Abstract
Introduction
We aimed to analyze the testicular histopathology of men who died with active COVID-19 infection.
Methods
We performed autopsy of eight consecutive men who died of COVID-19 pneumonia. Lung and testis tissue of all men were stained for SARS-CoV-2 nucleocapsid, angiotensin-converting enzyme 2 (ACE-2) receptor immunohistochemistry (IHC). H&E was performed to assess for spermatogenesis and evidence of testicle tissue damage. Reverse transcriptase polymerase chain reaction (RT-PCR) analysis for SARS-CoV-2 was performed on matched lung and bilateral testicular tissue samples from all men.
Results
Patient age ranged from 50–79 years. SARS-CoV-2 viral RNA was detected by RTPCR in testis tissue in one man. All eight testicle specimens that underwent IHC for ACE2 receptor showed uniformly strong immunoreactivity against all testicle cell populations. By H&E, all testis specimens showed no inflammation, vascular thrombosis, vasculitis, or morphological evidence of viral changes. One case showed diminished but not absent spermatogenesis, consistent with patient age.
Conclusions
Our results suggest that SARS-CoV-2 is unlikely to affect male fertility. Contrary to all prior histological studies, our results showed no evidence of damage to reproductive tissues that might impair fertility.
Introduction
Since the novel coronavirus, SARS-CoV-2, and its associated illness, COVID-19, began its worldwide spread in approximately December 2019, the scientific community has made great strides over a short period of time to better understand the virus pathogenesis.1 SARS-CoV-2 is acquired by hosts via respiratory droplets and enters cells via the angiotensin-converting enzyme 2 (ACE-2) receptor while using the transmembrane serine protease 2 (TMPRSS2) for viral S protein priming.2,3 ACE-2 is expressed within human host target cells, particularly alveolar epithelial type II cells in the lung, contributing to the life-threating pneumonia of COVID-19, and within the gastrointestinal tract, myocardium, genitourinary tract, and testes and epididymis.4–6
Epidemiological analyses of large COVID-19 cohorts have shown that approximately 60% of patients requiring hospitalization are male.7–10 While the precise mechanism of the predilection of COVID-19 for men remains unknown, anatomic, cell-surface, and/or sex hormone differences between genetic males and females must be considered as possible mechanisms. Among human tissues, testes have the second highest density of ACE-2 receptor expression, trailing only the small intestine.11 ACE-2 is expressed predominantly in Leydig cells — the testosterone-producing cells — but is also expressed in Sertoli cells and spermatogonia.6,12 High ACE-2 concentration in the testes represents a potential target for SARS-CoV-2 infection.
Prior studies using histological techniques to investigate the effects of SARS-CoV-2 in the testicle have all reported histological evidence of tissue injury and concluded that severe COVID-19 infection results in testicle tissue injury and may impair future fertility. While it is still too early to understand the long-term effects of SARS-CoV-2 infection of the testes, the potential implications are significant: 1) altered infection severity should the testes serve as a potential virus reservoir; 2) virus transmission, should virus be present in semen; 3) adverse effects on male fertility, should testicular infection cause alterations in spermatogenesis; and 4) teratogenicity, should spermatocytes themselves suffer alteration and/or harbor infection transmissible to a healthy ooyctye.
In the present case series, we assessed postmortem autopsy testicle and lung tissue samples using reverse transcription polymerase chain reaction (RT-PCR) to determine whether there is evidence of SARS-CoV-2 viral infection within testicle tissue and lung tissue to confirm presence of infection. We used histological analysis to assess for testicle tissue damage.
Methods
Patient selection and demographic data collection
This study was reviewed and approved by the Cedars-Sinai Institutional Review Board #00000739. Male autopsy patients known to be infected with COVID-19 (by nasal swab SARS-CoV-2 RT-PCR performed upon admission to our hospital), had subsequently succumbed to their disease, and for whom written approval for autopsy (that included testicle tissue) was obtained, were enrolled.
A total of eight consecutive patients who met these criteria underwent autopsy, followed by RT-PCR and immunohistochemistry (IHC) analysis of their testicle and lung tissues between May and September 2020. All testicle tissue was harvested in an open fashion from the testicle (not via a percutaneous biopsy). Care was taken to process all human tissues per our institution’s standardized autopsy tissue handling protocol, which includes immediate refrigeration of the deceased prior to autopsy and expeditious harvest of tissues. Patient demographic data was collected, including age, race/ethnicity, and the mean time interval (days) between initial diagnosis of COVID-19 infection and death.
