Abstract
Yersinia-induced reactive arthritis is highly associated with HLA-B27, the role of which in defense against the triggering bacteria remains unclear. The aim of this study was to examine the capacity of rats transgenic for HLA-B27 to mount a cytotoxic T-lymphocyte (CTL) response against Y. pseudotuberculosis and to determine the influence of the HLA-B27 transgene on this response. Rats transgenic for HLA-B*2705 and human β2-microglobulin of the 21-4L line, which do not spontaneously develop disease, and nontransgenic syngeneic Lewis (LEW) rats were infected with Y. pseudotuberculosis. Lymph node cells were restimulated in vitro, and the presence of for Y. pseudotuberculosis-specific CTLs against infected targets was determined. Infection of 21-4L rats triggered a CD8+ T cell-mediated cytotoxic response specific for Y. pseudotuberculosis. Analysis of this response demonstrated restriction by an endogenous major histocompatibility complex molecule. However, no restriction by HLA-B27 was detected. In addition, kinetics studies revealed a weaker anti-Yersinia CTL response in 21-4L rats than in nontransgenic LEW rats, and the level of cytotoxicity against 21-4L lymphoblast targets sensitized with Y. pseudotuberculosis was lower than that against nontransgenic LEW targets. We conclude that HLA-B27 transgenic rats mount a CTL response against Y. pseudotuberculosis that is not restricted by HLA-B27. Yet, HLA-B27 exerts a negative effect on the level of this response, which could contribute to impaired defense against Yersinia.
Gastrointestinal infection with two gram-negative species of the genus Yersinia, Y. enterocolitica and Y. pseudotuberculosis, is sometimes complicated with sterile reactive arthritis (ReA) (50). This manifestation belongs to the spondyloarthropathies (SpA) and occurs with increased frequency in patients bearing the HLA-B27 class I major histocompatibility complex (MHC) allele (1, 26). Yersinia-induced ReA is thought to be mediated by the immune system; however, the basis for its association with HLA-B27 and its exact mechanism are still poorly understood. Although live yersiniae are not recovered from arthritic joints, the presence of bacterial components (16, 18) and of Yersinia-specific T-cell clones, either CD4+ or CD8+ (20, 29, 55), has been demonstrated in the synovial fluid cells or tissue. This T-cell response seems inappropriate, since it apparently fails to efficiently clear Yersinia antigen (Ag), and it is likely to play a role in the induction or maintenance of synovitis (35, 40). Because of the association between Yersinia-induced ReA and HLA-B27, it is speculated that arthritis could be driven by a pathogenic HLA-B27-restricted Yersinia-specific CD8+ T cell (9, 35, 40). However an alternate hypothesis, that HLA-B27 could instead obstruct the presentation of a protective bacterial epitope to CD8+ T cells, thereby favoring bacterial persistence, is also proposed (40).
An essential role for T cells in defense against Yersinia is established in mice (3), and CD8+ lymphocytes mediate protection in adoptive transfer experiments (2). Furthermore, CD8+ cytotoxic T lymphocytes (CTLs) that recognize Yersinia-derived Ag presented by HLA-B27 are obtained from the joints of patients suffering from ReA (21, 52). Nevertheless, direct evidence that those T cells are actually generated in primary response to Yersinia infection is lacking. Class I presentation is shown to work efficiently for a limited number of bacteria that survive intracellularly, such as listeriae, mycobacteria, salmonellae, and chlamydiae (22, 45). However, yersiniae reside extracellularly after in vivo infection (4, 44). One major issue with the presentation of Ag derived from an extracellularly located bacterium by class I molecules is that such Ag presumably need to penetrate the cell in order to gain access to class I presentation pathway (39). We have previously demonstrated that infection of nontransgenic Lewis (LEW) rat with Y. pseudotuberculosis elicits a Yersinia-specific rat class I MHC-restricted CTL response in vivo (12). This response is mediated by an invasin- and virulence plasmid-dependent mechanism that permits the transfer of plasmid-encoded Yersinia outer membrane proteins (Yops) directly into eukaryotic cells and presumably involves the presentation of Yop-derived Ag by class I molecule at the surface of target cells (13).
Rats transgenic for HLA-B27 and human β2-microglobulin (hβ2m) are helpful for investigating the basis of HLA-B27-associated SpA. Several HLA-B*2705/hβ2m transgenic rat lines expressing high levels of HLA-B27 develop a spontaneous multisystem disease with striking resemblance to human SpA manifestations, including gut inflammation and sterile arthritis (19). In addition, a direct influence of the bacterial flora on those manifestations is established in HLA-B27/hβ2m transgenic disease-prone lines (37, 48).
Rats from other lines that express moderate levels of HLA-B27 remain healthy (47). One such line, the 21-4L line on the LEW background, has been used to demonstrate the capacity of HLA-B27 to restrict a CTL-mediated response against the male-specific minor histocompatibility (H) Ag H-Y (42). In the present study, we used the 21-4L line to examine the influence of HLA-B27 on the CTL-mediated response against Y. pseudotuberculosis after in vivo infection and its capacity to restrict a Yersinia-specific CD8+ CTL response.
MATERIALS AND METHODS
Rats.
Inbred nontransgenic LEW (RT11), brown Norway (BN; RT1n), and dark agouti (DA; RT1av1) rats 2 to 3 months old were purchased from Iffa Credo (L’Arbresle, France) or CERJ (Le Genest-St-Isle, France). The transgenic rat line 21-4L, bearing six copies each of the HLA-B*2705 and hβ2m on the inbred LEW background, was originally produced at University of Texas Southwestern (19). Rats either homozygous or hemizygous for this transgene locus were bred at CDTA (Orleans, France). The hemizygous 21-4L rats were maintained by breeding with nontransgenic LEW rats and typing offspring for the B27/hβ2m transgene by dot blot hybridization of tail genomic DNA, as previously described (19), or by flow cytometric (FCM) detection of HLA-B27 on the surface of peripheral blood leukocytes. Rats homozygous for RT1av1 and bearing the 21-4L transgene locus were bred by successive backcrossing of 21-4L rats with DA strain rats and typing of the offspring for the B27 transgene and for the absence of RT11 by FCM. Rats were bred and housed in conventional conditions. Study procedures were approved by the institutional animal care committee.
Bacterial strains and growth conditions.
