Abstract
Since the discovery of conspicuously spatially tuned neurons in the hippocampal formation over 50 years ago, characterizing which, where, and how neurons encode navigationally relevant variables has been a major thrust of navigational neuroscience. While much of this effort has centered on the hippocampal formation and functionally-adjacent structures, recent work suggests that spatial codes, in some form or another, can be found throughout the brain, even in areas traditionally associated with sensation, movement, and executive function. In this review, we highlight these unexpected results, draw insights from comparison of these codes across contexts, regions, and species, and finally suggest an avenue for future work to make sense of these diverse and dynamic navigational codes.
Keywords: navigation, distributed encoding, representational similarity analysis, spatial cognition, cross-species comparison
Introduction
Navigation is ubiquitous in natural behaviors. Targets of interest, such as a food source or a conspecific, are often located more than a simple arm's reach away. A field mouse relies on navigation when it goes out foraging and finds its way home to the safety of its nest. A red-winged blackbird finds its way through the brush until it locates the source of a mating call. A New York City cab driver picks up a fare in Greenwich Village and needs to determine the most efficient way to drop them off in Astoria. Interacting with the environment requires navigational and motor plans for getting from where one is to a specific target. A fundamental question in behavioral neuroscience, and specifically in navigation research, is how underlying navigational codes are instantiated and coordinated throughout the brain to support this complex, adaptive behavior.
A half-century has passed since the groundbreaking discovery of the first spatial codes in the hippocampal formation. In that time, the navigation literature has blossomed, providing a deep and rich foundational understanding of spatial codes in the neural activity of the broader hippocampal formation. More recently, there is growing interest in understanding how these codes inform and interact with similar and complementary codes throughout the brain, and how these brain-wide codes support navigation behavior. This interest has raised a set of important, unanswered questions: If navigation is a practically ubiquitous behavior, might we expect to find navigationally relevant variables, such as space, to be encoded throughout the brain? If the encoding of navigationally relevant variables is distributed across the brain, is it necessarily redundant across systems and structures? How might the unique strategies, sensory modalities, and objectives supported in different systems modulate the distributed navigational codes? How might we plot a path forward for understanding all the cross-structural, cross-behavioral, and cross-species similarities and differences in navigational encoding?
In this brief review, we address these questions in turn, in each section below. First, we explore the ways in which navigational codes differ across brain regions, supporting distinct behaviors. Next, we consider how different instantiations of navigational codes throughout the brain are modulated by sensory modality and vary across species. Finally, we propose a promising approach for future work to make sense of the diversity and ubiquity of navigational codes across contexts, regions, and species. At the outset, we would like to note that this review is intended to serve as a record of the work presented and discussed at the minisymposium on encoding of navigationally relevant variables at the 2022 meeting of the Society for Neuroscience. As such, this work is not meant to be exhaustive but is instead intended to compliment other treatments of this topic (Grieves and Jeffery, 2017; Behrens et al., 2018; Boorman et al., 2021; Sosa and Giocomo, 2021), while highlighting the particular bodies of work and perspectives presented by the authors.
Distributed navigational codes
Arguably, the most common tool in the behavioral neuroscientist's arsenal is the peristimulus time histogram, also called the “tuning curve.” Almost 50 years ago, O'Keefe and Dostrovsky (1971) reported a remarkably distinctive pattern in the tuning curves of dorsal hippocampal neurons in rodents. As animals foraged along the 2D surface of an environment, some neurons would ramp up their average activity if, and only if, the animal approached a particular location in the environment (O'Keefe, 1976) (Fig. 1A). When considered as a population, the preferences of these location-specific hippocampal neurons covered the entirety of the navigable space, collectively forming a map-like representation of the environment. This category of spatially tuned cells was given the name “place cell,” and investigations of spatial cognition were forever changed.
Figure 1.
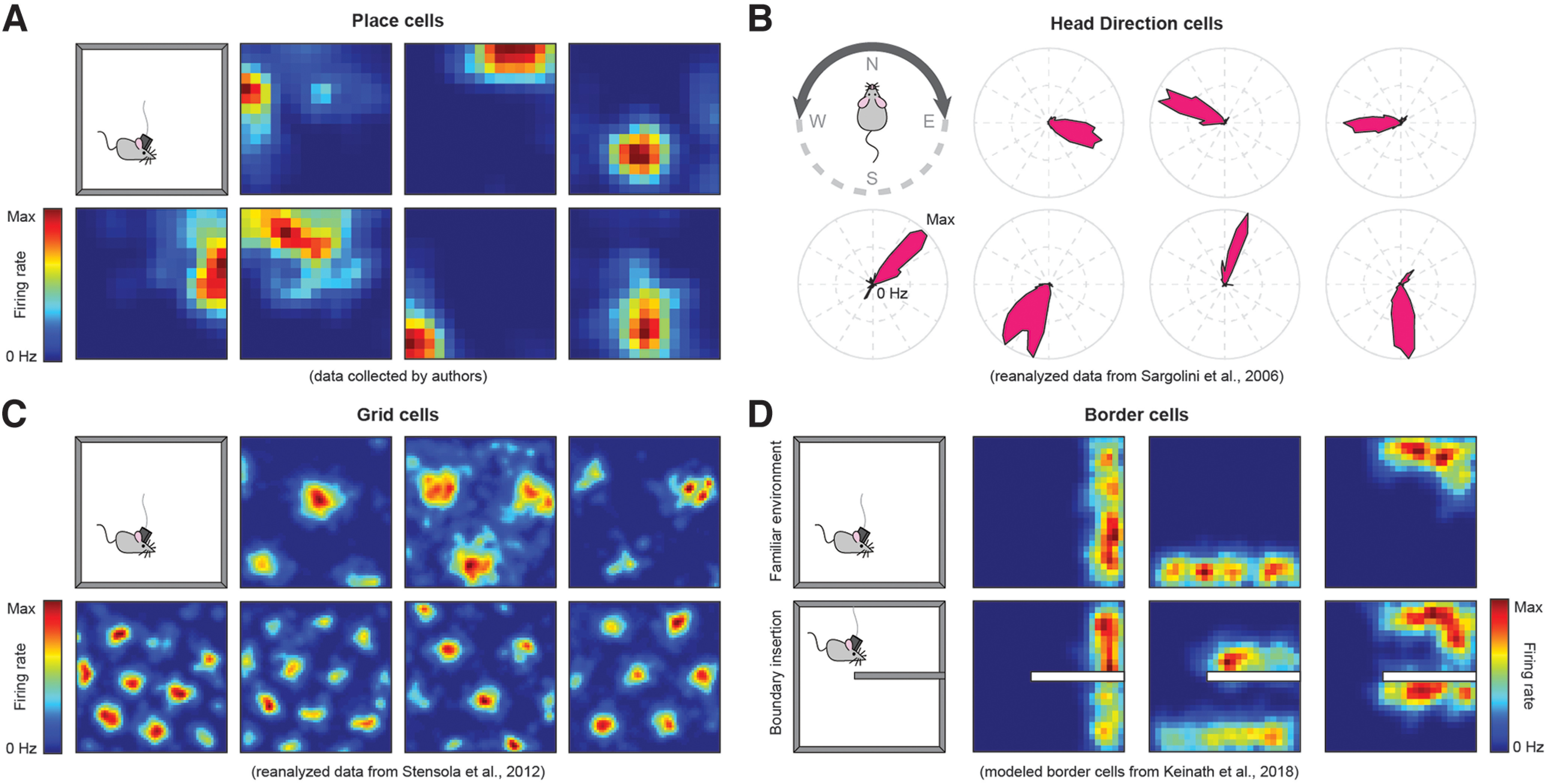
