Abstract
AIM:
To determine the features cited by motor phenotyping experts when identifying dystonia in people with cerebral palsy (CP)
METHOD:
Dystonia identification in CP, particularly when comorbid with spasticity, can be difficult. The dystonia diagnostic criterion standard remains subjective visual identification by expert consensus. For this qualitative study, we conducted an inductive thematic analysis of consensus-building discussions between three pediatric movement disorder physicians as they identified the presence or absence of dystonia in gait videos of 40 participants with spastic CP and periventricular leukomalacia.
RESULTS:
Unanimous consensus about the presence or absence of dystonia was achieved for 34 out of 40 videos. Two main themes were present during consensus-building discussions as videos were evaluated for dystonia: (1) unilateral leg or foot adduction that was variable over time, and (2) difficulty in identifying dystonia. Codes contributing to the first theme were more likely to be cited by a discussant when they felt dystonia was present (as opposed to absent) in a video (χ2 test, p=0.004).
DISCUSSION:
These results describe the gait features cited by experts during consensus-building discussion as they identify dystonia in ambulatory people with CP. Qualitative thematic analysis of these discussions could help codify the subjective process of dystonia diagnosis.
According to the 2013 international consensus, dystonia is defined as ‘sustained or intermittent muscle contractions causing abnormal, often repetitive, movements, postures, or both. Dystonic movements are typically patterned, twisting, and may be tremulous’.1 This definition is broad by necessity given the protean manifestations of dystonia in the settings of varying etiologies, voluntary movement triggers, and body parts involved.
Significant headway has been made in clinically operationalizing this consensus definition in the form of standardized scales such as the Dyskinesia Impairment Scale2–4 or diagnostic tools such as the Hypertonia Assessment Tool.5–7 However, again reflecting the difficulty in providing a rubric for dystonia diagnosis in all contexts, the Dyskinesia Impairment Scale and Hypertonia Assessment Tool do not clearly specify the type of movement that might qualify as dystonic in a given scenario, particularly for the arms and legs where dystonia may be most functionally impairing. The Dyskinesia Impairment Scale uses the wording ‘sustained muscle contractions causing abnormal posturing, involuntary and/or distorted voluntary movements’.4 The Hypertonia Assessment Tool uses the wording ‘involuntary movements or postures’.5,7
Despite the availability of these rigorously derived definitions, scales, and tools, dystonia is still often underdiagnosed or misdiagnosed.2,3,8–11 This is particularly true when dystonia is comorbid with spasticity as it is in cerebral palsy (CP).8,12 This has required the ongoing criterion standard of dystonia diagnosis to be visual identification by expert consensus discussion. This process is so inherently valuable that most movement disorder centers and professional conferences have patient video review sessions where movement phenomenology is debated before settling on group consensus. However, this subjective diagnostic practice is difficult to operationalize at all centers where numerous motor phenotyping experts may not be readily available.
Our objective was to characterize the features cited by motor phenotyping experts when identifying dystonia in people with CP. To do this, we used qualitative thematic analysis to analyze consensus-building discussions of pediatric movement disorder specialists as they assessed gait videos for lower extremity dystonia in people with spastic CP. Our hypothesis was that this approach would highlight specific motor features that experts use to identify gait dystonia in people with spastic CP. Codifying the currently subjective process of dystonia diagnosis could be a valuable supplement to the available scales and tools for dystonia diagnosis, particularly when describing how dystonia is identified to other practitioners and trainees.
METHOD
This qualitative study of how motor phenotyping experts identify dystonia was granted Human Subjects Research approval from the Washington University School of Medicine Institutional Review Board.
Characteristics of motor phenotyping expert participants
Three fellowship-trained pediatric movement disorder physicians with particular interest in caring for people with CP participated as motor phenotyping experts for this study (BRA, KU, and TP). All three completed pediatric neurology and movement disorders fellowship training at different institutions and shared no training mentors. All three currently see patients in the St. Louis Children’s Hospital Cerebral Palsy Center.
