Abstract
Proteases of Porphyromonas gingivalis are considered to be important virulence determinants of this periodontal bacterium. Several biochemical isoforms of arginine-specific proteases are derived from rgpA and rgpB. HRgpA is a heterodimer composed of the catalytic α chain noncovalently associated with a β adhesin chain derived from the C terminus of the initial full-length translation product. The catalytic α chain is also present as a monomer (RgpA) either free in solution or associated with membranes. rgpB lacks the coding region for the adhesin domain present in rgpA and yields only monomeric forms (RgpB) which again may be soluble or membrane associated. In this study, the catalytic chains of this unusual group of enzymes are shown to be differentially modified by the posttranslational addition of carbohydrate. A monoclonal antibody (MAb 1B5) raised to the monomeric RgpA did not react with the corresponding recombinant RgpA α chain expressed in Escherichia coli but was immunoreactive with P. gingivalis lipopolysaccharide. MAb 1B5 also reacted with the membrane-associated forms of RgpA and RgpB but not with the heterodimeric HRgpA and the soluble form of RgpB. RgpA treated with denaturants was capable of binding to MAb 1B5 whereas treatment with periodate abolished this binding, suggesting the presence of carbohydrate residues within the epitope. Chemical deglycosylation abolished immunoreactivity with MAb 1B5 and caused a ∼30% reduction in the size of the membrane-associated enzymes. Monosaccharide analysis of HRgpA and RgpA demonstrated 2.1 and 14.4%, respectively, carbohydrate by weight of protein. Furthermore, distinct differences were detected in their monosaccharide compositions, indicating that these protease isoforms are modified not only to different extents but also with different sugars. The variable nature of these additions may have a significant effect on the structure, stability, and immune recognition of these protease glycoproteins.
The irreversible tissue destruction which is characteristic of the destructive periodontal diseases is considered to be a consequence of the reaction by a susceptible host to a complex and variable microbial challenge presented by the subgingival plaque. Porphyromonas gingivalis, an anaerobic, gram-negative bacterium, is frequently isolated from the subgingival plaque of periodontal patients and is thought to be an important etiological agent in these conditions (28, 46). P. gingivalis produces several extracellular proteolytic enzyme activities with different peptide bond specificities which have a number of in vitro properties consistent with a role in the periodontal disease process (11). These include deregulatory effects on plasma cascades (21, 35, 49) and the specific and innate host defenses (45, 51), activation of matrix metalloproteases (13), degradation of connective tissue components (22), and interference with host cell function (37). Many of these actions have been shown to be a function of the activity of P. gingivalis proteases with specificity for Arg-x peptide bonds, and therefore there is some justification for regarding these enzymes as important virulence determinants in the periodontal diseases.
The extracellular Arg-x protease activity of P. gingivalis W50 is composed of three enzyme species (HRgpA, RgpA, and mt-RgpA), all derived from rgpA. These designations replace our earlier nomenclature of RI, RIA, and RIB, all derived from prpR1 (1, 10, 41). HRgpA is a heterodimer in which the catalytic α chain (Mr ∼ 54,000) is noncovalently associated with an adhesin chain (β), derived from the initial RgpA translation product, which is capable of mediating binding to the erythrocyte surface and host macromolecules. RgpA is the free monomeric catalytic chain, and membrane-type RgpA (mt-RgpA) is a highly posttranslationally modified form of this chain (Mr ∼ 70,000 to 80,000) which is exclusively associated with the membrane fraction (1, 10, 41). Two additional proteases with Arg-x specificity, RgpB and mt-RgpB (formerly RIIA and RIIB), were detected in the culture supernatant of an rgpA isogenic mutant of P. gingivalis W50 (42). These two forms, which closely resemble the monomeric proteases derived from rgpA, are produced from a second gene, rgpB (prR2), which lacks the coding region for the adhesin chain. RgpB and mt-RgpB may correspond to proteases which are normally cell associated in the wild-type strain W50. These enzymes have not been demonstrated in the culture supernatants of strain W50.
Analysis of the structures and properties of the RgpA and RgpB proteases of P. gingivalis has provided some insights into the molecular survival strategies adopted by an organism whose sole ecological site in the oral cavity is the microbial biofilm in the hostile environment of an inflamed periodontal pocket. For example, these enzymes have been described as extremely efficient C3 and C5 convertases whose activity leads to the fluid-phase inactivation of these key components of the host’s defensive armory (51). Furthermore, while a primary function of the β component of the HRgpA heterodimer may be to target the action of this isoform (39), analysis of the antibody response to this protease in humans or experimental animal models indicates that the β component may also have a role in subversion of the very significant, specific immune response of the colonized host (10, 17, 23). Shielding the catalytically active component of the molecule with a highly immunogenic protein partner may effectively divert the antibody response from regions of the molecule directly involved in proteolysis. A similar strategy has been described for Trypanosoma cruzi, in which a long C-terminal extension to the catalytic domain of the cysteine protease, cruzipain, has been suggested to perform this role (31).
In this work, we wished to extend our previous immunochemical investigations (8, 10) to examine the structure and immunogenicity of each of the RgpA and RgpB proteases by the development of monoclonal antibodies (MAbs) to the catalytic chain of each of these enzymes. To avoid complications arising from the immunogenicity of the β component of HRgpA, these experiments were performed with the single-chain RgpA isoform. The results of the study demonstrate the presence of immunogenic, covalently linked carbohydrate additions to the catalytic chain of some of the enzymes of this family of proteases. Glycosylation of these bacterial proteins may have an influence on the stability of not only the resulting glycoconjugate but also their immune recognition.
MATERIALS AND METHODS
Bacteria and growth conditions.
P. gingivalis W50 and W501 (rgpA) were cultured in brain heart infusion-hemin medium (34, 41) or blood agar base containing 5% defibrinated horse blood in an atmosphere of N2, H2, and CO2 (80:10:10; Don Whitley anaerobic work station). Escherichia coli XL-1 Blue MRF′ (Stratagene) and M15(pREP4) (Qiagen) were grown in Luria-Bertani (44) medium. If required, tetracycline was added to 20 μg/ml. In E. coli, plasmids were selected by incorporation of ampicillin (50 μg/ml) for pUC (53), pJF119 (16), and pGEX-derived constructs (Pharmacia) or kanamycin (25 μg/ml) for pREP4 (Qiagen). Plasmids were purified by the method of Clark-Curtiss and Curtiss (6) or by ion-exchange chromatography using Qiagen tips.
Generation of recombinant RgpA proteins.
A region containing the catalytic α domain of RgpA was expressed in E. coli as an N-terminal polyhistidine (His6) fusion protein to facilitate purification. Plasmid KpL is a subclone of the original rgpA clone, pJM2 (1), and contains the coding region for RgpA M1-T949. An internal fragment of the pKpL insert, corresponding to the coding region for RgpA G149-S737, was excised by SmaI/BamHI restriction digestion and blunted with Klenow DNA polymerase. Following gel purification, the 1,764-bp fragment was cloned into the SmaI site of the multiple cloning site of pQE30 under control of the Tn5 promoter (Qiagen) in E. coli XL-1 Blue to generate pQ3010. For expression of the recombinant protein, pQ3010 was used to transform E. coli M15, which harbors pREP4 containing lacI.
Variable levels of expression of the recombinant protein were obtained with this system. As an alternative, the entire coding region for the fusion protein, including the ribosome binding site and the ATG initiation codon, was removed from pQ3010 by restriction digestion with EcoRI/SalI and directionally cloned into pJF119EH (16), which contains lacIq for tighter control of expression under the tac promoter. Expression from the resulting construct, pJFQ3010, was performed in E. coli XL-1 Blue. A C-terminal His6 fusion protein of dihydrofolate reductase (DHFR) which was used as a control antigen was expressed from pQE16 (Qiagen) in E. coli M15.
