoints (see later). Of note, additional molecular mechanisms restraining TNF cytotoxicity have been reported, including the phosphorylation of RIPK1 by the MAPK-activated kinase MK2 (refs.55–57) or by the kinase Unc-51-like autophagy activating kinase 1 (ULK1)58, as well as the poly(ADP-ribosyl)ation of complex II by tankyrase 1 (ref.59). In contrast to the three cell death checkpoints described above, inactivation of these additional protective mechanisms does not switch the TNFR1 response from survival to death. It increases TNF cytotoxicity only in conditions of a previously compromised checkpoint, indicating that they do not regulate the most critical brake in the pathway but rather control additional layers of regulation, limiting the extent of cell death.
TNF-induced cell death in pathogen defence
Host–pathogen interactions are a major selective pressure acting on both organisms. While the host must adapt to survive infection by pathogens, pathogens must in turn develop mechanisms to avoid elimination by the host’s immune defences. This continuous pressure selects for multiple, layered and interconnected defence mechanisms in the host. Similarly, the pathogen has developed sophisticated strategies to evade host immunity by hijacking inflammatory signalling pathways or by blocking other antimicrobial defence mechanisms. The different TNFR1 cell death checkpoints appear to have evolved as a response of the host to this microbial hijacking. Indeed, TNF signalling aims to establish an inflammatory response, primarily by promoting inflammatory gene activation by the MAPK and NF-κB signalling pathways. Remarkably, the kinases that activate these signalling pathways are also the ones putting a break on TNF cytotoxicity. Consequently, when the pathogen tries to suppress inflammatory gene activation in the host by delivering virulence or effector factors that affect proper activation of these kinases, the cell will switch its response to induce cell death, thereby activating an alternative pathway to alert the immune system though the release of damage-associated molecular patterns. This is nicely illustrated in the context of infection by the mammalian pathogenic species of the Gram-negative genus Yersinia, which injects an acyltransferase, named ‘YopJ/P’, capable of inhibiting the catalytic activity of TAK1, IKKα and IKKβ in an attempt to escape host defences by preventing MAPK- and NF-κB-dependent expression of proinflammatory mediators60–63. As a consequence of this hijacking, RIPK1 is no longer blocked by MK2 and IKKα–IKKβ phosphorylation, and TNFR1-mediated and Toll-like receptor 4-mediated RIPK1 kinase activity-dependent and caspase 8-dependent apoptosis and/or pyroptosis are induced, releasing damage-associated molecular patterns to promote optimal antibacterial immunity22,23,31,56,64–66.
Cell death, in its multiple forms, is thus recognized as a host defence mechanism for the elimination of pathogens, stripping them of their replication niche and simultaneously alerting the immune system to kick in. As a consequence, microorganisms have developed multiple strategies to interfere with the different cell death pathways to avoid their eradication by the host (reviewed in67). However, host cells have, in turn, developed strategies to also cope with this by activating backup mechanisms. In this context, the TNFR1 cell death checkpoint that controls RIPK1 cleavage by caspase 8 appears to have evolved to ensure activation of necroptosis as a backup cell death mode when the apoptotic pathway is blocked by pathogenic caspase 8 inhibitors, such as the poxvirus-encoded serpin CrmA68 or the viral FLICE-inhibitory protein (vFLIP) identified in herpesviruses and in the human poxvirus Molluscum contagiosum virus69,70. As a response, several pathogens also express proteins that specifically target necroptosis by inhibiting RIPK1, RIPK3, MLKL or the effects downstream of MLKL67.
Cell death by TNF is, however, not always a beneficial response for the host. At least in some specific context, excessive activation of TNF-mediated cell death is indeed reported to drive, rather than prevent, microorganism pathogenicity and lethality, as seen upon infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), Mycobacterium tuberculosis and Bacillus anthracis71–74.
TNF-induced cell death in inflammatory disease
Although TNF-induced cell death can help to mount proper immune responses during microbial infection, it can also turn into a highly detrimental process at the origin of various (sterile) inflammatory diseases when aberrantly induced as a result of environmental factors and/or genetic mutations. It is now clear that TNF contributes to the pathogenesis of inflammatory disease not only by inducing expression of inflammatory mediators but also by triggering cell death. For instance, TNF induces a lethal septic shock in mice that is caused by RIPK1 kinase activity-dependent cell death, as genetic or pharmacological inhibition of RIPK1 enzymatic activity fully protects the mice from the cytokine storm, hypothermia and morbidity induced by TNF75–77. In this model, the triggering event was first believed to be necroptosis, but later studies suggested additional contribution of RIPK1 kinase activity-dependent apoptosis and pyroptosis. Indeed, caspase 8 heterozygosity was reported to partially rescue hypothermia, and GSDMD loss was reported to limit lethality23,78. The reason why TNF induces RIPK1 kinase activity-dependent cell death in vivo while most cells do not succumb to single TNF stimulation in vitro is not fully understood, but indicates that the in vivo inflammatory context somehow affects RIPK1 cell death checkpoints. It is tempting to speculate that the cytotoxicity originates from the co-sensing of multiple cytokines, which are provided by the inflammatory context. Indeed, a subclass of TNF family ligands, which includes CD40, TNF-like weak inducer of apoptosis (TWEAK) and lymphotoxin-β, activates the non-canonical NF-κB pathway upon binding of the ligands to their cognate receptors. Activation of this pathway involves the ligand-dependent degradation of a pool of TRAF2–TRAF3 and cIAP1–cIAP2, resulting in stabilization of NF-κB-inducing kinase (NIK), and finally in the activation of IKKα by NIK-mediated phosphorylation79. While single stimulation of cells with TNF or one of these additional ligands is mostly not toxic to cells, their combination may instead result in TNFR1-induced RIPK1 kinase activity-dependent and RIPK1 kinase activity-independent apoptosis or pyroptosis due to partial cIAP1 and cIAP2 degradation, affecting proper ubiquitylation of complex I, which consequently indirectly inactivates two of the aforementioned cell death checkpoints in the TNFR1 pathway55,80. In line with this idea, it is interesting to note that TWEAK and CD40L are upregulated in inflammatory bowel disease (IBD) and rheumatoid arthritis81,82, two TNF-driven human diseases for which RIPK1 inhibitors may be promising.
Of note, binding of TNF to TNFR2 also activates the non-canonical NF-κB pathway. Consequently, co-sensing of TNF by TNFR1 and TNFR2 also has the potential to switch the TNFR1 response from survival to RIPK1 kinase activity-dependent death. It is therefore possible that part of the discrepancy between the in vitro cytotoxic response and the in vivo cytotoxic response to TNF originates from difference in TNFR2 expression levels, or in the expression of membrane-bound TNF versus soluble TNF, as the latter is relatively poor at activating TNFR2. In addition to ligands of the TNF family, the co-sensing of TNF and interferon-γ was also recently reported to induce RIPK1 kinase activity-dependent cell death (apoptosis, pyroptosis and necroptosis) by activating the JAK–STAT1–IRF1 axis71. However, it remains unclear whether, and how, activation of this pathway affects the known cell death checkpoints downstream of TNFR1. Interestingly, the combination of neutralizing antibodies to TNF and interferon-γ was shown to protect mice from death during SARS-CoV-2 infection71, which may support the reported causative role of pyroptosis in hyperinflammation during severe COVID-19 (refs.83–85).
Mutations that either directly or indirectly inactivate some of the cell death checkpoints within the TNFR1 pathway are also sufficient to cause mouse and human inflammatory diseases, as highlighted by some examples in the non-exhaustive list of studies mentioned below (Tables 1 and 2). The in vivo inflammatory consequence of inactivating the caspase 8 checkpoint that prevents RIPK1-dependent cytotoxicity was initially revealed by genetic studies in mice that lack caspase 8 or FADD. Genetic full-body deletion of Casp8 or Fadd in mice results in embryonic lethality86,87, which can be rescued to birth by deletion of Ripk1 and to adulthood by deletion of Ripk3 or Mlkl88–91. Also, specific deletion of Casp8 or Fadd in the intestinal epithelium leads to the development of a severe intestinal pathology that is TNF dependent and rescued by RIPK3 or MLKL deficiency or by inhibition of RIPK1 kinase activity, providing evidence that intestinal inflammation results from necroptosis of FADD- or caspase 8-deficient intestinal epithelial cells (IECs)24,92–94. High levels of RIPK3 and increased necroptosis could be confirmed in the intestine of patients with Crohn’s disease93, and mutations in CASP8 have been identified in patients who develop autoimmune lymphoproliferative syndrome and also in patients with severe forms of very early onset IBD95,96. In IBD, aberrant cell death leads to impairment of the epithelial barrier and invasion of the underlying tissues by the microbiota, promoting inflammation. Deficiency of the adaptor protein myeloid differentiation primary response protein 88 (MyD88) and antibiotic treatment were shown to prevent colon inflammation in IEC-specific FADD-deficient mice, demonstrating that bacterially mediated Toll-like receptor activation by intestinal bacteria is essential for disease pathogenesis92. Follow-up studies in mice revealed that FADD prevents intestinal inflammation not only by inhibiting necroptosis but also by inhibiting caspase 8–GSDMD-mediated pyroptosis of epithelial cells24. How FADD simultaneously promotes and inhibits caspase 8 to respectively inhibit necroptosis but promote pyroptosis is currently unclear. Inducible deletion of Casp8 in the endothelium of 6-week-old mice causes fatal haemorrhagic lesions exclusively within the small intestine driven by microbial commensals and TNF. This phenotype is prevented in mice that lack MLKL, confirming that the haemorrhage is caused by unrestrained necroptosis in the small intestine97. Deficiency of FADD or caspase 8 in keratinocytes causes keratinocyte necroptosis and severe skin inflammation in mice, which is prevented by RIPK3 loss and is partly dependent on TNF94,98,99. Since RIPK3 also contributes to TNF-induced caspase 8 activation100, additional studies in MLKL-deficient mice will be required to formally demonstrate that keratinocyte necroptosis drives the inflammatory skin phenotype in these mice. Keratinocyte-specific RIPK1 deficiency also causes keratinocyte necroptosis and skin inflammation, which is only partially rescued in a TNFR1-deficient background, but is completely prevented by Ripk3 or Mlkl deficiency101,102. As genetic targeting of RIPK1 kinase activity does not lead to any overt phenotype, these results identify RIPK1 scaffold function as an inhibitor of RIPK3–MLKL-dependent necroptosis in keratinocytes.
