Abstract
Bordetella bronchiseptica and Bordetella parapertussis express a surface polysaccharide, attached to a lipopolysaccharide, which has been called O antigen. This structure is absent from Bordetella pertussis. We report the identification of a large genetic locus in B. bronchiseptica and B. parapertussis that is required for O-antigen biosynthesis. The locus is replaced by an insertion sequence in B. pertussis, explaining the lack of O-antigen biosynthesis in this species. The DNA sequence of the B. bronchiseptica locus has been determined and the presence of 21 open reading frames has been revealed. We have ascribed putative functions to many of these open reading frames based on database searches. Mutations in the locus in B. bronchiseptica and B. parapertussis prevent O-antigen biosynthesis and provide tools for the study of the role of O antigen in infections caused by these bacteria.
Lipopolysaccharide (LPS) is the major glycolipid molecule present on the cell surface of gram-negative bacteria. Most of our understanding of LPS has come from early studies of the Enterobacteriaceae (15, 35), in which the molecule has usually been described as having three domains: lipid A, core, and O antigen. Lipid A is often linked through 2-keto-3-deoxyoctulosonic acid to the core oligosaccharide, which consists of heptoses and hexoses. Linked to the core is the O-antigen polysaccharide, which consists of repeats of oligosaccharide units that in turn consist of one or more sugars.
The genus Bordetella contains several species, some of which are respiratory tract pathogens. B. pertussis and B. parapertussis cause whooping cough (6, 7, 12, 16, 17), and B. parapertussis is also found in ovine species (18–20). B. bronchiseptica infects many species of animals and is commonly associated with atrophic rhinitis in pigs, snuffles in rabbits, and kennel cough in dogs (3, 24, 30, 31). B. bronchiseptica has also been occasionally described as a respiratory tract pathogen in humans (10, 23, 29). All three pathogens are very closely related in terms of multilocus enzyme electrophoresis, DNA hybridization, and DNA sequence analyses (27, 34). The mechanistic bases for their different host ranges and pathogenicities are unknown but are likely to depend on differences in surface structures between the three pathogens.
The LPS molecules from the three bordetellae share basic structural features in that they each have a lipid A domain and a branched-chain core oligosaccharide (4, 5, 13), but there are also substantial differences. One of the most striking of these is that B. bronchiseptica and B. parapertussis synthesize a long-chain polysaccharide structure consisting of a homopolymer of 2,3-dideoxy-2,3-diN-acetylgalactosaminuronic acid (2,3-diNAcGalA), known as O antigen, whereas B. pertussis does not (9). This structural difference between the LPS molecules of the three main pathogenic bordetellae is substantial and likely to confer quite different surface properties on the different species. The genetic basis for this difference is unknown.
We report the identification of a DNA locus that is present in B. bronchiseptica and B. parapertussis but is absent from B. pertussis. Mutation of this locus results in the loss of O-antigen expression. We thus propose that this locus contains genes required for O-antigen biosynthesis and/or assembly and that its absence from B. pertussis explains the absence of O antigen in this species. The generation of defined O-antigen-deficient mutants will allow the investigation of the role of this domain of the LPS molecule in the pathogenesis of infections due to B. bronchiseptica and B. parapertussis.
MATERIALS AND METHODS
Bacterial strains and plasmids.
B. pertussis BP536, B. bronchiseptica CN7635E, and B. parapertussis CN2591 have been described (1). B. bronchiseptica RB50 was from Jeff Miller, University of California, Los Angeles (8). Escherichia coli XL1-Blue (Stratagene, Cambridge, United Kingdom) was used for the cloning and maintenance of pUC plasmids, and E. coli SM10λpir was used as the donor in conjugations with suicide vectors.
pUC18 was used as a general cloning vector and pEX100T (26) was used to generate allelic-exchange mutants in B. parapertussis and B. bronchiseptica. This vector is a ColE1 replicon and thus is unable to replicate in bordetellae. It also contains oriT and can thus be mobilized by E. coli SM10λpir. Conjugations were performed as previously described (1) on Bordet-Gengou plates supplemented with 15% defibrinated horse blood (Department of Clinical Veterinary Medicine, University of Cambridge) and 10 mM MgCl2; Bordetella strains RB50 and CN2591 were recipients and E. coli SM10λpir, carrying the appropriate plasmid, was the donor.
