Abstract
We have investigated the effect of preexisting immunity to homologous (Salmonella typhimurium) or heterologous (S. dublin) serotypes of Salmonella on the ability of an attenuated S. typhimurium aroA aroD vector (BRD509) to immunize mice against the heterologous antigen fragment C (FrgC). We studied two strains, BRD847 and BRD937, expressing FrgC carried on plasmids that differ only with respect to the promoter controlling FrgC expression, the nirB promoter in the case of BRD847 and the htrA promoter in the case of BRD937. Mice were preimmunized orally with S. typhimurium BRD509, S. dublin aroA aroD (BRD620), or saline. Forty-four days later, they were immunized orally with BRD847 or BRD937. Prior immunity to S. typhimurium severely depressed the serum immunoglobulin G (IgG) and IgA anti-FrgC response in both BRD847- and BRD937-immunized mice. Mice with existing immunity to S. dublin also had lower IgG anti-FrgC geometric mean titers (GMTs) than did mice preimmunized with saline, but this difference was significant only in the case of mice immunized with BRD937. However, in nonimmune mice or in mice preimmunized with S. typhimurium or S. dublin, the anti-FrgC IgG GMTs were always higher in mice in the BRD937 groups than in the equivalent BRD847 groups. This is reflected in the effect of prior immunity on the ability of oral immunization with BRD847 or BRD937 to protect mice from challenge with a lethal dose of tetanus toxin. All of the mice preimmunized with saline and then immunized with BRD847 or BRD937 survived challenge. Only 20% of the animals immunized with BRD847 and 60% of the mice in the BRD937 group survived tetanus toxin challenge if they were preimmunized with BRD509. Preexisting immunity to S. dublin did not affect the ability of BRD937 to immunize mice against tetanus, but it did reduce the efficiency of BRD847: only 60% percent of the mice survived challenge. The intestinal secretory IgA responses to FrgC were very similar in the BRD847 and BRD937 groups. Prior immunity did depress the IgA anti-FrgC titers but only significantly so in the mice preimmunized with BRD509. These results show that preexisting Salmonella immunity, particularly to homologous serotypes, can severely compromise the ability of live Salmonella vectors to deliver heterologous antigens to the mammalian immune system. However, the results also indicate that this may be overcome by the design of more powerful in vivo expression systems.
Defined nonreverting attenuated strains of Salmonella are being investigated as live vaccines against salmonellosis and as live vectors for delivering heterologous antigens to immune systems of humans and animals (3, 14, 21). Mutations in a number of different genes have been shown to attenuate Salmonella spp. and render them useful as live vaccine and vector strains (3, 14, 21).
A variety of antigens from a number of different organisms have been expressed in Salmonella carriers and have been used to immunize humans and animals (3, 14, 21). Immunization with recombinant Salmonella strains can induce antibody (secretory and circulating) and cellular response to the heterologous antigen. Live Salmonella vaccines can be administered orally, avoiding the need for injections and the possible risk of blood-borne infections that can arise from the reuse of needles, a practice common in the developing world.
Great variation in the immune response to different foreign antigen expressed in Salmonella vectors has been reported (3, 14, 21). A number of parameters that might influence the response to the foreign antigen have been examined; these include expression levels, constitutive versus inducible expression, carrier strain, cellular location of the antigen, and route and number of immunizations (21). One factor that might have a great effect on the efficacy of the Salmonella carriers, the existence of preexisting immunity to the carrier strain, has not been investigated in depth. The few studies performed to date have produced conflicting results; two studies showed prior immunity to the salmonella vector enhanced the response to the foreign antigen, and a third demonstrated a suppressive effect on the response to the heterologous antigen (1, 2, 29). Prior immunity is known to greatly reduce the effectiveness of live viral vectors (23, 30).
We have been developing a single-dose oral tetanus vaccine by using fragment C (FrgC)-expressing Salmonella (6, 8, 12, 21, 22). There is a great need for such a vaccine in the developed world, where nearly 1 million people die from tetanus every year (26). The disadvantages of using the current tetanus vaccine (tetanus toxoid) in the developing world are the need for a cold chain, the need to administer the vaccine by injection, and the need for multiple immunizations.
We have a great deal of experience with expressing FrgC in Salmonella and of the effects that of variables such as gene copy number and the type of promoter can have on the subsequent immune response following immunization (21). We can now consistently induce 100% protection from tetanus in mice by immunizing with a single oral dose of an attenuated Salmonella strain carrying a plasmid which encodes the FrgC gene under the control of the nirB or htrA promoter (6, 22).