SARS-CoV-2 RT-PCR of lung and testicle tissues
RNA was extracted from paired lung and bilateral testicular tissue blocks collected from all patients. Testicular tissue slides were macrodissected to remove non-seminiferous tissue (tunica, large blood vessels, etc.). Unstained formalin and paraffin embedded (FFPE) slides were processed using the AllPrep DNA/RNA FFPE Kit on the QIAcube Connect (Qiagen, Germantown, U.S.). Nucleic acid was screened for the presence of SARS-CoV-2 using real-time duplex RT-PCR for the SARS-CoV-2 nsp3 gene and an internal control (Accelerate Technologies, Singapore). Lung and testicle tissue samples were run in replicate (5x) and COVID-19 positivity was defined as amplification of the targeted region crossing the threshold in any of the replicates. Cycle threshold (CT) values were calculated in positive samples and averaged if positive in more than one replicate.
SARS-COV2 & ACE-2 immunohistochemistry
Human lung and testis tissue samples were fixed in 10% neutral buffered FFPE. Samples were sectioned 4 um on Superfrost Plus slides (12-550-15, Fisher Scientific). IHC staining was performed on an automated Ventana Discovery Ultra Instrument (Roche).
After antigen retrieval CC1 (Tris, pH8.0) (950-124, Roche Ventana), two primary antibodies were manually applied. All antibodies were diluted with Antibody Dilution Buffer (ADB250, Roche Ventana).
Rabbit anti-SARS-CoV-2 nucleocapsid protein (ab273167, abcam), 1:3000 1 hour at 37°C
Rabbit anti-ACE2 (aa140-172) (LS-B6672, LSBio) 1:800 32 minutes at 37°C
These steps were carried out using the detection system with DISC anti-Rabbit HQ (760-4815, Roche Ventana) for 12 minutes and DISC anti-HQ HRP (760-4820, Roche Ventana) for 12 minutes. Finally, DISC ChromoMap DAB Kit were applied (760-159, Roche Ventana).
Histological assessment by H&E staining
Representative sections of lung and testicle tissue harvested at autopsy were fixed in FFPE, stained with hematoxylin and eosin (H&E), and subsequently analyzed by light microscopy. Testicle tissue seminiferous tubules were analyzed for: 1) degree of spermatogenesis; 2) presence of interstitial Leydig cells; 3) presence of Sertoli cells; 4) percentage of tubules showing fibrosis; and 5) percentage of tubules showing complete fibrosis and atrophy. Testicle tissue sections were also surveyed for a wide range of potential histological abnormalities, including inflammation, granulomata, vasculitis, vascular thrombi, and infarction.
Results
Demographic data
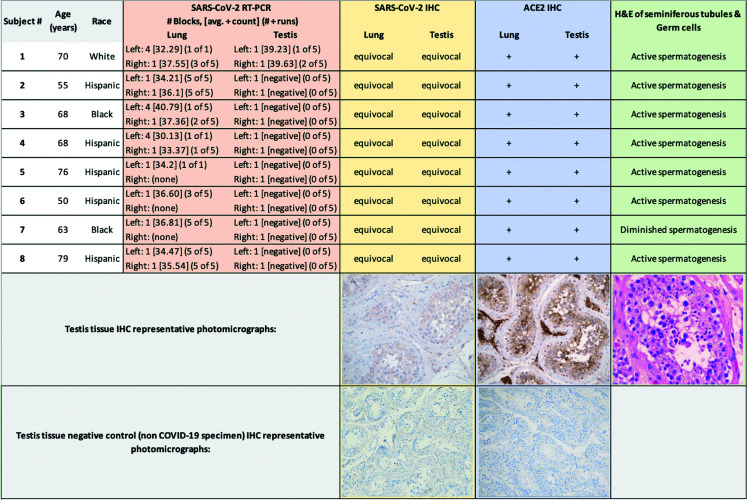
The average age of the eight male subjects in the cohort was 66 years (±9.8 years). Five men were Hispanic, two were black, and one was white (Figure 1). The average time from first positive COVID-19 test to death was 20.5 days (±9.5 days).