His6 recombinant proteins were purified under denaturing conditions by nickel chelate chromatography on Ni-nitrilotriacetic acid resin following solubilization of the cells in 6 M guanidine-HCl as instructed by the manufacturer (Qiagen). Correct identity of the resulting homogeneous protein preparation was confirmed by N-terminal sequence analysis of the first 20 residues as previously described (41). Throughout this report, the His6-RgpA fusion protein is referred to as recombinant RgpA α component (rec RgpA α).
An internal region (D784 to V1130) of the β domain of RgpA was expressed as a glutathione S-transferase (GST) N-terminal fusion protein in Spodoptera frugiperda (Sf9 insect cell line) via baculovirus technology and was purified by affinity chromatography on glutathione-agarose by using methods to be described elsewhere (8a). This is referred to as recombinant RgpA β component (rec RgpA β). Recombinant GST was expressed from pGEX-3X (Pharmacia) in E. coli XL-1 Blue.
P. gingivalis W50 proteases and LPS purifications.
HRgpA, RgpA, and mt-RgpA proteases were purified from the culture supernatant of P. gingivalis W50 as previously described (41). RgpB and mt-RgpB proteases were prepared in a similar manner from the culture supernatant of an isogenic rgpA (prpR1) mutant of P. gingivalis W50 (W501), again as described previously (42). Lipopolysaccharide (LPS) from P. gingivalis W50 was prepared by the method of Darveau and Hancock (12), and purity was determined via sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by silver staining (52).
MAb production.
Female BALB/c mice were immunized intraperitoneally and intramuscularly with 10 μg of P. gingivalis W50 RgpA emulsified with Freund’s incomplete adjuvant on three occasions at 4-week intervals. After a further 4 weeks, and 3 days prior to fusion, the mice were boosted with a further 10 μg of RgpA intravenously. Hybridomas were raised as described by Kohler and Milstein (24). Hybrid culture media and reagents were described previously (8). The NSI/Ag4.1 mouse myeloma cell line was used as the fusion partner and was grown in spinner cultures in RPMI 1640 (Bethesda Research Laboratories) plus 10% fetal calf serum (FCS) at 37°C in a 5% CO2 atmosphere and maintained in log-phase growth prior to fusion with spleen cells in a 1:3 ratio.
A two-stage screening approach was used for the detection of MAbs to the peptide chain of RgpA. The initial screen used an RgpA enzyme-linked immunoabsorption assay (ELISA) to determine which culture supernatants contained antibody reactive with the immunizing antigen. Positive hybridomas were maintained in culture, and the supernatants were then screened by ELISA for antibody reactive with RgpA, rec RgpA α, rec RgpA β, P. gingivalis W50 LPS, and the two control proteins, His6-DHFR and GST.
Ninety-six-well flat-bottomed microtiter plates were coated with P. gingivalis RgpA (5 μg/ml; 100 μl/well) and incubated for 4 h at room temperature. After the antigen was removed and the plates were washed with 0.5 mM sodium carbonate buffer (pH 9.6) blocking with 1% FCS proceeded for a further 2 h. The remaining antigen plates were prepared in the same way. Tissue culture supernatants from wells containing hybridomas were added to the wells (100 μl/well) of the screening assay plates and incubated for 2 h. Following washing of the plates with Tris-buffered saline–Tween 20 (0.5%), 100 μl of alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G antibody (1:5,000) in RPMI plus 1% FCS was added and incubated for 1 h. Bound secondary antibody was detected by using p-nitrophenyl phosphate, and color development was monitored at 405 nm. Positive binding was taken as twice the normal background value of RPMI 1640 control wells on each antigen plate. Hybridomas that gave strong signals by ELISA were then cloned by limit dilution and retested against the panel of antigens.
PAGE and immunochemistry.
PAGE in the presence of SDS (25) was carried out at 5°C in 7.5 or 12.5% (wt/vol) polyacrylamide slab gels. Samples of protease (10 to 20 μg) were first treated with 50 μl of leupeptin (1 mM) at 25°C for 20 min, heated at 100°C for 5 min, and dried in vacuo. PAGE in the presence of 8 M urea at pH 8.8 in 7.5% (wt/vol) slab gels was carried out as described by Marshall and Inglis (30). Western immunoblotting was performed following electroblotting onto nitrocellulose. N-terminal amino acid sequencing was performed following electroblotting of proteins onto polyvinylidene difluoride membranes. The rabbit antiserum to P. gingivalis W50 whole cells has been described previously (10).
Denaturant treatment of RgpA.
Samples of RgpA (10 μg) in sodium acetate buffer (0.2 M, pH 5.3) were incubated for 60 min in 6.4 M urea (55°C), 3.3% SDS (55°C), or 75% formic acid (22°C). The formic acid-treated RgpA was evaporated to dryness to remove the acid, and then aliquots of all samples (corresponding to 0.5 μg of RgpA) were subjected to SDS-PAGE and Western blotting onto nitrocellulose. For periodate oxidation, leupeptin-inhibited RgpA (0.5 μg) was subjected to SDS-PAGE, and electroblotted onto nitrocellulose, and the blots were then incubated in 1% periodic acid–7% acetic acid in the dark at 4°C. Leupeptin-inhibited RgpA acted as the control.
Biotinylation of glycosylated proteins.
The presence of covalently linked carbohydrate on individual proteases was determined by periodate oxidation at pH 5.5 followed by treatment with biotin hydrazide to biotinylate any resultant free aldehyde groups. Biotinylation of the proteases was then detected with streptavidin conjugated to alkaline phosphatase following Western blotting onto polyvinylidene difluoride membranes. Control samples were treated identically except that the periodate oxidation step was omitted. All procedures were performed as instructed by the manufacturer of the Glycotrack carbohydrate detection system (Oxford GlycoSystems, Abingdon, United Kingdom).
Deglycosylation procedures.
Proteases were treated with peptide-N4-(N-acetylglucosaminyl) asparagine amidase (PNGase F; Genzyme) to hydrolyze any asparagine-linked oligosaccharides at the β-aspartylglycosylamine bond between the innermost N-acetylglucosamine and asparagine residue. The proteases (1 mg/ml) were first denatured by heating at 100°C for 5 min in 0.5% SDS. Hydrolysis was performed for 18 h in Tris-HCl (50 mM, pH 8.0)–EDTA (50 mM)–β-mercaptoethanol (50 mM). CHAPS {3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate nonionic detergent was added to 2.5% (wt/vol) to protect the PNGase F from SDS denaturation. Ovalbumin was used as a positive control. The removal of susceptible asparagine-linked oligosaccharide was then determined by examining the mobility of the treated proteases on SDS-PAGE and by Western blotting using MAbs to the carbohydrate additions.
Proteases RgpA and mt-RgpA were subjected to chemical deglycosylation using a GlycoFree TM deglycosylation kit (Oxford GlycoSystems). The enzymes were first treated with anhydrous trifluoromethane sulfonic acid (TFMS), which effectively cleaves N- and O-linked glycans nonselectively from glycoproteins, leaving the primary structure of the protein intact. Toluene was used as a scavenger species to neutralize reactive groups formed during the deglycosylation reaction. Excess TFMS was later neutralized by reaction with pyridine.
Monosaccharide analysis of HRgpA and RgpA.
