Abstract
Purpose:
Waning of immunity after vaccination with the acellular Pertussis (aP) vaccine has been proposed as one of the main reasons for pertussis resurgence in the US. In this study, we estimated time-varying vaccine effectiveness after 5 doses of aP vaccine.
Methods:
We conducted a retrospective cohort study among children 5–9 years old (born between 2008 and 2012) living in King County, Washington, USA, who participated in the Washington State Immunization Information System. We estimated time-varying vaccine effectiveness after 5 doses of aP using smoothed scaled Schoenfeld residuals obtained from fitting Cox proportional hazards models to the data as well as piecewise constant Poisson regression.
Results:
There were 55 pertussis cases in this cohort, of whom 22 (40%) were fully-vaccinated and 33 (60%) were under-vaccinated. Vaccine effectiveness (VE) remained high for up to 42 months after the fifth dose (VE(t)= 89%; 95% CI: 64%, 97%) as estimated using survival analysis methods and up to 4 years (VE(t)=93%; 95% CI: 67%, 98%) as estimated using Poisson regression.
Conclusion:
We did not find evidence for waning of vaccine effectiveness for up to four years after 5 doses of aP among 5 to 9 years old children in King County, WA.
Keywords: Acellular pertussis vaccines, Vaccine waning effects, Vaccine effectiveness, Time-varying vaccine effectiveness, Schoenfeld residuals, Pertussis resurgence
Introduction
Despite availability of a vaccine since the 1940s, pertussis outbreaks continue to occur periodically even in countries with high vaccination coverage [1,2]. The WHO reported 151,074 pertussis cases globally in 2018 and 89,000 estimated deaths in 2008 [3]. A modeling study estimated an even larger death toll of 160,700 deaths among children under 5 years of age in 2014 [4]. Since the late 1970s, the US has experienced an increase in pertussis activity [5]. Incidence remains highest among infants and has been steadily increasing in school children, adolescents, and adults [6,7]. In 2012, reported pertussis cases in the US peaked at 48,277, the highest number of cases reported since 1955 [8].
One popular driver of pertussis resurgence is hypothesized to be waning of immunity after vaccination with acellular pertussis vaccines (aP) [9], which replaced the whole-cell pertussis (wP) vaccines in high-income countries after concerns about vaccine safety and reactogenicity [2]. However, estimates of duration of aP vaccine-derived immunity vary widely between studies and can depend on the study design used [10]. Several epidemiologic studies conducted in highly immunized populations concluded that immunity induced by acellular pertussis vaccines wanes within a few years and have called for vaccines with more long-lasting protection [11–14]. One meta-analysis of published studies on duration of immunity due to pertussis vaccines found that aP-induced immunity wanes after 4–12 years [15]. Another meta-analysis concluded that only 10% of children vaccinated by aP would be protected over 8 years [16]. In contrast, modeling studies have found evidence of long-lasting, slowly waning immunity lasting up to 70 years using population-level longitudinal time series data [17,18].
We estimated vaccine effectiveness of acellular pertussis vaccines over time in a population of 5–9 years old children living in King County, Washington, and registered in the Washington State Immunization Information System (WA-IIS) using survival analysis methods. Specifically, we used smoothed scaled Schoenfeld residuals to estimate the time trends of vaccine effectiveness for evidence of waning immunity over time [19].
Methods
Pertussis cases
Pertussis cases born between January 1, 2008, and December 31, 2012, (ages 5 to 9 years as of December 31, 2017) were obtained from the Public Health Seattle and King County (PHSKC) surveillance database. The clinical case definition of pertussis used is a cough illness lasting ≥ 2 weeks with at least one of the following: paroxysms of coughing or inspiratory “whoop” or post-tussive vomiting. Pertussis cases were classified as ‘suspected’, ‘probable’, and ‘confirmed’ by PHSKC [20]. A confirmed case is defined as a case of acute cough illness of any duration with isolation of B. pertussis from a clinical specimen or polymerase chain reaction (PCR) positive for B. pertussis. A probable case is defined as (in absence of a more likely diagnosis) an illness meeting the clinical criteria, or an illness with cough of any duration with at least one of the clinical symptoms and contact with a laboratory confirmed case (epidemiologic link). Suspected pertussis cases are cases with cough lasting ≥ 2 weeks with no other symptoms, or cough of any duration with one of the case-defining symptoms without lab confirmation or epidemiologic link, or an epidemiologic link with cough of any duration and no other symptoms and no lab confirmation, or PCR positive for B. pertussis but no documentation of cough or case-defining symptoms [20,21].
Pertussis remains severely underreported [2], and only cases that were symptomatic, sought medical help, and were reported to PHSKC were included. Information on age, sex, and home address for cases was available. We geocoded home addresses of cases to their census tract of residence using ArcGIS 10.1 [22].
