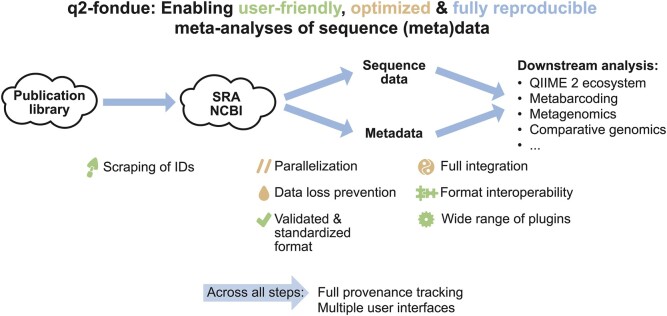
Abstract
Motivation
The volume of public nucleotide sequence data has blossomed over the past two decades and is ripe for re- and meta-analyses to enable novel discoveries. However, reproducible re-use and management of sequence datasets and associated metadata remain critical challenges. We created the open source Python package q2-fondue to enable user-friendly acquisition, re-use and management of public sequence (meta)data while adhering to open data principles.
Results
q2-fondue allows fully provenance-tracked programmatic access to and management of data from the NCBI Sequence Read Archive (SRA). Unlike other packages allowing download of sequence data from the SRA, q2-fondue enables full data provenance tracking from data download to final visualization, integrates with the QIIME 2 ecosystem, prevents data loss upon space exhaustion and allows download of (meta)data given a publication library. To highlight its manifold capabilities, we present executable demonstrations using publicly available amplicon, whole genome and metagenome datasets.
Availability and implementation
q2-fondue is available as an open-source BSD-3-licensed Python package at https://github.com/bokulich-lab/q2-fondue. Usage tutorials are available in the same repository. All Jupyter notebooks used in this article are available under https://github.com/bokulich-lab/q2-fondue-examples.
Supplementary information
Supplementary data are available at Bioinformatics online.
Graphical Abstract
1 Introduction
The increasing volume of publicly available nucleotide sequence data is driving a revolution in the life sciences, by enabling comparative studies to discover generalizable trends that are often inaccessible or underpowered in an individual study. Individual studies addressing similar biological questions can differ in many technical aspects, including (but not limited to) specific experimental design, employed sequencing technologies, definitions of the examined target variables and selection of potential covariates influencing the target. These inter-study variations can make individual study results biased (Serghiou et al., 2016) and even contradictory to one another (Ioannidis and Trikalinos, 2005). Meta-analysis allows the synthesis of findings from individual studies to reach a more complete understanding: identifying consistent and reproducible signatures across studies and resolving causes of variation among study results (Gurevitch et al., 2018).
Meta-analyses of nucleotide sequencing-based studies have intensified within the past decade (see Fig. 1), given the high potential of these data for reuse in comparative analyses. Meta-analyses of genome-wide association data have expanded our knowledge of human polygenic disorders and quantitative traits (Panagiotou et al., 2013). Comparative genomics has given insights into vertebrate genome evolution (Meadows and Lindblad-Toh, 2017) and the processes of genome function, speciation, selection and adaptation (Alföldi and Lindblad-Toh, 2013). Comparative analyses of global microbiome datasets have driven deepening insight into spatiotemporal and biogeographic variation in Earth’s microbial diversity (Tara Oceans Consortium Coordinators et al., 2015; Thompson et al., 2017). Re-analysis of the published genome and metagenome data has enabled the discovery of novel and candidate microbial clades, as in the Genome Taxonomy Database (Parks et al., 2017), and highlighted the abundance (Lloyd et al., 2018) and ecosystem-impact (Zamkovaya et al., 2021) of uncultured microbes, also known as ‘microbial dark matter’. Since meta-analyses can only be conducted if the original study data are publicly available, the recent increase in meta-analyses can be partly attributed to the ongoing open-science efforts of making sequencing data and accompanying metadata standardized and publicly available (Berman et al., 2014; McNutt et al., 2016; The Path to Open Data, 2019; Yilmaz et al., 2011). The Sequence Read Archive (SRA), established as part of the International Nucleotide Sequence Database Collaboration (INSDC) by the National Center for Biotechnology Information (NCBI), enables free access to sequence data (Kodama et al., 2012; Leinonen et al., 2011b), including sequences stored on ENA (Leinonen et al., 2011a) and DDBJ (Mashima et al., 2017). Since its creation in 2009, the SRA has gathered data at the petabyte scale and continues to scale its infrastructure to ensure efficient data storage and retrieval (Katz et al., 2021).
Fig. 1.
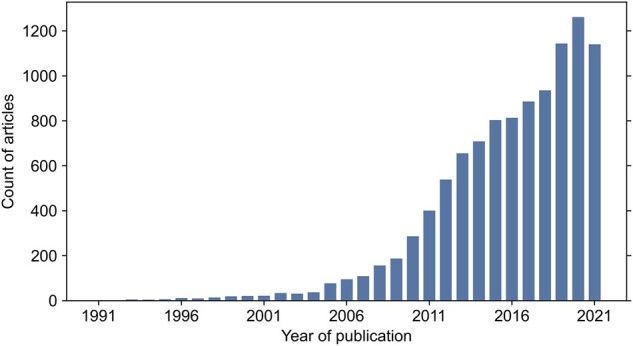
Increasing trend of sequencing-based meta-analyses over the past 30 years. Displayed article counts were retrieved from PubMed on February 21, 2022 with the search query ‘(meta-analysis) AND ((omics) OR (genom*) OR (microbio*) OR (transcriptom*))’ and a requirement for the article type to be a meta-analysis
A selection of tools to programmatically access data from the SRA has recently emerged. NCBI offers the sra-tools command-line toolkit (Leinonen et al., 2011b) for downloading and interacting with raw sequence data. The entrezpy Python library (Buchmann and Holmes, 2019) aids in automating the data download from NCBI’s Entrez databases by providing abstract classes allowing custom implementations. pysradb (Choudhary, 2019) makes use of the curated metadata database available through the SRAdb project (Zhu et al., 2013) to download data from the SRA, and grabseqs enables fetching of data from not only the SRA but also MG-RAST (Meyer et al., 2008) and iMicrobe (Youens-Clark et al., 2019). However, the wider application of these tools in large comparative analyses is hindered by several challenges, including technical complication and a steep learning curve for users with limited programming skills, and the difficulty of tracking data provenance necessary for reproducibility and traceability.