Media, chemicals, and reagents.
Bordetella strains were grown on Bordet-Gengou agar supplemented with 15% defibrinated horse blood. E. coli strains were grown on Luria-Bertani agar or in Luria-Bertani broth. All media were purchased from Difco or Oxoid. Antibiotic resistance was selected by using ampicillin at 100 μg/ml, chloramphenicol at 10 μg/ml (Bordetella) or 30 μg/ml (E. coli), and streptomycin at 200 μg/ml. Antibiotics and standard chemicals were purchased from Sigma. DNA restriction endonucleases and other modifying enzymes were bought from Boehringer Mannheim (Lewes, United Kingdom).
DNA preparations.
Plasmid DNA was purified using a plasmid DNA preparation kit (Qiagen, Crawley, United Kingdom). Chromosomal DNA was prepared in agarose blocks as described previously (25).
Southern hybridizations.
Southern hybridizations were performed using a digoxigenin hybridization and detection system from Boehringer Mannheim according to the manufacturer’s instructions.
LPS preparation, SDS-PAGE, and Western blot.
LPS was prepared as described previously (21) and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using the Tricine gel buffer system as described previously (14), followed by silver staining (32). Western blotting was performed as previously described (1).
DNA sequencing and sequence analysis.
B. bronchiseptica DNA was sequenced as a preliminary to the Bordetella genome sequencing project at the Sanger Centre, Hinxton, United Kingdom. B. parapertussis DNA was sequenced with an automated sequencer at the Department of Biochemistry, University of Cambridge. DNA sequence was analyzed with the Genetics Computer Group software package (Wisconsin Package, version 9.1; Genetics Computer Group, Madison, Wis.) and the annotation tool DIANA (2a), which allows the integration of the results of BLASTX, BLASTN, BLASTP, and FASTA searches against the EMBL, TREMBL, and SwissProt databases with Prosite, Pfam, and other protein motif searches. Gene prediction was based on codon-specific positional base preference and amino acid bias.
Nucleotide sequence accession numbers.
The DNA sequence and detailed annotations are available in the EMBL DNA sequence database under accession no. AJ007747.
RESULTS
Cloning of the O-antigen biosynthesis locus.
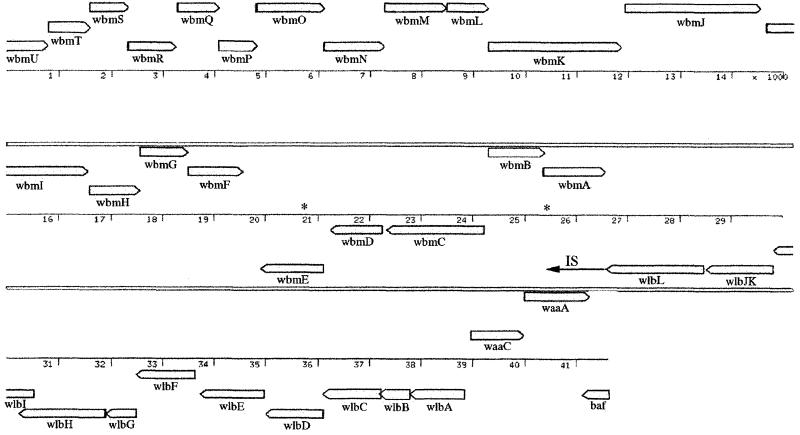
The identification and characterization of the wlb loci (previously bpl), required for LPS biosynthesis in the bordetellae, were described previously (2). Cosmid clones were isolated from B. bronchiseptica (pBgl-br) and B. parapertussis (pBgl-pa) that were apparently the same, each containing a 41,624-bp BglII fragment containing wlb as well as an extra 20 to 25 kb of DNA. It was noted that the DNA sequence of the 3′ end of wlb in B. bronchiseptica and B. parapertussis differed from that in B. pertussis. In place of the insertion sequence (IS) found at the 3′ end of the B. pertussis locus, B. bronchiseptica and B. parapertussis had DNA which we speculated may be required for O-antigen biosynthesis. We have now determined the DNA sequence of the entire 42-kb insert in pBgl-br, which contains both the B. bronchiseptica wlb locus and a putative O-antigen biosynthesis locus. One end of the insert (right end in Fig. 1) contained the wlb locus as previously reported whereas the other end of the insert encoded 21 additional open reading frames (ORFs) (Fig. 1 and Table 1), named wbm according to the proposed new nomenclature for LPS biosynthesis genes (22). In addition, 9.5 kb of DNA from the putative O-antigen locus from B. parapertussis was sequenced and was 99.6% identical to the analogous B. bronchiseptica region. The B. bronchiseptica wlb locus was highly homologous to the previously reported B. pertussis wlb locus. However, a significant difference in the two loci is the fact that wlbJ and wlbK, which are separate genes in B. pertussis, are fused into a single ORF in B. bronchiseptica. This may indicate that these genes have different functions in the two bordetellae, and we are currently investigating this by mutating these genes in the different bordetellae.
