SUMMARY
Maternal diabetes and/or obesity in pregnancy are undoubtedly associated with later disease-risk in the offspring. The placenta, interposed between the mother and the foetus, is a potential mediator of this risk through epigenetic mechanisms, including DNA methylation. In recent years, multiple studies have identified differentially methylated CpG sites in the placental tissue DNA in pregnancies complicated by diabetes and obesity. We reviewed all published original research relevant to this topic and analysed our findings with the focus of identifying overlaps, contradictions, and gaps. Most studies focused on the association of gestational diabetes and/or hyperglycaemia in pregnancy and DNA methylation in placental tissue at term. We identified overlaps in results related to specific candidate genes, but also observed a large research gap of pregnancies affected by type 1 diabetes. Other unanswered questions relate to analysis of specific placental cell types and the timing of DNA methylation change in response to diabetes and obesity during pregnancy. Maternal metabolism is altered already in the first trimester involving structural and functional changes in the placenta, but studies into its effects on placental DNA methylation during this period are lacking and urgently needed. Foetal sex is also an important determinant of pregnancy outcome, but only few studies have taken this into account. Collectively, we provide a reference work for researchers working in this large and evolving field. Based on the results of the literature review, we formulate suggestions for future focus of placental DNA methylation studies in pregnancies complicated by diabetes and obesity.
KEYWORDS: Pregnancy, placenta, epigenetic, DNA methylation, gestational diabetes, type 1 diabetes, type 2 diabetes, obesity, hyperglycaemia, hyperlipidaemia, foetal development, offspring
Introduction
Diabetes and obesity in pregnancy
The incidence of pregnancies with disturbed metabolic homoeostasis such as in women with Type 1 diabetes mellitus (T1DM), Type 2 diabetes mellitus (T2DM), gestational diabetes mellitus (GDM), and in overweight or obese women has been increasing worldwide [1–5]. Metabolism in these women is often characterized by hyperglycaemia and hyperlipidaemia because of various degrees of and/or combinations of insufficient or absent beta cell function, or insulin resistance. In most instances insulin resistance is associated with elevated concentrations of circulating insulin [6].
The risk of short-term complications of these pregnancies has been known for long. More recently, evidence has accumulated to demonstrate also long term consequences for mother and offspring throughout the life course [6–8]. Specifically, in utero exposure to disturbed metabolism of the mother increases offspring risk for adiposity, T2DM, metabolic syndrome and cardiovascular problems, also with an influence of foetal sex [9].
While the mechanisms underlying these adverse health conditions have not been fully understood, (epi)genetic transmission of risks has been demonstrated and the placenta has been implicated [10,11].
The placenta
The placenta is interposed between the maternal and foetal circulation and, hence, exposed to perturbations in both compartments albeit with different surfaces. The syncytiotrophoblast bathes in maternal blood in the intervillous space, whereas endothelial cells line the feto-placental vasculature and are under the influence of foetal circulating factors [12]. The placenta’s broad array of diverse functions has been long recognized to play a major role in regulating maternal metabolism as well as foetal growth and development. Structural and functional changes of the placenta in T1DM, T2DM, GDM and in obese women have been regarded as contributing to foetal phenotype, mostly foetal overgrowth, in these conditions either directly by modifying foetal metabolism or indirectly by changing maternal insulin secretion or insulin resistance or both [13,14]. Evidence suggests that maternal metabolic adaptations during pregnancy, especially of glucose metabolism, are also partly regulated by foetal sex, as the risk of developing GDM and GDM severity is increased in women with male newborns [9,15]. Moreover, mothers of male offspring have increased risk of developing T2DM later in life when compared to mothers of female offspring [16].
Epigenetic mechanisms in the placenta
Epigenetics is the study of ‘structural adaptations of chromosomal regions so as to register, signal or perpetuate altered activity states’[17]. ‘Epigenetic marks’ is a broad term that includes DNA methylation (DNAm), histone post-translational modifications, RNA modifications, and non-coding RNAs. These marks are modified by specific enzymes which are recruited by transcription factors [18].
The human genome contains ~28 million cytosine-phosphate-guanine (CpG) sites, comprising less than ~1% of the total genome. CpG content in the genome is lower than the expected ~4% due to natural deamination of methylated cytosine to thymine [19]. Therefore, most CpG sites are scattered at low density across the genome, while a subset occur at high density in hypomethylated regions known as CpG Islands [20]. Techniques that measure DNAm range from global (e.g., High-performance liquid chromatography (HPLC) to detect methylated cytosines as a percentage of all cytosines, or whole-genome sequencing), genome-wide arrays (e.g., Illumina arrays, which cover ~3% of all CpG sites) and locus-specific (e.g., bisulphite amplicon sequencing to detect DNAm at a specific genomic region) [21–24]. All of these, except global measurement by HPLC, require bisulphite conversion of DNA as the first step [25]. Due to the multiple different methodological approaches of studying DNAm, we specify throughout the review and in Tables 1 and 2 the approach that was used to generate the data.
Table 1.
Literature search on studies of DNA methylation in placenta tissue and diabetes and obesity in pregnancy.
| Sample size | Method | Genes | Results | Influence of foetal sex | Authors, year | |
|---|---|---|---|---|---|---|
| GDM | ||||||
| Placenta, 3rd trimester | ||||||
| 21 GDM 20 Ctrl |
RRBS | 2,799 CpG sites with changes of DNAm after adjustment for maternal BMI. Pathway analysis found DNAm changes related to T2D and insulin resistance pathways. | No data on foetal sex | Lu et al, 2022 [88] | ||
| 80 GDM 119 Ctrl |
PS | HTR2A | Increased pre-pregnancy BMI and GDM was independently associated with lower promoter DNAm in female but not male placentas. | Stratified for foetal sex | Horvatiček et al, 2022 [89] | |
| 34 GDM 46 Ctrl |
PS | LEP | Higher DNAm in placenta from women with GDM of South Asian ethnicity but not European ethnicity. | No data on foetal sex | Sletner et al, 2021 [90] | |
| 33 GDM 27 Ctrl |
PS | FTO | No association between FTO DNAm and GDM. | No data on foetal sex | Franzago et al, 2021 [91] | |
| 23 GDM 23 Ctrl |
MethylTarget Technique |
MEG3 | DNAm was increased in GDM, and positively associated with birthweight and fasting glucose levels. | No data on foetal sex | Chen et al, 2021 [92] | |
| 20 GDM 16 Ctrl |
450k EWAS | 662 CpGs with differential DNAm in GDM (without FDR). Pathway analysis found DNAm changes related to polyamines, amines, and vitamin B6 metabolism pathways. | Adjusted for foetal sexX/Y probes removed | Awamleh et al, 2021 [93] | ||
| 41 GDM 40 Ctrl |
MS-PCR | CYP24A1, CYP27B1 | No difference in DNAm between GDM and Ctrl. | No data on foetal sex | Wang et al, 2020 [94] | |
| 15 GDM 15 Ctrl |
MethylTarget Technique |
DLK1 | Higher DNAm at both foetal and maternal side. DNAm associated with birthweight and 2 hr glucose levels post OGTT. | No data on foetal sex | Zhao et al, 2019 [95] | |
| 6 GDM 6 Ctrl |
MS-PCR | G6PD, IGFBP, TKT, IGFBP2, IGFBP6 | Higher DNAm of IGFBP1, IGFBP2 and IGFBP6, which also were pos. associated with maternal glucose levels at fasting and 1 hr, but not at 2 hrs, post OGTT. | Only females | Steyn et al, 2019 [96] | |
| 32 GDM 31 Ctrl |
450k EWAS | OAS1, PPIA, POLR2G | 24,577 CpG sites with changes of DNAm (without FDR). Pathway analysis identified OAS1, PPIA and POLR2G as possible pathogenic genes of GDM, based on protein-protein interaction analysis. | No data on foetal sex | Zhang et al, 2018 [97] | |
| 20 GDM 20 Ctrl |
BS | PGC1A, PDX1 | Higher PGC1A DNAm in GDM. | No data on foetal sex | Wang et al, 2018 [44] | |
| 24 GDM 42 Ctrl |
PS | LPL | Out of 20 CpGs, one had lower DNAm in GDM, and one was positively associated with birth- and childhood weight Z-scores and fat mass, and negatively with lean mass. | Adjusted for foetal sex | Gagne-Ouellet et al, 2017 [98] |
|
| 18 GDM 32 Ctrl |
BS | SLC6A4 | Average DNAm was 27% lower in GDM and negatively associated with expression and fasting glucose levels. All were caesarean sections, and biopsies were from foetal side. | No association with foetal sex | Blazevic et al, 2017 [99] | |
| 56 GDM 974 Ctrl |
LC-MS/MS | Higher DNAm in GDM across the epigenome. The quintile with highest degree of DNAm has the strongest representation of GDM, whereas Q1-4 had equal GDM numbers. | Adjusted for foetal sex | Reichetzeder et al, 2016 [100] |
||
| 133 GDM 100 Ctrl |
PS, 450k EWAS |
PGC1A, PRDM16, BMP7, CTBP2 | GDM was associated with DNAm of PGC1A, PRDM16 and BMP7. DNAm of PGC1A and PRDM16 was associated with cord blood leptin. DNAm of PGC1A explained 0.8% of variation of cord leptin levels, independent of maternal fasting glucose. | Adjusted for foetal sex | Coté et al, 2016 [45] | |
| 36 GDM 40 Ctrl |
PS, MEDIP |
RBP4, GLUT3, RETN, PPARA | DNAm of 4699 DMRs were altered in GDM. Pathway analysis identified cell death and cell regulation, immune/inflammatory response, and nervous system development as top pathways. RBP4, GLUT3, RETN and PPARA were validated by PS. | No data on foetal sex | Rong et al, 2015 [101] | |
| 20 GDM 60 Ctrl |
PS | DLGAP2, LRP1B, BRD2 | No difference in average DNAm between GDM and Ctrl, but one CpG of DLGAP2 had higher DNAm in GDM. LRP1B and BRD2 DNAm associated with glucose levels in Ctrls. | Adjusted for foetal sex | Houde et al, 2015 [69] | |
| 41 GDM 41 Ctrl |