Study cohort
The study was conducted in a cohort of children registered in the WA-IIS, which is a lifetime registry that tracks immunization records for people of all ages in Washington State [23]. Healthcare providers voluntarily report patient immunizations to WA-IIS. Additionally, birth certificates of children born in King County are loaded into the registry every two weeks. As of 2018, 94% of public sites participating in the Vaccines for Children program share data with the WA-IIS, and 95% of birth records are entered into the WA-IIS within 30 days or less after birth. Data quality has improved over time and the study cohort was restricted to children born in King County after 2008 to ensure data completeness and accuracy. Ninety-nine percent of children aged 4 months - 5 years have 2 or more immunizations recorded in the WA-IIS [23]. Children born between January 1, 2008 and December 31, 2012, (children between the ages of 5 and 9 years in 2017) were followed up from January 1, 2013, to December 31, 2017 (N=134,950). Thus, children born in 2008 were followed up for 5 years (from ages 5 through 9), children born in 2009 were followed up for 4 years (from ages 5 through 8), and so on. Vaccination information including vaccine name and date of receipt was requested for all pediatric vaccines recommended from birth through 9 years of age [24]. Demographic information included date of birth, sex, and current residential address and county. Home addresses (or zip codes when home addresses were unavailable) of WA-IIS participants were geocoded to their census tract of residence by WA Department of Health staff. We calculated a census tract level neighborhood socioeconomic score (NSES) for each participant using the 2010 US census data [25] and the method described in Miles, et al (2016) [26]. Briefly, the NSES Index is on a scale from 0 to 100 and incorporates census tract-level median household income, percent of households with income below the Federal Poverty Line, educational attainment of adults age 25+, unemployment rate, and percent of households with children under 18 that are “female-headed” (no male present).
Linking surveillance and immunization datasets
Immunization records from the WA-IIS and surveillance data from PHSKC were linked based on a probabilistic matching algorithm that used participants’ first name, last name, date of birth, sex, and city of residence [27] (See Fig S1). Matching was performed using the fastLink package in R [28].
Censoring of registry participants
Participants were followed in time until they were diagnosed with pertussis, died, moved to another county or changed healthcare providers, or until the end of follow-up period on December 31, 2017, whichever came first. WA-IIS flags participants as “inactive” if they move out of state/city/change healthcare provider/die, but this variable is inconsistently recorded. For our analysis, we assumed that a participant is “active” unless they are marked as “inactive” or “died”. Because the inactive patients are not always flagged in the registry, if participant’s current address is not in King County, we assumed they moved out of the county (Moved or Gone Elsewhere [MOGE]) on the date their patient record was last updated. For “active” children, vaccination dates for pediatric vaccines were considered proxies for continued residence in King County, unless current county of residence was recorded as other than King County in the IIS, in which case they were censored at date MOGE. The flowchart in Fig S2 describes details of censoring. The following rules were used sequentially for censoring individuals in this study:
Participants diagnosed with pertussis were censored on the date of diagnosis.
For non-cases who were “Inactive” and whose current county of residence was not King County, follow-up ended on the date MOGE (date of last patient record update).
For non-cases who were “Inactive” and residents of King County, follow-up ended on date of last IIS record update.
For non-cases who were “Active” in the registry and whose current residence was King County, if no immunization (any pediatric immunization) was ever recorded for a participant, they were censored on their first birthday. Follow-up ended at two years after the last recorded vaccine if last vaccine was recorded at age < 36 months, or at the end of study period if last vaccine was recorded at age ≥ 36 months.
For non-cases who were “Active” in the registry and whose current address was not King County, follow-up ended on the date they moved out of King County or changed provider (date MOGE).
Study design
We used a retrospective cohort study to estimate time-varying aP vaccine effects. The Centers for Disease Control and Prevention (CDC) Advisory Committee for Immunization Practice (ACIP) recommendations for childhood aP doses were used to determine vaccination status [29]. Five doses of aP vaccine are recommended at ages 2, 4, and 6 months, 15–18 months, and 4–6 years. The main exposure of interest was aP vaccination categorized as fully-vaccinated (received 5+ doses by age 6 years) and under-vaccinated (received fewer than 5 doses by age 6 years). Participants may or may not have received all 5 doses in a timely manner but were considered fully-vaccinated as long as they received 5 or more doses by age 6 years. We assumed that if a child had no record of an aP dose in the WA-IIS, they did not receive it. Outcome was all suspected, probable, and confirmed pertussis cases as reported to PHSKC. We decided to include only cases diagnosed 15 days after vaccination because it might take up to 15 days for the vaccine to show any protective effect [13]. Ultimately, no cases had to be excluded due to this criterion because all cases occurred at least 15 days after last aP vaccination.
