Abstract
Moraxella catarrhalis expresses surface receptor proteins that specifically bind host transferrin (Tf) and lactoferrin (Lf) in the first step of the iron acquisition pathway. Acute- and convalescent-phase antisera from a series of patients with M. catarrhalis pulmonary infections were tested against Tf and Lf receptor proteins purified from the corresponding isolates. After the purified proteins had been separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting, we observed strong reactivity against Tf-binding protein B (TbpB; also called OMP1) and Lf-binding protein B (LbpB) but little or no reactivity against Tf-binding protein A (TbpA) or Lf-binding protein A (LbpA), using the convalescent-phase antisera. Considerable antigenic heterogeneity was observed when TbpBs and LbpBs isolated from different strains were tested with the convalescent-phase antisera. Comparison to the reactivity against electroblotted total cellular proteins revealed that the immune response against LbpB and TbpB constitutes a significant portion of the total detectable immune response to M. catarrhalis proteins. Preparations of affinity-isolated TbpA and LbpA reacted with convalescent-phase antisera in a solid-phase binding assay, but blocking with soluble TbpB, soluble LbpB, or extracts from an LbpA− mutant demonstrated that this reactivity was attributed to contaminants in the TbpA and LbpA preparations. These studies demonstrate the immunogenicity of M. catarrhalis TbpB and LbpB in humans and support their potential as vaccine candidates.
Moraxella catarrhalis is a common inhabitant of the human upper respiratory tract and is implicated as an important cause of upper respiratory infections in children and of lower respiratory tract infections in the elderly (33). M. catarrhalis has been shown to be responsible for approximately 15% of the episodes of otitis media in children on the basis of culture, and PCR analysis suggests that the frequency of colonization may be even higher (33). Several lines of evidence have confirmed this organism is a cause of infection in chronic obstructive pulmonary disease patients, and it has been reported to be responsible for up to 30% of exacerbations of disease in these patients (33). The recognition of M. catarrhalis as a significant human pathogen and the increasing prevalence of antibiotic resistance has prompted interest in development of immunotherapeutic approaches to combat the threat of disease caused by this organism.
A number of reports have explored the human immune response to M. catarrhalis infections as a logical first step in identifying potential vaccination targets (19, 25, 31, 43). This approach has led to the identification of a number of candidate antigens, including OMPB1 (12, 31, 43), CopB (1), UspA (2, 13), and CD (44). Since there are differences in the source of sera and the methodologies used in the various studies, it is difficult to make comparisons of the antigenicities of these candidate antigens in human infections. Nevertheless, the use of active or passive immunization in animal models has provided evidence for protective capacity of antibodies directed against these antigens (1, 2, 13, 44).
Several members of the family Neisseriaceae, including M. catarrhalis, have been shown to produce exquisitely host specific receptors to allow iron acquisition from transferrin (Tf) and lactoferrin (Lf) during the infectious process (24). The apparent inability of M. catarrhalis to utilize other potential iron sources such as heme-hemopexin, hemoglobin, and hemoglobin-haptoglobin (3) suggests an essential in vivo role of these surface-exposed receptor proteins, making them putative vaccine targets. The genes encoding these receptors, designated Tf-binding proteins A and B (TbpA and -B) and Lf-binding proteins A and B (LbpA and -B), have been recently identified (18, 34). The comigration of transferrin binding activity with reactivity against convalescent-phase sera (12) and inhibition of reactivity against convalescent-phase sera by soluble receptor protein (31) has been used to implicate TbpB as immunogenic protein during infections. Until relatively recently, the identity of the Lf receptor proteins in M. catarrhalis was uncertain (20), and there is no information on the immunogenicity of these proteins in humans. In this study, we assessed the abilities of both the Tf and Lf receptors to elicit an immune response during a natural pulmonary M. catarrhalis infection in humans and confirmed their identities by several different methods.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Seventeen clinical isolates from patients with pulmonary infections that were attributed to M. catarrhalis based on microbiological analysis of clinical samples (sputum, broncheoalveolar lavage fluid, and transtracheal aspirate) were collected in conjunction with the acute- and convalescent-phase sera used for this study. These isolates, designated n130 to n144, were collected by S. Ainsworth, Veteran Affairs Medical Center, Alexandria, La., and were from patients drawn from a region encompassing northwestern Louisiana and Western Texas. In an attempt to offset any geographical bias or features specifically associated with isolates causing pulmonary diseases, we included a limited selection of additional isolates: (i) three sputum isolates (n056, n057, and n105) obtained from C. Anand, Foothills Hospital, Calgary, Alberta, Canada; (ii) one pulmonary isolate (Q8) from M. Bergeron, University of Laval, Montreal, Quebec, Canada; (iii) one otitis media isolate (4223) obtained from T. Murphy, State University of New York, Buffalo; and (iv) three isolates (25240, 43627, and 43617) obtained from the American Type Culture Collection.
Strains stored in 30% glycerol suspensions at −70°C were used to inoculate chocolate agar plates and grown overnight at 37°C in an atmosphere containing 5% CO2. The cells were used to inoculate brain heart infusion broth cultures; after reaching mid-log to late log phase of growth, the cells were subcultured (to a starting A600 of 0.05) into broth containing 100 μM EDDA for iron limitation and incubated until late log-stationary phase of growth.
Isolation of receptor proteins.
To screen for reactivity against the native Tf and Lf receptor proteins (Fig. 1 and 2), the proteins were isolated directly from intact M. catarrhalis cells by modification of previously published procedures for isolation of receptor proteins from crude total membrane preparations (10). Cells collected after growth in iron-deficient media were resuspended in 50 mM Tris-HCl (pH 8)–1 M NaCl (1 ml of buffer per 1 to 4 ml of culture), and the receptor proteins were solubilized by the addition of EDTA (to 10 mM) and Sarkosyl (to 0.3%) and incubation for 1 h at room temperature. After centrifugation (10 min, 13,000 rpm) to remove insoluble debris, the supernatant was applied to an iron-loaded human Tf (hTf)-Sepharose or hLf-Sepharose resin and washed three times with solubilization buffer (containing 0.15% Sarkosyl) and then with 50 mM Tris-HCl, (pH 8) buffer prior to elution with sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer.
