Abstract
Individual, provider, clinic, and societal level barriers have been shown to undermine the potential impact of genetic testing. The current approach in the primary care setting places an exorbitant burden on both providers and patients. Current literature provides insight into how to address barriers across multiple levels (patient, provider, clinic, system) and at multiple stages in the testing process (identification, referral, counseling, and testing) but interventions have had limited success. After outlining the current approach to genetic testing in the primary care setting, including the barriers that prevent genetic testing uptake and the methods proposed to address these issues, we recommend integrating genetic testing into routine medical care through population-based testing. Success in efforts to increase the uptake of genetic testing will not occur without significant changes to the way genetic services are delivered. These changes will not be instantaneous but are critical in moving this field forward to realize the potential for cancer risk genetic assessment to reduce cancer burden.
Keywords: cancer, genetics, primary care, population testing, genetic testing
Introduction
There is a pressing need to better integrate genetic testing for hereditary cancer risk into primary care settings. Genetic testing is the future of preventative medicine, but currently remains an untapped resource for patients who may benefit from additional cancer surveillance or risk-reduction. The clinical impact of this testing depends on the effective identification of interested at-risk patients, successful delivery of testing, and follow-up care for individuals with positive results.
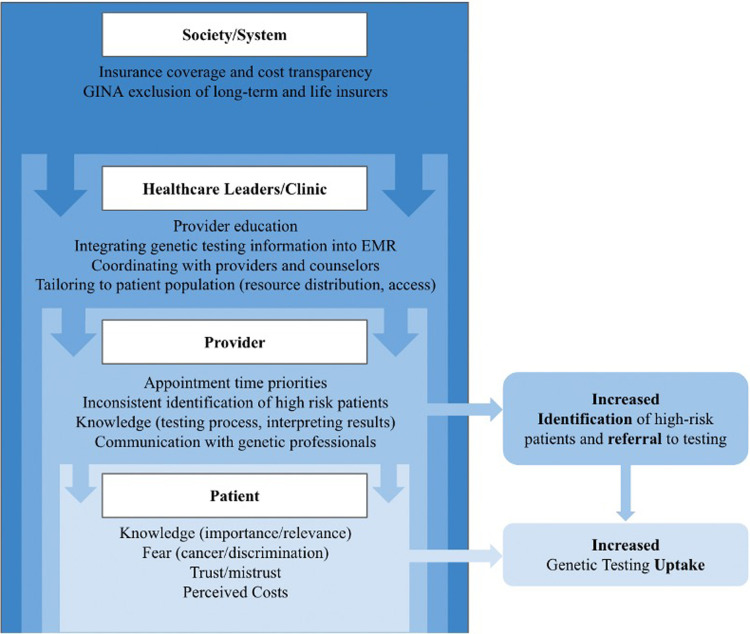
Individual interventions have had varying degrees of success in identifying strategies that are capable of increasing hereditary risk assessment and genetic testing uptake (1, 2). However, with existing barriers on the patient, provider, and systems level, targeted interventions have had limited efficacy. As such, we argue that until genetic testing for cancer is integrated as a routine part of medical care at a population level, interventions will continue to be limited in their capacity to increase genetic testing uptake and subsequent follow-up care. The successful integration of routine assessment for hereditary cancer risk into an existing healthcare setting will require input from, and action by, multiple stakeholders (Figure 1).
Figure 1.
Multilevel barriers to accessing genetic testing for monogenic, hereditary cancer risk.
The purpose of this paper is to provide an overview of the current model of genetic testing in the primary care setting, describe multilevel barriers to genetic testing, and assess proposed interventions from the existing body of literature. We conclude with suggestions for a future action plan that promotes population-based genetic testing and informs practical decisions about how to integrate genetic testing for cancer into the primary care setting.
Current primary care model of genetic testing for cancer risk
The current primary care model relies on a patient-provider interaction to start the testing process, an interaction that often occurs by chance. A patient may fill out a family history or the patient may ask a direct question about their risk that cues the provider to ask about family history and other risk factors. Sometimes, a patient-provider general discussion reveals clues that may lead an astute provider to pursue a cancer-risk conversation (3, 4). Any of these interactions would shape the provider's decision about appropriate testing and can lead to a subsequent referral to a genetics expert or to directly order a test, depending on the clinic's resources.
If a provider does determine that a patient would benefit from genetic testing, they can either directly order a test or refer the patient to a genetics professional. Even if they choose referral, extensive patient education may be needed regarding purpose, benefit, and risk of genetic testing to motivate the patient to complete the genetics consultation. If the provider orders the test directly, they are responsible for choosing the correct test, answering any patient concerns, including those surrounding privacy, cost, and explaining implications of positive and negative results (5–7). When results are ready, the test requisitioner must explain their implications, and then the primary care provider is responsible for integrating the genetic risk assessment into follow-up care. This places an unreasonable amount of responsibility on primary care providers who have minimal genetics training and have many competing priorities, leaving behind a large proportion of patients with a strong family history of hereditary cancer who would benefit from genetic testing (8, 9).