In order to provide consistent and reliable findings, meta-analyses must follow Findable, Accessible, Interoperable and Reusable (FAIR) Guiding Principles (Wilkinson et al., 2016). To this end, meta-analyses should be performed in a reproducible manner, making use of consistent workflows while keeping track of all the performed data retrieval and analysis steps. Despite increasingly facilitated access to sequencing data, reproducibility and provenance of primary and secondary studies remain challenging (Amann et al., 2019; Baker, 2016; Huang and Gottardo, 2013; Kim et al., 2018; Reichman et al., 2011). Here, we introduce an open-source software package q2-fondue (Functions for reproducibly Obtaining and Normalizing Data re-Used from Elsewhere) to expedite the initial acquisition of data from the SRA, while offering complete provenance tracking. q2-fondue simplifies retrieval of sequencing data and accompanying metadata in a validated and standardized format interoperable with the QIIME 2 ecosystem (Bolyen et al., 2019). By allowing access through multiple QIIME 2 user interfaces, it can be employed by users of different computational capabilities.
Here, we describe the q2-fondue software package and subsequently demonstrate its use in comparative analyses of marker gene, genome and metagenome sequencing studies. We anticipate q2-fondue will lower existing barriers to comparative analyses of nucleotide sequence data, facilitating more transparent, open and reproducible conduct of meta-analyses.
2 Implementation
2.1 Software overview
q2-fondue is an open source Python 3 package released under the BSD 3-clause license. It can be installed in a conda environment on any UNIX-based system as described in the installation instructions provided on the package website (https://github.com/bokulich-lab/q2-fondue). q2-fondue has been implemented as a QIIME 2 plugin, allowing the use of QIIME 2’s integrated data provenance tracking system, multiple user interfaces and streamlined interoperability with downstream sequence analysis plugins.
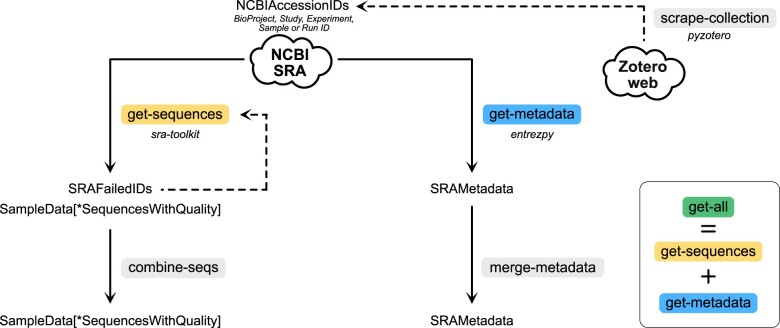
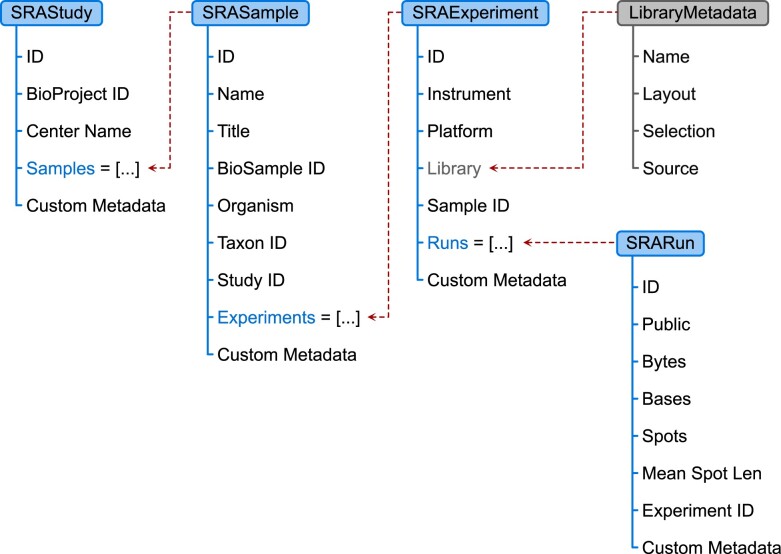
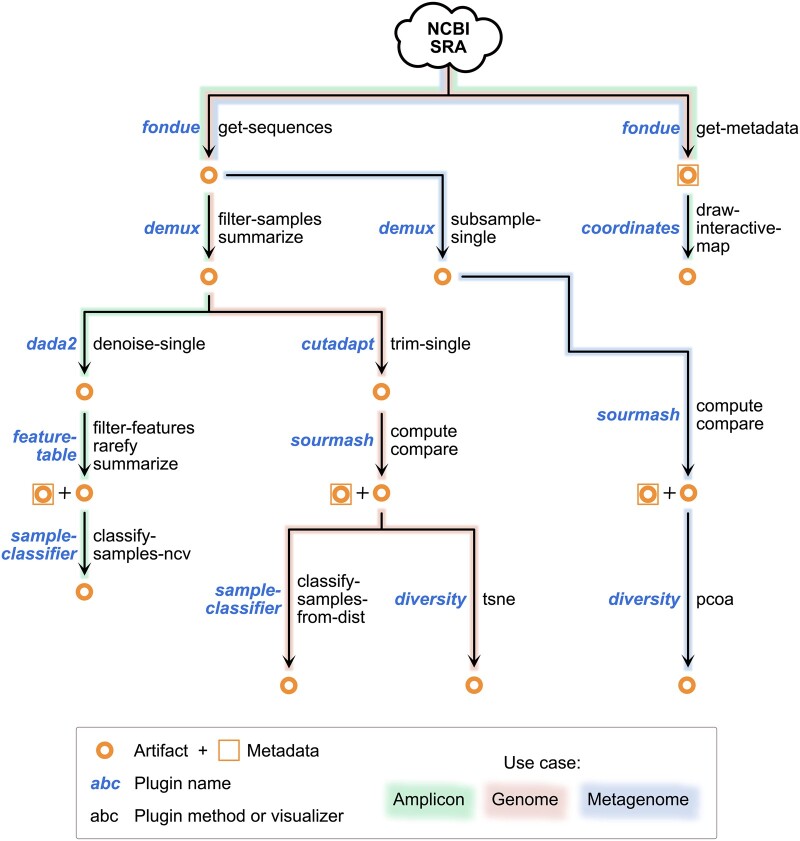
An overview of q2-fondue is shown in Figure 2. Two separate q2-fondue actions allow easy access to the SRA database: get-sequences and get-metadata fetch per-sample sequence data and corresponding metadata (e.g. sample and run information), respectively. The get-all pipeline wraps both of these actions to simultaneously retrieve sequences and metadata for a list of SRA accessions. These three actions, get-sequences, get-metadata and get-all, require a single input file containing accession IDs of one type to be fetched. Currently, q2-fondue supports BioProject, study, sample, experiment and run accession IDs. BioProject and study IDs are in a one-to-one relationship, where a study ID denotes the SRA record of the associated BioProject ID. All other IDs are in a one-to-many relationship in the listed order (e.g. one study ID, with its linked BioProject ID, can have many sample IDs associated with it—see Fig. 3 for an overview). Run IDs allow direct interaction with the SRA databases, while the other IDs are first translated into corresponding run IDs using a chain of E-Direct utilities (Kans, 2013; https://www.ncbi.nlm.nih.gov/books/NBK179288/). An E-Search query is executed to look up provided IDs in the BioProject (if BioProject ID was provided as input) or SRA (if study, sample or experiment ID was provided as input) database, followed by an E-Link query finalized by an E-Fetch query to retrieve the linked run IDs.