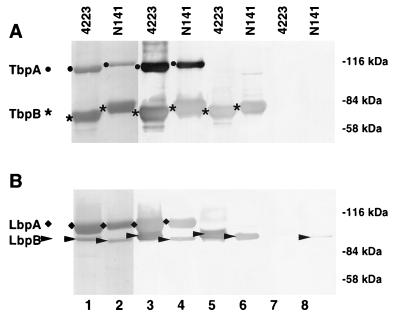
FIG. 1.
Assessment of the ability of Tf and Lf receptor proteins to elicit an immune response. Using hTf-Sepharose (A) or hLf-Sepharose (B) as the affinity matrix, we isolated receptor proteins from iron-starved cells of M. catarrhalis 4223 and N141. The proteins were eluted by boiling in Laemmli sample buffer and subjected to SDS-PAGE (7% gel), and Western blots were prepared. The blots were either stained for protein (lanes 1, 2) or blocked and then probed with rabbit polyclonal antiserum (lanes 3 and 4; 1/5,000 dilution), human convalescent-phase serum (lanes 5 and 6; 1/200 dilution), or human acute-phase serum (lanes 7 and 8; 1/200 dilution). The human sera were obtained from the patient with a pulmonary infection attributed to M. catarrhalis N141.
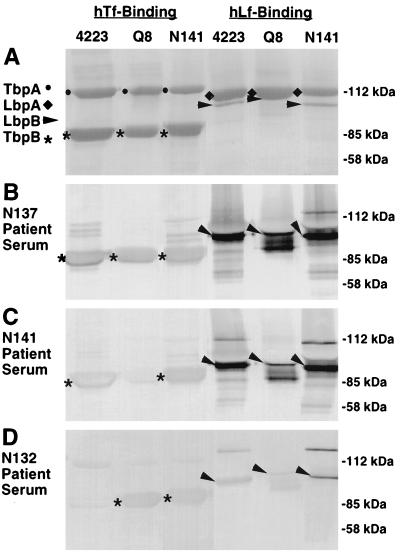
FIG. 2.
Analysis of host immune response to Tf and Lf receptor proteins from homologous and heterologous M. catarrhalis strains. Using hTf-Sepharose or hLf-Sepharose as the affinity matrix, we isolated receptor proteins from iron-starved cells of M. catarrhalis 4223, Q8, and N141. The proteins were eluted from the matrix by boiling in Laemmli sample buffer and subjected to SDS-PAGE (7% gel), and replicate Western blots were prepared. The blots were either stained for protein (A) or blocked and probed with convalescent-phase antisera (1/200 dilution) obtained from patients infected with M. catarrhalis N137 (B), N141 (C) or N132 (D).
Native Tf receptor complex (TbpA and TbpB) used in solid-phase binding assays (Fig. 5) was isolated from crude total membrane preparations by previously published procedures (10). Selective isolation of TbpA was achieved by exposure of the solubilized membrane proteins to apo-hTf-Sepharose, since TbpB does not bind to apo-Tf and thus remained in the eluant (45). The eluant was analyzed for the presence of TbpA and, if necessary, was exposed to additional apo-hTf-resin to remove all remaining TbpA. The TbpA-depleted eluant was applied to a column containing the iron-loaded form of hTf-Sepharose to isolate TbpB. Native Lf receptor (LbpA and LbpB) or LbpA for the solid-phase binding assays was isolated essentially as described previously (10) except that membranes prepared from an isogenic LbpB− mutant (9) was used for LbpA isolation. Bound receptor proteins were eluted from the various columns by the addition of 100 ml of buffer containing >2 M guanidine hydrochloride, dialyzed against three changes of 50 mM Tris buffer (pH 8.0), and then dialyzed against ammonium bicarbonate before lyophilization and storage at −20°C.
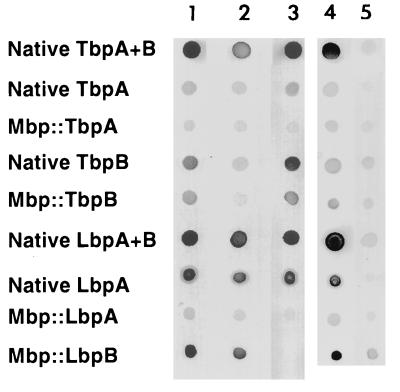
FIG. 5.
Solid-phase immunological analysis. Native Tf receptor proteins were affinity isolated with apo- or holo-hTf-Sepharose from M. catarrhalis 4223 (TbpA and TbpB, TbpA alone, or TbpB alone). Native Lf receptor proteins were affinity isolated with holo-hLf-Sepharose from strain N141 or from an N141 LbpB− isogenic mutant (9). The Mbp receptor protein fusions were affinity isolated by using an amylose resin. Approximately 50 ng of isolated protein was spotted onto the nitrocellulose matrix and incubated with a 1:1,000 dilution of convalescent-phase sera from the patient infected with M. catarrhalis N141 (sera alone; columns 1 and 4), the same sera preincubated with approximately 1 mg of either Mbp::TbpB (column 2) or Mbp::LbpB (column 3) or with 100 μl of a crude extract from the N141 LbpA− isogenic mutant (9) (column 5).
For production of maltose-binding protein (Mbp) fusion proteins (Mbp::Tbp and Mbp::Lbp) lacking a signal peptide, specific primers (Table 1) were designed to allow PCR amplification of the tbpA or tbpB gene from strain 4223 or the lbpA or lbpB gene from strain N141. Primers were designed to allow in-frame ligation to the malE gene of pMal-c2 (New England Biolabs, Mississauga, Ontario, Canada). Expression of the Mbp fusion proteins was induced by the addition of isopropyl-β-d-thiogalactoside (IPTG) to Escherichia coli DH5α cells containing the pMal-c2 derivative plasmid as described previously (8).
TABLE 1.
Oligonucleotide primer sequences used for PCR amplification
| Gene | Regiona | Oligonucleotide sequenceb |
|---|---|---|
| tbpA | 5′ | TCTAGAATTCTTTCTTTGGGTCTGCTTAACATC |
| tbpA | 3′ | CCCGGGAGATCTTTAAAACTTCATTTCAAGTGC |
| tbpB | 5′ | TCTAGAATTGGTTCAGGTGGTTCAAATCCACC |
| tbpB | 3′ | CCCGGGGGATCCTTACTTAACTTCTTGTTGTC |
| lbpA | 5′ | AGAAGGGGTTCTGGTGTGGCAGTTTTACCCCTA |
| lbpA | 3′ | TTAAGCTTAAAACTTCATTTCAAGACTGGC |
| lbpB | 5′ | AAATCTAGACGCTCTGATGACATCAGCGTC |
| lbpB | 3′ | TTAAGCTTATTTATCTTTAACAGCCCCAAAGAC |
5′, positive strand at the 5′ end of the gene; 3′, negative strand at the 3′ end of the gene.