HRgpA (0.2 mg) and RgpA (0.2 mg) proteases were dialyzed against 5% (vol/vol) aqueous acetic acid to remove salts and detergents and were then freeze-dried. Dulcitol (10 μg) was added to each protease sample, and the mixture was subjected to methanolysis in 200 μl of 0.5 M solution of HCl in methanol (Supelco, Bellefonte, Pa.) at 85°C for 4.5 h. After removal of excess methanolic-HCl under vacuo, the mixture was N-acetylated in a mixture of 200 μl of methanol, 25 μl of pyridine, and 25 μl of acetic anhydride at 22°C for 30 min. After removal of reagents under vacuo, the monosaccharides were analyzed by GC-MS (gas chromatography-mass spectrometry) following derivatization to their O-trimethylsilyl (O-TMS) ethers (20). The relative molar response factors for each monosaccharide with respect to dulcitol were calculated from analysis of standard monosaccharides.
Release of oligosaccharides by β elimination.
O-linked oligosaccharides in HRgpA and RgpA were released from the protein by treatment with 1 M NaBH4 (sodium borohydride) in 50 mM NaOH at 50°C for 16 h essentially as described by Lechner and Wieland (27). Oligosaccharides were separated by high-pressure liquid chromatography (HPLC) on a porous graphitized carbon (PGC) column (Shandon, Runcorn, Cheshire, United Kingdom), using a gradient of acetonitrile from 0 to 40% (vol/vol) in 0.1% trifluoroacetic acid in water. The absorbance of column effluent was monitored at 210 nm.
Release of oligosaccharides by hydrazinolysis and 2-AB (2-aminobenzamide) labelling.
The monosaccharides present were confirmed after release of the oligosaccharides from the proteases by hydrazinolysis and their analysis by GC-MS of O-TMS ethers of methyl glycosides.
Salt- and detergent-free solutions of proteases HRgpA (0.8 mg) and RgpA (0.15 mg) were dried in vacuo and then dried in a desiccator over P2O5 overnight. Hydrazinolysis of proteases was performed at 60°C for 4 h to release O-linked chains, using an Oxford GlycoSystems kit, and at 95°C for 4 h to release O- and N-linked chains. Released oligosaccharides were labelled with 2-AB according to the kit manufacturer’s instructions.
RESULTS
Antigens.
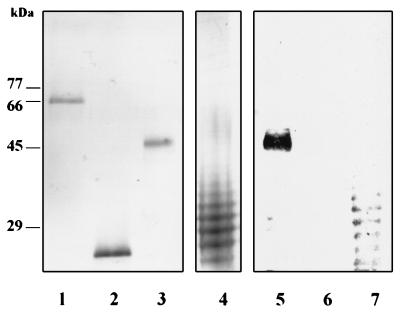
High yields of rec RgpA α were attained both from pQ3010 in E. coli M15(pREP4) and from JFQ3010 in E. coli XL-1 Blue. The recombinant protein was enzymatically inactive, in keeping with our previous experience of heterologous expression of this gene in E. coli (1). However, significant reductions in recombinant protein expression by these clones were observed following resuscitation of frozen stocks, and hence all purifications were performed on preparative scale batches of newly transformed cells. Recombinant RgpA α is composed of RgpA G149-S737, which incorporates the α domain of RgpA (Y228 to R719) and an N-terminally truncated propeptide region (G149 to R227), as well as some vector-derived sequences which mainly consist of the N-terminal His6 purification tag. Hence the molecular weight of the rec RgpA α (67,700) is significantly higher than that of the wild-type P. gingivalis RgpA. The immunizing antigen for the MAb protocol (RgpA) and the screening antigens rec RgpA α, DHFR, and P. gingivalis W50 LPS are shown in Fig. 1.
FIG. 1.
P. gingivalis W50 and recombinant antigens used in the MAb protocol and the specificity of MAb 1B5. Samples (2 μg) in lanes 1 to 4 were subjected to PAGE on an SDS–12.5% polyacrylamide gel and stained with Coomassie blue (lanes 1 to 3) or silver (lane 4). Lanes: 1, rec RgpA α; 2, DHFR; 3, P. gingivalis RgpA; 4, P. gingivalis LPS. Lanes 5 to 7, Western blot probed with MAb 1B5 of P. gingivalis RgpA (lane 5), rec RgpA α, and P. gingivalis LPS, respectively.
MAbs to RgpA are LPS reactive.
A total of six myeloma cell-spleen cell fusions were performed, and similar results were generated on each occasion. A consistent finding was that hybridoma supernatants which were positive in the primary and secondary screen ELISAs for antibody to P. gingivalis RgpA were negative in rec RgpA α ELISAs. For example, in Pg10 fusion, a total of 14 hybridoma supernatants contained antibody reactive with RgpA by ELISA in the primary screen. Of these, six proved stable to long-term culture. In the secondary screen, all six were still strongly positive in the RgpA ELISA but none were reactive with rec RgpA α. Significantly, however, all of these RgpA hybridomas were also strongly positive in the P. gingivalis W50 LPS ELISA. Throughout the six fusions, we did not obtain a single hybridoma which produced antibody reactive with the rec RgpA α. One of the Pg10 hybridomas (1B5) which was positive for both P. gingivalis RgpA and LPS was selected for further study and cloned by limit dilution. Subsequent isotyping demonstrated that MAb 1B5 is an immunoglobulin G2a.
To establish whether MAb 1B5 was reacting with contaminating LPS in the RgpA preparation, Western blots of RgpA, rec RgpA α, and LPS on nitrocellulose membranes were probed with this MAb (Fig. 1, lanes 5 to 7). MAb 1B5 reacted with a single band in the RgpA preparation coincident with the position of the protein band. No other immunoreactive components were present, suggesting that MAb 1B5 recognizes a determinant on RgpA which is not dissociated by SDS-PAGE. As predicted from the ELISA data, MAb 1B5 was immunoreactive with multiple bands in the P. gingivalis LPS preparation but did not recognize the rec RgpA α.
MAb 1B5 determinant on RgpA is periodate sensitive and stable to denaturants.
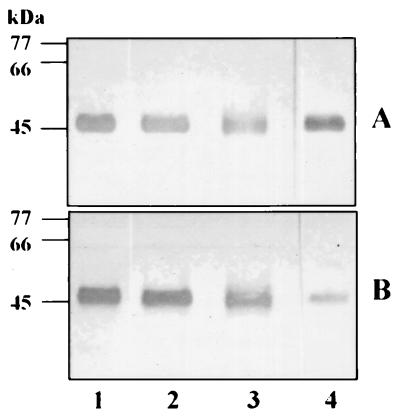
To determine whether the MAb 1B5 determinant on P. gingivalis RgpA represents a covalent modification, the stability of the immunoreactivity to denaturing conditions was examined (Fig. 2). The results were compared to the immunoreactivity of identical samples with rabbit P. gingivalis whole-cell antiserum which recognizes both the LPS and peptide chain of RgpA. There was no significant reduction in the immunoreactivity of MAb 1B5 with RgpA which had been treated with SDS or formic acid. Similar results were obtained with samples treated with 6.4 M urea or 6 M guanidine-HCl for 60 min at 55°C (not shown). However, following periodate oxidation of RgpA, there was a significant reduction in the immunoreactivity with MAb 1B5 but no change in the reactivity with whole-cell antiserum. Together with the results presented above, these data strongly suggest that the peptide chain of RgpA is covalently modified with periodate-sensitive carbohydrate residues which are common to the LPS of this organism. We have previously suggested that covalent modification of the α chain of mt-RgpA with carbohydrate common to the LPS of P. gingivalis accounted for the increased size of this enzyme on SDS-PAGE (10, 41). The modifications to RgpA and mt-RgpA were then further investigated by biotinylation of periodate-oxidized proteases, using biotin hydrazide.
FIG. 2.