Inclusion and exclusion criteria for gait videos
Videos were taken of patients seen in the St. Louis Children’s Hospital Cerebral Palsy Center between 1st January 2005 and 31st December 2018. Our goal was to identify dystonia from videos taken of a relatively homogenous patient population engaging in the same functionally relevant voluntary movement. This was done to ensure discussions could focus on movement features primarily related to dystonia while controlling for other clinical factors. Standardization of the voluntary movement assessed was necessary as dystonia may appear differently if triggered by different voluntary movements. We also sought patients with documented spasticity, since comorbid spasticity can make dystonia diagnosis particularly difficult in CP.8 Inclusion criteria were: (1) International Classification of Diseases (ICD-9 or ICD-10) diagnoses of spastic diplegic, triplegic, hemiplegic, or quadriplegic CP, (2) video taken during routine clinic visits to the Cerebral Palsy Center, and (3) presence of periventricular leukomalacia on brain magnetic resonance images (MRI) per radiology report. Exclusion criteria were histories of: (1) traumatic brain injury, (2) brain surgeries, (3) brain tumors, (4) encephalitis, (5) chemotherapy, (6) metabolic disorders, or (7) genetic disorders. Exclusion criteria were purposefully broad to focus on only a single type of brain injury, again to select as homogenous a patient population as possible. Videos were screened for clips of the patients independently walking barefoot down a 20-foot stretch of hallway, with knees to toes visible, in a straight line towards the camera. Videos were variable in length depending on the patient’s functional status and how long it took each patient to complete this standardized movement task. This gait task was chosen for its functional relevance and because it allowed the best visualization of the whole person during voluntary movement. Videos with patients using handheld walking aids were assessed, but videos with patients walking in orthoses were not.
Consensus-based video review
A single gait video clip from each selected patient was deidentified and shared via a secure server with the putative CP motor phenotyping experts (henceforth referred to as ‘discussants’). The discussants watched the videos together in real-time and then silently voted about the presence or absence of dystonia in the lower extremities in each video. Discussants were asked to focus on identifying dystonia in the lower extremities as some patients used handheld walking aids for ambulation, precluding comparable assessment of upper extremity dystonia across all patients. The results of the silent vote were then anonymously revealed and discussants engaged in consensus-building discussion about how they approached identifying dystonia in the video. Discussants subsequently openly voted about their revised opinions on the presence or absence of dystonia in the video. Of note, given that the discussants all see patients in the Cerebral Palsy Center from which the videos used in this study were obtained, three of the patients were recognized by at least one discussant. Codes generated from these videos were not included in further analysis.
Qualitative thematic analysis
Our goal was to use qualitative discussion data to quantify the frequency with which specific gait features were cited by experts when identifying dystonia. To do this, an inductive thematic analysis approach was used to extract semantic themes.13 That is, themes were determined directly from the discussion data without extrapolation to implied meanings beyond what was explicitly said by the discussants. This method was chosen to best characterize the real-time process of subjective dystonia identification without ascribing other features known to be associated with dystonia in other contexts. Themes were determined by coalescing the most codes extracted from consensus-building discussion statements. These codes were inductively determined by two investigators (SES and LG) who were not present for the live discussion. That is, codes were derived directly from the discussion transcript without preconceived assumptions about what codes might appear. Discussion transcripts were coded independently by two investigators (SES and LG). Only codes agreed upon by the two investigators were used for developing overarching themes. An abbreviated codebook is shown in Table 1.
Table 1:
Abbreviated codebook
| Code | Definition | Example(s) |
|---|---|---|
| Uncertainty | Statement indicates that the discussant is unsure about the presence or absence of dystonia | ‘I think I see …’ ‘I’m hedging’ |
| Unilateral | Specific mention of the movement feature occurring in one limb | ‘… the left foot looks …’ ‘… his right leg does …’ |
| Variability over time | Statement is about features occurring in just one portion of the video but not in other portions of the video | ‘Her right foot initially turns in more and then straightens out in the end’ ‘It disappears later on’ |
| Subtle | Discussant acknowledges that a movement feature is difficult to see or of low amplitude | ‘He’s got a little bit of crouch’ ‘It’s just so mild’ |
| Adduction | Either the leg or foot is noted to come towards the midline | ‘That left leg crosses in’ ‘The left knee comes in’ |
| Need chart information | Discussant expresses the desire for additional information about the participant to help with dystonia identification | ‘How old is she?’ ‘I want to know her diagnosis’ |
| Abnormal movement, non-specific | A movement is mentioned as being abnormal but not further characterized | ‘That’s a funny posture’ ‘I would call something in her feet’ |
| Selective dorsal rhizotomy | Discussant ponders whether or not the child has had or would benefit from a selective dorsal rhizotomy | ‘This patient has had a rhizotomy maybe’ ‘I just feel like a lot of our rhizotomy patients will have a gait that looks like that several years later when they get bigger’ |
| Foot | The foot is mentioned explicitly | |
| Leg | The leg is mentioned explicitly |
Statistical analysis
Comparisons of code frequency using χ2 tests were made between four discussant categories based on initial and final votes about the presence of dystonia in each video: no to no, no to yes, yes to no, and yes to yes (Graph Pad Prism 8, GraphPad Software, San Diego, CA, USA). Cohen’s κ was used to assess the interrater reliability for video-based dystonia diagnosis.