Restriction endonuclease recognition sites are underlined.
Collection and preparation of antisera.
Sera were collected from patients with pulmonary infections attributed to M. catarrhalis based on microbiological analysis of clinical samples (sputum, broncheoalveolar lavage fluid, or transtracheal aspirate). Sera were collected at the time which the symptoms were first reported (acute-phase sera) and after resolution of the infection after treatment (convalescent-phase sera) and stored at −20°C.
For the production of antiserum in New Zealand White rabbits, 50 μg of purified receptor protein emulsified in complete Freund’s adjuvant was used for the first intramuscular injection and boosted on days 14 and +29 with the same dose of protein emulsified in incomplete Freund’s adjuvant after the primary injection.
Western blot or solid-phase immunoblot analysis.
SDS-PAGE samples were boiled in Laemmli sample buffer and separated by SDS-PAGE using a 7% acrylamide gel with the Tris-HCl–glycine buffer system (28). Proteins were transferred to Immobilon-P (Millipore, Bedford, Mass.) and stained for protein (amido black) or probed with specific antisera as described previously (10).
Samples of purified receptor proteins were either directly applied onto Nitro ME-nitrocellulose (Micron Separations, Westboro, Mass.) membranes or placed into a dot blot apparatus with vacuum apparatus attached. The remaining binding sites on the blot were blocked by incubation for 30 min with Tris-buffered saline containing 0.5% blotting-grade nonfat dry milk blocker (Bio-Rad Laboratories). After removal of blocking buffer, the membranes were exposed to the indicated dilution of the patient acute- or convalescent-phase sera in blocking solution and incubated for 1 h. Where indicated, purified, recombinant fusion proteins (Mbp::TbpB or Mbp::LbpB) were added to the diluted antisera at a final concentration of 100 μg/ml and incubated for 1 h prior to exposure to the membrane. Similarly, 100 μl of crude cellular extracts of the LbpA− mutant obtained by French press lysis (10 mg of cellular protein/ml of buffer) was added per 5 ml of diluted antisera and incubated for 1 h prior to exposure to the membrane. After incubation, the diluted antisera was removed, the membrane washed and then exposed to a 1/2,000 dilution of horseradish peroxidase-conjugated goat anti-human immunoglobulin G (IgG)-IgM-IgA and incubated for 1 h. The second antibody solution was removed; the membrane washed and then developed with horseradish peroxidase color development reagent (Bio-Rad).
RESULTS
Analysis of the immune response to native Tf and Lf receptor proteins.
A total of 17 paired sera from patients with pulmonary infections attributable to M. catarrhalis were collected, and isolates from 15 of the 17 paired sera were available for analysis. To specifically evaluate the immune response against Tf and Lf receptor proteins from these isolates, we isolated the receptor proteins by affinity techniques. To facilitate analysis from a large number of strains, we adapted our affinity isolation techniques to isolate receptor proteins directly from intact cells (see Materials and Methods). Several additional strains, used for more extensive analysis of the receptor proteins (11, 18, 34), were also included for comparison. Figure 1 illustrates the results obtained with one of the clinical isolates from this series, N141, and with an otitis media isolate that we have worked with previously, 4223 (45). The modified procedure enabled us to obtain relatively pure preparations of Tf receptor proteins with an hTf-Sepharose resin (Fig. 1A, lanes 1 and 2, TbpA and TbpB) and relatively pure preparations of Lf receptor proteins with an hLf-Sepharose resin (Fig. 1B, lanes 1 and 2, LbpA and LbpB). We used SDS-polyacrylamide gels with a lower-percentage acrylamide (7%) to optimally resolve LbpB from LbpA, since these two proteins comigrate on conventional (10%) gels (11).
The ability of the Tf and Lf receptor proteins to elicit an immune response during the course of a natural infection in humans was assessed by Western blot analysis. Electroblotted receptor proteins were probed with either acute-phase or convalescent-phase sera from patients with a pulmonary infection attributed to M. catarrhalis. Figure 1 illustrates the results obtained with a representative set of paired antisera from a patient infected with strain N141. Significant reactivity against the lower-molecular-weight component of either the Tf receptor (Fig. 1A, lanes 5 and 6, TbpB) or Lf receptor (Fig. 1B, lanes 5 and 6, LbpB) from either the infecting strain N141 or strain 4223 was observed with the convalescent-phase antiserum. In contrast, little reactivity to either TbpA or LbpA was observed with either strain. When the acute-phase antiserum was tested, little reactivity was noted with the Tf (Fig. 1A, lanes 7 and 8) and Lf (Fig. 1B, lanes 7 and 8) receptor proteins.
In a preliminary attempt to address the potential cause of the lack of reactivity of TbpA and LbpA with the convalescent-phase antiserum, we decided to test the immunogenicity of the purified proteins in rabbits. Polyclonal antisera were prepared by immunizations with affinity-purified receptor preparations from strain 4223 emulsified in Freund’s adjuvant and tested against the electroblotted receptor preparations. In contrast to the convalescent-phase antiserum, the rabbit polyclonal serum reacted strongly with electroblotted TbpA from either the homologous strain (Fig. 1A, lane 3) or a heterologous strain (lane 4). Similarly, the rabbit antiserum prepared against purified Lf receptor reacted with LbpA from the homologous strain (lane 3) or the heterologous strain (lane 4). Thus, the lack of reactivity of TbpA and LbpA with the convalescent-phase antiserum does not appear to be due to some intrinsic property of these proteins.
The results obtained with the antisera from the patient infected with strain N141 are fairly representative. There was substantial reactivity against TbpB and LbpB from the infecting strain in all of the convalescent-phase antisera and little or no reactivity against these proteins in 14 of 17 of the acute-phase sera (data not shown). However, the acute-phase antisera from three of the patients had detectable reactivity against LbpB and/or TbpB, albeit less than that observed in the convalescent-phase antisera. This is perhaps not surprising, as most of the infections were acute exacerbations in elderly patients with chronic bronchitis, and we could not exclude the possibility of prior subclinical infection in these patients. We were unable to demonstrate reactivity of any of the convalescent antisera against TbpA or LbpA.