Barriers presented by the current model
Barriers, as it applies to genetic screening and testing, refers to the individual, provider, system, and policy level hurdles an individual must overcome to access genetic testing and navigate the process of genetic testing. Investigators have done extensive work identifying the multilevel barriers within the current model of genetic testing for cancer risk (Figure 1). Shen and colleagues (10) conducted a systematic review of the barriers to population genetic screening as a whole, while other studies have evaluated challenges specific to genetic testing for cancer (3, 6, 11–15).
Individual-level barriers include the perceived cost of genetic testing, knowledge and beliefs about the genetic testing process, fear related to being at an increased risk for cancer, fear of genetic discrimination, and distrust in the medical system (16–18). Additionally, patients may have confusion over different testing options (such as lab tests vs. direct-to-consumer genetic testing) (19). These challenges make it difficult for patients to ascertain definitive answers related to medical genetic testing, leading to reduced genetic testing uptake (17, 18).
At the level of the provider, primary care providers (PCPs) play an important role in helping patients overcome individual-level barriers. However, to do so they need to prioritize conversations about cancer risk during appointments, consistently identify high-risk patients, clarify how to obtain and order genetic testing, learn the nuances of result interpretation, improve communication with the clinical genetics services, and establish how to utilize test results to inform patient care (5, 6, 13, 14, 20–22). This highlights another issue – the need for extensive physician education on risk assessment and genetics, including continuing medical education. Even with improved educational opportunities, it seems unrealistic to expect primary care providers to gain that level of comfort with cancer genetics when it is only offered on a case-by-case basis.
An additional barrier is that many PCPs do not have a thorough understanding of the role and availability of genetics professionals to effectively collaborate with them or connect patients to these resources (13, 21). Furthermore, the current shortage of genetic counselors compounds the challenge, particularly for providers in rural areas. This lack of communication with or access to genetics professionals, along with providers' inability to consistently determine which patients are high-risk, contributes to an overall under-referral and under-utilization of genetic testing; an issue that is exacerbated in historically marginalized populations who have lower referral and genetic testing rates from their providers (14).
At the systems level, genetic testing processes have not been integrated fully into EHR systems. The nuanced nature of genetic testing information, referral recommendations, coordination of communication between providers and specialists, and post-test care pathways for patients identified with hereditary risk all pose challenges to the production of an effective EHR (23). Alongside these issues are challenges involving various test types and inconsistent nomenclatures from different labs.
Even if an individual patient overcomes these multilevel barriers, they may still face policy-level barriers related to lack of insurance coverage and the cost of genetic testing. The cost of genetic testing services is not well understood by patients or providers. Although the cost has decreased dramatically in recent history, out-of-pocket costs of genetic testing can still range anywhere from $100 to $2,000 depending on the type of test that is ordered and how much is covered by insurance (24). Additionally, it is unclear to patients and providers whether insurance will cover the cost of subsequent genetic counseling services and follow up care, which can expose patients to additional unexpected costs. Medicare and Medicaid have long been criticized for underinsuring preventative care. Medicare does not cover the cost of pre-diagnosis genetic testing for hereditary cancer risk, excluding one test for colorectal cancer, Cologuard™ (25). Research has shown that many patients who could benefit from genetic testing for hereditary cancers are being missed because Medicare will not cover the cost of pre-symptomatic genetic testing until after the cancer is diagnosed (15, 26).
Additionally, concerns about discrimination by insurers make individuals hesitant to undergo genetic testing (27). While the Genetic Information Nondiscrimination Act (GINA) prevents discrimination by health insurance companies based on genetic test results, it does not apply to long term care insurance, disability insurance, or life insurance (28).
Solutions from existing literature
All these factors are intricately intertwined and contribute to a low uptake rate of genetic testing for hereditary cancer, particularly amongst cancer-free patients. Failure to address these issues across multiple levels has contributed to an inability to reduce cancer mortality rates (12). A comprehensive, population-based, and standardized model that considers and balances challenges at each level and can be adapted to unique healthcare systems is critical to overcoming this problem on a large scale. Studies to date have failed to address multilevel barriers, target different aspects of the genetic testing process, and have largely been conducted in urban, high-resourced facilities under optimal conditions.