Fig. 2.
Overview of q2-fondue methods. get-sequences method can be used to fetch raw sequencing data (single- and/or paired-end), while get-metadata can download corresponding metadata. Both methods can be run simultaneously by using the get-all pipeline, which produces all outputs (SampleData, SRAFailedIDs and SRAMetadata). get-sequences, get-metadata and get-all are run with a list of one type of accession IDs (BioProject, study, sample, experiment or run ID). Sequences obtained from multiple fetches can be combined using combine-seqs and multiple metadata artifacts can be merged with merge-metadata. All accession IDs can be retrieved from Zotero web library collections with the scrape-collection action
Fig. 3.
Structure of the SRA metadata data classes used by the get-metadata action. Each of the classes represents a different level of metadata organization and can contain other nested metadata objects. As all the objects are linked together, metadata of the entire study and all its children can be retrieved directly from the SRAStudy object
All data-fetching actions support configurable parallelization to maximally reduce the processing time. The get-metadata method employs a multi-threading approach built into the Entrezpy modules (see Section 2.2.), while get-sequences uses multiple processes and queues to coordinate the data download with its pre- and post-processing steps. Note that parallelization does not improve download speed (for which network bandwidth is the limiting factor), but markedly decreases pre- and post-processing runtime (e.g. data validation steps) (Supplementary Fig. S1).
2.2 Sequence retrieval
The get-sequences action makes use of the sra-tools command-line toolkit (Leinonen et al., 2011b; https://github.com/ncbi/sra-tools) developed by NCBI. The prefetch tool is first used to reliably fetch SRA datafiles using the provided run IDs and the fasterq-dump utility is then executed to retrieve the corresponding sequences (single- or paired-end) in the FASTQ format. To follow QIIME 2’s naming convention those sequences are then renamed using their accession IDs, compressed and finally validated by QIIME 2’s built-in type validation system. The action keeps track of any errors that occurred while fetching the sequences and performs available storage checks on every iteration to ensure no data are lost when space is exhausted during download. There are three output files generated by the get-sequences action: two QIIME artifacts corresponding to single- and paired-end reads, respectively, and one table containing the list of IDs for which the download failed (if any) including the linked error messages.
2.2 Metadata retrieval
Retrieval of SRA metadata is possible through the get-metadata action. This action uses the Entrezpy package (Buchmann and Holmes, 2019) to interact with the SRA database by building on top of its built-in modules for different E-Direct utilities. Specifically, we implemented a new EFetchResult and EFetchAnalyzer that work in tandem to request and parse metadata for the provided run IDs. The result is represented as a table where a single row corresponds to one SRA run and columns reflect all the metadata fields found in the obtained response. In order to keep track of different metadata levels (study, sample, experiment and run), we introduced a set of cascading Python data classes to delineate the hierarchical relationship of the SRA metadata entries (Fig. 3) and to preserve this structure in the final study metadata. Moreover, tight integration with QIIME 2’s internal type validation system guarantees consistency of metadata generated by q2-fondue by ensuring the presence of all required metadata fields, as specified by NCBI.
2.2 Metadata retrieval
The q2-fondue package contains additional functions to simplify sequencing (meta)data retrieval and manipulation, particularly when multiple data fetches are necessary.
get-all allows the simultaneous download of sequences and related metadata.
merge-metadata concatenates metadata tables obtained from several independent get-metadata runs and allows the generation of a single, unified metadata artifact.
combine-seqs merges sequences obtained from multiple artifacts obtained from several get-sequences runs (or from other external sources) into a single sequence artifact.
scrape-collection retrieves accession IDs and associated DOI names from a Zotero web library collection (https://www.zotero.org) by using the pyzotero package (Hügel et al., 2019). This enables seamless workflows for collecting IDs of interest from a literature collection, automatically downloading the data, and processing downstream with q2-fondue and QIIME 2.
3 Materials and methods
The q2-fondue plugin can be used to facilitate comparative analysis of any nucleotide sequence data and metadata available on the SRA. To demonstrate some example use cases, we used q2-fondue and QIIME 2 to analyze publicly available marker gene, whole genome sequence and shotgun metagenome data. All analyses described below are available as fully reproducible and executable Jupyter notebooks (available in the Data Availability section). These examples are provided merely as method demonstrations to showcase seamless integration/interoperation of q2-fondue with downstream analyses and do not represent complete analyses of biological data. Additional steps and larger comparative analyses would be required to derive meaningful conclusions, and to eliminate potential biases from covariates, which were not controlled for in this demonstration analysis.
3.1 Marker gene amplicon sequence data analysis
Marker gene amplicon sequencing (e.g. of 16S rRNA genes) is currently the most popular method for high-throughput, untargeted profiling of microbial communities as well as non-microbial diet metabarcoding and environmental DNA applications. To demonstrate the use of q2-fondue for comparative cross-study analysis of marker gene amplicon sequence data, we selected three studies that analyzed the development of the infant gut microbiome in distinct geographical locations: Lewis et al. (2017) with BioProjectID PRJEB16321, Davis et al. (2017) with BioProjectID PRJEB15633 and McClorry et al. (2018) with BioProjectID PRJEB23239. All three studies sequenced 16S rRNA genes in the V4 region with the forward 515F primer and a read length of 251 to 253 nucleotides. McClorry et al. (2018) additionally used the reverse primer R806.