Effects of denaturants on immunoreactivity of P. gingivalis RgpA with MAb 1B5. P. gingivalis RgpA was treated with leupeptin (control; lane 1), 3.3% SDS (lane 2) or 75% formic acid (lane 3) as described in the text and then subjected to PAGE on a SDS–12.5% polyacrylamide gel prior to Western blotting. For periodate treatment (lane 4), leupeptin-inhibited RgpA was first subjected to SDS-PAGE and electroblotted onto nitrocellulose; the blots then treated with 1% periodic acid in 7% acetic acid. Immunoreactivities with anti-P. gingivalis whole-cell antiserum (A) and MAb 1B5 (B) are shown.
Proteases RgpA and mt-RgpA of P. gingivalis are glycoproteins.
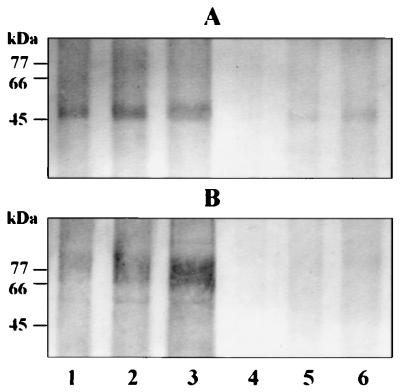
The two proteases were labelled in solution with biotin hydrazide following pretreatment with periodate or buffer control. The extent of labelling was then determined by using streptavidin conjugated to alkaline phosphatase. Significant periodate-dependent biotin labelling of both RgpA and mt-RgpA was detected with this system, with a lower limit of detection of approximately 75 ng of each enzyme (Fig. 3). However, we were unable to detect periodate-sensitive carbohydrate residues on either chain of the heterodimeric HRgpA protease of P. gingivalis by this method (not shown). We therefore examined the MAb 1B5 immunoreactivity of all of the arginine-specific proteases derived from rgpA and rgpB of this organism in order to establish whether the glycosylation process occurs selectively.
FIG. 3.
Detection of carbohydrate modifications to RgpA and mt-RgpA by using biotin hydrazide labelling following periodate oxidation. Increasing amounts (75, 150, and 300 ng) of RgpA (A) and mt-RgpA (B) were incubated with (lanes 1 to 3) and without (lanes 4 to 6) periodate prior to labelling with biotin hydrazide. Samples were then subjected to electrophoresis, and bound biotin was detected with streptavidin-alkaline phosphatase. The greater incorporation of biotin in the periodate-treated samples in lanes 1 to 3 than in lanes 4 to 6 reflects the presence of periodate-sensitive carbohydrate residues on RgpA and mt-RgpA.
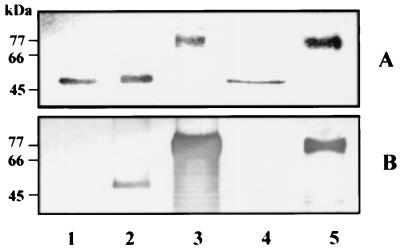
MAb 1B5 reacts differentially with the isoforms of the RgpA and RgpB proteases.
The MAb 1B5 reaction with HRgpA, RgpA, and mt-RgpA from the culture supernatant of P. gingivalis W50 and with RgpB and mt-RgpB from the supernatant of strain W501 (rgpA) was assessed by Western blot analysis (Fig. 4). RgpA and the two highly modified proteases mt-RgpA and mt-RgpB reacted very strongly with this MAb. However, there was no immunoreactivity with HRgpA or RgpB. Identical results (not shown) were obtained with two other MAbs (6B3 and 7B4) which we have shown previously to be reactive with P. gingivalis LPS (8, 10).
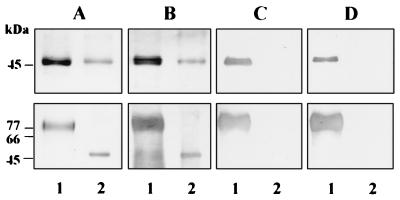
FIG. 4.
Immunoreactivity of the isoforms of the RgpA and RgpB proteases with MAb 1B5 HRgpA (lane 1), RgpA (lane 2), mt-RgpA (lane 3), RgpB (lane 4), and mt-RgpB (lane 5) (2 μg of each) were run on an SDS–12.5% polyacrylamide gel and stained with Coomassie blue (A) or Western blotted and reacted with MAb 1B5 (B).
Deglycosylation of RgpA and mt-RgpA.
Chemical deglycosylation of RgpA by using TFMS did not result in a discernible decrease in the molecular mass compared to the untreated enzyme on SDS-PAGE. However, mt-RgpA, which normally runs as a diffuse heterogeneous band of 70 to 80 kDa, showed a dramatic decrease in molecular mass to ∼54 kDa, the molecular mass of RgpA (Fig. 5A). This finding suggests that mt-RgpA contains approximately 20 to 30% (by weight) carbohydrate. As expected, the immunoreactivity of RgpA and mt-RgpA with MAb 1B5 and MAb 7B4 was completely abolished following deglycosylation with TFMS, while significant reactivity with the anti-whole-cell antiserum, which recognizes epitopes in the peptide chain, was maintained (Fig. 5B to D).
FIG. 5.
Effect of TFMS deglycosylation on SDS-PAGE mobility and immunoreactivity of RgpA (upper panels) and mt-RgpA (lower panels). Untreated (lanes 1) or TFMS-treated (lanes 2) proteases were run on SDS–12.5% polyacrylamide gels and stained with Coomassie blue (A) or Western blotted and developed by using anti-P. gingivalis whole-cell antiserum (B), MAb 1B5 (C), or MAb 7B4 (D).
Treatment of RgpA and mt-RgpA with PNGase F failed to alter the molecular weights of these two enzymes on SDS-PAGE and did not affect their immunoreactivity with MAb 1B5 (not shown). Asn-linked glycans representing all major eucaryotic oligosaccharide classes are hydrolyzed by PNGase F (29), which releases the oligosaccharide with an amino group attached, while the Asn in the protein is converted to Asp. Asn-linked oligosaccharides containing an α(1→6)-fucose substituent on the Asn-proximal N-acetylglucosamine residue are easily hydrolyzed, but the corresponding α(1→3)-fucose substituent found in plant glycoproteins completely blocks deglycosylation (50). RgpA may not contain Asn-GlcNAc linkages, or there may be substituents on GlcNAc which block deglycosylation by PNGase F.
Relationship of the α chains of HRgpA, RgpA, and mt-RgpA.
Although the catalytic component of HRgpA and RgpA are both derived from the α coding region of rgpA, the presence of the MAb 1B5 determinant only on RgpA suggested that the α chains of these two enzymes may be resolved by electrophoretic separations based on properties other than size. This was examined by using urea-PAGE, which separates peptides based on the charge/size ratio and which we have used previously to separate the α and β components of HRgpA (41). Based on migration patterns on these gels (Fig. 6), RgpA appears to be significantly more negatively charged than the α component of HRgpA. However, this charge difference does not appear to be a direct consequence of the glycan modifications to RgpA, since deglycosylation of RgpA with TFMS did not affect its mobility. Hence, the difference in mobilities of HRgpA and RgpA is more likely due to variation at the C terminus of the α chain of these two isoforms. Conversely, the mobility of deglycosylated mt-RgpA on 8 M urea-PAGE was identical to that of the RgpA samples, suggesting that the only difference between these two monomeric species is the extent of glycan addition.
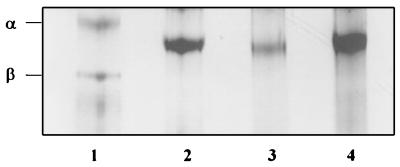
FIG. 6.
Analysis by 8 M urea-PAGE of HRgpA, RgpA, and deglycosylated RgpA and mt-RgpA. Leupeptin-inhibited HRgpA (lane 1) and RgpA (lane 2) and TFMS-treated RgpA (lane 3) and mt-RgpA (lane 4) were subjected to 8 M urea-PAGE in 7.5% acrylamide gels and then stained with Coomassie blue. Arrows indicate positions of the α and β chains of HRgpA.