Still, this is a prolific field of study with many suggested solutions existing at each level. Bednar and colleagues (2) conducted a comprehensive review of tested interventions over the past 20 years (2000–2020) and found over 60 interventions targeting different barriers throughout the testing process. Among the 16 interventions aimed at increasing genetic testing uptake, only 5 showed positive results (28–32), while several studies demonstrated high rates of genetic testing but with no supporting statistical analysis (33–35). Additionally, only 5 of the 16 studies recruited participants who had not previously received a cancer diagnosis (28, 34, 36–38), while only 2 of these 5 were aimed at the general public or a general clinic population (28, 38). The first was conducted by the Centers for Disease Control and Prevention who conducted a direct-to-consumer mass marketing campaign aimed to increase uptake in genetic testing for BRCA1 and BRCA2 variants (28). Although they did not report genetic testing rates, they report that there was a statistically significant increase in genetic testing uptake in target cities. The second, conducted by DeFrancesco et al., implemented family history screening methods based on NCCN guidelines in community-based OB/GYN clinics (38). Patients who were eligible were then offered genetic counseling and genetic testing. Although no statistical tests were performed, genetic testing uptake increased from 0.5% pre-implementation to 4.0% post-implementation.
While ideally patients will be captured prior to a cancer diagnosis, this has not always been the case under our current model of genetic testing. As such, interventions have been tested to address this issue, also with varying success. For example, Uyar et al. (31) recruited patients with ovarian cancer from an academic medical center to refer them to genetic counseling and genetic testing. This intervention included provider education, EHR integration, patient education and navigation, and tumor board documentation. After performing tests of significance, they found that genetic testing rates significantly increased from 27% pre-intervention to 82% post-intervention. We can certainly use this and other types of studies as a model for how to improve genetic testing uptake for all patients.
Effective aspects of all 16 interventions included direct patient engagement through mass marketing or online testing information, integration of a genetic counselor or health navigator into the genetic testing process, and physician education and support. Importantly, an aspect of physician support that will be critical as we move towards a population-based screening model is effective integration of genetic information in the EHR (39–43). Additionally, telemedicine and telephone delivery strategies (expanded since the onset of the COVID-19 pandemic) offer the opportunity to expand accessibility of genetic counseling services (44, 45), although reported success of these types of interventions varies (2) and concerns about equity in access remain (46).
Still, there are remaining limitations, including: a lack of follow-up time with patients, lack of detail in risk assessment strategies, largely White and high-income study populations (and other generalizability issues), and privacy concerns (39–41, 44, 45, 47, 48). None of these interventions have addressed larger systems-level issues such as insurance coverage and policies that protect against discrimination by long-term care insurance or life insurance.
To build on previous efforts, we have implemented the EDGE Study (NCT04746794) which is a multilevel intervention with input from varied stakeholders, to improve population-based screening methods for hereditary cancer (49). All active participants identified in participating clinics are given the opportunity to take an online familial risk assessment survey and are offered genetic testing if they are eligible. Components of this intervention include in clinic and online screening tools, free genetic testing, telephone genetic counseling, and clinical care pathways developed for patients who receive positive genetic test results. Results of this intervention are pending, but the main goal is to eliminate the primary care provider as the gatekeeper of genetic testing, drawing them into the clinical paradigm to implement well-defined care plans for patients identified with pathogenic variants that increase cancer risk.
Genetic testing, generally, has been historically inaccessible to underrepresented and underserved populations (6, 50, 51), particularly for individuals of low socioeconomic status and/or low health literacy. The genetic tests offered by the EDGE Study were free, which addresses individual-level barriers (perceived cost of genetic testing) and systems-level barriers (lack of insurance coverage). However, this is not a real-world model in current practice, and the costs associated with genetic testing will have to be addressed with larger societal interventions.
During the EDGE study, individuals are offered the opportunity to discuss their concerns or questions regarding genetic testing with research or clinic staff (49). PCPs do not have to prioritize the time in their visit because the familial risk assessment survey is taken online or while patients are waiting for their appointment to begin. The use of this online risk assessment is easily integrated into EHR and ensures consistent screening methods for all patients, as shown in other studies (9, 39). As a part of the EDGE Study, physicians participated in an educational course, earning continuing medical education credits, during which they learned more about the process of genetic testing and when genetic testing would be appropriate for their patients. If a patient receives a positive test result, the provider is given a gene-specific care pathway that outlines recommended cancer surveillance and/or prevention measures. EDGE has also created a protocol for healthcare systems to scan genetic test results into EHR as soon as they are released to providers. This type of tool can be implemented in diverse clinic environments to improve the communication of testing results.
Population-based testing as the future of preventative medicine
While these interventions have been successful in increasing genetic testing uptake, their success has ultimately been limited by the multilevel barriers prolific in our current testing model. Offering genetic testing on a case-by-case basis, as we do currently, relies on the ability of healthcare providers to identify eligible patients with fidelity and move them through the process with ease. Meeting these conditions has proven untenable in many healthcare systems, even with certain interventions. As such, we must consider shifting to a population-based model in which all patients are offered genetic testing for hereditary cancer risk as part of their routine medical care.