The get-all action of q2-fondue was used to retrieve metadata and sequence data of all three studies from the SRA. To normalize metadata features of interest across studies, namely age and health status, we employed the Python library pandas (McKinney, 2010; Reback et al., 2020). The downloaded single-read gene sequences were filtered according to the availability of metadata with the q2-demux QIIME 2 plugin (https://github.com/qiime2/q2-demux), denoised with the q2-dada2 QIIME 2 plugin (Callahan et al., 2016), https://github.com/qiime2/q2-dada2) and finally features were filtered by frequency, rarefied and summarized with the q2-feature-table QIIME 2 plugin (Bokulich et al., 2018b) https://github.com/qiime2/q2-feature-table). Finally, the processed metadata and sequence data were used to train two Random Forest classifiers with q2-sample-classifier (Bokulich et al., 2018a), https://github.com/qiime2/q2-sample-classifier) to predict an infant’s age and its health status from its gut microbiome. The infant’s age was reported in four binned age groups: 0–1, 1–4, 4–6 and older than 6 months. For the health status, an infant was identified as healthy if no disease-related features were reported, namely no indication of stunting, wasting, underweight, elevated C-reactive protein status, parasites or anemia. Both classifiers were trained and tested with 10-fold cross-validation using Random Forest classifiers grown with 500 trees. The performance of the trained classifiers was evaluated on the area under the curve (AUC) of the receiver operating characteristics (ROC) curve of the test set of each fold using scikit-learn implementations (Pedregosa et al., 2011). The entire analysis can be reproduced by executing the u1-amplicon.ipynb Jupyter notebook, available in the Data Availability section.
3.2 Comparative whole-genome sequence data analysis
The initial list of SARS-CoV-2 whole genome sequencing data accession IDs was generated based on the metadata obtained from the Nextstrain.org platform (Hadfield et al., 2018; https://data.nextstrain.org/files/ncov/open/metadata.tsv.gz, access date: February 08, 2022). Only records with available SRA accession IDs and the least amount of missing data (column QC_missing_data == ‘good’) were retained. Furthermore, to limit the scope of this use case only those geographical regions were considered where enough samples were collected (at least 2000 samples per region). The get-metadata action of q2-fondue was used to retrieve metadata of 37 500 randomly sampled genomes (12 500 genomes per location) from the SRA. Obtained metadata was then supplemented with the original Nextstrain metadata by merging the two datasets on a common SRA run ID and only samples sequenced using single-end reads on the Illumina NextSeq 500/550 platforms were retained. Finally, sequencing data for 500 randomly sampled genomes (250 genomes per location) was fetched from the SRA using q2-fondue’s get-sequences method. Reads shorter than 35 nt were discarded using the trim_single method from the q2-cutadapt plugin (Martin, 2011; https://github.com/qiime2/q2-cutadapt) and the quality of the sequences was evaluated using the summarize action from the q2-demux QIIME 2 plugin (https://github.com/qiime2/q2-demux). MinHash signatures were computed for every sample and compared using the q2-sourmash plugin (compute and compare methods, respectively) (Ondov et al., 2016; https://github.com/dib-lab/q2-sourmash). A t-SNE plot was generated from the resulting distance matrix using q2-diversity (tsne method with a learning rate of 125 and perplexity set to 18, https://github.com/qiime2/q2-diversity, Halko et al., 2011) and visualized using matplotlib and seaborn Python packages (Hunter, 2007; Waskom, 2021). Finally, to determine whether MinHash signatures are predictive of SARS-CoV-2 geographic origin, k-nearest-neighbors classification with 10-fold cross-validation was applied through the q2-sample-classifier plugin (Bokulich et al., 2018a; https://github.com/qiime2/q2-sample-classifier). The entire analysis can be reproduced by executing the u2-genome.ipynb Jupyter notebook, available in the Data Availability section.
3.3 Shotgun metagenome sequence data analysis
To fetch metadata for all the shotgun metagenome samples from the Tara Oceans Expedition (Tara Oceans Consortium Coordinators et al., 2015) we used q2-fondue’s get-metadata action with the following BioProject IDs: PRJEB1787, PRJEB4352, PRJEB4419, PRJEB9691, PRJEB9740 and PRJEB9742. After the removal of missing values, the resulting metadata table was randomly sampled to 100 records and used as input to the draw-interactive-map action from the q2-coordinates plugin (Bokulich and Caporaso, 2018; https://github.com/bokulich-lab/q2-coordinates) to visualize values of the sensors used during the expedition according to their geographical location. Additionally, sequences corresponding to 10 samples at two different locations were fetched using the get-sequences action. To reduce the computational time required for this use case demonstration, the reads were subsampled to 20% of the original read count and the resulting artifact (containing single-end reads) was used to calculate and compare MinHash signatures (see the previous section). A PCoA analysis was performed on the resulting distance matrix using the pcoa action from the q2-diversity plugin (https://github.com/qiime2/q2-diversity;Halko et al., 2011) and the PCoA plot was visualized using matplotlib and seaborn Python packages (Hunter, 2007; Waskom, 2021). The entire analysis can be reproduced by executing the u3-metagenome.ipynb Jupyter notebook, available in the Data Availability section.
4 Results
Any meta-analysis can be carried out using raw experimental data, its associated metadata or a combination of both. To demonstrate the versatility of q2-fondue in all those scenarios, and seamless integration/interoperability with downstream bioinformatics tools, we performed three example use case data analyses using amplicon, whole genome, and shotgun metagenome sequencing data and related metadata. All three use cases employ QIIME 2 plugins to process received data and illustrate how q2-fondue can immensely increase data analysis reproducibility and transparency by including details on the raw data fetching in the QIIME 2 provenance. These analyses are only meant to demonstrate some example use cases for q2-fondue and should not be interpreted as biologically meaningful results.
4.1 Use Case 1: amplicon sequence data analysis
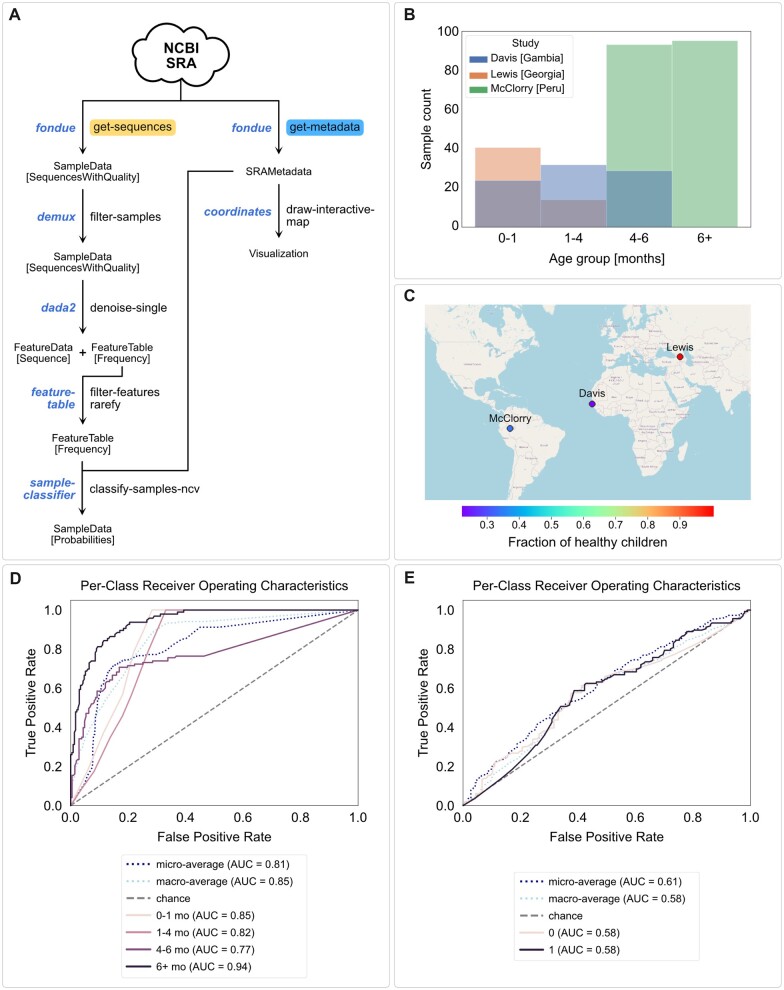
As a demonstration of q2-fondue’s capacities in enabling the collection and analysis of amplicon sequencing data, we selected three infant gut microbiome development studies from distinct geographical locations: the study by Lewis et al. (2017) from Georgia, Davis et al. (2017)’s study from Gambia and McClorry et al. (2018)’s study from Peru. We used the BioProject IDs reported by those studies to fetch the corresponding raw metadata and sequencing data. This provided us with 350 sequence samples each annotated with 148 metadata features.