FIG. 1.
Arrangement of genes in the insert in pBgl-br and the different frames of the coding sequences. wbmA to wbmU are novel, while the remaining genes have been previously described (1), including wlb (LPS band A biosynthesis locus), waaC (heptosyltransferase), waaA (2-keto-3-deoxyoctulosonic acid transferase), and baf (Bvg accessory factor). The DNA to the left of wlbL is present in B. bronchiseptica. The region to the left of wlbL extending to wbmH is also present in B. parapertussis. DNA sequence information beyond this region is not available although whole-genome DNA sequencing of B. parapertussis is under way. In B. pertussis, the wbm locus is replaced by an IS (arrow). The positions of the BstEII restriction sites used for the construction of deletion mutants are indicated by asterisks. wbm contains at least three different transcriptional units, suggested by the direction of the genes. The coding sequences of wbmA and wbmB, wbmH to wbmF, wbmL and wbmK, wbmO to wbmM, wbmP and wbmQ, and wbmR to wbmU may comprise translationally linked units; the coding sequences of adjacent genes within these units overlap. Position numbers are in thousands.
TABLE 1.
Putative functions of proteins encoded by wbmA to wbmU based on similarities to previously characterized proteinsa
| Protein | Length (no. of amino acids) | Putative function |
|---|---|---|
| WbmA | 405 | Glycosyltransferase |
| WbmB | 361 | Unknown |
| WbmC | 628 | Amidotransferase |
| WbmD | 340 | ? (possible membrane protein) |
| WbmE | 393 | ? (possibly secreted) |
| WbmF | 357 | Nucleotide sugar epimerase/dehydratase |
| WbmG | 310 | Nucleotide sugar epimerase/dehydratase |
| WbmH | 313 | Nucleotide sugar epimerase/dehydratase |
| WbmI | 636 | Amidotransferase |
| WbmJ | 878 | Glycosyltransferase |
| WbmK | 866 | Unknown |
| WbmL | 263 | O-antigen exporter (membrane protein) |
| WbmM | 402 | O-antigen exporter (membrane protein) |
| WbmN | 389 | O-antigen exporter (ATP binding) |
| WbmO | 432 | Unknown |
| WbmP | 239 | Unknown |
| WbmQ | 274 | Formyltransferase |
| WbmR | 309 | Formyltransferase |
| WbmS | 239 | Unknown |
| WbmT | 262 | Unknown |
| WbmU | >274 | Formyltransferase |
Conceptual translations of the B. bronchiseptica cosmid DNA sequence were compared to sequences in the GenBank and EMBL databases as described in Materials and Methods. Full annotations for these ORFs are contained in GenBank under accession no. AJ007747.
Of the 21 wbm genes in the putative O-antigen locus, three encode proteins that exhibit homology to sugar epimerases and dehydratases involved in O-antigen biosynthesis in other organisms (Table 1). Three others encode proteins that are similar to formyltransferases. Most interestingly, WbmL, WbmM, and WbmN are similar to proteins that comprise ATP-binding cassettes (ABC) transporter systems for the export of O antigen from various bacterial species (11, 28). These similarities provided strong evidence that this locus is indeed responsible for O-antigen biosynthesis in B. bronchiseptica and B. parapertussis.
Mutagenesis of the O-antigen locus.
