Abstract
AbstractAs a result of treatment and diagnosis, adults with primary or metastatic brain tumors experience comorbidities that impacts their health and well-being. The Children’s Oncology Group has guideline recommendations for childhood survivors of brain tumors; however, guidelines for monitoring long-term sequela among adult brain tumor survivors are lacking. The purpose of this review is to present the screening recommendations for the long-term complications after brain tumor treatment from a multidisciplinary panel of healthcare professionals. Chronic complications identified include cognitive dysfunction, vasculopathy, endocrinopathy, ophthalmic, ototoxicity, physical disability, sleep disturbance, mood disorder, unemployment, financial toxicity, and secondary malignancy. We invited specialists across disciplines to perform a literature search and provide expert recommendations for surveillance for long-term complications for adult brain tumor survivors. The Brain Tumor Center Survivorship Committee recommends routine screening using laboratory testing, subjective assessment of symptoms, and objective evaluations to appropriately monitor the complications of brain tumor treatments. Effective monitoring and treatment should involve collaboration with primary care providers and may require referral to other specialties and support services to provide patient-centered care during neuro-oncology survivorship. Further research is necessary to document the incidence and prevalence of medical complications as well as evaluate the efficacy of screening and neuro-oncology survivorship programs.
Keywords: adults, brain tumor survivorship, long-term complications, management, toxicity
Annually 88 000 adults are diagnosed with primary benign or malignant brain tumors and over 200 000 patients with secondary or metastatic brain tumors.1 Surgery, radiation therapy (RT), and chemotherapy (CTX) are necessary treatments to prevent neurological deterioration and extend overall survival for patients with brain tumors. With improved survival, especially among adults with metastatic BT, more survivors may experience chronic symptoms, comorbidities, and psychosocial issues that impact their overall well-being, daily functioning, and quality of life (QOL). Commonly identified complications among BT survivors include cognitive dysfunction, vasculopathy, endocrinopathy, ophthalmic sequela, ototoxicity, physical disability, sleep disturbance, mood disorder, unemployment, financial toxicity, secondary malignancy, and care partner fatigue. While there are guidelines for childhood survivors of BT, there are limited guidelines for screening, monitoring, and managing treatment-related complications for individuals diagnosed as adults with BT. This review aims to present the results of the literature review and expert recommendations from a multidisciplinary panel of healthcare professionals for assessing and screening for complications of treatment during adult neuro-oncology survivorship.
Methods
A multidisciplinary committee composed of physicians, advanced practice providers, nurses, rehabilitation counselors, clinical psychologists, social workers, audiologists, and neuropsychologists was assembled. The committee focused on patients diagnosed with intracranial BT diagnosed as adults (age 18 or older). Adult complications of spinal cord tumors and spinal cord radiation or surgery are beyond the scope of this work.
The committee reviewed the National Comprehensive Cancer Network (NCCN)2 and Children’s Oncology Group Guideline3 to provide a foundation for a focused literature review. The databases used during the literature review were PubMed, EMBASE, and CINAHL. The committee structured literature review and meetings based on the COG guidelines to review possible complications for adult BT survivors. To bridge the literature gap in the adult screening, experts in the adult specialties in radiation oncology, neuropsychology, endocrinology, vascular neurology, otolaryngology, ophthalmology, sleep medicine, and rehabilitation medicine were invited to discuss their perspectives and recommendations on the screening and management of BT survivors. When multiple screening tools were available, the committee discussed the quality of the literature, clinical availability of the tool, and patient response burden to develop the suggested recommendations. Detailed transcriptions were gathered from these meetings and used by committee members to summarize the recommendations in the following sections.
Cognitive Function
Cognitive dysfunction is the most common neurologic symptom in adult patients with primary or metastatic BT; however, the NCCN guidelines do not suggest screening methods in patients with CNS malignancies.2 The majority of patients with primary BT have cognitive impairment in multiple domains at the time of tumor discovery.4 Tumor location and characteristics may determine the type of cognitive symptoms, often caused by compression of brain structures, edema, and/or disruption of neural circuitry. Additionally, patient attributes (e.g., age, cognitive reserve, medical and psychiatric histories) modulate cognitive symptom onset and severity. Brain-directed treatments and other co-occurring factors (eg, seizures, medications, cerebrovascular disease, and medical/psychiatric conditions) also contribute to cognitive dysfunction. Memory, executive functioning, and processing speed commonly impact patient functional status more than any BT-related symptom.5
Response biases can skew patient reports (eg, deficit unawareness), resulting in under- or over-endorsement of cognitive symptoms; thus, cognitive assessment requires a combination of patient-reported, observer-reported, and performance-based strategies. A thoroughly conducted interview allows for ascertaining detailed baseline historical, medical, developmental, and psychosocial factors influencing the patient’s cognitive status. Inclusion of the care partners’ observation is beneficial as collateral information to provide additional insight into reported cognitive symptoms.
Psychometric instruments such as the MD Anderson Symptom Inventory-Brain Tumor (MDASI-BT),6 Functional Assessment of Cancer Therapy—Cognitive (FACT-Cog),7 and FACT—Brain Tumor8 have been explicitly developed for tumor treatment outcomes to provide information on the presence of cognitive symptoms and their effects on functional status.
Brief cognitive screening instruments offer a standardized way of monitoring the presence, severity, and trends of cognitive dysfunction throughout the disease trajectory. Cognitive screening tools used in BT patients, including the Montreal Cognitive Assessment (MoCA), Clinical Trial Battery, and the computer-based CNS Vital Signs, have been explored in BT outcomes research. Only the MoCA is widely available for use in clinical practice.
Screening instruments can lack sufficient sensitivity in detecting cognitive symptoms, especially if mild in severity.9 Neuropsychological evaluation (NPE) is a more comprehensive, tailored assessment of cognitive symptoms. NPE, while providing greater sensitivity and scope, is resource-intensive in terms of testing time, materials, and appropriate expertise (eg, neuropsychologist) in test administration and test score interpretation. Its incremental value in clinical management increases with time over the disease course, with more comprehensive evaluation particularly useful in differentiating co-occurring contributory factors, informing treatment recommendations, and determining readiness to return to activities such as employment and school.
Our group recommends routine screening of subjective and objective cognitive functions to evaluate the change over time and help guide additional evaluations or interventions (See Table 1). NPE can identify domains of impairments and help to guide cognitive rehabilitation, emphasizing compensatory-based treatment.10
Table 1.
Suggested Screening for cognitive impairment
| Ideal Patient Screening | Brief Patient Screening | Potential Contributing Factors |
|---|---|---|
| Subjective: MDASI – BT FACT – Cog FACT – BT Objective: NPE |
Subjective: Have you noted any changes in your thinking? Have family members expressed any personality or behavioral concerns? Objective: FACT – BT |
Medication side effects Emotional distress Symptoms: Pain, fatigue, sleep disturbance Use of alcohol or other agents that alter cognition Screening endocrinopathies Vitamin deficiencies (B1, B12, D) |
Suggested recommendation:
• Cognitive screening every 6–12 months from the time of BT diagnosis with:
◦ Montreal Cognitive Assessment (MoCA) or other cognitive screening tools.
◦ Patient-reported symptoms and care partner’s observations.
• If cognitive impairment is identified, evaluation of potential contributing factors and consideration for NPE and cognitive rehabilitation.