Statistical analysis
We estimated vaccine effectiveness using the Cox proportional hazards model and time-varying vaccine effectiveness using smoothed scaled Schoenfeld residuals [30]. Vaccine effectiveness for pertussis disease, VE(t), is the measure of how protective the vaccine is against disease at time t [31]. We first constructed a diagnostic log-minus-log plot of Kaplan-Meier estimates of survival curves for the fully vaccinated and under-vaccinated groups to check the proportionality of hazards assumption. Vaccination status was defined as fully-vaccinated (5 doses of aP vaccine at age 6) or under-vaccinated (fewer than 5 doses of aP vaccine at age 6). We fit an ordinary Cox proportional hazards (PH) model to the data to estimate the hazard ratio (HR) of pertussis comparing fully-vaccinated children to under-vaccinated children, adjusted for age and NSES index, and computed the scaled Schoenfeld residuals. Schoenfeld residuals are the scaled differences between actual and computed covariate values at each event time [30]. Adding the smoothed Schoenfeld residuals to the Cox PH coefficient estimates yields a time-varying estimate of vaccine effectiveness, given by VE(t) = 1 - eβ(t). A hypothesis test for departure from the proportional hazards assumption was conducted, where the null hypothesis was H0: β(t) = β for all times t. The analysis was done using packages survminer [32], survival [33], and kyotil [34] in R Studio version 3.4.1 [35].
As an alternative method, we estimated vaccine effectiveness for each year post vaccination using incidence rates, denoted by VEIR(t). We partitioned the data into years since vaccination and fitted a separate Poisson regression model for each year to obtain a piecewise constant estimate of VEIR(t).
Ethical Review
This study was reviewed by the Washington State Institutional Review Board and the PHSKC Research Administrative Review Committee.
Results
The study included 134,852 children 5 years to 9 years old living in King County, WA, and registered in the WA-IIS who were followed between January 1, 2013 and December 31, 2017. The median duration of follow-up was 905 days (range: 1 – 1827 days). A total of 5,305 (3.9%) participants were lost to follow up. About half of the participants were female (49%). A slightly higher proportion of children who were under-vaccinated resided in census tracts of lowest NSES quantile compared to fully-vaccinated children (23.3% vs. 19.8%). Under-vaccination rates improved with successive birth cohorts (Table 1). Of the WA-IIS participants, 1,738 (1.3%) had no recorded doses of aP (1,363 of whom had received at least one other pediatric vaccine while 375 had no pediatric vaccines recorded in the registry) and 21,777 (16.1%) were partially vaccinated. Of those partially-vaccinated, 2,390 (11%) received 1 dose, 1,461 (6.7%) received 2 doses, 2,573 (11.8%) received 3 doses, and 15,353 (70.5%) received 4 doses of aP by age 6 years. There were 55 pertussis cases in this cohort. Of these, 22 (40%) were fully-vaccinated and 33 (60%) were under-vaccinated (Table 1). Of the 33 under-vaccinated cases, 19 (57%) had no aP vaccine doses recoded in the WA-IIS (Table S1).
Table 1:
Characteristics of Washington State Immunization Information System participants aged 5 to 9 years born or living in King County, WA between 2008 and 2012 by the 5-dose acellular pertussis vaccination status
| Characteristics | Under-vaccinated (N= 18,620) | Fully-vaccinated (N=116,232) |
|---|---|---|
| Sex | N (%) | N (%) |
| Male | 9,412 (50.5) | 59,505 (51.2) |
| Female | 9,208 (49.5) | 56,727 (48.8) |
| NSES score | ||
| Q1 (lowest) | 4,346 (23.3) | 22,993 (19.8) |
| Q2 | 3,897 (20.9) | 22,191 (19.1) |
| Q3 | 3,645 (19.6) | 23,949 (20.6) |
| Q4 | 3,437 (18.5) | 22,527 (19.4) |
| Q5 (highest) | 3,237 (17.4) | 24,264 (20.9) |
| Pertussis cases | 33 (58.2) | 22 (41.8) |
| Median age (years) | 8 | 7 |
| Birth cohort | ||
| 2008 | 5,902 (31.7) | 22,794 (19.6) |
| 2009 | 5,394 (29.0) | 22,308 (19.2) |
| 2010 | 4,557 (24.5) | 22,260 (11.9) |
| 2011 | 1,547 (8.4) | 24,405 (20.9) |
| 2012 | 1,220 (6.5) | 24,465 (21.0) |
| Total aP doses | ||
| 0 | 1,738 | 0 |
| 1 | 2,390 | 0 |
| 2 | 1,461 | 0 |
| 3 | 2,573 | 0 |
| 4 | 10,458 | 4,895 |
| 5+ | 0 | 11,1337 |
NSES: Neighborhood socioeconomic status; aP: acellular pertussis
Fully-vaccinated: 5+ doses by age 6 years; Under-vaccinated: <5 doses by age 6 years
Col % reported for sex, Neighborhood SES score, and birth cohort; row% for pertussis cases
4,895 children were considered fully-vaccinated with 4 doses of aP per ACIP recommendations that children who receive dose 4 at age 4 years or older and at least 6 months after dose 3 need not receive dose 5 [29].