Table 1.
A selection of studies in mouse models demonstrating that inflammation results from unrestrained cell death
| Genotype | Phenotype | Expected inactivated CDC | Rescue background | Refs. |
|---|---|---|---|---|
| Casp8−/− | Embryonic lethality | Caspase 8 checkpoint |
Ripk3−/− Mlkl−/− |
88,89,91 |
| Casp8IEC-KO | Severe intestinal pathology; enterocyte necroptosis |
Ripk3−/− Mlkl−/− Tnfr1IEC-KO (colon) Ripk1D138N/D138N |
24,93,94 | |
| Cdh5–CreERT2 Casp8fl/fl | Fatal necroptotic haemorrhage in the small intestine |
Mlkl−/− Tnf−/− LPS-Rs administration |
97 | |
| Casp8E-KO | Severe skin inflammation; keratinocyte necroptosis |
Ripk3−/− Tnf−/− (partial rescue) |
94,98 | |
| Fadd−/− | Embryonic lethality | Caspase 8 checkpoint |
Ripk1−/− Mlkl−/− |
90,91 |
| FaddIEC-KO | Severe intestinal pathology; enterocyte necroptosis and pyroptosis |
Ripk3−/− Mlkl−/− (colon) Tnf−/− (colon) Tnfr1IEC-KO (colon) Ripk1D138N/D138N Mlkl−/−Gsdmd−/− |
24,92 | |
| FaddE-KO | Severe skin inflammation; keratinocyte necroptosis |
Ripk3−/− Tnf−/− (partial rescue) Tnfr1−/− (partial rescue) |
99 | |
| Ripk1D325A/D325A | Embryonic lethality | Caspase 8 checkpoint |
Tnfr1−/− Ripk3−/−Casp8−/− Mlkl−/−Fadd−/− Ripk3−/−Fadd−/− Ripk1D138N/D138N |
51,52,54 |
| Ciap2−/−Ciap1−/− | Embryonic lethality |
IKK checkpoint NF-κB checkpoint |
Tnfr1−/− Mlkl−/−Casp8−/− |
35,106 |
| CreERT2Ciap1−/− Ciap2fl/fl | Lethal upon tamoxifen injection |
Tnfr1−/− Ripk3−/−Casp8−/− |
106 | |
| Xiap1−/− | Ileal inflammation | Unknown |
Tnf−/− Tnfr1−/− Tnfr2−/− Ripk3−/− |
109 |
| Sharpincpdm/cpdm | Chronic proliferative dermatitis (inflammatory skin lesions, multi-organ inflammation) | IKK checkpoint |
Tnf−/− Ripk1K45A/K45A Tnfr1−/− Ripk3−/−Casp8+/− Tnfr1E-KO (skin) FaddE-KORipk3−/− (skin) TraddE-KORipk3−/− (skin) Mlkl−/−Casp8−/− (skin) |
38,39,77,112–115 |
| Hoip−/− | Embryonic lethality |
IKK checkpoint NF-κB checkpoint |
Tnfr1−/− (partial rescue) Mlkl−/−Casp8−/− |
38,40 |
| HoipE-KO | Severe skin inflammation; keratinocyte necroptosis |
Tnfr1−/− (partial rescue) Mlkl−/−Casp8−/− Tnfr1−/−Mlkl−/− |
37 | |
| Hoil1−/− | Embryonic lethality |
IKK checkpoint NF-κB checkpoint |
Tnfr1−/− (partial rescue) Mlkl−/−Casp8−/− Ripk3−/−Casp8−/− (partial) Ripk3−/−Casp8−/−Ripk1−/− |
38 |
| Hoil1E-KO | Severe skin inflammation; keratinocyte necroptosis |
Tnfr1−/− (partial rescue) Ripk3−/−Casp8−/− |
37 | |
|
OtulinC129A OtulinL272P |
Embryonic lethality |
IKK checkpoint NF-κB checkpoint |
Tnfr1−/− (partial rescue) Ripk3−/−Casp8−/− Ripk1D138N/D138N (partial rescue) |
49,117 |
| OtulinE-KO | Severe skin inflammation; keratinocyte necroptosis |
Tnfr1−/− Tnfr1E-KO Ripk1D138N/D138N Ripk3−/− (partial rescue) Mlkl−/− (partial rescue) Ripk3−/−Fadd−/− Fadd/MlklE-KO |
117,118 | |
| Ikbkg−/− | Embryonic lethality |
IKK checkpoint NF-κB checkpoint |
– | 142 |
| IkbkgE-KO | Severe skin inflammation; keratinocyte cell death | Tnfr1−/− | 146 | |
| IkbkgIEC-KO | Severe intestinal pathology; enterocyte apoptosis |
Tnfr1−/− Tnfr1E-KO FaddIEC-KORipk3−/− Ripk3−/− (partial rescue) Ripk1D138N/D138N |
153,154 | |
| Ikkb−/− | Embryonic lethality |
IKK checkpoint NF-κB checkpoint |
– | 140 |
| IkkbE-KO | Severe skin inflammation; keratinocyte cell death |
Tnfr1−/− Tnfr1E-KO Ripk3−/− (partial rescue) Ripk3E-KO (partial rescue) Mlkl−/− (partial rescue) FaddE-KORipk3−/− Ripk1D138N/D138N |
150,151 | |
| Ikka−/−Ikkb−/− | Embryonic lethality |
IKK checkpoint NF-κB checkpoint |
– | 141 |
| Ikka/IkkbIEC-KO | Severe intestinal pathology; enterocyte apoptosis | 153 | ||
| Ripk1K376R/K376R | Embryonic lethality | IKK checkpoint |
Mlkl−/−Fadd−/− Ripk3−/−Fadd−/− Ripk3−/−Casp8−/− Tnfr1−/− (partial rescue) Tnfr1−/−Ripk3−/− |
42,43 |
| Tbk1−/− | Embryonic lethality (C57BL/6 background) | IKK checkpoint |
Ripk1D138N/+ Ripk1D138N/D138N Ripk3−/− (partial rescue) |
29,155 |
| Viable (129 background), spontaneous inflammation in multiple tissues | 156 | |||
| Rela+/− | Cutaneous ulceration from TNF exposure; severe dextran sodium sulfate-induced colitis | NF-κB checkpoint | Anti-TNF antibodies | 152 |
| Cflar−/− | Embryonic lethality | NF-κB checkpoint | Ripk3−/−Fadd−/− | 158,159 |
| CflarIEC-KO | Severe intestinal pathology; enterocyte death | Tnfr1−/− (partial rescue) | 94,160,161 | |
| CreERT2CflarE-KO | Severe skin inflammation; keratinocyte apoptosis | Anti-TNF antibodies (partial rescue) | 94,162 | |
| Ripk1IEC-KO | Severe intestinal pathology; enterocyte apoptosis |
NF-κB checkpoint Unknown checkpoint |
Tnfr1−/− (partial rescue) Casp8−/− (colon) FaddIEC-KO (partial rescue) FaddIEC-KO Ripk3−/− |
101,166 |
| Ripk1E-KO | Severe skin inflammation; keratinocyte necroptosis |
Tnfr1−/− (partial rescue) Ripk3−/− Mlkl−/− |
101,102 |
Cflar, gene encoding FLICE-like inhibitory protein (FLIP); CDC, cell death checkpoint; CreERT2, tamoxifen-inducible Cre expression; E-KO, epidermis-specific knockout; IEC-KO, intestinal epithelial cell-specific knockout; Ikbkg, gene encoding nuclear factor-κB essential modulator (NEMO); IKK, inhibitor of nuclear factor-κB kinase; LPS-Rs, lipopolysaccharide from Rhodobacter sphaeroides, which acts as an inhibitor of Toll-like receptor 4 signalling; NF-κB, nuclear factor-κB; TNF, tumour necrosis factor.
Table 2.