Cerebrovascular Complications
Cerebrovascular disease is a known long-term complication of BT treatments, secondary to RT and CTX. RT-induced complications include stroke, moya-moya disease, occlusive vasculopathy, cavernomas, and Stroke-like migraine attacks after RT (SMART syndrome). Risk factors for vasculopathy11,12 are summarized in Table 2. RT-induced vasculopathy predominantly affects larger arteries, such as the internal carotid arteries and Circle of Willis.13 Small vessel involvement is also well documented clinically by both ischemic and hemorrhagic stroke or radiographically by microbleeds and white matter hyperintensity. The time from treatment to the development of cerebrovascular complications in adults varies widely and is likely impacted by pre-existing cerebrovascular disease. In one series, the reported median interval between completion of RT to stroke was 3.2 years; however, the range was 0.5–30 years.14 Small vessel changes, including lacunar infarcts and progressive white matter hyperintensity, occur earlier (<6 months) than large vessel stroke.15
Table 2.
Cerebrovascular Risk Factors, Screening Among Adult Brain Tumor Survivors
| High-risk factors |
| RT to sellar/parasellar, prepontine cistern, posterior fossa |
| RT dose ≥ 50 Gy |
| Age > 55 |
| Genetic risk factors (eg, neurofibromatosis type 1) |
| Concomitant chemotherapy (eg, cisplatin) |
| Extent of RT fields |
| Suggested screening recommendation |
| Small vessel |
| MRI brain (containing a minimum of T2, T2 Flair, T1 and DWI/ADC sequences) |
| High risk: consideration of screening of imaging at year 1, 3, and then 5-year intervals from the time of radiation. |
| Large vessel: intracranial (occlusive vasculopathy as well as aneurysm |
| High-resolution VWI-MRI head is preferred every 3–5 years based on imaging and clinical factors. If VWI-MRI is not available consider CTA head or MRA head |
| High risk: consideration of vessel imaging at 1, 3, and 5 years after RT and continue every 5 years if no vasculopathy is identified |
| Secondary vascular pathology (cavernomas, microhemorrhages) |
| Cavernomas and microhemorrhages: consideration of including T2* imaging (ex: GRE, SWI) at least every 5 years |
| Management |
| Vasculopathy |
| Referral to vascular neurology for consideration of antiplatelet agents and secondary stroke risk factor modification. |
| Moderate aerobic exercise 30 min 3–4 times a week |
Given the risk of vasculopathy, optimization of reversible vascular risk factors (diabetes, hypertension, etc.) is recommended for BT survivors treated with cranial RT. Vascular imaging should be considered among high-risk individuals and those presenting symptoms (ie, TIA, stroke, and amaurosis fugax) to assess vascular flow and secondary lesions. The ideal option for intracranial large vessel imaging is the ventricular mass index (VMI)—MRI, which gives high-resolution pictures of the vessel wall compared to the Carotid-Intima Media Thickness test (CIMT).16 If VMI-MRI is unavailable, a CTA head is preferred over standard magnetic resonance angiography (MRA) head for intracranial large vessel evaluation, renal function permitting. Vasculopathy affecting small vessels occur rather acutely due to abundant endothelial cells, which are extremely sensitive to RT.17 Unfortunately, these small vessels cannot be imaged with available technology. Structural brain MRIs can identify chronic small vessel ischemia areas as lacune or T2 hyperintensity. It is well understood that vasculopathy risk may accumulate over time. Clinical or imaging evidence of vasculopathy or ischemia should prompt referral to a vascular neurologist for evaluation and treatment.
The cerebrovascular risks from CTX during active treatment are related to endothelial toxicity and abnormalities in coagulation and hemostasis factors.18 Stroke-like events and stroke have been reported after methotrexate treatment, with a 40-fold increase among long-term survivors from pediatric cancer groups.19,20 This group does not recommend screening BT survivors treated alone with chemotherapy, immunotherapy, or targeted agents, as most known cases occur in the acute setting during treatment, and incidence in survivorship is rare.
Suggested recommendation:
• Management of vascular risk factors treated with cranial RT.
• Consideration of vascular imaging among high-risk individuals and those presenting symptoms.
• If there is evidence of vasculopathy by vessel imaging or clinical presentation, patients should be referred to a vascular neurologist for further evaluation and management.
Endocrinopathy
BT survivors are at risk for endocrinopathies related to the BT, surgery, RT, CTX, and other medications. The timing of the onset and type of endocrinopathy is related to the location and kind of BT and the treatment modalities employed. Most endocrinopathies are related to direct and indirect effects on the functioning of the hypothalamic-pituitary-end-organ axis. However, CTX can directly affect the gonads, leading to primary hypogonadism and infertility.21,22 Corticosteroids, frequently used in patients with BT, can also result in pituitary dysfunction. Although the effects are reversible if the corticosteroids are withdrawn, even relatively short-term use can have long-term adverse effects on obesity, insulin resistance, and the subsequent risk of diabetes and osteoporosis. More recently, the increasing use of immune checkpoint inhibitors (ICI) has led to a diverse list of immune-mediated endocrinopathies that may be secondary (pituitary) or primary (end-organ) in etiology resulting in specific endocrine surveillance guidelines.23
Tumors and surgical procedures that involve the hypothalamus or pituitary are likely to result in endocrine dysfunction in the short-term. However, retrospective data have also demonstrated delayed pituitary dysfunction after surgical intervention, even when BT is distant from the pituitary and hypothalamus.24 The effects of cranial RT on the endocrine systems may be undetected, given that symptoms of endocrine dysfunction can be subtle, non-specific, and gradual in onset. While the risk correlates with the RT dose to the hypothalamus and less so to the pituitary, the overall incidence of RT-induced pituitary dysfunction for tumors distant from the pituitary range from 38% to 80%.23,25 Endocrine deficiencies may occur as early as three months or greater than ten years after RT or neurosurgical procedures.26 Deficiencies of growth hormone, gonadotropins, thyroid-stimulating hormone, and adrenocorticotropin (ACTH) can occur singly or in combinations.
Our group recommends yearly screening of pituitary function, including thyroid and ACTH deficiency. TSH alone is insufficient to screen for thyroid dysfunction and requires free T4 in patients who received brain RT. Adrenal insufficiency can be a life-threatening condition and is diagnosed by the “gold standard” cosyntropin stimulation test. However, an AM cortisol value > 13 ug/dl is reassuring for normal function.27 Those with abnormal values should be referred to endocrinology for formal evaluation. Additionally, screening for pituitary deficiencies should be done whenever there is clinical suspicion, regardless of prior treatment. Growth hormone deficiency has great importance in children but can also be a factor in determining QOL issues in adults. Deficiencies of sex steroids can have wide-ranging effects, including an increased risk of osteoporosis. Our group recommends screening for testosterone and estrogen as clinically indicated. These recommendations are also summarized in Table 3.
Table 3.