Y. pseudotuberculosis IP2777 (serogroup I [43]) and YPIII (serogroup III [7]), harboring virulence plasmid pYV (wild types), and their derivative strains cured of virulence plasmid (denoted by a “c” [43]), were used in this study. Yersinia strains were grown in Luria broth (LB) medium, sometimes supplemented with 20 mM sodium oxalate–20 mM MgCl2 (Ca2+-deficient LB medium), at 28 or 37°C. Strains of Escherichia coli 25922 obtained from the American Type Culture Collection (Rockville, Md.) and Salmonella typhimurium (patient isolate) were grown in LB medium at 37°C. The actual number of bacteria in the inoculum was determined by plating serial dilutions of the inoculum on LB agar and counting CFU after incubation for 48 h at 28 or 37°C.
Tissue culture medium, cell lines, MAbs, and other reagents.
The tissue culture medium was RPMI 1640 with l-glutamine, 5% fetal calf serum (FCS), penicillin, streptomycin, 0.02 mM 2-mercaptoethanol, and 5 mM HEPES unless otherwise stated. The L929 cell line was a gift from D. S. Finbloom, Bethesda, Md. The C1R human lymphoblastoid cell line transfected with HLA-B*2705 (C1R-B27 [21]) was provided by D. Yu, Los Angeles, Calif. Epstein-Barr virus-transformed B-lymphocyte (B-EBV) cell lines from an HLA-B*2705+ SpA patient and a healthy donor were provided by A. Toubert, Paris, France. The mouse anti-rat Ag monoclonal antibodies (MAbs) used and their specificities, references of which are cited in reference 12, were as follows: R73, immunoglobulin G1 (IgG1); T-cell receptor alpha/beta chain (TCRα/β); OX34, IgG2a, CD2; OX8, IgG1, CD8α; OX35, IgG2a, CD4; OX33, IgG1, B-cell-specific CD45 epitope; OX18, IgG1, RT1 class I (several loci); OX6, IgG1, RT1-B (MHC class II locus); and OX42, IgG2a, C3bR (macrophages). Other murine MAbs used were as follows: B1.23.2, IgG2a, monomorphic HLA-B and C (38); ME1, IgG1, several HLA-B loci (11); and TM1, IgG2b, HLA-B27 (49). The anti-RT11 MAb YR2/69 (rat IgG2b [27]) was a gift from G. W. Butcher (The Brahabam Institute, Cambridge, United Kingdom). Irrelevant isotype-matched MAbs served as negative controls. Goat anti-mouse (GAM) fluorescein isothiocyanate (FITC)-labeled IgG was from Eurobio (Les Ulis, France). Goat anti-rat (GAR) IgG-FITC was from Caltag (Burlingame, Calif.). Concanavalin A (ConA), α-methyl-d-mannoside, and dimethyl sulfoxide (DMSO) were purchased from Sigma (St-Quentin Fallavier, France).
Infection of animals.
Y. pseudotuberculosis grown overnight at 28°C in Ca2+-deficient LB medium was centrifuged and resuspended in sterile phosphate-buffered saline (PBS). For intragastric infection, rats were given 109 bacteria in 0.5 ml of PBS for 3 consecutive days. For intraperitoneal (i.p.) infection, rats received 105 bacteria in 0.5 ml of PBS either once or daily for 3 consecutive days.
Generation of CTLs.
Peripheral and mesenteric lymph node (LN) cells from infected rats were resuspended in culture medium containing 10% ConA-stimulated rat spleen and LN cell supernatant plus 50 mM α-methyl-d-mannoside and restimulated in 96-well U-bottom culture dishes (105 cells/well) with LN cells from naive rats (3 × 105 cells/well) that had been in vitro infected with Y. pseudotuberculosis as follows. Bacteria grown overnight at 28°C in LB medium followed by 4 h of incubation at 37°C in Ca2+-deficient LB medium, to induce expression of virulence plasmid Yops (46), were washed and resuspended in tissue culture medium without antibiotics and then incubated with LN cells at a ratio of 50 bacteria/cell for 2 h at 37°C. Infected stimulator cells were treated for 1 h with gentamicin (100 μg/ml), washed, and irradiated (3,000 rads) before use.
Proliferation assays.
Purified LN T cells from infected rats were restimulated for 3 to 5 days in 96-well U-bottom culture dishes (2 × 105 cells/well) with irradiated LN cells from naive rats (105 cells/well) that had been infected in vitro with Y. pseudotuberculosis or control bacteria or left uninfected. [3H]thymidine was added during the last 12 h of culture, and radioactivity incorporated into the cells was determined by liquid scintillation counting.
Cell-mediated cytotoxicity assay.
Rat LN cell ConA-induced lymphoblasts (ConA blasts) and bone marrow-derived macrophage (BMDM) targets were obtained as previously described (12, 23, 28). Infection of target cells was performed immediately before labeling, using a procedure similar to that described above for infection of stimulator cells. For labeling of target cells, 106 cells were centrifuged at 200 × g, the supernatant was removed by aspiration, 50 μCi of sodium [51Cr]chromate (DuPont NEN, Boston, Mass.) was added to the cell pellet, and the cells were incubated for 3 h at room temperature (ConA blasts) or for 2 h at 37°C (BMDM, C1R, and B-EBV cells). Labeled cells were washed three times by centrifugation at 200 × g for 7 min, counted, and resuspended in culture medium containing 10% FCS.
For cytotoxicity assays, restimulated LN cells harvested after 5 days of culture were resuspended at 5 × 106 cells/ml in culture medium containing 10% FCS. Labeled target cells were diluted in the same medium to 5 × 104 cells/ml. Threefold dilutions of effector cells (100 μl) and target cells (100 μl) were dispensed in triplicate into 96-well U-bottom culture dishes. Medium without effectors (100 μl) or 1 N HCl (100 μl) was also added to triplicate wells of targets to determine spontaneous and maximal lysis, respectively. Effector and target cells in the plates were incubated for 4 h at 37°C. Supernatant was harvested from each well (50 μl) and counted in a 1450 Microbeta counter (Wallac, Turku, Finland). Percent specific lysis was computed by the formula 100 × (isotope release by effector cells − spontaneous release)/(maximum release − spontaneous release). The ratio of spontaneous to maximal 51Cr release from lysed targets was routinely <30% and averaged 20%. The standard deviation among triplicate assays was always <5% specific lysis.
Flow cytometric detection of cell surface Ag.
All procedures were carried at 4°C in Dulbecco’s PBS–5% FCS/0.01% NaN3 with two washes after each 15-min incubation. Briefly, 1 × 105 to 5 × 105 cells were incubated with saturating concentrations of the primary MAb and then incubated with FITC-conjugated monoclonal GAM or GAR IgG. FCM was carried out as described previously (12).
In vitro magnetic cell depletions.