Examples of cell types exhibiting distinct patterns of tuning to different spatial variables. A, Heat maps represent the firing rates of seven example place cells. Note the tuning to distinct locations throughout the environment. B, Radial plots of the firing rate for seven example head direction cells. Preferred orientations span all directions. C, Heat maps represent the firing rates of seven example grid cells. D, Heat maps represent the firing rates of three example synthetic allocentric border cells in an open field and when a boundary is inserted. Note the duplication of firing nearby and at a similar allocentric direction to the inserted boundary.
Following the discovery of place cells, our knowledge of spatial codes has expanded to include numerous other spatial cell types defined by striking tuning curve patterns. Allocentric head direction cells spike as the navigator's heading approaches a particular preferred orientation (Taube et al., 1990) (Fig. 1B). The activity of grid cells covers the environment with tessellating fields whose vertices form a hexagonal grid (Hafting et al., 2005) (Fig. 1C). Allocentric border cells fire when a navigational boundary is nearby, in a particular allocentric direction (Savelli et al., 2008; Solstad et al., 2008) (Fig. 1D). Relatedly, egocentric boundary cells spike when a navigational boundary is located at a particular egocentric distance and direction to the navigator (Wang et al., 2018; Hinman et al., 2019; LaChance et al., 2019; Mao et al., 2021). Importantly, the majority of these cell types were discovered within the broader hippocampal formation and functionally adjacent structures, leading some to speculate that these codes alone, or a small subset of these codes, are responsible for instantiating the navigator's cognitive map and making flexible navigation possible (O'Keefe and Nadel, 1978). However, a growing body of evidence suggests that tuning to navigationally relevant variables, and in particular the stereotyped patterns of activity described above, is far more broadly distributed throughout the brain than previously appreciated. Below we discuss some of these cases in additional detail, while Table 1 lists brain areas, including those discussed, where navigationally relevant neural activity has been found.
Table 1.
Areas with reported navigationally relevant encoding
| Brain area | Navigational variable | Species | Reference(s) |
|---|---|---|---|
| Cerebellum | Map of visual space | Human | van Es et al., 2019 |
| Cerebellum | Direction and self-motion during navigation | Rodents | Rondi-Reig et al., 2014 |
| Brainstem | Drivers of head direction tuning | Rat | Bassett et al., 2007 |
| Brainstem | 3D position of visually pursued target | Macaque | Duffy, 2003 |
| Tegmentum | Head direction tuning | Rat | Sharp et al., 2001 |
| Anterior thalamus | Head direction tuning | Rat |
Stackman and Taube, 1997; Shinder and Taube, 2011; Jankowski et al., 2013; |
| Rostral thalamus | Border/perimeter tuning | Rat | Matulewicz et al., 2019 |
| Nucleus reuniens | Head direction tuning | Rat | Taube, 1995; Jankowski et al., 2014 |
| Thalamus | Spatial orientation of target object | Human | Hulme et al., 2010 |
| Striatum | Spatial orientation of target object | Macaque | Yoo et al., 2018 |
| Striatum | Head direction tuning | Rat | Wiener, 1993 |
| Ventral striatum and PFC | Spatial orientation of target object | Macaque | Strait et al., 2016 |
| Dorsal striatum | Space and task-relevant spatial cues | Rat | Schmitzer-Torbert and Redish, 2008 |
| Hippocampus | Place cells | Rat | O'Keefe and Dostrovsky, 1971 |
| Hippocampus | Position, egocentric boundary distance, head direction |
Macaque | Mao et al., 2021 |
| Hippocampal formation | Egocentric boundary distance | Rat | Wang et al., 2018; Hinman et al., 2019; LaChance et al., 2019 |
| Entorhinal cortex | Grid cells | Rat | Hafting et al., 2005 |
| Entorhinal cortex | Border/perimeter tuning | Rat | Savelli et al., 2008; Solstad et al., 2008 |
| Subiculum | Grid-like spatial tuning | Rat | Boccara et al., 2010 |
| Subiculum | Position tuning | Rat | Moser et al., 2008 |
| Subiculum | Head direction tuning | Rat | Taube et al., 1990 |
| Piriform cortex | Space tuning | Rat | Poo et al., 2022 |
| Somatosensory cortex | Space, head direction, border, and grid tuning | Rat | Long and Zhang, 2021 |
| Retrosplenial cortex | Head direction tuning | Rat | Chen et al., 1994 |
| Parahippocampal place area and retrosplenial cortex | Landmark position | Human | Epstein, 2008 |
| Parahippocampal, retrosplenial cortex, frontal lobe | Spatial anchoring of cognitive maps | Human | Epstein et al., 2017 |
| Parahippocampal, retrosplenial, and parietal cortex | Landmark/target vector representation | Human | Polti et al., 2022 |
| OFC | Current and/or place and goal position | Rat | Basu et al., 2021; Wikenheiser et al., 2021 |
| Frontal cortices | Grid-like spatial tuning | Human | Doeller et al., 2010; Jacobs et al., 2013; Constantinescu et al., 2016 |
Column (1) indicates the brain area in which the encoding was detected. Column (2) indicates the category of navigationally relevant variable encoded by neurons in the corresponding structure. Column (3) indicates the species in which the encoding was identified. Column (4) a citation demonstrating the report of neuronal activity in the corresponding structure.