Suggested Adult Brain Tumor Survivorship Guideline
| Complication | Brain Tumor Treatment | Suggested Screening Recommendations | Frequency |
|---|---|---|---|
| Eye | Cranial radiation close to optic nerve or known ocular pathology related to tumor or treatment | Neuro-ophthalmology evaluation | Annually |
| All brain tumor | Comprehensive eye examination | Every 1-2 years | |
| Hearing | All brain tumor | Hearing screen question: Do you have any difficulty with hearing? | Annually |
| Patients with cisplatin >200 mg/m2 or carboplatin >1500 mg/m2 | Audiology testing | Post treatment, 2 years after, then every 5 years | |
| Patient with Cranial RT | Post treatment baseline with audiogram. If detected hearing loss, annual audiogram. If normal, proceed with survey screening annually as below. |
Annually | |
| Radiosurgery near CN VIII | Audiology testing | Annually | |
| Cognition | Any | MoCA | Every 6 -12 months |
| Hormones/Endocrine | All brain tumor | Annual survey for symptoms | Every 6 -12 months |
| Brain Radiation | TSH, free T4, AM cortisol Men – Testosterone Women of child-bearing age – clinic screening with question of experiencing irregular menses |
Annually | |
| Immunotherapy | Per NCCN guidelines | Per NCCN guidelines | |
| Chemotherapy | Men: Testosterone Women of child-bearing age – clinic screening with question of experiencing irregular menses |
Men: Test once post treatment Women: Annually |
|
| Mood | Any | PHQ9 and GAD | Every 6 months |
| Sleep | Any | Insomnia single question: Do you have problems falling sleep or staying asleep for three or more nights per week? | Annually |
| STOP BANG | Every 5 years | ||
| RLS single question: “When you try to relax in the evening or sleep at night, do you ever have unpleasant, restless feelings in your legs that can be relieved by walking or movement?” | Annually | ||
| Balance/ Coordination | Any | Survey questions and exam: Have you had any difficulty taking care of yourself, walking, balancing, or falling? Have you had any difficulty taking your own medications? | Annually |
| Tandem stance (5-10 seconds), Romberg, Single leg stance | Annually | ||
| Cerebrovascular | Brain Radiation | Brain Vessel Imaging (CTA vs MRI) | 1 year from XRT, then 3 years. If no vasculopathy every 5 years. If cardiovascular risk factors every 3 years. |
| MRI brain with T2* imaging | Every 5 years | ||
| Employment | Any | If you are currently in work or school: Are you having difficulty preforming tasks at work or school? How often are you missing work? Have you received negative feedback on job/school performance? Is it taking you longer to complete tasks? Are you behind at work or putting in extra hours to keep up? Do you have concerns your employment or enrollment are in jeopardy? |
Every 6 months |
| Financial | Any | Over the last 6 months: Are you feeling more financially stressed? Are you struggling to meet monthly expenses? |
Every 6 months |
| Support/Caregivers | Any | Subjective Assessment/Survey Clinical Screening: Caregiver Needs Screen in Neuro-Oncology Family Caregivers (CNS) Distress Thermometer Kingston Caregiver Stress Scale (KCSS) |
Every 6 months |
| Secondary Malignancy | Chemotherapy or XRT | Physical exam | Annually |
Suggested recommendation:
• For patients treated with brain RT, annual screening of pituitary function with TSH, free T4, and morning cortisol.
• Estrogen and testosterone screening may be considered as clinically indicated.
Ophthalmic Sequelae
Considerations of ocular pathology in the setting of BT survivors are dependent not only on the type and location of the BT but also on accompanied treatment modalities. Preoperative counseling and expectation management are imperative given the poor potential of vision recovery after insult. While local compression and/or resection of BT are often the main source of morbidity in this patient population, RT and CTX have risks of ocular toxicity.28–30Table 4 summarizes ophthalmic complications commonly observed in BT survivors. Lastly, ICI medications also have possible ocular side effects, including retinal and optic nerve toxicity, anterior uveitis, and myasthenia gravis.31 These reactions are exceedingly rare, less than 1%; however, they should be considered ocular manifestations that can occur 1 week to 52 weeks from starting ICI.32 Collaboration and routine follow-up with ophthalmology specialists are essential to minimize ocular toxicity post-treatment during survivorship.
Table 4.
Summary of Ophthalmic Sequela in Adult Brain Tumor Survivors
| Complication | Manifestation |
|---|---|
| Visual field defect: Any damage along the posterior visual pathway may result in a contralateral homonymous visual defect | Patients typically complain of vision loss, often just in the eye with the temporal visual field loss, although they may present with difficulty reading or navigating |
| Optic neuropathy: Local compression/edema may acutely lead to optic nerve injury | Decreased vision, dyschromatopsia, and visual field loss |
| Cranial nerve palsy (III, IV, and VI) | Binocular diplopia is the most common complaint |
| Dry eye syndrome: Dose-dependent; exposure of ~34 Gy cumulative radiation carries a ~5% risk of severe DES | Complaints of foreign body sensation, stinging/burning eye pain, blurry vision worsened with reading or visual tasks |
| Cataract: Dose-dependent, risk increases with as little ~2–5 Gy in one fraction | Patients will complain of gradual decreased visual acuity or glare |
| Radiation retinopathy: Dose-dependent; exposure to less than ~25 Gy cumulative radiation is unlikely to develop significant retinopathy | Patients typically complain of gradual decreased visual acuity |
| Radiation optic neuropathy: Radiation doses from 50 to 60 Gy assumes a risk of ~5% within 10 years | Characterized by painless, progressive, rapid vision loss/dyschromatopsia over several days to weeks. May present acutely or years post-exposure (peak incidence 1.5 years) |
Suggested recommendation:
• For those who underwent cranial RT or have known ophthalmic BT sequela, perform an annual vision examination with consideration of referral to neuro-ophthalmology/ophthalmology.
Ototoxicity
Adult BT survivors experience ototoxicity as tinnitus and/or progressive, irreversible hearing loss. Ototoxicity is a common complication of platinum CTX and cranial RT, with more than 50% of patients who receive a combined modality developing treatment-induced hearing loss.33 However, this prevalence varies depending on the treatment regimen, patient age, baseline hearing levels, the presence of other confounding factors such as co-medications, renal toxicity, concomitant noise exposure, and genetic susceptibility.34 Hearing loss affects speech recognition and ease of communication; thus is associated with increased stress, social isolation, loneliness, impaired memory and cognition, and risk for dementia.35 Early identification of hearing loss and rehabilitation is crucial as it reduces the negative impacts on communication, QOL, and influences cognitive rehabilitation.36
Our group recommends long-term surveillance due to the risk of progressive hearing loss for any BT survivor treated with cranial RT or ototoxic chemotherapy. While audiology screening is the standard of care for children treated with ototoxic therapy, most adults with cancer do not receive baseline or post-treatment hearing evaluations.38 Survivorship programs provide an essential opportunity to address unmet hearing needs for patients who received ototoxic cancer therapy.
Suggested recommendation:
• For patients who received cisplatin > 200 mg/m2, carboplatin > 1500 mg/m2, formal hearing evaluation post-treatment, two years, and every 5 years.
• For patients treated with posterior fossa stereotactic radiosurgery (SRS), especially around the 8th cranial nerve, audiology screening post-treatment followed by annual testing.
• For patients who received cranial RT of 30 Gy or greater, baseline audiogram after treatment, if normal annual survey screening.
• Audiology and otolaryngology consultation for ongoing hearing surveillance for any BT survivor who has symptoms of hearing loss, tinnitus, or an abnormal hearing screen.