Selected subsets of T cells were obtained after restimulation by negative selection using the following combinations of MAbs: OX33 and OX42 to purify all T cells, plus either OX8 or OX35 to purify CD4+ or CD8+ T cells, respectively. All procedures were carried out at 4°C in PBS–4% FCS as follows. Cells were incubated with saturating concentrations of MAb for 15 min, washed, and further incubated with GAM IgG-conjugated microbeads (Dynabeads M-450; Dynal, Oslo, Norway) at a ratio of 20 microbeads/cell. Cells were washed and sorted with a magnet (Dynal MPC).
Light microscopy of cytospin smears.
Blast cells were incubated with live bacteria at a ratio of 50 bacteria/cell for 1 h at 37°C, followed by 1 h at 37°C with gentamicin, and spun down at 70 × g for 8 min in a Cytospin III (Shandon SA, Eragny sur Oise, France). Bacteria and lymphoblasts were examined under a light microscope after May-Grünwald-Giemsa staining.
Prediction of putative HLA-B2705*-binding epitope.
We used the program developed by K. C. Parker (National Institutes of Health, Bethesda, Md.) (4a) to test for the presence of putative HLA-B*2705-binding 9-or 10-mer peptides carried by Y. pseudotuberculosis Yops. This program was initially established to predict the relative binding strengths of nonapeptides to the HLA-A2 molecule (32) and has been adapted for other HLA molecules, including B*2705. The algorithm used derives a score for each 9- or 10-mer sequence that is an estimate of the half-time dissociation (in minutes) of HLA complexes containing the peptide at 37°C, as a result of calculations based on the observed anchor residues preferences (33, 36).
Statistical analysis.
Levels of cytolysis of nontransgenic LEW and 21-4L transgenic lymphoblast targets were compared by using a Student paired t test. A P value less than 0.05 was considered significant.
RESULTS
HLA-B27 transgenic rats mount a specific CTL response against Y. pseudotuberculosis.
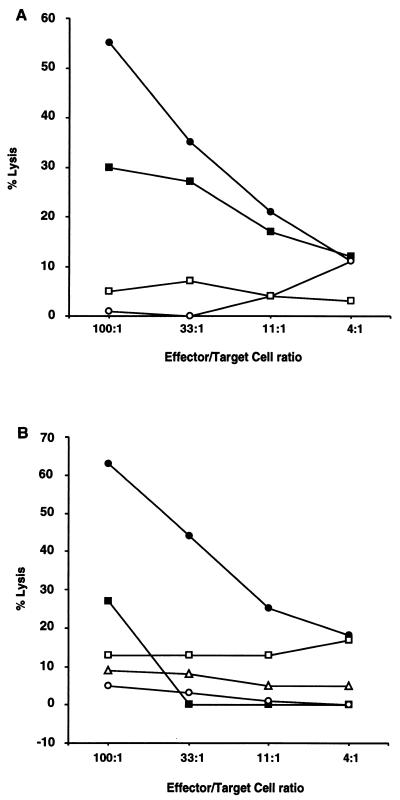
HLA-B27/hβ2m transgenic rats of the healthy 21-4L line infected with Y. pseudotuberculosis either intragastrically or i.p. develop a specific cellular immune response characterized by a proliferative (not shown) and a CTL response. As shown (Fig. 1A), CTLs obtained from LN cells of 21-4L rats infected in vivo 2 to 12 weeks before with virulent Y. pseudotuberculosis killed infected ConA blasts and infected BMDM from nontransgenic LEW rats.
FIG. 1.
Evidence for specific CTL response against Y. pseudotuberculosis in HLA-B27/hβ2m transgenic rats. (A) LN cells from an 21-4L rat infected i.p. with Y. pseudotuberculosis IP2777(pYV+) were restimulated in vitro with nontransgenic LEW LN cells infected with IP2777(pYV+). CTLs were tested 5 days later for lysis of LEW ConA blast (circles) and BMDM (squares) targets infected in vitro with IP2777 (pYV+) (solid symbols) or uninfected (open symbols). (B) LN cells from an 21-4L rat infected intragastrically with strain IP2777(pYV+) were restimulated in vitro with 21-4L LN cells infected with IP2777(pYV+) and tested for lysis of 21-4L lymphoblast targets uninfected (open circles) or infected with IP2777(pYV+) (solid circles), plasmid-cured strain IP2777c(pYV−) (solid squares), S. typhimurium (open triangles), or E. coli (open squares).
When used as stimulatory cells, in vitro-infected 21-4L LN cells efficiently restimulated effector cells present in the LN cell population from infected nontransgenic LEW (not shown) and 21-4L (Fig. 1B) rats, and infected 21-4L lymphoblasts were efficiently killed by LEW (not shown) or 21-4L (Fig. 1B) CTLs. As we have previously shown with nontransgenic LEW CTLs (12), the CTL response raised in 21-4L rats against blast targets was dependent on the presence of the virulence plasmid of Y. pseudotuberculosis, since Y. pseudotuberculosis strains cured of their virulence plasmid failed to sensitize nontransgenic LEW blast targets (not shown), or 21-4L blast targets for killing upon in vitro infection (Fig. 1B).
This CTL response was specific for Yersinia, since lymphoblasts infected with E. coli or with S. typhimurium were not efficiently killed by CTLs derived from 21-4L rats (Fig. 1B).
The anti-Y. pseudotuberculosis CTL response is mediated by CD8+ T cells and is restricted by rat class I MHC.
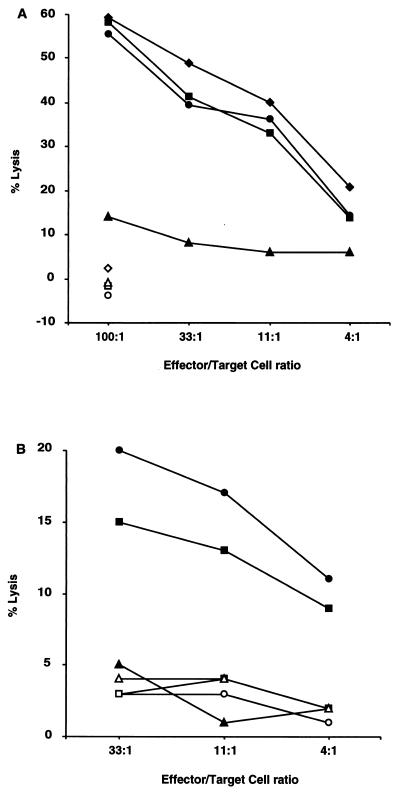
Studied by FCM, in vitro-restimulated LN cells from Y. pseudotuberculosis-infected 21-4L rats were mostly (>90%) composed of HLA-B27+ CD2+ TCRα/β+ T cells, of which one-third expressed the CD8 molecule and two-thirds expressed the CD4 molecule (data not shown). Sorting experiments revealed that CD8+ T cells were responsible for the anti-Yersinia CTL response (Fig. 2A).