Autoinflammatory diseases caused by mutations in genes encoding essential TNF signalling proteins
| Gene symbol | Protein name | Disease mechanism | Patient phenotype | OMIM entry | Refs. |
|---|---|---|---|---|---|
| CASP8 | Caspase 8 | Homozygous, loss of function | Autoimmune lymphoproliferative syndrome; very early onset IBD | 607271 | 95,96 |
| IKBKG | NEMO | Loss of function | Incontinentia pigmenti in heterozygous females (lethal in males); immunodeficiency; EDA-ID | 308300 | 147 |
| IKBKG | NEMO | Carboxy-terminal deletions in NEMO | Inflammatory skin and intestinal disease; ectodermal dysplasia with anhidrosis and immunodeficiency | NA | 139 |
| IKBKG | NEMO | NEMO lacking the domain encoded by exon 5 | Severe autoinflammatory syndrome; NDAS | 301081 | 148,149 |
| OTULIN | OTULIN | Homozygous, loss of function | Early-onset recurrent fever; neutrophilic dermatitis/panniculitis, joint swelling; ORAS | 617099 | 122–125 |
| OTULIN | OTULIN | Compound heterozygous variants | Atypical late-onset ORAS | NA | 126 |
| RBCK1 | HOIL1 | Homozygous, loss of function | Severe multi-organ autoinflammation, immunodeficiency, invasive bacterial infections, muscular amylopectinosis | 615895 | 120 |
| RELA | RelA | Haploinsufficiency | Fever, abdominal pain, mucocutaneous lesions | 618287 | 152 |
| RIPK1 | RIPK1 | Homozygous, loss of function | Recurrent infections, early-onset IBD, progressive polyarthritis, immunodeficiency | 618108 | 168,169 |
| RIPK1 | RIPK1 | Heterozygous mutation of the RIPK1 caspase 8 cleavage site | Early-onset periodic fever syndrome and lymphadenopathy; CRIA | 618852 | 52,53,103 |
| RNF31 | HOIP | Homozygous, loss of function | Severe multi-organ autoinflammation, immunodeficiency | NA | 119,121 |
| TBK1 | TBK1 | Homozygous, loss of function | Chronic and systemic autoinflammation | NA | 157 |
| TNFAIP3 | A20 | Haploinsufficiency | Early-onset severe multiorgan autoinflammatory syndrome; HA20 | 616744 | 137,138 |
| XIAP | XIAP | Loss of function | Pathogen-associated hyperinflammation, fever, severe IBD; XLP2 | 300635 | 107 |
Details of the genetic disorders can be found in OMIM. CRIA, cleavage-resistant receptor-interacting serine/threonine protein kinase 1 (RIPK1)-induced autoinflammatory syndrome; EDA-ID, anhidrotic ectodermal dysplasia with immune deficiency; HA20, haploinsufficiency of A20; IBD, inflammatory bowel disease; NA, not available; ORAS, OTULIN-related autoinflammatory syndrome; NDAS, nuclear factor-κB essential modulator (NEMO) deleted exon 5–autoinflammatory syndrome; TBK1, TANK-binding kinase 1; XIAP, X-linked inhibitor of apoptosis protein; XLP2, X-linked lymphoproliferative syndrome 2.
More recent studies specifically targeted the caspase 8 checkpoint by the generation of mice expressing a caspase 8 cleavage-resistant variant of RIPK1 (Ripk1D325A). The mutation induces embryonic lethality in mice, which is prevented by loss of RIPK1 kinase activity, loss of TNFR1 or loss of both MLKL (or RIPK3) and FADD (or caspase 8), but not by loss of MLKL or RIPK3 alone, confirming combined induction of apoptosis and necroptosis51,52,54. Importantly, patients with pathogenic mutations in RIPK1 that prevent caspase 8 cleavage were also identified, and were shown to experience early-onset autoinflammatory disease, so-called cleavage-resistant RIPK1-induced autoinflammatory syndrome, which is caused by hypersensitivity of patients’ cells to RIPK1 activation, apoptosis and necroptosis52,53,103.
By serving as anchoring sites for the kinases IKKα, IKKβ, IKKε and TBK1, the ubiquitin chains conjugated to TNFR1 complex I by cIAP1, cIAP2 and LUBAC indirectly control the two checkpoints that counteract caspase 8-dependent cell death, either in an RIPK1 kinase activity-dependent manner or in an RIPK1 kinase activity-independent manner (Fig. 3). Consequently, mutations that affect proper regulation of ubiquitin signalling can trigger aberrant TNF-mediated cell death and result in inflammatory disorders (reviewed in104,105). This is, for instance, the case upon deletion of cIAP1 and cIAP2, which results in embryonic lethality caused by TNFR1 signalling35. Further studies demonstrated that deletion of cIAP1 and cIAP2 in adult mice causes inflammation and lethality by the release of a brake on caspase 8-dependent cell death106. Deficiency in ubiquitin ligase X-linked inhibitor of apoptosis protein (XIAP) is the cause of X-linked lymphoproliferative syndrome 2, a severe inflammatory disease107. With use of gene-targeted mice, XIAP was shown to prevent TNF- and RIPK3-dependent cell death by regulating ubiquitylation of RIPK1, which might explain the hyperinflammation in patients with X-linked lymphoproliferative syndrome 2 (refs.108–110).
The notion that linear ubiquitin chains protect against cell death-driven inflammation is supported by the phenotypes of LUBAC- and OTULIN-deficient mice. Mutation in Sharpin, in so-called Cpdm mice, causes chronic proliferative dermatitis characterized by inflammatory skin lesions, multi-organ inflammation and immune system dysregulation, which is fully caused by TNF-mediated RIPK1 kinase activity-dependent cell death39,77,111–115. Cpdm mice lacking RIPK3 or MLKL show a delayed onset of the dermatitis and only a partial amelioration of the multi-organ pathology, indicating a contribution of necroptosis to the phenotype. However, epidermal deletion of FADD together with deficiency in RIPK3 completely prevented keratinocyte death and skin inflammation, demonstrating that FADD-mediated apoptosis of keratinocytes is the driver of skin inflammation in Cpdm mice39,115. Importantly, genetic ablation of MyD88 or depletion of the microbiota by antibiotics rescued the skin inflammation in Cpdm mice, demonstrating that the death of keratinocytes affects barrier integrity and induces inflammatory skin disease through the sensing of pathogen-associated molecular patterns from microorganisms that have breached the barrier116. Mutations in Hoip (also known as Rnf31) or Hoil1 (also known as Rbck1) lead to embryonic lethality in mice which is partially dependent on TNFR1 and RIPK1 enzymatic activity but is prevented by co-deletion of Casp8 or Mlkl, but not Ripk3 (refs.38,40). Also knock-in mice that express catalytically inactive OTULIN (C129A) or a hypomorphic L272P mutation die at midgestation as a result of cell death mediated by TNFR1 and RIPK1 kinase activity, and these mice can be rescued by the combined loss of caspase 8 and RIPK3 expression49,117. Studies in tissue-specific LUBAC- and OTULIN-deficient mice further confirmed TNF- and RIPK1 kinase activity-mediated cell death as a driver of inflammatory pathology37,117,118. Homozygous loss-of-function mutations in HOIP, HOIL1 and OTULIN have been identified in humans. These mutations are rare and cause a neonatal potentially fatal multi-organ autoinflammatory condition119–125. Most patients with OTULIN-related autoinflammatory syndrome (ORAS; also known as otulipenia) are successfully treated with TNF-blocking agents, identifying TNF as the main driver of the autoinflammatory condition122–124. Recently, two new compound heterozygous variants in OTULIN were identified in a patient who developed a fulminant atypical late-onset ORAS, which differs clinically from classical ORAS, but is also triggered by perturbed TNF signalling126.
The role of M1-linked ubiquitylation in preventing cell death driven-inflammation is further demonstrated by mutations affecting the protein A20. The anti-inflammatory properties of A20 are commonly attributed to its ability to suppress inflammatory NF-κB signalling, but gene-targeting studies in mice have demonstrated that A20 primarily suppresses inflammation by preventing cell death45,47,48,127–133. In the TNFR1 pathway, A20 represses RIPK1 kinase activity-dependent and RIPK1 kinase activity-independent cell death induction by binding and stabilizing the M1-linked ubiquitin chains associated with TNFR1 complex I (refs.44,45,48,134). Single-nucleotide polymorphisms in TNFAIP3, the gene encoding A20, have been linked to many inflammatory and autoimmune diseases135,136. Importantly, rare heterozygous loss-of-function variants have been shown to cause a severe autoinflammatory syndrome, named ‘HA20’ (haploinsufficiency of A20)137,138, which can be treated in most patients with cytokine inhibitors, including infliximab137,138. Patients with carboxy-terminal deletions in NEMO, which impair interactions with A20, also develop an autoinflammatory phenotype that resembles HA20 (refs.139).