Analysis of TbpB and LbpB heterogeneity.
The convalescent-phase antisera were tested against receptor proteins isolated from a collection of 23 strains, including all of the clinical isolates from the pulmonary infections, to assess the immunological heterogeneity of the TbpB proteins. The results demonstrated that there was considerable antigenic heterogeneity among TbpBs from different strains, and although the individual antisera appeared to provide a preliminary basis for grouping of the strains, the analysis did not result in any consistent groupings (data not shown). Figure 2 illustrates representative data for convalescent-phase antisera from three patients against TbpBs from three of the strains. The convalescent-phase antiserum from a patient infected with strain N137 demonstrated strong reactivity against TbpBs from all three strains (4223, Q8, and N141), whereas the antisera from the patients infected with strain N141 and strain N132 reacted predominantly with TbpBs from two of the strains (4223 and N141 or Q8 and N141, respectively). These results suggest that several variable immunodominant epitopes are present on TbpBs from different M. catarrhalis strains. Although immunization of rabbits with purified Tf receptor proteins provided antiserum that could cross-react with receptor proteins from all strains (1/5,000 dilution [data not shown]), the distinctive pattern of reactivity due to the immunodominant epitopes was evident at higher dilutions (1:20,000).
The analysis of antigenic heterogeneity was also performed on the Lbp proteins isolated from different strains. In most cases, the reactivity to LbpB was more intense than the reactivity of the same sera to TbpB from that strain, using equivalent serum dilutions (Fig. 2). As with the TbpBs, there was evidence of antigenic heterogeneity between LbpB proteins from different strains, although the differences were not as apparent when similar dilutions of the antiserum were used (Fig. 2).
Recombinant Tbp and Lbp analysis.
Affinity isolation of the Tf and Lf receptor proteins was performed for the serological analysis to facilitate identification of antibodies directed specifically against these proteins. The identification of the individual receptor proteins (TbpA, TbpB, LbpA, and LbpB) was based on their relative mobility in SDS-polyacrylamide gels as demonstrated in previous studies (10, 11, 41). However, the similar migration of LbpA and LbpB in SDS-polyacrylamide gels and the appearance of multiple immunoreactive bands, even in the affinity-isolated samples (Fig. 2), raised some concern about the identification of the specific proteins. To definitively confirm the identity of the immunoreactive bands with convalescent-phase sera, we prepared recombinant receptor proteins with the genes encoding the receptor proteins from M. catarrhalis (18, 34).
The regions encoding each of the mature receptor proteins were PCR amplified from chromosomal DNA and cloned in frame to the malE gene of the pMal-c2 vector. SDS-polyacrylamide gels of cells containing the expressed Mbp fusions were either stained for protein (Fig. 3, lanes 1 to 4) or electroblotted and incubated with convalescent-phase antiserum (lanes 5 and 6) from the patient infected with strain N141. As occurred with the native proteins, the convalescent-phase antiserum reacted with recombinant TbpB (lane 6) and LbpB (lane 8) but not with recombinant TbpA (lane 5) or LbpA (lane 7). Some of the samples exhibited multiple immunoreactive bands (lane 8), which we attribute to breakdown products and/or complexes of the recombinant receptor fusion proteins since they are absent in the other samples. When tested after electroblotting, only Mbp::TbpB and Mbp::LbpB retained ligand binding activity (data not shown), supporting our prior designations for these proteins based on their functional attributes (11, 45).
FIG. 3.
Reactivity of convalescent-phase sera to recombinant Tf and Lf receptors. The regions encoding the mature forms of the individual Tf and Lf receptor proteins were PCR amplified and ligated in frame with the malE gene of the pMal-c2 vector for production of Mbp fusion proteins. Expression of the Mbp fusions was induced by the addition of IPTG, and the cells were boiled in Laemmli sample buffer prior to SDS-PAGE (7% gel). The electroblotted proteins were either stained for protein (lanes 1 to 4) or probed with convalescent-phase antiserum (1:500) from the patient with a pulmonary infection attributed to M. catarrhalis N141. Lanes 1 and 5, Mbp::TbpA (strain 4223 tbpA gene); lanes 2 and 6, Mbp::TbpB (strain 4223 tbpB gene); lanes 3 and 7, Mbp::LbpA (strain N141 lbpA gene); lanes 4 and 8, Mbp::LbpB (strain N141 lbpB gene). Symbols are as in Fig. 1 and 2.
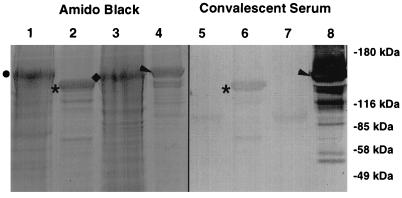
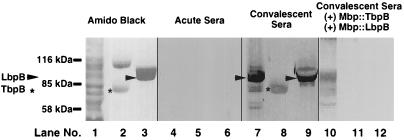
Although our analysis confirmed that an immune response was generated against TbpB and LbpB, we wanted to assess this response relative to the response against other M. catarrhalis proteins. To this end, we isolated total cellular proteins (Fig. 4, lane 1), Tf receptor proteins (lane 2), and Lf receptor proteins (lane 3) from M. catarrhalis and tested them for reactivity against acute-phase (lanes 4 to 6) and convalescent-phase (lanes 7 to 9) sera. The convalescent-phase antiserum reacted with a number of different protein bands in the sample representing total cellular proteins (lane 7). There was a strong immunoreactive band that comigrated with the affinity-purified LbpB (lane 9) and a weaker band of immunoreactivity that comigrated with affinity-purified TbpB (lane 8). To demonstrate that the reactivity was directed toward TbpB and LbpB, respectively, we preincubated the convalescent-phase sera with amylose resin-affinity purified Mbp::TbpB and Mbp::LbpB. Subsequently, the convalescent-phase sera demonstrated little or no reactivity with either the purified native TbpB (lane 11) or LbpB (lane 12). In addition, the reactivity of the sera with the total cellular proteins decreased dramatically at the regions that comigrated with the TbpB and LbpB (lane 10), confirming the identity of these immunoreactive regions as TbpB and LbpB.
FIG. 4.