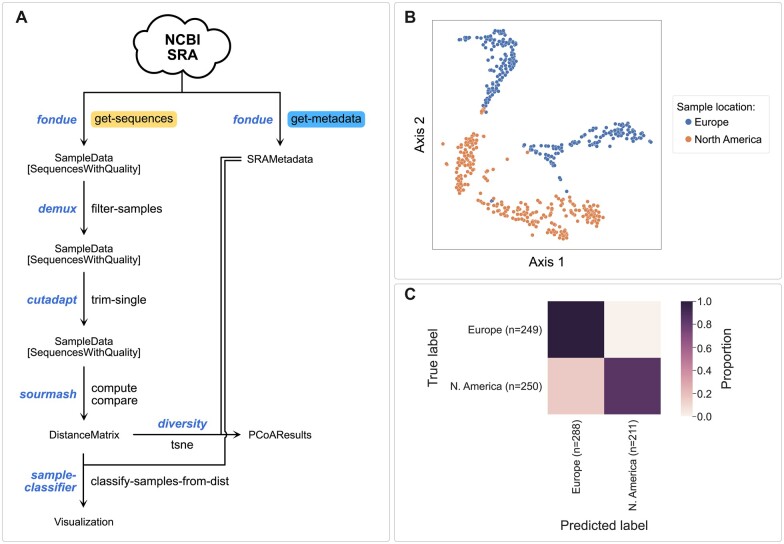
After performing filtering, normalization and denoising steps on the raw 16S rRNA gene sequences (see Fig. 4A for an overview of plugins and actions used throughout this use case), a total of 3880 amplicon sequencing variants (ASVs) were identified for 330 samples. The available metadata was used to define binned age groups. The distribution of samples per age group as well as the analyzed age range differ markedly between studies (Fig. 4B). We further defined a binary health status which denotes whether the sample stems from a healthy or unhealthy infant (see Methods for more details). Across all studies, 194 unhealthy and 136 healthy infant samples were identified. Figure 4C displays the fraction of healthy infants in each of the three geographic locations covered by the selected studies. It shows that the fraction of healthy infants is very different between studies and geographical locations with Lewis et al. (2017) having analyzed only healthy infants in Georgia and Davis et al. (2017) and McClorry et al. (2018) having analyzed mainly unhealthy infants in Gambia and Peru, respectively.
Fig. 4.
Analysis of amplicon sequencing data from three infant gut microbiome development studies. (A) Overview of QIIME 2 actions used during the amplicon data analysis. (B) Counts of samples in the defined age groups per study. (C) Fraction of healthy infants in the geographic locations covered by the selected studies. (D and E) ROC curves of Random Forest classifiers predicting age groups (D) and health status (E), indicating better predictive accuracy for age groups (macro averaged AUC = 0.85) than health status (macro averaged AUC = 0.58) which is not to be trusted as the individual studies differ in many covariates that were not adjusted for in this demonstration
Finally, we trained two Random Forest classifiers with 10-fold cross-validation on the processed microbiome sequence data to predict the age group and health status of each sample, respectively. The classifiers were evaluated on the test set of each fold and revealed a better performance in predicting age groups (macro averaged AUC = 0.85, Fig. 4D) than health status (macro averaged AUC = 0.58, Fig. 4E). This initial result would, however, require further careful analysis as the individual studies differ in many variables (e.g. age range, health status and geographical location) which we did not account for in this demonstration. Hence, differences in predicted age bins and health status could be artificially inflated by the differences in geolocation or study design. The classifiers trained here might not be capturing age-specific or health-specific features but rather features stemming from the particular study setups.
4.2 Use Case 2: Whole genome sequence data analysis
To illustrate how q2-fondue can be used as an entry-point to analysis of whole genome sequencing data we turned to one of the most rapidly growing datasets of the recent years: the SARS-CoV-2 genome dataset. We used all of the pre-processed metadata obtained through the Nextstrain.org platform (Hadfield et al., 2018) to identify samples that have been deposited in the SRA. We subsampled genomes of SARS-CoV-2 variants from two geographic locations: Europe and North America. We then fetched the corresponding SRA metadata using q2-fondue, which was used to prepare our final list of genomes. To simplify the analysis and reduce technical variability, we focused only on samples sequenced using single-end reads on the Illumina NextSeq 500/550 platforms. Following the quality control step, we used the sourmash tool to readily compare viral genomes to one another by computing their MinHash signatures (Ondov et al., 2016). The resulting distance matrix was then used to generate a t-SNE plot visualizing how sampled genomes group together. Figure 5B shows that the samples taken at different locations group together to form distinct geographic clusters. Finally, we used k-nearest-neighbors clustering to quantitatively compare genome MinHash signatures to predict SARS-CoV-2 geographic origin (Fig. 5C). We found that it was possible to classify the SARS-CoV-2 origin with an accuracy of 92%. This result is intended as a simple demonstration of the capability of q2-fondue in conjunction with other QIIME 2 plugins but should not be considered as final. Additional factors may need to be taken into account to get a complete picture of the relationship between geographic location and viral genome MinHash signatures: other sequencing platforms should be included and more samples from other continents should be added, as well as a careful evaluation of covariates that could bias these results. An overview of plugins and actions applied in this use case can be found in Figure 5A.
Fig. 5.