FIG. 2.
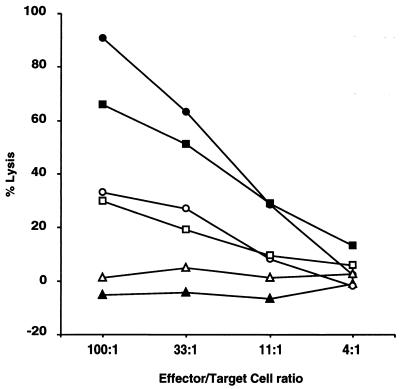
The anti-Y. pseudotuberculosis CTL response in 21-4L rats is mediated by CD8+ T cells and is genetically restricted. (A) LN cells from an 21-4L rat infected i.p. with Y. pseudotuberculosis IP2777(pYV+) were restimulated in vitro with 21-4L LN cells infected with IP2777(pYV+) and tested for lysis of 21-4L targets infected with IP2777(pYV+) (closed symbols) or uninfected (open symbols). Immunomagnetic sorting was performed to negatively select for all the T cells (squares), CD8+ T cells (diamonds), and CD4+ T cells (triangles), that were compared to unsorted cells (circles). (B) LN cells from a 21-4L rat infected i.p. with strain IP2777(pYV+) were restimulated in vitro with 21-4L LN cells infected with IP2777(pYV+) and tested for lysis of nontransgenic LEW (circles), 21-4L (squares), or BN (triangles) lymphoblast targets infected with strain IP2777(pYV+) (closed symbols) or uninfected (open symbols).
Nontransgenic lymphoblast targets from inbred strains of rats other than LEW, such as BN (Fig. 2B) or DA (not shown), infected with Y. pseudotuberculosis were not killed by CTLs from 21-4L rats, suggesting a specificity of this CTL response for LEW MHC and/or HLA-B27.
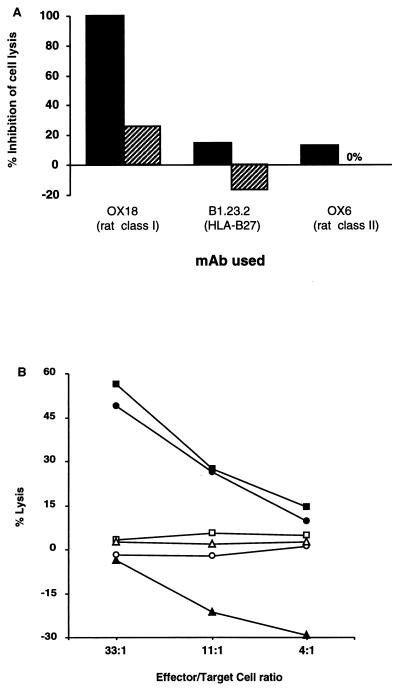
To further address the issue of MHC restriction, we conducted blocking experiments that used preincubation of target cells with anti-rat MHC MAbs. These experiments revealed that at least part of the CTL response was dependent on rat class I but not class II MHC molecules (Fig. 3A). Indeed, when CTLs from 21-4L rat were assayed in the presence of anti-rat class I MAb OX18, complete blocking of nontransgenic LEW target cytolysis was observed (Fig. 3A). However, only partial blocking was achieved against 21-4L targets. This observation could be explained by a decreased affinity of OX18 MAb for rat class I heavy chains complexed with hβ2m (19). However, it also suggested the possibility that CTLs restricted by the HLA-B27 molecule were present in the effector T-cell population (Fig. 3A).
FIG. 3.
The anti-Y. pseudotuberculosis CTL response in 21-4L rats is not restricted by HLA-B27. (A) LN cells from an 21-4L rat infected i.p. with Y. pseudotuberculosis IP2777(pYV+) were restimulated in vitro with 21-4L LN cells infected with IP2777(pYV+) and tested for lysis of IP2777(pYV+)-infected nontransgenic LEW (solid bars) or 21-4L (hatched bars) lymphoblast targets that were preincubated with saturating concentrations of anti-rat class I MHC (OX18), anti-HLA-B27 (B1.23.2), anti-rat class II MHC (OX6), or isotype-matched control MAb at an effector/target cell ratio of 100. Percent inhibition = (% lysis with control mAb − % lysis with relevant MAb)/% lysis without mAb × 100. (B) LN cells from 21-4L rat infected intragastrically with strain IP2777(pYV+) were restimulated in vitro with 21-4L LN cells infected with IP2777(pYV+) and tested for lysis of nontransgenic LEW (circles), 21-4L (squares), or RT1av1 21-4L backcross (DA B27+; triangles) rat lymphoblast targets infected with strain IP2777(pYV+) (closed symbols) or uninfected (open symbols).
Absence of HLA-B27-restricted anti-Y. pseudotuberculosis CTL response.
Surprisingly, preincubation of target cells with any of three anti-B27 MAbs tested, e.g., B1.23.2 (Fig. 3A), ME1, and TM1 (not shown), failed to demonstrate any blocking of the anti-Yersinia CTL response against sensitized 21-4L blast targets, although all these antibodies remained bound to HLA-B27 expressed at the surface of in vitro-infected targets throughout the CTL assay, as studied by FCM (not shown). The lack of blocking by anti-B27 MAbs was also observed in an inhibition assay combining anti-rat class I and anti-HLA-B27 MAbs at concentrations known to inhibit both RT1-A-restricted and HLA-B27-restricted anti-H-Y cytolysis in 21-4L rat (not shown) (42).
Given the lack of blocking of the CTL response with anti-B27 MAbs, we tested for the presence of anti-Yersinia HLA-B27-restricted CTLs in the population of restimulated 21-4L LN cells, using targets that express HLA-B27 but lack LEW rat class I MHC molecules, as described previously (42). However, we failed to detect a CTL response against any of the following sensitized targets: lymphoblast targets from RT1av1 21-4L backcross rats (DA B27+ [Fig. 3B]), C1R-B27 lymphoblastoid cells, or HLA-B27+ B-EBV-transformed cells from an SpA patient or from a healthy donor (not shown). Altogether, these data argue against the presence of a B27-restricted T-cell population among anti-Yersinia CTLs generated in 21-4L rats.
The presence of the HLA-B27 transgene impairs the CTL response against Y. pseudotuberculosis.
