Abstract
Introduction
Unresectable and metastatic small cell carcinoma of the prostate (SCPC) is a rare and aggressive disease that is under-represented in clinical trials. We carried out a retrospective chart review of metastatic or unresectable SCPC patients at British Columbia (BC) Cancer centers, studying diagnosis and treatment patterns.
Methods
Drug-dispensing records from the six BC Cancer centers were obtained from 2002–2017. For each patient, information was collected on baseline information prior to therapy and for each line of treatment. Treatments at each line were compared regarding time to progression and overall survival by Kaplan-Meier curves.
Results
Forty-one patients received treatment; 65.6% had metastatic disease and 61% had pure small cell carcinoma. Median time from treatment to death was 10 months (95% confidence interval [CI] 6–16). Patients with initially prostate-confined disease had a better median overall survival (mOS) of 21 months (95% CI 13–34) compared to those with initially locally advanced (mOS 19 months, 95% CI 5–37) and metastatic disease (mOS 8 months, 95% CI 6–10) (log-rank p=0.0364). All patients received either cisplatin- or carboplatin-based combination chemotherapy as the first-line treatment and 36.7% received second-line therapy. Time to second-line therapy was eight months for those who presented with metastatic SCPC, compared to 13 months for those with initial non-metastatic SCPC.
Conclusions
This single-province, multi-institution cohort reports data on unresectable and metastatic SCPC and highlights the poor prognosis of this rare disease entity.
Introduction
Small cell prostate cancer (SCPC) is not only clinically, biologically, and histologically different from prostatic adenocarcinoma, but a more aggressive variant as well. Surveillance, Epidemiology, and End Results (SEER) data suggests approximately 60% of men present with metastases, with a median survival of 18 months from diagnosis. 1 Metastatic small cell prostate cancer (mSCPC) typically emerges from patients with high-grade metastatic adenocarcinoma that have been treated with androgen deprivation therapy. This transformation to SCPC occurs approximately 18–25 months from time of diagnosis of prostatic adenocarcinoma, though this can be extremely variable.2,3 When this transformation occurs, these cells are no longer responsive to hormonal manipulations to control the disease.
Metastatic SCPC is a rare entity, occurring in only 0.5–2% of men with prostate cancer,4,5 which has precluded many prospective trials. Data has historically been gathered from case series or single-arm clinical trials, among which enrollment criteria have substantially differed, treatment regimens and sequences have not been standardized, and histological diagnoses have not been necessarily required to make a firm diagnosis. Prognosis and natural history of this disease have been similarly scant in literature.6 A more in-depth analysis of “real-world” practice patterns from a Canadian perspective would be useful, given the paucity of guidelines on diagnosis and management of this rare entity. We therefore carried out a retrospective chart review of metastatic or unresectable SCPC patients at British Columbia (BC) Cancer centers, studying practice patterns around diagnosis and management.
Methods
We initially identified patients with SCPC by reviewing drug-dispensing records for patients treated at the six BC Cancer centers with the following protocols available on the BC Cancer website: palliative therapy of extensive-stage genitourinary small cell tumors with a platinum and etoposide (GUSCPE) (created in August 2002); and therapy of genitourinary small cell tumors with a platinum and etoposide with radiation (GUSCPERT) (created in September 2002). All consecutive patients who received these regimens between January 1, 2002, and December 31, 2017, and treated at the regional BC Cancer centers were included. Patients who received these regimens outside of the BC Cancer centers (i.e., community oncology network clinics) were also included if they received them between January 1, 2013, and December 31, 2017. This inclusion criteria assumed that all patients who had nonresectable, locally advanced or metastatic SCPC and were eligible for treatment would have received systemic therapy under the two protocols. We then reviewed each case individually to identify and only include those who had histology-proven small cell carcinoma originating from prostate. The study was reviewed and approved by the BC Cancer Research Ethics Board (H18-00043).
Baseline demographic and laboratory information prior to therapy initiation for each patient was collected from electronic charts. The site of metastatic burden, if applicable, was documented. All information regarding first-line mSCPC treatment was recorded; the same was done for subsequent lines of treatment. The time interval between lines of therapy was recorded, and inclusion criteria included all patients who had completed and had progressed on at least one line of therapy in the unresectable or metastatic setting for SCPC at one of the six BCCA centers. Exclusion criteria included patients not treated at a BCCA centre, with nonmetastatic disease, or disease not treated as SCPC (on clinical or pathological grounds).