RESULTS
Seven hundred and twenty-one patients met the inclusion criteria. Of these patients, 640 were excluded for having the listed disease processes or surgeries. The videos of the remaining 81 patients were screened, with 40 patients having videos of independent barefoot ambulation in a straight line towards the camera. Of these 40 patients, three were documented to have spastic hemiplegia, four were documented to have spasticity in both lower extremities but with clearly asymmetric involvement, and all others had documented spasticity in both lower extremities without clear asymmetry (Table 2). A single gait video clip was selected for each of these 40 patients for further analysis. Video duration ranged from 2 to 24 seconds depending on the length of time it took each of these patients to traverse the same 20-foot length of hallway. Most videos (28 out of 40) were between 5 and 10 seconds’ duration. There was no significant difference in video duration between videos unanimously classified as displaying dystonia (mean 7.3s, 95% CI 5.1–9.4) versus not (mean 8.6s, 95% CI 6.4–10.7) (Student’s t-test, p=0.89). There was also no significant difference in video duration between videos where the classic dystonia descriptor of variability of movement over time was mentioned during consensus-building discussion (mean 8.0s, 95% CI 5.7–10.3) versus not mentioned (mean 8.1s, 95% CI 6.0–10.3) (Student’s t-test, p=0.83).
Table 2:
Patient characteristics
| Participant | Sex | Gestational age at birth (wks) | Brain injury/CP etiology | GMFCS level | Age (y:mo) | Walking aid used? | Leg spasticity laterality | Discussant Initial to final vote |
||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||||||
| 1 | M | 30 | PVL | III | 16:5 | Y | L=R | Y to Y | Y to N | N to N |
| 2 | M | 25 | PVL | III | 18:10 | Y | L=R | N to N | N to N | N to N |
| 3 | F | Term | PVL (unknown etiology) | I | 6:8 | N | L<R | N to Y | Y to Y | N to Y |
| 4 | F | Preterm (adopted) | PVL | I | 17:10 | N | L=R | N to N | N to N | N to N |
| 5 | M | 36 | HIE, PVL, IPH | III | 13:5 | Y | L=R | N to Y | Y to Y | N to Y |
| 6 | F | Term | Stroke, PVL | II | 8:4 | N | L | Y to Y | N to Y | Y to Y |
| 7 | F | 25 | PVL | II | 12:10 | N | L=R | N to N | N to N | Y to N |
| 8 | F | 33 | PVL | III | 18:8 | Y | L=R | Y to Y | Y to Y | N to Y |
| 9 | M | 30 | PVL | III | 12:7 | Y | L=R | Y to Y | Y to Y | Y to Y |
| 10 | M | 36 | PVL (unknown etiology) | I | 8:4 | N | L=R | Y to Y | Y to Y | N to N |
| 11 | F | 27 | PVL | III | 17:8 | Y | L=R | N to Y | Y to Y | Y to Y |
| 12 | M | 30 | PVL | I | 3:7 | N | L=R | N to N | N to N | N to N |
| 13 | F | Preterm (adopted) | Stroke, PVL | II | 18:8 | N | L<R | N to N | N to N | N to N |