All of the foregoing results were obtained in assays using 21-4L transgenic rats hemizygous for the B27/hβ2m transgene, which reproducibly mount a strong specific CTL response against Y. pseudotuberculosis IP2777 or YPIII. Surprisingly, this anti-Y. pseudotuberculosis CTL response was repeatedly weak or negative in 21-4L rats homozygous for the B27/hβ2m transgene. Indeed, when comparing the CTL response in homozygous 21-4L rats and in nontransgenic LEW rats, in which infections and restimulations were conducted in parallel, we observed a much weaker anti-Yersinia CTL response in homozygous 21-4L rats than in nontransgenic LEW rats (Fig. 4).
FIG. 4.
Homozygous HLA-B27/hβ2m transgenic rats of the 21-4L line mount a weaker anti-Y. pseudotuberculosis CTL response than nontransgenic LEW rats. LN cells from nontransgenic LEW (solid symbols) or homozygous 21-4L (open symbols) rats infected intragastrically 3 weeks earlier with Y. pseudotuberculosis YPIII(pYV+) were restimulated in vitro with YPIII(pYV+)-infected nontransgenic LEW or 21-4L LN cells, respectively, and tested for lysis of YPIII(pYV+)-infected nontransgenic LEW (circles) or 21-4L (squares) or uninfected (triangles) lymphoblast targets.
Furthermore, when comparing the levels of cytolysis of sensitized nontransgenic LEW and 21-4L transgenic lymphoblast targets by anti-Yersinia CTLs, we observed that the 21-4L targets were reproducibly killed at lower levels than the nontransgenic targets (Fig. 2B, 4, and 5). This difference was significant when restimulated LN cells were from nontransgenic LEW rats, infected either with strain IP2777 or with strain YPIII (P < 0.04 at an effector/target cell ratio of 100), but not when effector cells were from 21-4L rats (Fig. 5). This difference in cytolysis levels between nontransgenic LEW and 21-4L blast targets was not explained by a decreased attachment of Y. pseudotuberculosis to 21-4L blast targets, as studied by light microscopic examination of cytospin smears (not shown).
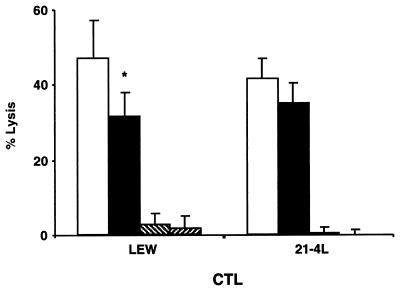
FIG. 5.
The average lysis level of 21-4L targets by anti-Y. pseudotuberculosis CTL is lower than that of nontransgenic LEW targets. LN cells from nontransgenic LEW or 21-4L rats infected with Y. pseudotuberculosis(pYV+) were restimulated in vitro with Y. pseudotuberculosis(pYV+)-infected syngeneic LN cells and tested in parallel for lysis of infected nontransgenic LEW (open bars) or 21-4L (closed bars) or uninfected (hatched bars) lymphoblast targets at effector/target cell ratio of 100. Results are expressed as mean specific percent of lysis + standard error of the mean of targets in 7 (nontransgenic LEW CTLs) and 13 (21-4L CTLs) separate experiments. ∗, P < 0.04, nontransgenic LEW versus 21-4L targets, for nontransgenic LEW CTLs.
Candidate target Ags for anti-Yersinia CTL carry a putative HLA-B27-binding epitope.
We have previously established in nontransgenic LEW rats that among Yersinia-derived proteins, virulence plasmid-encoded Yops which are translocated inside eukaryotic cytosol are presumably recognized as Ag by rat RT1-A-restricted CTLs (12). It was of interest to determine if these Yops carry suitable epitopes likely to be presented by the HLA-B27 molecule. Therefore, we used an HLA peptide binding prediction program to test for putative binding sites among all Y. pseudotuberculosis Yops known to be translocated, except YopM, for which full sequence is not available. Results of this search suggest that several peptides derived from Yops, including YopE, which is the major candidate Ag for LEW RT1-A-restricted CTL response, are likely to bind with high affinity to HLA-B*2705, just like several known HLA-B27-binding sequences (Table 1). Additional putative HLA-B*2705-binding peptide sequences were also identified by examining several nontranslocated Yops, such as YopB, YopK, and LcrV (data not shown). Hence, the absence of a B27-restricted response does not seem attributable to lack of potential B27-presented peptide Ag.
TABLE 1.
Putative HLA-B*2705-binding epitopes in Y. pseudotuberculosis Yop proteinsa
| Protein origin | Peptide sequence | Scored | Reference |
|---|---|---|---|
| Yop protein | |||
| YopO (previously YpkA) | KTYRIIDNQV | 9,000 | 15 |
| YopH | QRFGMPDYF | 5,000 | 8 |
| YopE | QRMFSEGSHK | 2,000 | 14 |
| YopD | TRHEAQAIK | 2,000 | 17 |
| YopJ | IRTKTAIER | 1,000 | 15 |
| Known HLA-B27-binding motifb | |||
| Human translation factor eIF2 | KRFEKHWRL | 30,000 | 6 |
| Human proteasome subunit C5 | RRFMPYYVY | 15,000 | 6 |
| Human immunodeficiency virus type 1 gp120 | GRAFVTIGK | 2,000 | 6 |
| Rat H-Y Ag | KQYQKSTER | 1,500 | 41 |
| Human ribosomal protein S25 | HRAQVIYTR | 1,000 | 6 |
| Control sequencec | |||
| HLA-A29 endogenous ligand | KEFQEHYEY | 225 | 6 |
Putative HLA-B*2705-binding 9- or 10-mer peptides carried by Y. pseudotuberculosis Yop proteins were identified by using the HLA Peptide Binding Predictions program (4a). Only one selected sequence with the highest estimate score is shown for each Yop tested.
Scores derived with the same program for several known HLA-B*2705-binding sequences.
Score derived with the same program for a non-HLA-B27-binding sequence.
DISCUSSION
We previously established that upon in vivo infection, nontransgenic LEW rats mount a specific anti-Y. pseudotuberculosis CTL response that is restricted by LEW class I MHC molecule RT1-A (12). In this study, we used healthy rats of the 21-4L line, transgenic for HLA-B*2705/hβ2m on a LEW inbred background, to investigate the effect of HLA-B27 on the anti-Yersinia CTL response. The question of whether an anti-Yersinia HLA-B27-restricted CTL response arises appears critical for understanding the mechanism of Yersinia-induced ReA, a human disease that is closely associated with the presence of HLA-B27.