Physical Function
Impaired physical functioning, such as weakness, gait, and balance disorders, are prevalent in greater than 50% of BT survivors.36,37 gait and balance disorders are multifactorial due to the primary tumor, surgical resection, and/or treatment sequelae. Our group recommends a comprehensive neurological evaluation combined with questions focused on functioning during routine surveillance since a decline in physical functioning affects QOL and individuals’ risk for falls or injury (see Table 5). Gait speed measured with the 10-m walk test (10MWT) can be performed in the clinic to help identify patients at elevated risk for falls (speed cut-off < 0.7 m/s).38 Increased difficulty in performing activities of daily living or medication management is also indicative of possible BT-related sequelae. Routine evaluation of gait, balance, and physical functioning can detect subtle changes that could indicate tumor recurrence, new or worsening hydrocephalus, or late-delayed RT effects that may require medical interventions.41 Careful review of brain imaging to evaluate underlying structural causes with any change in function. Once the diagnostic workup is completed, we recommend referral to rehabilitation services. Patients with drastic changes in function or worsening spasticity should be referred to a physiatrist for a comprehensive evaluation, treatment, and rehabilitation therapy management.
Table 5.
Summary of Proposed Evaluation and Recommendation in Physical Function
| Subjective Questionnaire: In The Past Three Months, Have You Had Difficulty With | Specific Exam Maneuver | Referral Services as Indicated by Subjective and/or Physical Exam |
|---|---|---|
| Taking care of yourself (bathing, toileting, dressing)? | See the cognitive function section, strength testing, and balance testing as below | Occupational therapy |
| Walking, balancing, or falling? | Tandem stance < 10 s or single leg stance < 5 s, visuospatial neglect (letter cancellation test), 10MWT (gait speed < 0.7 m/s) | Physiatry (physical medicine and rehabilitation) and/or physical therapy |
| Taking medications without assistance? | Fine motor coordination in addition to cognitive screening. | Occupational therapy (fine/gross motor coordination, functional cognition) and/or speech therapy (swallow and/or cognitive therapy) |
Suggested recommendation:
• For all BT survivors, annual subjective assessment of independence and mobility.
• For all BT survivors, annual comprehensive neurological examination evaluating strength, coordination, vision, balance, tone, and cerebellar signs.
Sleep Disturbance
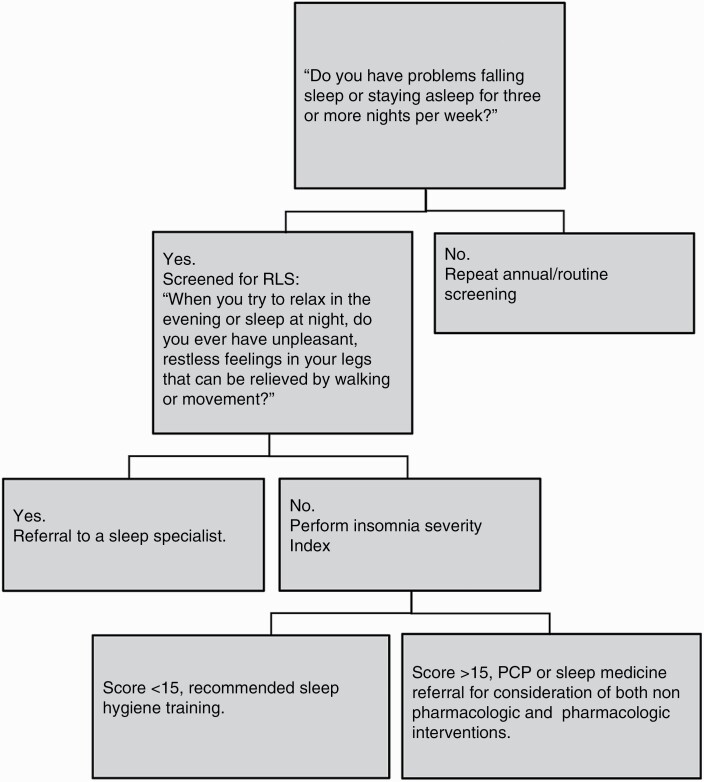
Sleep disturbance is one of the most commonly reported symptoms among patients with BT. Sleep disturbance, in this review, is the perceived or actual alterations in sleep resulting in impaired daytime functioning. Sleep disturbance encompasses insomnia, sleep-related breathing such as Obstructive Sleep Apnea (OSA), movement disorders like restless legs syndrome (RLS), and dissatisfaction with sleep quality. Because of its close association with psychological well-being, cognitive functioning, and QOL,39 our group recommends routine screening of sleep issues. The proposed sleep disturbance screening algorithm is described in Figure 1. The questionnaires included in the algorithm have shown sensitivity in neuro-oncology literature.45 Insomnia Severity Index (ISI) is a relatively short 7-question instrument that has demonstrated validity and internal consistency in cancer patients, including BT survivors. While a single cut-off for clinically significant insomnia has not been reported in BT survivors, data was extrapolated from other cancer types. If the ISI score is greater than 15, discussion of pharmacologic treatment and non-pharmacologic treatment with their PCP or referral to a sleep specialist is advised.40,41 Lastly, multiple studies have found an association between OSA and malignant BT.42,43 Given the health consequences of untreated OSA, it is prudent to include screening for OSA using the well-validated STOP-BANG tool every five years, regardless of sleep disturbance.
Figure 1.
Algorithm of sleep disturbance screening and surveillance.
Suggested recommendation:
• Annual screening from the time of BT diagnosis, independent of treatment, using ISI.
• STOP-BANG questionnaire screening every five years to screen for OSA.
Mood
Clinically important symptoms of depression, anxiety, and suicidal ideation are common complications that adversely affect patients with BT.44–46 Depression rates in survivors of BT are among the highest compared to survivors of other cancers.45 Within the first year of survivorship, the prevalence of depression among BT survivors ranges from 15% to 28%, with higher rates among patients with glioma.44,45,47 After treatment, the prevalence of depression increases to 38–42% and anxiety to 48%, with comorbid depression and anxiety at 31–34%.46 Suicidal ideation, reported in 10–12% of BT survivors, is associated with depression and anxiety severity, history of psychiatric disorders, and poorer health-related QOL.48 Other long-term side effects, including sleep disturbance, fatigue, and cognitive deficits overlap with depression and anxiety.49–51 Thus, careful evaluation is required to determine whether an underlying mood disorder is present and requires treatment.
The etiology of mood problems in BT survivors is multifactorial and can be influenced by tumor location, treatment, medications (eg, antiepileptic therapy, corticosteroid use), adjustment-related distress in response to the diagnosis, prognosis, and social and environmental factors (eg, family psychiatric history).47 Anxiety and depression symptoms are closely associated with diminished QOL, worse survival outcomes, and complications, such as deep vein thrombosis, seizure, systemic infection, and adverse drug reactions.44,49–51 Thus, our group recommends screening for these symptoms at the time of diagnosis, throughout the disease trajectory, and every six months into survivorship to inform appropriate treatment and mental health care. This screening can often be done in partnership with their PCP, given United States Preventive Services Task Force guidelines for screening all adults.52 Standard self-report measures such as Hospital Anxiety and Depressional Scale (HADS),53 9-item Patient Health Questionnaire (PHQ-9),54 and a 7-item Generalized Anxiety Disorder (GAD-7)55 have been used in cancer survivors, including BT survivors, to screen for depression and anxiety symptoms. A score of ≥ 7 on the HADS depression subscale56,57 and ≥ 8 on the HADS anxiety subscale,53 or a score of ≥ 10 on the PHQ-956,57 or GAD -758 warrant further investigation for a mood disorder.