Tuning to head direction, for instance, has been reported in both subcortical and cortical structures. In one experiment, systematically rotating an environment and distal cues allowed researchers to show that 10% of recorded striatal neurons showed tuning to the orientation of the head while an additional 20% were tuned to the onset of angular movements (Wiener, 1993). In another study, ∼12.5% of recorded dorsal tegmental neurons were found to have distinctively preferred head orientation, and an additional 73% were tuned to the angular velocity of the head (Sharp et al., 2001). In the retrosplenial cortex, one report showed 8.5% of recorded neurons are tuned to head direction (Chen et al., 1994). Neurons in both the anterior thalamic nucleus and the ventral midline thalamic nucleus reuniens are also known to be selective to head direction (Taube, 1995; Jankowski et al., 2014).
A widely distributed sensitivity to head direction alone may be expected, as many behaviors and functions (navigational and otherwise) depend on how an agent is oriented. An important question then is which other canonical navigational variables appear to be distributed. In a recent study, investigators recorded the activity of neurons in the piriform cortex while rats used olfactory cues to navigate a plus-shaped maze (Poo et al., 2022). Not only were there neurons that encoded the odor identity, but this odor code was dissociable from the spatial code. The animal's current location, their goal location, and the location of the intertrial zones were all reliably decodable from the activity of piriform neurons. In another recent report of navigational tuning in sensory areas, researchers found neurons in the somatosensory cortex with activity that closely resembled hippocampal-like place fields (Long and Zhang, 2021). The same study also reported proportions of neurons with head direction, border, and grid cell-like activity in the somatosensory cortex, albeit to a lesser extent.
In hindsight, it may not be altogether surprising to find neurons tuned to navigationally relevant stimuli in brain regions most commonly linked to sensory information and motor control. For one, the ubiquity of navigation in natural behaviors may depend on coordination of navigationally relevant codes across many distributed signals. After all, many (but not all) of the inputs that inform a navigator's map are sensory-driven, and the navigational behaviors using those maps are ultimately motor-dependent. Moreover, navigationally relevant variables may offer a broad and efficient architecture for performing computations on a diverse range of information. For example, primarily executive brain structures also instantiate and rely on navigational codes (Behrens et al., 2018). The Hayden and Zimmermann laboratories are investigating navigational encoding by neurons across a diverse collection of prefrontal cortices in freely foraging nonhuman primates. It is becoming clear that, even within a single structure, encoding the breadth of navigational variables appears to be distributed across available neuronal populations. In rodents, recent reports show spatial tuning in neurons of the orbitofrontal cortex (OFC) during both goal-directed and open-foraging behaviors (Basu et al., 2021; Wikenheiser et al., 2021). In humans, results from fMRI have suggested grid cell-like activity in cingulate and broader frontal cortices (Doeller et al., 2010; Jacobs et al., 2013; Constantinescu et al., 2016).
Together, evidence for the ubiquity of navigational codes throughout the brain is mounting. While in some ways this ubiquity might be justified by gesturing at the ways in which navigation pervades many, if not most, behaviors as we have here, fundamental questions nevertheless remain. What is the advantage of all of this ostensible redundancy? Are these navigational codes instantiated de novo or inherited from upstream structures? Are coding differences organized functionally? Do these codes differentially support distinct behaviors? We address these questions in the following sections.
Same, but different? Shared and unique features of navigation-related representations across structures
It is now well established that spatial, movement, and other navigationally relevant representations are present in brain regions outside of the hippocampal formation (Hok et al., 2005; Saleem et al., 2018; Esteves et al., 2021; Flossmann and Rochefort, 2021; Sauer et al., 2022). To what extent are such representations similar to or different from canonical hippocampal representations of space? Although we are not yet able to fully answer this question, one trend that seems to emerge is that cortical and hippocampal formation representations of space frequently share common organizational principles but exhibit revealing differences in the content of their representations. A common representational format may facilitate coordination between hippocampal formation and cortical regions during periods of learning or decision-making, while differences in what is encoded may reflect functional specializations related to how representations ultimately influence behavior.
During attentive states, ensemble activity in the rodent hippocampus is concentrated within cycles of ongoing theta-frequency local field potential oscillations (Wikenheiser and Redish, 2015). Theta cycles are thought to constitute discrete units of processing and representation (Colgin, 2013), and the ordering of place cell activity within theta cycles reflects the arrangement of place fields in the environment. Ensemble “theta sequences” within each theta cycle trace out trajectories beginning near the animal's current position and extending forward to varying degrees (Skaggs et al., 1996; Foster and Wilson, 2007). The expression of discrete trajectories within theta cycles and the relative independence of representations from one cycle to the next suggest a means of serially representing potential courses of action while preserving the separation of different options (Kay et al., 2020). Representations of this sort may be important for planning and decision-making.
Intriguingly, simultaneous recordings in rat hippocampus and mPFC suggest that hippocampal theta may act as a cross-structural organizing force (Hyman et al., 2005; Jones and Wilson, 2005a). Hippocampal and prefrontal theta oscillations are coherent during behaviorally critical moments, such as learning and decision-making, and many neurons in rodent mPFC phase-lock to hippocampal theta, especially when prefrontal-hippocampal theta coherence is elevated (Jones and Wilson, 2005b; Benchenane et al., 2010). Cells in mPFC frequently show spatial modulation, albeit with weaker temporal stability and larger, less organized firing fields compared with dorsal hippocampus. Like hippocampal place cells, spatially tuned mPFC neurons show direction-specific responses as rats traverse linear tracks, and location can be decoded from mPFC ensembles on a theta-cycle timescale (Zielinski et al., 2019).