Binding of NEMO to the M1-linked ubiquitin chains associated with complex I permits the recruitment and activation of the kinases IKKα, IKKβ, IKKε and TBK1 to TNFR1 complex I. While these kinases prevent RIPK1 kinase activity-dependent apoptosis and pyroptosis by phosphorylating RIPK1, IKKα–IKKβ additionally repress RIPK1 kinase activity-independent apoptosis through the NF-κB-dependent expression of prosurvival molecules, including FLIP (Fig. 3). Disruption of the genes encoding NEMO (Ikbkg), IKKα (Ikka; also known as Chuk) and/or IKKβ (Ikkb; also known as Ikbkb) in mice results in early lethality with massive cellular death in several organs, such as the liver, the skin and, in the case of mice lacking IKKα and IKKβ, the nervous system140–145. Moreover, specific loss of NEMO in keratinocytes causes severe and lethal skin inflammation in mice that requires TNF146. In humans, NEMO deficiency causes embryonic lethality in males and incontinentia pigmenti in heterozygous females, a genetic disease characterized by development of skin lesions among other symptoms147. Also patients with NEMO deleted exon 5–autoinflammatory syndrome have recently been described. In contrast to patients with loss-of-function NEMO mutations who exhibit immunodeficiency, patients with the NEMO spliced mutant develop a severe autoinflammatory disease involving uveitis, panniculitis and hepatitis148,149. TNF also causes skin inflammation in mice with epidermis-specific knockout of Ikkb or both Rela and Rel (which encode NF-κB subunits) by inducing RIPK1 kinase activity-dependent necroptosis of keratinocytes150,151. In humans, a heterozygous mutation in RELA, causing RelA haploinsufficiency, induces chronic mucocutaneous ulceration, which can be suppressed by anti-TNF therapy152. Fibroblasts from such patients have impaired NF-κB activation and exhibit increased cell death in response to TNF. Similarly, Rela heterozygous mice show impaired NF-κB activation, develop cutaneous ulceration from TNF exposure and exhibit severe gastrointestinal inflammation upon exposure to dextran sodium sulfate, which is suppressed by TNF inhibition152. NEMO deficiency in IECs triggers intestinal pathology caused by TNF-induced apoptosis153. Inhibition of RIPK1 kinase activity or combined deficiency of FADD and RIPK3 prevents IEC death and colitis development in these mice, suggesting that RIPK1 inhibitors could be useful for the treatment of colitis in patients with NEMO mutations and possibly in IBD154. However, it remains puzzling that inactivation of the kinase activity of RIPK1 is sufficient to fully prevent pathology in these mice. Indeed, with a defect in NF-κB activation, the IECs should still be sensitized to RIPK1 kinase activity-independent apoptosis.
According to the specific role of TBK1 in repressing RIPK1 kinase activity in the TNFR1 pathway, biallelic loss of Tbk1 is embryonic lethal, and viability is rescued by TNF deficiency or by complementation with kinase-inactive RIPK1 (refs.28,29,155). Interestingly, loss of Tbk1 in mice with a 129 genetic background was reported to be viable, but was shown to cause inflammation in multiple tissues156. Transferring this allele onto the C57BL/6 background, however, also resulted in embryonic lethality156. In agreement, biallelic loss-of-function mutations in TBK1 cause an early-onset autoinflammatory syndrome in humans that was shown to depend on TNF and RIPK1 kinase activity-dependent cell death. Hence, autoinflammation in these patients is suppressed with anti-TNF therapy157.
FLIP plays a central role in NF-κB-dependent cell survival, as shown by the phenotypes of FLIP-deficient mice. Genetic deletion of Cflar (which encodes FLIP) results in embryonic lethality, due to a defect in the vascular development of the yolk sac158, and combined deletion of Fadd and Ripk3 is required for prevention of the lethal phenotype of FLIP-deficient mice159. IEC-specific FLIP-deficient mice develop severe colitis due to IEC apoptosis and necroptosis, which can be suppressed by loss of TNFR1 or by treatment with neutralizing anti-TNF antibodies94,160,161. Postnatal deletion of Cflar in keratinocytes induces severe skin inflammation in mice due to TNF-dependent keratinocyte apoptosis94,162. Interestingly, loss of FLIP expression in skin epidermis could be shown in patients with different skin diseases associated with epidermal cell apoptosis162.
Finally, full-body Ripk1 ablation causes postnatal lethality which is rescued by caspase 8 and RIPK3 deficiency, demonstrating a key function for RIPK1 in inhibiting cell death and subsequent inflammation163–165. RIPK1 deficiency in IECs in mice induces a severe pathology caused by TNF-mediated IEC apoptosis101,166. Whereas RIPK1 contributes to the NF-κB-dependent checkpoint by serving as a ubiquitylated substrate for NEMO recruitment, in vitro studies suggest a more prominent anti-apoptotic role of RIPK1 through another, and yet to be discovered, additional cell death checkpoint in the TNF pathway167. In agreement, patients with RIPK1 deficiency experience inflammatory diseases, including IBD168,169.
Perspective: cell death-blocking drugs
Although experimental studies in mice genetically altered to lack (or express mutant versions of) essential proteins of the apoptotic, necroptotic and pyroptotic apparatus have provided formal proof of the concept that aberrant cell death could instigate inflammatory disease development, functional validation using specific inhibitors will be required to establish the importance of proinflammatory cell death for the pathogenesis of human diseases. RIPK1 and RIPK3 inhibitors, as well as GSDMD inhibitors, are currently under investigation as potential therapies for human inflammatory diseases. Such inhibitors may become an alternative treatment for patients with autoimmune diseases, especially for those patients who do not respond to or show adverse effects of anti-TNF treatment. Indeed, one third of patients with rheumatoid arthritis will discontinue treatment with an anti-TNF drug in the first year of therapy, mostly because of inefficacy or adverse events170, and similar efficacy profiles have been shown in patients with IBD and psoriasis171,172.
RIPK1 has a unique hydrophobic pocket that allosterically regulates its kinase activity, which enabled the development of small-molecule kinase inhibitors that dock into that pocket173,174. Some of these RIPK1-targeting compounds have entered clinical trials for the treatment of inflammatory disorders, such as ulcerative colitis, psoriasis and rheumatoid arthritis (reviewed in16). Also blood–brain barrier-permeant RIPK1 inhibitors have entered clinical trials for the treatment of neurodegenerative diseases including amyotrophic lateral sclerosis, Alzheimer disease and multiple sclerosis (reviewed in17). However, the first results from two such trials using the RIPK1 inhibitor GSK2982772 did not show clinical efficacy in a small group of patients with ulcerative colitis or rheumatoid arthritis175,176. One explanation for this could be the lack of proper patient stratification, highlighting the need to stratify patients on the basis of detection of specific markers. In this respect, antibodies targeting RIPK1 phosphorylation at S166 and phosphorylated MLKL may become useful. However, the requirement of RIPK1 enzymatic activity for TNF-induced cell death may also be different between mice and humans, raising the important question of the exact function of RIPK1 kinase activity, as no lethal substrate apart from RIPK1 has been identified so far. RIPK3 kinase inhibitors are also being considered for the treatment of inflammatory diseases, but so far no such inhibitors have been selected for therapeutic development, mainly because of the surprising observation that such compounds may assemble a caspase 8–FADD–RIPK1 complex that induces apoptotic cell death177.
As described earlier herein, TNF can trigger caspase 8-dependent GSDMD cleavage22–24. Since the discovery of GSDMD as a central mediator of pyroptosis178,179, GSDMD inhibition has been proposed as a novel therapeutic strategy to prevent inflammatory pathology in different diseases (reviewed in180). Disulfiram (Antabuse), a US Food and Drug Administration (FDA)-approved drug used to treat alcohol addiction, was shown to inhibit pyroptosis and inflammatory cytokine secretion in human and mouse cells, and in mouse models of LPS-induced septic shock181 and multiple sclerosis182. Necrosulfonamide was shown to be efficacious in sepsis183, and dimethyl fumarate was reported to suppress pathology in mouse models of familial Mediterranean fever, sepsis and multiple sclerosis184. All three currently available GSDMD inhibitors (disulfiram, necrosulfonamide and dimethyl fumarate) covalently modify reactive cysteines and hence are not specific, and specific small-molecular inhibitors of GSDMD will need to be discovered. As secondary necrosis–pyroptosis can also be induced via caspase 3-mediated cleavage of GSDME185,186, and via caspase 8-induced cleavage of GSDMC187, other GSDM inhibitors need to be considered.
Future research should also investigate whether ninjurin 1 inhibition could be beneficial in suppressing TNF-mediated inflammation. Indeed, a recent study revealed that plasma membrane rupture, a common feature of pyroptotic and necroptotic cell death, but also happening during secondary necrosis of apoptotic cells that are not engulfed and removed in a timely manner, is actively regulated and mediated by ninjurin 1 (ref.188). Proof-of-principle studies have already demonstrated that an antibody targeting the amino-terminal extracellular region of ninjurin 1, which is shown to be critical for its cytotoxicity, impairs plasma membrane rupture in pyroptotic macrophages188.
Finally, preclinical studies in mice have also made clear that the different cell death signalling pathways do not operate in isolation but are highly interconnected whereby intervention in one module may be unable to confer protection but instead may engage a backup cell death pathway. This intimate crosstalk between cell death pathways may ultimately compromise the use of single inhibitory drugs and may require multiple agents to simultaneously inhibit multiple cell death modules or to target central signalling hubs.
