Eggs of oviparous animals must be prepared to develop rapidly and robustly until hatching. The balance between sugars, fats, and other macromolecules must therefore be carefully considered when loading the egg with nutrients. Clearly, packing too much or too little fuel would lead to suboptimal conditions for development. While many studies have measured the overall energy utilization of embryos, little is known of the identity of the molecular-level processes that contribute to the energy budget in the fi rst place [1]. Here, we introduce Drosophila embryos as a platform to study the energy budget of embryogenesis. We demonstrate through three orthogonal measurements — respiration, calorimetry, and biochemical assays — that Drosophila melanogaster embryogenesis utilizes 10 mJ of energy generated by the oxidation of the maternal glycogen and triacylglycerol (TAG) stores (Figure 1). Normalized for mass, this is comparable to the resting metabolic rates of insects [2]. Interestingly, alongside data from earlier studies, our results imply that protein, RNA, and DNA polymerization require less than 10% of the total ATPs produced in the early embryo.
Figure 1. Measuring energy utilization during Drosophila embryogenesis using calorimetry, respirometry, and biochemical assays.
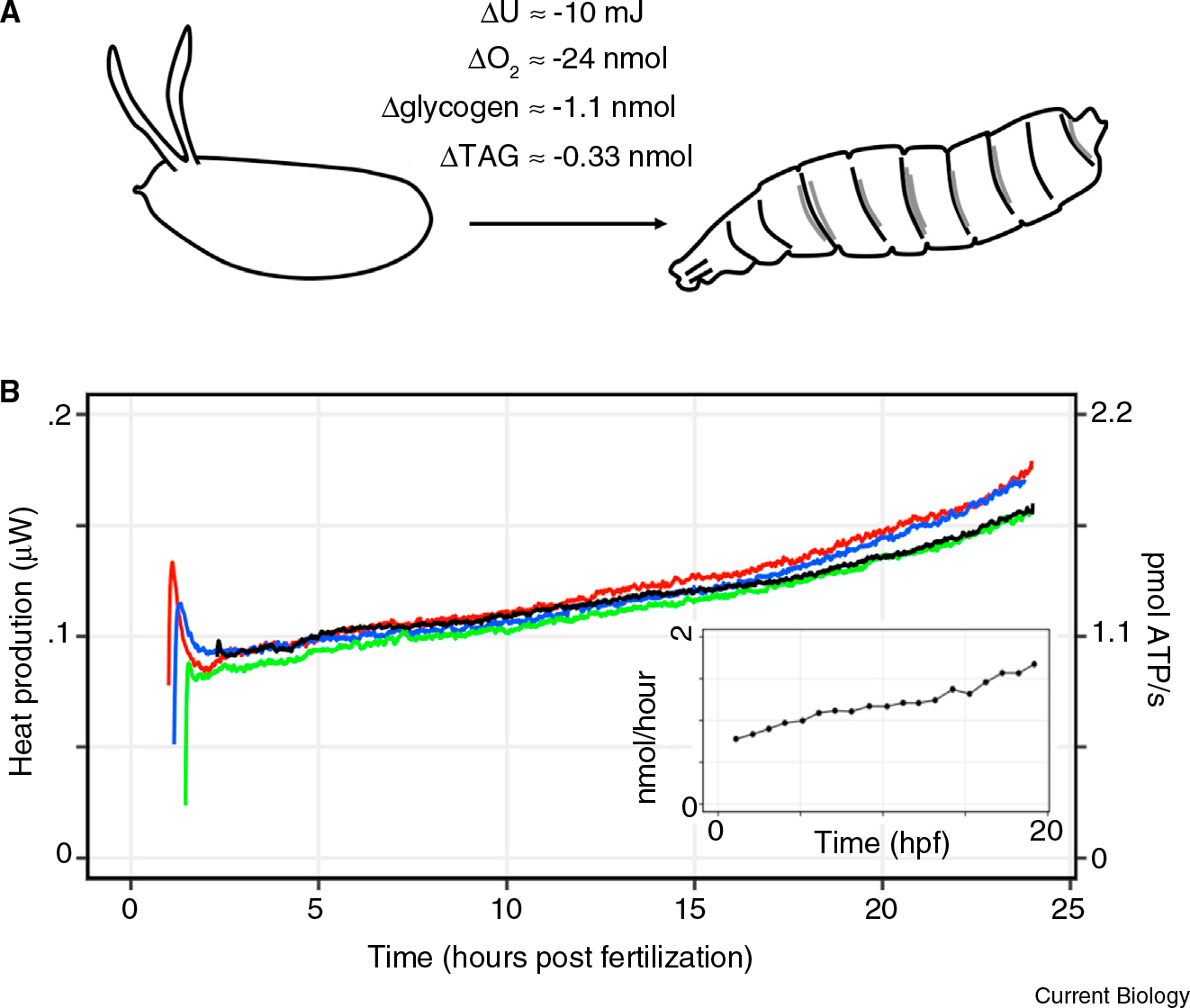
(A) Schematic of energy balance in Drosophila embryogenesis. The zygote burns fuel to develop into a larva, and dissipates heat to the environment in the process. The measurements of net energy usage, oxygen consumption, and fuel source depletion during Drosophila embryogenesis are shown. (B) Heat dissipation rate (μJ/second) and the calculated equivalent in the rate of ATP turnover per embryo throughout embryogenesis. The rate of ATP production was calculated as described in the main text. The different colors represent separate experiments. The spike observed at the beginning of the time course is from introducing the embryo-containing ampoules into the thermal bath at 22°C. The inset shows the representative measurements of oxygen consumption rate during Drosophila embryogenesis at 25°C, adapted from [6]. Hpf denotes hours post fertilization. The experimental setup of the calorimeter and the respirometer is shown in Figure S1.