PS, 450k EWAS | CCDC181, HLA-H/J, HLA-DOA, SNRPN | DNAm did not differ between GDM and Ctrl (after FDR). Four selected CpGs of top 20 could not be validated in a separate cohort. | Adjusted for foetal sex X/Y probes removed |
Binder et al, 2015 [102] | |
| 25 GDM 18 Ctrl |
450k EWAS | 1708 CpGs had more than 5% higher or lower DNAm in GDM (after FDR). Pathway analysis identified endocytosis, MAPK signalling and metabolic processes. | No data on foetal sex X/Y probes removed |
Finer et al, 2015 [103] | ||
| 7 GDM 7 Ctrl |
PS, 450k EWAS |
DGKZ, ARMCX6, TBR1, DCAF11 | 2021 CpGs (981 genes) showed differential DNAm in GDM. DGKZ, ARMCX6, DCAF11 and TBR1 were validated by PS. | Only males | Petropoulos et al, 2015 [104] |
|
| 28 GDM 30 Ctrl |
BS, 385k Island EWAS | GLUT3, RBP4, PGC1A | GLUT3 DNAm was higher and RBP4 and PGC1A DNAm was lower in GDM. 5% of the DMRs (total 10,424) were located on autosomes. | No data on foetal sex X/Y probes included |
Liu et al, 2014 [40] | |
| 27 GDM 99 Ctrl |
PS | LPL | DNAm was lower in GDM. Two CpGs were negatively associated with 2 hr glucose levels, post OGTT. DNAm at one intron CpG explained up to 26% of LPL mRNA. | Adjusted for foetal sex | Houde et al, 2014 [105] | |
| 30 GDM 14 Ctrl |
450k EWAS | DNAm of 8657 CpGs (3271 genes) were changed in GDM (without FDR). Pathway analysis identified cardiovascular disease as top hit. | Adjusted for foetal sex X/Y probes removed |
Ruchat et al, 2013 [106] | ||
| 16–29 diet-treated GDM 18–34 insulin-treated GDM 21–50 Ctrl |
PS | H19, MEG3, IT1, MEST, NESPAS, LEP, PEG3, APC, SNRPN, NR3C1, PPARA, DUFB6, OCT4, IL10, ALU, LINE1 | Lower DNAm of MEST, NESPAS, NR3C1, PPAPA, ALU and LINE1 in GDM compared to controls. However, the control group were all non-smokers, whereas the GDM group had smokers, which may confound the results, since smoking affects foetal DNAm [107]. | No association with foetal sex | El Hajj et al, 2013 [49] | |
| Placenta and Decidua, 3rd trimester | ||||||
| 40 GDM 40 Ctrl |
MS-PCR | ESR1 | DNAm of ESR1 was not detected in placenta of GDM and Ctrls, but in decidua of Ctrls. | Adjusted for foetal sex | Knabl et al, 2015 [108] | |
| FPECs, 3rd trimester | ||||||
| 9 GDM 9 Ctrl |
450k EWAS | 2617 CpGs (2063 genes) in dAEC and 1568 CpGs (1360 genes) in dVEC showed DNAm changes in GDM (without FDR). Six genes altered by GDM in both dAEC and dVEC were associated with actin reorganization processes. | Adjusted for foetal sex X/Y probes removed |
Cvitic et al, 2018 [109] | ||
| 5 GDM 9 Ctrl |
450k EWAS | ICAM1 | No difference in DNAm between GDM and Ctrl. | Adjusted for foetal sex X/Y probes removed |
Diaz-Perez et al, 2016 [110] |
|
| GDM and T2DM | ||||||
| Placenta, 3rd trimester | ||||||
| 16 GDM 7 T2DM 23 Ctrl |
EPI-JET | PGC1A | In GDM and T2DM, DNAm at one CpG was higher in male offspring placentas. | Stratified for foetal sex | Jiang et al, 2020 [46] | |
| 14 GDM 3 T2DM 17 Ctrl |
450k EWAS | PIWIL3, CYBA, STM1, GSTM5, KCNE1, NXN | DNAm was changed in GDM at 465 CpGs of male offspring, at 247 CpGs of female offspring, and at 277 CpGs when sexes were combined (without FDR). DNAm changes were found at loci related to mitochondrial function, DNA repair, inflammation, oxidative stress. DNAm was negatively associated with mRNA and protein levels for PIWIL3, CYBA, GSTM1, GSTM5, KCNE1 and NXN. | Stratified for foetal sex | Alexander et al, 2018 [39] | |
| 2 GDM 3 T2DM 12 Ctrl |
MS-PCR | DLL1, NOTCH1 | No difference in DNAm between GDM, T2DM and Ctrls, but DNAm associated with other pregnancy complications. | No data on foetal sex | Shimanuki et al, 2015 [111] | |
| Obesity and pre-pregnancy BMI | ||||||
| Placenta, 1st trimester | ||||||
| 15 Obese 15 Lean |
EPIC EWAS | BRCA1 | No difference in DNAm between obese and Ctrls. | Adjusted for foetal sex X/Y probes removed |
Hoch et al, 2020 [112] | |
| Placenta, 3rd trimester | ||||||
| 11 Obese 12 Ctrl |
MethylFlash ELISA | Global DNAm was incr. in obese pregnancies. | No data on foetal sex | Shen et al, 2022 [113] | ||
| 437 | EPIC EWAS | CRHBP, CCDC97 | Higher early pregnancy BMI associated with higher DNAm in CRHBP and with lower DNAm of CCDC97, in paired analysis of placenta and cord blood. | Adjusted for foetal sex X/Y probes removed |
Ghildayal et al, 2021 [114] | |
| 301 | 450k EWAS | The Horvath Clock | Negative association between placental epigenetic age acceleration and maternal pre-pregnancy BMI in male offspring only. | Adjusted and stratified for foetal sex | Workalemahu et al, 2021 [56] | |
| 301 | 450k EWAS | EGFL7, VEZT, AC092377.1 | Each 1 kg/m2 increase in maternal pre-pregnancy BMI was associated with 0.09% higher EGFL7 DNAm, 0.13% higher VEZT DNAm, and 0.07% lower AC092377.1 DNAm (after FDR). EGFL7 DNAm associated negatively with mRNA expression. The 3-phosphoinositide degradation pathway was enriched with pre-pregnancy BMI-associated DNAm. | Adjusted for foetal sex | Shrestha et al, 2020 [115] | |
| 72 Mothers 63 Fathers |
PS | C19MC | Lower DNAm associated with maternal BMI and with offspring size at 6 yrs. | Adjusted for foetal sex | Prats-Puig et al, 2020 [116] | |
| 12 Obese 18 Ctrl |
PS | LEP, LEPR, ADIPOQ, ADIPOR1 | Higher LEP DNAm at foetal side only. Lower ADIPOQ DNAm and higher ADIPOR1 DNAm at maternal side only. | No data on foetal sex | Nogues et al, 2019 [52] | |
| 10 Obese 10 Ctrl |
MEDIP | DNAm. 21% higher and hydroxyDNAm 31% lower, in obese compared to Ctrls. Enrichment in DNAm and hydroxyDNAm at chromosomes 17 and 19. | No data on foetal sex | Mitsuya et al, 2017 [117] | ||
| 20 Obese 20 Ctrl |
PS | LEP, ADIPOQ | No difference in LEP DNAm in obese compared to Ctrls. ADIPOQ DNAm was not detected in any of the groups. | No data on foetal sex | Haghiac et al, 2014 [51] | |
| GDM and Obesity | ||||||
| Placenta, 3rd trimester | ||||||
| 7–8 GDM 17–18 Obese |
LUMA | Global DNAm was associated with GDM and obesity in opposite directions. Global DNAm was negatively associated with newborn body length and head circumference. | Non-adjusted and adjusted for foetal sex | Nomura et al, 2014 [118] | ||
| 47 GDM 135 Obese 353 Ctrl |
PS | LEP | DNAm was higher in GDM and in GDM and obesity combined. Obesity alone did not have effect. DNAm was higher in male offspring placentas (all groups together). | Adjusted for foetal sex | Lesseur et al, 2014 [48] | |
If FDR is not stated in the table, it was not stated in the original paper.
Abbreviations: GDM: Gestational Diabetes Mellitus, Ctrl: Control, DNAm: DNA methylation, BMI: body mass index, DMR: differentially methylated region, OGTT: oral glucose tolerance test, FDR: false discovery rate, RRBS: reduced representation bisulphite sequencing, LC-MS/MS: liquid chromatography with tandem mass spectrometry, LUMA: Luminometric Methylation Assay, EWAS: epigenome wide association study, MEDIP: methylated DNA immunoprecipitation, BS, bisulphite sequencing, MS-PCR: methylation-specific PCR, PS: pyrosequencing.
Table 2.
Literature search on studies of DNA methylation in placenta tissue and hyperglycaemia and dyslipidemia in pregnancy.
| Sample size | Method | Genes | Results | Influence of foetal sex | Authors, year, PMID |
|---|---|---|---|---|---|
| Dyslipidemia | |||||
| Placenta, 3rd trimester | |||||
| 262 | 450k EWAS | STK11, MOGAT2, DHRS12, BRD1, ECI2, SRM, ALX4, MICA, RPTOR, FAAH, HECTD2 | 11 CpGs in 11 genes associated with maternal dyslipidemia (after FDR). | Adjusted for foetal sex | Ouidir et al, 2020 [119] |
| 69 | PS | LDLR, LRP1, SCARB1 | Maternal cholesterol changes were negatively associated with LDLR DNAm and positively associated with LRP1 DNAm. LDLR and LRP1 DNAm was associated with cord blood triglyceride and leptin levels. Mediation analysis supported a causal relationship between cholesterol changes, LRP1 DNAm, and cord blood leptin level. | Adjusted for foetal sex | Guay et al, 2019 [120] |
| 262 | 450k EWAS | The Horvath Clock | Low maternal HDL cholesterol associated with accelerated placental epigenetic ageing among mothers with normal pre-pregnancy weight and a female foetus. | Adjusted and stratified for foetal sex | Shrestha et al, 2019 [55] |
| Hyperglycaemia | |||||
| Placenta, 3rd trimester | |||||
| 259 | EPIC EWAS | LEP | Maternal glycaemia associated with LEP DNAm, neonatal leptinemia, and adiposity and skinfolds at age 3 years. DNAm levels at cg15758240 mediates 0.8% of the association between maternal glycaemia and neonatal leptinemia. | Adjusted for foetal sex X/Y probes removed |
Gagné-Ouellet et al, 2020 [50] |
| 430 | EPIC EWAS |
CHRNA4, MICALL2/ UNCX, DLGAP2, ENTPD2, DP1P |
DNAm at 188 CpG sites was associated with Matsuda index (after FDR). Mendelian randomization analyses found five loci where DNAm may causally influence maternal insulin sensitivity, including the maternally imprinted gene DLGAP2. | Adjusted for foetal sex X/Y probes removed |
Hivert et al, 2020 [65] |
| 12 decreased GI 12 increased GI |
PS, 450k EWAS |
PLIN1, CPT1B, SSTR4, CIDEA | Negative association between DNAm and miRNA expression*. | No data on foetal sex X/Y probes removed |
Yan et al, 2019 [121] |
| 448 | EPIC EWAS | PDE4B, TNFRSF1B, LDLR, BLM | DNAm of PDE4B, NFRSF1B, LDLR, and BLM associated (after FDR) with 2 hr glucose post OGTT. DNAm and mRNA expression of PDE4B, TNFRSF1B and LDLR was negatively correlated. In an independent replication the results were consistent in direction. | Adjusted for foetal sexX/Y probes removed | Cardenas et al, 2018 [122] |
| 24 GDM 34 Ctrl |
PS | PGC1A | In combined groups, DNAm associated positively with fasting, 1 hr, and 2 hr glucose levels post OGTT. | Adjusted for foetal sex | Xie et al, 2015 [47] |
| 34 IGT 106 NGT |
PS | IGF1R, IGFBP3, IGF1, INSR | DNAm of IGF1R and IGFBP3 were lower in IGT compared to NGT and associated negatively with fasting (IGF1R) and 2 hr glucose levels (IGF1R and IGFBP3) post OGTT. | Adjusted for foetal sex | Desgagné et al, 2014 [123] |
| 26 IGT 74 NGT |
PS | ABCA1 | DNAm at maternal side was positively associated with 2 hr glucose levels post OGTT. | Adjusted for foetal sex (partly) | Houde et al, 2013 [124] |
| 98 | PS | ADIPOQ | Foetal side DNAm associated negatively with 2hr glucose post OGTT. Maternal side DNAm associated negatively with HOMA-IR. DNAm at both sides associated negatively with maternal adiponectin levels | No data on foetal sex | Bouchard et al, 2012 [53] |
If FDR is not stated in the table, it was not stated in the paper.