Suggested recommendation:
• Routine screening at the time of diagnosis, then every six months using validated measures such as PHQ-9 and GAD-7 for all BT patients in partnership with PCP.
• Referrals to mental health specialists for further evaluation and interventions.
Financial Toxicity and Employment Status
The economic burden of BT survivors accumulates during their cancer diagnosis trajectory. The cost of treatment is estimated to be as high as $138 767 for those who received both CTX and RT, with a cumulative cost ranging from $262 877 to $274 416 at five years.59 Contributing factors to financial toxicity involve high unemployment and loss of income from the patient and their informal care partner, insurance reimbursement with large segments of out-of-pocket from medications/durable medical equipment, and medical expenses for hospital/physician services.60,61 Furthermore, the cost of transportation, home care services, and childcare potentiate this financial crisis resulting in acquiring loans in approximately 25–50% of patients.61 Our group recommends screening, assessing, evaluating, and managing financial toxicity as financial burden impacts overall survival, symptom burden, and QOL of cancer survivors. We suggest screening by asking if they are experiencing medical financial hardship. If so, this is followed by a collaboration with patient navigators, social workers, or providers in assessing and adjusting the current treatment plan and identifying resources for financial assistance.62
Assessing employment status and successful return to work is crucial in maintaining financial stability long-term. Employment is a valuable contributor to QOL, maintenance of identity, and physical and emotional health. In addition to providing financial security, employment for many BT survivors is necessary for accessing essential healthcare benefits.
BT survivors are at risk of employment disruption and job loss due to the impact of treatment and disease-related symptoms on work capacity.63 Decreased productivity, absenteeism, and job performance problems can occur, with fatigue and cognitive impairment as key symptoms leading to job challenges.63 Patients often under-report employment problems; therefore, our group recommends explicitly asking if they have any difficulty performing job duties or how often they are missing work (Table 3). Educating patients about disability rights, developing employment support strategies, and referral to Vocational Rehabilitation (VR) are fundamental parts of survivorship counseling. VR can facilitate services for individuals with disabilities that have difficulty maintaining employment or returning to work through job accommodation and modification, employment retention, and funding for job training, equipment, and other medical services.
Suggested recommendation:
• Subjective assessment of medical financial hardship at the time of tumor diagnosis and every six months for all BT patients.
• Collaboration between social workers, patient navigators, and providers in adjusting the current treatment plan and identifying resources for financial assistance.
• Subjective assessment of employment status, productivity, and absenteeism every six months and early referral to vocational rehabilitation.
Caregiver Burden and Support
Care partners must monitor and often mitigate debilitating physiological, behavioral, and cognitive symptoms of BT survivors. Although caregiving can be very fulfilling, it is demanding and stressful, especially when care partners have multiple competing responsibilities and care recipients have unmet care needs.64 Hence, most BT care partners experience significant distress.65 Caregiving demands may influence partner’s physical and emotional health, their ability to provide care, and even the recipient’s survival.66,67 It is imperative that clinicians regularly assess and address care partner needs. Best practices for supporting BT patients include frequent monitoring using patient-reported outcome measures and tailored supportive care. Our group recommends the same practices for care partners. Because caregivers are not patients of record, most health systems do not have the efficient infrastructure and trained staff to ensure that their needs are regularly reflected in care plans.
Assessment measures developed for general cancer populations do not capture neurological and cognitive difficulties. They, therefore, have limited generalizability to BT patients. Caregiver Needs Screen in Neuro-Oncology Family Caregivers [CNS]68 is one of the best disease-specific validated measures. CNS is a 30-item, participant-centered self-report that takes 5–7 min to administer. Other measures applicable in neuro-oncology are caregiver needs assessments developed for people living with dementia. A comprehensive review of the reliability, validity, and relevance of these measures identified Partnering for Better Health: Living with Dementia [PBH-LCI]69 as a current gold standard.70 Furthermore, most BT care partners report higher distress than their care recipients but do not endorse these symptoms spontaneously. We recommend that clinicians must iteratively assess and address caregiver stress.71,72 Clinic visits are usually very taxing and not an indicator of overall stress.64 The best measurement approach is to prompt caregivers to reflect on their stress levels during the past week at home. A distress thermometer is a gold standard measure of stress in oncology. Another practical measure is KCSS, a brief, valid, and reliable measure that assesses caregiving, family, and financial issues.73 Care for BT survivors requires care partner support which includes educating clinicians about the importance of caregiver assessment74 and, ideally, creating caregiver support programs and/or facilitating caregiver-to-caregiver peer support.75
Suggested recommendation:
• Subjective assessment of BT care partner’s unmet needs and stress using Caregiver Needs Screen in Neuro-Oncology Family Caregivers (CNS), Distress Thermometer, or Kingston Caregiver Stress Scale (KCSS) at the time of tumor diagnosis and every 6 months.
Second Malignancy
BT survivors are at risk for secondary malignancies induced by ionizing RT. These can be detected several years after therapy within the radiation treatment field and differ in histology from the original tumor.76 After brain irradiation, the most common secondary malignancy is meningioma, followed by glioma and sarcoma.77–80 The precise incidence of secondary malignancy for adult BT survivors is challenging given the overall lack of long-term follow-up, ascertainment bias, and primary data concentration on the pediatric population. Secondary malignancies often develop away from the primary tumor location in the lower dose radiation regions. For instance, one study noted 12% of secondary malignancies in the irradiated volume (the planning target volume), 66% in the beam-bordering region, and 22% in the areas located more than 5 cm from the irradiated volume. There was no threshold dose for the risk of secondary malignancy.81 The limited literature on the adult BT population, compared to the general population, suggests that the 30-year cumulative risk for secondary malignancy ranges from 2.7% to 8.5% in irradiated patients.78,82–85 However, this incidence cannot be attributed to RT alone, as patients with primary BT already have an increased risk of subsequent secondary malignancy.84,86 Furthermore, most of the data on the adult population are from patients treated for pituitary adenoma with older RT techniques.78,82,83 Current techniques allow for an overall decreased volume of the irradiated brain.
There is a paucity of data regarding whether early detection and surveillance for secondary malignancy improve outcomes.87 Some studies suggest treatment can improve survival after secondary malignancy.88 Thus, it may be helpful to detect secondary malignancy earlier. Our group recommends counseling on the risk of secondary malignancy, routine imaging surveillance as indicated for tumor surveillance or vasculopathy screening, and annual neurological exam. If a secondary malignancy is suspected, given varied RT techniques utilized, it is important to verify whether the secondary malignancy correlates with the irradiated field with the recorded treatment plan.
Suggested recommendation:
• Upfront counseling on the risk of secondary malignancy for BT patients at the time of cranial RT.
• Annual neurologic exam and routine imaging as indicated for tumor surveillance or vasculopathy screening.
Conclusion
As therapeutic and diagnostic strategies improve, especially for patients with metastatic brain tumors, the population of adult BT survivors will continue to grow. At this time, most BT survivorship research has focused on childhood survivors of primary BT, limiting the data to guide survivorship in adult BT survivors. We formed a multidisciplinary committee to review the available literature and develop recommendations for surveillance of adult BT survivors. These guidelines will advance future research into treatment complications in adult BT survivorship.