The Drosophila melanogaster embryo develops into a larva with about 105 cells in 24 hours at room temperature. Since the embryo does not perform work on the environment, the change in internal energy (U) of the embryo is equal to the heat (Q) dissipated over time (ΔU=Q). Therefore, we measured the energy required for embryogenesis through isothermal calorimetry, using the setup previously employed to measure heat dissipation from frog embryos (Figure S1 in Supplemental Information, published with this article online) [3]. The thermal dissipation rate increased linearly from roughly 100 nW to 170 nW throughout embryogenesis. From fertilization until hatching, Drosophila embryos dissipate about 10 mJ of energy (Figure 1). Roughly, this is as much energy as you need to lift a finger.
Glycogen, a polysacharride of glucose, and TAG, a lipid composed of fatty acids and glycerol, are common fuel sources in animal metabolism. To quantify the fuel usage throughout embryogenesis, we measured the glycogen and TAG contents from embryos at the fi rst and last hours of embryogenesis. Similar to previous reports, we found that both fuel sources decreased signifi cantly (Figure 1A) [4]. Previous studies have shown that the rest of the macromolecules remain relatively constant or increase in time (protein [4]; DNA and RNA [5]). Thus, heat dissipation during embryogenesis comes mainly from the breakdown of glucose and TAG.
From known enthalpies of combustion (, and ), we estimated the heat that would have been dissipated from the complete oxidation of the depleted glycogen and TAG stores. For the calculation of , the fatty acid chains composing TAG were approximated as palmitate. Then, the corresponding total heat dissipation is , where Δ[glucose] = 1.1 ± 0.1 nmol and Δ[TAG] = 0.33 ± 0.03 nmol (± represents 2 standard deviations of the mean with n ≥ 7). The calculated value of 13.4 mJ is similar to the 10 mJ obtained from calorimetry. We also calculated the amount of oxygen required for the complete oxidation of the carbon fuel sources by stoichiometry:
Glucose oxidation:
TAG oxidation:
Then, the total calculated O2 consumption is 6Δ[glucose] + 72.5Δ[TAG] = 30.3 ± 2 nmol, which is similar to the results from respirometry (~24 nmol per embryo (Figure 1B inset, adapted from [6])). Note that the respiration and fuel depletion measurements were done in 25°C, whereas the isothermal calorimetry was done in 22°C. To compare the values obtained from these separate experiments, we assumed that the net energy usage during embryogenesis is independent of temperature, as was shown in frog embryos [3]. The 30% discrepancy between the fuel depletion and heat dissipation/respiration measurements can result either from inaccuracies in measurements or from the shunting of the fuel sources towards anabolic pathways. Nevertheless, most of the depleted glycogen and TAG is oxidized to generate ATP. Indeed, it is well known that Drosophila embryogenesis arrests in response to hypoxia [7]. Thus, through three orthogonal measurements, we conclude that Drosophila embryos use 10 mJ of energy, which is generated by the oxidation of the maternally deposited carbon fuel sources (Figure 1).