Analysis of carbohydrate in HRgpA and RgpA.
The monosaccharide composition of proteases HRgpA and RgpA was determined after consecutive methanolysis, N-acetylation, and conversion of N-acetyl-O-methyl glycosides to O-TMS ethers followed by GC-MS. RgpA contained Ara, Rha, Fuc, Man, Gal, Glc, GalN(Ac), GlcN(Ac), and N-acetylneuraminic acid (NANA) totalling 14.4% by weight of protein. HRgpA contained Rha, Man, Gal, Glc, GalN(Ac), and GlcN(Ac) (i.e., lacking Ara, Fuc, and NANA) totalling 2.1% by weight of protein. The molar ratios of monosaccharides are shown in Table 1. The presence of these monosaccharides was also confirmed after release of several O-linked oligosaccharides from the proteases by β elimination and analysis by methanolysis as previously described. The sugar which is covalently linked to Ser/Thr residues in proteins is reduced to its corresponding alditol during the β-elimination reaction. This gives a characteristic GC retention time and MS fingerprint for each monosaccharide. Figure 7 shows the GC total ion chromatogram of monosaccharides from an O-linked oligosaccharide released from RgpA by β elimination and purified by HPLC on a PGC column. This shows that GalN(Ac) is the monosaccharide linked to Ser/Thr in RgpA, as it occurs as its alditol (Fig. 7, peak 6). In addition to confirming that most of the monosaccharides detected previously are released by β elimination, these data also indicate that the sugars in RgpA are present predominantly in O-linked chains (results not shown). However, Ara was detected only in oligosaccharides released by hydrazine under extremely harsh conditions (95°C for 4 h), which suggests it is present in N-linked oligosaccharide (results not shown). A control sample of RgpA (0.2 mg) which was not treated with hydrazine but directly labelled with 2-AB followed by subsequent purification and derivatization for GC-MS did not reveal the presence of any monosaccharides.
TABLE 1.
GC-MS analysis of O-TMS ethers of methyl glycosides showing the molar ratios of monosaccharides in the oligosaccharides of HRgpA, RgpA, and LPS of P. gingivalis W50 after methanolysis
| Oligosaccharide | Molar ratio
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ara | Rha | Fuc | Man | Gal | Glc | GalN(Ac) | GlcN(Ac) | NANA | |
| HRgpA | 1 | 3.6 | 1.7 | 3 | 2 | 1 | |||
| RgpA | 2.4 | 1.8 | 2.8 | 1 | 11.8 | 4 | 18 | 6.4 | 15 |
| LPSa | 1 | 2.4 | 5 | 1.8 | 1 | ||||
| Core region of LPSb | 1 | 2.5 | 3.7 | ||||||
Two unidentified sugars are also present.
One unidentified sugar (which is also present in LPS).
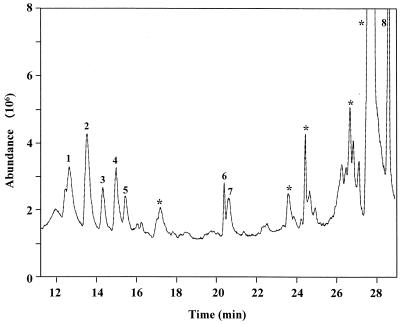
FIG. 7.
GC total-ion chromatogram of the O-TMS ethers of methyl glycosides of monosaccharides from an O-linked oligosaccharide released from RgpA by β elimination. Peaks: 1 to 3, Gal; 4 and 5, Glc; 6, GalN(Ac)-ol; 7, GlcN(Ac); 8, NANA. ∗, no fragmentation ions consistent with monosaccharides were present.
Table 1 also presents the molar ratios of monosaccharides found in the P. gingivalis LPS after methanolysis, N-acetylation, and GC-MS. Rha, Gal, Glc, GalN(Ac), and GlcN(Ac) are all present in P. gingivalis LPS, but Ara, Fuc, Man and NANA, which are components of the oligosaccharides of RgpA, are absent.
DISCUSSION
It is widely acknowledged that most eucaryotic proteins do not exist in a free form but are modified by the covalent attachment of carbohydrate chains. Glycosylation of a protein can have significant effects on the intrinsic properties of the resulting glycoprotein by altering the stability, resistance to proteolysis, and tertiary structure. Furthermore, the large size of oligosaccharides may allow them to cover functionally important areas of the protein, to modulate the interaction of the glycoconjugate with other molecules, and to affect the rate of processes which involve conformational changes. In addition to the modulation of protein function, oligosaccharides also serve an important role as recognition markers (14).
The presence of glycoproteins in procaryotes has been demonstrated and accepted only recently. However, there are now numerous examples of microbial glycoproteins. These include cell wall proteins in the archaea (32), secreted or cell wall-associated enzymes of clostridia and flavobacteria (18, 33, 43), and proteins from human pathogens involved in interactions with host cell components such as the pilin proteins of pathogenic neisseriae (48) and Pseudomonas aeruginosa (5), a platelet aggregation-associated protein of Streptococcus sanguis (15), and surface antigens of Mycobacterium tuberculosis (19). Given the importance of carbohydrate modifications to protein function, stability, and recognition, it is highly likely that glycosylation of bacterial proteins is of major relevance to the molecular basis of infectious disease processes. An example of this potential involvement was described recently for Chlamydia trachomatis. The major outer membrane protein of this intracellular pathogen is an N-linked glycoprotein containing a high-mannose-type oligosaccharide which was shown to have a direct role in mediating the attachment and infectivity of the bacterium to an epithelial cell line (25).
In the present investigation, several lines of evidence suggested that the catalytic chains of the RgpA and RgpB arginine-specific proteases of P. gingivalis undergo variable degrees of glycosylation. MAbs raised to RgpA reacted with the homologous antigen and P. gingivalis LPS but not with heterologously expressed recombinant protein on Western blots following SDS-PAGE. The periodate sensitivity of the immunoreaction indicated that the epitope contained carbohydrate residues with cis-hydroxyl groups. To ascertain that the reactivity was a consequence of covalently linked determinants rather than nonspecific complex formation with LPS, RgpA was subjected to a number of harsh denaturant treatments, all of which failed to influence the MAb recognition. Furthermore, the determinants were removed from RgpA and mt-RgpA by reaction with anhydrous TMFS, which nonspecifically cleaves the O- and N-linked glycans without destroying the protein. The presence of carbohydrate additions to RgpA and mt-RgpA was also demonstrated by labelling free aldehydes generated by periodate oxidation with biotin hydrazide.
Analysis of HRgpA and RgpA to determine the monosaccharide composition after consecutive methanolysis, N-acetylation, derivatization, and GC-MS showed that HRgpA contained Rha, Man, Gal, Glc, GalN(Ac), and GlcN(Ac) totalling 2.1% by weight of protein, whereas RgpA contained Ara, Rha, Fuc, Man, Gal, Glc, GalN(Ac), GlcN(Ac) and NANA totalling 14.4% by weight of protein. The constituent monosaccharides were confirmed by the analysis of oligosaccharides released from HRgpA and RgpA by β elimination. Analysis of the subsequently purified oligosaccharides by methanolysis and GC-MS unambiguously identified the monosaccharides covalently bound to these enzymes. The released oligosaccharides could be purified by HPLC on a PGC column, and the oligosaccharide-specific reducing end could be identified as the corresponding alditol. No monosaccharides were detected in enzyme samples which had not been subjected to hydrazinolysis prior to 2-AB labelling.