Our group recognizes the limitations of this review. There is an active project to evaluate care partners’ and patients’ perceptions regarding survivorship screening. The screening does require time and effort from patients and their care partners as well as medical providers. Optimal care of these complicated patients requires partnership with PCP as screening guidelines overlap with general health screening.49 The majority of experts from this group were from a single-institution, and insurance coverage for screening at our institute has not been a barrier. A broader more diverse group of experts across the globe should be considered in revising these guidelines.
Despite the limitations of this review, comprehensive monitoring of the unique complications in adult BT survivors is necessary in addition to routine tumor surveillance. The comprehensive screening and surveillance recommendations developed through this multidisciplinary group are summarized in Table 3. On-going thorough symptom assessment during survivorship is required due to the variability of onset and progressive nature of the medical conditions. Recognition and early detection of treatment complications are intended to improve health and maximize the QOL of BT survivors. Further research is necessary to document the incidence and prevalence of medical complications as well as evaluate the efficacy of screening and neuro-oncology survivorship programs.
Acknowledgments
Our group would like to acknowledge Dr Gavriel Kohlberg and Dr Flavia Consens, Corrine Hoeppner, ARNP, Katie Sofie, MSW, Mandy Myers-Little, and Samantha Shula, RNs, for contributing immensely to the Alvord Brain Tumor Survivorship program.
Contributor Information
Karl Cristie F Figuracion, ITHS TL1 Training Program University of Washington School of Nursing, Seattle, Washington 98105, USA; Alvord Brain Tumor Center, Department of Radiation Oncology, University of Washington, Seattle, Washington 98105, USA.
Lia M Halasz, Department of Radiation Oncology, School of Medicine, University of Washington, Seattle, Washington 98105, USA.
Ny-Ying Lam, Department of Rehabilitation Medicine, School of Medicine, University of Washington, Seattle, Washington 98105, USA.
Myron Goldberg, Department of Rehabilitation Medicine, School of Medicine, University of Washington, Seattle, Washington 98105, USA.
Joe Stuckey, Department of Rehabilitation Medicine, School of Medicine, 98105 University of Washington, Seattle, Washington 98105, USA.
Richard A Failor, Department of Metabolism, Endocrinology and Nutrition, University of Washington, Seattle, Washington, 98105, USA.
Lindsey M Knowles, Department of Rehabilitation Medicine, University of Washington, Seattle, Washington 98105, USA.
Samantha Artherholt, Department of Rehabilitation Medicine, University of Washington, Seattle, Washington 98105, USA.
Brian Chou, Department of Ophthalmology, School of Medicine, University of Washington, Seattle, Washington 98105, USA.
Courtney E Francis, Department of Ophthalmology, School of Medicine, University of Washington, Seattle, Washington 98105, USA.
Kristin Knight, Oregon Health and Science University, Portland, Oregon 97239, USA.
Maninder Kaur, Loma Linda University Health, Loma Linda, California, USA.
Tatiana Sadak, Biobehavioral Nursing and Health Informatics, School of Nursing, University of Washington, Seattle, Washington 98105, USA.
Tresa McGranahan, Department of Neurology, School of Medicine, University of Washington, Seattle, Washington 98105, USA.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Ms. Karl Cristie F. Figuracion is currently supported by the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number TL1 TR002318, Jonas Scholar 2021-2023, and Oncology Nursing Foundation. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health. Dr. Lia M. Halasz is receiving funding from BioMimetix and Kuni Foundation. Ms. Kristy Knight is supported by Alex’s Lemonade Stand Foundation. Dr. Tresa McGranahan has clinical trial support from the Kuni Foundation, Novocure, BioMimetix, Denovo Biopharma and Institut de Recherches Internationales Servier (I.R.I.S.).
Conflict of interest statement. There is no conflict of interest when writing this manuscript, other than we are clinicians who collaborate routinely in managing these complications in our current institution.
References
- 1. Ostrom QT, Cioffi G, Waite K, et al. CBTRUS Statistical Report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021;23(12 Suppl 2):iii1–iii105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Comprehensive Cancer Network. Survivorship (Version 1.2022). https://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Accessed May 3, 2022.
- 3. Hudson MM, Bhatia S, Casillas J, Landier W, Section On hematology/oncology CSOGASOFPHO. Long-term follow-up care for childhood, adolescent, and young adult cancer survivors. Pediatrics. 2021;148(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. van Kessel E, Baumfalk AE, van Zandvoort MJ, et al. Tumor-related neurocognitive dysfunction in patients with diffuse glioma: a systematic review of neurocognitive functioning prior to anti-tumor treatment. J. Neuro-oncol. 2017;134(1):9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neuro-oncol. 2006;76(3):283–91. [DOI] [PubMed] [Google Scholar]
- 6. Armstrong TS, Mendoza T, Gning I, et al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neuro-oncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 7. Wagner L, Sweet, J., Butt, Z., et al. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy–cognitive function instrument. J Support Oncol. 2009;7(6):W32–W39. [Google Scholar]
- 8. Weitzner MA, Meyers CA, Gelke CK, et al. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–11 61. [DOI] [PubMed] [Google Scholar]
- 9. Robinson GA, Biggs V, Walker DG. Cognitive screening in brain tumors: short but sensitive enough? Front Oncol. 2015;5:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sohlberg MM. Cognitive Rehabilitation Manual: Translating Evidence-based Recommendations into Practice. Oxford University Press; 2012. [Google Scholar]
- 11. Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2006;24(33):5277–5282. [DOI] [PubMed] [Google Scholar]
- 12. Xu J, Cao Y. Radiation-induced carotid artery stenosis: a comprehensive review of the literature. Int. Neurol. 2013;2(4):183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bowers DC, Mulne AF, Reisch JS, et al. Nonperioperative strokes in children with central nervous system tumors. Cancer. 2002;94(4):1094–101. [PubMed] [Google Scholar]
- 14. Kreisl TN, Toothaker T, Karimi S, DeAngelis LM. Ischemic stroke in patients with primary brain tumors. Neurology. 2008;70(24):2314–20. [DOI] [PubMed] [Google Scholar]
- 15. Morris B, Partap S, Yeom K, et al. Cerebrovascular disease in childhood cancer survivors: A Children’s Oncology Group Report. Neurology. 2009;73(22):1906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tanyildizi Y, Keweloh S, Neu MA, et al. Radiation-induced vascular changes in the intracranial irradiation field in medulloblastoma survivors: an MRI study. Radiother Oncol. 2019;136:50–55. [DOI] [PubMed] [Google Scholar]
- 17. Ghazaleh D, Beran A, Berry B, Ghannam M. Occlusive radiation cerebral vasculopathy implies medical complexity: a case report. J Med Case Rep. 2019;13(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saynak M, Cosar-Alas R, Yurut-Caloglu V, et al. Chemotherapy and cerebrovascular disease. J Balkan Union Oncol. 2008;13(1):31. [PubMed] [Google Scholar]