Figure 1.
Demographic data and representative photomicrographs of testis immunohistochemistry stains.
SARS-CoV-2 RT-PCR
SARS-CoV-2 viral RNA was detected in pulmonary tissue in all patients and testicular tissue from one of eight COVID-19+ subjects. All RT-PCR trials were repeated five times. Pulmonary tissue positivity ranged from 26.9–40.8 CT. In the one patient whose testicular tissue was positive by RT-PCR, both the left and right testicle had detectable viral RNA, and at an average of 39.6 and 39.2 CT, respectively. This subject’s RT-PCR findings were confirmed with three repeat trials. Also of note, this same patient also had the highest CT values (37.55 CT in three of five runs) in his lung tissues (Figure 1).
SARS-COV-2 & ACE-2 IHC
SARS-CoV-2
Seven of eight lung and testicle tissue samples were studied by IHC using antibody against SARS-CoV-2 nucleocapsid. No testicular tissue-positive control was available from the antibody manufacturer (Abcam PLC, Cambridge, U.K.) for the Rabbit anti-SARS-CoV-2 nucleocapsid protein. Negative controls were stained and demonstrated uniform mild staining.
All COVID-19+ cases lung and testicular tissue cell populations (including Sertoli cells, Leydig cells, and germinal epithelium) showed little to no additional immunoreactivity, as compared to negative controls (Figures 1, 2). Because lung and testicle tissue immunoreactivity was equivocal to the negative control specimens, these results were adjudicated by our genitourinary pathologists (DL and WT) as indicating either absence of SARS-CoV-2 viral protein or that its presence was lower than the level of detection for IHC.
Figure 2.
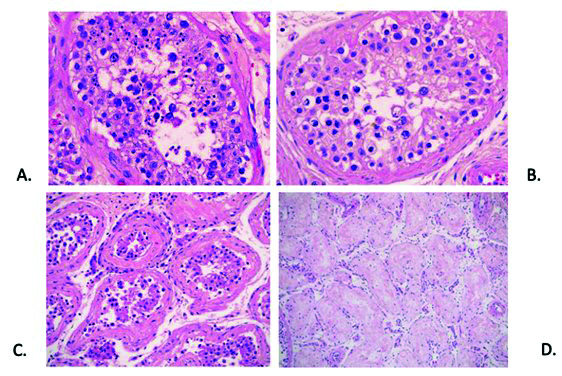
Representative photomicrographs demonstrating histological features of testicular tissue (H&E stain): (A) Seminiferous tubules showing normal levels of spermatogenesis with intraluminal spermatids (400 × magnification); (B) Seminiferous tubules showing diminished levels of spermatogenesis, with no intraluminal spermatids (400 × magnification); (C) Seminiferous tubules with thickened and fibrotic basement membranes (200× magnification); (D) Seminiferous tubules with complete fibrosis and atrophy, with no Sertoli cells or germinal epithelium (100× magnification).
ACE-2
All subjects’ lung and testicle tissues studied by IHC using antibodies for ACE-2 receptor showed uniformly strong immunoreactivity among both lung and testicle tissue cell populations (including Sertoli cells [2+], Leydig cells [2+], and germinal epithelium [2+] (Figures 1, 3). No positive controls were run for the Rabbit anti-ACE2; however, the tissues tested had very strong internal control cell positivity, therefore, can be used for study analysis.
Figure 3.
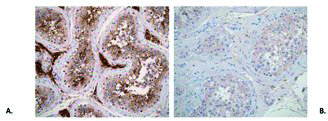
Representative photomicrographs demonstrating: (A) Immunohistochemical reaction of antibodies against ACE-2 receptor in testicular tissue. Note strong reactivity in Leydig cells, Sertoli cells, and germinal epithelium (200 × magnification); (B) Immunohistochemical reaction of SARS-CoV-2 nucleocapsid protein in testicular tissue. Note weak reaction in Leydig cells, Sertoli cells, and germinal epithelium (200 × magnification).