To place 10 mJ in the context of molecular processes, we fi rst convert it into its ATP equivalent. From canonical catabolic pathways, we assume that each mole of glucose and TAG generates 30 and 335 moles of ATP, respectively. Then, the ratio between the heat of combustion and the amount of ATP produced per mole of the two fuel sources are roughly equivalent:
Since we know the total heat production , we can calculate the total ATP production as follows:
We can similarly calculate the amount of ATP produced at any time interval from the heat dissipation measurements. During 2–6 hours of embryogenesis, the embryo dissipates 1.4 mJ of heat, thereby producing 15 nmol of ATP. During the same period, 0.24 nmol of amino acids are polymerized by ribosomes [8]. Assuming that each amino acid polymerization requires 4 ATPs, the total ATP required for translation between 2–6 hours post fertilization is 1 nmol, which accounts for 6.7% of the total ATP produced. Even fewer ATPs are required for DNA and RNA polymerization. By the 6th hour, 0.06 and 0.03 nmol of dNTPs and NTPs are polymerized into DNA and RNA, respectively [5]. Assuming that attaching each dNTP/NTP to the growing DNA/RNA strand requires 2 ATP equivalents, a total of 0.18 nmol of ATP is required for nucleic acid polymerization. Thus, about 1.2% of the total ATP produced in the first 6 hours of embryogenesis is consumed by DNA and RNA polymerization.
The overall energy usage in Drosophila embryos, normalized for mass by Kleiber’s Law (Watt/mass¾), falls within the trend of the resting metabolic rates of insects [2]. Normalized for volume (Watt/volume), the ATP production rate of embryos is comparable to that of immortalized baby mouse kidney cells grown in culture [9]. However, the major biosynthetic processes such as protein, DNA, and RNA polymerization consume less than 10% of the total ATP produced. Thus, the major energy sinks in cellular processes remain to be determined. While a recent study mapped the origin of the oscillations in the heat dissipation of zebrafish embryogenesis to the biochemical activity of cell cycle oscillators [10], most of the energy produced was left unaccounted. Indeed, countless biochemical processes are required to maintain and create order in the cell, all of which use energy that must be included in the energy budget of embryogenesis. In this light, we propose that the advanced understanding of Drosophila development can be leveraged to gain quantitative insights into the energy budget of embryogenesis.
Supplementary Material
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information contains one figure and experimental procedures, and can be found with this article online at https://doi.org/10.1016/j.cub.2019.05.025.
REFERENCES
- 1.Needham J (1932). Chemical embryology. Annu. Rev. Biochem. 1, 507–526. [Google Scholar]
- 2.Gillooly JF, Brown JH, and West GB (2001). Effects of size and temperature on metabolic rate. 293, 2248–2252. [DOI] [PubMed] [Google Scholar]
- 3.Nagano Y, and Ode KL (2014). Temperature-independent energy expenditure in early development of the African clawed frog Xenopus laevis. Phys. Biol. 11, 046008. [DOI] [PubMed] [Google Scholar]
- 4.Tennessen JM, Bertagnolli NM, Evans J, Sieber MH, Cox J, and Thummel CS (2014). Coordinated metabolic transitions during Drosophila embryogenesis and the onset of aerobic glycolysis. G3 (Bethesda). 4, 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson K, and Lengyel J (1981). Changing rates of DNA and RNA synthesis in Drosophila embryos. Dev. Biol. 138, 127–138. [DOI] [PubMed] [Google Scholar]
- 6.Lints CV, Lints FA, and Zeuthen E (1967). Oxygen consumption during development of the egg in genotypes of Drosophila melanogaster with contributions to the gradient diver technique. Biol. Inst. Carlsb. Found. 36, 35–66. [PubMed] [Google Scholar]
- 7.DiGregorio PJ, Ubersax JA, and O’Farrell PH (2001). Hypoxia and nitric oxide induce a rapid, reversible cell cycle arrest of the Drosophila syncytial divisions. J. Biol. Chem. 276, 1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santon J, and Pellegrini M (1981). Rates of ribosomal protein and total protein synthesis during Drosophila early embryogenesis. Dev. Biol. 85, 252–257. [DOI] [PubMed] [Google Scholar]
- 9.Fan J, Kamphorst JJ, Mathew R, Chung MK, White E, Shlomi T, and Rabinowitz JD (2013). Glutamine-driven oxidative phosphorylation is a major ATP source in transformed mammalian cells in both normoxia and hypoxia. Mol. Syst. Biol. 9, 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodenfels J, Neugebauer KM, and Howard J (2019). Heat oscillations driven by the embryonic cell cycle reveal the energetic costs of signaling. Dev. Cell 48, 646–658.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