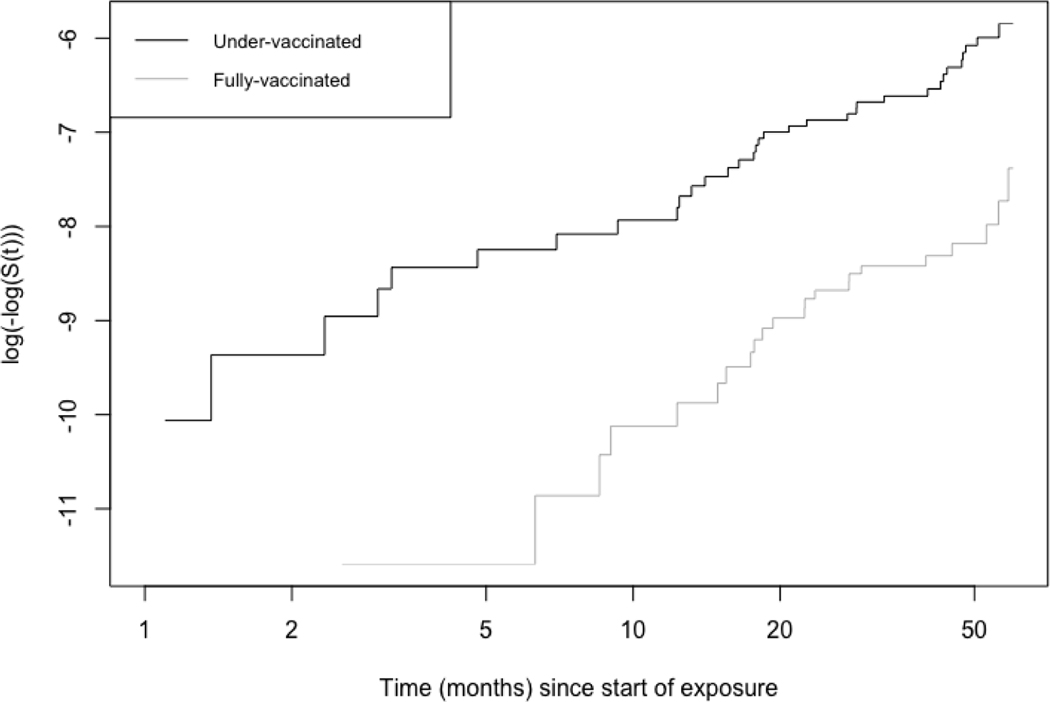
Fig 1 shows the results of the log-minus-log plots of Kaplan-Meier estimates of the survival curves for the aP fully vaccinated and under-vaccinated participants. Separation between the curves indicates that being fully vaccinated with 5 doses of aP protects better against pertussis. It appears that the curves slowly approach each other at the end of 5 years, but this is difficult to infer based on cumulative incidence curves.
Figure 1:
log-minus-log plots of Kaplan-Meier estimates of the survival curves for participants who were fully-vaccinated vs. under-vaccinated with the acellular Pertussis vaccine in King County between January 1, 2013, and December 31, 2017.
Survival curve for fully-vaccinated individuals is in grey, under-vaccinated is in black. The two curves are nearly parallel. Exposure here is being fully-vaccinated or under-vaccinated with 5 doses of the acellular pertussis vaccine. Time at risk begins at age 5 years. S(t): Survival at time t
Fig 2 shows smoothed plots of VE(t) for the 5-dose aP vaccine series estimated using Schoenfeld residuals after fitting a Cox PH model to the data. Table 2 gives the effectiveness estimates and approximate 95% confidence intervals for roughly six-month time intervals throughout the follow-up period. Testing for a linear association between scaled Schoenfeld residuals and time showed no significant time-dependent waning effects (individual test for vaccination status, p:0.44) (Fig S3). This indicates that there is no statistically significant waning of vaccine effects for the aP vaccine. Vaccine effectiveness remained high for up to 42 months after the fifth dose, with a VE(t) of 89% (95% CI: 64%, 97%) (Table 2). However, VE(t) estimates for the fifth year are not statistically significant. A downward curve and large 95% confidence intervals towards the end of the study are due to very few events occurring among children who contributed more than 4 years of follow-up (Fig 2).
Figure 2:
Estimated protective effectiveness of 5-dose acellular Pertussis vaccine series over time.