Pathology reports for each patient were used to determine the proportion of patients that had their diagnosis made on pathological grounds vs. clinical features alone. The percentage of patients diagnosed with de novo vs. treatment-emergent (i.e., transdifferentiated from prostatic adenocarcinoma) disease was established. Where applicable, the disease setting at time of transformation was recorded.
All treatments were documented, including radiation to prostate/pelvis, prophylactic cranial irradiation, and systemic therapy. Information regarding the first-line and later-line therapy was documented. Treatments at each line were compared regarding time to progression and overall survival (OS) by Kaplan Meier curves. All statistical analyses were performed on SAS v.9.
Results
In total, we identified 41 patients who received treatment for SCPC who met eligibility. The baseline characteristics are described in Table 1. Most patients had metastatic (65.9%) or locally advanced (26.8%) cancers; 61% had pure small cell carcinoma; the rest had mixed histology. A total of 24 patients had Gleason scores associated with their histology (58.5%). Many patients presented with bone or lymph node metastases, followed by liver and lung. Median prostate-specific antigen (PSA) at the time of diagnosis was 2.3 (interquartile range 0.27, 11.7), relatively low compared with typical patients with an initial diagnosis of metastatic castrate-sensitive prostate cancer (mCSPC). Median Gleason score, if the cancer had adenocarcinomatous pathology, was 9 (range 6–10). Twenty patients (48.7%) had de novo disease, while 21 patients (51.2%) had treatment-emergent disease.
Table 1.
Baseline characteristics at time of diagnosis of unresectable or metastatic small cell prostate carcinoma (u/mSCPC)
| n=41 | Value, n (%) |
|---|---|
| Age (median, interquartile range) | 68 (62–72) |
| ECOG | |
| 0 | 6 (18.8) |
| 1 | 11 (34.4) |
| 2 | 9 (28.1) |
| 3 | 6 (18.8) |
| Unknown | 9 (28.1) |
| Stage of disease | |
| Prostate-confined | 3 (7.3) |
| Locally advanced | 11 (26.8) |
| Metastatic | 27 (65.9) |
| Diagnosis of pure small cell carcinoma | 25 (61) |
| Diagnosis by pathology | 37 (90.2) |
| Burden of disease at time of u/mSCPC diagnosis | |
| Bone | 19 (46.3) |
| Visceral | 12 (29.3) |
| Liver | 7 (17.1) |
| Lymph nodes | 20 (48.8) |
| Lung | 5 (12.2) |
| Labs at time of u/mSCPC diagnosis | |
| PSA (median, interquartile range), ug/L | 2.3 (0.27,11.7) |
| Hb (median, interquartile range), g/L | 121 (112.5, 132) |
| LDH (median, interquartile range), U/L | 323.5 (206, 562.5) |
| Albumin (median, interquartile range), g/L | 37 (32, 40) |
| ALP (median, interquartile range), U/L | 87.5 (71, 285) |
| Median Gleason score where applicable (i.e., of adenocarcinomatous portion of disease) | 9 (6,10) |
| De novo vs. transformation | |
| De novo diagnosis of SCPC | 20 (48.7%) |
| Transformation from adenocarcinoma to SCPC | 21 (51.2%) |
| Recurrence after treatment of prostate-confined disease | 4 (9.8%) |
| mCSPC | 5 (12.1%) |
| nmCRPC | 3 (7.3%) |
| mCRPC | 9 (22.0%) |
ALP: alkaline phosphatase; ECOG: Eastern Cooperative Oncology Group; Hb: hemoglobin; LDH: lactate dehydrogenase; mCSPC: metastatic castrate-sensitive prostate cancer; mCRPC: metastatic castrate-resistant prostate cancer; nmCRPC: nonmetastatic castrate-resistant prostate cancer; PSA: prostate-specific antigen.
In terms of survival outcomes, median time from treatment of mSCPC to death was 10 months (95% confidence interval [CI] 6–16 months). Patients with initially prostate-confined disease had the best median overall survival (mOS) of 21 months (95% CI 13–34 months), whereas those with initially locally advanced disease (mOS 19 months, 95% CI 5–37 months) and metastatic disease (mOS 8 months, 95% CI 6–10 months) had significantly decreased mOS (log-rank p=0.0364) (Figure 1). Patients with de novo mSCPC had OS of 16.5 months (95% CI 9–28 months), while those with treatment-emergent mSCPC had 6 months (95% CI 5–10 months) (log-rank p=0.0223).