One recent study identified clear theta sequences in mPFC ensembles of rats performing a spatial decision-making task (Tang et al., 2021). Interestingly, the trajectory representation observed in the hippocampus was related to, but not always identical to, the theta sequence representation occurring simultaneously in mPFC. Before rats committed to a decision, hippocampal sequences alternately represented both possible future choices, while mPFC theta sequences were biased toward the option that rats would ultimately select. Once rats passed the choice point and locked in their choice, sequences in both structures generally represented the rat's actual choice trajectory. These data show that, while the formatting and organization of navigation-related representations may be shared across brain regions, what is represented need not be redundant. Such differences may have functional importance, suggesting that hippocampus encodes possible future actions, which are accumulated, integrated, or otherwise evaluated by prefrontal structures to eventually reach a decision (Shadlen and Shohamy, 2016; Bakkour et al., 2019).
Like mPFC, more lateral portions of the rodent frontal cortex, such as the OFC, have long been linked to decision-making (Wallis, 2007; Klein-Flügge et al., 2022). OFC neurons represent the probability and magnitude of potential outcomes, along with the qualitative aspects of impending rewards, including gustatory and olfactory features. When valuable resources are localized to distinct places, as they frequently are in natural settings, there is evidence that OFC neurons encode information about outcome location alongside other outcome attributes. For instance, when rats use an odor cue presented from a central port to decide whether to search for reward at a dispenser to the left or right of the start location, many OFC neurons encode the direction of impending movements (Feierstein et al., 2006; Roesch et al., 2006). Suppressing outputs from the ventral hippocampus substantially weakens this representation of spatial goals (Wikenheiser et al., 2017). These data suggest that even within the confines of the rodent operant chamber, where minimal navigation or movement is required, circuits involved in decision-making represent information about the location of outcomes and the actions necessary to obtain them. Similar results have been observed in some primate studies of decision-making (e.g., Strait et al., 2016), where subject movement is even more limited, although cortical value representations that do not integrate the sensorimotor properties of outcomes have also been reported (e.g., Padoa-Schioppa and Assad, 2006).
Even stronger evidence linking OFC representations with navigation-related signals comes from freely moving rodents performing spatial tasks (Shapiro et al., 2014; Wikenheiser and Schoenbaum, 2016). One recent study (Riceberg et al., 2022) directly compared simultaneously recorded hippocampal and OFC ensembles as rats performed a spatial reversal-learning task on a plus maze. Here, rats first learned to approach a rewarded location, beginning from one of two distinct start locations on each trial. Once proficient, the reward location reversed, such that rats were rewarded for approaching a previously unrewarded location. Before each trial began, ensembles in either hippocampus or OFC could be used to decode which location the rat would travel to later in the trial, suggesting that pretrial activity in both structures reflected future spatial goal locations. Nevertheless, representations differed as rats executed trajectories from start point to goal location. Distinct sets of hippocampal place cells fired during the two partially distinct trajectories beginning from the two start locations but ending at the same goal. In contrast, OFC neurons tended to generalize across trajectories that linked different start points to the same destination. Once again, hippocampal theta oscillations played a role in organizing activity in both structures. As rats learned to reverse reward contingencies for the first time, many OFC neurons phase-locked to hippocampal theta.
These results highlight the growing appreciation that, while the encoding of navigational variables may be distributed across the brain, the similarities and differences in content and implementation among these codes are crucial to understanding how these codes might cooperate or act in isolation to support distinct navigation behaviors. Some insight into untangling these relationships can be gained by comparing these codes across species with differing sensory and navigational capacities.
Graded spatial codes in support of a variety of navigational strategies
The presence of spatial codes throughout the cortical mantle may reflect a diversity of navigational strategies. For instance, when macaques are required to navigate to the location of briefly presented targets by accumulating optic flow velocity signals (Alefantis et al., 2022), they innately track these targets with their eyes (Lakshminarasimhan et al., 2020). Similarly, when navigating complex mazes, humans' gaze patterns show a rapid and sequential prospection of the future path to be taken (Zhu et al., 2022), a strategy evocative of neural forward replay/preplay (Dragoi and Tonegawa, 2011). In addition to demonstrating an embodied mnemonic strategy in keeping track of evolving goals, these eye movements may substantially shape aspects of the neural code for spatial navigation. Indeed, in support of this conjecture, Noel et al. (2022) recently recorded single-unit spiking activity from the dorsomedial superior temporal area (MSTd), parietal area 7a, and dorsolateral prefrontal cortex (dlPFC) as macaques navigated in virtual reality to “catch fireflies” (i.e., hidden targets). As one may anticipate, eye movement-related variables were most frequently encoded in MSTd (Komatsu and Wurtz, 1988), sensorimotor variables (e.g., linear and angular velocity) were preferentially encoded in parietal area 7a, and latent variables (e.g., distance and angle to the hidden target) were preferentially encoded in dlPFC. Importantly, however, all areas showed multiplexing of spatial variables (see Hardcastle et al., 2017), and all areas, including areas traditionally thought of as sensory alone such as MSTd, coded for latent spatial variables (i.e., vector coding of spatial goals). Analysis of the statistical dependencies between neurons (i.e., noise correlations) demonstrated a strong functional coupling between MSTd and dlPFC, which was predictive of the degree to which eye movements tracked the hidden spatial goals (Noel et al., 2022). Together, in addition to showing a gradation of spatial codes throughout the cortex, these results suggest that a putative reason why spatial codes could be found even in MSTd may be precisely to support the tracking of hidden spatial goals with our eyes, a behavioral strategy that some, but not all animals, may use.