Genome MinHash signatures are predictive of SARS-CoV-2 geographic origin. (A) Overview of QIIME 2 actions used during the genome data analysis. (B) t-SNE analysis of the SARS-CoV-2 genome MinHash distance matrix shows that virus samples can be grouped into distinct geographic clusters based on only genome hash signatures. (C) The same distance matrix can be used to reliably predict virus origin from genome hashes. K-nearest-neighbors clustering approach with 10-fold cross-validation was used to classify samples—a confusion matrix constructed from all test sets is shown
4.3 Use case 3: shotgun metagenome sequence data analysis
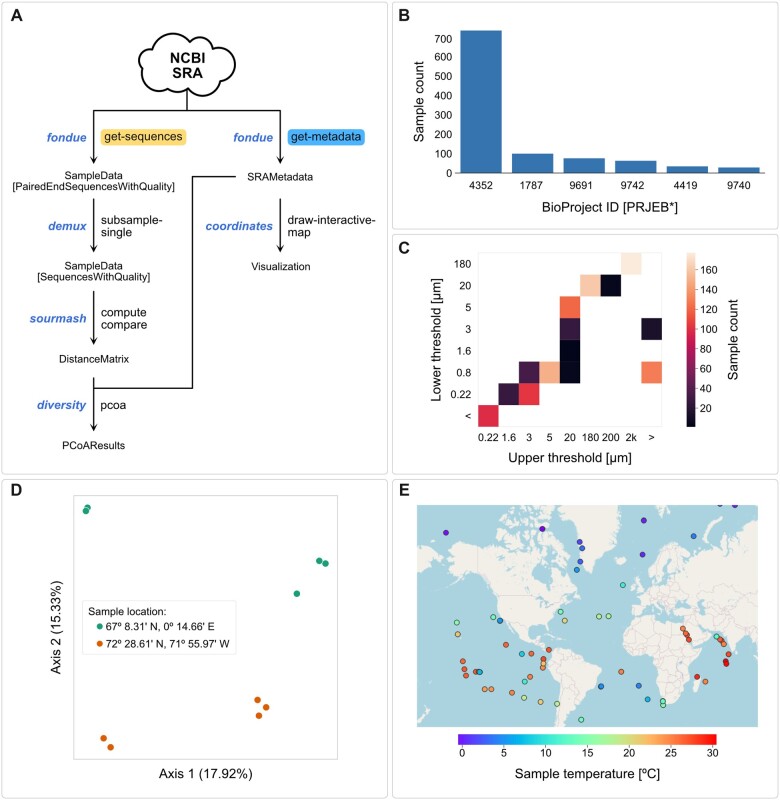
We used the Tara Ocean expedition dataset (Tara Oceans Consortium Coordinators et al., 2015) to illustrate how geographic location included in sample metadata deposited in the SRA can be used to display sample properties, using q2-fondue and QIIME 2 (see Fig. 6A for an overview of plugins and actions used throughout this use case). We fetched metadata for six BioProjects containing 1049 ocean samples obtained through size fractionation followed by shotgun metagenome sequencing (Fig. 6B and C). As geographical coordinates of every sample are included in the SRA metadata, we could directly draw an array of interactive maps visualizing various sample properties using the q2-coordinates plugin (Bokulich and Caporaso, 2018). As an example, Figure 6D illustrates sample temperatures across the globe. Moreover, we randomly selected 10 samples collected at two distinct locations and used the corresponding sequences to calculate and compare their MinHash signatures. Using PCoA analysis of the resulting distance matrix, we could show that the samples can be separated by location when using only their genome hash signatures (Fig. 6E). More interactive visualizations can be found in the Jupyter notebook accompanying this manuscript (see Data Availability section).
Fig. 6.
Tara Oceans expedition (meta)data analysis. (A) Overview of QIIME 2 actions used during the metagenome analysis. (B) Counts of samples in the retrieved dataset according to BioProject ID. (C) Counts of samples corresponding to different fractions obtained through size fractionation. (D) PCoA analysis of the metagenome MinHash signatures of 10 samples taken at two randomly selected locations. Fraction of explained variance is shown for two plotted dimensions. (E) Temperature of samples taken at different geographical locations. Only 100 randomly selected samples are shown
4.4 Integration with QIIME 2 ecosystem
Since q2-fondue is a QIIME 2 plugin, it tightly integrates with and benefits from the rest of the QIIME 2 ecosystem. Sequences obtained through the get-sequences action can be directly passed into any other QIIME 2 action that accepts this data type (see Fig. 7 for an overview of actions applied in this study). In addition to defining format checks for SRA metadata objects, q2-fondue has implemented transformer functions to allow the metadata downloaded through the use of get-metadata action to serve as input to any QIIME 2 action that requires sample metadata. Furthermore, integration with QIIME 2’s built-in provenance tracking system ensures that data fetching from the SRA is also included in the provenance graph (stored directly in all data outputs), enabling researchers to track and completely reproduce the entire analysis pipeline from data download to final visualizations.
Fig. 7.
Overview of q2-fondue integration with other QIIME 2 plugins and actions as applied in the three use cases presented in this study. This is only a limited demonstration of possible downstream uses for three different nucleotide sequence data types, not an exhaustive list
5 Discussion
Declining costs and increasing throughput of nucleotide sequencing have fueled an exponential increase in published sequence data over the past two decades (Stephens et al., 2015). These data have an immense reuse potential, which has led to a growing trend of sequencing-based meta-analyses (Fig. 1), paving the way to additional discoveries regarding general biological trends (Abbas et al., 2019; Panagiotou et al., 2013; Thompson et al., 2017). However, such studies remain technically challenging and data acquisition and management are significant bottlenecks.
We developed q2-fondue to lower these hurdles, and to facilitate reproducible acquisition and management of metadata and nucleotide sequence datasets from the SRA (see Table 1 for a summary of the most important features). Its integration with the QIIME 2 framework offers complete provenance tracking of the entire process, multiple user interfaces, and thorough input/output data validation, allowing to conduct meta-analyses in a reproducible manner. Furthermore, q2-fondue outputs can be directly used with a wide range of QIIME 2 plugins, offering the user a smooth incorporation with any sequence-based analysis that is (or will become) available within the QIIME 2 ecosystem. Even though the main target of the QIIME 2 framework is microbiome research, q2-fondue itself is intended to be agnostic to the research field and non-microbiome researchers can equally profit from its most important features (see Table 1) when performing downstream analyses without QIIME 2 (see tutorials at https://github.com/bokulich-lab/q2-fondue). Finally, q2-fondue’s integration with QIIME 2 offers users unparalleled support through the QIIME 2 forum—an exchange platform between users and plugin developers (with a current total of 5700 signed-up members).
Table 1.