In this study, we determined the effect of prior immunization with the carrier strain alone (Salmonella typhimurium aroA aroD) or with a heterologous Salmonella serotype, S. dublin aroA aroD, on the circulating and secretory antibody response to FrgC and protection from tetanus in mice immunized with S. typhimurium aroA aroD expressing FrgC. The effect on the anti-FrgC response of expressing FrgC from different promoters in the face of prior immunity was also examined.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
S. typhimurium BRD509 (SL1344 aroA aroD), BRD847 (BRD509/pTETnir15), and BRD937 (BRD509/pTEThtrA1) and plasmids pTETnir15 and pTEThtrA1 have been described previously (6, 22, 27). The S. dublin aroA aroD strain (BRD620) was a gift from Cliff Hayward. Bacteria were routinely cultured aerobically in L broth or on L agar (9). Ampicillin was included in the growth medium used for BRD847 and BRD937.
Immunization.
For immunization of mice, salmonellae were grown statically overnight in L broth, recovered by centrifugation, and resuspended in sterile phosphate-buffered saline (PBS; pH 7.2) to approximately 1 × 1010 to 5 × 1010 CFU/ml. Female BALB/c mice (6 to 8 weeks old; Charles River, Margate, United Kingdom) were orally immunized with salmonella suspension (0.2 ml) administered by gavage tube as described previously (9). Viable counts were performed on all vaccines.
Measurement of the systemic and local anti-FrgC antibody response.
Anti-FrgC specific serum immunoglobulin G (IgG), IgA, and IgM antibody titers were measured by enzyme-linked immunosorbent assay (ELISA) as previously described (20). Briefly, 96-well enzyme immunoassay/radio immunoassay plates (Costar, High Wycombe, United Kingdom) were coated with recombinant FrgC (50 μl; 2.5 μg/ml in PBS, overnight, 4°C), washed three times with PBS containing 0.05% (vol/vol) Tween 20 (PBST; Sigma), and then blocked with PBS containing 1% bovine serum albumin (BSA). After being washed, plates were incubated with serial dilutions of serum for 2 h at 37°C. All samples and reagents were diluted in PBST containing 0.1% BSA. Plates were washed, incubated with biotin-conjugated goat anti-mouse IgG, IgA, or IgM as appropriate (Sigma), and washed again. Then horseradish peroxidase-conjugated streptavidin (Dako, High Wycombe, United Kingdom) was added, and bound antibodies were visualized by adding o-phenylenediamine substrate (0.04% o-phenylenediamine in citrate-phosphate buffer [pH 5] containing 0.01% H2O2). After color development, the reaction was stopped with 3 M H2SO4 and absorbance was read at 490 nm. Absorbance values were plotted against dilutions, and titers were determined as the reciprocal of the highest sample dilution giving an absorbance of 0.3 optical density unit.
To determine the intestinal antibody response, fresh fecal pellets (two to four per mouse) were collected into microcentrifuge tubes. One milliliter of a solution consisting of 1% (wt/vol) BSA (Sigma) and 1 mM phenylmethyl sulfonyl fluoride (Sigma) in PBS was added to each tube. After being incubated overnight at 4°C, the tubes were vortexed to disrupt all solid material and then centrifuged at 16,000 × g for 5 min. The supernatant was recovered and stored at −20°C until analysis. The fecal extracts were assayed by ELISA for FrgC-specific IgA as described previously (22). The protocol for the ELISA was essentially as described above. To correct for variation in IgA present in each sample, the total IgA concentration of each fecal sample was determined by an IgA-specific capture ELISA as described previously (22). Final results were calculated by dividing the FrgC-specific IgA endpoint titer by the total IgA (micrograms) in the fecal sample (22).
Tetanus toxin challenge.
Mice were challenged with 0.01 μg (50 × the 50% lethal dose [LD50]) of purified tetanus toxin as previously described, and fatalities were recorded for 4 days (6).
RESULTS
We examined the effect of prior immunity to Salmonella spp. on the efficacy of an attenuated S. typhimurium strain, BRD509, to deliver a foreign antigen, FrgC of tetanus toxin, to the murine immune system. BRD509 is an aroA aroD mutant of S. typhimurium. The systemic and local antibody response to FrgC and protection from tetanus toxin were analyzed in mice with and without preexisting Salmonella immunity. Although most Salmonella strains are closely related, immunity to Salmonella infection is largely serotype specific (15–18, 24). To analyze if any influence of preexisting Salmonella immunity on the immune response to a foreign antigen expressed by a Salmonella carrier is serotype specific or nonspecific, we preimmunized mice either with the S. typhimurium carrier strain alone (BRD509) or with BRD620, an S. dublin aroA aroD mutant. S. typhimurium and S. dublin belong to different serogroups, B (S. typhimurium) and D (S. dublin). The effects of different expression systems were also examined. Two derivatives of BRD509, BRD847, and BRD937 which express FrgC were used in this study (6, 22). Both strains possess a plasmid that encodes the FrgC gene. The two plasmids, pTETnir15 and pTEThtrA1, are identical except that the FrgC gene is controlled by different promoters (6, 22). The nirB promoter controls FrgC expression in strain BRD847 (BRD509/pTETnir15); in strain BRD937 (BRD509/pTEThtrA1), FrgC is expressed from the htrA promoter (6, 22). These promoters are induced by different environmental cues, the former by anaerobiosis and the latter by stress such as elevated temperature (6, 22). There is also evidence that both promoters are upregulated as bacteria enter cells (11). A single oral immunization of mice with either BRD847 or BRD937 induces high levels of serum and secretory antibodies to FrgC and complete and long-lasting immunity to tetanus toxin and S. typhimurium.