A population-based testing model will address some testing barriers by design and others through systematization. Currently, providers fail to identify patients who meet eligibility criteria, and the eligibility criteria fail to capture all individuals who would benefit from genetic testing (52). These barriers are made obsolete through population-based testing. Many remaining barriers can be addressed through effective systematization using the lessons learned from existing interventions. Routinization of the genetic testing process will destigmatize genetic testing. Routine testing should increase providers' ability to order and interpret genetic test results, allow clinics to establish clear workflows, and improve patients' understanding of genetic testing. However, this will rely on investment in infrastructure, integration with EHR systems, hiring of appropriate personnel, and extensive education programs (53) – all shown to be effective through previous intervention efforts.
Studies evaluating population-based models have found that population-based testing identifies carriers that would be missed by traditional criteria, does not result in increased psychosocial concerns, can result in clinically actionable information, and can be cost-effective if implemented at 30 years old or earlier (52, 54–56). In population testing, genetic counseling is generally reserved for the post-test setting for those identified with positive results (i.e., pathogenic variants), similar to providing specialized counseling for an individual with high cholesterol on routine screening.
Until the scaffolding exists to establish population-based testing, streamlined genetic testing for patients with certain cancers (ie breast, ovarian, and cancers under 50) must remain a priority for interventions. Additionally, it is possible that individuals opt out of genetic testing as a preventative tool but still need a route to testing should they develop cancer. As such, there must be an effective clinical care pathway to receive streamlined genetic testing for those with a personal history of cancer. Hopefully, as the fear and stigma associated with genetic testing decreases and population-based testing expands, the need for streamlined testing will become less significant.
Many cancer prevention or surveillance tools are used in routine medical care (pap smears, low-dose CT scans, hepatitis C screenings, colonoscopies, mammograms) but genetic testing for cancer risk has not been utilized in the same way, thus limiting our capacity to prevent and detect hereditary cancer. Genetic indicators of risk should not be treated as though they are significantly different from other risk indicators and genetic testing should be integrated into primary care accordingly.
Discussion
The joint guidelines put forth by the American College of Medical Genetics (ACMG) and the National Society of Genetic Counselors (NSGC), the President's Cancer Panel, and the extensive work in intervention research highlight the urgency of increasing genetic testing uptake rates for individuals who are eligible for, and interested in, genetic testing (57, 58). There is also strong empirical evidence synthesized in National Comprehensive Cancer Network (NCCN) screening guidelines, which highlight specific genes that could be included for genetic testing (Table 1) (59, 60). As cancer continues to burden individuals in the U.S., effective and creative ways to holistically address the multilevel barriers that prevent people from accessing genetic testing are crucial.
Table 1.
Synthesis of genes and associated cancers.
| Group Name | Gene | Main Cancers |
|---|---|---|
| Breast and Ovarian Cancer | BRCA1 | breast, ovarian, prostate, and pancreatic |
| BRCA2 | breast, ovarian, prostate, and pancreatic | |
| PALB2 | breast, ovarian, and pancreatic | |
| ATM | breast, ovarian, prostate, and pancreatic | |
| NBN | breast and ovarian | |
| BRIP1 | breast and ovarian | |
| RAD51C | breast and ovarian | |
| RAD51D | breast and ovarian | |
| Li Fraumeni | TP53 | breast, colon, central nervous system, bone, and soft tissue |
| Breast and Gastric | CDH1 | breast and gastric |
| Colon (Lynch) | MLH1 | colon, uterine, ovarian, pancreatic, urothelial |
| MSH2 | colon, uterine, ovarian, pancreatic, urothelial | |
| MSH6 | colon, uterine, ovarian, pancreatic, urothelial | |
| PMS2 | colon and uterine | |
| EPCAM | colon, uterine, ovarian, pancreatic, urothelial | |
| Cowden Syndrome | PTEN | breast, uterine, thyroid, endometrial, kidney, and melanoma |
| Breast and Colon | CHEK2 | breast and colon |
| Breast | BARD1 | breast |
| NF1 | Breast and peripheral nerve | |
| Colon | APC | colorectal |
| Colon (Juvenile Polypsis Syndrome) | BMPR1A | colon and stomach |
| SMAD4 | colon and stomach | |
| Colon (Adenomatous Polypsis) | GREM1 | colon |
| POLD1 | colon | |
| POLE | colon | |
| Colon (MUTYH-Associated Polypsis) | MUTYH | colon |
| Colon (Peutz-Jeghers Syndrome) | STK11 | breast, ovarian, pancreatic, colon, stomach, small intestine, cervix, uterus, testes, and lung |
| Pancreatic | CDKN2A | pancreas and melanoma |
The current model in the primary care setting is ineffective and fails to provide testing to eligible, interested patients. It places the burden of identifying high-risk patients on the provider or on the motivated patient, ignoring larger systemic and societal barriers an individual must overcome to access genetic testing and exacerbating existing healthcare disparities. Primary care providers, in our current medical landscape, are not given the tools and information to facilitate genetic testing for their patients. An existing body of work offers solutions to address multilevel barriers. However, there are limitations and testing uptake remains low. As demonstrated in previous work and by the methodology of the EDGE Study, future interventions must simultaneously address these barriers at as many levels as possible. Most of these barriers could be eliminated through a systematic population-based model.
