Introduction
Soft tissue sarcomas account for approximately 6% of childhood cancers and almost 50% of these are rhabdomyosarcomas, a highly malignant tumor developing from skeletal muscle cells that have failed to fully differentiate.1 Following neuroblastoma and Wilms tumor, rhabdomyosarcoma is the third most common extracranial solid pediatric malignancy.
Fifteen to 20% of rhabdomyosarcomas emerge from the genitourinary system and sites include prostate, bladder, and the paratesticular region. Rhabdomyosarcoma can be generally divided into two main histological subsets: alveolar and embryonal. Usually, the incidence of alveolar rhabdomyosarcoma is higher during adolescence, whereas embryonal rhabdomyosarcomas are more common during early childhood. 2 Botryoid and spindle cell rhabdomyosarcoma constitute less common variants of embryonal rhabdomyosarcoma with a superior prognosis.2 With this case report, we aimed to delineate a therapeutic decision-making process in a boy presenting with rhabdomyosarcoma of the bladder considering the most contemporary therapy options.
Case report
In November 2016, a five-year-old boy of Syrian origin presented with lower abdominal pain, dysuria, hematuria, and progressive urinary retention with reduced urinary flow within the last three days. Abdominal ultrasound suggested a hyperechoic structure at the lower base of the bladder (Figures 1A, 1B), as well as free pelvic fluid in the rectovesical pouch. A magnetic resonance imaging (MRI) confirmed suspicious ultrasound findings resulting in a transurethral resection of bladder tumor (TURBT) both to obtain histological specimens and treat the progressive urinary retention. The pathology report revealed a botryoid embryonal rhabdomyosarcoma.
Figure 1.
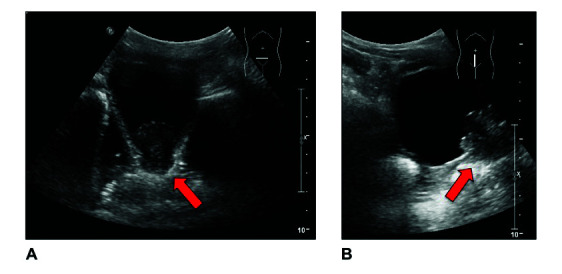
Initial abdominal ultrasound showing a cauliflower-like, hyperechoic tumor with approximately 3.0 cm in diameter and presumed infiltration of the bladder wall and the urethra in the (A) transverse and (B) sagittal scan plane.
After TURBT, the voiding problems were completely resolved, pointing towards an anatomical issue rather than neurological damage to the bladder causing the initial urinary symptoms. A multidisciplinary tumor board opted for the initiation of weekly systemic chemotherapy with ifosfamide, vincristine, and actinomycin D according to the Cooperative Soft Tissue Sarcoma Study Group (CWS) guidance.
After seven weeks of chemotherapy, a restaging MRI suggested a stable disease. Subsequent cystoscopy confirmed tumor infiltration of the bladder neck, including the seminal colliculus, precluding orthotopic continent urinary diversion. The patient was referred for local treatment via proton radiation therapy (total dose of 55.8 Gy) five months after diagnosis, which was administered without any clinically relevant toxicity.
After radiation therapy and 12 cycles of chemotherapy, a staging MRI showed only partial response (Figures 2A, 2B). A subsequent transurethral biopsy confirmed a residual tumor mass, which led to the inevitable decision to perform a nerve-sparing radical cystectomy to complete local tumor treatment.
Figure 2.

Magnetic resonance imaging of the abdomen after completion of chemotherapy and proton therapy showing residual disease with a thickened bladder outlet and residual irregularities in the proximal urethra in the (A) sagittal and (B) coronal plane.
Ultimately, the diagnosis was pathologically confirmed (Figures 3A, 3B).
Figure 3.

Hematoxylin and eosin stains of the cystectomy specimen showing polytope residual infiltrates of a botryoid embryonal rhabdomyosarcoma. Bars represent (A) 200 μm and (B) 50 μm, respectively. (A) A regular urothelium on the surface overlying a malignant neoplasm, which is arranged in form of a cambium layer (subepithelial condensation of the tumor), the classic feature of a botryoid rhabdomyosarcoma. The higher magnification (B) shows polymorphic tumor cells (rhabdomyoblasts) in the lamina propria with broad eosinophilic cytoplasm and pleomorphic nuclei.