Groups of 10 mice were preimmunized orally with ∼1010 CFU of either BRD509 or BRD620; control mice received saline. Forty-four days later, mice were separated into groups of five and immunized orally with ∼1010 CFU of either BRD847 or BRD937.
Effect of prior Salmonella immunity on the serum antibody response to FrgC.
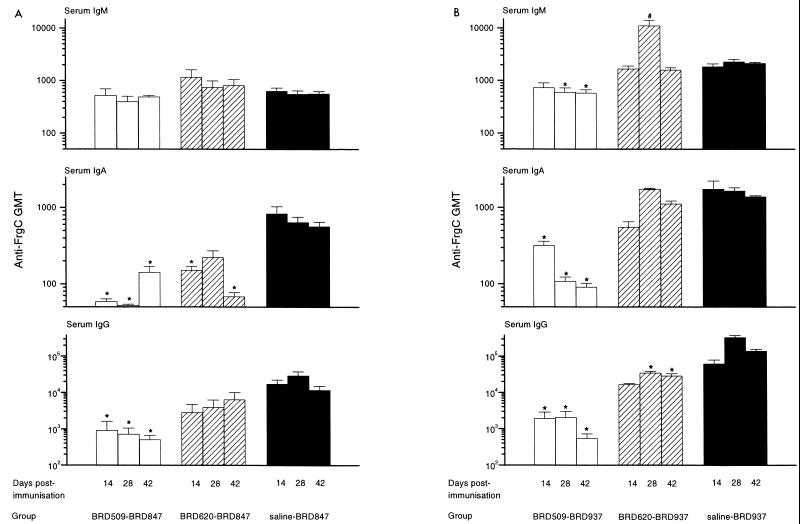
Serum samples were taken 14, 28, and 42 days after immunization with BRD847 or BRD937. The titers of IgG, IgA, and IgM anti-FrgC antibodies in the sera from individual mice were determined by ELISA, and the results are shown in Fig. 1. The anti-FrgC response was examined at several time points to see if prior immunity affected the kinetics of the anti-FrgC response as well as the magnitude of the response.
FIG. 1.
Effect of prior immunity on the serum antibody response to FrgC in BRD847 or BRD937 mice. Mice were preimmunized orally with BRD509, BRD620, or saline and then immunized orally 44 days later with either BRD847 (A) or BRD937 (B). Serum samples were taken from mice on days 14, 28, and 42 after immunization, and IgG, IgA, and IgM serum anti-FrgC titers were determined. Bars represent anti-FrgC GMTs; error bars represent standard errors of the means. ∗, GMT significantly lower (P < 0.05) than the GMT of sera taken at the same time from mice preimmunized with saline; #, GMT significantly higher (P < 0.05) than the GMT of sera taken at the same time from mice preimmunized with saline.
At all time points, the IgG anti-FrgC antibodies were significantly reduced (P < 0.05) in the sera of mice immunized with BRD847 or BRD937 (hereafter referred to as BRD847 or BRD937 mice) if they were previously immunized with the carrier strain BRD509. For example, at the peak of the response (28 days), the geometric mean titers (GMTs) of anti-FrgC IgG in the sera from the control mice (the saline-BRD937 group) and the BRD509-BRD937 mice were 329,570 and 2,060, respectively. The difference in the mean anti-FrgC titers between mice preimmunized with the homologous carrier (BRD509) or saline was greater for the BRD937 group (30- 260-fold) than for the BRD847 group (20- 40-fold). However, in both the saline and BRD509 mice, the anti-FrgC IgG titers were higher in the sera of BRD937 mice than those of the mice in the equivalent BRD847 groups. The kinetics of the IgG anti-FrgC response induced by BRD847 was altered by preimmunization with BRD509 because the peak response in the BRD509-BRD847 mice occurred at 14 days rather than 28 days as for the saline-BRD847 mice. The pattern of the serum IgA response was also different in these mice.