If the presence and gradation of spatial codes throughout the brain reflect the need to support a variety of navigational strategies, then we might expect these codes to qualitatively differ across species. Indeed, while primates use their eyes to make observations about their sensory milieu, rodents preferentially sample from their environment via olfaction, their vibrissa, and by moving their heads. Instead, eye movements in freely moving rodents appear to be largely compensatory for head rotations (Michaiel et al., 2020). Thus, we may expect that navigational strategies allow for “far sensing” in primates (e.g., eye and head movements sampling from an environment “out there”), while they may more heavily rely on “near-sensing” (e.g., vibrissa) in rodents. In this context, it is interesting to note that only recently have researchers started to quantify movement-related variables during freely moving and open arena scenarios in macaques (Bala et al., 2020), as is traditional in the rodent literature. In this vein, Mao et al. (2021) recorded from the hippocampal formation of macaques, while tracking their bodies — and importantly, their eyes — in 3D. The authors reported that, contrary to observations from the rodent literature, only a small minority of neurons tiled space in a grid-like fashion (1% in hippocampus and 2% in entorhinal cortex) or encoded the location they occupied (for results in marmosets suggesting the presence of place fields, but a differential relationship to theta cycles, see Courellis et al., 2019). Instead, Mao et al. (2021) found a greater fraction of neurons that coded for the location where monkeys were looking (“spatial view” cell) (Georges-François et al., 1999) or where they were facing (“facing location cells”; 16% in hippocampus and 25% in entorhinal cortex). Furthermore, eye movements strongly modulated neural activity in both areas. Of course, these are only initial efforts, and future studies ought to more directly compare spatial codes across species while using comparable technology (i.e., eye, head, and body tracking). Nonetheless, these initial results do suggest that while coarse-grain principles may be similar across species (e.g., primates, bats, and rodents all possessing grid and place cells), their fine grain implementation may depend on the precise navigational challenges faced and strategies used.
Interestingly, a growing body of literature argues that cells traditionally considered to encode physical properties may not necessarily be specialized for the mapping of physical space, but instead may instantiate a more abstract cognitive map (e.g., Behrens et al., 2018). For instance, grid cells may emerge from summarizing 2D spaces, in particular driven by the transition structure between states in this space (Stachenfeld et al., 2017). However, we just noted that grid cells are not apparent when conditioning on the current location of macaques (Mao et al., 2021). Instead, grid-like cells have been reported when head-fixed monkeys freely view images (Killian et al., 2012; for a similar observation in humans via fMRI, see Julian et al., 2018). Thus, “spatial” codes may be most readily apparent when different animals are allowed to use their natural strategies and associated sensory modalities to sample from the environment; eye movements, the visual system, and exploration from afar in primates, and olfaction, vibrissa, and nearby body-centric exploration in rodents. This suggestion does not contradict or oppose the conceptualization of grid cells as reflecting transition probabilities (and “spatial” codes in general underpinning cognitive maps) (Behrens et al., 2018) but simply emphasizes the active role of agents (and the properties of their particular biological sensors) in building these transition probabilities by actively sampling from the environment.
Future work contrasting spatial codes across species and brain areas will also be informative regarding their functional role. For instance, while place- (Ekstrom et al., 2003) and grid-like (Jacobs et al., 2013) cells have been recorded from humans, albeit in a virtual environment (for interesting work suggesting striking differences in spatial codes between real and virtual environments, see Aghajan et al., 2015), it is not entirely obvious that place and grid cells ought to have a strictly overlapping set of functions in humans and others. Likely, the role of these cells in encoding space is preserved across species. But if spatial codes also scaffold our ability to structure abstract spaces (i.e., cognition), then one would expect strong distinctions between species, and perhaps especially so in the connectivity pattern between “spatial codes” in the hippocampal formation with “spatial-like codes” throughout the rest of the brain.
Representational similarity analysis (RSA) can help us make sense of the multiplicity of dynamic spatial codes
So far, we have highlighted how recent work is leading to a growing appreciation for the ubiquity of dynamic spatial codes throughout the brain, and in particular in extrahippocampal regions not traditionally associated with spatial coding. Yet even within the hippocampal formation field, things are changing. Whereas perhaps at one time “space” was conceptually sufficient to organize our thoughts about neural codes with a reasonable amount of specificity (O'Keefe and Nadel, 1978), the explanatory power of “space” has become increasingly fuzzy as the field has progressed in recent years. Mounting work has demonstrated influences of unexpected variables on hippocampal representations, prompting debate over whether it is our definition of “space” or our belief that the hippocampal formation encodes space that needs revision (Bernardi et al., 2020; O'Keefe and Krupic, 2021). The discovery of “spatial codes” in many regions not traditionally associated with space, which take different forms across different contexts in the same region, across different regions in the same brain, and across different brains (species) makes it clear that “spatial coding” can be associated with a whole array of implementations at the neural level. At the same time, we have seen a proliferation of “spatial mapping theories” (in both physical and abstract senses) which attempt to provide normative explanations for activity in a variety of brain regions (Stachenfeld et al., 2017; Whittington et al., 2020; Gardner and Schoenbaum, 2021). Often expressed in the form of computational models, these theories make predictions that frequently differ from one another in only subtle, quantitative ways. All in all, these advances make it clear that, to keep moving forward and make sense of this spatial coding milieu, we need to perform high-powered experiments specifically designed to quantitatively compare spatial codes across contexts, regions, species, and computational models (Schrimpf et al., 2020).
RSA provides an ideal framework for exactly these kinds of experiments (Kriegeskorte et al., 2008). At its highest level, RSA proposes that measurements of neural activity, behavior, and model output be abstracted away from raw values into distance matrices (representational distance matrices [RDMs]), which capture the similarity of a given measure for all pairwise comparisons between conditions in the experiment. These distance matrices can then be quantitatively related to one another by leveraging a number of analytical tools to uncover whether and how representational structure differs between levels of explanation, contexts, regions, species, and models (Nili et al., 2014; Lu and Ku, 2020). Because in this case the unit of analysis is the RDM, this framework also encourages a particular type of experimental design, one that maximizes the number of comparisons across conditions so long as statistical power within each comparison can be maintained.