Vaccine effectiveness estimated for children 5 –9 years of age. Exposure starts at age 5 when children are supposed to have received dose 5 of aP vaccine. 95% confidence intervals are shown as dashed lines.
Table 2:
Estimated vaccine effectiveness over time VE(t) with 95% confidence intervals (CI) for 5-dose series of the acellular Pertussis vaccine among 5 to 9 years old children living in King County, Washington, followed from January 1, 2013, through December 31, 2017.
| Time since vaccination (months) | Vaccine Effectiveness (VE(t)) | 95% Confidence Intervals |
|---|---|---|
| 5.5 | 0.90 | 0.76, 0.96 |
| 12.1 | 0.79 | 0.51, 0.91 |
| 17.8 | 0.76 | 0.41, 0.91 |
| 24.5 | 0.83 | 0.63, 0.93 |
| 30.2 | 0.89 | 0.71, 0.96 |
| 35.8 | 0.90 | 0.71, 0.97 |
| 42.6 | 0.89 | 0.64, 0.97 |
| 48.2 | 0.83 | 0.39, 0.95 |
| 53.8 | 0.69 | −0.85, 0.95 |
| 60 | 0.23 | −11.04, 0.95 |
Cox proportional hazards models adjusted for age and neighborhood socio-economic score were fit to estimate vaccine effectiveness (VE(t)) and associated 95% confidence intervals. VE(t) estimates at 6 month intervals since vaccination with 5-dose series are displayed here.
In the piecewise constant Poisson regression analysis, we observed high vaccine effectiveness for up to four years after vaccination with no evidence of waning (VEIR(t) =93%; 95% CI: 67%, 98%), and VEIR(t) estimate for the fifth year is not significant (Table 3).
Table 3:
Piecewise constant 1- incidence rate ratio (1-IRR) estimates with 95% confidence intervals (CI) for 5 doses of aP vaccine among 5–9 year old children living in King County, WA, between January 1, 2013, and December 31, 2017
| Time since vaccination (years) | Cases in vaccinated group | Person-time at risk in vaccinated group | Cases in under-vaccinated group | Person-time at risk in under-vaccinated group | VEIR(t) | 95% CI |
|---|---|---|---|---|---|---|
| 1 | 4 | 1,213,657 | 8 | 259,261 | 0.91 | 0.70, 0.97 |
| 2 | 10 | 937,463 | 12 | 206,559 | 0.82 | 0.58, 0.92 |
| 3 | 3 | 678,022 | 4 | 156,973 | 0.81 | 0.19, 0.96 |
| 4 | 2 | 421,552 | 7 | 102,776 | 0.93 | 0.67, 0.98 |
| 5 | 3 | 165,978 | 2 | 41,096 | 0.64 | −1.14, 0.94 |
VEIR(t): Vaccine effectiveness at time t
Poisson regression models are adjusted for age and neighborhood SES index.
Person time at risk measured in months
Discussion
Using survival analysis methods, we estimated time-varying vaccine effects after the fifth dose of aP vaccine among children aged 5 to 9 years in King County, WA. We found that contrary to other studies [11,13,14], vaccine effectiveness after 5 doses of aP in this age group is high and does not wane for close to 4 years after vaccination. We compared the smoothed nonparametric VE(t) estimates with yearly VEIR(t) estimates from Poisson regression method and found the estimates to be consistent.
The survival analysis method based in using smoothed scaled Schoenfeld residuals allowed us to visualize how vaccine effectiveness varies over time. Additionally, we could test the departure from the proportional hazards assumption. An advantage of using Schoenfeld residuals over yearly Poisson regression models to estimate time-varying vaccine effectiveness is that one need not arbitrarily group data into time periods and assume a piecewise constant model for each time period, providing VE(t) estimates continuously over time. For example, VE(t) estimates were most reliable for 3.5 years as estimated using Schoenfeld residuals, after which the confidence intervals widen considerably. Based on the Poisson model, we concluded that VEIR(t) is high and reliable at least up to 4 years. In this case, the difference of the two estimates was not large. The survival analysis method can be easily implemented in R statistical software using package kyotil [34] and has been used to estimate time-varying cholera vaccine efficacy [19,36].
Our estimate of vaccine effectiveness against symptomatic disease between 83% and 90% after 5 doses of aP in four years of follow-up is similar to other studies [10,37]. Several modeling studies have shown that current pertussis dynamics can be explained by long-lasting aP immunity. One study estimated a 10% risk of vaccine protection waning within 10 years and a 55% chance of lifelong protection in vaccinated individuals in Massachusetts [38]. Studies that used age-structured pertussis incidence data from Thailand and Sweden also found evidence for long-lasting protection of aP vaccine at the population scale [18,39]. While our observational study could only estimate effects of waning for up to 4 years post-vaccination, it supports the overall conclusions of these modeling studies that aP immunity does not wane rapidly.