*Yan et al did not include continuous data on glycaemic index (GI).
Abbreviations: DNAm: DNA methylation, GI: glycaemic index, OGTT: oral glucose tolerance test, IGT: impaired glucose tolerance, NGT: Normal glucose tolerance, FDR: false discovery rate, EWAS: epigenome wide association study, PS: pyrosequencing.
Due to its early separation from the embryo at the blastocyst stage, the placenta has a unique DNAm profile, with several similarities to human tumours [26], such as low global methylation [27], partially methylated domains [28], and tumour-suppressor methylation [29]. Alterations of DNAm signatures often parallel transcriptional, morphological and functional changes of the placenta [30–32]. Importantly, specific placental DNAm patterns have been associated with maternal exposures [33] and offspring outcomes [34]. Hence, measuring placental DNAm, including cell-free DNA in maternal circulation, is useful for assessing placental health [35].
In this review we summarize current knowledge on variation of placental epigenetic profiles in pregnancies in women with diabetes and in obese women. Since maternal hyperglycaemia and hyperlipidaemia are hallmarks of metabolic changes in these conditions, we have included placental DNAm related to these perturbations. As the influence of foetal sex is increasingly being acknowledged, we also note how foetal sex was integrated into the statistical models. We focused on DNAm as the most stable epigenetic variation, which can be measured with high reproducibility. The list of genes with identified changes in DNAm in any of these conditions provides an up to date overview and shall help readers to easily assess whether their gene of interest shows DNAm changes. We conclude by highlighting areas for future research that have emerged from conducting this review.
Results
Literature search results
Using PubMed, we conducted a literature search covering publications between 1960 and 12 July 2022 of peer-reviewed original research of placental DNAm in pregnancies complicated by diabetes or obesity. We used following search terms: DNA methylation OR epigenetic* AND placenta AND human AND diabetes OR obes* OR BMI OR GDM OR hyperglycaemia OR hyperlipidaemia OR dyslipidemia. In total, 233 papers were identified using these search terms. All were screened for suitability to be covered in this review (Figure 1). We included all studies regardless of aim and sample size with following inclusion criteria: original research papers, in English language and matching subject criteria. The screening excluded 90 papers not within the subject area, 82 review papers, and nine non-English language papers. In the end, 52 papers were found suitable and were included (Figure 1). These were divided into two groups: case-control studies (Table 1) and studies of continuous glucose/lipid measurements (Table 2). Finally, we merged all data on DNAm differences of specific genes/gene regions, and sorted on gene annotations to provide an overview of candidate genes investigated in multiple studies, as well as to assess whether specific genes and differential DNAm associated with exposures were replicated across studies (Table 3).
Figure 1.
Overview of literature search strategy and screening of included and excluded original research papers.
Table 3.
Differentially methylated genes identified in literature search.
| Genes | Exposure | Authors | Imprinted gene |
Genes | Exposure | Authors | Imprinted gene |
||
|---|---|---|---|---|---|---|---|---|---|
| ABCA1 | Hyperglycaemia | Houde et al [124] | LDLR | Dyslipidemia | Guay et al [120] | ||||
| AC092377.1 | Pre-pregnancy BMI | Shrestha et al [115] | LDLR | Hyperglycaemia | Cardenas et al [122] | ||||
| ADIPOQ | Hyperglycaemia | Bouchard et al [53] | LEP | GDM | el Hajj et al [66] | ||||
| ADIPOQ | Obesity | Haghiac et al [51] | LEP | GDM and Obesity | Lesseur et al [48] | ||||
| ADIPOQ | Obesity | Nogues et al [52] | LEP | Hyperglycaemia | Gagné-Ouellet et al [50] | ||||
| ADIPOR1 | Obesity | Nogues et al [52] | LEP | Obesity | Haghiac et al [51] | ||||
| ALU repeat | GDM | el Hajj et al [66] | LEP | Obesity | Nogues et al [52] | ||||
| ALX4 | Dyslipidemia | Ouidir et al [119] | LEP | GDM | Sletner et al [90] | ||||
| APC | GDM | el Hajj et al [66] | LEPR | Obesity | Nogues et al [52] | ||||
| ARMCX6 | GDM | Petropoulos et al [104] | LINE1 repeat | GDM | el Hajj et al [66] | ||||
| BDP1P | Hyperglycaemia | Hivert et al [65] | LIT1 | GDM | el Hajj et al [66] | X | |||
| BLM | Hyperglycaemia | Cardenas et al [122] | LPL | GDM | Gagné-Ouellet et al [50] | ||||
| BMP7 | GDM | Cote et al [45] | LPL | GDM | Houde et al [105] | ||||
| BRCA1 | Obesity | Hoch et al [112] | LRP1 | Dyslipidemia | Guay et al [120] | ||||
| BRD1 | Dyslipidemia | Ouidir et al [119] | LRP1B | GDM | Houde et al [69] | ||||
| BRD2 | GDM | Houde et al [69] | MEG3 | GDM | el Hajj et al [66] | X | |||
| C19MC | Pre-pregnancy BMI | Prats-Puig et al [116] | MEG3 | GDM | Chen et al [92] | X | |||
| CCDC181 | GDM | Binder et al [102] | MEST | GDM | el Hajj et al [66] | X | |||
| CCDC97 | Obesity | Ghildayal et al [114] | MICA | Dyslipidemia | Ouidir et al [119] | ||||
| CHRNA4 | Hyperglycaemia | Hivert et al [65] | MICALL2/UNCX | Hyperglycaemia | Hivert et al [65] | ||||
| CIDEA | Hyperglycaemia | Yan et al [121] | MOGAT2 | Dyslipidemia | Ouidir et al [119] | ||||
| CPT1B | Hyperglycaemia | Yan et al [121] | NDUFB6 | GDM | el Hajj et al [66] | ||||
| CRHBP | Obesity | Ghildayal et al [114] | NESPAS | GDM | el Hajj et al [66] | X | |||
| CTBP2 | GDM | Cote et al [45] | NOTCH1 | GDM and T2DM | Shimanuki et al [111] | ||||
| CYBA | GDM and T2DM | Alexander et al [39] | NR3C1 | GDM | el Hajj et al [66] | ||||
| CYP24A1 | GDM | Wang et al [44] | NXN | GDM and T2DM | Alexander et al [39] | ||||
| CYP27B1 | GDM | Wang et al [44] | OAS1 | GDM | Zhang et al [97] | ||||
| DCAF11 | GDM | Petropoulos et al [104] | OCT4 | GDM | el Hajj et al [66] | ||||
| DGKZ | GDM | Petropoulos et al [104] | PDE4B | Hyperglycaemia | Cardenas et al [122] | ||||
| DHRS12 | Dyslipidemia | Ouidir et al [119] | PDX1 | GDM | Wang et al [44] | ||||
| DLGAP2 | GDM | Houde et al [69] | X | PEG3 | GDM | el Hajj et al [66] | X | ||
| DLGAP2 | Hyperglycaemia | Hivert et al [65] | X | PGC1a | GDM | Wang et al [44] | |||
| DLK1 | GDM | Zhao et al [95] | X | PGC1a | GDM | Cote et al [45] | |||
| DLL1 | GDM and T2DM | Shimanuki et al [111] | PGC1a | GDM | Liu et al [40] | ||||
| ECI2 | Dyslipidemia | Ouidir et al [119] | PGC1a | GDM and T2DM | Jiang et al [46] | ||||
| EGFL7 | Pre-pregnancy BMI | Shrestha et al [115] | PGC1a | Hyperglycaemia | Xie et al [47] | ||||
| ENTPD2 | Hyperglycaemia | Hivert et al [65] | PIWIL3 | GDM and T2DM | Alexander et al [39] | ||||
| ESR1 | GDM | Knabl et al [108] | PLIN1 | Hyperglycaemia | Yan et al [121] | ||||
| FTO | GDM | Franzago et al [91] | POLR2G | GDM | Zhang et al [97] | ||||
| FAAH | Dyslipidemia | Ouidir et al [119] | PPARA | GDM | Rong et al [101] | ||||
| G6PD | GDM | Steyn et al [96] | PPARA | GDM | el Hajj et al [66] | ||||
| GLUT3 | GDM | Rong et al [101] | PPIA | GDM | Zhang et al [97] | ||||
| GLUT3 | GDM | Liu et al [40] | PRDM16 | GDM | Cote et al [45] | ||||
| GSTM1 | GDM and T2DM | Alexander et al [39] | RBP4 | GDM | Rong et al [101] | ||||
| GSTM5 | GDM and T2DM | Alexander et al [39] | RBP4 | GDM | Liu et al [40] | ||||
| H19 | GDM | el Hajj et al [66] | X | RETN | GDM | Rong et al [101] | |||
| HECTD2 | Dyslipidemia | Ouidir et al [119] | RPTOR | Dyslipidemia | Ouidir et al [119] | ||||
| HLA-DOA | GDM | Binder et al [102] | SCARB1 | Dyslipidemia | Guay et al [120] | ||||
| HLA-H/J | GDM | Binder et al [102] | SLC6A4 | GDM | Blazevic et al [99] | ||||
| HTR2A | GDM | Horvatiček et al [89] | SNRPN | GDM | el Hajj et al [66] | X | |||
| ICAM-1 | GDM | Diaz-Perez et al [110] | SNRPN | GDM | Binder et al [102] | X | |||
| IGF1 | Hyperglycaemia | Desgagné et al [123] | SRM | Dyslipidemia | Ouidir et al [119] | ||||
| IGF1R | Hyperglycaemia | Desgagné et al [123] | X | SRM | Dyslipidemia | Ouidir et al [119] | |||
| IGFBP1 | GDM | Steyn et al [96] | SSTR4 | Hyperglycaemia | Yan et al [121] | ||||
| IGFBP2 | GDM | Steyn et al [96] | STK11 | Dyslipidemia | Ouidir et al [119] | ||||
| IGFBP3 | Hyperglycaemia | Desgagné et al [123] | TBR1 | GDM | Petropoulos et al [104] | ||||
| IGFBP6 | GDM | Steyn et al [96] | TKT | GDM | Steyn et al [96] | ||||
| IL10 | GDM | el Hajj et al [66] | TNFRSF1B | Hyperglycaemia | Cardenas et al [122] | ||||
| INSR | Hyperglycaemia | Desgagné et al [123] | VEZT | Pre-pregnancy BMI | Shrestha et al [115] | ||||
| KCNE1 | GDM and T2DM | Alexander et al [39] | |||||||
Abbreviations: GDM: Gestational Diabetes Mellitus, T2DM: Type 2 Diabetes Mellitus, BMI: body mass index.