To confirm whether or not the locus was required for O-antigen biosynthesis, a 4,895-bp BstEII restriction fragment was replaced by a chloramphenicol resistance gene cassette, deleting DNA between positions 20613 and 25507 (numbering as in the database sequence). This deletion encompassed wbmB to wbmD and the N-terminal half of wbmE. The mutated construct was moved into the chromosomes of B. bronchiseptica and B. parapertussis through allelic exchange as described previously (1). Chloramphenicol-resistant B. bronchiseptica and B. parapertussis organisms which contained the expected chromosomal DNA rearrangements were recovered, as was confirmed by Southern hybridization (data not shown).
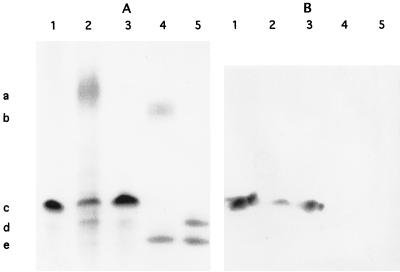
LPS was prepared from several mutants and analyzed by SDS-PAGE, followed by silver staining (Fig. 2). All the mutants were devoid of O antigen, suggesting that the presence of a wild-type locus was required for O-antigen expression. The B. parapertussis O-antigen mutant produced an LPS structure not seen in the wild type. This structure was not recognized by a monoclonal antibody that recognizes the band A trisaccharide of B. pertussis and B. bronchiseptica LPS (Fig. 2). Previously, it was demonstrated that B. parapertussis is probably deficient in an enzyme required for the addition of the terminal GlcNAc to band A and thus is unable to synthesize the entire band A structure (2). It was hypothesized that wild-type B. parapertussis synthesizes a disaccharide band A that is inefficiently transferred to the band B acceptor structure (2). Furthermore, it was hypothesized that in B. parapertussis, the addition of O antigen to produce full-length molecules occurs with high efficiency. Thus, the band B–band A structure was present in very small amounts, if at all. In the O-antigen mutant, the band B–truncated band A structure did not contain further substitutions of O antigen and thus accumulated, possibly constituting the novel band observed in the B. parapertussis O-antigen mutant LPS.
FIG. 2.
(A) SDS-PAGE–silver stain analysis of LPS from B. pertussis Tohama I (lane 1), B. bronchiseptica wild type (lane 2) and mutant (lane 3), and B. parapertussis wild type (lane 4) and mutant (lane 5). The markers indicate the migratory positions of the O antigen of B. bronchiseptica (a, lane 2) and B. parapertussis (b, lane 4), band A of B. pertussis (c, lane 1) and B. bronchiseptica (c, lanes 2 and 3), band B of B. pertussis (d, lane 1) and B. bronchiseptica (d, lanes 2 and 3), the novel structure expressed by the B. parapertussis mutant (d, lane 5), and the truncated band B of B. parapertussis (e, lanes 4 and 5). Wild-type B. bronchiseptica and B. parapertussis both expressed O antigen whereas the mutants did not. (B) Western blot analysis of a replica of the gel shown in panel A with monoclonal antibody BL-2, which recognizes an epitope in band A. This analysis demonstrates that the B. bronchiseptica O-antigen mutant was not affected in band A expression and that the novel structure expressed by the B. parapertussis O antigen mutant was not a full band A structure.
For LPS preparations, the B. bronchiseptica and B. parapertussis strains were grown in the presence of 50 mM MgSO4. This modulated the activity of the Bvg two-component regulatory system (33). Under this condition, B. bronchiseptica and B. parapertussis produced the simple LPS banding patterns seen in Fig. 2. Under nonmodulating conditions, additional LPS structures were produced in these strains (data not shown).
The O-antigen locus is absent from B. pertussis.
These data suggest that the DNA immediately downstream of the wlb locus in B. bronchiseptica and B. parapertussis is required for O-antigen biosynthesis. This region is replaced by an IS in B. pertussis, but it was unclear whether the O-antigen locus was simply present elsewhere in the B. pertussis genome. To investigate this, DNA from the left end (positions 1 to 1355), the middle (positions 2617 to 7684), and the right end (positions 17941 to 22820) of the B. parapertussis wbm locus were used as probes in Southern hybridizations of genomic DNA from all three Bordetella species that had been restricted with the enzyme EcoRI. Hybridizing DNA fragments were present in the B. bronchiseptica and B. parapertussis genomes but were not present in the genome of B. pertussis (data not shown). Furthermore, the complete genome sequence of B. pertussis Tohama I has been initiated and is nearing completion. Searches of this sequence with the DNA sequence from the B. bronchiseptica O-antigen locus did not show any similarities, which substantiates the claim that the locus is absent from B. pertussis.