Selection of the most significant issues faced by users when retrieving large amounts of sequencing data, together with their user-friendly solutions offered by q2-fondue
| Problem | Solution offered by q2-fondue |
|---|---|
| Plethora of accession ID types complicates retrieval of sequences/metadata. | Conversion between different accession IDs is performed automatically. All associated parent and child accession IDs are recorded in the final metadata table. |
| Potential data loss on space exhaustion when fetching large amounts of runs. | q2-fondue keeps track of available disk space and will abort without data loss when the amount of space is insufficient. |
| Sequencing data requires pre-processing/name normalization before it can be used in downstream analyses. | q2-fondue takes care of renaming/standardizing all the files after retrieval. |
| Merged datasets and subsequent data analysis steps are not always reproducible. | Tight integration with QIIME 2 ensures that every data fetching and analysis detail is recorded in provenance stored together with every single output. |
| Diversity of metadata fetched from multiple studies complicates its application in subsequent analyses. | Metadata retrieved by q2-fondue is normalized into a single table with standardized columns. |
| Network issues and other errors lead to data loss and require cumbersome, repeated data fetches. | Data retrieval can be automatically repeated in case of encountered errors. In case of repeated failures, all errors are reported and can be investigated by the user once the download is finished. No data loss occurs. |
| Parallelization of custom SRA access scripts is complicated and time-consuming. | q2-fondue takes care of data retrieval/processing in a parallel way, making use of multiple threads and CPUs available on the user's system. |
Despite its ease of use, q2-fondue does not free the user of their due diligence in checking the details on the extracted datasets in the accompanying publications, where mismatches with obtained run metadata or sequences could be detected. The same holds for following best practices when performing meta-analyses or comparative analyses with data obtained using q2-fondue. The user must investigate sources of heterogeneity among individual studies included in the analysis (Thompson, 1994) and follow statistical procedures to ensure that the detected signals are not an artifact of the different study setups (Gurevitch et al., 2018).
The q2-fondue demonstrations shown here represent only a few possible (although simplified) use cases for the software, and we envision many other possible applications for the analysis of diverse nucleotide sequence data types.
5.1 Future plans
The q2-fondue package remains under active development, and several additional functionality upgrades are planned in the future. As q2-fondue operates on large amounts of sequencing data we will introduce several performance-enhancing updates that will allow better management of free storage space available during download as well as streamline downloading large numbers of accession IDs to avoid multiple re-fetches.
q2-fondue’s metadata retrieval action already greatly simplifies downloading metadata of multiple projects and formatting those as a single result table. Several additional functions are planned to assist with the management and integration of diverse study and sample metadata. Moreover, we acknowledge that additional features may be needed, particularly for accessing non-microbial datasets (as the demonstrations in this publication focus on microbial datasets). We are motivated to further improve q2-fondue to encompass diverse use cases and invite feature requests via q2-fondue’s GitHub repository or the QIIME 2 forum.
Finally, to unlock the potential of sequencing data stored in and processed by other repositories we will add support for (meta)data retrieval from various other databases (e.g. MGnify; Mitchell et al., 2020). Altogether, we hope that q2-fondue can become the tool of choice for interacting with the SRA and other similar repositories, while at the same time seamlessly integrating with the whole QIIME 2 ecosystem, hence enabling a wide range of available analysis types.
Supplementary Material
Acknowledgements
We thank Evan Bolyen (Northern Arizona University) for insightful discussions on metadata processing and working with the SRA and SRA Toolkit. We also thank Anton Lavrinienko (ETH Zürich) for his valuable comments on the manuscript.
Funding
This work was partially supported by the grant [#2021-362] of the Strategic Focus Area ‘Personalized Health and Related Technologies (PHRT)’ of the ETH Domain (Swiss Federal Institutes of Technology) and the Swiss National Science Foundation [310030_204275]. L.F. gratefully acknowledges the support of the Swiss Government Excellence Ph.D. Scholarships for foreign students.
Conflict of Interest: none declared.
Contributor Information
Michal Ziemski, Laboratory of Food Systems Biotechnology, Institute of Food, Nutrition, and Health, ETH Zürich, Zürich 8092, Switzerland.
Anja Adamov, Laboratory of Food Systems Biotechnology, Institute of Food, Nutrition, and Health, ETH Zürich, Zürich 8092, Switzerland.
Lina Kim, Laboratory of Food Systems Biotechnology, Institute of Food, Nutrition, and Health, ETH Zürich, Zürich 8092, Switzerland.
Lena Flörl, Laboratory of Food Systems Biotechnology, Institute of Food, Nutrition, and Health, ETH Zürich, Zürich 8092, Switzerland.
Nicholas A Bokulich, Laboratory of Food Systems Biotechnology, Institute of Food, Nutrition, and Health, ETH Zürich, Zürich 8092, Switzerland.
Data Availability
An introductory tutorial with background information and examples for the usage of q2-fondue can be accessed at https://github.com/bokulich-lab/q2-fondue/blob/main/tutorial/tutorial.md. All Jupyter notebooks used in this article are available under https://github.com/bokulich-lab/q2-fondue-examples.
References
- Abbas A.A. et al. (2019) Redondoviridae, a family of small, circular DNA viruses of the human oro-respiratory tract that are associated with periodontitis and critical illness. Cell Host Microbe, 25, 719–729.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alföldi J., Lindblad-Toh K. (2013) Comparative genomics as a tool to understand evolution and disease. Genome Res., 23, 1063–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann R.I. et al. (2019) Toward unrestricted use of public genomic data. Science, 363, 350–352. [DOI] [PubMed] [Google Scholar]
- Baker M. (2016) 1,500 Scientists lift the lid on reproducibility. Nature, 533, 452–454. [DOI] [PubMed] [Google Scholar]
- Berman F. et al. (2014) Building global infrastructure for data sharing and exchange through the research data alliance. D-Lib Mag., 20, 10.1045/january2014-berman. [DOI] [Google Scholar]
- Bokulich N.A. et al. (2018) q2-sample-classifier: machine-learning tools for microbiome classification and regression. J. Open Source Softw., 3, 934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich N.A. et al. (2018) Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome, 6, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokulich N., Caporaso G. (2018) Nbokulich/q2-Coordinates: 2018.11. Zenodo. 10.5281/zenodo.2124295. [DOI] [Google Scholar]
- Bolyen E. et al. (2019) Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol., 37, 852–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann J.P., Holmes E.C. (2019) Entrezpy: a python library to dynamically interact with the NCBI entrez databases. Bioinformatics (Oxford, England), 35, 4511–4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan B.J. et al. (2016) DADA2: high-resolution sample inference from illumina amplicon data. Nat. Methods, 13, 581–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S. (2019) Pysradb: a python package to query next-generation sequencing metadata and data from NCBI sequence read archive. F1000Research, 8, 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J.C.C. et al. (2017) Growth and morbidity of Gambian infants are influenced by maternal milk oligosaccharides and infant gut microbiota. Sci. Rep., 7, 40466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevitch J. et al. (2018) Meta-analysis and the science of research synthesis. Nature, 555, 175–182. [DOI] [PubMed] [Google Scholar]
- Hadfield J. et al. (2018) Nextstrain: real-time tracking of pathogen evolution. Bioinformatics, 34, 4121–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halko N. et al. (2011) An algorithm for the principal component analysis of large data sets. ArXiv:1007.5510 [Cs, Stat]. http://arxiv.org/abs/1007.5510.