Acknowledgements
The G.v.L. laboratory is supported by the Vlaams Instituut voor Biotechnologie, by Ghent University (BOF) and by grants from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (G090322N, G026520N, G012618N and EOS-G0H2522N), the Charcot Foundation, the Belgian Foundation against Cancer and the FOREUM Foundation for Research in Rheumatology. Research in the laboratory of M.J.M.B. is supported by the Vlaams Instituut voor Biotechnologie, by Ghent University (iBOF ATLANTIS) and by grants from the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (G035320N, G044518N, EOS G0G6618N and EOS G0I5722N) and the Flemish Government (Methusalem BOF16/MET_V/007 to P. Vandenabeele).
Glossary
- Necroptosis
A programmed form of necrosis whose execution relies on the activation of receptor-interacting serine/threonine protein kinase 3 (RIPK3) and subsequent phosphorylation of mixed lineage kinase domain-like protein (MLKL) by RIPK3, ultimately leading to the translocation of phosphorylated MLKL to the plasma membrane, where it either directly or indirectly causes plasma membrane rupture.
- Pyroptosis
A highly inflammatory form of programmed necrosis, usually caused by microbial infection, that relies on the proteolytic activation of the pore-forming molecule gasdermin D by caspase 1 and caspase 11 (or caspase 8). It is classically activated downstream of the inflammasome pathways and is associated with the release of biologically active IL-1β and IL-18.
- Secondary necrosis
A lytic, or necrotic, form of cell death that occurs when apoptotic cells are not efficiently removed by efferocytosis. It involves proteolytic activation of the pore-forming molecule gasdermin E by the effector caspase 3.
Author contributions
The authors contributed equally to all aspects of the article.
Peer review
Peer review information
Nature Reviews Immunology thanks I. Aksentijevich and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
OMIM: www.omim.org
Contributor Information
Geert van Loo, Email: geert.vanloo@irc.vib-ugent.be.
Mathieu J. M. Bertrand, Email: mathieu.bertrand@irc.vib-ugent.be
References
- 1.Anderton H, Wicks IP, Silke J. Cell death in chronic inflammation: breaking the cycle to treat rheumatic disease. Nat. Rev. Rheumatol. 2020;16:496–513. doi: 10.1038/s41584-020-0455-8. [DOI] [PubMed] [Google Scholar]
- 2.Carswell EA, et al. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl Acad. Sci. USA. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas: with a report of ten original cases. Am. J. Med. Sci. 1893;105:487–511. doi: 10.1097/00000441-189305000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus) Proc. R. Soc. Med. 1910;3:1–48. doi: 10.1177/003591571000301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shear MJ, Perrault A. Chemical treatment of tumors. IX. Reactions of mice with primary subcutaneous tumors to injection of a hemorrhage-producing bacterial polysaccharide. J. Natl Cancer Inst. 1944;44:461–476. [Google Scholar]
- 6.Pennica D, et al. Human tumour necrosis factor: precursor structure, expression and homology to lymphotoxin. Nature. 1984;312:724–729. doi: 10.1038/312724a0. [DOI] [PubMed] [Google Scholar]
- 7.Marmenout A, et al. Molecular cloning and expression of human tumor necrosis factor and comparison with mouse tumor necrosis factor. Eur. J. Biochem. 1985;152:515–522. doi: 10.1111/j.1432-1033.1985.tb09226.x. [DOI] [PubMed] [Google Scholar]
- 8.Fransen L, et al. Molecular cloning of mouse tumour necrosis factor cDNA and its eukaryotic expression. Nucleic Acids Res. 1985;13:4417–4429. doi: 10.1093/nar/13.12.4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal BB, Eessalu TE, Hass PE. Characterization of receptors for human tumour necrosis factor and their regulation by gamma-interferon. Nature. 1985;318:665–667. doi: 10.1038/318665a0. [DOI] [PubMed] [Google Scholar]
- 10.Loetscher H, et al. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990;61:351–359. doi: 10.1016/0092-8674(90)90815-V. [DOI] [PubMed] [Google Scholar]
- 11.Schall TJ, et al. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990;61:361–370. doi: 10.1016/0092-8674(90)90816-W. [DOI] [PubMed] [Google Scholar]
- 12.Smith CA, et al. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990;248:1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- 13.Heller RA, et al. Complementary DNA cloning of a receptor for tumor necrosis factor and demonstration of a shed form of the receptor. Proc. Natl Acad. Sci. USA. 1990;87:6151–6155. doi: 10.1073/pnas.87.16.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balkwill F. Tumour necrosis factor and cancer. Nat. Rev. Cancer. 2009;9:361–371. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 15.Beutler B, et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 16.Martens S, Hofmans S, Declercq W, Augustyns K, Vandenabeele P. Inhibitors targeting RIPK1/RIPK3: old and new drugs. Trends Pharmacol. Sci. 2020;41:209–224. doi: 10.1016/j.tips.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Mifflin L, Ofengeim D, Yuan J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat. Rev. Drug Discov. 2020;19:553–571. doi: 10.1038/s41573-020-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting AT, Bertrand MJM. More to life than NF-kappaB in TNFR1 signaling. Trends Immunol. 2016;37:535–545. doi: 10.1016/j.it.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delanghe T, Dondelinger Y, Bertrand MJM. RIPK1 kinase-dependent death: a symphony of phosphorylation events. Trends Cell Biol. 2020;30:189–200. doi: 10.1016/j.tcb.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Lork M, Verhelst K, Beyaert R. CYLD, A20 and OTULIN deubiquitinases in NF-kappaB signaling and cell death: so similar, yet so different. Cell Death Differ. 2017;24:1172–1183. doi: 10.1038/cdd.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 22.Orning P, et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Science. 2018;362:1064–1069. doi: 10.1126/science.aau2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Demarco B, et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci. Adv. 2020;6:eabc3465. doi: 10.1126/sciadv.abc3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarzer R, Jiao H, Wachsmuth L, Tresch A, Pasparakis M. FADD and caspase-8 regulate gut homeostasis and inflammation by controlling MLKL- and GSDMD-mediated death of intestinal epithelial cells. Immunity. 2020;52:978–993. doi: 10.1016/j.immuni.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Du F, Wang X. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Wilson NS, Dixit V, Ashkenazi A. Death receptor signal transducers: nodes of coordination in immune signaling networks. Nat. Immunol. 2009;10:348–355. doi: 10.1038/ni.1714. [DOI] [PubMed] [Google Scholar]
- 27.Dondelinger Y, et al. NF-kappaB-independent role of IKKalpha/IKKbeta in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol. Cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 28.Lafont E, et al. TBK1 and IKKepsilon prevent TNF-induced cell death by RIPK1 phosphorylation. Nat. Cell Biol. 2018;20:1389–1399. doi: 10.1038/s41556-018-0229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu D, et al. TBK1 suppresses RIPK1-driven apoptosis and inflammation during development and in aging. Cell. 2018;174:1477–1491. doi: 10.1016/j.cell.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Du J, et al. RIPK1 dephosphorylation and kinase activation by PPP1R3G/PP1gamma promote apoptosis and necroptosis. Nat. Commun. 2021;12:7067. doi: 10.1038/s41467-021-27367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dondelinger Y, et al. Serine 25 phosphorylation inhibits RIPK1 kinase-dependent cell death in models of infection and inflammation. Nat. Commun. 2019;10:1729. doi: 10.1038/s41467-019-09690-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 33.Bertrand MJ, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 34.Vince JE, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 35.Moulin M, et al. IAPs limit activation of RIP kinases by TNF receptor 1 during development. EMBO J. 2012;31:1679–1691. doi: 10.1038/emboj.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mahoney DJ, et al. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc. Natl Acad. Sci. USA. 2008;105:11778–11783. doi: 10.1073/pnas.0711122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taraborrelli L, et al. LUBAC prevents lethal dermatitis by inhibiting cell death induced by TNF, TRAIL and CD95L. Nat. Commun. 2018;9:3910. doi: 10.1038/s41467-018-06155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peltzer N, et al. LUBAC is essential for embryogenesis by preventing cell death and enabling haematopoiesis. Nature. 2018;557:112–117. doi: 10.1038/s41586-018-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rickard JA, et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. Elife. 2014;3:e03464. doi: 10.7554/eLife.03464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peltzer N, et al. HOIP deficiency causes embryonic lethality by aberrant TNFR1-mediated endothelial cell death. Cell Rep. 2014;9:153–165. doi: 10.1016/j.celrep.2014.08.066. [DOI] [PubMed] [Google Scholar]
- 41.O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NF-kappaB-independent cell-death switch in TNF signaling. Curr. Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang X, et al. Ubiquitination of RIPK1 suppresses programmed cell death by regulating RIPK1 kinase activation during embryogenesis. Nat. Commun. 2019;10:4158. doi: 10.1038/s41467-019-11839-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang Y, et al. K63-linked ubiquitination regulates RIPK1 kinase activity to prevent cell death during embryogenesis and inflammation. Nat. Commun. 2019;10:4157. doi: 10.1038/s41467-019-12033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Draber P, et al. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13:2258–2272. doi: 10.1016/j.celrep.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Priem D, et al. A20 protects cells from TNF-induced apoptosis through linear ubiquitin-dependent and -independent mechanisms. Cell Death Dis. 2019;10:692. doi: 10.1038/s41419-019-1937-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wertz IE, et al. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature. 2015;528:370–375. doi: 10.1038/nature16165. [DOI] [PubMed] [Google Scholar]