Diabetes and obesity phenotypes
As outlined in Figure 2a, more than half (52%) of the 52 included studies focused on GDM. When combing with the other glucose intolerance phenotypes (T2DM and GDM combined studies (6%) and hyperglycaemia studies (15%)) 73% percent of the studies covered were focusing on aspects of glucose levels in pregnancy. A smaller proportion of studies (17%) focused on obesity, and only 4% of the papers focused on GDM and obesity combined. Important to notice, we were unable to find a study on placental DNAm with a focus on T1DM in pregnancy.
Figure 2.
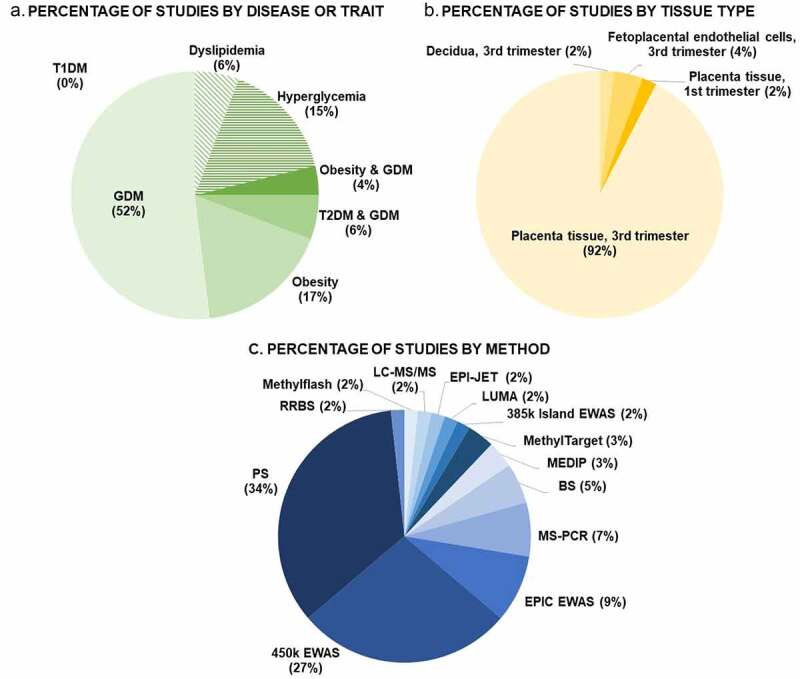
Percental overviews of number of studies conducted stratifying: A. by disease or trait, B. by placental tissue type, and C. by methodology. Abbreviations: GDM: Gestational Diabetes Mellitus, T1DM: Type 1 Diabetes Mellitus, T2DM: Type 2 Diabetes Mellitus, RRBS: reduced representation bisulphite sequencing, LC-MS/MS: liquid chromatography with tandem mass spectrometry, LUMA: Luminometric Methylation Assay, EWAS: epigenome wide association study, MEDIP: methylated DNA immunoprecipitation, BS, bisulphite sequencing, MS-PCR: methylation-specific PCR, PS: pyrosequencing.
Tissue and cell type specificity in Placenta DNAm
Of the 52 papers included, the vast majority (92%) studied DNAm in total placental tissue, mainly collected at term (Figure 2b). Hence, the DNAm results conducted in these studies provide an average DNAm percentage for all placental cell types [36]. Only one study had been performed in first trimester placenta whole tissue biopsies. Only three studies had focused on specific cell types (all at term); one study of DNAm in decidua and two studies in feto-placental endothelial cells (Figure 2b). No studies investigated DNAm in trophoblasts, although this placental cell type is the primary target of alterations in the maternal circulation and has crucial functions for placental growth and development.
Methods of DNAm measurements
Almost half of DNAm studies in the placenta that were included in this review were performed with genome-wide methods. The two most common approaches were pyrosequencing (34% of studies) and the Illumina 450 K array (27% of studies). (Figure 2c).
Sex-specific differences
Only nine of the 52 studies included sex differences as their outcome in the analyses (Table 1+2), which is unfortunate since the foetal sex has an impact on placental DNAm. Indeed, DNAm plays a key role in X-chromosome inactivation, a process that achieves dosage compensation for X-encoded gene products between female and male cells [37]. However, differential sex chromosome dosage complicates genome-wide epigenomic assessments as sex-specific methylation patterns on the X chromosome largely reflect the effects of X-chromosome inactivation [38]. Therefore, the sex chromosomes are frequently excluded from statistical analyses to avoid sex bias. Almost half of the EWAS studies investigating GDM removed both X-and Y-chromosome probes prior to statistical analysis (Table 1). One study, which segregated the influence of foetal sex on placental DNAm in GDM, removed only X-chromosome associated probes [39]. Another study included all probes in the analysis and report in total 10,424 differentially methylated regions (DMRs) in GDM placenta, out of which only 5% were annotated to autosomal chromosomes [40]. In none of the EWAS studies investigating DNAm in dyslipidemia were X-and Y-chromosomes probes removed, although all studies report adjusted analyses for foetal sex. In contrast, in EWAS studies of hyperglycaemia effects X-and Y-chromosome probes were always removed prior to statistical analysis and, except one, all adjusted for foetal sex (Table 2).
Discussion and future perspectives
Similarities and differences of results across maternal phenotypes
Four different genes (PGC1A, PPARA, LEP and ADIPOQ) were studied in three or more separate papers (Table 3). Both PPARA (nuclear receptor peroxisome proliferator activated receptor-alpha) and PGC1A (PPAR-gamma coactivator-1-alpha) play important roles in transcriptional regulation of energy metabolism including regulation of mitochondrial biogenesis and liver gluconeogenesis [41]. Indeed, in multiple studies PGC1A DNAm was directly associated with PGC1A mRNA expression and increased PGC1A promoter DNAm is positively associated with T2DM and physical inactivity [42,43]. Regarding PGC1A, four different studies were identified, of which two examined GDM pregnancies, one examined both GDM and T2DM and one examined various levels of glycaemia. Interestingly, all four studies showed an increase in placenta PGC1A promotor DNAm with hyperglycaemia and/or GDM compared to controls [44–47], however, one study observed increased PGC1A DNAm in only placentas linked to male offspring [46]. Regarding PPARA, notably all three studies conducted in GDM versus control cohorts consistently showed decreased PPARA DNAm. Even though cohort size in these studies ranged from rather small sample size of 40 placentas to up to 233 samples, the results demonstrate a reproducible and consistent effect of hyperglycaemia in pregnancy on placenta PGC1A and PPARA promotor DNAm.
Two other candidate genes, LEP (leptin) and ADIPOQ (adiponectin), were also targets in several studies. LEP was investigated in placentas from both GDM and obese pregnancies compared to controls. In two studies, LEP DNAm are found to be increased in GDM pregnancies independent of obesity [48], however, other studies did not find differences [49], or even contradictory results of decreased DNAm associated with glucose levels in 2nd trimester [50]. In obese versus lean pregnancies, one study found no differences in whole placenta tissue [51], whereas another study observed increased LEP DNAm when studying the foetal side of the placenta only [52]. Two studies have investigated ADIPOQ DNAm in obese versus lean pregnancies with apparently contradictory results: Nogues et al. found decreased DNAm at the maternal side only, but the average DNAm percentage was less than 5% [52] raising doubts about the presence of ADIPOQ in placenta. This was indeed concluded in another study, which failed to detect any DNAm of ADIPOQ in placenta [51]. A third study conducted in a significantly larger cohort (n = 98) found ADIPOQ DNAm at foetal side negatively associated with 2 hr glucose post OGTT, ADIPOQ DNAm at maternal side negatively associated with HOMA-IR, and that ADIPOQ DNAm at both sides negatively associated with maternal serum levels of adiponectin [53].
The Horvath epigenetic age acceleration model takes advantage of 62 CpGs in blood cells that are known to be highly associated with biological age. Indeed it has been speculated that offspring of hyperglycaemic and obese pregnancies have an older biological age as compared to their nominal age [54]. We observed that one study of dyslipidemia in women due to altered HDL cholesterol concentrations was associated with accelerated placental epigenetic ageing among women with normal pre-pregnancy weight and a female foetus [55]. This suggests an association between dyslipidemia and placental ageing that may vary by maternal obesity status and foetal sex. In addition, another study observed a negative association between placental epigenetic age acceleration and maternal pre-pregnancy BMI in male offspring only [56]. Whether this can be due to the male sex-associated placentas being more premature remains to be further investigated.
Placenta and imprinted genes
Imprinted genes are characterized by monoallelic expression as a result of epigenetic silencing of one allele based on its parent of origin [57]. Different from all other genes, epigenetic marks of imprinted genes escape erasure during the early stages of blastocyst development and their DNAm levels are stable throughout pregnancy [58]. Based on offspring phenotypes in human imprinting disorders such as Beckwith-Wiedemann or Russel-Silver syndrome, paternally expressed genes are considered to favour foetal growth, whereas maternally expressed genes restrict foetal growth [59,60].
To date close to 100 imprinted genes have been identified in humans [61]. The specific number expressed in human placenta in a strictly monoallelic fashion is unknown, but lower than originally thought [62] and maybe in the range of about 50 to 70. Also the C19MC gene cluster of 52 miRNAs is imprinted in human placenta exclusively expressed from the paternal allele [63]. These imprinted genes and gene clusters play key roles in placental development and function [64].
Pregnancies in women with diabetes or elevated BMI are often associated with altered placental and foetal phenotypes compared to pregnancies in healthy women. Hence, one could predict DNAm changes in imprinted genes with these conditions as a result of both maternal and foetal metabolic changes. Studies have so far limited themselves to maternal exposures and have focused on GDM. They have included only 10 genes imprinted in placenta (Table 4). Whereas the majority of maternally expressed genes were unaltered except for DLG associated protein 2 (DLGAP2) with increased DNAm levels in GDM, the three other imprinted genes affected by GDM were paternally expressed [65] (Table 4). In addition, reduced MEST DNAm was strongly associated with GDM [66] (Table 4). MEST is thought to be involved in angiogenesis regulation [67]. Hence, its lower DNAm in GDM may contribute to placental hypervascularization in some pregnancies in women with GDM.
Table 4.