Testicle tissue H&E
All testicles were grossly unremarkable and of normal size. In all eight cases, testicular tissue was analyzed by light microscopy to reveal no active inflammation, vascular thrombosis, vasculitis, or morphological evidence of viral changes. Seven of eight cases showed seminiferous tubules with normal degrees of spermatogenesis; one case showed diminished levels of spermatogenesis. Most cases (seven of eight) showed varying degrees of tubular fibrosis and atrophy, similar to what one would expect to see in normal testicular tissue (Figures 1, 4). Interstitial Leydig cells were present in appropriate numbers in all cases.
Figure 4.
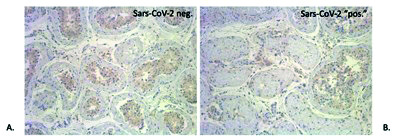
Representative photomicrographs demonstrating immunohistochemical reaction using antibody against SARS-CoV-2 (nucleocapsid protein) in testicular tissue from (A) a patient with polymerase chain reaction (PCR)-negative results from testicular tissue and (B) from a patient with PCR-positive results from the testicular tissue. Note that no significant increase in staining is identified and only non-specific background staining is observed in either PCR-negative or positive samples (100 × magnification).
Discussion
In the present case series of eight men who succumbed to complications related to COVID-19 infection, we found RT-PCR positivity for SAR-CoV-2 in one man and completely normal for age testicular histology in all eight men. These results differ from all previous work investigating COVID-19 and its effect on testicular histology.
Although histological analysis by IHC for SARS-CoV-2 in all subjects yielded visible immunoreactive staining, we were compelled to interpret them as “equivocal” because the IHC results of the one testicle tissue RT-PCR-positive case were not significantly different from the other seven subjects whose testicle tissue was RT-PCR-negative for SARS-CoV-2, and, from COVID-negative (control) subjects; this was true of lung tissue as well. We reviewed the appearance of our SARS-CoV-2 IHC results with those published from prior studies and our results appeared to be similar.13–15 It should be noted that prior studies using SARS-CoV-2 either did not also perform RT-PCR, or when they did, they did not address how some specimens could be RT-PCR-negative but be “positive” by SARS-CoV-2 IHC.14–16 To date, there is no well-established positive control for SARS-CoV-2 IHC, and we did not feel that our IHC results were conclusive, despite repeated attempts to troubleshoot our IHC results.
Prior work assessing effects of COVID-19 on testicular infection cited evidence of damage to testicular tissues and spermatogenesis that would presumably impair future fertility; 13,15,16 however, histopathological review of all our testicle tissue specimens to assess for alterations in spermatogenesis found all our COVID-19 subjects to have normal, age-specific spermatogenesis and tissue architecture. Our work found normal (for age) testis tissue architecture and preserved spermatogenesis in our entire cohort of men with SARS-CoV-2 RT-PCR-positive lung tissue who died of COVID-19.
Prior studies have reported testicular SARS-CoV-2 infection as well. In June 2020, Bian et al published their autopsy findings from 91 COVID-19 cases. They reported that among other tissues, SARS-CoV-2 is detectable in testis tissue via RT-PCR, IHC, and transmission electron microscopy (TEM).13 The authors commented that their autopsy specimens showed various degrees of spermatogenic cell reduction and injury.13 They do not report the age of the subjects with abnormal spermatogenesis. It should also be noted that all tissue was obtained for analysis from a subset of cases (54/91), and then only via percutaneous biopsy, which introduces the potential tissue contamination with viral RNA from non-testis tissue that could be harboring the virus.