- 47.Polykratis A, et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat. Cell Biol. 2019;21:731–742. doi: 10.1038/s41556-019-0324-3. [DOI] [PubMed] [Google Scholar]
- 48.Martens A, et al. Two distinct ubiquitin-binding motifs in A20 mediate its anti-inflammatory and cell-protective activities. Nat. Immunol. 2020;21:381–387. doi: 10.1038/s41590-020-0621-9. [DOI] [PubMed] [Google Scholar]
- 49.Heger K, et al. OTULIN limits cell death and inflammation by deubiquitinating LUBAC. Nature. 2018;559:120–124. doi: 10.1038/s41586-018-0256-2. [DOI] [PubMed] [Google Scholar]
- 50.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Newton K, et al. Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis. Nature. 2019;574:428–431. doi: 10.1038/s41586-019-1548-x. [DOI] [PubMed] [Google Scholar]
- 52.Lalaoui N, et al. Mutations that prevent caspase cleavage of RIPK1 cause autoinflammatory disease. Nature. 2020;577:103–108. doi: 10.1038/s41586-019-1828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao P, et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature. 2020;577:109–114. doi: 10.1038/s41586-019-1830-y. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Dowling JP, Zhang J. RIPK1 can mediate apoptosis in addition to necroptosis during embryonic development. Cell Death Dis. 2019;10:245. doi: 10.1038/s41419-019-1490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dondelinger Y, et al. MK2 phosphorylation of RIPK1 regulates TNF-mediated cell death. Nat. Cell Biol. 2017;19:1237–1247. doi: 10.1038/ncb3608. [DOI] [PubMed] [Google Scholar]
- 56.Menon MB, et al. p38(MAPK)/MK2-dependent phosphorylation controls cytotoxic RIPK1 signalling in inflammation and infection. Nat. Cell Biol. 2017;19:1248–1259. doi: 10.1038/ncb3614. [DOI] [PubMed] [Google Scholar]
- 57.Jaco I, et al. MK2 phosphorylates RIPK1 to prevent TNF-induced cell death. Mol. Cell. 2017;66:698–710. doi: 10.1016/j.molcel.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu W, et al. The autophagy-initiating kinase ULK1 controls RIPK1-mediated cell death. Cell Rep. 2020;31:107547. doi: 10.1016/j.celrep.2020.107547. [DOI] [PubMed] [Google Scholar]
- 59.Liu L, et al. Tankyrase-mediated ADP-ribosylation is a regulator of TNF-induced death. Sci. Adv. 2022;8:eabh2332. doi: 10.1126/sciadv.abh2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukherjee S, et al. Yersinia YopJ acetylates and inhibits kinase activation by blocking phosphorylation. Science. 2006;312:1211–1214. doi: 10.1126/science.1126867. [DOI] [PubMed] [Google Scholar]
- 61.Mittal R, Peak-Chew SY, McMahon HT. Acetylation of MEK2 and I kappa B kinase (IKK) activation loop residues by YopJ inhibits signaling. Proc. Natl Acad. Sci. USA. 2006;103:18574–18579. doi: 10.1073/pnas.0608995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haase R, Richter K, Pfaffinger G, Courtois G, Ruckdeschel K. Yersinia outer protein P suppresses TGF-beta-activated kinase-1 activity to impair innate immune signaling in Yersinia enterocolitica-infected cells. J. Immunol. 2005;175:8209–8217. doi: 10.4049/jimmunol.175.12.8209. [DOI] [PubMed] [Google Scholar]
- 63.Paquette N, et al. Serine/threonine acetylation of TGFbeta-activated kinase (TAK1) by Yersinia pestis YopJ inhibits innate immune signaling. Proc. Natl Acad. Sci. USA. 2012;109:12710–12715. doi: 10.1073/pnas.1008203109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peterson LW, et al. RIPK1-dependent apoptosis bypasses pathogen blockade of innate signaling to promote immune defense. J. Exp. Med. 2017;214:3171–3182. doi: 10.1084/jem.20170347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peterson LW, et al. Cell-extrinsic TNF collaborates with TRIF signaling to promote yersinia-induced apoptosis. J. Immunol. 2016;197:4110–4117. doi: 10.4049/jimmunol.1601294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sarhan J, et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA. 2018;115:10888–10897. doi: 10.1073/pnas.1809548115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tummers B, Green DR. The evolution of regulated cell death pathways in animals and their evasion by pathogens. Physiol. Rev. 2022;102:411–454. doi: 10.1152/physrev.00002.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Q, et al. Target protease specificity of the viral serpin CrmA. Analysis of five caspases. J. Biol. Chem. 1997;272:7797–7800. doi: 10.1074/jbc.272.12.7797. [DOI] [PubMed] [Google Scholar]
- 69.Thome M, et al. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature. 1997;386:517–521. doi: 10.1038/386517a0. [DOI] [PubMed] [Google Scholar]
- 70.Bertin J, et al. Death effector domain-containing herpesvirus and poxvirus proteins inhibit both Fas- and TNFR1-induced apoptosis. Proc. Natl Acad. Sci. USA. 1997;94:1172–1176. doi: 10.1073/pnas.94.4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karki R, et al. Synergism of TNF-alpha and IFN-gamma triggers inflammatory cell death, tissue damage, and mortality in SARS-CoV-2 infection and cytokine shock syndromes. Cell. 2021;184:149–168. doi: 10.1016/j.cell.2020.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roca FJ, Whitworth LJ, Redmond S, Jones AA, Ramakrishnan L. TNF induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial-lysosomal-endoplasmic reticulum circuit. Cell. 2019;178:1344–1361. doi: 10.1016/j.cell.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Hauwermeiren F, et al. Bacillus anthracis induces NLRP3 inflammasome activation and caspase-8-mediated apoptosis of macrophages to promote lethal anthrax. Proc. Natl Acad. Sci. USA. 2022 doi: 10.1073/pnas.2116415119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simpson DS, et al. Interferon-gamma primes macrophages for pathogen ligand-induced killing via a caspase-8 and mitochondrial cell death pathway. Immunity. 2022;55:423–441. doi: 10.1016/j.immuni.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duprez L, et al. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 76.Newton K, et al. Activity of protein kinase RIPK3 determines whether cells die by necroptosis or apoptosis. Science. 2014;343:1357–1360. doi: 10.1126/science.1249361. [DOI] [PubMed] [Google Scholar]
- 77.Berger SB, et al. Cutting edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. J. Immunol. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newton K, et al. RIPK3 deficiency or catalytically inactive RIPK1 provides greater benefit than MLKL deficiency in mouse models of inflammation and tissue injury. Cell Death Differ. 2016;23:1565–1576. doi: 10.1038/cdd.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Varfolomeev E, et al. Cellular inhibitors of apoptosis are global regulators of NF-kappaB and MAPK activation by members of the TNF family of receptors. Sci. Signal. 2012;5:ra22. doi: 10.1126/scisignal.2001878. [DOI] [PubMed] [Google Scholar]
- 80.Vince JE, et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J. Cell Biol. 2008;182:171–184. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Croft M, Siegel RM. Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat. Rev. Rheumatol. 2017;13:217–233. doi: 10.1038/nrrheum.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kawashima R, et al. Interleukin-13 damages intestinal mucosa via TWEAK and Fn14 in mice-a pathway associated with ulcerative colitis. Gastroenterology. 2011;141:2119–2129. doi: 10.1053/j.gastro.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 83.Lucas C, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature. 2020;584:463–469. doi: 10.1038/s41586-020-2588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rodrigues TS, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2021;218:e20201707. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ferreira AC, et al. SARS-CoV-2 engages inflammasome and pyroptosis in human primary monocytes. Cell Death Discov. 2021;7:43. doi: 10.1038/s41420-021-00428-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Varfolomeev EE, et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/S1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- 87.Zhang J, Cado D, Chen A, Kabra NH, Winoto A. Fas-mediated apoptosis and activation-induced T-cell proliferation are defective in mice lacking FADD/Mort1. Nature. 1998;392:296–300. doi: 10.1038/32681. [DOI] [PubMed] [Google Scholar]
- 88.Kaiser WJ, et al. RIP3 mediates the embryonic lethality of caspase-8-deficient mice. Nature. 2011;471:368–372. doi: 10.1038/nature09857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Oberst A, et al. Catalytic activity of the caspase-8-FLIP(L) complex inhibits RIPK3-dependent necrosis. Nature. 2011;471:363–367. doi: 10.1038/nature09852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang H, et al. Functional complementation between FADD and RIP1 in embryos and lymphocytes. Nature. 2011;471:373–376. doi: 10.1038/nature09878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alvarez-Diaz S, et al. The pseudokinase MLKL and the kinase RIPK3 have distinct roles in autoimmune disease caused by loss of death-receptor-induced apoptosis. Immunity. 2016;45:513–526. doi: 10.1016/j.immuni.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Welz PS, et al. FADD prevents RIP3-mediated epithelial cell necrosis and chronic intestinal inflammation. Nature. 2011;477:330–334. doi: 10.1038/nature10273. [DOI] [PubMed] [Google Scholar]
- 93.Gunther C, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weinlich R, et al. Protective roles for caspase-8 and cFLIP in adult homeostasis. Cell Rep. 2013;5:340–348. doi: 10.1016/j.celrep.2013.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lehle AS, et al. Intestinal inflammation and dysregulated immunity in patients with inherited caspase-8 deficiency. Gastroenterology. 2019;156:275–278. doi: 10.1053/j.gastro.2018.09.041. [DOI] [PubMed] [Google Scholar]