While RSA has enjoyed widespread adoption and success in human fMRI and EEG designs, it was originally pioneered explicitly for comparison across different levels of explanation and computational models. In keeping with these origins, RSA is very well suited for designs across all levels of systems neuroscience. This is especially true of designs using high-yield neural recordings, such as calcium imaging and high-density probes (Dombeck et al., 2010; Ghosh et al., 2011; Berényi et al., 2014; Jun et al., 2017), as is increasingly common in rodent and nonhuman primate research. Because these approaches can provide unprecedented power when characterizing neural similarity across conditions at the level of the population, they open the door to RSA designs which leverage high-precision RDMs for high-confidence quantitative characterizations between measurements. Moreover, whereas relatively noisy measurements might limit the interpretability of individual voxels or channels with fMRI or EEG, the yield and power of these nonhuman imaging and electrophysiological techniques makes it possible to apply the whole toolbox of RSA analyses not only at the level of the population but also at the level of individual cells and subpopulations. Finally, whereas prior work with conventional recording techniques has often had to rely on the pooling of recordings across subjects into pseudo-populations, the power of these new techniques combined with an RSA approach also allows us to quantitatively partition contributions of within-subject and between-subject variability to the heterogeneity we observe in neural recordings. Therefore, together, use of RSA designs is well motivated by recent advances in neural recording technology.
So far, we have described in general the advantages of an RSA approach to addressing the mounting need for quantitative comparison across measured and theorized spatial codes. To illustrate how this might be borne out in practice, here we elaborate an ongoing research program motivated by the RSA framework. In this program, we have chosen to characterize one property that is fundamental to any spatial code: how that spatial code is determined by the shape (or reshaping) of the space it is mapping. Prior work has shown that when a familiar environment is reshaped, hippocampal and entorhinal spatial codes deform in some interesting, stereotyped ways (Gothard et al., 1996; O'Keefe and Burgess, 1996; Barry et al., 2007; Stensola et al., 2012; Dabaghian et al., 2014; Krupic et al., 2015). Moreover, researchers have noted provocative similarities between these coding changes, measured in rodents (O'Keefe and Burgess, 1996; Krupic et al., 2015; Keinath et al., 2018), and spatial memory, measured in humans in similar paradigms (Hartley et al., 2004; Bellmund et al., 2020; Keinath et al., 2021; de Cothi et al., 2022). Thus, there is good reason to think that characterizing coding and mnemonic changes in response to environmental deformations will be fruitful to compare across codes, species, and levels of explanation using this framework.
Yet while ground-breaking, prior work has been for various reasons limited in its power to adjudicate between alternatives at the resolution we need today. To overcome these limitations, we have combined high-yield miniature microscope calcium imaging in freely behaving mice with a novel deformation paradigm designed with RSA in mind. In this paradigm, a familiar large square environment can be thought of as a 3 × 3 grid, akin to a tic-tac-toe board (Fig. 2). New configurations of this environment can be created by closing off partitions of this imaginary grid, allowing us to sample many possible configurations. Once measured, we can compare the spatial code within each partition to that of every other partition both within the same configuration and across all other configurations (Fig. 2). This yields a large and rich RDM, which can later be compared with other assays with the RSA toolbox. So far, we have demonstrated the power of this design by collecting a proof-of-concept dataset from dorsal hippocampus in young mice and comparing this code with predictions from different computational models. In the future, this program can be extended to include many other assays, including some which speak to the complexities of spatial coding raised in this review. These assays include spatial codes in other mouse brain regions, spatial codes in the human brain, and spatial memory assayed in mice and humans, with further comparison of these assays across navigational context, lifespan, sex, and in health and disease.
Figure 2.
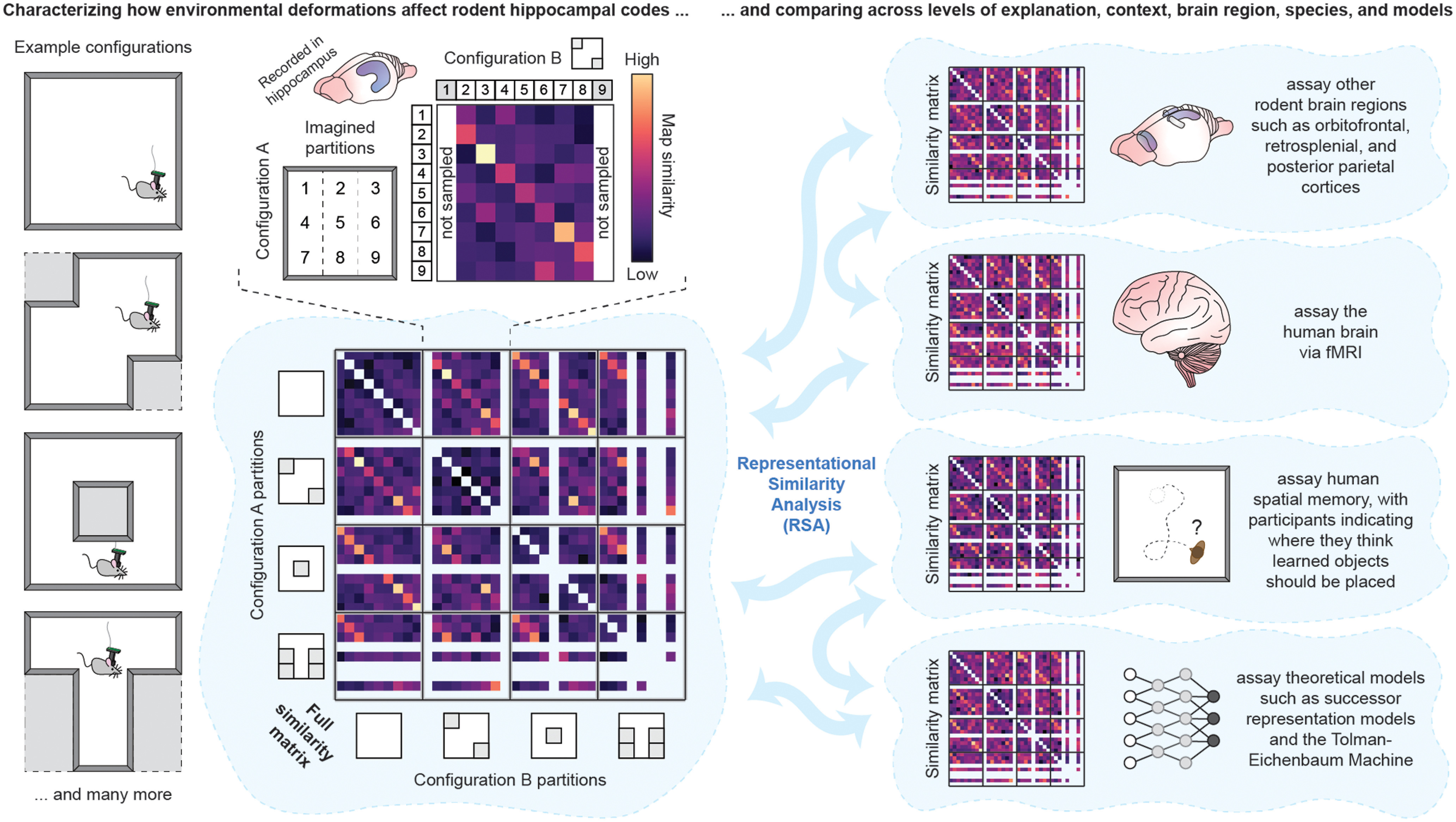
Schematic of an example RSA design comparing the effects of environmental deformations on spatial coding and spatial memory across assays of interest.