Pre-immunization with BRD509 also reduced the anti-FrgC serum IgA and IgM titers, although the magnitude of the decrease was less than for the IgG titers. The effect was greatest for the IgA response. The reduction in the IgA anti-FrgC GMT was significant (P < 0.05) for both the BRD847 and BRD937 groups at all time points; however, the difference in IgM anti-FrgC GMTs was not significant for any of the time points for the BRD847 group and for only the 28- and 42-day sera for the BRD937 group.
Preimmunization with S. dublin BRD620 also negatively affected the serum antibody response to FrgC in mice immunized BRD847 and BRD937. However, the effect was much lower than that seen for mice immunized with the homologous serotype. For the BRD620-BRD937 mice, the IgG anti-FrgC GMTs were significantly lower at the 28- and 42-day time points than for the saline-BRD937 mice. In contrast, there was no significant difference in titers between the saline-BRD847 and BRD620-BRD847 groups at any time point. However, BRD620-BRD937 mice mounted a stronger anti-FrgC antibody response than BRD620-BRD847 mice, and although immunity to S. dublin reduced the anti-FrgC IgG GMT in the sera of BRD937 mice 4- to 10-fold compared to the saline-BRD937 group, the GMTs were still higher than those of the saline-BRD847 group. Preimmunization with BRD620 also altered the kinetics of the IgG anti-FrgC response.
Preexisting immunity to S. dublin significantly compromises the IgA anti-FrgC response in mice immunized with BRD847. Immunization with BRD620 did not significantly reduce the IgA anti-FrgC GMTs in BRD937 mice, but it did affect the development of the response such that the peak of the response was delayed until day 28 rather than day 14 as was seen in the saline-preimmunized group. The IgM anti-FrgC titers were elevated in the mice preimmunized with BRD620 in both the BRD847 and BRD937 groups, although except for the 28-day sera from the BRD620-BRD937 mice, this increase was slight and nonsignificant.
Effect of prior immunity on the intestinal IgA anti-FrgC response.
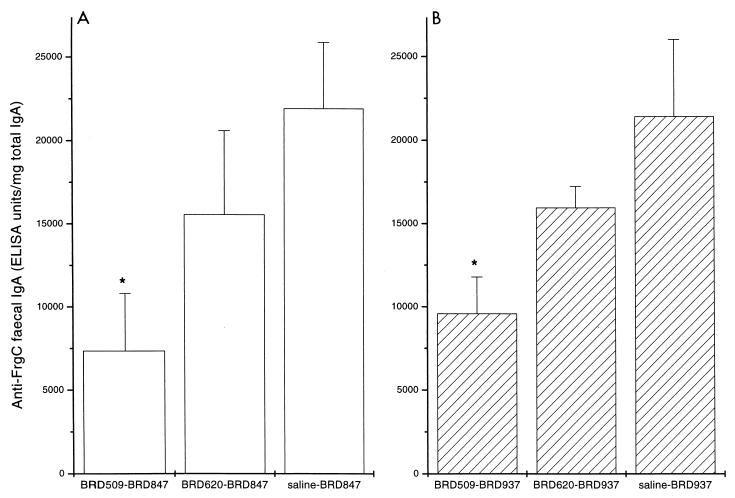
The intestinal anti-FrgC IgA response was measured in fecal extracts. ELISAs were performed on extracts from fecal pellets obtained 28 and 42 days after the second immunization because previous experiments had demonstrated that was when the peak response occurred in mice immunized with BRD847 and BRD937 (22). In the present study the strongest response was seen in the 28-day postimmunization fecal extract samples; the results for these samples are shown in Fig. 2. Similar specific anti-FrgC IgA titers were seen in the mice preimmunized with saline and then BRD847 or BRD937. Preimmunization with both the homologous and the heterologous carrier strain reduced the intestinal response to FrgC in mice subsequently immunized with BRD847 or BRD937. As with the serum responses, the lowest anti-FrgC titers were measured in the samples taken from mice preimmunized with the homologous carrier strain. For both the BRD847 and BRD937 groups, the titers anti-FrgC IgA antibodies measured in fecal extracts of mice preimmunized with BRD509, but not BRD620, were significantly lower (P < 0.05) than those of mice in the saline group. The fold decrease in the fecal anti-FrgC IgA titers of mice preimmunized with BRD509 or BRD620 was very similar in the BRD847 and BRD937 groups.
FIG. 2.