| 14 | M | 28 | PVL | III | 19:1 | Y | L=R | N to N | N to N | N to N |
| 15 | M | 31 | PVL | II | 15:6 | N | L=R | Y to Y | Y to Y | Y to Y |
| 16 | M | 31 | PVL | II | 16:1 | N | L=R | Y to Y | Y to Y | N to Y |
| 17 | F | 25 | PVL, IVH, PHH | II | 8:2 | N | L | Y to Y | Y to Y | Y to Y |
| 18 | M | 32 | PVL | II | 11:1 | N | L=R | Y to N | N to N | N to N |
| 19 | M | 27 | PVL | II | 11:1 | N | L=R | Y to N | Y to N | N to N |
| 20 | M | 33 | PVL | I | 9:6 | N | L=R | N to N | N to N | N to N |
| 21 | M | 35 | HIE, PVL | II | 8:11 | N | L<R | Y to Y | N to Y | Y to Y |
| 22 | F | Preterm | PVL | II | 24:5 | N | L=R | N to N | N to N | Y to N |
| 23 | F | 24 | PVL | I | 22:4 | N | L=R | N to N | N to N | N to N |
| 24 | F | Preterm (adopted) | PVL | I | 13:2 | N | L=R | Y to Y | N to N | N to N |
| 25 | F | 39 | HIE (placental abruption), PVL | II | 6:0 | N | L=R | Y to Y | Y to Y | Y to Y |
| 26 | F | 24 | PVL | II | 16:5 | N | L=R | N to N | N to N | Y to N |
| 27 | F | 26 | PVL, IVH | II | 4:5 | N | L=R | Y to Y | Y to Y | Y to Y |
| 28 | F | Term | PVL (unknown etiology) | III | 8:10 | Y | L=R | N to Y | Y to Y | Y to Y |
| 29 | M | 31 | PVL | III | 7:8 | Y | L=R | Y to N | N to N | N to N |
| 30 | F | Unknown (adopted) | PVL (unknown etiology) | I | 18:4 | N | L | N to N | N to Y | Y to Y |
| 31 | F | 28 | PVL | II | 6:2 | N | L=R | N to N | N to N | N to N |
| 32 | M | 27 | PVL, IVH | I | 9:2 | N | L=R | N to N | N to N | N to N |
| 33 | F | 28 | HIE (placental abruption), PVL, IVH | III | 12:11 | Y | L=R | Y to Y | Y to Y | Y to Y |
| 34 | F | 40 | PVL, IPH | I | 7:2 | N | L=R | N to Y | N to N | Y to Y |
| 35 | F | 28 | PVL, IVH | II | 23:2 | N | L=R | N to N | Y to N | N to N |
| 36 | F | 34 | HIE (placental abruption), PVL | I | 17:7 | N | L=R | N to N | N to N | N to N |
| 37 | F | 40 | PVL (unknown etiology) | II | 19:4 | N | L=R | N to N | N to N | N to N |
| 38 | M | 33 | PVL | I | 6:5 | N | L<R | Y to Y | Y to Y | N to Y |
| 39 | F | 28 | PVL | III | 13:11 | Y | L=R | N to N | N to N | N to N |
| 40 | M | 33 | PVL | III | 22:5 | Y | L=R | N to N | N to N | Y to Y |
CP, cerebral palsy; GMFCS, Gross Motor Function Classification System;14 M/F, male or female; PVL, periventricular leukomalacia; L=R, spasticity noted in both legs without documentation of asymmetry; Y/N, discussant’s yes/no vote about presence or absence of dystonia; HIE, hypoxic–ischemic encephalopathy; IPH, intraparenchymal hemorrhage; IVH, intraventricular hemorrhage; PHH, post-hemorrhagic hydrocephalus; L>R or L<R, spasticity documented in both legs; but with L either affected more (>) or less (<) than the R; L or R, spasticity documented in just one leg.