To prevent further tumor progression, the patient was bridged with an additional cycle of trofosfamide and idarubicin until surgery was performed five months after completion of radiation therapy. The surgery was uneventful and a MAINZ I (mixed augmentation ileum and cecum) pouch was chosen as a continent urinary diversion. The procedure was performed by a very high-volume surgeon (MF) and was adapted to a previously described technique for nerve-sparing cystoprostatectomy.3 No radiotherapy-related technical difficulties were experience intraoperatively.
The final pathological report revealed residual infiltrates of a vital, botryoid rhabdomyosarcoma at the bladder neck. The perioperative course was mainly uneventful. Few minor complications were documented (two urinary tract infections, hematuria, edema, emesis, and constipation). Of note, the educational process to teach intermittent self-catheterization to the patient and, particularly, to his parents proved difficult and we decided to extend the inpatient period until catheterization was appropriately feasible. Hence, the patient was discharged nine weeks after surgery.
Given an R0 resection, an interdisciplinary tumor board opted for regular followup without adjuvant therapy. Followup was performed per an institutional protocol without further complications. At last day of followup in May 2022, the patient was alive, with no evidence of disease. Functional urinary outcome was assessed using selected questions regarding urinary function from the validated cystectomy-specific Bladder Cancer Index (BCI).4 At the time of last followup, the patient reported no leakage at all while awake and when sleeping, translating into patient-reported total control of urinary leakage both day and night according to the BCI.
Overall, patient-reported bother related to the catheterizable pouch was very small; however, there were moderate problems regarding diversion-related body odor, limited exercise, and very limited social activities with friends due to difficulties with the pouch. Due to the prepubescent age of the patient, valid assessment of erectile function was not yet possible.
Discussion
The current diagnostic and treatment algorithm of genitourinary rhabdomyosarcomas in children consists of biopsy and staging followed by chemotherapy. Risk stratification is recommended, including tumor stage, group, and histology.5 The chemotherapy regimen is usually composed of vincristine, actinomycin D, and cyclophosphamide (VAC).6 Later modifications and additive use of novel chemotherapeutic agents have not significantly improved survival outcomes compared to VAC alone.7
Recommendations differ regarding further therapy following chemotherapy to provide local tumor control. There are two different concepts, with a certain geographical difference regarding treatment paradigms. European trials evaluate overall survival as an endpoint and employ initial standard induction chemotherapy followed by intensified chemotherapy in poor responders. To obtain local control, surgical resection is preferred over radiation therapy, but the main purpose is to reduce the use of local therapy as far as possible. In contrast, North American trials primarily investigate event-free survival, aiming for local control shortly after chemotherapy, mostly preferring radiation therapy over extirpative surgical resection.8
To compare the different approaches, survival outcomes of an international cohort of 322 patients with localized bladder or prostate embryonal rhabdomyosarcoma were evaluated after stratification by varying treatment protocols.9 Interestingly, there was no significant difference in failure-free and overall survival between different treatment algorithms. In these regards, the outcome of our case is premature.
Partial bladder-preserving surgery as opposed to radical surgery was not feasible, given the infiltration of the bladder neck and proximal urethra. Incomplete resection may lead to a significant decrease in overall survival and we, therefore, refrained from partial cystectomy. On the other hand, radiation therapy has been demonstrated to cause significant treatment-related morbidity.10 Consequently, different promising options for local therapy, such as brachytherapy or proton therapy, are being investigated. Arguably, surgical therapy of an irradiated bladder goes in hand with a higher complication rate compared to primary urinary diversion. Thus, we firmly believe that radiogenic complications may be avoided by upfront surgery if a complete resection is feasible.
Notwithstanding pending high-level evidence for the superiority of proton therapy over established intensity-modulated radiation therapy, preliminary data demonstrated favorable toxicity rates for proton radiation compared to photon radiation, without a significant decrease in overall survival.11 Similarly, initial studies on brachytherapy combined with conservative surgery in patients with prostate and/or bladder neck rhabdomyosarcoma show equally positive results.12 Unfortunately, proton therapy in our patient did not result in complete response. Thus, we opted for a nerve-sparing radical cystectomy and the patient underwent urinary diversion with a MAINZ I pouch.
Particularly in younger children, if radical extirpative surgery is inevitable, urinary diversion should provide the best possible quality of life. Several variants of urinary diversion after cystectomy in children with rhabdomyosarcoma have been described in the literature. Among others, Stein et al presented options for urinary diversion in patients with bladder or prostate rhabdomyosarcoma, including uretero-sigmoidostomy, rectosigmoid pouch, continent cutaneous ileocecal pouch, urethral ileocecal pouch, and incontinent colon conduit.13 An orthotopic neobladder is considered to be the gold standard in terms of quality of life, but in our case, we were not able to preserve the external urethral sphincter, which is essential to ensure continence after orthotopic bladder substitution.