Effect of prior immunity on the intestinal IgA antibody response to FrgC in BRD847 or BRD937 mice. Mice were preimmunized orally with BRD509, BRD620, or saline and then immunized orally 44 days later with either BRD847 (A) or BRD937 (B). Extracts from fecal pellets on day 28 after immunization were prepared, and the IgA anti-FrgC titer was determined and corrected for variation in the total IgA present. Bars represent anti-FrgC GMTs; error bars represent standard errors of the means. ∗, GMT significantly lower (P < 0.05) than the GMT of fecal response of mice preimmunized with saline.
Effect of prior immunity on protection from tetanus.
To evaluate if the reduction in the antibody response to FrgC seen in the mice preimmunized with the Salmonella carriers was significant with regard to protective immunity, we challenged mice with tetanus toxin 53-days after the second immunization and recorded deaths for 4 days; the results are shown in Table 1. Mice that did not have immunological experience of S. typhimurium prior to immunization with BRD847 or BRD937 were fully protected against tetanus, confirming the previous report (22). Preexisting immunity to S. typhimurium greatly reduced the ability of BRD847 and BRD937 to induce protection against tetanus challenge. The effect was greatest in the BRD847 group: only 20% of the mice preimmunized with BRD509 survived tetanus toxin challenge. The BRD509-BRD937 mice were not as affected: 60% of the animals in this group were immune to tetanus. This finding reflects the higher mean anti-FrgC titers in the sera of BRD937 immunized mice. Immunity to BRD620 did not affect the ability of BRD937 to induce protection against tetanus (at the challenge dose of tetanus toxin used). Immunity to BRD620 did, however, compromise the effectiveness of BRD847 at inducing protection to tetanus, as only 60% of the BRD620-BRD847 mice were protected from tetanus challenge.
TABLE 1.
Effect of prior immunity on protection from tetanusa
| Group
|
Tetanus toxin challenge survivors (%) | |
|---|---|---|
| 1st immunization | 2nd immunization | |
| Saline | BRD847 | 100 |
| BRD620 | BRD847 | 60 |
| BRD509 | BRD847 | 20 |
| Saline | BRD937 | 100 |
| BRD620 | BRD937 | 100 |
| BRD509 | BRD937 | 60 |
| Saline | Saline | 0 |
Mice were immunized as described in the text; 53 days after the second immunization, mice were challenged with 50 LD50 of tetanus toxin, and the fatalities were recorded for 4 days.
DISCUSSION
Preexisting immunity to S. typhimurium had a major negative effect on the immune response to FrgC in mice immunized orally with an S. typhimurium aroA aroD vector expressing FrgC. This was reflected in reduced serum and fecal anti-FrgC antibody titers and protection against tetanus toxin compared to mice with no preexisting S. typhimurium immunity. The kinetics of the serum anti-FrgC response was also altered in some cases. The magnitude of the anti-FrgC response in the mice with preexisting immunity to S. typhimurium was dependent on the promoter used by the immunizing strain to express FrgC. The serum anti-FrgC antibody response was much stronger in mice immunized with strain BRD937, in which FrgC expression is under the control of the htrA promoter, than in mice immunized with BRD847, which expresses FrgC from the nirB promoter. This was also the case in the control mice preimmunized with saline. This confirms the superior immunogenicity of BRD937 with regard to the anti-FrgC response as was seen in a previous study (22).
Protection from tetanus toxin is mediated by serum antibodies. It is our experience that BRD847 and BRD937 induce the highest anti-FrgC antibody responses of a number of Salmonella-FrgC constructs that we have investigated (6, 22). A single oral dose of either BRD847 or BRD937 will provide solid long-lasting protection against tetanus. However, in the face of preexisting S. typhimurium immunity, both BRD847 and BRD937 were seriously compromised in the ability to induce immunity to tetanus. As would be expected from the serum anti-FrgC titers, preimmunization with BRD509 had a less dramatic effect on immunity to tetanus in mice immunized with BRD937 than on mice immunized with BRD847.
Existing immunity to a heterologous Salmonella serotype, S. dublin, also depressed the anti-FrgC response in mice receiving BRD847 or BRD937, although the effect was less severe than that seen in mice immune to S. typhimurium. The mean serum IgG anti-FrgC titers in mice given BRD620 and then BRD937 were significantly lower than in mice receiving BRD937 alone. However, the level of serum anti-FrgC antibodies in all of the BRD620-BRD937 mice was still sufficient to protect them from tetanus. In contrast, although the mean IgG anti-FrgC titers were not significantly lower in BRD620-BRD847 mice group than in saline-BRD847 mice, not all mice in the former group had anti-FrgC antibodies sufficient to protect them from the effects of tetanus toxin.
Preexisting Salmonella immunity also impaired the development of a mucosal antibody response to FrgC. The mean fecal IgA anti-FrgC titers were lower in the Salmonella-preimmunized mice than in the controls. As with the serum response, mice preimmunized with the S. typhimurium strain had the lowest anti-FrgC titers.