Deglycosylation of RgpA with TFMS does not significantly influence its migration on SDS-PAGE. In contrast, the glycan moiety of mt-RgpA is a major constituent. In earlier reports, we had suggested that mt-RgpA of P. gingivalis was a highly glycosylated form of the RgpA α chain principally on the basis of its staining by the periodic acid-Schiff reagent on SDS-polyacrylamide gels (41). The glycoprotein nature of mt-RgpA was further substantiated in the present work through its reactivity with the RgpA MAbs and by chemical deglycosylation with TMFS, which reduced the molecular mass of this isoform by approximately 30 kDa to that of an unmodified RgpA α chain. Hence, RgpA and mt-RgpA are both glycoproteins comprising the same RgpA α chain with different amounts of glycan addition.
In contrast to the monomeric forms, the heterodimeric HRgpA, the third isoenzyme product of rgpA, contains less carbohydrate. The lack of recognition of this form by the RgpA and anti-LPS MAbs may be explained by the absence of Ara, Fuc, and NANA in the carbohydrate moiety. This suggests a difference in glycosylation of the α chain of all three enzymes, although all are derived from the same rgpA coding sequence. Ara appears to be present in the N-linked sugar in RgpA, which could indicate that some sites for glycan addition within the HRgpA isoform are masked. In either case, it is clear that the maturation pathway of rgpA-derived enzymes contains a critical branch point which determines the glycosylation status of the final products of this gene. We are unable to demonstrate at which stage in the growth of the culture these processes occur. Throughout, we have used late-stationary-phase cultures in order to maximize extracellular enzyme yields.
Previous analysis of a rgpA isogenic mutant of P. gingivalis W50 had demonstrated the production of two more monomeric Arg-x specific proteases (RgpB and mt-RgpB) derived from rgpB. On the basis of their kinetic behavior and charge and mass characteristics, these enzymes appeared almost indistinguishable from RgpA and mt-RgpA, respectively. Thus, mt-RgpB appeared to represent a highly posttranslationally modified form analogous to mt-RgpA derived from rgpA, and as expected, mt-RgpB was strongly reactive with the RgpA and LPS MAbs (42). However, the presence of these major modifications does not appear to be required for membrane association because unmodified RgpB can be isolated from the membrane fraction of P. gingivalis W50/BE1 (7).
RgpB did not appear to contain carbohydrate modifications on the basis of immunochemical recognition. However, RgpB isolated from an rgpA isogenic mutant of P. gingivalis W50 (42) was found to contain Ara, Rha, Fuc, Gal, Glc, GlcA, and GlcN(Ac) totalling 10% by weight of protein, raising the possibility that there may be glycan additions to the RgpB isoform which are not cross-reactive with the MAbs used in this investigation. The data in the present report are supported by a recent investigation of the structure and activity of RgpB from P. gingivalis H66. Different isoforms of RgpB were detected by chromatofocusing, which the authors suggested may reflect variability in a posttranslational addition to the peptide chain of this enzyme (40). A scheme of the putative RgpA and RgpB protease maturation pathway based on the findings of this and earlier studies is shown in Fig. 8.
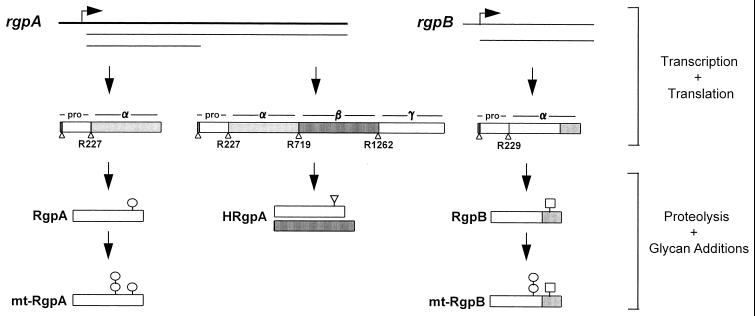
FIG. 8.
Proposed maturation pathways leading to the generation of five differentially modified enzymes from rgpA and rgpB of P. gingivalis. HRgpA, RgpA, and mt-RgpA are all products of rgpA. The catalytic chain of the monomeric enzymes may be derived either from the translation product of the full-length rgpA transcript or via translation of a truncated transcript. Variable levels of glycan addition then leads to the formation of either RgpA or mt-RgpA. RgpB and mt-RgpB are derived from translation of an rgpB transcript. RgpB may acquire different carbohydrate modifications compared to RgpA. mt-RgpB is glycosylated to a similar extent as mt-RgpA. The rgpA and rgpB loci on the P. gingivalis chromosome are shown as thick bold lines with the direction of transcription denoted by arrows; mRNA transcripts are shown as thin lines below the genes. Rectangular boxes represent the translation products of the genes and are organized into domains corresponding to propeptide (pro), catalytic domain (α), adhesin domain (β), and C-terminal extension (γ). Sequence differences at the C termini of the α domains of RgpA and RgpB are shown by additional shading on RgpB. Triangles denote proteolytic processing sites on the initial translation products. Carbohydrate modifications to the mature enzymes are indicated by circles (for those which contain the MAb 1B5 determinant), squares, and inverted triangles. Carbohydrate symbols can represent single or multiple oligosaccharide side chains and in the case of HRgpA may be present on either chain or both chains. The double-circle symbols on mt-RgpA and mt-RgpB signify the high level (>30%) of posttranslational addition to these two isoforms.
To our knowledge, this is the first report of bacterial glycoproteins in which the glycan addition contains antigenic determinants common to the LPS of the organism. The chemical structure of lipid A from P. gingivalis LPS has been determined (36), and lipid A was found to be a glucosamine β-(1-6)-disaccharide-1-monophosphate acylated by 3-hydroxy-15-methyl hexadecanoic acid and 3-hexadecanoyloxy-15-methyl hexadecanoic acid at the 2 and 2′ positions, respectively. In this work, we have analyzed the monosaccharides present in LPS (Table 1) and shown the presence of Rha, Gal, Glc, GalN(Ac), and GlcN(Ac) and also detected two other sugars which were not present in RgpA. Furthermore, analysis of sugars in the core region of LPS showed the absence of GalN(Ac) and GlcN(Ac). Therefore, RgpA has distinct sugar modifications which could not have arisen from LPS contamination.
It is not unprecedented for LPS to play a significant role in the functional stabilization of a gram-negative extracellular protein. The extracellular hemolysin of pathogenic E. coli (HlyA) purified from culture supernatants has been shown to contain LPS by both chemical and immunological techniques (3, 4, 38), and in strains harboring the hlyCABD operon, mutations in rfaP and rfaC, genes involved in LPS core biosynthesis, result in a significant reduction in the biological activity of the resultant extracellular HlyA (2, 47). Since chaotropic agents can restore the hemolytic activity of the culture supernatant HlyA in such mutants, it has been suggested that association with LPS containing a complete core may be essential for the formation or maintenance of an active conformation of HlyA, which thereby prevents its aggregation or degradation.
The critical distinction between the HlyA-LPS macromolecule and the RgpA and mt-RgpA glycoproteins described in the present report lies in the stability of these molecules to denaturing conditions. In the case of HlyA and LPS, the macromolecule is readily dissociated into its component parts by the sample preparation procedures routinely used for reducing SDS-PAGE. Conversely, in the case of RgpA and mt-RgpA, we were unable to remove the glycan components with any of the harsh denaturing conditions used except for treatment with anhydrous TFMS, which is a well-established procedure for the removal of covalently attached carbohydrate from the protein backbone of glycoconjugates.