Analysis of immune response to M. catarrhalis total cellular proteins. The Tf receptor proteins from M. catarrhalis 4223 (lanes 2, 5, 8, and 11) or the Lf receptors from strain N141 (lanes 3, 6, 9, and 12) were isolated by using either hTf-Sepharose or hLf-Sepharose as the affinity matrix. The receptor proteins or strain N141 whole cells (lanes 1, 4, 7, and 10) were boiled in Laemmli sample buffer and subjected to SDS-PAGE (7% gel), and replicate Western blots were prepared. The blots were either stained for protein (lanes 1 to 3) or blocked and probed with either acute-phase sera from the patient infected with M. catarrhalis N141 (lanes 4 to 6), the convalescent-phase sera alone (lanes 7 to 9), or the convalescent-phase sera containing approximately 1 mg each of Mbp::TbpB and Mbp::LbpB purified by amylose affinity chromatography.
Solid-phase immunological and functional analysis.
Since the A constituents of the Tf and Lf receptors lose their ligand binding capacity after SDS-PAGE and Western blotting, we were concerned that our inability to detect anti-TbpA or anti-LbpA antibodies could be due to conformationally dependent epitopes destroyed by the denaturing conditions of SDS-PAGE. Therefore, we sought to provide the receptor proteins with a more native conformation for detection of convalescent-phase serum reactivity. The affinity isolation methods provide pure preparations of receptor proteins, and although they require relatively harsh conditions for elution of the bound proteins, we have been able to restore ligand binding in the purified receptor preparations, suggesting that native conformation is at least partially restored. However, receptor isolated from wild-type strains contain both the A and B components, and thus we needed to separate the individual receptor proteins by biochemical or genetic means.
For the preparation of LbpA, we fortunately had a recently constructed LbpB− isogenic mutant (9) in which the wild-type lbpB gene had been replaced with an insertionally inactivated copy. LbpA isolated from this strain with an hLf-Sepharose affinity column retained substantial Lf binding activity in solid-phase binding assays (data not shown). We also were able to readily purify the recombinant fusion proteins containing mature LbpA and LbpB (Mbp::LbpA and Mbp::LbpB, respectively), but only the fusion containing LbpB retained significant Lf binding activity in solid-phase binding assays (data not shown). These various LbpA and LbpB preparations, along with native receptor complex (LbpA and LbpB) isolated from the wild-type parent, were tested in a solid-phase binding assay for reactivity against the convalescent-phase antiserum (Fig. 5). In this assay, excess Mbp::TbpB or Mbp::LbpB was premixed with the convalescent-phase antiserum to bind antibody against these proteins. The loss of reactivity of the antiserum against Mbp::LbpB, Mbp::TbpB, and native TbpB (Fig. 5) demonstrated that this preincubation was effective at eliminating the binding activity.
The solid-phase binding assay demonstrated that there was substantial reactivity of the convalescent-phase antiserum against purified receptor complex (native LbpA and LbpB) and LbpA purified from the isogenic mutant (native LbpA), even in the presence of excess competing Mbp::LbpB (Fig. 5). There was relatively little reactivity against Mbp::LbpA under these conditions, initially suggesting most of the reactive antibody was directed against conformationally dependent epitopes, since this latter LbpA preparation was deficient in Lf binding activity. To exclude the possibility that the reactivity observed against the native LbpA preparations was due to a contaminant (i.e., lipopolysaccharide) isolated from M. catarrhalis, the convalescent antiserum was preincubated with crude extracts obtained from an LbpA− isogenic mutant of M. catarrhalis (14). As illustrated in Fig. 5, preincubation with the LbpA− mutant essentially eliminated reactivity against native receptor complex and LbpA, demonstrating that there was no reactivity directed against LbpA and that any apparent reactivity was due to some contaminant in the affinity-isolated LbpA preparation.
The preferential binding of TbpB to the iron-loaded form of hTf (45) enabled us to use affinity methods to purify and separate native TbpA and TbpB from M. catarrhalis. This preparation of TbpA retained substantial hTf binding activity in solid-phase binding assays, unlike electroblotted TbpA. As an alternate source of the individual Tf receptor proteins, we used recombinant fusion proteins containing the intact mature TbpA and TbpB proteins fused to Mbp which were isolated and purified with an amylose affinity column. The convalescent-phase serum demonstrated significant levels of reactivity with native receptor complex (TbpA and TbpB), native TbpB, Mbp::TbpB, and to a lesser extent native TbpA (Fig. 5). Although the method of production of native TbpA resulted in relatively pure preparations based on SDS-PAGE analysis (45), we could not exclude the possibility that trace amounts of TbpB were responsible for the reactivity observed against the native TbpA preparation. Therefore, the sera were preincubated with excess amounts of Mbp::TbpB, or Mbp::LbpB, as a control. The pretreatment with Mbp::TbpB resulted in a substantial decrease in the reactivity against the native TbpA preparation, indicating that contaminating TbpB must have been responsible for a substantial amount of the observed reactivity. Although there was a weak but detectable reactivity remaining against the native TbpA preparation in the presence of excess competing Mbp::TbpB, this was also detected in the native TbpB preparation, suggesting that it may be due to reactivity against contaminants in these preparations. Unfortunately, we do not have isogenic TbpA− or TbpB− strains of M. catarrhalis that would have allowed us to address this question.
DISCUSSION
Tf receptors in pathogenic gram-negative bacteria from the families Pasteurellaceae and Neisseriaceae play a critical role in acquisition of iron from Tf (7, 15, 23, 27), which appears to be an essential process for survival in vivo (16). Lf receptors in members of the Neisseriaceae are similarly required for acquisition of iron from Lf (8, 9, 29, 35), but the importance of this function in vivo has not been established. The role that these receptor proteins play and their requisite surface accessibility suggest that they may serve as ideal candidates for vaccines and has led to considerable effort at evaluating their utility.