Larger systemic and societal barriers, as well as the interaction between these levels, will take more time and effort to address, with input necessary from multiple stakeholders. Future interventions should target the challenges in expanding to population-based genetic testing and can start from the ground up - working within the system to address patient, provider, and clinic barriers while pushing for systemic- and policy-level change.
Acknowledgments
We would like to acknowledge the work of both Da Laina Cameron and Heather Harris, who played essential roles as project coordinator and data manager for the EDGE Study. They both coordinated the submission of this paper as well as reviewed the text. We would also like to acknowledge the mentorship and guidance of Deborah J. Bowen. She was a devoted leader in the field of public health and bioethics as well as a wonderful mentor and colleague.
Funding
This research was funded by the National Cancer Institute of the National Institutes of Health (NIH), Grant Office ID: A140201, Funding Source ID: 1U01CA232795-01A1.
Author contributions
Conceptualization, DJB, ED, TT, CW; resources, DJB; writing—original draft preparation, ED, TT, DJB; writing—review and editing, ED, TT, DJB, CW, ES, BD, MBV, BS, MR, BN, JMB; visualization, ED, TT; supervision, DJB, CW, ES; project administration, ED, TT; funding acquisition, DJB; All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors of this paper are all investigators on the Early Detection of Genetic Risk Study, which is described in this paper. The authors declare no commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
EDGE Study Team
Beth Devine, Barbara Norquist, Brian Shirts, Mariebeth Velasquez, Michael Raff, Jeannine M. Brant.
References
- 1.McCuaig JM, Armel SR, Care M, Volenik A, Kim RH, Metcalfe KA. Next-generation service delivery: a scoping review of patient outcomes associated with alternative models of genetic counseling and genetic testing for hereditary cancer. Cancers. (2018) 10(11):1–36. 10.3390/cancers10110435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bednar EM, Nitecki R, Krause KJ, Rauh-Hain JA. Interventions to improve delivery of cancer genetics services in the United States: a scoping review. Genet Med. (2022) 24(6):1176–86. 10.1016/j.gim.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 3.Hamilton JG, Abdiwahab E, Edwards HM, Fang M-L, Jdayani A, Breslau ES. Primary care providers’ cancer genetic testing-related knowledge, attitudes, and communication behaviors: a systematic review and research agenda. J Gen Intern Med. (2017) 32(3):315–24. 10.1007/s11606-016-3943-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoffel EM, Carethers JM. Current approaches to germline cancer genetic testing. Annu Rev Med. (2020) 71(1):85–102. 10.1146/annurev-med-052318-101009 [DOI] [PubMed] [Google Scholar]
- 5.Evenson SA, Hoyme HE, Haugen-Rogers JE, Larson EA, Puumala SE. Patient and physician perceptions of genetic testing in primary care. S D Med. (2016) 69(11):487–93. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/28810112 [PubMed] [Google Scholar]
- 6.Valencia OM, Samuel SE, Viscusi RK, Riall TS, Neumayer LA, Aziz H. The role of genetic testing in patients with breast cancer: a review. JAMA Surg. (2017) 152(6):589–94. 10.1001/jamasurg.2017.0552 [DOI] [PubMed] [Google Scholar]
- 7.Harding B, Webber C, Ruhland L, Dalgarno N, Armour CM, Birtwhistle R, et al. Primary care providers’ lived experiences of genetics in practice. J Community Genet. (2019) 10(1):85–93. 10.1007/s12687-018-0364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Childers CP, Childers KK, Maggard-Gibbons M, Macinko J. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. (2017) 35(34):3800–6. 10.1200/JCO.2017.73.6314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McCarthy AM. Persistent underutilization of BRCA1/2 testing suggest the need for new approaches to genetic testing delivery. J Natl Cancer Inst. (2019) 111(8):751–3. 10.1093/jnci/djz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen EC, Srinivasan S, Passero LE, Allen CG, Dixon M, Foss K, et al. Barriers and facilitators for population genetic screening in healthy populations: a systematic review (2022). Available at: https://www.researchsquare.com/article/rs-1315303/v1 (Accessed May 8, 2022). [DOI] [PMC free article] [PubMed]