Figure 1.
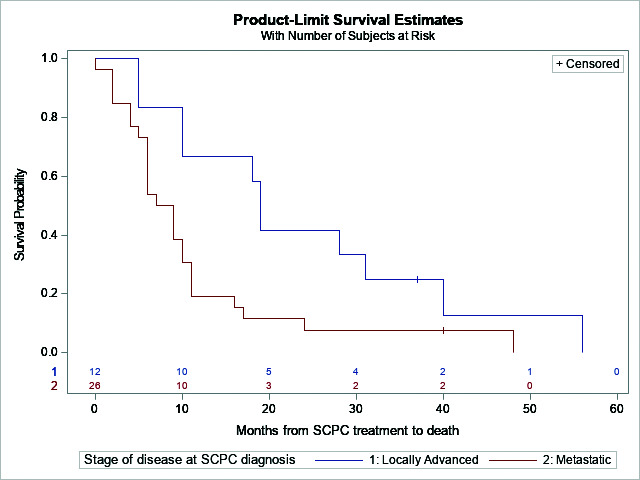
Overall survival probability of small cell prostate cancer (SCPC) by stage of disease.
Table 2 illustrates treatments administered for SCPC. All patients received either cisplatin-or carboplatin-based combination chemotherapy as the first-line treatment; 34.1% received either concurrent or sequential local radiation therapy to prostate and 36.7% received second-line systemic therapy. Few patients received third-line systemic therapy or beyond. Median number of treatment lines was 1 (range 1–7). Only one patient had prophylactic cranial radiation therapy. Table 3 illustrates time to subsequent systemic therapies after the first-line treatment for SCPC. Patients with metastatic SCPC had eight months before the patient progressed to the second-line treatment, while patients with initially non-metastatic SCPC had 13 months before the second-line treatment.
Table 2.
Treatments employed for SCPC
| n=41 | Value, n (%) |
|---|---|
| Radiation to prostate | 14 (34.1) 8 concurrent, 6 post-chemotherapy consolidation |
| Prophylactic cranial irradiation (n=41) | 1 (2.4) |
| Agent used in first-line | |
| Platinum-based treatment | 100% |
| Percentage of patients embarking on second-line therapy (n=41) | 15 (36.7) |
| Agent used in second-line (c=15) | |
| Platinum re-challenge | 7 (46.7) |
| Irinotecan | 2 (13.3) |
| Topotecan | 1 (6.7) |
| CDV | 3 (20) |
| Etoposide | 2 (13.3) |
| Percentage of patients embarking on third-line therapy (n=41) | 8 (19.5) |
| Agent used in third-line (n=8) | |
| Platinum re-challenge | 3 (37.5) |
| Irinotecan | 1 (12.5) |
| Topotecan | 1 (12.5) |
| CDV | 0 (0) |
| Etoposide | 3 (37.5) |
| Percentage of patients embarking on >third-line therapy (n=41) | 3 (7.3) |
| Median number of small cell prostate cancer treatment lines, n (range) | 1 (1, 7) |
CDV: cyclophosphamide, vincristine, and dexamethasone. SCPC: small cell prostate carcinoma.
Table 3.
Time to subsequent therapy after first-line treatment for SCPC
| Value, n (%) | |
|---|---|
| Time to second-line of treatment (months) | All: 12 Nonmetastatic: 13 Metastatic: 8 |
| Time to third-line of treatment (months) | 7 |
| Time to fourth-line of treatment (months) | 1 patient: 7 months |
SCPC: small cell prostate carcinoma.