As TbpB is a largely exposed surface lipoprotein anchored by its fatty acyl tails (24), it may be a reasonable target for immunotherapy. The bactericidal activity of antisera prepared against receptor complex from Neisseria meningitidis in rabbits was directed against TbpB (17), suggesting that it may be a more useful target for vaccine development. Further, studies indicate that recombinant TbpBs (produced in E. coli) from N. meningitidis (38), Haemophilus influenzae (30), and M. catarrhalis (34) retain their propensity to bind Tf and ability to generate strain-specific bactericidal and protective anti-TbpB antibodies. Thus, industrial production of TbpB suitable for vaccination purposes may be an achievable goal. The homologue of TbpB involved in Lf iron acquisition has only recently been identified and characterized (8, 11, 29, 35); thus, there is little information on its potential as a vaccine antigen. Nevertheless, recent studies have shown that recombinant LbpB from M. catarrhalis is capable of inducing bactericidal antibodies (11), suggesting that its potential as a vaccine antigen may parallel that of TbpB.
The ability to produce functional antibody in animals supports the potential utility of TbpB and LbpB for human vaccines, but evidence from the human host is ultimately required. Previous studies have demonstrated that convalescent-phase sera from patients infected with H. influenzae (26) or N. meningitidis (6, 21) contains antibody directed against TbpBs. Similarly, convalescent-phase antisera from a natural infection by M. catarrhalis reacted strongly with OMPB1 (43), which was shown to comigrate with Tf binding activity (12), suggesting that the antibody was directed against TbpB. However, comigration is not necessarily a good basis for identification, as the experience with initial identification of TbpB (42) and subsequent confusion with a major 70-kDa protein (4) demonstrate. The use of affinity-purified protein as target (Fig. 1 and 2) or as a blocking agent (31) provides more convincing evidence for a human anti-TbpB response. Furthermore, the use of recombinant TbpB protein as a target for antibody (Fig. 3) or to competitively block reactivity in the binding assays (Fig. 4 and 5) enables us to conclusively state that there is a substantial human antibody response directed against M. catarrhalis TbpB. Our results (Fig. 1 to 5) also enable us to definitively conclude that there is a substantial antibody response directed against LbpB after natural M. catarrhalis infection. The recent identification of LbpB from N. meningitidis (8, 29, 35) should facilitate similar studies with receptor from that species.
Although there are studies demonstrating an antibody response against TbpB and LbpB in humans, there is no evidence for production of functional human antibody against these proteins. However, recombinant TbpBs from veterinary pathogens have been successfully used in active immunization experiments in the native host (36, 40), which, by inference, suggests that production of functional antibody in humans is possible.
A considerable degree of genetic and antigenic heterogeneity has been demonstrated for TbpBs from N. meningitidis (32), H. influenzae (30), and M. catarrhalis (34), probably a consequence of their surface exposure and immunogenicity. Antigenic variation clearly has important implications regarding consideration of TbpB as a vaccine antigen; thus, it is noteworthy that despite the considerable variation, two meningococcal TbpBs were capable of producing functional antibody that cross-reacted with a representative collection of meningococcal strains (39). Analysis of the human antibody response to M. catarrhalis TbpBs also demonstrates the antigenic heterogeneity of these proteins (Fig. 2). Analysis of the predicted amino acid sequences of TbpBs and the bactericidal activity of guinea pig antisera suggests that there may be two broad groups of TbpBs (34), reminiscent of the situation with meningococcal TbpBs (37). In this context, it is interesting that although some of the human antisera readily discriminated between the representative strains from these two groups (4223 and Q8; N141 and N137 antisera [Fig. 2]), other antisera were capable of recognizing both TbpBs (N137 antiserum).
Since LbpBs have only recently been identified (8, 11, 29, 35), there is less information available on genetic and antigenic heterogeneity among these proteins. Sequence comparisons among LbpBs from M. catarrhalis revealed comparatively high levels of identity (77% among LbpBs from three strains) (11). Surprisingly, there is complete identity among the published sequences of meningococcal LbpBs (8, 29, 35). To exclude the possibility that this is a consequence of use of the same strains (even though different strain names were reported), sequences from additional strains will be required. The analysis of human antisera from patients with M. catarrhalis infections demonstrated the presence of cross-reactive antibody against heterologous Lbps (Fig. 1 and 2), but the functionality of this antibody is not known. Preliminary evidence for cross-reactive bactericidal activity of antisera raised against M. catarrhalis LbpBs in guinea pigs are encouraging (11), but the results indicate that in spite of greater sequence similarity, there still is sufficient antigenic heterogeneity to necessitate inclusion of several representative LbpBs for broad-spectrum coverage.
The critical roles that TbpA and LbpA play in iron acquisition from Tf and Lf suggest that they might be preferred targets for immunotherapy. However, several features have limited their consideration for vaccine development. Unlike TbpB and LbpB, recombinant TbpA and LbpA have failed to produce bactericidal antibodies (18, 34). TbpA requires export and assembly in the outer membrane to attain a native conformation capable of binding ligand (22), which may also be necessary for induction of functional antibody (5, 6). This requirement may ultimately limit consideration of intact recombinant protein as a vaccine antigen.
Analysis of convalescent human antisera from patients with meningococcal (6, 21) or M. catarrhalis (Fig. 1, 2, and 4) infections has failed to detect anti-TbpA or anti-LbpA antibody against electroblotted proteins. Since this could also be attributed to failure of electroblotted TbpA and LbpA to attain the appropriate conformation necessary for binding antibody, attempts have been made to use purified TbpA or LbpA in solid-phase binding assays (6) (Fig. 5). Indeed some antibody binding activity is detected in preparations of purified TbpA or LbpA (6) (Fig. 5), albeit less than that observed for TbpB or LbpB. Substantial loss of antibody binding by prior denaturation of the receptor protein (6) provides additional evidence for the antibody being directed at conformational epitopes in TbpA.
One disadvantage of using purified receptor proteins isolated from the original bacterium to evaluate reactivity against human antisera is that it is difficult to totally exclude the contribution of contaminants in the receptor preparation, in spite of the apparent purity by conventional analyses. Thus, addition of extracts from an LbpA− isogenic mutant to dilutions of the human convalescent-phase antiserum effectively eliminated reactivity against purified LbpA (Fig. 5). This finding indicates that the reactivity was due not to LbpA but to some contaminant in the preparation, even though there were no contaminants evident in SDS-PAGE analysis of the affinity-purified LbpA preparation (data not shown). Similarly, although there was no TbpB detected in the purified preparation of TbpA (data not shown), addition of excess recombinant TbpB significantly reduced the detected reactivity against the TbpA preparation (Fig. 5). It is certainly possible that the remaining reactivity was directed against TbpA, but since we did not have an available TbpA− isogenic mutant of M. catarrhalis, we could not exclude the possibility that it was due to other contaminants in the TbpA preparation.