Of the other studies that have investigated the effect of prior immunity on the efficacy of Salmonella vectors, our results are in agreement with one and conflict with the other two (1, 2, 29). In terms of experimental design and vectors used, our study is closest to that of Bao and Clements (2). They reported that preexisting immunity to either S. typhimurium or S. dublin (induced by immunization with an aroA mutant of each strain) augmented the local and systemic antibody response to the B subunit of Escherichia coli heat-labile enterotoxin (LTB) in mice immunized with an S. dublin aroA carrier expressing LTB (2). The conflicting findings of our study and that of Bao and Clements (2) is unlikely to be explained by differences in the Salmonella carrier strains because both studies used strains that were attenuated due to mutations in the prechorismate (aromatic) pathway. The slight differences between the two studies in the preimmunization and immunization protocols would seem to be too minor to account for the vast differences seen. More likely, the opposing findings of the two studies may be accounted for by differences in the properties and cellular locations of the heterologous antigens studied: FrgC in our study and LTB in the work of Bao and Clements (2).
LTB is a good mucosal immunogen, whereas FrgC is not (10, 19, 19a, 20, 28). The two antigens also differ with respect to dependence on the viability of the Salmonella carrier to induce an immune response. Cardenas et al. (4) reported that mice immunized orally on four occasions with heat-inactivated S. dublin expressing LTB developed serum and intestinal anti-LTB titers that were statistically indistinguishable from those in mice similarly immunized with the viable strain. In contrast, two oral or intravenous doses of heat-killed S. typhimurium expressing FrgC failed to immunize any mice against tetanus toxin challenge (the antibody response was not determined [12]).
The locations of FrgC and LTB within the Salmonella cell are different. FrgC resides within the cytoplasm, whereas LTB is secreted into the periplasm (13, 25). It is likely that LTB, but not FrgC, is able to leak from the Salmonella cell. LTB needs to escape from the periplasm of enterotoxigenic E. coli to exert its effect but lacks specialized machinery to do so (13). As LTB is a good mucosal immunogen, LTB that has escaped from the Salmonella cell could induce a local and systemic antibody response. If this hypothesis is correct, then it is not surprising that LTB responses still occur when mice are immunized with S. dublin expressing LTB even in the face of preexisting anti-Salmonella immunity. The enhanced immunogenicity of LTB in the preimmunized mice may be due to a bystander effect arising from the anamnestic immune response to the Salmonella carrier.
Whittle and Verma reported that intraperitoneal priming with a S. dublin aroA strain enhanced the subsequent antibody response to a viral B-cell epitope when they were immunized intraperitoneally with the same S. dublin strain expressing the B-cell epitope genetically fused to flagellin (29). It is not known if the route of immunization or the fact that only a B-cell epitope was expressed are important to the outcome of the study.
Our results are in agreement with those of Attridge et al. (1), who found that immunization with S. stanley significantly compromised the serum antibody response to K88 in mice subsequently immunized with S. stanley expressing K88. They found that the effect was less severe if the same preimmunizing but a different K88-expressing strain (S. strasbourg) was used.
Further studies with Salmonella strains expressing other heterologous antigens will be necessary to determine if prior Salmonella immunity generally suppresses or augments the antibody response to foreign antigens. It will also be necessary to examine if prior immunity affects the cellular response to foreign antigens as well as the antibody response. Longer-term studies in which the period between preimmunization and immunization is extended are needed to see if the detrimental effect on the immune response to the foreign antigen wanes with time.
Attenuated strains of S. typhi are being developed as improved oral typhoid vaccines and also as live carriers to deliver heterologous antigens. A number of stable attenuated S. typhi mutants have been constructed and are showing great promise in human volunteers. Several S. typhi strains that express FrgC have now been constructed and are awaiting human trials (7, 22). Our findings have serious implications for the development of a Salmonella-based oral tetanus vaccine. For a number of reasons described above, such a vaccine would be ideal for immunizing against tetanus in the developing world. Unfortunately, the people living in such areas may have been exposed to S. typhi. If our findings on the negative effect of prior Salmonella immunity on the response to a Salmonella-carried foreign antigen also apply to humans, then the S. typhi-FrgC vaccine may not be efficacious in individuals living in areas where typhoid is endemic. However, our results also suggest possible ways to overcome this problem. Although immunity to a heterologous serotype also reduce the effectiveness of the S. typhimurium carrier, the effect was much less than that seen in mice which were immune to S. typhimurium. It is possible that a carrier unrelated to S. typhi, for example, one derived from a paratyphoid strain, may be effective in individuals in areas where typhoid is endemic. We found that the more immunogenic the strain, the lower the effects of prior immunity. By searching for new promoter and expression systems for FrgC, it may be possible to construct strains that are able to induce protective immunity even in the face of preexisting immunity to the carrier itself.