Patients’ characteristics and discussants’ decisions about the presence or absence of dystonia in these clips are shown in Table 2. Patients’ ages at the time of video recording ranged from 6 to 24 years old (mean 13y 4mo, 95% CI 11.6–15.1). Handheld walking aids were used in 10 videos. Twelve participants were categorized in Gross Motor Function Classification System14 (GMFCS) level I, 16 in GMFCS level II, and 12 in GMFCS level III.
Before assessing the features cited by discussants when they identified dystonia, we first confirmed that discussants had comparable assessments of dystonia in these video clips. Discussants were able to reach consensus about the presence or absence of dystonia for 34 videos with high interrater reliability (Cohen’s κ=0.80). Dystonia was unanimously identified in 15 videos (example shown in Video S1, online supporting information) and unanimously not identified in 19 (example shown in Video S2, online supporting information). To ensure that no single discussant was swaying the group during consensus-building discussion, we confirmed that discussants did not differ from each other in the frequency with which they initially or ultimately classified a video as having dystonia (χ2, p=0.992) (Fig. 1).
Figure 1:
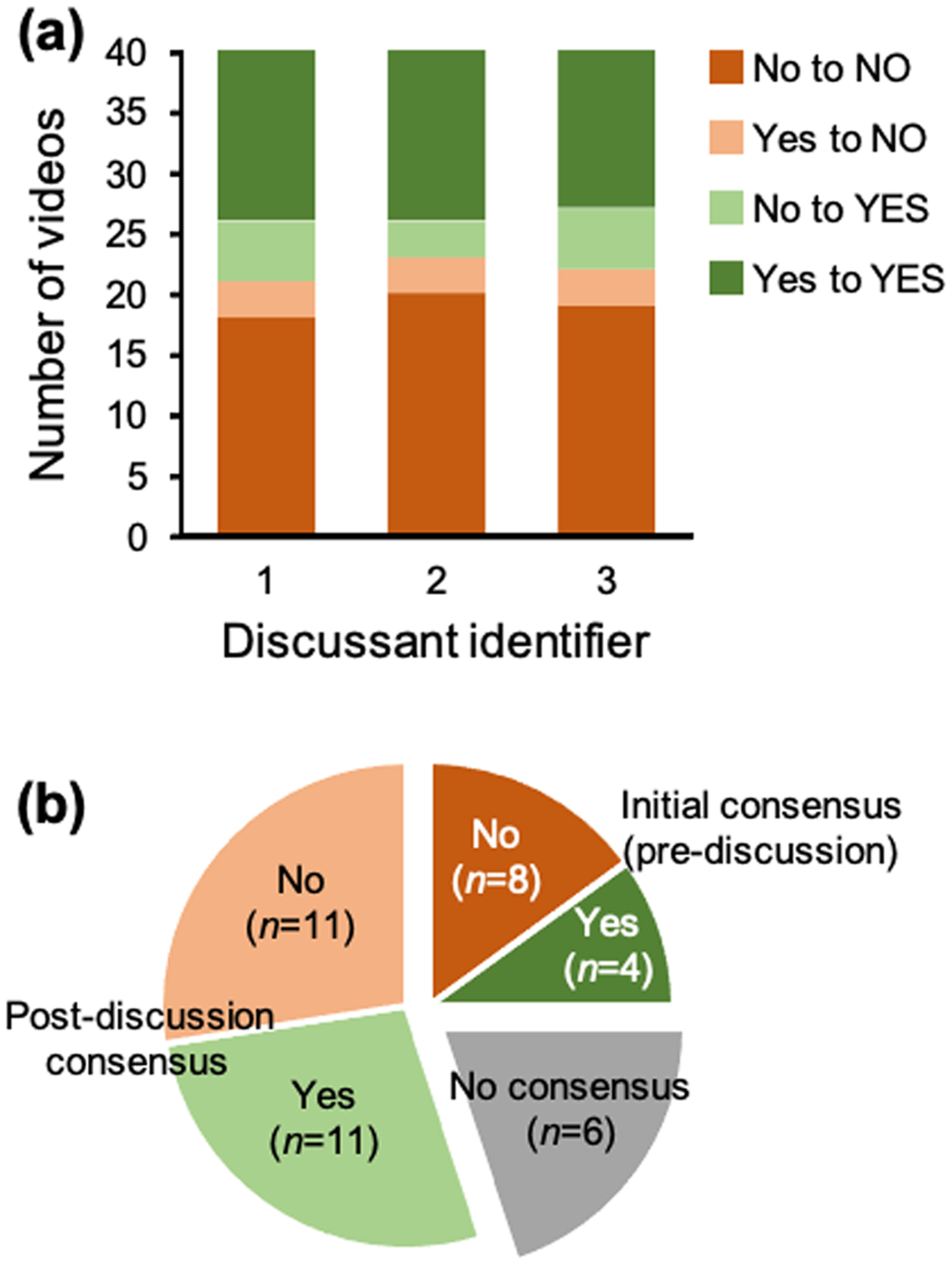
Voting results by discussant and across all videos. (a) Frequencies of initial and final yes and no votes about the presence or absence of dystonia in participant videos, separated by discussant. (b) Consensus voting results about the presence or absence of dystonia across all participant videos.
The consensus-building discussion transcript was ultimately parsed into 519 codes, representing 54 individual codes that were identified as occurring at least once. These codes described movement features as well as discussants’ perceptions about the dystonia identification process. The most frequently used codes were expressions of uncertainty, movement variability over time, and movement abnormalities that were identified as being unilateral at any given time during the video, subtle, in the foot, in the leg, or involving adduction. The next most frequently used codes occurred after an apparent inflection point in the code frequency distribution and were also not as useful in defining movement qualities that could prompt dystonia identification. These codes included requests for additional chart information, characterizing a movement as non-specifically abnormal, and pondering whether the participant may have had a selective dorsal rhizotomy (Table 1 and Fig. 2). Less frequent codes describing specific movements included foot inversion (n=6), foot plantar flexion (n=3), and leg extension (n=4).