- Huang Y., Gottardo R. (2013) Comparability and reproducibility of biomedical data. Brief. Bioinformatics, 14, 391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügel S. et al. (2019) Urschrei/Pyzotero: Zenodo Release. Zenodo. 10.5281/zenodo.2917290. [DOI] [Google Scholar]
- Hunter J.D. (2007) Matplotlib: a 2D graphics environment. Comput. Sci. Eng., 9, 90–95. [Google Scholar]
- Ioannidis J.P.A., Trikalinos T.A. (2005) Early extreme contradictory estimates may appear in published research: the Proteus phenomenon in molecular genetics research and randomized trials. J. Clin. Epidemiol., 58, 543–549. [DOI] [PubMed] [Google Scholar]
- Kans J. (2013) Entrez direct: e-utilities on the Unix command line. In: Entrez Programming Utilities Help [Internet].National Center for Biotechnology Information (US), Bethesda (MD: ). [Google Scholar]
- Katz K. et al. (2022) The sequence read archive: a decade more of explosive growth. Nucleic Acids Res., 50, D387–D390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.-M. et al. (2018) Experimenting with reproducibility: a case study of robustness in bioinformatics. GigaScience, 7, 1–8. 10.1093/gigascience/giy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama Y. on behalf of the International Nucleotide Sequence Database Collaboration. et al. (2012) The sequence read archive: explosive growth of sequencing data. Nucleic Acids Res., 40, D54–D56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen R. et al. (2011a) The European nucleotide archive. Nucleic Acids Res., 39, D28–D31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen R. et al. ; International Nucleotide Sequence Database Collaboration. (2011b) The sequence read archive. Nucleic Acids Res., 39, D19–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Z.T. et al. (2017) The fecal microbial community of breast-fed infants from Armenia and Georgia. Sci. Rep., 7, 40932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd K.G. et al. (2018) Phylogenetically novel uncultured microbial cells dominate earth microbiomes. MSystems, 3, e00055–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet. J., 17, 10. [Google Scholar]
- Mashima J. et al. (2017) DNA data bank of Japan. Nucleic Acids Res., 45, D25–D31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClorry S. et al. (2018) Anemia in infancy is associated with alterations in systemic metabolism and microbial structure and function in a sex-specific manner: an observational study. Am. J. Clin. Nutr., 108, 1238–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney W. (2010) Data Structures for Statistical Computing in Python. pp. 56–61. 10.25080/Majora-92bf1922-00a. [DOI]
- McNutt M. et al. (2016) Liberating field science samples and data. Science, 351, 1024–1026. [DOI] [PubMed] [Google Scholar]
- Meadows J.R.S., Lindblad-Toh K. (2017) Dissecting evolution and disease using comparative vertebrate genomics. Nat. Rev. Genet., 18, 624–636. [DOI] [PubMed] [Google Scholar]
- Meyer F. et al. (2008) The metagenomics RAST server—a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics, 9, 386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A.L. et al. (2020) MGnify: the microbiome analysis resource in 2020. Nucleic Acids Res., 48, D570–D578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondov B.D. et al. (2016) Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol., 17, 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panagiotou O.A. et al. (2013) The power of meta-analysis in genome wide association studies. Annu. Rev. Genomics Hum. Genet., 14, 441–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D.H. et al. (2017) Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol., 2, 1533–1542. [DOI] [PubMed] [Google Scholar]
- Pedregosa F. et al. (2011) Scikit-learn: machine learning in python. In: Machine Learning in Python, Vol. 6, 2826–2830. 10.48550/ARXIV.1201.0490. [DOI] [Google Scholar]
- Reback J. et al. MomIsBestFriend (2020) Pandas-Dev/Pandas: Pandas 1.0.3. Zenodo. 10.5281/zenodo.3715232. [DOI] [Google Scholar]
- Reichman,O.J. et al. (2011) Challenges and opportunities of open data in ecology. Science , 331, 703–705. 10.1126/science.1197962. [DOI] [PubMed] [Google Scholar]
- Serghiou S. et al. (2016) Field-wide Meta-analyses of observational associations can map selective availability of risk factors and the impact of model specifications. J. Clin. Epidemiol., 71, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens Z.D. et al. (2015) Big data: astronomical or genomical? PLoS Biol., 13, e1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesant S. et al. ; Tara Oceans Consortium Coordinators. (2015) Open science resources for the discovery and analysis of Tara oceans data. Sci. Data, 2, 150023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2019) The path to open data. Nat. Rev. Nephrol., 15, 521. [DOI] [PubMed] [Google Scholar]
- Thompson L.R. et al. ; Earth Microbiome Project Consortium. (2017) A communal catalogue reveals earth’s multiscale microbial diversity. Nature, 551, 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S.G. (1994) Why sources of heterogeneity in meta-analysis should be investigated. BMJ (Clinical Research Ed.), 309, 1351–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskom M. (2021) Seaborn: statistical data visualization. J. Open Source Softw., 6, 3021. [Google Scholar]
- Wilkinson M.D. et al. (2016) The FAIR guiding principles for scientific data management and stewardship. Sci. Data, 3, 160018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz P. et al. (2011) Minimum information about a marker gene sequence (MIMARKS) and minimum information about any (x) sequence (MIxS) specifications. Nat. Biotechnol., 29, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youens-Clark K. et al. (2019) iMicrobe: tools and data-driven discovery platform for the microbiome sciences. GigaScience, 8. 10.1093/gigascience/giz083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamkovaya T. et al. (2021) A network approach to elucidate and prioritize microbial dark matter in microbial communities. ISME J., 15, 228–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y. et al. (2013) SRAdb: query and use public next-generation sequencing data from within R. BMC Bioinformatics, 14, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
An introductory tutorial with background information and examples for the usage of q2-fondue can be accessed at https://github.com/bokulich-lab/q2-fondue/blob/main/tutorial/tutorial.md. All Jupyter notebooks used in this article are available under https://github.com/bokulich-lab/q2-fondue-examples.