21-4L rats hemizygous for the B27/hβ2m transgene, like nontransgenic LEW rats, mount an anti-Yersinia CTL response. However, we found no evidence for the presence of HLA-B27-restricted CTLs in this transgenic line. This finding is intriguing, since it has been consistently shown in the same line of rats that HLA-B27 is a restriction molecule for anti-H-Y CTLs (41, 42). None of three different anti-B27 MAbs could block the anti-Yersinia CTL response generated in 21-4L rats. Nevertheless, this negative result does not entirely rule out the possibility that complexes of HLA-B27 and Yersinia-derived peptides recognized by putative B27-restricted CTLs assume a conformation that alters binding of all the MAbs tested, and bacterium-derived peptides presented by HLA-B27 may indeed display special characteristics such as an unusual length (5) and thereby modify anti-B27 MAb affinity (53). However, none of the Y. pseudotuberculosis-sensitized HLA-B27+ targets, which lack LEW RT11 molecules, were killed by anti-Yersinia CTLs from 21-4L rats. This result adds compelling evidence for the absence of HLA-B27-restricted CTL population in these rats, since DA B27+ rat lymphoblasts and human C1R-B27 and HLA-B27+ lymphoblasts presenting exogenously added HLA-B27-restricted H-Y peptides were all efficiently killed by anti-H-Y CTLs from 21-4L rats (41, 42).
The foregoing results fail to support the hypothesis that HLA-B27-restricted CTLs are generated in response to Yersinia infection and thereby mediate pathogenesis of ReA. On the contrary, we observed a much weaker anti-Yersinia CTL response in 21-4L rats homozygous for the B27 transgene than in nontransgenic LEW rats. Because of the copy number-dependent expression of transgenic class I molecules, homozygosity for the transgene may amplify any effect of HLA-B27 in the 21-4L line (47). The anti-Yersinia CTL response in rats is thought to be directed against plasmid-encoded Yops that are translocated to eukaryotic cytosol during interaction between the bacteria and target cells, and YopE is a major candidate Ag for CTL response in LEW rat (12, 13). Several explanations can be proposed to explain the impaired CTL response in homozygous 21-4L rats compared with nontransgenic LEW rat. First, it could result from a competition between HLA-B27 and rat class I molecules for the presentation of Yersinia-derived Ag. This interpretation is also supported by the lower specific lysis of 21-4L lymphoblast targets by anti-Yersinia LEW CTLs compared with nontransgenic LEW targets. Such a competition effect involving HLA-B27 has been described elsewhere (34, 51). Indeed, the presence of putative HLA-B27-binding epitopes carried by Yops, including YopE, is intriguing with respect to the absence of B27-restricted CTL. If numerous B27-restricted epitopes were generated by processing of Yops after their translocation into the target cytosol, competition among them and also with rat class I-restricted epitopes could affect the level of presentation of all epitopes, such that none of the B27-restricted epitopes would be presented above the threshold necessary to trigger a CTL response (30, 54, 56). Second, HLA-B27 may negatively influence the function or the repertoire of anti-Yersinia T cells. Indeed, it can be speculated either that Yersinia-specific B27-restricted CD8+ T cells have no cytolytic function, but rather produce inhibitory cytokines (25), or that HLA-B27 molecules expressed in the thymus in combination with self-peptides clonally delete T cells which are involved in a CTL response against the bacteria (24). Any of these mechanisms would in turn impair the cellular immune defense against the bacteria. Interestingly, this interpretation is supported by earlier reports of weak anti-Yersinia T-cell proliferative response (50) and prolonged persistence of bacteria in gut mucosa (10) of HLA-B27+ patients in the setting of Yersinia-induced ReA, and by the higher mortality and morbidity from infection with Yersinia that was observed in HLA-B27 transgenic mice as compared to nontransgenic littermates (31).
Since HLA-B27 may impair appropriate CTL responses during infection with Yersinia, it could favor persistence of infection. Low-grade infection could lead to alternate pathways of bacterial Ag processing and thereby contribute to the emergence of additional Yersinia epitopes, especially upon handling by professional antigen-presenting cells. This possibility was not addressed in our experiments but could explain why only a very low frequency of B27-restricted anti-Yersinia CTLs have been obtained from the joints of ReA patients (21, 52).
In conclusion, our observations fail to support the hypothesis that B27-restricted CTLs appear in the course of Yersinia infection but are more consistent with the hypothesis that HLA-B27 may decrease the capacity to mount an effective CD8+ T-cell response to Yersinia infection and to properly eliminate the bacteria (40).
ACKNOWLEDGMENTS
This work was supported by an SFR grant. G.F. was supported in part by a grant from ARCR10/96.
We are indebted to J. D. Taurog and to R. E. Hammer, who kindly provided HLA-B27/hβ2m transgenic rats of the 21-4L line. We thank J. D. Taurog for critical review of the manuscript.
REFERENCES
- 1.Aho K, Ahvonen P, Lassus A, Sievers K, Tiilikainen A. HL-A 27 in reactive arthritis. A study of Yersinia arthritis and Reiter’s disease. Arthritis Rheum. 1974;17:521–526. doi: 10.1002/art.1780170505. [DOI] [PubMed] [Google Scholar]
- 2.Autenrieth I B, Tingle A, Reske-Kunz A, Heesemann J. T lymphocytes mediate protection against Yersinia enterocolitica in mice: characterization of murine T-cell clones specific for Y. enterocolitica. Infect Immun. 1992;60:1140–1149. doi: 10.1128/iai.60.3.1140-1149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autenrieth I B, Vogel U, Preger S, Heymer B, Heesemann J. Experimental Yersinia enterocolitica infection in euthymic and T-cell-deficient athymic nude C57BL/6 mice: comparison of time course, histomorphology, and immune response. Infect Immun. 1993;61:2585–2595. doi: 10.1128/iai.61.6.2585-2595.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autenrieth I G, Firshing R. Penetration of M cells and destruction of Peyer’s patches by Yersinia enterocolitica: an ultrastructural and histological study. J Med Microbiol. 1996;44:285–294. doi: 10.1099/00222615-44-4-285. [DOI] [PubMed] [Google Scholar]
- 4a.BioInformatics & Molecular Analysis Section. HLA peptide binding predictions. [Online.] http://bimas.dcrt.nih.gov/molbio/. National Institutes of Health, Bethesda, Md. [7 June 1999, last date accessed.]