DISCUSSION
We have identified a locus from B. bronchiseptica and B. parapertussis, called wbm, that is required for the expression of the O-antigen domains of their LPS molecules. Mutation of this locus led to the generation of B. bronchiseptica and B. parapertussis mutants lacking O antigen. These mutants will provide excellent tools for the study of the role of O antigen in infections caused by these bacteria.
The wbm locus contains 21 ORFs, some of which are similar to sequences corresponding to previously identified O-antigen biosynthetic functions. A previous study defined the O-antigen polysaccharide from several B. bronchiseptica and B. parapertussis strains as consisting of a homopolymer of 2,3-dideoxy-2,3-di-N-acetylgalactosaminuronic acid (9). The authors suggested that O antigen is identical in all B. bronchiseptica and B. parapertussis strains. The mutagenesis data presented here, coupled with the putative functions assigned from similarity searches, strongly support the view that several of the ORFs in the wbm locus are involved in the biosynthesis of this homopolymer. Full annotations of the ORFs with the best matches to sequences in the databases are given in a GenBank entry (accession no. AJ007747). Speculative attempts to match each of the putative functions with the known structure of the O antigen indicate that there are more genes in the locus than are required to biosynthesize such a relatively simple polymer. This may suggest that other polysaccharides are present in the bordetellae, and their biosynthesis may depend on enzymes encoded by genes in the wbm locus.
It is unknown whether the entire wbm locus is required for O-antigen biosynthesis. We suggest that the ORFs from wlb up to and including the putative ABC transporter system (i.e., wbmA to wbmN) are highly likely to be required because (i) the ABC transporter is most similar to previously characterized polysaccharide export systems, (ii) other ORFs in this region are homologous to previously identified O-antigen biosynthesis functions, and (iii) deletion of the region encompassing wbmB to wbmE abrogates O-antigen expression. The functions of the seven ORFs outside this area are unknown. Three of them have strong similarities to formyltransferases, and the other four are not similar to any other proteins in the sequence databases. This region of DNA is, however, not present in B. pertussis, which may suggest that these ORFs do function in O-antigen biosynthesis. The fact that the entire wbm locus is absent from B. pertussis might suggest that there is more unique DNA in this region of the chromosome adjacent to the DNA that we have cloned. If this is so, then either this DNA is part of a much larger O-antigen biosynthesis region or the O-antigen biosynthesis locus forms part of a larger locus peculiar to B. bronchiseptica and B. parapertussis that encodes functions besides O-antigen biosynthesis.
Our data support previous hypotheses that explain the absence of band A from B. parapertussis LPS (2) by demonstrating that, in the absence of O-antigen transfer to the LPS molecule, a structure consistent with band B containing a truncated, disaccharide band A is synthesized. B. parapertussis synthesized a band B that, on SDS-PAGE, appeared to be truncated when compared to the band Bs of B. pertussis and B. bronchiseptica. This altered band B was not recognized by BL-8, a monoclonal antibody that recognizes B. pertussis and B. bronchiseptica band B (2), and thus it was not possible to confirm that the novel structure synthesized by the B. parapertussis O antigen mutant contained a substituted band B without detailed structural analysis.
The fact that the O-antigen biosynthesis locus is not present in B. pertussis provides an explanation for why this bacterium is never seen to express O antigen. Recent work suggests that B. pertussis and B. bronchiseptica evolved from a common ancestor which was more similar to B. bronchiseptica (34). During this evolution it appears that the O-antigen locus was lost from B. pertussis and replaced by an IS. The genetic rearrangement leading to this may have occurred as a consequence of the insertion of the IS followed by recombination between this copy of the IS and a second copy elsewhere in the chromosome, with concomitant genetic rearrangement. The evolutionary consequences of this event for B. pertussis pathogenesis and host range are unknown.
ACKNOWLEDGMENTS
This work was supported by The Wellcome Trust, project grant no. 045666. Sequencing of DNA from cosmid pBgl-br was performed as part of the Bordetella genome sequencing project supported by the Beowulf Genomics initiative of The Wellcome Trust, grant no. 054672.