Differentially methylated imprinted genes.
| Genes | Protein/transcript | Chr. locus | Parental origin | Exposure | Change with exposure | Authors | Function (related to development of placenta and foetus) |
|---|---|---|---|---|---|---|---|
| C19MC | Chromosome 19 microRNA cluster | 19q13.41 | P | Pre-pregnancy BMI | ↓ | Prats-Puig et al [116] | miRNA cluster consisting of 46 genes, encoding 59 mature miRNAs, that are primate-specific and exclusively expressed in the placenta, embryonic stem cells and few cancers [63]. |
| DLGAP2 | DLG Associated Protein 2 | 8p23.3 | M | GDM | ↑ | Houde et al [69] | Methylation may causally influence maternal insulin sensitivity [65]. |
| DLGAP2 | Hyperglycaemia | ↑ | Hivert et al [65] | ||||
| DLK1 | Delta Like Non-Canonical Notch Ligand 1 | 14q32.2 | P | GDM | ↑ | Zhao et al [95] |
DLK1-MEG3 imprinting locus associated with T1DM risk [125]. Predominant placental expression in vascular endothelial cells and pericytes, may play a pivotal role in development of these cells in placenta [126]. Hypomethylated DLK1 and H19 detected in Beckwith-Wiedeman-Syndrome [127]. Placental expression at term, but not in first trimester, tended to correlate with birthweight [57]. |
| H19 | long non-coding RNA | 11p15 | M | GDM | ns. | el Hajj et al [66] | Placental expression in first trimester, but not at term, correlated with crown rump length and tended to correlate with birth weight [57]. |
|
LIT1/ KCNQ1OT1 |
non-coding RNA | 11p15.5 | P | GDM | ns. | el Hajj et al [66] | Major genetic locus of Beckwith-Wiedeman-Syndrome [128]. Placental expression also altered in intrauterine growth restriction [128]. |
| MEG3 | Maternally Expressed 3 | 14q32.3 | M | GDM | ns. | el Hajj et al [66] | Reduced expression in Intrauterine growth restriction [129]. Expression not correlated with birth weight or placental weight [57]. DLK1-MEG3 imprinting locus associated with T1DM risk [125]. |
| MEG3 | GDM | ↑ | Chen et al [92] | ||||
| MEST | Mesoderm Specific Transcript | 7q32.2 | P/biallelic | GDM | ↓ | el Hajj et al [66] | Monoallelic expression in 81% of term placenta samples [57]. Differentially methylated in placentas of Small and large for gestational age [130]. No correlation between methylation and expression [130]. No expression correlation with birth weight [57]. Reduced placental methylation and increased expression in second trimester idiopathic spontaneous abortion [131]. |
| NESPAS | long non-coding RNA | 20q13.32 | P | GDM | ↓ | el Hajj et al [66] | Antisense to NESP; encodes neuroendocrine secretory protein 55 in endocrine and brain tissues, considered neuron-specific [132]. Nothing known in placenta. |
| PEG3 | Paternally Expressed 3 | 19q13.4 | P/biallelic | GDM | ns. | el Hajj et al [66] | Monoallelic expression in 88% of term placenta samples [57]. Cord blood PEG3 methylation associates with placental weight [133]. Reduced expression in Intrauterine growth restriction [129]. No correlation between expression and birth weight [57]. |
| SNRPN | SmallNuclear RibonucleoproteinPolypeptide N | 15q11.2 | P | GDM | ns. | el Hajj et al [66] | DNAm variation regulate normal placentation and placental disorders, is potentially susceptible to folic acid supplementation, and may be useful as novel foetal DNA marker in maternal plasma [134]. |
| SNRPN | GDM | ns. | Binder et al [102] |
Abbreviations: ns: non-significant, GDM: Gestational Diabetes Mellitus, T1DM: Type 1 Diabetes Mellitus, DNAm: DNA methylation.
The placenta is not only under the influence of maternal and foetal exposures, but itself can also modulate maternal and foetal metabolic, endocrine and inflammatory conditions thereby establishing a feedforward/feedback loop between mother/placenta and foetus/placenta [68]. Thus, in general DNAm of placental genes may have the potential to also influence maternal conditions. Interestingly, among 188 CpGs, whose DNAm levels associated with maternal insulin sensitivity in an EWAS, were 14 CpGs at 12 imprinted genes, nine maternally and three paternally expressed, respectively [65]. Mendelian randomization found five of these negatively associated with maternal insulin sensitivity among which was DLGAP2 [65] (Table 4). Therefore, higher placental DNAm of DLGAP2 contributes to insulin resistance in the pregnant woman, which may explain DLGAP2 increased DNAm in placentas of women with GDM [69].
Cellular composition and methods for placental DNAm data analysis
Placental phenotypes in adverse metabolic conditions are often accompanied by changes in cellular composition of the placenta. Hypervascularization is a common adaptive response to feto-placental transient or chronic hypoxia often found in pregnancies complicated by maternal diabetes or obesity [6,70,71]. Thus, cellular heterogeneity of the placenta may give rise to differential epigenetic patterns in the tissue sample obtained [72]. Variation of cellular composition, but also of position dependent environmental effects on the tissue within placental tissue are major confounders making selection of representative samples important [31]. Position effects of samples have been clearly shown in the imprinted IGF2/H19 region, with increasing methylation the further away the placenta sample was obtained from cord insertion [33]. Cell heterogeneity of the placenta may also be gene-specific, as previously documented for the repetitive LINE-1 region, where DNAm were similar across sampling sites [73]. At present, there is still no consensus on how placenta samples preferably should be obtained, Therefore, the study design of sample positioning, regarding both foetal versus maternal side, central versus posterior location, as well as single versus pooled multiple samples from each placenta, is important to report. This has already been emphasized [35,70,71,74], but positions of sampling sites have not been documented in most studies, which is a considerable limitation of studies using total placental tissue. Bioinformatic methods have been developed to account for potential alterations of cellular composition using deconvolution/cell type specific methods [31]. Reference-based algorithms have been developed to correct for cellular heterogeneity and have also been applied to human placental tissue [75]. Further, a recent study of purified placental cell types allows estimation of cell composition from whole placenta EWAS data [76].
Diabetes or obesity-associated changes in placental cellular composition certainly vary between individual pregnancies adding to confounding. Thus, deconvolution of data that is appropriate in a normal pregnancy may not be suitable for situations with more complex changes in cell composition and cellular phenotype. It remains to be demonstrated whether above or any future methods based on bioinformatics can fully capture the complexity of these changes and correct for them properly.
Perspectives
The focus of studies has so far been on the end of gestation, likely because of easy tissue availability and the association of DNAm with placental health [35].
The early pregnancy period, in particular the first trimester, is understudied. At the molecular and cellular level the placenta responds to maternal diabetes and obesity already at this early stage in pregnancy [77,78]. One can predict changes in DNAm associated with these conditions and, hence, there is an urgent need for these studies. However, early pregnancy placenta samples are difficult to avail. Usually, they are obtained from spontaneous or planned pregnancy terminations, which, for obvious ethical, and in some countries also legal reasons, are very restricted. Even when sampling is possible, general tissue availability is limited, pregnancies are often clinically and metabolically poorly characterized, and pregnancy outcome is unknown. Placental biopsies are normally only obtained by chorionic villus sampling on medical indications (e.g., suspicion of chromosomal/genetic abnormalities) and the amount of tissue is very limited. Whenever feasible, such studies will help to understand how placental trajectories are established that ultimately contribute to foetal development and neonatal outcome [79–81]. Notably, the IGF2/IGF2R axis including H19 is an important target to study, because their transcript levels associated not only with crown-rump length of the foetus in the first trimester, but these associations also track throughout pregnancy to include birth weight [57].
Causal effects of placental DNAm on maternal or foetal phenotype have been hypothesized, but only tested in one study employing Mendelian randomization [65]. This method of genetic epidemiology based on genetic variation needs to be used more widely in order to avoid over-interpretation of statistical exposure-phenotype associations [10]. Associations cannot establish causality and also do not allow for determining directionality. This is particularly important, because of potential bidirectional and distinct effects at the maternal-placental and foetal-placental interface. Quantifying the degree of DNAm of placental genes in the total cell free DNA pool in the maternal circulation may hold promise for being developed into a suitable early biomarker of GDM, perhaps combined with other anamnestic or laboratory parameters predictive of GDM [82].
DNA can not only be methylated to 5-methylcytosine within CpG dinucleotides, but also 5-hydroxymethylated to form 5-hydroxy-methylcytosine. Hydroxymethylation has its own epigenetic function and, in collaboration with 5-methylcytosine, regulates gene transcription in the human placenta [83,84]. Placental hydroxymethylation levels are higher than in most somatic tissues [85] and allelic placental hydroxymethylation is enriched in imprinted domains [84]. Nothing is known about potential placental gene modifications by hydroxymethylation in diabetes and obesity, despite their enrichment in genes involved in regulation of metabolic processes in the placenta [84].
Although the influence of foetal sex on placental responses during pregnancy as well as pregnancy outcome and disease risk later in life is highly suggested, only few studies performed DNAm analysis stratified by foetal sex. Such analyses would provide insights into in utero events driven by foetal sex and potentially shed light on different disease risk development between male and female adults. Besides molecular causes such as X-and Y-chromosome regulated processes, sexual dimorphism might arise due to maternal, placental and/or foetal hormonal differences during pregnancy, which should be taken into account. Specifically, early placental choriogonadotropin (hCG), maternal leptin, oestrogen and progesterone have been associated with risk for GDM and differ between pregnancies of male vs female foetuses [86].
Conclusion
With this review, we have summarized current knowledge on variation of placental DNAm profiles in pregnancies affected by diabetes, obesity, hyperglycaemia and hyperlipidaemia. We observe interesting overlaps in DNAm variation between several studies including a consistent higher DNAm degree at the PGC1A promoter, and lower DNAm degree at the PPARA gene region. Also, the DNAm of the imprinted gene DLGAP2 was found increased both with GDM, and when examining the association by continuous glucose measurements. In addition, available evidence suggests that GDM is associated with higher LEP DNAm, independent of obesity, reinforcing the complexity of GDM effects including different mechanisms linked to hyperglycaemia versus maternal obesity. To the best of our knowledge the effect of maternal T1DM on placental DNAm has not been investigated so far despite established alterations in placental phenotype in T1DM [87]. We furthermore identified missing, yet highly relevant, research of specific placental cell types and in samples obtained at earlier time points than at delivery. Maternal and foetal outcomes directly linked to placental DNAm variation need to be established with consideration of foetal sex. For future approaches there is great potential in conducting Mendelian randomization studies in large sample sizes, to identify causal pathways linking maternal metabolic health during pregnancy with placental DNAm and short- as well as long-term offspring outcome. Introduction of uniformed statistical protocols for DNAm analysis i.e., removal or inclusion of X-and Y-linked probes and adjustment for foetal sex and other known confounders is also a point for improvement as it would enable better comparisons of results between the studies and potentially increase reproducibility. The few studies reporting DNAm variations stratified by foetal sex indeed show sex-specific alterations although we acknowledge the small sample size which is a frequent limitation in these studies. Finally, importance of placental sampling positioning and the overlap between maternal versus foetal DNAm patterns across placenta, cord blood and maternal blood remains unclear, and should be prioritized in future research.
Funding Statement
The Danish Diabetes Academy supported by the Novo Nordisk Foundation, and The Danish Diabetes Association (Diabetesforeningen). LH is partly employed at the Novo Nordisk Foundation Center for Basic Metabolic Research, which is an independent research center at the University of Copenhagen, partially funded by an unrestricted donation from the Novo Nordisk Foundation (NNF18CC0034900).