Figure 2:
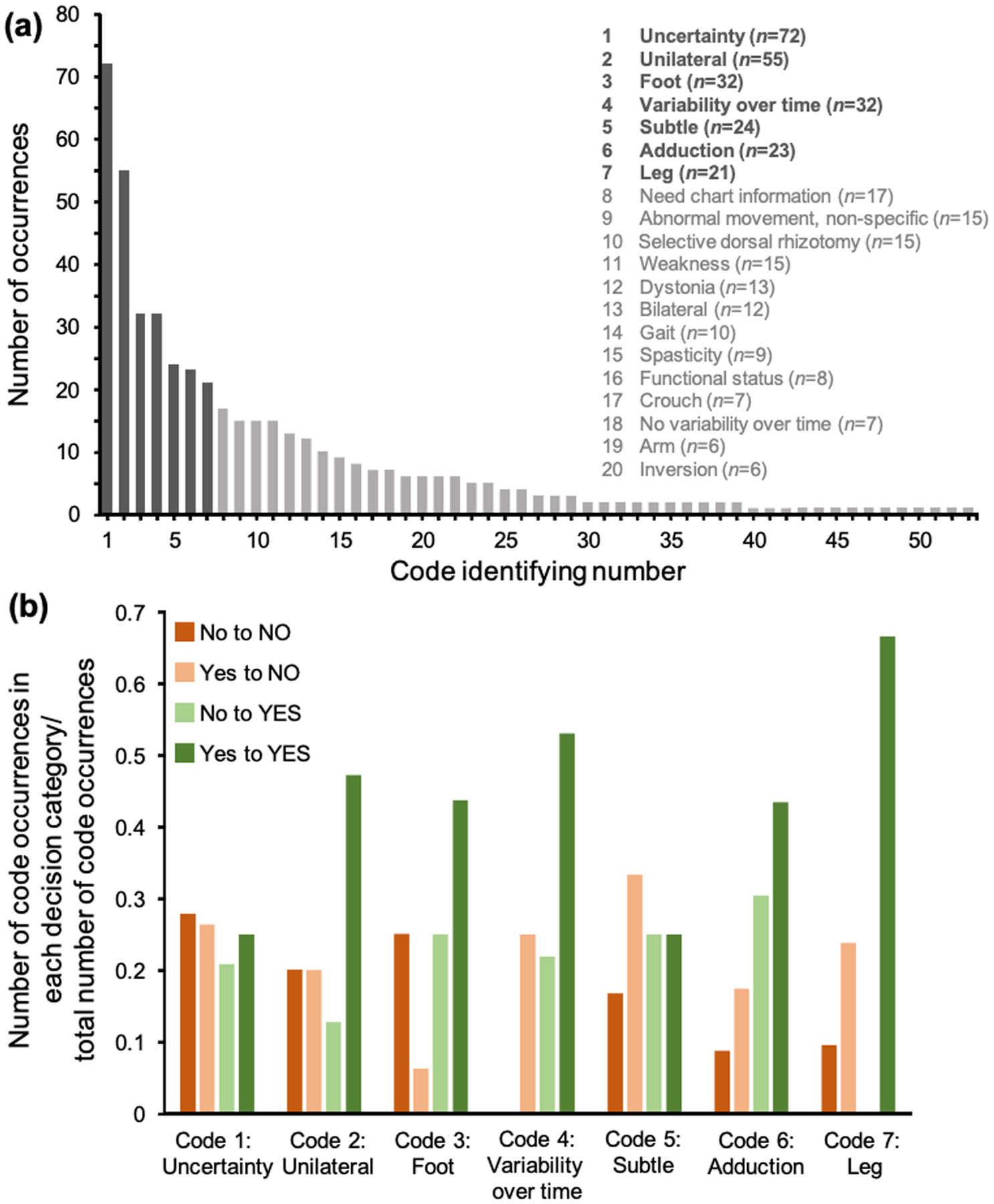
Coding of consensus-building discussions. (a) Frequencies of individual codes (total of 54 individual codes with the top 20 codes indicated). There was a natural inflection point in the frequency distribution following the first seven codes. (b) Frequencies of top codes separated by discussant voting category. Codes were used at significantly different frequencies by discussants depending on how they voted about the presence or absence of dystonia in a video (χ2, p=0.004).
Codes were used at significantly different frequencies by discussants depending on how they voted about the presence or absence of dystonia in a video (χ2, p=0.004). When taken in aggregate, codes describing movements as being variable over time (n=32), unilateral (n=55), in the foot (n=32), in the leg (n=21), or involving adduction (n=23) occurred more commonly when a discussant’s initial and final post-discussion vote was that dystonia was present in the video: that is, to affirm the identification of dystonia. Almost half of the time these codes were cited, they were cited by this group of discussants (81 out of 163 total code mentions). This was a significant difference compared with any of the other three discussant voting groups, which each cited these codes only 14% to 18% of the times they were used (χ2, p<0.00001, Fig. 2). These codes also commonly occurred together within any given single statement. At least three of these codes were present together in 18% of discussants’ statements containing any one of these codes, and all codes were present together in 7% of the statements. An example of a statement sharing all of these codes was, ‘I felt like her left foot intermittently turns in and that her right leg came in sharply once in the middle of the video’. As indicated in this example, descriptions of unilateral involvement often varied over the course of the video (i.e. a participant may have dystonia described in one leg initially and then have it described in the other leg subsequently). The presence of asymmetric leg spasticity did not significantly affect the frequency with which discussants noted unilateral leg dystonia (χ2, p=0.09). Therefore, the primary theme derived from this study is that, when identifying dystonia, discussants cited unilateral foot or leg adduction that was variable over time. Codes attributable to this primary theme represented 31% of the total codes identified (163 out of 519 codes).