REFERENCES
- 1.Allen A, Maskell D. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. doi: 10.1046/j.1365-2958.1996.354877.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen A G, Thomas R T, Cadisch J T, Maskell D J. Molecular and functional analysis of the lipopolysaccharide biosynthesis locus wlb from Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Mol Microbiol. 1998;29:27–38. doi: 10.1046/j.1365-2958.1998.00878.x. [DOI] [PubMed] [Google Scholar]
- 2a.Barrell, B. G., et al. Unpublished data.
- 3.Burns E H, Jr, Norman J M, Hatcher M D, Bemis D A. Fimbriae and determination of host species specificity of Bordetella bronchiseptica. J Clin Microbiol. 1993;31:1838–1844. doi: 10.1128/jcm.31.7.1838-1844.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caroff M, Chaby R, Karibian D, Perry J, Deprun C, Szabó L. variations in the carbohydrate regions of Bordetella pertussis lipopolysaccharides: electrophoretic, serological, and structural features. J Bacteriol. 1990;172:1121–1128. doi: 10.1128/jb.172.2.1121-1128.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaby R, Caroff M. Lipopolysaccharide of Bordetella pertussis endotoxin. In: Wardlaw A C, Parton R, editors. Pathogenesis and immunity in pertussis. New York, N.Y: John Wiley; 1988. pp. 247–271. [Google Scholar]
- 6.Cherry J D. Historical review of pertussis and the classical vaccine. J Infect Dis. 1996;174:S259–S263. doi: 10.1093/infdis/174.supplement_3.s259. [DOI] [PubMed] [Google Scholar]
- 7.Cherry J D. Pertussis: the trials and tribulations of old and new pertussis vaccines. Vaccine. 1992;10:1033–1038. doi: 10.1016/0264-410x(92)90113-x. [DOI] [PubMed] [Google Scholar]
- 8.Cotter P A, Miller J F. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–3390. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiFabio J L, Caroff M, Karibian D, Richards J C, Perry M B. Characterization of the common antigenic lipopolysaccharide O chains produced by Bordetella bronchiseptica and Bordetella parapertussis. FEMS Microbiol Lett. 1992;97:275–282. doi: 10.1016/0378-1097(92)90348-r. [DOI] [PubMed] [Google Scholar]
- 10.Gueirard P, Weber C, Le Coustumier A, Guiso N. Human Bordetella bronchiseptica infection related to contact with infected animals: persistence of bacteria in host. J Clin Microbiol. 1995;33:2002–2006. doi: 10.1128/jcm.33.8.2002-2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo D, Bowden M G, Pershad R, Kaplan H B. The Myxococcus xanthus rfbABC operon encodes an ATP-binding cassette transporter homolog required for O-antigen biosynthesis and multicellular development. J Bacteriol. 1996;178:1631–1639. doi: 10.1128/jb.178.6.1631-1639.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heininger U, Stehr K, Schmittgrohe S, Lorenz C, Rost R, Christenson P D, Uberall M, Cherry J D. Clinical characteristics of illness caused by Bordetella parapertussis compared with illness caused by Bordetella pertussis. Pediatr Infect Dis J. 1994;13:306–309. doi: 10.1097/00006454-199404000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Lebbar S, Caroff M, Szabo L, Merienne C, Szilogyi L. Structure of a hexasaccharide proximal to the hydrophobic region of lipopolysaccharides present in Bordetella pertussis endotoxin preparations. Carbohydr Res. 1994;259:257–275. doi: 10.1016/0008-6215(94)84061-x. [DOI] [PubMed] [Google Scholar]
- 14.Lesse A J, Campagnari A A, Bittner W E, Apicella M A. Increased resolution of lipopolysaccharides and lipooligosaccarides utilizing tricine-sodium dodecyl sulphate-polyacrylamide gel electrophoresis. J Immunol Methods. 1990;126:109–117. doi: 10.1016/0022-1759(90)90018-q. [DOI] [PubMed] [Google Scholar]
- 15.Luderitz O, Galanos C, Risse H J, Ruschmann E, Schlecht S, Schmidt G, Shulte-Holthausen H, Wheat R, Westphal O. Structural relationships of Salmonella O and R antigens. Ann N Y Acad Sci. 1966;133:349–374. doi: 10.1111/j.1749-6632.1966.tb52376.x. [DOI] [PubMed] [Google Scholar]