Existing immunity to tetanus toxin, or derivatives thereof, should not affect the ability of a Salmonella carrier expressing FrgC to induce anti-FrgC response. In fact, Chabalagoity et al. (5) found that the anti-tetanus toxoid serum antibody response was greater in mice that had been preimmunized with tetanus toxoid than in mice with no prior immunity to tetanus toxoid.
ACKNOWLEDGMENTS
We thank Susan Humphreys and Andrew Stevenson for critical reading of the manuscript.
This work was supported by grant P05639 from the BBSRC.
REFERENCES
- 1.Attridge S R, Davies R, LaBrooy J T. Oral delivery of foreign antigens by attenuated Salmonella: consequences of prior exposure to the vector strain. Vaccine. 1997;15:155–162. doi: 10.1016/s0264-410x(96)00158-2. [DOI] [PubMed] [Google Scholar]
- 2.Bao J X, Clements J D. Prior immunologic experience potentiates the subsequent antibody response when Salmonella strains are used as vaccine carriers. Infect Immun. 1991;59:3841–3845. doi: 10.1128/iai.59.10.3841-3845.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cardenas L, Clements J D. Oral immunization using live attenuated Salmonella spp. as carriers of foreign antigens. Clin Microbiol Rev. 1992;5:328–342. doi: 10.1128/cmr.5.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cardenas L, Dasgupta U, Clements J D. Influence of strain viability and antigen dose on the use of attenuated mutants of Salmonella as vaccine carriers. Vaccine. 1994;12:833–840. doi: 10.1016/0264-410x(94)90293-3. [DOI] [PubMed] [Google Scholar]
- 5.Chabalagoity J A, Villareal-Ramos B, Khan C M, Chatfield S N, de Hormaeche R D, Hormaeche C E. Influence of preimmunization with tetanus toxoid on immune responses to tetanus toxin fragment C-guest antigen fusions in a Salmonella vaccine carrier. Infect Immun. 1995;63:2564–2569. doi: 10.1128/iai.63.7.2564-2569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatfield S N, Charles I G, Makoff A J, Oxer M D, Dougan G, Pickard D, Slater D, Fairweather N F. Use of the nirB promoter to direct the stable expression of heterologous antigens in Salmonella oral vaccine strains: development of a single-dose oral tetanus vaccine. Bio/Technology. 1992;10:888–892. doi: 10.1038/nbt0892-888. [DOI] [PubMed] [Google Scholar]
- 7.Chatfield S N, Fairweather N, Charles I, Pickard D, Levine M, Hone D, Posada M, Strugnell R A, Dougan G. Construction of a genetically defined Salmonella typhi Ty2 aroA, aroC mutant for the engineering of a candidate oral typhoid-tetanus vaccine. Vaccine. 1992;10:53–60. doi: 10.1016/0264-410x(92)90420-o. [DOI] [PubMed] [Google Scholar]
- 8.Clare J J, Rayment F B, Ballantine S P, Sreekrishna K, Romanos M A. High-level expression of tetanus toxin fragment C in Pichia pastoris strains containing multiple tandem integrations of the gene. Bio/Technology. 1991;9:455–460. doi: 10.1038/nbt0591-455. [DOI] [PubMed] [Google Scholar]
- 9.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 10.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everest P, Frankel G, Li J, Lund P, Chatfield S, Dougan G. Expression of LacZ from the htrA, nirB and groE promoters in a Salmonella vaccine strain: influence of growth in mammalian cells. FEMS Microbiol Lett. 1995;126:97–101. doi: 10.1111/j.1574-6968.1995.tb07398.x. [DOI] [PubMed] [Google Scholar]
- 12.Fairweather N F, Chatfield S N, Makoff A J, Strugnell R A, Bester J, Maskell D J, Dougan G. Oral vaccination of mice against tetanus by use of a live attenuated Salmonella carrier. Infect Immun. 1990;58:1323–1326. doi: 10.1128/iai.58.5.1323-1326.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairweather N F, Lyness V A, Maskell D J. Immunization of mice against tetanus with fragments of tetanus toxin synthesized in Escherichia coli. Infect Immun. 1987;55:2541–2545. doi: 10.1128/iai.55.11.2541-2545.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hormaeche C E, Anjam Khan C M, Mastroenii P, Villareal B, Roberts M, Dougan G, Chatfield S. Salmonella vaccines: mechanisms of immunity and their use as carriers of recombinant antigens. In: Ala Aldeen D A, Hormaeche C E, editors. Molecular and clinical aspects of bacterial vaccine development. Chichester, England: John Wiley & Sons Ltd.; 1995. pp. 119–154. [Google Scholar]