- 11.Hyatt C, Russo J, McDougall C. Genetic counseling perspective of engagement with urology and primary care. Can J Urol. (2019) 26(5 Suppl 2):52–3. Retrieved from https://canjurol.com/html/free-articles/Cdn_JU26-I5S2_27_DrHyatt.pdf [PubMed] [Google Scholar]
- 12.Giri VN, Morgan TM, Morris DS, Berchuck JE, Hyatt C, Taplin M-E. Genetic testing in prostate cancer management: considerations informing primary care. CA Cancer J Clin. (2022) 72(4):360–71. 10.3322/caac.21720 [DOI] [PubMed] [Google Scholar]
- 13.Muessig KR, Zepp JM, Keast E, Shuster EE, Reyes AA, Arnold B, et al. Retrospective assessment of barriers and access to genetic services for hereditary cancer syndromes in an integrated health care delivery system. Hered Cancer Clin Pract. (2022) 20(1):7. 10.1186/s13053-022-00213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang S, Axilbund JE, O’Leary E, Michalski ST, Evans R, Lincoln SE, et al. Underdiagnosis of hereditary breast and ovarian cancer in Medicare patients: genetic testing criteria miss the Mark. Ann Surg Oncol. (2018) 25(10):2925–31. 10.1245/s10434-018-6621-4 [DOI] [PubMed] [Google Scholar]
- 15.Zhen JT, Syed J, Nguyen KA, Leapman MS, Agarwal N, Brierley K, et al. Genetic testing for hereditary prostate cancer: current status and limitations. Cancer. (2018) 124(15):3105–17. 10.1002/cncr.31316 [DOI] [PubMed] [Google Scholar]
- 16.Guo F, Hirth JM, Fuchs EL, Cofie LE, Brown V, Kuo Y-F, et al. Knowledge, attitudes, willingness to pay, and patient preferences about genetic testing and subsequent risk management for cancer prevention. J Cancer Educ. (2022) 37(2):362–9. 10.1007/s13187-020-01823-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blomen CL, Pott A, Volk AE, Budäus L, Witzel I. Communication processes about predictive genetic testing within high-risk breast cancer families: a two-phase study design. Res Square. (2021) 11:1–10. 10.21203/rs.3.rs-365468/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng H. The problem with direct-to-consumer genetic tests. Scientific American Blog Network. Available at: https://blogs.scientificamerican.com/observations/the-problem-with-direct-to-consumer-genetic-tests/ (Accessed Sep 12, 2022).
- 19.Tai-Seale M, McGuire TG, Zhang W. Time allocation in primary care office visits. Health Serv Res. (2007) 42(5):1871–94. 10.1111/j.1475-6773.2006.00689.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faucett WA, Peay H, Coughlin CR, 2nd. Genetic testing: consent and result disclosure for primary care providers. Med Clin North Am. (2019) 103(6):967–76. 10.1016/j.mcna.2019.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burke W. Genetic testing in primary care. Annu Rev Genomics Hum Genet. (2004) 5:1–14. 10.1146/annurev.genom.5.061903.180029 [DOI] [PubMed] [Google Scholar]
- 22.Hayward J, McDermott J, Qureshi N, Newman W. Pharmacogenomic testing to support prescribing in primary care: a structured review of implementation models. Pharmacogenomics. (2021) 22(12):761–76. 10.2217/pgs-2021-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NIH. What is the cost of genetic testing, and how long does it take to get the results?. MedlinePlus (2021). Available at: https://medlineplus.gov/genetics/understanding/testing/costresults/#:∼:text=The%20cost%20of%20genetic%20testing%20can%20range%20from%20under%20%24100,screening%2C%20costs%20vary%20by%20state (Accessed Apr 10, 2021).
- 24.Klug M. Genetic tests and medicare: Coverage and oversight. SMP National Resource Center The Sentinel; (2019). [Google Scholar]
- 25.Gross AL, Blot WJ, Visvanathan K. BRCA1 And BRCA2 testing in medically underserved medicare beneficiaries with breast or ovarian cancer. JAMA. (2018) 320(6):597–8. 10.1001/jama.2018.8258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bélisle-Pipon J-C, Vayena E, Green RC, Cohen IG. Genetic testing, insurance discrimination and medical research: what the United States can learn from peer countries. Nat Med. (2019) 25(8):1198–204. 10.1038/s41591-019-0534-z [DOI] [PubMed] [Google Scholar]
- 27.Jacobellis J, Martin L, Engel J. Genetic Alliance, Genetics & Public Policy Center, National Coalition for Health Professional Education in Genetics. Genetic Information Nondiscrimination Act. Genetic Information Nondiscrimination Act (2010). Available at: http://ginahelp.org/ (Accessed Apr 10, 2022).