- 19. Rollins N, Winick N, Bash R, Booth T. Acute methotrexate neurotoxicity: findings on diffusion-weighted imaging and correlation with clinical outcome. Am J Neuroradiol. 2004;25(10):1688–1695. [PMC free article] [PubMed] [Google Scholar]
- 20. Grisold W, Oberndorfer S, Struhal W. Stroke and cancer: a review. Acta Neurol Scand. 2009;119(1):1–16. [DOI] [PubMed] [Google Scholar]
- 21. Molina JR, Barton DL, Loprinzi CL. Chemotherapy-induced ovarian failure. Drug Saf. 2005;28(5):401–416. [DOI] [PubMed] [Google Scholar]
- 22. Servitzoglou M, De Vathaire F, Oberlin O, et al. Dose–effect relationship of alkylating agents on testicular function in male survivors of childhood lymphoma. Pediat Hematol Oncol. 2015;32(8):613–23. [DOI] [PubMed] [Google Scholar]
- 23. Barroso-Sousa R, Barry WT, Garrido-Castro AC, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol. 2018;4(2):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schneider HJ, Rovere S, Corneli G, et al. Endocrine dysfunction in patients operated on for non-pituitary intracranial tumors. Eur J Endocrinol. 2006;155(4):559–566. [DOI] [PubMed] [Google Scholar]
- 25. Couto-Silva AC, Brauner R, Adan LF. Endocrine sequelae after radiotherapy in childhood and adolescence. Arquivos brasileiros de endocrinologia e metabologia. 2005;49(5):825–832. [DOI] [PubMed] [Google Scholar]
- 26. Chow EJ, Liu W, Srivastava K, et al. Differential effects of radiotherapy on growth and endocrine function among acute leukemia survivors: a childhood cancer survivor study report. Pediat Blood Cancer. 2013;60(1):110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary–adrenal insufficiency: a meta-analysis. J Clin Endocrinol Metab. 2008;93(11):4245–4253. [DOI] [PubMed] [Google Scholar]
- 28. Mayo C, Martel MK, Marks LB, et al. Radiation dose–volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S28–35. [DOI] [PubMed] [Google Scholar]
- 29. Chodick G, Bekiroglu N, Hauptmann M, et al. Risk of cataract after exposure to low doses of ionizing radiation: a 20-year prospective cohort study among US radiologic technologists. Am J Epidemiol. 2008;168(6):620–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Indaram M, Ali FS, Levin MH. In search of a treatment for radiation-induced optic neuropathy. Cur Treat Opt Neurol. 2015;17(1):325. [DOI] [PubMed] [Google Scholar]
- 31. Kim JM, Materin MA, Sznol M, et al. Ophthalmic immune-related adverse events of immunotherapy: a single-site case series. Ophthalmology. 2019;126(7):1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Parikh RA, Chaon BC, Berkenstock MK. Ocular Complications of Checkpoint Inhibitors and Immunotherapeutic Agents: A Case Series. Ocul Immunol Inflamm. 2020:1–6. [DOI] [PubMed] [Google Scholar]
- 33.Theunissen EA, Zuur CL, Jóźwiak K, et al. Prediction of hearing loss due to cisplatin chemoradiotherapy. JAMA Otolaryngol–Head Neck Surg. 2015;141(9):810–815. [DOI] [PubMed]
- 34. Zuur CL, Simis YJ, Lansdaal PE, et al. Risk factors of ototoxicity after cisplatin-based chemo-irradiation in patients with locally advanced head-and-neck cancer: a multivariate analysis. Int J Radiat Oncol Biol Phys. 2007;68(5):1320–1325. [DOI] [PubMed] [Google Scholar]
- 35. Lin FR, Ferrucci L, Metter EJ, et al. Hearing loss and cognition in the Baltimore longitudinal study of aging. Neuropsychology. 2011;25(6):763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ness KK, Morris EB, Nolan VG, et al. Physical performance limitations among adult survivors of childhood brain tumors. Cancer. 2010;116(12):3034–3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sloane K, Vachani C, Hampshire MK, et al. Late effects in survivors of central nervous system tumors: reports by patients and proxies. J Cancer Survivorship. 2016;10(2):234–240. [DOI] [PubMed] [Google Scholar]
- 38. Middleton A, Fritz SL, Lusardi M. Walking speed: the functional vital sign. J Aging Phys Act. 2015;23(2):314–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jeon MS, Dhillon HM, Descallar J, et al. Prevalence and severity of sleep difficulty in patients with a CNS cancer receiving palliative care in Australia. Neuro-oncol Pract. 2019;6(6):499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Armstrong TS, Gilbert MR. Practical strategies for management of fatigue and sleep disorders in people with brain tumors. Neuro-oncology. 2012;14(Suppl 4):iv65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Howell D, Oliver T, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;25(4):791–800. [DOI] [PubMed] [Google Scholar]
- 42. Cho JH, Lim YC, Han K-D, et al. The incidence of malignant brain tumors is increased in patients with obstructive sleep apnea: A national health insurance survey. PloS One. 2020;15(11):e0241598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen J-C, Hwang J-H. Sleep apnea increased incidence of primary central nervous system cancers: a nationwide cohort study. Sleep Med. 2014;15(7):749–754. [DOI] [PubMed] [Google Scholar]
- 44. Rooney AG, Carson A, Grant R. Depression in cerebral glioma patients: a systematic review of observational studies. J Natl Cancer Inst. 2011;103(1):61–76. [DOI] [PubMed] [Google Scholar]
- 45. Krebber A, Buffart L, Kleijn G, et al. Prevalence of depression in cancer patients: a meta‐analysis of diagnostic interviews and self‐report instruments. Psycho‐Oncology. 2014;23(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arnold SD, Forman LM, Brigidi BD, et al. Evaluation and characterization of generalized anxiety and depression in patients with primary brain tumors. Neuro-oncology. 2008;10(2):171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wellisch DK, Kaleita TA, Freeman D, Cloughesy T, Goldman J. Predicting major depression in brain tumor patients. Psychooncology. 2002;11(3):230–238. [DOI] [PubMed] [Google Scholar]
- 48. Litofsky NS, Farace E, AndersonF, Jr., et al. Depression in patients with high-grade glioma: results of the Glioma Outcomes Project. Neurosurgery. 2004;54(2):358–366; discussion 366–357. [DOI] [PubMed] [Google Scholar]
- 49. Noll KR, Sullaway CM, Wefel JS. Depressive symptoms and executive function in relation to survival in patients with glioblastoma. J Neuro-oncology. 2019;142(1):183–191. [DOI] [PubMed] [Google Scholar]
- 50. Miovic M, Block S. Psychiatric disorders in advanced cancer. Cancer. 2007;110(8):1665–1676. [DOI] [PubMed] [Google Scholar]
- 51. Hickmann A-K, Nadji-Ohl M, Haug M, et al. Suicidal ideation, depression, and health-related quality of life in patients with benign and malignant brain tumors: a prospective observational study in 83 patients. Acta Neurochir. 2016;158(9):1669–1682. [DOI] [PubMed] [Google Scholar]
- 52. Siu AL, Force USPST, Bibbins-Domingo K, et al. Screening for depression in adults: US preventive services task force recommendation statement. JAMA. 2016;315(4):380–387. [DOI] [PubMed] [Google Scholar]
- 53. Gehrke AK, Baisley MC, Sonck AL, et al. Neurocognitive deficits following primary brain tumor treatment: systematic review of a decade of comparative studies. J Neuro-oncology. 2013;115(2):135–142. [DOI] [PubMed] [Google Scholar]