- 5.Boisgérault F, Mounier J, Tieng V, Stolzenberg M C, Khalil-Daher I, Schmid M, Sansonetti P, Charron D, Toubert A. Alteration of HLA-B27 peptide presentation after infection of transfected murine L cells by Shigella flexneri. Infect Immun. 1998;66:4484–4490. doi: 10.1128/iai.66.9.4484-4490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boisgérault F, Tieng V, Stolzenberg M-C, Dulphy N, Khalil I, Tamouza R, Charron D, Toubert A. Differences in endogenous peptides presented by HLA-B*2705 and B*2703 allelic variants. Implications for susceptibility to spondylarthropathies. J Clin Investig. 1996;98:2764–2770. doi: 10.1172/JCI119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bölin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982;37:506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bölin I, Wolf-Watz H. The plasmid-encoded Yop2b protein of Yersinia pseudotuberculosis is a virulence determinant regulated by calcium and temperature at the level of transcription. Mol Microbiol. 1988;2:237–245. doi: 10.1111/j.1365-2958.1988.tb00025.x. [DOI] [PubMed] [Google Scholar]
- 9.Burmester G R, Daser A, Kamradt T, Krause A, Mitchison N A, Sieper J, Wolf N. Immunology of reactive arthritides. Annu Rev Immunol. 1995;13:229–250. doi: 10.1146/annurev.iy.13.040195.001305. [DOI] [PubMed] [Google Scholar]
- 10.de Koning J, Heesemann J, Hoogkamp-Korstanje J A A, Festen J J M, Houtman P M, van Oijen P L M. Yersinia in intestinal biopsy specimens from patients with seronegative spondyloarthropathy: correlation with specific serum IgA antibodies. J Infect Dis. 1989;159:109–112. doi: 10.1093/infdis/159.1.109. [DOI] [PubMed] [Google Scholar]
- 11.Ellis S A, Taylor C, McMichael A J. Recognition of HLA-B27 and related antigens by a monoclonal antibody. Hum Immunol. 1982;5:49–59. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- 12.Falgarone G, Blanchard H S, Virecoulon F, Simonet M, Breban M. Coordinate involvement of invasin and Yop proteins in a Yersinia pseudotuberculosis-specific class I-restricted cytotoxic T cell-mediated response. J Immunol. 1999;162:2875–2883. [PubMed] [Google Scholar]
- 13.Fällman M, Persson C, Wolf-Watz H. Yersinia proteins that target host cell signaling pathways. J Clin Investig. 1997;99:1153–1157. doi: 10.1172/JCI119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forsberg A, Wolf-Watz H. The virulence protein Yop5 of Yersinia pseudotuberculosis is regulated at transcriptional level by plasmid-pIB1-encoded trans-acting elements controlled by temperature and calcium. Mol Microbiol. 1988;2:121–133. doi: 10.1111/j.1365-2958.1988.tb00013.x. [DOI] [PubMed] [Google Scholar]
- 15.Galyov E E, Håkansson S, Wolf-Watz H. Characterization of the operon encoding the YpkA Ser/Thr protein kinase and the YopJ protein of Yersinia pseudotuberculosis. J Bacteriol. 1994;176:4543–4548. doi: 10.1128/jb.176.15.4543-4548.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granfors K, Jalkanen S, Von Essen R, Lahesmaa-Rantala R, Isomäki O, Pekkola-Heino K, Merilahti-Palo R, Saario R, Isomäki H, Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med. 1989;320:216–221. doi: 10.1056/NEJM198901263200404. [DOI] [PubMed] [Google Scholar]
- 17.Håkansson S, Bergman T, Vanooteghem J-C, Cornelis G R, Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61:71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hammer M, Zeidler H, Klimsa S, Heesemann J. Yersinia enterocolitica in the synovial membrane of patients with Yersinia-induced arthritis. Arthritis Rheum. 1990;33:1795–1800. doi: 10.1002/art.1780331206. [DOI] [PubMed] [Google Scholar]
- 19.Hammer R E, Maika S D, Richardson J A, Tang J-P, Taurog J D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 20.Hermann E, Lohse A W, Van der Zee R, Van Eden W, Mayet W J, Probst P, Poralla T, Meyer zum Büschenfelde K-H, Fleischer B. Synovial fluid-derived Yersinia-reactive T cells responding to human 65-kDa heat-shock protein and heat-stressed antigen-presenting cells. Eur J Immunol. 1991;21:2139–2143. doi: 10.1002/eji.1830210923. [DOI] [PubMed] [Google Scholar]
- 21.Hermann E, Yu D T Y, Meyer zum Büschenfelde K-H, Fleisher B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 22.Kaufmann S H E. Immunity to intracellular microbial pathogens. Immunol Today. 1995;16:338–342. doi: 10.1016/0167-5699(95)80151-0. [DOI] [PubMed] [Google Scholar]
- 23.Keller R, Keist R. Induction of interleukin 1 secretion and of tumoricidal activity in macrophages are not closely related phenomena. Eur J Immunol. 1987;17:1665–1668. doi: 10.1002/eji.1830171124. [DOI] [PubMed] [Google Scholar]
- 24.Kisielow P, Blüthmann H, Staerz U, Stenmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 25.Le Gros G, Erard F. Non-cytotoxic, IL-4, IL-5, IL-10 producing CD8+ T cells: their activation and effector functions. Curr Opin Immunol. 1994;6:453–457. doi: 10.1016/0952-7915(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 26.Leirisalo R M, Skylv G, Kousa M. Follow-up study of Reiter’s disease and reactive arthritis. Factors influencing the natural course and the prognosis. Clin Rheumatol. 1987;2:73–82. doi: 10.1007/BF02203388. [DOI] [PubMed] [Google Scholar]
- 27.Leong J M, Fournier R S, Isberg R R. Identification of the integrin binding domain of the Yersinia pseudotuberculosis invasin protein. EMBO J. 1990;9:1979–1989. doi: 10.1002/j.1460-2075.1990.tb08326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livingstone A M, Powis S J, Diamond A G, Butcher G W, Howard J C. A trans-acting major histocompatibility complex-linked gene whose alleles determine gain and loss changes in the antigenic structure of a classical class I molecule. J Exp Med. 1989;170:777–795. doi: 10.1084/jem.170.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mertz A K H, Ugrinovic S, Lauster R, Wu P, Grolms M, Böttcher U, Appel H, Yin Z, Schiltz E, Batsford S, Schauer-Petrowski C, Braun J, Distler A, Sieper J. Characterization of the synovial T cell response to various recombinant Yersinia antigens in Yersinia enterocolitica-triggered reactive arthritis. Arthritis Rheum. 1998;41:315–326. doi: 10.1002/1529-0131(199802)41:2<315::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Milligan G N, Morrison L A, Gorka J, Braciale V L, Braciale T J. The recognition of a viral antigenic moety by class I MHC-restricted cytolytic T lymphocytes is mediated by the availability of the endogenously processed antigen. J Immunol. 1990;145:3188–3193. [PubMed] [Google Scholar]