Author Contributions
LH and GD developed the ideas presented in this review, with contributions from BN, SC, RS and PD. LH, BN, SC and GD wrote the manuscript, with contributions from RS and PD. All authors critically revised the manuscript and had access to the final version.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data Availability Statement
Data is contained within the article and can be accessed from the corresponding authors on reasonable request.
References
- [1].Ben-Haroush A, Yogev Y, Hod M.. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabet Med. [Internet]. 2004;21(2):103–113. Available from: http://www.ncbi.nlm.nih.gov/pubmed/14984444 [DOI] [PubMed] [Google Scholar]
- [2].Damm P, Kühl C, Bertelsen A, et al. Predictive factors for the development of diabetes in women with previous gestational diabetes mellitus. Am J Obstet Gynecol. [Internet]. 1992;167(3):607–616. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1530012 [DOI] [PubMed] [Google Scholar]
- [3].Wang H, Li N, Chivese T, et al. IDF diabetes atlas: estimation of global and regional gestational diabetes. Diabetes Res Clin Pract. [Internet] 2022;183:109050.Available fromhttp://www.ncbi.nlm.nih.gov/pubmed/34883186 [DOI] [PubMed] [Google Scholar]
- [4].Chivese T, Hoegfeldt CA, Werfalli M, et al. IDF Diabetes Atlas: the prevalence of pre-existing diabetes in pregnancy - A systematic reviewand meta-analysis of studies published during 2010-2020. Diabetes Res Clin Pract. [Internet]. 2022;183:109049. http://www.ncbi.nlm.nih.gov/pubmed/34883190 [DOI] [PubMed] [Google Scholar]
- [5].Poston L, Caleyachetty R, Cnattingius S, et al. Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol. [Internet]. 2016;4(12):1025–1036. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27743975 [DOI] [PubMed] [Google Scholar]
- [6].McIntyre HD, Catalano P, Zhang C, et al. Gestational diabetes mellitus. Nat Rev Dis Prim. [Internet]. 2019;5:47. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31296866 [DOI] [PubMed] [Google Scholar]
- [7].Fernandez-Twinn DS, Hjort L, Novakovic B, et al. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. [Internet]. 2019;62(10):1789–1801. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31451874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Damm P, Houshmand-Oeregaard A, Kelstrup L, et al. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia. [Internet]. 2016;59(7):1396–1399. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27174368 [DOI] [PubMed] [Google Scholar]
- [9].Retnakaran R, Shah BR. Fetal sex and the natural history of maternal risk of diabetes during and after pregnancy. J Clin Endocrinol Metab. [Internet]. 2015;100(7):2574–2580. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25993641 [DOI] [PubMed] [Google Scholar]
- [10].Richmond RC, Timpson NJ, Felix JF, et al. Using genetic variation to explore the causal effect of maternal pregnancy adiposity on future offspring adiposity: a mendelian randomisation study. PLoS Med. [Internet]. 2017;14(1):e1002221. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28118352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hjort L, Novakovic B, Grunnet LG, et al. Diabetes in pregnancy and epigenetic mechanisms-how the first 9 months from conception might affect the child’s epigenome and later risk of disease. lancet Diabetes Endocrinol [Internet] 2019; Available from: http://www.ncbi.nlm.nih.gov/pubmed/31128973 [DOI] [PubMed]
- [12].Gauster M, Desoye G, Tötsch M, et al. The placenta and gestational diabetes mellitus. Curr Diab Rep. [Internet]. 2012;12(1):16–23. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22102097 [DOI] [PubMed] [Google Scholar]
- [13].Ringholm L, Mathiesen ER, Kelstrup L, et al. Managing type 1 diabetes mellitus in pregnancy--from planning to breastfeeding. Nat Rev Endocrinol. [Internet]. 2012;8(11):659–667. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22965164 [DOI] [PubMed] [Google Scholar]
- [14].Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obs Gynecol. [Internet]. 2007;50(4):972–979. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17982340 [DOI] [PubMed] [Google Scholar]
- [15].Verburg PE, Tucker G, Scheil W, et al. Sexual dimorphism in adverse pregnancy outcomes - a retrospective Australian population study 1981-2011. PLoS One. [Internet]. 2016;11:e0158807. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27398996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Retnakaran R, Shah BR. Sex of the baby and future maternal risk of Type 2 diabetes in women who had gestational diabetes. Diabet Med. [Internet]. 2016;33(7):956–960. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26470996 [DOI] [PubMed] [Google Scholar]
- [17].Bird A. Perceptions of epigenetics.Nat. 2007;447(7143):396–398. [DOI] [PubMed] [Google Scholar]
- [18].Hyun K, Jeon J, Park K, et al. Writing, erasing and reading histone lysine methylations. Exp Mol Med. [Internet]. 2017;49(4):e324. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28450737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. [Internet]. 2001;409(6822):860–921. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11237011 [DOI] [PubMed] [Google Scholar]
- [20].Bird AP. CpG-rich Islands and the function of DNA methylation. Nature. [Internet]. 1986;321(6067):209–213. Available from: http://www.ncbi.nlm.nih.gov/pubmed/2423876 [DOI] [PubMed] [Google Scholar]
- [21].Pidsley R, Wong CC Y, Volta M, et al. A data-driven approach to preprocessing Illumina 450K methylation array data. BMC Genomics. [Internet]. 2013;14(1):293. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23631413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Novakovic B, Wong NC, Sibson M, et al. DNA methylation-mediated down-regulation of DNA methyltransferase-1 (DNMT1) is coincident with, but not essential for, global hypomethylation in human placenta. J Biol Chem. [Internet]. 2010;285(13):9583–9593. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20071334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lister R, Pelizzola M, Dowen RH, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. [Internet]. 2009;462(7271):315–322. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19829295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Masser DR, Berg AS, Freeman WM. Focused, high accuracy 5-methylcytosine quantitation with base resolution by benchtop next-generation sequencing. Epigenetics Chromatin. [Internet]. 2013;6(1):33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24279302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Clark SJ, Harrison J, Paul CL, et al. High sensitivity mapping of methylated cytosines. Nucleic Acids Res. [Internet]. 1994;22(15):2990–2997. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8065911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Smith ZD, Shi J, Gu H, et al. Epigenetic restriction of extraembryonic lineages mirrors the somatic transition to cancer. Nature. [Internet]. 2017;549(7673):543–547. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28959968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Ehrlich M, Gama-Sosa MA, Huang LH, et al. Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res. [Internet]. 1982;10(8):2709–2721. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7079182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Schroeder DI, Blair JD, Lott P, et al. The human placenta methylome. Proc Natl Acad Sci U S A. [Internet]. 2013;110(15):6037–6042. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23530188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chiu RWK, Chim SSC, Wong IHN, et al. Hypermethylation of RASSF1A in human and rhesus placentas. Am J Pathol. [Internet]. 2007;170(7271):941–950. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17322379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nelissen ECM, van Montfoort APA, Dumoulin JCM, et al. Epigenetics and the placenta. Hum Reprod Update. [Internet]. 2009;17(7271):397–417. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20959349 [DOI] [PubMed] [Google Scholar]
- [31].Januar V, Desoye G, Novakovic B, et al. Epigenetic regulation of human placental function and pregnancy outcome: considerations for causal inference. Am J Obstet Gynecol. 2015;213(4):S182–96. [DOI] [PubMed] [Google Scholar]
- [32].Bhattacharya A, Freedman AN, Avula V, et al. Placental genomics mediates genetic associations with complex health traits and disease. Nat Commun. [Internet]. 2022;13(1):706. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35121757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Loke YJ, Galati JC, Morley R, et al. Association of maternal and nutrient supply line factors with DNA methylation at the imprinted IGF2/H19 locus in multiple tissues of newborn twins. Epigenetics. 2013;8:1069–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Paquette AG, Houseman EA, Green BB, et al. Regions of variable DNA methylation in human placenta associated with newborn neurobehavior. Epigenetics. [Internet]. 2016;11(8):603–613. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27366929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wilson SL, Robinson WP. Utility of DNA methylation to assess placental health. Placenta. [Internet]. 2018;64(Suppl 1):S23–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29273273 [DOI] [PubMed] [Google Scholar]
- [36].Khankin EV, Royle C, Karumanchi SA. Placental vasculature in health and disease. Semin Thromb Hemost. [Internet]. 2010;36(3):309–320. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20490981 [DOI] [PubMed] [Google Scholar]
- [37].Lyon MF. Gene action in the X-chromosome of the mouse (Mus musculus L.). Nature. [Internet]. 1961;190(4773):372–373. Available from: http://www.ncbi.nlm.nih.gov/pubmed/13764598 [DOI] [PubMed] [Google Scholar]
- [38].Duncan CG, Grimm SA, Morgan DL, et al. Dosage compensation and DNA methylation landscape of the X chromosome in mouse liver. Sci Rep [Internet]. 2018;8:10138. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29973619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Alexander J, Teague AM, Chen J, et al. Offspring sex impacts DNA methylation and gene expression in placentae from women with diabetes during pregnancy. PLoS One. [Internet]. 2018;13(2):e0190698. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29470513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Liu L, Zhang X, Rong C, et al. Distinct DNA methylomes of human placentas between pre-eclampsia and gestational diabetes mellitus. Cell Physiol Biochem. [Internet]. 2014;34: 1877–1889. Available from http://www.ncbi.nlm.nih.gov/pubmed/25503509 [DOI] [PubMed] [Google Scholar]
- [41].Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. [Internet]. 2003;100(14):8466–8471. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12832613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Barres R, Osler ME, Yan J, et al. Non-CpG methylation of the PGC-1alpha promoter through DNMT3B controls mitochondrial density. Cell Metab. [Internet]. 2009;10(3):189–198. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19723495 [DOI] [PubMed] [Google Scholar]
- [43].Barrès R, Yan J, Egan B, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15(3):405–411. [DOI] [PubMed] [Google Scholar]
- [44].Wang L, Fan H, Zhou L, et al. Altered expression of PGC-1 α and PDX1 and their methylation status are associated with fetal glucose metabolism in gestational diabetes mellitus. Biochem Biophys Res Commun. [Internet]. 2018;501(1):300–306. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29730292 [DOI] [PubMed] [Google Scholar]
- [45].Côté S, Gagné-Ouellet V, Guay S-P, et al. PPARGC1α gene DNA methylation variations in human placenta mediate the link between maternal hyperglycemia and leptin levels in newborns. Clin Epigenetics. [Internet]. 2016;8(1):72. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27340502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jiang S, Teague AM, Tryggestad JB, et al. Role of metformin in epigenetic regulation of placental mitochondrial biogenesis in maternal diabetes. Sci Rep. [Internet]. 2020;10(1):8314. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32433500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Xie X, Gao H, Zeng W, et al. Placental DNA methylation of peroxisome-proliferator-activated receptor-γ co-activator-1α promoter is associated with maternal gestational glucose level. Clin Sci (Lond). [Internet]. 2015;129(4):385–394. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25875376 [DOI] [PubMed] [Google Scholar]