In contrast to these modeling studies and our results, some observational studies have concluded that despite providing good protection against pertussis, immunity from aP vaccines wanes steadily between 27% to 42% annually [12–14]. While vaccine immunity clearly wanes over time, their interpretation of the rate at which immunity wanes could be problematic. Klein et al. estimated that risk of pertussis increased by 42% for every year since vaccination of fifth dose, interpreting the annual increase in odds of pertussis as rapid decrease of vaccine effectiveness [14]. Simply interpreting increasing yearly odds of pertussis as rapid loss of vaccine-derived immunity does not take into account the complex transmission dynamics of pertussis [17,40]. One study did not include a vaccine naive or under-vaccinated comparison group in their estimation of waning vaccine effectiveness and interpreted an increase in pertussis incidence per year post-vaccination in fully vaccinated individuals as strong evidence of rapid waning [13]. In our study, we compared vaccine effectiveness between fully vaccinated and under-vaccinated groups and estimated VE(t) with minimum assumptions [19].
Further evidence of slowly waning immunity at the population level in our study is that there were only 55 pertussis cases in a cohort of over 130,000 children and incidence in the vaccinated group was quite low. If indeed protection after the fifth dose waned quickly, we would expect to see many more cases of pertussis given how highly transmissible it is [17]. At the individual level, infection with pertussis despite vaccination is possible due to loss of immunity because of underlying medical conditions or immune-suppression, failure for vaccine to take effect (primary vaccine failure) or increased contact in certain age groups such as school children [18,41]. This might explain the small number of cases observed in epidemiological and clinical studies of vaccine effectiveness. Another reason for a low case count in our study could be that the 5-year surveillance period did not include any large pertussis outbreaks. However, the long interepidemic period itself may be due to long-lasting vaccine-induced immunity [42].
Our study has limitations. The small number of cases may have limited our ability to reliably estimate smoothed VE(t). Our case definition was highly specific, where confirmed cases had clinical diagnosis with laboratory confirmation or an epidemiologic link and probable cases had to have prolonged cough with paroxysms, vomiting, or a whooping sound. Thus, cases in our study may have been more severe and vaccine effectiveness may have been over-estimated [43] compared to studies which relied on more sensitive but perhaps less specific polymerase-chain reaction (PCR) diagnostic techniques for case detection [44]. Our comparison group consisted of partially vaccinated children and not just vaccine-naive children. Most of the partially vaccinated children had received at least 4 doses of aP vaccine, and presumably had good protection against pertussis. Including partially vaccinated children in the comparison group likely underestimated the VE(t) estimates. One could recategorize vaccination groups as unvaccinated, partially vaccinated, and fully vaccinated, but estimation of VE(t) might be difficult given the small number of cases. We were not able to follow-up all children in the cohort for the entire 5 years post vaccination because we did not have WA-IIS records for birth cohorts before 2008 or after 2017. Pertussis cases may have been misclassified as under-vaccinated because healthcare providers failed to report doses to the IIS, which may have resulted in overestimation of vaccine effectiveness. If children had shorter or longer follow-up time than what we assigned by our censoring algorithm, VE(t) could be biased in either direction. The results of this study are generalizable only to countries that administer acellular pertussis vaccines and use the same schedule as the US. Even though WA-IIS is a population-based registry, results may not be generalizable to those who do not participate in the registry or are not captured by the PHSKC surveillance system.
To summarize, we found that the 5-dose aP vaccine series provides good protection against pertussis disease and contrary to oft cited evidence, vaccine effectiveness does not wane rapidly. Our results suggest that changing current vaccination schedule to delay dose 5 or administer booster doses before age 10 might not be necessary as some studies have proposed [11,45].
Supplementary Material
Highlights.
Immunity after 5 doses of DTaP vaccine does not wane rapidly in young children
Vaccine effectiveness against pertussis was ~90% in fully vaccinated children.
We used survival analysis methods to estimate time-varying vaccine effects.
Acknowledgements
The authors would like to thank Dr. Youyi Fong for his helpful feedback on using the R package kyotil.