- 54. Rooney AG, McNamara S, Mackinnon M, et al. Screening for major depressive disorder in adults with cerebral glioma: an initial validation of 3 self–report instruments. Neuro–oncology. 2013;15(1):122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pringle A, Taylor R, Whittle I. Anxiety and depression in patients with an intracranial neoplasm before and after tumour surgery. Br J Neurosur. 1999;13(1):46–51. [DOI] [PubMed] [Google Scholar]
- 56. Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. J Gen Int Med. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pranckeviciene A, Bunevicius A. Depression screening in patients with brain tumors: a review. CNS Oncol. 2015;4(2):71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bunevicius A, Deltuva V, Tamasauskas S, et al. Screening for psychological distress in neurosurgical brain tumor patients using the patient health questionnaire‐2. Psycho‐Oncology. 2013;22(8):1895–1900. [DOI] [PubMed] [Google Scholar]
- 59. Jiang S, Hill K, Patel D, et al. Direct medical costs of treatment in newly-diagnosed high-grade glioma among commercially insured US patients. J Med Econ. 2017;20(12):1237–1243. [DOI] [PubMed] [Google Scholar]
- 60. Aly A, Singh P, Korytowsky B, et al. Survival, costs, and health care resource use by line of therapy in US Medicare patients with newly diagnosed glioblastoma: a retrospective observational study. Neurooncol Pract. 2020;7(2):164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Haider SA, Asmaro K, Kalkanis SN, et al. The economic impact of glioma survivorship: The cost of care from a patient perspective. Neurology. 2020;95(11):e1575–e1581. [DOI] [PubMed] [Google Scholar]
- 62. Yabroff KR, Bradley CJ, Shih YT. Improving the process of screening for medical financial hardship in oncology Practice. Cancer Epidemiol Biomarkers Prev. 2021;30(4):593–596. [DOI] [PubMed] [Google Scholar]
- 63. Silvaggi F, Leonardi M, Raggi A, et al. Employment and work ability of persons with brain tumors: a systematic review. Front Human Neurosci. 2020;14:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Boele FW, van Uden-Kraan CF, Hilverda K, et al. Neuro-oncology family caregivers’ view on keeping track of care issues using eHealth systems: it’s a question of time. J Neurooncol. 2017;134(1):157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Trad W, Koh ES, Daher M, et al. Screening for psychological distress in adult primary brain tumor patients and caregivers: considerations for cancer care coordination. Front Oncol. 2015;5:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Boele FW, Given CW, Given BA, et al. Family caregivers’ level of mastery predicts survival of patients with glioblastoma: a preliminary report. Cancer. 2017;123(5):832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Choi CW, Stone RA, Kim KH, et al. Group-based trajectory modeling of caregiver psychological distress over time. Ann Behav Med. 2012;44(1):73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Boele FW, Terhorst L, Prince J, et al. Psychometric evaluation of the caregiver needs screen in neuro-oncology family caregivers. J Nurs Meas. 2019;27(2):162–176. [DOI] [PubMed] [Google Scholar]
- 69. Sadak T, Korpak A, Borson S. Measuring caregiver activation for health care: validation of PBH-LCI:D. Geriatr Nurs. 2015;36(4):284–292. [DOI] [PubMed] [Google Scholar]
- 70. Kipfer S, Pihet S. Reliability, validity and relevance of needs assessment instruments for informal dementia caregivers: a psychometric systematic review. JBI Evid Synth. 2020;18(4):704–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Piil K, Jakobsen J, Christensen KB, et al. Needs and preferences among patients with high-grade glioma and their caregivers—a longitudinal mixed methods study. Eur J Cancer Care (Engl). 2018;27(2):e12806. [DOI] [PubMed] [Google Scholar]
- 72. Renovanz M, Maurer D, Lahr H, et al. Supportive care needs in glioma patients and their caregivers in clinical practice: results of a multicenter cross-sectional study. Front Neurol. 2018;9:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sadak T, Korpak A, Wright JD, et al. Psychometric evaluation of Kingston Caregiver Stress Scale. Clin Gerontol. 2017;40(4):268–280. [DOI] [PubMed] [Google Scholar]
- 74. Sherwood P, Cwiklik M, Donovan HS. Neuro-oncology family caregiving: review and directions for future research. CNS Oncol. 2016;5(1):41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Page MS, Chang SM. Creating a caregiver program in neuro-oncology. Neurooncol Pract. 2017;4(2):116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cahan WG, Woodard HQ, Higinbotham NL, et al. Sarcoma arising in irradiated bone: report of eleven cases. Cancer: Interdiscipl Int J Am Cancer Soc. 1998;82(1):8–34. [DOI] [PubMed] [Google Scholar]
- 77. Goshen Y, Stark B, Kornreich L, et al. High incidence of meningioma in cranial irradiated survivors of childhood acute lymphoblastic leukemia. Pediat Blood Cancer. 2007;49(3):294–297. [DOI] [PubMed] [Google Scholar]
- 78. Minniti G, Traish D, Ashley S, et al. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J Clin Endocrinol Metabol. 2005;90(2):800–804. [DOI] [PubMed] [Google Scholar]
- 79. Strojan P, Popović M, Jereb B. Secondary intracranial meningiomas after high-dose cranial irradiation: report of five cases and review of the literature. Int J Radiat Oncol Biol Phys. 2000;48(1):65–73. [DOI] [PubMed] [Google Scholar]
- 80. Vinchon M, Leblond P, Caron S, et al. Radiation-induced tumors in children irradiated for brain tumor: a longitudinal study. Child’s Nervous Syst. 2011;27(3):445–453. [DOI] [PubMed] [Google Scholar]
- 81. Diallo I, Haddy N, Adjadj E, et al. Frequency distribution of second solid cancer locations in relation to the irradiated volume among 115 patients treated for childhood cancer. Int J Radiat Oncol Biol Phys. 2009;74(3):876–883. [DOI] [PubMed] [Google Scholar]
- 82. Tsang R, Laperriere N, Simpson W, et al. Glioma arising after radiation therapy for pituitary adenoma. A report of four patients and estimation of risk. Cancer. 1993;72(7):2227–2233. [DOI] [PubMed] [Google Scholar]
- 83. Norberg L, Johansson R, Rasmuson T. Intracranial tumours after external fractionated radiotherapy for pituitary adenomas in northern Sweden. Acta Oncol. 2010;49(8):1276–1282. [DOI] [PubMed] [Google Scholar]
- 84. Kutsenko A, De Gonzalez AB, Curtis RE, Rajaraman P. Risk of second benign brain tumors among cancer survivors in the surveillance, epidemiology, and end results program. Cancer Causes Contr. 2014;25(6):659–668. [DOI] [PubMed] [Google Scholar]
- 85. Baker KS, DeFor TE, Burns LJ, et al. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21(7):1352–1358. [DOI] [PubMed] [Google Scholar]
- 86. Van Varsseveld N, Van Bunderen C, Ubachs D, et al. Cerebrovascular events, secondary intracranial tumors, and mortality after radiotherapy for nonfunctioning pituitary adenomas: a subanalysis from the Dutch National Registry of Growth Hormone Treatment in Adults. J Clin Endocrinol Metab. 2015;100(3):1104–1112. [DOI] [PubMed] [Google Scholar]
- 87. Bowers DC, Verbruggen LC, Kremer LCM, et al. Surveillance for subsequent neoplasms of the CNS for childhood, adolescent, and young adult cancer survivors: a systematic review and recommendations from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2021;22(5):e196–e206. [DOI] [PubMed] [Google Scholar]
- 88. Paulino AC, Mai WY, Chintagumpala M, et al. Radiation-induced malignant gliomas: is there a role for reirradiation? Int J Rad Oncol Biol Phys. 2008;71(5):1381–1387. [DOI] [PubMed] [Google Scholar]