While the program we describe is ongoing, the quality of the preliminary results in preparation from our proof-of-concept hippocampal recordings are encouraging. Thus, we join other fields of neuroscience (Kriegeskorte et al., 2008; Popal et al., 2019; Freund et al., 2021) in suggesting that an RSA framework offers one fruitful avenue for future work aiming to make sense of spatial codes across brain regions, navigational contexts, species, and models in a principled, quantitative way.
Open questions for future directions
One important open question is whether navigational codes are distributed because they are inherited from the upstream output of the hippocampal formation or whether they are encoded de novo within each structure. While this is a largely unanswered question, there are a few studies that have attempted to adjudicate between these two points (Wikenheiser et al., 2017; Tingley and Buzsáki, 2018). For example, lateral septum neurons showed phase codes to be downstream transformations of hippocampal spatial rate code outputs. However, using purely computational approaches, it is challenging to know for certain whether the relationships between similarly encoded parameters are necessarily indicative of downstream readouts and not lagged de novo encoding. Further work using highly controlled projection suppression techniques is desperately needed.
Two recent studies (Bota et al., 2021; Esteves et al., 2021) demonstrate the promise of combined inactivation and recording approaches for understanding the role of hippocampal function in extrahippocampal spatial representations. Esteves et al. (2021) imaged the dorsal cortical surface of mice running on a cue-rich treadmill, and reported that a large fraction of neurons showed apparent spatial tuning. Lesions of the dorsal hippocampus decreased the proportion of spatially tuned neocortical cells by half, suggesting that a substantial portion of the observed spatial tuning in cortex is ultimately derived from hippocampus.
Between the extremes of direct inheritance of spatial representations from the hippocampus and parallel computation of spatial representations outside of the hippocampus, a range of other possibilities exist. Bota et al. (2021) used calcium imaging in freely moving rats to identify ACC neurons with place cell-like activity patterns. Combining imaging with chemogenetic inactivation of dorsal hippocampus, the authors found that, while the development of ACC spatial tuning depended on intact hippocampal function, established ACC spatial representations persisted when hippocampus was inactivated. As techniques for combining manipulation and observation of neural activity increase in sophistication, future experiments in this vein will improve our understanding of how and when the hippocampus is necessary for spatial encoding in other parts of the brain.
A second major unanswered question — and one that we have alluded to throughout the review — concerns the function of navigation-related and spatial representations outside of the hippocampus. Although we have largely focused here on studies that describe the properties of such representations, knowing how they contribute to behavior is of great importance. Advances in three domains will be necessary for progress.
First, causal manipulations of neural activity will prove critical for ascribing specific functions to observed representations. Although interpreting the behavioral effects produced by manipulations of neural activity requires care (Jazayeri and Afraz, 2017), the increasing sophistication of such methods affords great opportunities. Contemporary optogenetic and chemogenetic approaches offer unprecedented control over neural activity in both time and space yet still fall short of the temporal and spatial resolution necessary to re-create realistic neural representations. Emerging holographic optogenetic methods (Adesnik and Abdeladim, 2021), which approach the temporal scale of milliseconds and the spatial scale of individual neurons, may eventually aid progress here. Second, new behavioral tasks that isolate potential cognitive functions of extrahippocampal spatial representations are needed. The link between hippocampal function and spatial abilities was forged by integrating detailed observations of the striking spatial tuning in hippocampal neurons and the distinctive pattern of deficits on cleverly designed navigation tasks that resulted from hippocampal damage. New behavioral assays will likely be required to understand the potentially distinct functions to which spatial representations outside the hippocampus contribute. Finally, new theoretical frameworks for understanding the utility of brain-wide spatial encoding will need to be developed and elaborated to guide the design of behavioral tasks. Indeed, this process is already underway, with diverse classes of models providing new ways of interpreting hippocampal activity, understanding the ubiquity of spatially organized representations throughout the brain, and generating testable experimental predictions (Stachenfeld et al., 2017; Behrens et al., 2018; Piray and Daw, 2021). As these models continue to develop, they will be invaluable in guiding experimentation.
In conclusion, the goal of this review has been threefold. First, we aimed to highlight recent results characterizing navigational codes in regions outside of the hippocampal formation, especially those in regions more traditionally associated with sensation, movement, and executive function. Second, by juxtaposing these codes with those observed in the hippocampal formation, we argued that navigation might be better understood as being supported by distributed brain-wide encoding of navigationally relevant variables, rather than by a particular code in a particular region. Furthermore, comparing the form and content of these codes across regions, navigational contexts, and even species suggests that some of these differences might be particular to given sensory capacities and navigational strategies, while commonalities might signal general organizing principles of these codes. Finally, we proposed one powerful experimental and analytical framework, RSA, for untangling the complex relationships among these diverse spatial codes and navigation behavior, including a more detailed look at one of our research programs using this approach. We hope that this review will motivate comparative approaches to understanding navigational codes in the future.