Funding
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number [R37 AI032042]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflicts of Interest
The authors have no conflict of interest, financial or otherwise.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Domenech de Celles M, Magpantay FMG, King AA, Rohani P. The pertussis enigma: reconciling epidemiology, immunology and evolution. Proc R Soc B 2016; 283:20152309-. Available at: http://rspb.royalsocietypublishing.org/content/283/1822/20152309?etoc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rohani P, Scarpino SV. Pertussis Epidemiology, Immunology, and Evolution. 1st ed. Oxford University Press, 2019. [Google Scholar]
- 3.World Health Organization. Immunization, Vaccines and Biologicals: National Passive Surveillance Pertussis. 2020. Available at: https://www.who.int/immunization/monitoring_surveillance/burden/vpd/surveillance_type/passive/pertussis/en/. Accessed 5 January 2020. [Google Scholar]
- 4.Yeung KHT, Duclos P, Nelson EAS, Hutubessy RCW. An update of the global burden of pertussis in children younger than 5 years: a modelling study. Lancet Infect Dis 2017; 17:974–980. Available at: 10.1016/S1473-3099(17)30390-0. [DOI] [PubMed] [Google Scholar]
- 5.Rohani P, Drake JM. The decline and resurgence of pertussis in the US. Epidemics 2011; 3:183–188. Available at: 10.1016/j.epidem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka M. Trends in Pertussis Among Infants in the United States, 1980–1999. Jama 2003; 290:2968. Available at: 10.1001/jama.290.22.2968. [DOI] [PubMed] [Google Scholar]
- 7.Paisley RD, Blaylock J, Hartzell JD. Whooping cough in adults: An update on a reemerging infection. Am J Med 2012; 125:141–143. Available at: 10.1016/j.amjmed.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 8.CDC | National Center for Immunization and Respiratory Diseases | Division of Bacterial Diseases. 2012 Final Pertussis Surveillance Report. 2013. Available at: http://www.cdc.gov/pertussis/downloads/pertuss-surv-report-2012.pdf%5Cnhttp://www.cdc.gov/mmwr/preview/mmwrhtml/mm6233a6.htm?s_cid=mm6233a6_w. [Google Scholar]
- 9.Gambhir M, Clark TA, Cauchemez S, Tartof SY, Swerdlow DL, Ferguson NM. A Change in Vaccine Efficacy and Duration of Protection Explains Recent Rises in Pertussis Incidence in the United States. PLoS Comput Biol 2015; 11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crowcroft NS, Schwartz KL, Chen C, et al. Pertussis vaccine effectiveness in a frequency matched population-based case-control Canadian Immunization Research Network study in Ontario, Canada 2009–2015. Vaccine 2019; 37:2617–2623. [DOI] [PubMed] [Google Scholar]
- 11.Witt MA, Katz PH, Witt DJ. Unexpectedly limited durability of immunity following acellular pertussis vaccination in preadolescents in a north American outbreak. Clin Infect Dis 2012; 54:1730–1735. [DOI] [PubMed] [Google Scholar]
- 12.Baxter R, Bartlett J, Rowhani-Rahbar A, Fireman B, Klein NP. Effectiveness of pertussis vaccines for adolescents and adults: case-control study. BMJ 2013; 347:f4249. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23873919. [DOI] [PubMed] [Google Scholar]
- 13.Zerbo O, Bartlett J, Goddard K, Fireman B, Lewis E, Klein NP. Acellular pertussis vaccine effectiveness over time. Pediatrics 2019; 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein NP, Bartlett J, Fireman B BR. Waning Protection after Fifth Dose of Acellular Pertussis Vaccine in Children. Pediatrics 2016; 137:e20153326. [DOI] [PubMed] [Google Scholar]
- 15.Wendelboe AM, Van Rie A, Salmaso S, Englund JA. Duration of immunity against pertussis after natural infection or vaccination. Pediatr Infect Dis J 2005; 24:58–61. [DOI] [PubMed] [Google Scholar]
- 16.Mcgirr A, Fisman DN. Duration of pertussis immunity after DTaP immunization: A meta-analysis. Indian J Pract Pediatr 2015; 17:15. [DOI] [PubMed] [Google Scholar]
- 17.Domenech De Cellès M, Rohani P, King AA. Duration of Immunity and Effectiveness of Diphtheria-Tetanus-Acellular Pertussis Vaccines in Children. JAMA Pediatr 2019; 173:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackwood JC, Cummings DAT, Broutin H, Iamsirithaworn S, Rohani P. Deciphering the impacts of vaccination and immunity on pertussis epidemiology in Thailand. Proc Natl Acad Sci U S A 2013; 110:9595–600. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3677483&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durham K l, Longini IM, Halloran ME, Clemens JD, Nizam A, Rao M. Estimation of Vaccine Efficacy in the Presence of Waning : Application to Cholera Vaccines. Am J Epidemiol 1998; 147:948–959. [DOI] [PubMed] [Google Scholar]
- 20.CDC. Pertussis / Whooping Cough (Bordetella pertussis) 2014 Case Definition. 2014. Available at: https://wwwn.cdc.gov/nndss/conditions/pertussis/case-definition/2014/. Accessed 29 June 2018. [Google Scholar]