In our patient, functional outcome was assessed by regular visits in our outpatient pediatric urological clinic and both patient and his mother reported satisfactory catheterization and trouble-free pouch management.
Conclusions
The relatively low number of cases, differing histology, and multiple treatment protocols for pediatric rhabdomyosarcoma result in challenging decision-making. Failure of proton beam therapy, which can generally be considered a novel treatment option to gain local tumor control, inevitably requires surgical resection. In the scenario, salvage nerve-sparing radical cystectomy in combination with a continent cutaneous urinary diversion allows for relatively low treatment related-morbidity, with adequate functional outcome and good quality of life.
Footnotes
Competing interests: The authors do not report any competing personal or financial interests related to this work.
This paper has been peer-reviewed.
References
- 1.Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Rudzinski ER, Anderson JR, Hawkins DS, et al. The World Health Organization classification of skeletal muscle tumors in pediatric rhabdomyosarcoma: A report from the Children’s Oncology Group. Arch Pathol Lab Med. 2015;139:1281–7. doi: 10.5858/arpa.2014-0475-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schlegel PN, Walsh PC. Neuroanatomical approach to radical cystoprostatectomy with preservation of sexual function. J Urol. 1987;138:1402–6. doi: 10.1016/S0022-5347(17)43655-X. [DOI] [PubMed] [Google Scholar]
- 4.Gilbert SM, Dunn RL, Hollenbeck BK, et al. Development and validation of the Bladder Cancer Index: A comprehensive, disease specific measure of health-related quality of life in patients with localized bladder cancer. J Urol. 2010;183:1764–9. doi: 10.1016/j.juro.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Saltzman AF, Cost NG. Current treatment of pediatric bladder and prostate rhabdomyosarcoma. Curr Urol Rep. 2018;19:11. doi: 10.1007/s11934-018-0761-8. [DOI] [PubMed] [Google Scholar]
- 6.Maurer HM, Crist W, Lawrence W, et al. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61:209–20. doi: 10.1002/1097-0142(19880115)61:2<209::AIDCNCR2820610202>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 7.Lager JJ, Lyden ER, Anderson JR, et al. Pooled analysis of phase ii window studies in children with contemporary high-risk metastatic rhabdomyosarcoma: A report from the soft tissue sarcoma committee of the Children’s Oncology Group. J Clin Oncol. 2006;24:3415–22. doi: 10.1200/JCO.2005.01.9497. [DOI] [PubMed] [Google Scholar]
- 8.Dasgupta R, Fuchs J, Rodeberg D. Rhabdomyosarcoma. Semin Pediatr Surg. 2016;25:276–83. doi: 10.1053/j.sempedsurg.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 9.Rodeberg DA, Anderson JR, Arndt CA, et al. Comparison of outcomes based on treatment algorithms for rhabdomyosarcoma of the bladder/prostate: Combined results from the Children’s Oncology Group, German Cooperative Soft Tissue Sarcoma Study, Italian Cooperative Group, and International Society of Pediatric Oncology Malignant Mesenchymal Tumors Committee. Int J Cancer. 2011;128:1232–9. doi: 10.1002/ijc.25444. [DOI] [PubMed] [Google Scholar]
- 10.Heyn R, Haeberlen V, Newton WA, et al. Second malignant neoplasms in children treated for rhabdomyosarcoma. Intergroup rhabdomyosarcoma study committee. J Clin Oncol. 1993;11:262–70. doi: 10.1200/JCO.1993.11.2.262. [DOI] [PubMed] [Google Scholar]
- 11.Ladra MM, Szymonifka JD, Mahajan A, et al. Preliminary results of a phase 2 trial of proton radiotherapy for pediatric rhabdomyosarcoma. J Clin Oncol. 2014;32:3762–70. doi: 10.1200/JCO.2014.56.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martelli H, Haie-Meder C, Branchereau S, et al. Conservative surgery plus brachytherapy treatment for boys with prostate and/or bladder neck rhabdomyosarcoma: A single-team experience. J Ped Surg. 2009;44:190–6. doi: 10.1016/j.jpedsurg.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Stein R, Frees S, Schröder A, et al. Radical surgery and different types of urinary diversion in patients with rhabdomyosarcoma of bladder or prostate — a single-institution experience. J Ped Urol. 2013;9:932–9. doi: 10.1016/j.jpurol.2013.01.008. [DOI] [PubMed] [Google Scholar]