Discussion
To our knowledge, this study is the first to report on the clinical characteristics and outcomes of patients diagnosed with SCPC in BC, Canada. Although presumed to be rare,7,8 one study shows that treatment-emergent SCPC is present in nearly one-fifth of patients with metastatic castrate-resistent prostate cancer (mCRPC).9 Genomic analysis further shows that the transformation from adenocarcinoma to SCPC is likely one of the major mechanisms for resistance to hormonal manipulations9 and may be a distinct subset from other types of mCRPC that develop DNA repair mutations. 10,11 Once diagnosed, patients with SCPC have invariably poor survival and it is associated with liver and other soft tissue metastases, also shown in our cohort.9,10 A low PSA with even a relatively heavy burden of disease, and with new liver or visceral metastases, may be indicative of SCPC; similar to our study, other studies have shown that the median PSA is 2–4 mg/mL2. Recognition and diagnosis of SCPC transformed from adenocarcinoma can be challenging, however, and literature reports that approximately 40% present as mixed histology.12
Our study adds to the existing case reports and cohort studies that have reported on outcomes from other parts of the world.1,13,14 For example, Horne et al presented on 800 patients with SCPC from the National Cancer Database, U.S., diagnosed between 2004 and 2015.15 Although the data only exist in abstract form, it reports that only 45% patients received chemotherapy, in contrast to our cohort in which everyone received chemotherapy. Despite the disparities in treatment receipt, the OS outcomes were similar to those of our cohort when stratified by staging. Our study also expands upon the pre-existing Canadian data reported on outcomes for SCPC. One Canadian study by Ahmed et al assessed the small cell cancers of bladder and prostate.16 In their study, 14 patients with SCPC were included, all but one patient with metastatic disease. The outcomes from our cohort are consistent with Ahmed et al, with OS of approximately 10 months. Our study, however, includes a larger group of patients with initially prostate-confined disease and with a focus on SCPC.
Comparable to recommended systemic therapies for small cell lung cancer (SCLC), patients in our cohort have received platinum and etoposide as first-line treatment. No level 1 evidence exists to guide clinicians on optimal treatment modalities of SCPC, due to the lack of relevant prospective studies. A phase 2 trial enrolled “anaplastic” CRPC in which SCPC was included, and administered first-line carboplatin and docetaxel and second-line etoposide and cisplatin to 120 patients, and showed a median survival of 16 months.17 Given that over 70% of patients in this study went on to receive second-line treatment, in comparison to 36% in our cohort, the numerically better OS in this study is likely due to inclusion of patients who are well enough to enrol in a prospective study. Response rates are thought to be short, in the order of 5–6 months, and lower than SCLC, in the order of 30–60%, demonstrating the limitations in the current treatment options for SCPC.17,18 If most treatments are extrapolated from SCLC, other treatment options, such as an addition of programmed-death ligand-1 (PD-L1) inhibitors to chemotherapy, may need to be considered and studied in a trial;19,20 however, funding under a public payer and obtaining level 1 evidence to support their use remain a challenge.
As with other studies, ours illustrates that clinical characteristics may be limited in prognosticating and predicting response to treatment for SCPC. In prior literature, factors such as age, N1 status, and receipt of radiation were associated with survival in patients with nonmetastatic SCPC.15 Factors such as treatment in a non-academic facility, stage 4, and Gleason 8–10 may also predict poorer outcomes.21 Further studies to elucidate genomic landscape that may identify driver mutations and potential biomarkers are likely critical to improving outcomes. One study identified ONECUT2 as a candidate master transcriptional regulator of poorly differentiated SCPC through regulating tumor hypoxia signaling.22 Another study showed gene signatures of SCPC that resemble SCLC, with at least a subset exhibiting preserved androgen receptor signalling.23
Our study is significantly limited by a small number of patients, retrospective cohort design, and a lack of information on the treatment decision-making processes for each patient.
Conclusions
While no conclusion can be drawn regarding an optimal treatment algorithm based on our data, it reports on the Canadian, single-province, multi-institution cohort on an uncommon but aggressive subset of prostate cancer, SCPC, with outcomes following standards of care in heterogeneous stages and clinical settings.