- 16.Nennig M E, Shinefield H R, Edwards K M, Black S B, Fireman B H. Prevalence and incidence of adult pertussis in an urban population. JAMA. 1996;275:1672–1674. [PubMed] [Google Scholar]
- 17.Pichichero M E, Treanor J. Economic impact of pertussis. Arch Pediatr Adolesc Med. 1997;151:35–40. doi: 10.1001/archpedi.1997.02170380039006. [DOI] [PubMed] [Google Scholar]
- 18.Porter J F, Connor K, Donachie W. Differentiation between human and ovine isolates of Bordetella parapertussis using pulsed-field gel electrophoresis. FEMS Microbiol Lett. 1996;135:131–135. doi: 10.1111/j.1574-6968.1996.tb07977.x. [DOI] [PubMed] [Google Scholar]
- 19.Porter J F, Connor K, Donachie W. Isolation and characterization of Bordetella parapertussis-like bacteria from ovine lungs. Microbiology. 1994;140:255–261. doi: 10.1099/13500872-140-2-255. [DOI] [PubMed] [Google Scholar]
- 20.Porter J F, Connor K, Vanderzee A, Reubsaet F, Ibsen P, Heron I, Chaby R, Leblay K, Donachie W. Characterization of ovine Bordetella parapertussis isolates by analysis of specific endotoxin (lipopolysaccharide) epitopes, filamentous hemagglutinin production, cellular fatty-acid composition and antibiotic-sensitivity. FEMS Microbiol Lett. 1995;132:195–201. doi: 10.1111/j.1574-6968.1995.tb07833.x. [DOI] [PubMed] [Google Scholar]
- 21.Preston A, Maskell D, Johnson A, Moxon E R. Altered lipopolysaccharide characteristic of the I69 phenotype in Haemophilus influenzae results from mutations in a novel gene, isn. J Bacteriol. 1996;178:396–402. doi: 10.1128/jb.178.2.396-402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R H, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 23.Reina J, Bassa A, Llompart I, Borrell N, Gomez J, Serra A. Pneumonia caused by Bordetella bronchiseptica in a patient with a thoracic trauma. Infection. 1991;19:46–48. doi: 10.1007/BF01643760. [DOI] [PubMed] [Google Scholar]
- 24.Rutter J M. Quantitative observations on Bordetella bronchiseptica infection in atrophic rhinitis of pigs. Vet Rec. 1981;108:451–454. doi: 10.1136/vr.108.21.451. [DOI] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Schweizer H P, Hoang T T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 27.Stibitz S, Aaronson W, Monack D, Falkow S. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel 2-component system. Nature. 1989;338:266–269. doi: 10.1038/338266a0. [DOI] [PubMed] [Google Scholar]
- 28.Sugiyama T, Kido N, Kato Y, Koide N, Yoshida T, Yokochi T. Generation of Escherichia coli O9a serotype, a subtype of E. coli O9, by transfer of the wb* gene cluster of Klebsiella O3 into E. coli via recombination. J Bacteriol. 1998;180:2775–2778. doi: 10.1128/jb.180.10.2775-2778.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tamion F, Girault C, Chevron V, Pestel M, Bonmarchand G. Bordetella bronchiseptica [sic] pneumonia with shock in an immunocompetent patient. Scand J Infect Dis. 1996;28:197–198. doi: 10.3109/00365549609049077. [DOI] [PubMed] [Google Scholar]
- 30.Thrusfield M V, Aitken C G G, Muirhead R H. A field investigation of kennel cough—efficacy of different treatments. J Small Anim Pract. 1991;32:455–459. [Google Scholar]
- 31.Thrusfield M V, Aitken C G G, Muirhead R H. A field investigation of kennel cough—incubation period and clinical signs. J Small Anim Pract. 1991;32:215–220. [Google Scholar]
- 32.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 33.Uhl M A, Miller J F. Bordetella pertussis BvgAS virulence control system. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C: ASM Press; 1995. pp. 333–349. [Google Scholar]
- 34.van der Zee A, Mooi F, Van Embden J, Musser J. Molecular evolution and host adaptation of Bordetella spp.: phylogenetic analysis using multilocus enzyme electrophoresis and typing with three insertion sequences. J Bacteriol. 1997;179:6609–6617. doi: 10.1128/jb.179.21.6609-6617.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Westphal O, Jann K, Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]