- 21.Washington State Department of Health. Pertussis Reporting and Surveillance Guidelines. 2016. [Google Scholar]
- 22.ESRI. ESRI 2012. ArcGIS Desktop: Release 10.1 Redlands, CA: Environmental Systems Research Institute. 2012; [Google Scholar]
- 23.Washington State Department of Health. Washington State Department of Health: Immunization Data –Technical Technical Notes. 2015. [Google Scholar]
- 24.National Center for Immunization and Respiratory Diseases. Centers for Disease Control and Prevention. Child and Adolescent Immunization Schedule; (birth through 18 years). 2017. Available at: https://www.cdc.gov/vaccines/schedules/hcp/imz/childadolescent.html. Accessed 30 July 2021. [Google Scholar]
- 25.US Census Bureau. US Census Bureau: American Community Survey. 2017. Available at: https://data.census.gov/cedsci/table?q=CensuspopulationKingCountyWashingon&hidePreview=false&table=DP05&tid=ACSDP1Y2017.DP05&g=0500000US53033&vintage=2017&layer=county&cid=DP05_0001E&lastDisplayedRow=23. Accessed 9 April 2019. [Google Scholar]
- 26.Miles JN, Weden MM, Lavery D, Escarce JJ, Cagney KA, Shih RA. Constructing a Time-Invariant Measure of the Socio-economic Status of U.S. Census Tracts. J Urban Heal 2016; 93:213–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sayers A, Ben-Shlomo Y, Blom AW, Steele F. Probabilistic record linkage. Int J Epidemiol 2016; 45:954–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enamorado T, Fifield B, Imai K. Fast Probabilistic Record Linkage with Missing Data. 2018; [Google Scholar]
- 29.National Center for Immunization and Respiratory Diseases. Center for Disease Control and Prevention. Diphtheria, Tetanus, and Pertussis Vaccine Recommendations. 2016. Available at: https://www.cdc.gov/vaccines/vpd/dtap-tdap-td/hcp/recommendations.html. Accessed 1 January 2017. [Google Scholar]
- 30.Schoenfeld D. Partial Residuals for The Proportional Hazards Regression Model A. Biometrika 1982; 69:239–241. [Google Scholar]
- 31.Halloran ME, Struchiner CJ, Longini IM. Design and Analysis of Vaccine Studies. Springer New York, New York, 2010. [Google Scholar]
- 32.Kassambara A, Marcin K, Przemyslaw B, Scheipl F. Package ‘survminer’. 2019; Available at: http://www.sthda.com/english/rpkgs/survminer/. [Google Scholar]
- 33.Therneau T. Package ‘ survival ‘. 2020; Available at: https://github.com/therneau/survival. [Google Scholar]
- 34.Fong Y, Sebestyen K, Becker J, et al. Package ‘ kyotil ‘. 2019; [Google Scholar]
- 35.RStudio Team (2015). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA. [Google Scholar]
- 36.Fong Y, Halloran ME, Park JK, Marks F, Clemens JD, Chao DL. Efficacy of a bivalent killed whole-cell cholera vaccine over five years: A re-analysis of a cluster-randomized trial. BMC Infect Dis 2018; 18:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fulton TR, Phadke VK, Orenstein WA, Hinman AR, Johnson WD, Omer SB. Protective Effect of Contemporary Pertussis Vaccines: A Systematic Review and Meta-analysis. Clin Infect Dis 2016; 62:1100–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Cellès MD, Magpantay FMG, King AA, Rohani P. The impact of past vaccination coverage and immunity on pertussis resurgence. Sci Transl Med 2018; 10:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rohani P, Zhong X, King A. Contact Network Structure Explains the Changing Epidemiology of Pertussis. Science (80- ) 2010; 1781:982–986. [DOI] [PubMed] [Google Scholar]
- 40.Chit A, Zivaripiran H, Shin T, et al. Acellular pertussis vaccines effectiveness over time: A systematic review, meta-analysis and modeling study. PLoS One 2018; 13:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King AA, Domenech de Cellès M, Magpantay FMG, Rohani P. Pertussis immunity and the epidemiological impact of adult transmissio: statistical evidence from Sweden and Massachusetts. In: Rohani P, Scarpino SV., eds. Pertussis: Epidemiology,Immunology, and Evolution. Oxford University Press, 2019: 225–240. [Google Scholar]
- 42.Lavine JS, Rohani P. Resolving pertussis immunity and vaccine effectiveness using incidence time series. Expert Rev Vaccines 2012; 11:1319–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO meeting on case definition of pertussis. Geneva, 10–11 January, 1991. Geneva: 1991. [Google Scholar]
- 44.Lievano FA, Reynolds MA, Waring AL, et al. Issues associated with and recommendations for using PCR to detect outbreaks of pertussis. J Clin Microbiol 2002; 40:2801–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Misegades LK, Winter K, Harriman K, et al. Association of Childhood Pertussis With Receipt of 5 Doses of Pertussis Vaccine by Time Since Last Vaccine Dose, California, 2010. Jama 2012; 308:2126. Available at: 10.1001/jama.2012.14939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.