- 96.Chun HJ, et al. Pleiotropic defects in lymphocyte activation caused by caspase-8 mutations lead to human immunodeficiency. Nature. 2002;419:395–399. doi: 10.1038/nature01063. [DOI] [PubMed] [Google Scholar]
- 97.Bader SM, et al. Endothelial caspase-8 prevents fatal necroptotic hemorrhage caused by commensal bacteria. Cell Death Differ. 2022 doi: 10.1038/s41418-022-01042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kovalenko A, et al. Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J. Exp. Med. 2009;206:2161–2177. doi: 10.1084/jem.20090616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bonnet MC, et al. The adaptor protein FADD protects epidermal keratinocytes from necroptosis in vivo and prevents skin inflammation. Immunity. 2011;35:572–582. doi: 10.1016/j.immuni.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 100.Dondelinger Y, et al. RIPK3 contributes to TNFR1-mediated RIPK1 kinase-dependent apoptosis in conditions of cIAP1/2 depletion or TAK1 kinase inhibition. Cell Death Differ. 2013;20:1381–1392. doi: 10.1038/cdd.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dannappel M, et al. RIPK1 maintains epithelial homeostasis by inhibiting apoptosis and necroptosis. Nature. 2014;513:90–94. doi: 10.1038/nature13608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Devos M, et al. Sensing of endogenous nucleic acids by ZBP1 induces keratinocyte necroptosis and skin inflammation. J. Exp. Med. 2020;217:e20191913. doi: 10.1084/jem.20191913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tapiz IRAJ, et al. Characterization of novel pathogenic variants leading to caspase-8 cleavage-resistant RIPK1-induced autoinflammatory syndrome. J. Clin. Immunol. 2022 doi: 10.1007/s10875-022-01298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kupka S, Reichert M, Draber P, Walczak H. Formation and removal of poly-ubiquitin chains in the regulation of tumor necrosis factor-induced gene activation and cell death. FEBS J. 2016;283:2626–2639. doi: 10.1111/febs.13644. [DOI] [PubMed] [Google Scholar]
- 105.Beck DB, Werner A, Kastner DL, Aksentijevich I. Disorders of ubiquitylation: unchained inflammation. Nat. Rev. Rheumatol. 2022;18:435–447. doi: 10.1038/s41584-022-00778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J, et al. Ubiquitin ligases cIAP1 and cIAP2 limit cell death to prevent inflammation. Cell Rep. 2019;27:2679–2689. doi: 10.1016/j.celrep.2019.04.111. [DOI] [PubMed] [Google Scholar]
- 107.Rigaud S, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444:110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 108.Yabal M, et al. XIAP restricts TNF- and RIP3-dependent cell death and inflammasome activation. Cell Rep. 2014;7:1796–1808. doi: 10.1016/j.celrep.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 109.Wahida A, et al. XIAP restrains TNF-driven intestinal inflammation and dysbiosis by promoting innate immune responses of Paneth and dendritic cells. Sci. Immunol. 2021;6:eabf7235. doi: 10.1126/sciimmunol.abf7235. [DOI] [PubMed] [Google Scholar]
- 110.Lawlor KE, et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.HogenEsch H, et al. A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. Am. J. Pathol. 1993;143:972–982. [PMC free article] [PubMed] [Google Scholar]
- 112.Gerlach B, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 113.Tokunaga F, et al. SHARPIN is a component of the NF-kappaB-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 114.Ikeda F, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-kappaB activity and apoptosis. Nature. 2011;471:637–641. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumari S, et al. Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis. Elife. 2014;3:e03422. doi: 10.7554/eLife.03422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sharma BR, et al. Innate immune adaptor MyD88 deficiency prevents skin inflammation in SHARPIN-deficient mice. Cell Death Differ. 2019;26:741–750. doi: 10.1038/s41418-018-0159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hoste E, et al. OTULIN maintains skin homeostasis by controlling keratinocyte death and stem cell identity. Nat. Commun. 2021;12:5913. doi: 10.1038/s41467-021-25944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schunke H, Gobel U, Dikic I, Pasparakis M. OTULIN inhibits RIPK1-mediated keratinocyte necroptosis to prevent skin inflammation in mice. Nat. Commun. 2021;12:5912. doi: 10.1038/s41467-021-25945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Boisson B, et al. Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. J. Exp. Med. 2015;212:939–951. doi: 10.1084/jem.20141130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boisson B, et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oda H, et al. Second case of HOIP deficiency expands clinical features and defines inflammatory transcriptome regulated by LUBAC. Front. Immunol. 2019;10:479. doi: 10.3389/fimmu.2019.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Damgaard RB, et al. The deubiquitinase OTULIN is an essential negative regulator of inflammation and autoimmunity. Cell. 2016;166:1215–1230. doi: 10.1016/j.cell.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Damgaard RB, et al. OTULIN deficiency in ORAS causes cell type-specific LUBAC degradation, dysregulated TNF signalling and cell death. EMBO Mol. Med. 2019;11:e9324. doi: 10.15252/emmm.201809324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Q, et al. Biallelic hypomorphic mutations in a linear deubiquitinase define otulipenia, an early-onset autoinflammatory disease. Proc. Natl Acad. Sci. USA. 2016;113:10127–10132. doi: 10.1073/pnas.1612594113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nabavi M, et al. Auto-inflammation in a patient with a novel homozygous OTULIN mutation. J. Clin. Immunol. 2019;39:138–141. doi: 10.1007/s10875-019-00599-3. [DOI] [PubMed] [Google Scholar]
- 126.Zinngrebe J, et al. Compound heterozygous variants in OTULIN are associated with fulminant atypical late-onset ORAS. EMBO Mol. Med. 2022;14:e14901. doi: 10.15252/emmm.202114901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee EG, et al. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289:2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Vereecke L, et al. Enterocyte-specific A20 deficiency sensitizes to tumor necrosis factor-induced toxicity and experimental colitis. J. Exp. Med. 2010;207:1513–1523. doi: 10.1084/jem.20092474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vereecke L, et al. A20 controls intestinal homeostasis through cell-specific activities. Nat. Commun. 2014;5:5103. doi: 10.1038/ncomms6103. [DOI] [PubMed] [Google Scholar]
- 130.Catrysse L, et al. A20 prevents chronic liver inflammation and cancer by protecting hepatocytes from death. Cell Death Dis. 2016;7:e2250. doi: 10.1038/cddis.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kattah MG, et al. A20 and ABIN-1 synergistically preserve intestinal epithelial cell survival. J. Exp. Med. 2018;215:1839–1852. doi: 10.1084/jem.20180198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Slowicka K, et al. Physical and functional interaction between A20 and ATG16L1-WD40 domain in the control of intestinal homeostasis. Nat. Commun. 2019;10:1834. doi: 10.1038/s41467-019-09667-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vetters J, et al. The ubiquitin-editing enzyme A20 controls NK cell homeostasis through regulation of mTOR activity and TNF. J. Exp. Med. 2019;216:2010–2023. doi: 10.1084/jem.20182164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Razani B, et al. Non-catalytic ubiquitin binding by A20 prevents psoriatic arthritis-like disease and inflammation. Nat. Immunol. 2020;21:422–433. doi: 10.1038/s41590-020-0634-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martens A, van Loo G. A20 at the crossroads of cell death, inflammation, and autoimmunity. Cold Spring Harb. Perspect. Biol. 2019;12:a036418. doi: 10.1101/cshperspect.a036418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Priem D, van Loo G, Bertrand MJM. A20 and cell death-driven inflammation. Trends Immunol. 2020;41:421–435. doi: 10.1016/j.it.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 137.Aeschlimann FA, et al. A20 haploinsufficiency (HA20): clinical phenotypes and disease course of patients with a newly recognised NF-kB-mediated autoinflammatory disease. Ann. Rheum. Dis. 2018;77:728–735. doi: 10.1136/annrheumdis-2017-212403. [DOI] [PubMed] [Google Scholar]
- 138.Zhou Q, et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 2016;48:67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zilberman-Rudenko J, et al. Recruitment of A20 by the C-terminal domain of NEMO suppresses NF-kappaB activation and autoinflammatory disease. Proc. Natl Acad. Sci. USA. 2016;113:1612–1617. doi: 10.1073/pnas.1518163113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li ZW, et al. The IKKbeta subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J. Exp. Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li Q, Estepa G, Memet S, Israel A, Verma IM. Complete lack of NF-kappaB activity in IKK1 and IKK2 double-deficient mice: additional defect in neurulation. Genes Dev. 2000;14:1729–1733. doi: 10.1101/gad.14.14.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rudolph D, et al. Severe liver degeneration and lack of NF-kappaB activation in NEMO/IKKgamma-deficient mice. Genes Dev. 2000;14:854–862. doi: 10.1101/gad.14.7.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takeda K, et al. Limb and skin abnormalities in mice lacking IKKalpha. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 144.Makris C, et al. Female mice heterozygous for IKK gamma/NEMO deficiencies develop a dermatopathy similar to the human X-linked disorder incontinentia pigmenti. Mol. Cell. 2000;5:969–979. doi: 10.1016/S1097-2765(00)80262-2. [DOI] [PubMed] [Google Scholar]