- 15.Hormaeche C E, Joysey H S, Desilva L, Izhar M, Stocker B A. Immunity conferred by Aro− Salmonella live vaccines. Microb Pathog. 1991;10:149–158. doi: 10.1016/0882-4010(91)90075-l. [DOI] [PubMed] [Google Scholar]
- 16.Hormaeche C E, Mastroeni P, Harrison J A, Demarco de Hormaeche R, Svenson S, Stocker B A. Protection against oral challenge three months after i.v. immunization of BALB/c mice with live Aro Salmonella typhimurium and Salmonella enteritidis vaccines is serotype (species)-dependent and only partially determined by the main LPS O antigen. Vaccine. 1996;14:251–259. doi: 10.1016/0264-410x(95)00249-z. [DOI] [PubMed] [Google Scholar]
- 17.Hsu H S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989;53:390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lindberg A A, Segall T, Weintraub A, Stocker B A D. Antibody response and protection against challenge in mice vaccinated intraperitoneally with a live aroA O4,O9 hybrid Salmonella dublin strain. Infect Immun. 1993;61:1211–1221. doi: 10.1128/iai.61.4.1211-1221.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nashar T O, Webb H M, Eaglestone S, Williams N A, Hirst T R. Potent immunogenicity of the B subunits of Escherichia coli heat-labile enterotoxin-receptor-binding is essential and induces differential modulation of lymphocyte subsets. Proc Natl Acad Sci USA. 1996;93:226–230. doi: 10.1073/pnas.93.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Roberts, M. Unpublished observation.
- 20.Roberts M, Bacon A, Rappuoli R, Pizza M, Cropley I, Douce G, Dougan G, Marinaro M, McGhee J, Chatfield S. A mutant pertussis toxin molecule that lacks ADP-ribosyltransferase activity, PT-9K/129G, is an effective mucosal adjuvant for intranasally delivered proteins. Infect Immun. 1995;63:2100–2108. doi: 10.1128/iai.63.6.2100-2108.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts M, Chatfield S N, Dougan G. Salmonella as carriers of heterologous antigens. In: O’Hagan D T, editor. Novel delivery systems for oral vaccines. Boca Raton, Fla: CRC Press; 1994. pp. 27–58. [Google Scholar]
- 22.Roberts M, Li J, Bacon A, Chatfield S. Oral vaccination against tetanus: comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters. Infect Immun. 1998;66:3080–3087. doi: 10.1128/iai.66.7.3080-3087.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rooney J F, Wohlenberg C, Cremer K J, Moss B, Notkins A L. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J Virol. 1988;62:1530–1534. doi: 10.1128/jvi.62.5.1530-1534.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segall T, Lindberg A A. Oral vaccination of calves with an aromatic-dependent Salmonella dublin (O9,12) hybrid expressing O4,12 protects against S. dublin (O9,12) but not against Salmonella typhimurium (O4,5,12) Infect Immun. 1993;61:1222–1231. doi: 10.1128/iai.61.4.1222-1231.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spangler B A. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanfield J P, Galazka A. Neonatal tetanus in the world today. Bull W H O. 1984;62:647–669. [PMC free article] [PubMed] [Google Scholar]
- 27.Strugnell R, Dougan G, Chatfield S, Charles I, Fairweather N, Tite J, Li J L, Beesley J, Roberts M. Characterization of a Salmonella typhimurium aro vaccine strain expressing the P.69 antigen of Bordetella pertussis. Infect Immun. 1992;60:3994–4002. doi: 10.1128/iai.60.10.3994-4002.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi I, Marinaro M, Kiyono H, Jackson R J, Nakagawa I, Fujihashi K, Hamada S, Clements J D, Bost K L, McGhee J R. Mechanisms for mucosal immunogenicity and adjuvancy of Escherichia coli labile enterotoxin. J Infect Dis. 1996;173:627–635. doi: 10.1093/infdis/173.3.627. [DOI] [PubMed] [Google Scholar]
- 29.Whittle B L, Verma N K. The immune response to a B-cell epitope delivered by Salmonella is enhanced by prior immunological experience. Vaccine. 1997;15:1737–1740. doi: 10.1016/s0264-410x(97)00119-9. [DOI] [PubMed] [Google Scholar]
- 30.Yei S, Mittereder N, Tang K, O’Sullivan C, Trapnell B C. Adenovirus-mediated gene transfer for cystic fibrosis: quantitative evaluation of repeated in vivo vector administration to the lung. Gene Ther. 1994;1:192–200. [PubMed] [Google Scholar]