Given the importance of glycosylation of eucaryotic proteins to their stability, structure, resistance to proteolysis, and recognition, the modifications to the proteases described in this report are likely to have a functional role in the properties of these enzymes. In this regard, it is perhaps relevant that the half-life of HRgpA, which appears to carry lower levels of glycan modifications, is some 50-fold lower than that of both RgpA and mt-RgpA at pH 8.0 and 30°C (41). Furthermore, in light of our inability to generate MAbs to the peptide chain of RgpA following immunization with this enzyme, it is possible that these glycan additions influence the immune recognition of RgpA and mt-RgpA in a manner similar to the way in which the β chain of HRgpA diverts the recognition of the catalytic chain of this isoform.
In conclusion, the data in this report indicate that glycosylation of the catalytic chains of the RgpA and RgpB proteases is a selective process during the maturation of these enzymes. Ongoing analysis of the biochemical nature and sites of these modifications and characterization of the mechanism of addition may allow the generation of mutants impaired in this system in order to study the physiological relevance of glycoprotein synthesis in this periodontal pathogen.
ACKNOWLEDGMENT
This work was supported by the Medical Research Council (grant PG9318173).
REFERENCES
- 1.Aduse-Opoku J, Muir J, Slaney J M, Rangarajan M, Curtis M A. Characterization, genetic analysis, and expression studies of a protease antigen (PrpRI) of Porphyromonas gingivalis W50. Infect Immun. 1995;63:4744–4754. doi: 10.1128/iai.63.12.4744-4754.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauer M E, Welch R A. Pleiotropic effects of a mutation in rfaC on Escherichia coli hemolysin. Infect Immun. 1997;65:2218–2224. doi: 10.1128/iai.65.6.2218-2224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohach G A, Snyder I S. Chemical and immunological analysis of the complex structure of Escherichia coli α-hemolysin. J Bacteriol. 1985;164:1071–1080. doi: 10.1128/jb.164.3.1071-1080.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bohach G A, Snyder I S. Composition of affinity-purified α-hemolysin of Escherichia coli. Infect Immun. 1986;53:435–437. doi: 10.1128/iai.53.2.435-437.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castric P. PilO, a gene required for glycosylation of Pseudomonas aeruginosa 1244 pilin. Microbiology. 1995;141:1247–1254. doi: 10.1099/13500872-141-5-1247. [DOI] [PubMed] [Google Scholar]
- 6.Clark-Curtiss J E, Curtiss R., III Analysis of recombinant DNA using Escherichia coli minicells. Methods Enzymol. 1983;101:347–362. doi: 10.1016/0076-6879(83)01026-5. [DOI] [PubMed] [Google Scholar]
- 7.Collinson L M, Rangarajan M, Curtis M A. Altered expression and modification of proteases from an avirulent mutant of Porphyromonas gingivalis W50 (W50/BE1) Microbiology. 1998;144:2487–2496. doi: 10.1099/00221287-144-9-2487. [DOI] [PubMed] [Google Scholar]
- 8.Cridland J C, Booth V, Ashley F P, Curtis M A, Wilson R F, Shepherd P. Preliminary characterisation of antigens recognised by monoclonal antibodies raised to Porphyromonas gingivalis and by sera from patients with periodontitis. J Periodontal Res. 1994;29:339–347. doi: 10.1111/j.1600-0765.1994.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 8a.Curtis, M. A., et al. Unpublished data.
- 9.Curtis M A, Ramakrishnan M, Slaney J M. Characterisation of the trypsin-like enzymes of Porphyromonas gingivalis W83 using a radiolabelled active-site-directed inhibitor. J Gen Microbiol. 1993;139:949–955. doi: 10.1099/00221287-139-5-949. [DOI] [PubMed] [Google Scholar]
- 10.Curtis M A, Aduse-Opoku J, Slaney J M, Rangarajan M, Booth V, Cridland J, Shepherd P. Characterization of an adherence and antigenic determinant of the Argl protease of Porphyromonas gingivalis which is present on multiple gene products. Infect Immun. 1996;64:2532–2539. doi: 10.1128/iai.64.7.2532-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Curtis M A. Analysis of the protease and adhesin domains of the PrpRI of Porphyromonas gingivalis. J Periodontal Res. 1997;32:133–139. doi: 10.1111/j.1600-0765.1997.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 12.Darveau R P, Hancock R E W. Procedure for isolation of lipopolysaccharides from both rough and smooth Pseudomonas aeruginosa and Salmonella typhimurium strains. J Bacteriol. 1983;155:831–838. doi: 10.1128/jb.155.2.831-838.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeCarlo A A, Windsor L J, Bodden M K, Harber G J, Birkedal-Hansen B, Birkedal-Hansen H. Activation and novel processing of matrix metalloproteinases by a thiol-proteinase from the oral anaerobe Porphyromonas gingivalis. J Dent Res. 1997;76:1260–1270. doi: 10.1177/00220345970760060501. [DOI] [PubMed] [Google Scholar]
- 14.Dwek R A. Glycobiology: towards understanding the function of sugars. Biochem Soc Trans. 1995;23:1–25. doi: 10.1042/bst0230001. [DOI] [PubMed] [Google Scholar]
- 15.Erickson P R, Herzberg M C. Evidence for the covalent linkage of carbohydrate polymers to a glycoprotein from Streptococcus sanguis. J Biol Chem. 1993;268:23780–23783. [PubMed] [Google Scholar]
- 16.Furste J P, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- 17.Genco C A, Odusanya B M, Potempa J, Mikolajczyk-Pawlinska J, Travis J. A peptide domain on gingipain R which confers immunity against Porphyromonas gingivalis infection in mice. Infect Immun. 1998;66:4108–4114. doi: 10.1128/iai.66.9.4108-4114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerwig G J, de Waard P, Kamerling J P, Vliegenthart J F G, Morgenstern E, Lamed R, Bayer E A. Novel O-linked carbohydrate chains in the cellulase complex (cellulosome) of Clostridium thermocellum. J Biol Chem. 1989;246:1027–1035. [PubMed] [Google Scholar]
- 19.Herrmann J L, Gaora P O, Gallagher A, Thole J E R, Young D B. Bacterial glycoproteins: a link between glycosylation and proteolytic cleavage of a 19 kDa antigen from Mycobacterium tuberculosis. EMBO J. 1996;15:3547–3554. [PMC free article] [PubMed] [Google Scholar]
- 20.Hounsell E F. Characterization of the glycosylation status of proteins. Mol Biotechnol. 1994;2:45–60. doi: 10.1007/BF02789289. [DOI] [PubMed] [Google Scholar]
- 21.Imamura T, Pike R N, Potempa J, Travis J. Pathogenesis of periodontitis: a major arginine-specific cysteine proteinase from Porphyromonas gingivalis induces vascular permeability enhancement through activation of the kallikrein/kinin pathway. J Clin Investig. 1994;94:361–367. doi: 10.1172/JCI117330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadowaki T, Yoneda M, Okamoto K, Maeda K, Yamamoto K. Purification and characterization of a novel arginine-specific cysteine proteinase (argingipain) involved in the pathogenesis of periodontal disease from the culture supernatant of Porphyromonas gingivalis. J Biol Chem. 1994;269:21371–21378. [PubMed] [Google Scholar]