At present we have no conclusive evidence for antibody against TbpA or LbpA from M. catarrhalis in convalescent-phase human sera. In this context, it is interesting that cattle immunized with purified (and functional) preparations of TbpA isolated from the cattle pathogen Pasteurella haemolytica exhibited little or no anti-TbpA antibody response, whereas a robust anti-TbpB response was detected in animals immunized with recombinant TbpB, using the same adjuvant (36). This finding suggests that TbpA may not be immunogenic in the native host, which could possibly be a selected attribute of this protein since, unlike TbpB, it is not subject to the same degree of antigenic variation. Immunization experiments with TbpAs from other veterinary pathogens (in their native hosts) would be useful to establish whether this is a general property of TbpAs. In addition, it may be warranted to reevaluate the evidence for antibody against TbpA from N. meningitidis in convalescent-phase antisera (6), as this also has implications on the immunogenicity of TbpA in the native host and the utility of TbpA as a vaccine antigen. More extensive studies on the immune response against this protein may be essential if TbpA or its derivatives are to be considered for vaccination purposes.
ACKNOWLEDGMENTS
This research was supported by grants MT10350 and UI-13041 from the Medical Research Council of Canada.
We thank Sheena Loosmore and Robin Harkness from Pasteur-Merieux Connaught for their advice and support.
REFERENCES
- 1.Aebi C, Cope L D, Latimer J L, Thomas S E, Slaughter C A, McCracken G H, Jr, Hansen E J. Mapping of a protective epitope of the CopB outer membrane protein of Moraxella catarrhalis. Infect Immun. 1998;66:540–548. doi: 10.1128/iai.66.2.540-548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi C, Maciver I, Latimer J L, Cope L D, Stevens M K, Thomas S E, McCracken G H, Jr, Hansen E J. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect Immun. 1997;65:4367–4377. doi: 10.1128/iai.65.11.4367-4377.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aebi C, Stone B, Beucher M, Cope L D, Maciver I, Thomas S E, McCracken G H, Jr, Sparling P F, Hansen E J. Expression of the CopB outer membrane protein by Moraxella catarrhalis is regulated by iron and affects iron acquisition from transferrin and lactoferrin. Infect Immun. 1996;64:2024–2030. doi: 10.1128/iai.64.6.2024-2030.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ala’Aldeen D A, Davies H A, Wall R A, Borriello S P. The 70 kilodalton iron regulated protein of Neisseria meningitidis is not the human transferrin receptor. FEMS Microbiol Lett. 1990;69:37–42. doi: 10.1016/0378-1097(90)90409-j. [DOI] [PubMed] [Google Scholar]
- 5.Ala’Aldeen D A A, Borriello S P. The meningococcal transferrin-binding proteins 1 and 2 are both surface exposed and generate bactericidal antibodies capable of killing homologous and heterologous strains. Vaccine. 1996;14:49–53. doi: 10.1016/0264-410x(95)00136-o. [DOI] [PubMed] [Google Scholar]
- 6.Ala’Aldeen D A A, Stevenson P, Griffiths E, Gorringe A R, Irons L I, Robinson A, Hyde S, Borriello S P. Immune responses in humans and animals to meningococcal transferrin-binding proteins: implications for vaccine design. Infect Immun. 1994;62:2984–2990. doi: 10.1128/iai.62.7.2984-2990.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson J A, Sparling P F, Cornelissen C N. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonnah R A, Schryvers A B. Preparation and characterization of Neisseria meningitidis mutants deficient in the production of the human lactoferrin binding proteins LbpA and LbpB. J Bacteriol. 1998;180:3080–3090. doi: 10.1128/jb.180.12.3080-3090.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonnah R A, Wong H, Loosmore S M, Schryvers A B. Characterization of Moraxella (Branhamella) catarrhalis lbpB, lbpA, and lactoferrin receptor orf3 isogenic mutants. Infect Immun. 1999;67:1517–1520. doi: 10.1128/iai.67.3.1517-1520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonnah R A, Yu R-H, Schryvers A B. Biochemical analysis of lactoferrin receptors in the Neisseriaceae: identification of a second bacterial lactoferrin receptor protein. Microb Pathog. 1995;19:285–297. doi: 10.1016/s0882-4010(96)80002-7. [DOI] [PubMed] [Google Scholar]
- 11.Bonnah R A, Yu R-H, Wong H, Schryvers A B. Biochemical and immunological properties of lactoferrin binding proteins from Moraxella (Branhamella) catarrhalis. Microb Pathog. 1998;24:89–100. doi: 10.1006/mpat.1997.0173. [DOI] [PubMed] [Google Scholar]
- 12.Campagnari A A, Ducey T F, Rebmann C A. Outer membrane protein B1, and iron-repressible protein conserved in the outer membrane of Moraxella (Branhamella) catarrhalis, binds human transferrin. Infect Immun. 1996;64:3920–3924. doi: 10.1128/iai.64.9.3920-3924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D X, McMichael J C, VanDerMeid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chin N, Frey J, Chang C F, Chang Y F. Identification of a locus involved in the utilization of iron by Actinobacillus pleuropneumoniae. FEMS Microbiology Lett. 1996;143:1–6. doi: 10.1111/j.1574-6968.1996.tb08452.x. [DOI] [PubMed] [Google Scholar]
- 15.Cornelissen C N, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling P F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 17.Danve B, Lissolo L, Mignon M, Dumas P, Colombani S, Schryvers A B, Quentin-Millet M J. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine. 1993;11:1214–1220. doi: 10.1016/0264-410x(93)90045-y. [DOI] [PubMed] [Google Scholar]
- 18.Du R, Wang Q, Yang Y-P, Schryvers A B, Chong P, England D, Klein M H, Loosmore S M. Cloning and expression of the Moraxella catarrhalis lactoferrin receptor genes. Infect Immun. 1998;66:3656–3664. doi: 10.1128/iai.66.8.3656-3665.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faden H, Hong J, Murphy T. Immune response to outer membrane antigens of Moraxella catarrhalis in children with otitis media. Infect Immun. 1992;60:3824–3829. doi: 10.1128/iai.60.9.3824-3829.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fedorova N D, Highlander S K. Plasmids for heterologous expression in Pasteurella haemolytica. Gene. 1997;186:207–211. doi: 10.1016/s0378-1119(96)00704-4. [DOI] [PubMed] [Google Scholar]