Footnotes
Competing interests: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Deorah S, Rao MB, Raman R, et al. Survival of patients with small cell carcinoma of the prostate during 1973–2003: A population-based study. BJU Int. 2012;109:824–30. doi: 10.1111/j.1464-410X.2011.10523.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Epstein JI. Small cell carcinoma of the prostate: A morphologic and immunohistochemical study of 95 cases. Am J Surg Pathol. 2008;32:65–71. doi: 10.1097/PAS.0b013e318058a96b. [DOI] [PubMed] [Google Scholar]
- 3.Tetu B, Ro JY, Ayala AG, et al. Small cell carcinoma of the prostate. Part I. A clinicopathologic study of 20 cases. Cancer. 1987;59:1803–9. doi: 10.1002/1097-0142(19870515)59:10<1803::AIDCNCR2820591019>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Miyoshi Y, Uemura H, Kitami K, et al. Neuroendocrine differentiated small cell carcinoma presenting as recurrent prostate cancer after androgen deprivation therapy. BJU Int. 2001;88:982–3. doi: 10.1046/j.1464-4096.2001.00936.x. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka M, Suzuki Y, Takaoka K, et al. Progression of prostate cancer to neuroendocrine cell tumor. Int J Urol. 2001;8:431–6. doi: 10.1046/j.1442-2042.2001.00347.x. discussion 437. [DOI] [PubMed] [Google Scholar]
- 6.Nadal R, Schweizer M, Kryvenko ON, et al. Small cell carcinoma of the prostate. Nat Rev Urol. 2014;11:213–9. doi: 10.1038/nrurol.2014.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beltran H, Tagawa ST, Park K, et al. Challenges in recognizing treatment-related neuroendocrine prostate cancer. J Clin Oncol. 2012;30:e386–9. doi: 10.1200/JCO.2011.41.5166. [DOI] [PubMed] [Google Scholar]
- 8.Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015;161:1215–28. doi: 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: A multi-institutional prospective study. J Clin Oncol. 2018;36:2492–2503. doi: 10.1200/JCO.2017.77.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirano D, Okada Y, Minei S, et al. Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy. Eur Urol. 2004;45:586–92. doi: 10.1016/j.eururo.2003.11.032. discussion 592. [DOI] [PubMed] [Google Scholar]
- 11.Beltran H, Prandi D, Mosquera JM, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Mede. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbas F, Civantos F, Benedetto P, et al. Small cell carcinoma of the bladder and prostate. Urology. 1995;46:617–30. doi: 10.1016/S0090-4295(99)80290-8. [DOI] [PubMed] [Google Scholar]
- 13.Rauf A, Smith SF, Mukherjee R, et al. Not such a small diagnosis: Small cell carcinoma of the prostate. J Surg Case Rep. 2020;2020:rjaa117. doi: 10.1093/jscr/rjaa117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar K, Ahmed R, Chukwunonso C, et al. Poorly differentiated small-cell-type neuroendocrine carcinoma of the prostate: A case report and literature review. Case Rep Oncol. 2018;11:676–81. doi: 10.1159/000493255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horne ZD, Smith RP, Beriwal S, et al. Small cell carcinoma of the prostate: National patterns of care and outcomes. J Clin Oncol. 2019;37:9. doi: 10.1200/JCO.2019.37.7_suppl.9. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed S, Neufeld S, Kroczak TJ, et al. Small cell cancer of the bladder and prostate: A retrospective review from a tertiary cancer center. Cureus. 2015;7:e296. doi: 10.7759/cureus.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aparicio AM, Harzstark AL, Corn PG, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–30. doi: 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papandreou CN, Daliani DD, Thall PF, et al. Results of a phase 2 study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate. J Clin Oncol. 2002;20:3072– 80. doi: 10.1200/JCO.2002.12.065. [DOI] [PubMed] [Google Scholar]
- 19.Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide vs. platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomized, controlled, openlabel, phase 3 trial. Lancet. 2019;394:1929–39. doi: 10.1016/s0140-6736(19)32222-6. [DOI] [PubMed] [Google Scholar]
- 20.Horn L, Mansfield AS, Szczesna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379:2220–9. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 21.Xu KM, Liu Y, Gillespie T, et al. Small cell carcinoma of the prostate: A report of outcomes of localized disease using the National Cancer Database. Clin Genitourin Cancer. 2021;19:e193–9. doi: 10.1016/j.ijrobp.2019.06.1786. [DOI] [PubMed] [Google Scholar]
- 22.Guo H, Ci X, Ahmed M, et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat Comm. 2019;10:278. doi: 10.1038/s41467-018-08133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai HK, Lehrer J, Alshalalfa M, et al. Gene expression signatures of neuroendocrine prostate cancer and primary small cell prostatic carcinoma. BMC Cancer. 2017;17:759. doi: 10.1186/s12885-017-3729-z. [DOI] [PMC free article] [PubMed] [Google Scholar]