- 31.Nickerson C L, Luthra H S, Savarirayan S, David C S. Susceptibility of HLA-B27 transgenic mice to Yersinia enterocolitica infection. Hum Immunol. 1990;28:382–396. doi: 10.1016/0198-8859(90)90033-l. [DOI] [PubMed] [Google Scholar]
- 32.Parker K C, Bednarek M A, Coligan J E. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chain. J Immunol. 1994;152:163–175. [PubMed] [Google Scholar]
- 33.Parker K C, Biddison W E, Coligan J E. Pocket mutations of HLA-B27 show that anchor residues act cumulatively to stabilize peptide binding. Biochemistry. 1994;33:7736–7743. doi: 10.1021/bi00190a029. [DOI] [PubMed] [Google Scholar]
- 34.Pazmany L, Rowland-Jones S, Huet S, Hill A, Sutton J, Murray R, Brooks J, McMichael A. Genetic modulation of antigen presentation by HLA-B27 molecules. J Exp Med. 1992;175:361–369. doi: 10.1084/jem.175.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Probst P, Hermann E, Fleischer B. Role of bacteria-specific T cells in the immunopathogenesis of reactive arthritis. Trends Microbiol. 1994;2:329–332. doi: 10.1016/0966-842x(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 36.Rammensee H G, Friede T, Stevanoviic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 37.Rath H C, Herfarth H H, Ikeda J S, Grenther W B, Hamm Jr T E, Taurog J D, Hammer R E, Wilson K H, Sartor R B. Normal luminal bacteria, especially Bacteroides species, mediates chronic colitis, gastritis, and arthritis in HLA-B27/human β2 microglobulin transgenic rats. J Clin Investig. 1996;98:945–953. doi: 10.1172/JCI118878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rebaï N, Malissen B. Structural and genetic analyses of HLA class I molecules using monoclonal xenoantibodies. Tissue Antigens. 1983;22:107–117. doi: 10.1111/j.1399-0039.1983.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 39.Rock K L. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 40.Sieper J, Braun J. Pathogenesis of spondylarthropathies. Persistent bacterial antigens, autoimmunity, or both? Arthritis Rheum. 1995;38:1547–1554. doi: 10.1002/art.1780381105. [DOI] [PubMed] [Google Scholar]
- 41.Simmons W A, Summerfield S G, Roopenian D C, Slaughter C A, Zuberi A R, Gaskell S J, Bordoli R S, Hoyes J, Moomaw C R, Colbert R A, Leong L Y-W, Butcher G W, Hammer R E, Taurog J D. Novel HY peptide antigen presented by HLA-B27. J Immunol. 1997;159:2750–2759. [PubMed] [Google Scholar]
- 42.Simmons W A, Taurog J D, Hammer R E, Breban M. Sharing of an HLA-B27-restricted H-Y antigen between rat and mouse. Immunogenetics. 1993;38:351–358. doi: 10.1007/BF00210477. [DOI] [PubMed] [Google Scholar]
- 43.Simonet M, Falkow S. Invasin expression in Yersinia pseudotuberculosis. Infect Immun. 1992;60:4414–4417. doi: 10.1128/iai.60.10.4414-4417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun. 1990;58:841–845. doi: 10.1128/iai.58.3.841-845.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starnbach M N, Bevan M J, Lampe M F. Protective cytotoxic T lymphocytes are induced during murine infection with Chlamydia trachomatis. J Immunol. 1994;153:5183–5189. [PubMed] [Google Scholar]
- 46.Straley S C, Skrzypek E, Plano G V, Bliska J B. Yops of Yersinia spp. pathogenic for humans. Infect Immun. 1993;61:3105–3110. doi: 10.1128/iai.61.8.3105-3110.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taurog J D, Maika S D, Simmons W A, Breban M, Hammer R E. Susceptibility of inflammatory disease in HLA-B27 transgenic rat lines correlates with the level of expression of B27. J Immunol. 1993;150:4168–4178. [PubMed] [Google Scholar]
- 48.Taurog J D, Richardson J A, Croft J T, Simmons W A, Zhou M, Fernandez-Sueiro J L, Balish E, Hammer R E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thurau S R, Wildner G, Kuon W, Weiss E H, Riethmuller G. Expression and immunogenicity of HLA-B27 in high-transfection recipient P815: a method to induce monoclonal antibodies directed against HLA-B27. Tissue Antigens. 1989;33:511–519. doi: 10.1111/j.1399-0039.1989.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 50.Toivanen A, Granfors K, Lahesmaa-Rantala R, Leino R, Stahlberg T, Vuento R. Pathogenesis of Yersinia-triggered reactive arthritis: immunological, microbiological and clinical aspects. Immunol Rev. 1985;86:47–70. doi: 10.1111/j.1600-065x.1985.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 51.Tussey L G, Rowland-Jones S, Zheng T, Androlewicz M J, Cresswell P, Frelinger J A, McMichael A J. Different MHC class I alleles compete for presentation of overlapping viral epitopes. Immunity. 1995;3:65–77. doi: 10.1016/1074-7613(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 52.Ugrinovic S, Mertz A, Wu P, Braun J, Sieper J. A single nonamer from the Yersinia 60-kDa heat shock protein is the target of HLA-B27-restricted CTL response in Yersinia-induced reactive arthritis. J Immunol. 1997;159:5715–5723. [PubMed] [Google Scholar]
- 53.Urban R G, Chicz R M, Lane W S, Strominger J L, Rehm A, Kenter M J H, UytdeHaag F G C M, Ploegh H, Uchanska-Ziegler B, Ziegler A. A subset of HLA-B27 molecules contains peptides much longer than nonamers. Proc Natl Acad Sci USA. 1994;91:1534–1538. doi: 10.1073/pnas.91.4.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villanueva M S, Fischer P, Feen K, Pamer E G. Efficiency of MHC class I antigen processing: a quantitative analysis. Immunity. 1994;1:479–489. doi: 10.1016/1074-7613(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 55.Viner N J, Bailey L C, Life P F, Bacon P A, Gaston J S H. Isolation of Yersinia-specific T cell clones from the synovial membrane and synovial fluid of a patient with reactive arthritis. Arthritis Rheum. 1991;34:1151–1157. doi: 10.1002/art.1780340911. [DOI] [PubMed] [Google Scholar]
- 56.Viola A, Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. doi: 10.1126/science.273.5271.104. [DOI] [PubMed] [Google Scholar]