- [48].Lesseur C, Armstrong DA, Paquette AG, et al. Maternal obesity and gestational diabetes are associated with placental leptin DNA methylation. Am J Obstet Gynecol. [Internet]. 2014;211(6):654.e1–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24954653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].El HN, Pliushch G, Schneider E, et al. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes. [Internet]. 2013;62: 1320–1328. Available from http://www.ncbi.nlm.nih.gov/pubmed/23209187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Gagné-Ouellet V, Breton E, Thibeault K, et al. Mediation analysis supports a causal relationship between maternal hyperglycemia and placental DNA methylation variations at the leptin gene locus and cord blood leptin levels. Int J Mol Sci. [Internet]. 2020;21(1):329. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31947745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Haghiac M, Basu S, Presley L, et al. Patterns of adiponectin expression in term pregnancy: impact of obesity. J Clin Endocrinol Metab. [Internet]. 2014;99(9):3427–3434. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24796925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Nogues P, Dos Santos E, Jammes H, et al. Maternal obesity influences expression and DNA methylation of the adiponectin and leptin systems in human third-trimester placenta. Clin Epigenetics. [Internet]. 2019;11(1):20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30732639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Bouchard L, Hivert MF, Guay SP, et al. Placental adiponectin gene DNA methylation levels are associated with mothers’ blood glucose concentration. Diabetes. [Internet]. 2012;61(5):1272–1280. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22396200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hjort L, Vryer R, Grunnet LG, et al. Telomere length is reduced in 9- to 16-year-old girls exposed to gestational diabetes in utero. Diabetologia. 2018;61(4):870–880. [DOI] [PubMed] [Google Scholar]
- [55].Shrestha D, Workalemahu T, Tekola-Ayele F. Maternal dyslipidemia during early pregnancy and epigenetic ageing of the placenta. Epigenetics. [Internet]. 2019;14(10):1030–1039. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31179827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Workalemahu T, Shrestha D, Tajuddin SM, et al. Maternal cardiometabolic factors and genetic ancestry influence epigenetic aging of the placenta. J Dev Orig Health Dis. [Internet]. 2021;12(1):34–41. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31948495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Moore GE, Ishida M, Demetriou C, et al. The role and interaction of imprinted genes in human fetal growth. Philos Trans R Soc Lond B Biol Sci. [Internet]. 2015;370(1663):20140074. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25602077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fowden AL, Coan PM, Angiolini E, et al. Imprinted genes and the epigenetic regulation of placental phenotype. Prog Biophys Mol Biol. [Internet]. 2011;106(1):281–288. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21108957 [DOI] [PubMed] [Google Scholar]
- [59].Bressan FF, De Bem THC, Perecin F, et al. Unearthing the roles of imprinted genes in the placenta. Placenta. [Internet]. 2009;30(10):823–834. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19679348 [DOI] [PubMed] [Google Scholar]
- [60].Coan PM, Burton GJ, Ferguson-Smith AC. Imprinted genes in the placenta--a review. Placenta. [Internet]. 2005;26: A:S10–20. Available from http://www.ncbi.nlm.nih.gov/pubmed/15837057 [DOI] [PubMed] [Google Scholar]
- [61].Allach El Khattabi L, Backer S, Pinard A, et al. A genome-wide search for new imprinted genes in the human placenta identifies DSCAM as the first imprinted gene on chromosome 21. Eur J Hum Genet. [Internet]. 2019;27(1):49–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30206355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Pilvar D, Reiman M, Pilvar A, et al. Parent-of-origin-specific allelic expression in the human placenta is limited to established imprinted loci and it is stably maintained across pregnancy. Clin Epigenetics. [Internet]. 2019;11(1):94. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31242935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Noguer-Dance M, Abu-Amero S, Al-Khtib M, et al. The primate-specific microRNA gene cluster (C19MC) is imprinted in the placenta. Hum Mol Genet. [Internet]. 2010;19(18):3566–3582. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20610438 [DOI] [PubMed] [Google Scholar]
- [64].Wagschal A, Feil R. Genomic imprinting in the placenta. Cytogenet Genome Res. [Internet]. 2006;113(1–4):90–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16575167 [DOI] [PubMed] [Google Scholar]
- [65].Hivert M-F, Cardenas A, Allard C, et al. Interplay of placental DNA methylation and maternal insulin sensitivity in pregnancy. Diabetes. [Internet]. 2020;69: 484–492. Available from http://www.ncbi.nlm.nih.gov/pubmed/31882564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].El Hajj N, Pliushch G, Schneider E, et al. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes. 2013;62(4):1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Mayer W, Hemberger M, Frank HG, et al. Expression of the imprinted genes MEST/Mest in human and murine placenta suggests a role in angiogenesis. Dev Dyn. [Internet]. 2000;217(1):1–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10679925 [DOI] [PubMed] [Google Scholar]
- [68].Hiden U, Maier A, Bilban M, et al. Insulin control of placental gene expression shifts from mother to foetus over the course of pregnancy. Diabetologia. [Internet]. 2006;49(1):123–131. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16344925 [DOI] [PubMed] [Google Scholar]
- [69].Houde -A-A, Ruchat S-M, Allard C, et al. LRP1B, BRD2 and CACNA1D: new candidate genes in fetal metabolic programming of newborns exposed to maternal hyperglycemia. Epigenomics. [Internet]. 2015;7(7):1111–1122. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26586120 [DOI] [PubMed] [Google Scholar]
- [70].Desoye G, Wells JCK. Pregnancies in diabetes and obesity: the capacity-load model of placental adaptation. Diabetes. [Internet]. 2021;70(4):823–830. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33741605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Desoye G. The human placenta in diabetes and obesity: friend or foe? the 2017 norbert freinkel award lecture. Diabetes Care. [Internet]. 2018;41(7):1362–1369. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29934479 [DOI] [PubMed] [Google Scholar]
- [72].Konwar C, Del Gobbo G, Yuan V, et al. Considerations when processing and interpreting genomics data of the placenta. Placenta. [Internet]. 2019;84:57–62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30642669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Non AL, Binder AM, Barault L, et al. DNA methylation of stress-related genes and LINE-1 repetitive elements across the healthy human placenta. Placenta. [Internet]. 2012;33(3):183–187. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22222044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hogg K, Price EM, Robinson WP. Improved reporting of DNA methylation data derived from studies of the human placenta. Epigenetics. [Internet]. 2014;9(3):333–337. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24394602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Houseman EA, Kile ML, Christiani DC, et al. Reference-free deconvolution of DNA methylation data and mediation by cell composition effects. BMC Bioinformatics. [Internet]. 2016;17(1):259. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27358049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Yuan V, Hui D, Yin Y, et al. Cell-specific characterization of the placental methylome. BMC Genomics. [Internet]. 2021;22(1):6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33407091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Majali-Martinez A, Weiss-Fuchs U, Miedl H, et al. Type 1 diabetes mellitus and the first trimester placenta: hyperglycemia-induced effects on trophoblast proliferation, cell cycle regulators, and invasion. Int J Mol Sci. [Internet]. 2021;22(20):10989. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34681648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lassance L, Haghiac M, Leahy P, et al. Identification of early transcriptome signatures in placenta exposed to insulin and obesity. Am J Obstet Gynecol. [Internet]. 2015;212(5):647.e1–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25731694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Novakovic B, Gordon L, Robinson WP, et al. Glucose as a fetal nutrient: dynamic regulation of several glucose transporter genes by DNA methylation in the human placenta across gestation. J Nutr Biochem. [Internet]. 2013;24(1):282–288. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22901689 [DOI] [PubMed] [Google Scholar]
- [80].Novakovic B, Yuen RK, Gordon L, et al. Evidence for widespread changes in promoter methylation profile in human placenta in response to increasing gestational age and environmental/stochastic factors. BMC Genomics. [Internet]. 2011;12: 529. Available from http://www.ncbi.nlm.nih.gov/pubmed/22032438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Desoye G, Cervar-Zivkovic M. Diabetes mellitus, obesity, and the placenta. Obstet Gynecol Clin North Am. [Internet]. 2020;47(1):65–79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32008672 [DOI] [PubMed] [Google Scholar]
- [82].Del Vecchio G, Li Q, Li W, et al. Cell-free DNA methylation and transcriptomic signature prediction of pregnancies with adverse outcomes. Epigenetics. [Internet]. 2021;16(6):642–661. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33045922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Green BB, Houseman EA, Johnson KC, et al. Hydroxymethylation is uniquely distributed within term placenta, and is associated with gene expression. FASEB J. [Internet]. 2016;30: 2874–2884. Available from http://www.ncbi.nlm.nih.gov/pubmed/27118675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hernandez Mora JR, Sanchez-Delgado M, Petazzi P, et al. Profiling of oxBS-450K 5-hydroxymethylcytosine in human placenta and brain reveals enrichment at imprinted loci. Epigenetics. [Internet]. 2018;13(2):182–191. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28678681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Nestor CE, Ottaviano R, Reddington J, et al. Tissue type is a major modifier of the 5-hydroxymethylcytosine content of human genes. Genome Res. [Internet]. 2012;22(3):467–477. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22106369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Stern C, Schwarz S, Moser G, et al. Placental endocrine activity: adaptation and disruption of maternal glucose metabolism in pregnancy and the influence of fetal sex. Int J Mol Sci. [Internet]. 2021;22(23):12722. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34884524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Higgins M, Felle P, Mooney EE, et al. Stereology of the placenta in type 1 and type 2 diabetes. Placenta. [Internet]. 2011;32(8):564–569. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21621839 [DOI] [PubMed] [Google Scholar]
- [88].Lu S, Wang J, Kakongoma N, et al. DNA methylation and expression profiles of placenta and umbilical cord blood reveal the characteristics of gestational diabetes mellitus patients and offspring. Clin Epigenetics. [Internet]. 2022;14(1):69. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35606885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Horvatiček M, Perić M, Bečeheli I, et al. Maternal metabolic state and fetal sex and genotype modulate methylation of the serotonin receptor type 2A gene (HTR2A) in the Human Placenta. Biomedicines. [Internet]. 2022;10(2):467. Available from: http://www.ncbi.nlm.nih.gov/pubmed/35203678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sletner L, Moen AEF, Yajnik CS, et al. Maternal glucose and LDL-cholesterol levels are related to placental leptin gene methylation, and, together with nutritional factors, largely explain a higher methylation level among ethnic South Asians. Front Endocrinol (Lausanne). [Internet]. 2021;12: 809916. Availablefrom http://www.ncbi.nlm.nih.gov/pubmed/35002980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Franzago M, Fraticelli F, Marchioni M, et al. Fat mass and obesity-associated (FTO) gene epigenetic modifications in gestational diabetes: new insights and possible pathophysiological connections. Acta Diabetol. [Internet]. 2021;58(8):997–1007. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33743080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Chen C, Jiang Y, Yan T, et al. Placental maternally expressed gene 3 differentially methylated region methylation profile is associated with maternal glucose concentration and newborn birthweight. J Diabetes Investig. [Internet]. 2021;12(6):1074–1082. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33090678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Awamleh Z, Butcher DT, Hanley A, et al. Exposure to Gestational Diabetes Mellitus (GDM) alters DNA methylation in placenta and fetal cord blood. Diabetes Res Clin Pract. [Internet]. 2021;174:108690. Available from: http://www.ncbi.nlm.nih.gov/pubmed/33549677 [DOI] [PubMed] [Google Scholar]