- 145.Schmidt-Supprian M, et al. NEMO/IKK gamma-deficient mice model incontinentia pigmenti. Mol. Cell. 2000;5:981–992. doi: 10.1016/S1097-2765(00)80263-4. [DOI] [PubMed] [Google Scholar]
- 146.Nenci A, et al. Skin lesion development in a mouse model of incontinentia pigmenti is triggered by NEMO deficiency in epidermal keratinocytes and requires TNF signaling. Hum. Mol. Genet. 2006;15:531–542. doi: 10.1093/hmg/ddi470. [DOI] [PubMed] [Google Scholar]
- 147.Smahi A, et al. Genomic rearrangement in NEMO impairs NF-kappaB activation and is a cause of incontinentia pigmenti. The International Incontinentia Pigmenti (IP) Consortium. Nature. 2000;405:466–472. doi: 10.1038/35013114. [DOI] [PubMed] [Google Scholar]
- 148.Lee Y, et al. Genetically programmed alternative splicing of NEMO mediates an autoinflammatory disease phenotype. J. Clin. Invest. 2022;132:e128808. doi: 10.1172/JCI128808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.de Jesus AA, et al. Distinct interferon signatures and cytokine patterns define additional systemic autoinflammatory diseases. J. Clin. Invest. 2020;130:1669–1682. doi: 10.1172/JCI129301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pasparakis M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 151.Kumari S, et al. NF-kappaB inhibition in keratinocytes causes RIPK1-mediated necroptosis and skin inflammation. Life Sci. Alliance. 2021;4:e202000956. doi: 10.26508/lsa.202000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Badran YR, et al. Human RELA haploinsufficiency results in autosomal-dominant chronic mucocutaneous ulceration. J. Exp. Med. 2017;214:1937–1947. doi: 10.1084/jem.20160724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 154.Vlantis K, et al. NEMO prevents RIP kinase 1-mediated epithelial cell death and chronic intestinal inflammation by NF-kappaB-dependent and -independent functions. Immunity. 2016;44:553–567. doi: 10.1016/j.immuni.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bonnard M, et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19:4976–4985. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marchlik E, et al. Mice lacking Tbk1 activity exhibit immune cell infiltrates in multiple tissues and increased susceptibility to LPS-induced lethality. J. Leukoc. Biol. 2010;88:1171–1180. doi: 10.1189/jlb.0210071. [DOI] [PubMed] [Google Scholar]
- 157.Taft J, et al. Human TBK1 deficiency leads to autoinflammation driven by TNF-induced cell death. Cell. 2021;184:4447–4463. doi: 10.1016/j.cell.2021.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yeh WC, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/S1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 159.Dillon CP, et al. Survival function of the FADD-CASPASE-8-cFLIP(L) complex. Cell Rep. 2012;1:401–407. doi: 10.1016/j.celrep.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Piao X, et al. c-FLIP maintains tissue homeostasis by preventing apoptosis and programmed necrosis. Sci. Signal. 2012;5:ra93. doi: 10.1126/scisignal.2003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wittkopf N, et al. Cellular FLICE-like inhibitory protein secures intestinal epithelial cell survival and immune homeostasis by regulating caspase-8. Gastroenterology. 2013;145:1369–1379. doi: 10.1053/j.gastro.2013.08.059. [DOI] [PubMed] [Google Scholar]
- 162.Panayotova-Dimitrova D, et al. cFLIP regulates skin homeostasis and protects against TNF-induced keratinocyte apoptosis. Cell Rep. 2013;5:397–408. doi: 10.1016/j.celrep.2013.09.035. [DOI] [PubMed] [Google Scholar]
- 163.Dillon CP, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rickard JA, et al. RIPK1 regulates RIPK3-MLKL-driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 165.Kaiser WJ, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc. Natl Acad. Sci. USA. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Takahashi N, et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature. 2014;513:95–99. doi: 10.1038/nature13706. [DOI] [PubMed] [Google Scholar]
- 167.Gentle IE, et al. In TNF-stimulated cells, RIPK1 promotes cell survival by stabilizing TRAF2 and cIAP1, which limits induction of non-canonical NF-kappaB and activation of caspase-8. J. Biol. Chem. 2011;286:13282–13291. doi: 10.1074/jbc.M110.216226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cuchet-Lourenco D, et al. Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis, and intestinal inflammation. Science. 2018;361:810–813. doi: 10.1126/science.aar2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li Y, et al. Human RIPK1 deficiency causes combined immunodeficiency and inflammatory bowel diseases. Proc. Natl Acad. Sci. USA. 2019;116:970–975. doi: 10.1073/pnas.1813582116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Silva-Fernandez L, Hyrich K. Rheumatoid arthritis: when TNF inhibitors fail in RA-weighing up the options. Nat. Rev. Rheumatol. 2014;10:262–264. doi: 10.1038/nrrheum.2014.34. [DOI] [PubMed] [Google Scholar]
- 171.Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin. Transl. Gastroenterol. 2016;7:e135. doi: 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Esposito M, et al. Survival rate of antitumour necrosis factor-alpha treatments for psoriasis in routine dermatological practice: a multicentre observational study. Br. J. Dermatol. 2013;169:666–672. doi: 10.1111/bjd.12422. [DOI] [PubMed] [Google Scholar]
- 173.Degterev A, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat. Chem. Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Xie T, et al. Structural basis of RIP1 inhibition by necrostatins. Structure. 2013;21:493–499. doi: 10.1016/j.str.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 175.Weisel K, et al. A randomized, placebo-controlled experimental medicine study of RIPK1 inhibitor GSK2982772 in patients with moderate to severe rheumatoid arthritis. Arthritis Res. Ther. 2021;23:85. doi: 10.1186/s13075-021-02468-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Weisel K, et al. A randomised, placebo-controlled study of RIPK1 inhibitor GSK2982772 in patients with active ulcerative colitis. BMJ Open Gastroenterol. 2021;8:e000680. doi: 10.1136/bmjgast-2021-000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mandal P, et al. RIP3 induces apoptosis independent of pronecrotic kinase activity. Mol. Cell. 2014;56:481–495. doi: 10.1016/j.molcel.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shi J, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 179.Kayagaki N, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 180.Liu X, Xia S, Zhang Z, Wu H, Lieberman J. Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov. 2021;20:384–405. doi: 10.1038/s41573-021-00154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hu JJ, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020;21:736–745. doi: 10.1038/s41590-020-0669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li S, et al. Gasdermin D in peripheral myeloid cells drives neuroinflammation in experimental autoimmune encephalomyelitis. J. Exp. Med. 2019;216:2562–2581. doi: 10.1084/jem.20190377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rathkey JK, et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol. 2018;3:eaat2738. doi: 10.1126/sciimmunol.aat2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Humphries F, et al. Succination inactivates gasdermin D and blocks pyroptosis. Science. 2020;369:1633–1637. doi: 10.1126/science.abb9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rogers C, et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun. 2017;8:14128. doi: 10.1038/ncomms14128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wang Y, et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature. 2017;547:99–103. doi: 10.1038/nature22393. [DOI] [PubMed] [Google Scholar]
- 187.Hou J, et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020;22:1264–1275. doi: 10.1038/s41556-020-0575-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kayagaki N, et al. NINJ1 mediates plasma membrane rupture during lytic cell death. Nature. 2021;591:131–136. doi: 10.1038/s41586-021-03218-7. [DOI] [PubMed] [Google Scholar]
- 189.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 191.Zhang DW, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 192.Cho YS, et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.He S, et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-alpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 194.Sun L, et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213–227. doi: 10.1016/j.cell.2011.11.031. [DOI] [PubMed] [Google Scholar]
- 195.Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–247. doi: 10.1016/S0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 196.Haworth C, et al. Expression of granulocyte-macrophage colony-stimulating factor in rheumatoid arthritis: regulation by tumor necrosis factor-alpha. Eur. J. Immunol. 1991;21:2575–2579. doi: 10.1002/eji.1830211039. [DOI] [PubMed] [Google Scholar]
- 197.Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl Acad. Sci. USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Elliott MJ, et al. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 199.Elliott MJ, et al. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/S0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 200.Elliott MJ, et al. Repeated therapy with monoclonal antibody to tumour necrosis factor alpha (cA2) in patients with rheumatoid arthritis. Lancet. 1994;344:1125–1127. doi: 10.1016/S0140-6736(94)90632-7. [DOI] [PubMed] [Google Scholar]
- 201.Lipsky PE, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N. Engl. J. Med. 2000;343:1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 202.Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat. Med. 2003;9:1245–1250. doi: 10.1038/nm939. [DOI] [PubMed] [Google Scholar]
- 203.Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat. Rev. Drug Discov. 2013;12:147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]