- 28.Genetic testing for breast and ovarian cancer susceptibility: evaluating direct-to-consumer marketing—Atlanta, Denver, Raleigh-Durham, and Seattle, 2003. JAMA. (2004) 292(7):796. 10.1001/jama.292.7.796 [DOI] [PubMed] [Google Scholar]
- 29.Watson CH, Ulm M, Blackburn P, Smiley L, Reed M, Covington R, et al. Video-assisted genetic counseling in patients with ovarian, fallopian and peritoneal carcinoma. Gynecol Oncol. (2016) 143(1):109–12. 10.1016/j.ygyno.2016.07.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown J, Athens A, Tait DL, Crane EK, Higgins RV, Naumann RW, et al. A comprehensive program enabling effective delivery of regional genetic counseling. Int J Gynecol Cancer. (2018) 28(5):996–1002. 10.1097/IGC.0000000000001256 [DOI] [PubMed] [Google Scholar]
- 31.Uyar D, Neary J, Monroe A, Nugent M, Simpson P, Geurts JL. Implementation of a quality improvement project for universal genetic testing in women with ovarian cancer. Gynecol Oncol. (2018) 149(3):565–9. 10.1016/j.ygyno.2018.03.059 [DOI] [PubMed] [Google Scholar]
- 32.Bednar EM, Sun CC, Camacho B, Terrell J, Rieber AG, Ramondetta LM, et al. Disseminating universal genetic testing to a diverse, indigent patient population at a county hospital gynecologic oncology clinic. Gynecol Oncol. (2019) 152(2):328–33. 10.1016/j.ygyno.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bednar EM, Oakley HD, Sun CC, Burke CC, Munsell MF, Westin SN, et al. A universal genetic testing initiative for patients with high-grade, non-mucinous epithelial ovarian cancer and the implications for cancer treatment. Gynecol Oncol. (2017) 146(2):399–404. 10.1016/j.ygyno.2017.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Neill SC, White DB, Sanderson SC, Lipkus IM, Bepler G, Bastian LA, et al. The feasibility of online genetic testing for lung cancer susceptibility: uptake of a web-based protocol and decision outcomes. Genet Med. (2008) 10(2):121–30. 10.1097/GIM.0b013e31815f8e06 [DOI] [PubMed] [Google Scholar]
- 35.Frey MK, Lee SS, Gerber D, Schwartz ZP, Martineau J, Lutz K, et al. Facilitated referral pathway for genetic testing at the time of ovarian cancer diagnosis: uptake of genetic counseling and testing and impact on patient-reported stress, anxiety and depression. Gynecol Oncol. (2020) 157(1):280–6. 10.1016/j.ygyno.2020.01.007 [DOI] [PubMed] [Google Scholar]
- 36.Butrick M, Kelly S, Peshkin BN, Luta G, Nusbaum R, Hooker GW, et al. Disparities in uptake of BRCA1/2 genetic testing in a randomized trial of telephone counseling. Genet Med. (2015) 17(6):467–75. 10.1038/gim.2014.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halbert CH, Kessler L, Troxel AB, Stopfer JE, Domchek S. Effect of genetic counseling and testing for BRCA1 and BRCA2 mutations in African American women: a randomized trial. Public Health Genomics. (2010) 13(7–8):440–8. 10.1159/000293990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeFrancesco MS, Waldman RN, Pearlstone MM, Karanik D, Bernhisel R, Logan J, et al. Hereditary cancer risk assessment and genetic testing in the community-practice setting. Obstet Gynecol. (2018) 132(5):1121–9. 10.1097/AOG.0000000000002916 [DOI] [PubMed] [Google Scholar]
- 39.Mittendorf KF, Kauffman TL, Amendola LM, Anderson KP, Biesecker BB, Dorschner MO, et al. Cancer health assessments reaching many (CHARM): a clinical trial assessing a multimodal cancer genetics services delivery program and its impact on diverse populations. Contemp Clin Trials. (2021) 106:106432 10.1016/j.cct.2021.106432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David SP, Dunnenberger HM, Ali R, Matsil A, Lemke AA, Singh L, et al. Implementing primary care mediated population genetic screening within an integrated health system. J Am Board Fam Med. (2021) 34(4):861–5. 10.3122/jabfm.2021.04.200381 [DOI] [PubMed] [Google Scholar]
- 41.Petry N, Baye J, Aifaoui A, Wilke RA, Lupu RA, Savageau J, et al. Implementation of wide-scale pharmacogenetic testing in primary care. Pharmacogenomics. (2019) 20(12):903–13. 10.2217/pgs-2019-0043 [DOI] [PubMed] [Google Scholar]