- 23.Kelly C G, Booth V, Kendal H, Slaney J M, Curtis M A, Lehner T. The relationship between colonisation and haemagglutination inhibiting and B-cell epitopes of Porphyromonas gingivalis. Clin Exp Immunol. 1997;110:285–291. doi: 10.1111/j.1365-2249.1997.tb08329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohler G, Milstein C. Continuous culture of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 25.Kuo C-C, Takahashi N, Swanson A F, Ozeki Y, Hakomori S-I. An N-linked high-mannose type oligosaccharide, expressed at the major outer membrane protein of Chlamydia trachomatis, mediates attachment and infectivity of the microorganism to HeLa cells. J Clin Investig. 1996;98:2813–2818. doi: 10.1172/JCI119109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 27.Lechner J, Wieland F. Analysis of bacterial glycoproteins. Methods Mol Biol. 1993;14:119–129. doi: 10.1385/0-89603-226-4:119. [DOI] [PubMed] [Google Scholar]
- 28.Maiden M J F, Carman R J, Curtis M A, Gillett I R, Griffiths G S, Sterne J A C, Wilton J M A, Johnson N W. Detection of high-risk groups and individuals for periodontal diseases: laboratory markers based on the microbiological analysis of subgingival plaque. J Clin Periodontol. 1990;17:1–13. doi: 10.1111/j.1600-051x.1990.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 29.Maley F, Trimble R B, Tarentino A L, Plummer T H., Jr Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem. 1989;180:195–204. doi: 10.1016/0003-2697(89)90115-2. [DOI] [PubMed] [Google Scholar]
- 30.Marshall R C, Inglis A S. Protein oligomer composition, preparation of monomers and constituent chains. In: Darbre A, editor. Practical protein chemistry. Chichester, United Kingdom: John Wiley & Sons Ltd.; 1987. pp. 1–66. [Google Scholar]
- 31.Martinez J, Campetella O, Frasch A C C, Cazzulo J J. The reactivity of sera from chagasic patients against different fragments of cruzipain, the major cysteine proteinase from Trypanosoma cruzi, suggests the presence of defined antigenic and catalytic domains. Immunol Lett. 1993;35:191–196. doi: 10.1016/0165-2478(93)90090-o. [DOI] [PubMed] [Google Scholar]
- 32.Mescher M F, Strominger J L. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc Natl Acad Sci USA. 1976;73:2687–2691. doi: 10.1073/pnas.73.8.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messner P, Christain R, Kolbe J, Schulz G, Sleytr U B. Analysis of a novel linkage unit of O-linked carbohydrates from the crystalline surface layer glycoprotein of Clostridium thermohydrosulphuricum S102-70. J Bacteriol. 1992;174:2236–2240. doi: 10.1128/jb.174.7.2236-2240.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millar D J, Scott E E, Slaney J M, Benjamin S U P, Curtis M A. Production and characterisation of monoclonal antibodies to the principle sonicate antigens of Porphyromonas gingivalis W50. FEMS Immunol Med Microbiol. 1993;7:211–222. doi: 10.1111/j.1574-695X.1993.tb00401.x. [DOI] [PubMed] [Google Scholar]
- 35.Nilsson T, Carlsson J, Sundqvist G. Inactivation of key factors of the plasma proteinase cascade systems by Bacteroides gingivalis. Infect Immun. 1985;50:467–471. doi: 10.1128/iai.50.2.467-471.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogawa T. Chemical structure of lipid A from Porphyromonas (Bacteroides) gingivalis lipopolysaccharide. FEBS Lett. 1993;332:197–201. doi: 10.1016/0014-5793(93)80512-s. [DOI] [PubMed] [Google Scholar]
- 37.Okamoto K, Misumi Y, Kadowaki T, Yoneda M, Yamamoto K, Ikehara Y. Structural characterisation of argingipain, a novel arginine-specific cysteine protease as a major periodontal pathogenic factor from Porphyromonas gingivalis. Arch Biochem Biophys. 1995;316:917–925. doi: 10.1006/abbi.1995.1123. [DOI] [PubMed] [Google Scholar]
- 38.Ostolaza H, Bartoleme B, Serra J, de la Cruz F, Goni F. α-Hemolysin from E. coli: purification and self-aggregation properties. FEBS Lett. 1991;280:195–198. doi: 10.1016/0014-5793(91)80291-a. [DOI] [PubMed] [Google Scholar]
- 39.Pike R N, Potempa J, McGraw W, Coetzer T H T, Travis J. Characterization of the binding activities of proteinase-adhesin complexes from Porphyromonas gingivalis. J Bacteriol. 1996;178:2876–2882. doi: 10.1128/jb.178.10.2876-2882.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potempa J, Mikolajczyk-Pawlinska J, Brassell D, Nelson D, Thorgersen I B, Enghild J J, Travis J. Comparative properties of two cysteine proteinases (gingipains R), the products of two related but individual genes of Porphyromonas gingivalis. J Biol Chem. 1998;273:21648–21657. doi: 10.1074/jbc.273.34.21648. [DOI] [PubMed] [Google Scholar]
- 41.Rangarajan M, Smith S J, U S, Curtis M A. Biochemical characterisation of the arginine-specific proteases of Porphyromonas gingivalis W50 suggests a common precursor. Biochem J. 1997;323:701–709. doi: 10.1042/bj3230701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rangarajan M, Aduse-Opoku J, Slaney J M, Young K A, Curtis M A. The prpR1 and prR2 arginine-specific protease genes of Porphyromonas gingivalis W50 produce five biochemically distinct enzymes. Mol Microbiol. 1997;23:955–965. doi: 10.1046/j.1365-2958.1997.2831647.x. [DOI] [PubMed] [Google Scholar]
- 43.Reinhold B B, Hauer C R, Plummer T H, Reinhold V N. Detailed structure analysis of a novel specific O-linked glycan from the prokaryote Flavobacterium meningosepticum. J Biol Chem. 1995;270:13197–13203. doi: 10.1074/jbc.270.22.13197. [DOI] [PubMed] [Google Scholar]
- 44.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Harbor Laboratory Press; 1989. [Google Scholar]
- 45.Schenkein H A, Fletcher H M, Bodnar M, Macrina F L. Increased opsonisation of a PrtH defective mutant of Porphyromonas gingivalis W83 is caused by reduced degradation of complement-derived opsonins. J Immunol. 1995;154:5331–5337. [PubMed] [Google Scholar]
- 46.Slots J, Bragd L, Wikström M, Dahlen G. The occurrence of Actinobacillus actinomycetemcomitans, Bacteroides gingivalis and Bacteroides intermedius in destructive periodontal disease in adults. J Clin Periodontal. 1986;13:576–577. doi: 10.1111/j.1600-051x.1986.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 47.Stanley P L D, Diaz P, Bailey M J A, Gygi D, Juarez A, Hughes C. Loss of activity in the secreted form of Escherichia coli haemolysin caused by an rfaP lesion in core lipopolysaccharide assembly. Mol Microbiol. 1993;10:781–787. doi: 10.1111/j.1365-2958.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 48.Stimson E, Virji M, Makepeace K, Dell A, Morris H R, Payne G, Saunders J R, Jennings M P, Barker S, Panico M, Blench I, Moxon E R. Meningococcal pilin: a glycoprotein substituted with digalactosyl 2,4-diacetamido-2,4,6-trideoxyhexose. Mol Microbiol. 1995;17:1201–1214. doi: 10.1111/j.1365-2958.1995.mmi_17061201.x. [DOI] [PubMed] [Google Scholar]
- 49.Sundqvist G, Carlsson J, Herrmann B, Tarnvik A. Degradation of human immunoglobulins G and M and complement factors C3 and C5 by black-pigmented Bacteroides. J Med Microbiol. 1985;19:85–94. doi: 10.1099/00222615-19-1-85. [DOI] [PubMed] [Google Scholar]
- 50.Tretter V, Altmann F, Marz L. Peptide-N4-(N-acetyl-beta-glucosaminyl) asparagine amidase-F cannot release glycans with fucose attached alpha-1→3 to the asparagine-linked N-acetylglucosamine residue. Eur J Biochem. 1991;199:647–652. doi: 10.1111/j.1432-1033.1991.tb16166.x. [DOI] [PubMed] [Google Scholar]
- 51.Wingrove J A, DiScipio R G, Chen Z, Potempa J, Travis J, Hugli T E. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992;267:18902–18907. [PubMed] [Google Scholar]
- 52.Wray W, Boulikas T, Wray V P, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- 53.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]