The secondary key theme was difficulty. Multiple codes documented the difficulty discussants had in identifying dystonia including expressions of uncertainty, subtle movements, need for additional chart information, non-specific descriptors for abnormal movements, using the word ‘dystonia’ or ‘dystonic’ to define why dystonia was present, and the need to perform an examination. Codes attributable to this secondary theme also represented 31% of the total (159 out of 519 codes).
DISCUSSION
We identified two themes cited by experts when identifying dystonia in gait videos of people with spastic CP. Unilateral and variable foot or leg adduction was the most commonly cited motor feature. The difficulty of identifying dystonia in participants with CP was also frequently cited, supporting the need for studies such as this one. Qualitative thematic analysis of motor phenotyping consensus-building discussions, as we have done here, could help codify the subjective process of visual dystonia identification by expert consensus. This codification could serve as a valuable adjunct to the validated scales and tools currently available to help diagnose dystonia, particularly when training practitioners to identify dystonia.
Gait features cited by experts when identifying dystonia
Unilateral lower extremity abnormalities were more commonly cited when a discussant identified dystonia in a video both pre- and post-discussion compared with the three other discussant voting groups (i.e. the discussant changed their vote from yes to no or vice versa following discussion, or the discussant voted that dystonia was not present in the video both pre- and post-discussion). This occurred despite the assessed voluntary movement (gait) involving both legs and despite most participants having documented motor impairment affecting both legs. This may be because the participants were all affected by dystonia in one limb more than the other, because the limb engaging in the active swing portion of gait is more likely to demonstrate visualizable dystonia, or because experts found it easiest to pick up on differences in positioning between the limbs when identifying dystonia.
Although numerous movement patterns have been associated with dystonia at large,15 lower limb adduction was commonly cited when identifying dystonia in this study population. Sustained foot inversion has been described as a dystonic posture but leg adduction has not.15 In the context of the reviewed video clips, the leg adduction that may have prompted dystonia identification was probably variable over time and, therefore, dynamic and of short duration. If subtle and fleeting, leg adduction may have previously been missed as a movement associated with dystonia in people with CP. The concept of dystonic ‘scissoring’ involving leg adduction has historically been noted in those with CP, but can be difficult to differentiate from spasticity.16 In these videos, it is possible that the variability in leg adduction over time allowed for differentiation from spasticity.
As expected on the basis of the 2013 consensus definition,1 variability of movements over time was commonly cited when identifying dystonia. Video reviewers could potentially have been biased towards identifying variability in videos of longer duration, but this was not observed in our data set.
Limitations
Our participants were all independently ambulatory with or without an assistive device (GMFCS levels I–III14) and had diagnoses of spastic CP with periventricular leukomalacia noted on their brain MRIs. Focusing on a relatively homogenous participant population probably aided extraction of discussion themes specific to dystonia identification. However, our results may only apply to this population. Conducting similar analyses with other groups of people with CP with different motor functional statuses, different patterns of brain injury, and different CP etiologies, may yield different movements that are most commonly associated with dystonia by motor phenotyping experts.
Choosing independent ambulation as the voluntary movement used to trigger dystonia was experimentally valuable for many reasons: (1) gait is a functionally important voluntary movement, thus increasing the likelihood that any dystonia identified by motor phenotyping experts would have functional impact; (2) gait is a relatively standardized movement across participants that involves the whole body, allowing for the greatest likelihood of capturing dystonia in the video; and (3) other standardized examination measures or voluntary movements were not reliably captured across all patients during routine clinic visits in this retrospective study. Future efforts could involve having motor phenotyping experts prospectively review videos of standardized maneuvers (such as performance of the Hypertonia Assessment Tool5–7) in participants with CP. This might additionally alleviate some of the uncertainty expressed during consensus-building discussion about need for the examination to identify dystonia and would serve as a cross-validation of expert evaluation of videos.
The videos assessed were not different in length between participants who displayed or did not display dystonia. However, the videos were, overall, short in duration, raising questions about the sensitivity of using these video clips to assess variability of movements over time, a key feature of dystonia. Although experts were still able to comment upon variability, and did so more frequently in videos they felt displayed dystonia, future studies could use longer video clips or could work towards quantifying the degree of movement variability present in these video clips.
The motor phenotyping experts participating in this study were fellowship-trained pediatric movement disorder physicians who had trained in different locations but who all currently practice in the same location. Although codes generated from videos where the participant was recognized by a discussant were excluded and although discussants were otherwise blinded to participant identity, there may still be some bias present when reviewing patients’ videos from one’s own practice center. Furthermore, experts from different centers may identify dystonia differently from experts at a single center. Future studies could utilize online tele-consensus discussion between experts who practice at different centers.