- 21.Ferreirós C M, Ferrón L, Criado M T. In vivo human immune response to transferrin-binding protein 2 and other iron-regulated proteins of Neisseria meningitidis. FEMS Immunol Med Microbiol. 1994;8:63–68. doi: 10.1111/j.1574-695X.1994.tb00426.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez G C, Yu R-H, Rosteck P, Schryvers A B. Sequence, genetic analysis, and expression of Actinobacillus pleuropneumoniae transferrin receptor genes. Microbiology. 1995;141:2405–2416. doi: 10.1099/13500872-141-10-2405. [DOI] [PubMed] [Google Scholar]
- 23.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 25.Helminen M E, Maciver I, Latimer J L, Cope L D, McCracken G H, Jr, Hansen E J. A major outer membrane protein of Moraxella catarrhalis is a target for antibodies that enhance pulmonary clearance of the pathogen in an animal model. Infect Immun. 1993;61:2003–2010. doi: 10.1128/iai.61.5.2003-2010.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland J, Langford P R, Towner K J, Williams P. Evidence for in vivo expression of transferrin-binding proteins in Haemophilus influenzae type b. Infect Immun. 1992;60:2986–2991. doi: 10.1128/iai.60.7.2986-2991.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irwin S W, Averill N, Cheng C Y, Schryvers A B. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbp1 and tbp2, from Neisseria meningitidis. Mol Microbiol. 1993;8:1125–1133. doi: 10.1111/j.1365-2958.1993.tb01657.x. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lewis L A, Rohde K, Gipson M, Behrens B, Gray E, Toth S I, Roe B A, Dyer D W. Identification and molecular analysis of lbpBA, which encodes the two-component meningococcal lactoferrin receptor. Infect Immun. 1998;66:3017–3023. doi: 10.1128/iai.66.6.3017-3023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loosmore S M, Yang Y P, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S C, Klein M H. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 31.Mathers K E, Goldblatt D, Aebi C, Yu R H, Schryvers A B, Hansen E J. Characterisation of an outer membrane protein of Moraxella catarrhalis. FEMS Immunol Med Microbiol. 1997;19:231–236. doi: 10.1111/j.1574-695X.1997.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 32.Mazarin V, Rokbi B, Quentin-Millet M J. Diversity of the transferrin binding protein Tbp2 of Neisseria meningitidis. Gene. 1995;158:145–146. doi: 10.1016/0378-1119(95)00151-u. [DOI] [PubMed] [Google Scholar]
- 33.Murphy T F. Branhamella catarrhalis: epidemiology, surface antigenic structure, and immune response. Microbiol Rev. 1996;60:267–279. doi: 10.1128/mr.60.2.267-279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myers L E, Yang Y-P, Du R-P, Wang Q, Harkness R E, Schryvers A B, Klein M H, Loosmore S M. The transferrin binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect Immun. 1998;66:4183–4192. doi: 10.1128/iai.66.9.4183-4192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pettersson A, Prinz T, Umar A, van der Blezen J, Tommassen J. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol Microbiol. 1998;27:599–610. doi: 10.1046/j.1365-2958.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- 36.Potter A A, Schryvers A B, Ogunnariwo J A, Hutchens W, Lo R Y C, Watts T. Protective capacity of Pasteurella haemolytica transferrin-binding proteins TbpA and TbpB in cattle. 1999. Microb. Pathog., in press. [DOI] [PubMed] [Google Scholar]
- 37.Rokbi B, Mazarin V, Maitre-Wilmotte G, Quentin-Millet M J. Identification of two major families of transferrin receptors among Neisseria meningitidis strains based on antigenic and genomic features. FEMS Microbiol Lett. 1993;110:51–58. doi: 10.1111/j.1574-6968.1993.tb06294.x. [DOI] [PubMed] [Google Scholar]
- 38.Rokbi B, Mignon M, Caugant D A, Quentin-Millet M J. Heterogeneity of tbpB, the transferrin-binding protein B gene, among serogroup B from Neisseria meningitidis strains of the ET-5 complex. Clin Diagn Lab Immunol. 1997;4:522–529. doi: 10.1128/cdli.4.5.522-529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rokbi B, Mignon M, Maitre-Wilmotte G, Lissolo L, Danve B, Caugant D A, Quentin-Millet M-J. Evaluation of recombinant transferrin binding protein B variants from Neisseria meningitidis for their ability of induce cross reactive and bactericidal antibodies against a genetically diverse collection of serogroup B strains. Infect Immun. 1997;65:55–63. doi: 10.1128/iai.65.1.55-63.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rossi-Campos A, Anderson C, Gerlach G-F, Klashinsky S, Potter A A, Willson P J. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine. 1992;10:512–518. doi: 10.1016/0264-410x(92)90349-o. [DOI] [PubMed] [Google Scholar]
- 41.Schryvers A B, Lee B C. Comparative analysis of the transferrin and lactoferrin binding proteins in the family Neisseriaceae. Can J Microbiol. 1989;35:409–415. doi: 10.1139/m89-063. [DOI] [PubMed] [Google Scholar]
- 42.Schryvers A B, Morris L J. Identification and characterization of the transferrin receptor from Neisseria meningitidis. Mol Microbiol. 1988;2:281–288. doi: 10.1111/j.1365-2958.1988.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 43.Sethi S, Hill S L, Murphy T F. Serum antibodies to outer membrane proteins (OMPs) of Moraxella (Branhamella) catarrhalis in patients with bronchiectasis: identification of OMP B1 as an important antigen. Infect Immun. 1995;63:1516–1520. doi: 10.1128/iai.63.4.1516-1520.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y P, Myers L E, McGuinness U, Chong P, Kwok Y, Klein M H, Harkness R E. The major outer membrane protein, CD, extracted from Moraxella (Branhamella) catarrhalis is a potential vaccine antigen that induces bactericidal antibodies. FEMS Immunol Med Microbiol. 1997;17:187–199. doi: 10.1111/j.1574-695X.1997.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 45.Yu R-H, Schryvers A B. The interaction between human transferrin and transferrin binding protein 2 from Moraxella (Branhamella) catarrhalis differs from that of other human pathogens. Microb Pathog. 1993;15:443–445. doi: 10.1006/mpat.1993.1092. [DOI] [PubMed] [Google Scholar]