- 42.Kho AN, Rasmussen LV, Connolly JJ, Peissig PL, Starren J, Hakonarson H, et al. Practical challenges in integrating genomic data into the electronic health record. Genet Med. (2013) 15(10):772–8. 10.1038/gim.2013.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirts BH, Salama JS, Aronson SJ, Chung WK, Gray SW, Hindorff LA, et al. CSER And eMERGE: current and potential state of the display of genetic information in the electronic health record. J Am Med Inform Assoc. (2015) 22(6):1231–42. 10.1093/jamia/ocv065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinney AY, Butler KM, Schwartz MD, Mandelblatt JS, Boucher KM, Pappas LM, et al. Expanding access to BRCA1/2 genetic counseling with telephone delivery: a cluster randomized trial. J Natl Cancer Inst. (2014) 106(12):1–12. 10.1093/jnci/dju328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lerman C, Schwartz MD, Miller SM, Daly M, Sands C, Rimer BK. A randomized trial of breast cancer risk counseling: interacting effects of counseling, educational level, and coping style. Health Psychol. (1996) 15(2):75–83. 10.1037/0278-6133.15.2.75 [DOI] [PubMed] [Google Scholar]
- 46.Uhlmann WR, McKeon AJ, Wang C. Genetic counseling, virtual visits, and equity in the era of COVID-19 and beyond. J Genet Couns. (2021) 30(4):1038–45. 10.1002/jgc4.1469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lemke AA, Amendola LM, Thompson J, Dunnenberger HM, Kuchta K, Wang C, et al. Patient-reported outcomes and experiences with population genetic testing offered through a primary care network. Genet Test Mol Biomarkers. (2021) 25(2):152–60. 10.1089/gtmb.2020.0275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Foulkes WD, Knoppers BM, Turnbull C. Population genetic testing for cancer susceptibility: founder mutations to genomes. Nat Rev Clin Oncol. (2016) 13(1):41–54. 10.1038/nrclinonc.2015.173 [DOI] [PubMed] [Google Scholar]
- 49.Bowen DJ, Wang C, Cole AM, Norquist BM, Knerr S, Devine B, et al. Design of a study to implement population-based risk assessment for hereditary cancer genetic testing in primary care. Contemp Clin Trials. (2021) 101:106257. 10.1016/j.cct.2020.106257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. (2017) 1–19. 10.1155/2017/2819372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peterson EB, Chou W-YS, Gaysynsky A, Krakow M, Elrick A, Khoury MJ, et al. Communication of cancer-related genetic and genomic information: a landscape analysis of reviews. Transl Behav Med. (2018) 8(1):59–70. 10.1093/tbm/ibx063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frey MK, Finch A, Kulkarni A, Akbari MR, Chapman-Davis E. Genetic testing for all: overcoming disparities in ovarian cancer genetic testing. Am Soc Clin Oncol Educ Book. (2022) 42:1–12. 10.1200/EDBK_350292 [DOI] [PubMed] [Google Scholar]
- 53.Matloff E. Population genetic testing: Save lives and money, while avoiding financial toxicity. Forbes (2022). Available at: https://www.forbes.com/sites/ellenmatloff/2022/08/04/population-genetic-testing-save-lives-and-money-while-avoiding-financial-toxicity/?utm_campaign=Monthly (Accessed Sep 12, 2022).
- 54.Manchanda R, Loggenberg K, Sanderson S, Burnell M, Wardle J, Gessler S, et al. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish community: a randomized controlled trial. J Natl Cancer Inst. (2015) 107(1):379. 10.1093/jnci/dju379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guzauskas GF, Garbett S, Zhou Z, Spencer SJ, Smith HS, Hao J, et al. Cost-effectiveness of population-wide genomic screening for hereditary breast and ovarian cancer in the United States. JAMA Netw Open. (2020) 3(10):e2022874. 10.1001/jamanetworkopen.2020.22874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Narod SA, Gojska N, Sun P, Tryon A, Kotsopoulos J, Metcalfe K, et al. The screen project: guided direct-to-consumer genetic testing for breast cancer susceptibility in Canada. Cancers. (2021) 13(8):1–11. 10.3390/cancers13081894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hampel H, Bennett RL, Buchanan A, Pearlman R, Wiesner GL. Guideline development group, American college of medical genetics and genomics professional practice and guidelines committee and national society of genetic counselors practice guidelines committee. A practice guideline from the American college of medical genetics and genomics and the national society of genetic counselors: referral indications for cancer predisposition assessment. Genet Med. (2015) 17(1):70–87. 10.1038/gim.2014.147 [DOI] [PubMed] [Google Scholar]
- 58.Mitchell EP. Closing gaps in cancer screening: the president’s cancer panel report. J Natl Med Assoc. (2022) 114(1):1–2. Retrieved from https://prescancerpanel.cancer.gov/report/cancerscreening/ [DOI] [PubMed] [Google Scholar]
- 59.Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. (2021) 19(1):77–102. 10.6004/jnccn.2021.0001 [DOI] [PubMed] [Google Scholar]
- 60.Weiss JM, Gupta S, Burke CA, Axell L, Chen L-M, Chung DC, et al. NCCN Guidelines® insights: genetic/familial high-risk assessment: colorectal, version 1.2021. J Natl Compr Canc Netw. (2021) 19(10):1122–32. 10.1164/jnccn.2021.0048 [DOI] [PubMed] [Google Scholar]