CONCLUSION
Our results mark a critical step in delineating the specific movement features that are relevant to identifying dystonia in those with CP. Given that motor phenotyping has largely been seen as a subjective effort by putative experts, an analysis of the words used to describe movement disorders may contain our best hope of making what these experts do explicit. This will be beneficial both to teach others and for the education of the experts themselves about their own diagnostic practices. Furthermore, efforts to make the process of dystonia diagnosis more transparent are critical for ensuring this debilitating tone abnormality is diagnosed early and appropriately in those with CP.
Supplementary Material
Video S1: Person with gait dystonia.
Video S2: Person without gait dystonia.
What this paper adds.
Dystonia identification is visually difficult, even for experts.
Unilateral lower extremity variable adduction could represent gait dystonia in cerebral palsy (CP).
Qualitative thematic analysis helps delineate expert-cited dystonia features in CP.
ACKNOWLEDGMENTS
BRA receives research funding from the National Institute of Neurological Disorders and Stroke. TSP receives research funding from the National Institute of Neurological Disorders and Stroke and reports consulting fees from Teva Pharmaceuticals. KU, LG, HM, and SES report no disclosures. Funding supporting this work is from the National Institutes of Neurological Disorders and Stroke (5K12NS098482-02). The authors have stated that they had no interests that might be perceived as posing conflict or bias.
REFERENCES
- 1.Albanese A, Bhatia K, Bressman SB, et al. Phenomenology and classification of dystonia: a consensus update. Mov Disord 2013; 28: 863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monbaliu E, de Cock P, Ortibus E, et al. Clinical patterns of dystonia and choreoathetosis in participants with dyskinetic cerebral palsy. Dev Med Child Neurol 2016; 58: 138–44. [DOI] [PubMed] [Google Scholar]
- 3.Monbaliu E, Ortibus E, Roelens F, et al. Rating scales for dystonia in cerebral palsy: reliability and validity. Dev Med Child Neurol 2010; 52: 570–5. [DOI] [PubMed] [Google Scholar]
- 4.Monbaliu E, Ortibus E, De Cat J, et al. The Dyskinesia Impairment Scale: a new instrument to measure dystonia and choreoathetosis in dyskinetic cerebral palsy. Dev Med Child Neurol 2012; 54: 278–83. [DOI] [PubMed] [Google Scholar]
- 5.Jethwa A, Mink J, Macarthur C, et al. Development of the Hypertonia Assessment Tool (HAT): a discriminative tool for hypertonia in children. Dev Med Child Neurol 2010; 52: e83–7. [DOI] [PubMed] [Google Scholar]
- 6.Rice J, Skuza P, Baker F, et al. Identification and measurement of dystonia in cerebral palsy. Dev Med Child Neurol 2017; 59: 1249–55. [DOI] [PubMed] [Google Scholar]
- 7.Knights S, Datoo N, Kawamura A, et al. Further evaluation of the scoring, reliability, and validity of the hypertonia assessment tool (HAT). J Child Neurol 2014; 29: 500–4. [DOI] [PubMed] [Google Scholar]
- 8.Lin J-P, Lumsden DE, Gimeno H, Kaminska M. The impact and prognosis for dystonia in childhood including dystonic cerebral palsy: a clinical and demographic tertiary cohort study. J Neurol Neurosurg Psychiatry 2014; 85: 1239–44. [DOI] [PubMed] [Google Scholar]
- 9.Eggink H, Kremer D, Brouwer OF, et al. Spasticity, dyskinesia and ataxia in cerebral palsy: Are we sure we can differentiate them? Eur J Paediatr Neurol 2017; 21: 703–6. [DOI] [PubMed] [Google Scholar]
- 10.Albanese A, Sorbo F Del, Comella C, et al. Dystonia rating scales: critique and recommendations. Mov Disord 2013; 28: 874–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart K, Harvey A, Johnston LM. A systematic review of scales to measure dystonia and choreoathetosis in children with dyskinetic cerebral palsy. Dev Med Child Neurol 2017; 59: 786–95. [DOI] [PubMed] [Google Scholar]
- 12.Rice J, Skuza P, Baker F, et al. Identification and measurement of dystonia in cerebral palsy. Dev Med Child Neurol 2017; 59: 1249–55. [DOI] [PubMed] [Google Scholar]
- 13.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3: 77–101. [Google Scholar]
- 14.Palisano R, Rosenbaum P, Walter S, et al. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 2008; 39: 214–23. [DOI] [PubMed] [Google Scholar]
- 15.Sanger TD, Ferman D. Similarity of involuntary postures between different children with dystonia Mov Disord Clin Pract 2017; 4: 870–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JP, Nardocci N. Recognizing the common origins of dystonia and the development of human movement: a manifesto of unmet needs in isolated childhood dystonias. Front Neurol 2016; 7: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1: Person with gait dystonia.
Video S2: Person without gait dystonia.