In September 2020, Yang et al reported their autopsy pathology findings from 12 COVID-19 positive men.15 Among their cohort, testis tissue was SARS-CoV-2 RT-PCR-positive in one of 12 men. Overall, 10 of 12 men were RT-PCR-positive in lung tissue, and none of the men had detectable SARS-CoV-2 in testis tissue TEM, including the subject who was SARS-CoV-2 RT-PCR-positive in testis tissue. The authors reported that H&E staining showed significant injury to Sertoli cells and seminiferous tubules, reduced number of Leydig cells, and mild inflammatory infiltrates in the interstitium in eight of 12 COVID-19-positive subjects.15 Similarly, in February 2021, Ma et al published their findings from a pathological analysis of testis tissue from five men who died of COVID-19.17 Two of five men were RT-PCR-positive in testis tissue for SARS-CoV-2 and four of five men demonstrated profound germ cell loss, which the authors assert may have deleterious effects on fertility potential.17
Achua et al detected presence of SARS-CoV-2 viral particles via TEM in the testicular tissue of one of four men who died of COVID-19, as well as in the biopsy of a live patient who had recently seroconverted.16 The authors found varying degrees of abnormal spermatogenesis in three of their six COVID-19 subjects and all three of their non-COVID controls, all of which may have been attributed to normal aging.16 They also stained all six of their subjects’ testis tissue for ACE-2 in an effort to bridge the connection between ACE-2 expression and tissue infection. They found density of ACE-2 expression to be inversely related to level of spermatogenesis, asserting that COVID-19 patients with increased testicular ACE-2 expression are at greater risk for adverse fertility outcomes of infection.16
Additionally, Li et al studied the semen of men affected by COVID-19 at varying stages of infection.14 They found that six of 38 men with COVID-19 had RT-PCR evidence of SARS-CoV-2 in their semen; two of the six men with virus in their semen had recovered from infection, while the remaining four were acutely infected. Conversely, Pan et al found no RT-PCR evidence of SARS-CoV-2 in the semen of six men who had recovered from COVID-19 infection and had reported symptoms of orchitis during their infection.18 Presence of SARS-CoV-2 virus in semen raises the possibility of sexual transmission of COVID-19 as well as teratogenicity should infected sperm fertilize an egg; however, there is little to no clinical evidence to support either claim.
Strengths of our study include our strict methods. Our autopsy tissue-handling techniques guard against IHC findings confounded by tissue decomposition. Limitations of our work include our small sample size and our inability to assess severity and duration of illness other than time from first diagnosis to death. Our RT-PCR results should also be interpreted with caution, as our CT of 39.6 would fall into the indeterminate range by some laboratory standards but was found to be durable on repeat testing and significantly different from the rest of the study cohort.
Conclusions
Contrary to all prior work related to SARS-CoV-2 in the testes, the present work yielded no evidence of testicle tissue injury or abnormal spermatogenesis. Our study was limited to male subjects deceased from COVID-19. Whether or not testicular infection in men who recover from COVID-19 correlates with worse disease outcomes, sexual virus transmission, worse fertility outcomes, or teratogenicity requires further research.
Footnotes
Competing interests: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. JAMA. 2020;2019:1–13. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Roujian, Zhao Xiang, Li Juan, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–74. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–7. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185–92. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verma S, Saksena S, Hooman SA. ACE2 receptor expression in testes: Implications in COVID-19 pathogenesis. Biol Reprod. 2020;103:449–451. doi: 10.1093/biolre/ioaa080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO clinical characterization protocol: Prospective, observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Meng-Yuan, Li Lin, Yue Zhang X-SW. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z, Xu X. scRNA-seq Profiling of Human Testes Reveals the Presence of the ACE2 Receptor, a target for SARS-CoV-2 infection in spermatogonia, Leydig and Sertoli cells. Cells. 2020;9:920. doi: 10.3390/cells9040920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bian XW, Yao XH, Ping YF, et al. Autopsy of COVID-19 patients in China. Natl Sci Rev. 2020;7:1414–8. doi: 10.1093/nsr/nwaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Jin M, Bao P, et al. Clinical characteristics and results of semen tests among men with coronavirus disease 2019. JAMA Netw Open. 2020;3:e208292. doi: 10.1001/jamanetworkopen.2020.8292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang M, Chen S, Huang B, et al. Pathological findings in the testes of COVID-19 patients: Clinical implications. Eur Urol Focus. 2020;6:1124–9. doi: 10.1016/j.euf.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achua JK, Chu KY, Ibrahim E, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections on testis. World J Mens Health. 2021;39:65–74. doi: 10.5534/wjmh.200170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma X, Guan C, Chen R, et al. Pathological and molecular examinations of postmortem testis biopsies reveal SARS-CoV-2 infection in the testis and spermatogenesis damage in COVID-19 patients. Cell Mol Immunol. 2021;18:487–9. doi: 10.21203/rs.3.rs-56526/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan F, Xiao X, Guo J, et al. No evidence of severe acute respiratory syndrome-coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020;113:1135–9. doi: 10.1016/j.fertnstert.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]