- [94].Wang Y, Wang T, Huo Y, et al. Placenta expression of vitamin D and related genes in pregnant women with gestational diabetes mellitus. J Steroid Biochem Mol Biol. [Internet]. 2020;204:105754. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32946925 [DOI] [PubMed] [Google Scholar]
- [95].Zhao B-H, Jiang Y, Zhu H, et al. Placental delta-like 1 gene DNA methylation levels are related to mothers’ blood glucose concentration. J Diabetes Res. [Internet]. 2019:9521510. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31886292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Steyn A, Crowther NJ, Norris SA, et al. Epigenetic modification of the pentose phosphate pathway and the IGF-axis in women with gestational diabetes mellitus. Epigenomics. [Internet]. 2019;11(12):1371–1385. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31583916 [DOI] [PubMed] [Google Scholar]
- [97].Zhang Y, Zhang T, Chen Y. Comprehensive analysis of gene expression profiles and DNA methylome reveals Oas1, Ppie, Polr2g as pathogenic target genes of gestational diabetes mellitus.Sci Rep [Internet]. 2018;8(1):16244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Gagné-Ouellet V, Houde -A-A, Guay S-P, et al. Placental lipoprotein lipase DNA methylation alterations are associated with gestational diabetes and body composition at 5 years of age. Epigenetics. [Internet]. 2017;12(8):616–625. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28486003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Blazevic S, Horvaticek M, Kesic M, et al. Epigenetic adaptation of the placental serotonin transporter gene (SLC6A4) to gestational diabetes mellitus. PLoS One. [Internet]. 2017;12(6):e0179934. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28650965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Reichetzeder C, Dwi Putra SE, Pfab T, et al. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenetics. [Internet]. 2016;8(1):82. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27462376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Rong C, Cui X, Chen J, et al. DNA methylation profiles in placenta and its association with gestational diabetes mellitus. Exp Clin Endocrinol Diabetes. [Internet]. 2015;123(5):282–288. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25962407 [DOI] [PubMed] [Google Scholar]
- [102].Binder AM, LaRocca J, Lesseur C, et al. Epigenome-wide and transcriptome-wide analyses reveal gestational diabetes is associated with alterations in the human leukocyte antigen complex. Clin Epigenetics. [Internet]. 2015;7(1):79. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26244062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Finer S, Mathews C, Lowe R, et al. Maternal gestational diabetes is associated with genome-wide DNA methylation variation in placenta and cord blood of exposed offspring. Hum Mol Genet. [Internet]. 2015;24(11):3021–3029. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25634562 [DOI] [PubMed] [Google Scholar]
- [104].Petropoulos S, Guillemin C, Ergaz Z, et al. Gestational diabetes alters offspring dna methylation profiles in human and rat: identification of key pathways involved in endocrine system disorders insulin signaling, diabetes signaling, and ILK signaling. Endocrinology. [Internet]. 2015;156: 2222–2238. Available from http://www.ncbi.nlm.nih.gov/pubmed/25514087 [DOI] [PubMed] [Google Scholar]
- [105].Houde AA, St-Pierre J, Hivert MF, et al.,Placental lipoprotein lipase DNA methylation levels are associated with gestational diabetes mellitus and maternal and cord blood lipid profiles.J Dev Orig Heal Dis [Internet] 2014; 5:132–141. Available from.http://www.ncbi.nlm.nih.gov/pubmed/24847699. [DOI] [PubMed] [Google Scholar]
- [106].Ruchat SM, Houde AA, Voisin G, et al. Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics. [Internet]. 2013;8(9):935–943. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23975224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Reese SE, Zhao S, Wu MC, et al. DNA methylation score as a biomarker in newborns for sustained maternal smoking during pregnancy. Environ Health Perspect. [Internet]. 2017;125(4):760–766. Available from: http://www.ncbi.nlm.nih.gov/pubmed/27323799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Knabl J, Hiden U, Hüttenbrenner R, et al. GDM alters expression of placental estrogen receptor α in a cell type and gender-specific manner. Reprod Sci. [Internet]. 2015;22(12):1488–1495. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25947892 [DOI] [PubMed] [Google Scholar]
- [109].Cvitic S, Novakovic B, Gordon L, et al. Human fetoplacental arterial and venous endothelial cells are differentially programmed by gestational diabetes mellitus, resulting in cell-specific barrier function changes. Diabetologia. [Internet]. 2018;61:2398–2411. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30091044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Díaz-Pérez FI, Hiden U, Gauster M, et al. Post-transcriptional down regulation of ICAM-1 in feto-placental endothelium in GDM. Cell Adh Migr. [Internet]. 2016;10(1–2):18–27. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26761204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Shimanuki Y, Mitomi H, Fukumura Y, et al. Alteration of Delta-like ligand 1 and Notch 1 receptor in various placental disorders with special reference to early onset preeclampsia. Hum Pathol. [Internet]. 2015;46(8):1129–1137. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26014475 [DOI] [PubMed] [Google Scholar]
- [112].Hoch D, Bachbauer M, Pöchlauer C, et al. Maternal obesity alters placental cell cycle regulators in the first trimester of human pregnancy: new insights for BRCA1. Int J Mol Sci. [Internet]. 2020;21(2):468. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31940810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Shen W-B, Ni J, Yao R, et al. Maternal obesity increases DNA methylation and decreases RNA methylation in the human placenta. Reprod Toxicol. [Internet]. 2022;107:90–96. Available from: http://www.ncbi.nlm.nih.gov/pubmed/34890771 [DOI] [PubMed] [Google Scholar]
- [114].Ghildayal N, Fore R, Lutz SM, et al.Early-pregnancy maternal body mass index is associated with common DNA methylation markers in cord blood and placenta: a paired-tissue epigenome-wide association study.Epigenetics.[Internet] 2021;1–11.Available fromhttp://www.ncbi.nlm.nih.gov/pubmed/34384032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Shrestha D, Ouidir M, Workalemahu T, et al. Placental DNA methylation changes associated with maternal prepregnancy BMI and gestational weight gain. Int J Obes (Lond). [Internet]. 2020;44(6):1406–1416. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32071425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Prats-Puig A, Xargay-Torrent S, Carreras-Badosa G, et al. Methylation of the C19MC microRNA locus in the placenta: association with maternal and chilhood body size. Int J Obes (Lond). [Internet]. 2020;44(1):13–22. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31554916 [DOI] [PubMed] [Google Scholar]
- [117].Mitsuya K, Parker AN, Liu L, et al. Alterations in the placental methylome with maternal obesity and evidence for metabolic regulation. PLoS One. [Internet]. 2017;12(10):e0186115. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29045485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Nomura Y, Lambertini L, Rialdi A, et al. Global methylation in the placenta and umbilical cord blood from pregnancies with maternal gestational diabetes, preeclampsia, and obesity. Reprod Sci. [Internet]. 2014;21(1):131–137. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23765376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Ouidir M, Zeng X, Workalemahu T, et al. Early pregnancy dyslipidemia is associated with placental DNA methylation at loci relevant for cardiometabolic diseases. Epigenomics. [Internet]. 2020;12(11):921–934. Available from: http://www.ncbi.nlm.nih.gov/pubmed/32677467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Guay S-P, Houde -A-A, Breton E, et al. DNA methylation at LRP1 gene locus mediates the association between maternal total cholesterol changes in pregnancy and cord blood leptin levels. J Dev Orig Health Dis. [Internet]. 2020;11(4):369–378. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31753053 [DOI] [PubMed] [Google Scholar]
- [121].Yan W, Zhang Y, Wang L, et al. Maternal dietary glycaemic change during gestation influences insulin-related gene methylation in the placental tissue: a genome-wide methylation analysis. Genes Nutr. [Internet]. 2019;14(1):17. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31086609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Cardenas A, Gagné-Ouellet V, Allard C, et al. Placental DNA methylation adaptation to maternal glycemic response in pregnancy. Diabetes. [Internet]. 2018;67(8):1673–1683. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29752424 [DOI] [PubMed] [Google Scholar]
- [123].Desgagné V, Hivert M-F, St-Pierre J, et al. Epigenetic dysregulation of the IGF system in placenta of newborns exposed to maternal impaired glucose tolerance. Epigenomics. [Internet]. 2014;6(2):193–207. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24811788 [DOI] [PubMed] [Google Scholar]
- [124].Houde -A-A, Guay S-P, Desgagné V, et al. Adaptations of placental and cord blood ABCA1 DNA methylation profile to maternal metabolic status. Epigenetics . [Internet]. 2013;8(12):1289–1302. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24113149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Wallace C, Smyth DJ, Maisuria-Armer M, et al. The imprinted DLK1-MEG3 gene region on chromosome 14q32.2 alters susceptibility to type 1 diabetes. Nat Genet. [Internet]. 2010;42(1):68–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19966805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Kagami M, Matsuoka K, Nagai T, et al. Paternal uniparental disomy 14 and related disorders: placental gene expression analyses and histological examinations. Epigenetics. [Internet]. 2012;7(10):1142–1150. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22917972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Bliek J, Alders M, Maas SM, et al. Lessons from BWS twins: complex maternal and paternal hypomethylation and a common source of haematopoietic stem cells. Eur J Hum Genet. [Internet]. 2009;17(12):1625–1634. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19513094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Gou C, Liu X, Shi X, et al. Placental expressions of CDKN1C and KCNQ1OT1 in monozygotic twins with selective intrauterine growthrestriction. Twin Res Hum Genet. [Internet]. 2017;20(5):389–394. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28803575 [DOI] [PubMed] [Google Scholar]
- [129].McMinn J, Wei M, Schupf N, et al. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta. [Internet]. 2006;27(6–7):540–549. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16125225 [DOI] [PubMed] [Google Scholar]
- [130].Deyssenroth MA, Marsit CJ, Chen J, et al. In-depth characterization of the placental imprintome reveals novel differentially methylated regions across birth weight categories. Epigenetics. [Internet]. 2020;15(1–2):47–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31403346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Vasconcelos S, Ramalho C, Marques CJ, et al. Altered expression of epigenetic regulators and imprinted genes in human placenta and fetal tissues from second trimester spontaneous pregnancy losses. Epigenetics. [Internet]. 2019;14(12):1234–1244. Available from: http://www.ncbi.nlm.nih.gov/pubmed/31221015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Dent CL, Isles AR. Brain-expressed imprinted genes and adult behaviour: the example of Nesp and Grb10. Mamm Genome. [Internet]. 2014;25(1–2):87–93. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23974804 [DOI] [PubMed] [Google Scholar]
- [133].Haggarty P, Hoad G, Horgan GW, et al. DNA methyltransferase candidate polymorphisms, imprinting methylation, and birth outcome.PLoS One. 2013;8(7):e68896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Rahat B, Mahajan A, Bagga R, et al. Epigenetic modifications at DMRs of placental genes are subjected to variations in normal gestation, pathological conditions and folate supplementation. Sci Rep. [Internet]. 2017;7(1):40774. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28098215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article and can be accessed from the corresponding authors on reasonable request.