Footnotes
We thank all of the individuals within our respective research groups for influential contributions to shaping thoughts on this question.
The authors declare no competing financial interests.
References
- Adesnik H, Abdeladim L (2021) Probing neural codes with two-photon holographic optogenetics. Nat Neurosci 24:1356–1366. 10.1038/s41593-021-00902-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajan ZM, Acharya L, Moore JJ, Cushman JD, Vuong C, Mehta MR (2015) Impaired spatial selectivity and intact phase precession in two-dimensional virtual reality. Nat Neurosci 18:121–128. 10.1038/nn.3884 [DOI] [PubMed] [Google Scholar]
- Alefantis P, Lakshminarasimhan KJ, Avila E, Noel JP, Pitkow X, Angelaki DE (2022) Sensory evidence accumulation using optic flow in a naturalistic navigation task. J Neurosci 42:5451–5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A, Palombo DJ, Zylberberg A, Kang YH, Reid A, Verfaellie M, Shadlen MN, Shohamy D (2019) The hippocampus supports deliberation during value-based decisions. Elife 8:e46080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bala PC, Eisenreich BR, Yoo SB, Hayden BY, Park HS, Zimmermann J (2020) Automated markerless pose estimation in freely moving macaques with OpenMonkeyStudio. Nat Commun 11:4560. 10.1038/s41467-020-18441-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry C, Hayman R, Burgess N, Jeffery KJ (2007) Experience-dependent rescaling of entorhinal grids. Nat Neurosci 10:682–684. 10.1038/nn1905 [DOI] [PubMed] [Google Scholar]
- Bassett JP, Tullman ML, Taube JS (2007) Lesions of the tegmentomammillary circuit in the head direction system disrupt the head direction signal in the anterior thalamus. J Neurosci 27:7564–7577. 10.1523/JNEUROSCI.0268-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Gebauer R, Herfurth T, Kolb S, Golipour Z, Tchumatchenko T, Ito HT (2021) The orbitofrontal cortex maps future navigational goals. Nature 599:449–452. 10.1038/s41586-021-04042-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Muller TH, Whittington JC, Mark S, Baram AB, Stachenfeld KL, Kurth-Nelson Z (2018) What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100:490–509. 10.1016/j.neuron.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Bellmund JL, de Cothi W, Ruiter TA, Nau M, Barry C, Doeller CF (2020) Deforming the metric of cognitive maps distorts memory. Nat Hum Behav 4:177–188. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI (2010) Coherent theta oscillations and reorganization of spike timing in the hippocampal-prefrontal network upon learning. Neuron 66:921–936. 10.1016/j.neuron.2010.05.013 [DOI] [PubMed] [Google Scholar]
- Berényi A, Somogyvári Z, Nagy AJ, Roux L, Long JD, Fujisawa S, Stark E, Leonardo A, Harris TD, Buzsáki G (2014) Large-scale, high-density (up to 512 channels) recording of local circuits in behaving animals. J Neurophysiol 111:1132–1149. 10.1152/jn.00785.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi S, Benna MK, Rigotti M, Munuera J, Fusi S, Salzman CD (2020) The geometry of abstraction in the hippocampus and prefrontal cortex. Cell 183:954–967.e21. 10.1016/j.cell.2020.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser MB (2010) Grid cells in pre- and parasubiculum. Nat Neurosci 13:987–994. 10.1038/nn.2602 [DOI] [PubMed] [Google Scholar]
- Boorman ED, Sweigart SC, Park SA (2021) Cognitive maps and novel inferences: a flexibility hierarchy. Curr Opin Behav Sci 38:141–149. 10.1016/j.cobeha.2021.02.017 [DOI] [Google Scholar]
- Bota A, Goto A, Tsukamoto S, Schmidt A, Wolf F, Luchetti A, Hayashi Y (2021) Shared and unique properties of place cells in anterior cingulate cortex and hippocampus. bioRxiv 437441. 10.1101/2021.03.29.437441. [DOI] [Google Scholar]
- Chen LL, Lin LH, Green EJ, Barnes CA, McNaughton BL (1994) Head-direction cells in the rat posterior cortex. Exp Brain Res 101:8–23. 10.1007/BF00243212 [DOI] [PubMed] [Google Scholar]
- Colgin LL (2013) Mechanisms and functions of theta rhythms. Annu Rev Neurosci 36:295–312. [DOI] [PubMed] [Google Scholar]
- Constantinescu AO, O'Reilly JX, Behrens TE (2016) Organizing conceptual knowledge in humans with a gridlike code. Science 352:1464–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courellis H, Nummela SU, Metke M, Bussell R, Diehl G, Cauwenberghs G, Miller CT (2019) Spatial encoding in primate hippocampus during free-navigation. PLoS Biol 17:e3000546. 10.1371/journal.pbio.3000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabaghian Y, Brandt VL, Frank LM (2014) Reconceiving the hippocampal map as a topological template. Elife 3:e03476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cothi W, Nyberg N, Griesbauer EM, Ghanamé C, Zisch F, Lefort JM, Fletcher L, Newton C, Renaudineau S, Bendor D, Grieves R, Duvelle É, Barry C, Spiers HJ (2022) Predictive maps in rats and humans for spatial navigation. Curr Biol 32:3676–3689.e5. 10.1016/j.cub.2022.06.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, Barry C, Burgess N (2010) Evidence for grid cells in a human memory network. Nature 463:657–661. 10.1038/nature08704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dombeck DA, Harvey CD, Tian L, Looger LL, Tank DW (2010) Functional imaging of hippocampal place cells at cellular resolution during virtual navigation. Nat Neurosci 13:1433–1440. 10.1038/nn.2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S (2011) Preplay of future place cell sequences by hippocampal cellular assemblies. Nature 469:397–401. 10.1038/nature09633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy CJ (2003) Pursuit neurons encode 3D space: is the cortex nervous and tensor? Trends Neurosci 26:237–240. 10.1016/S0166-2236(03)00069-9 [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I (2003) Cellular networks underlying human spatial navigation. Nature 425:184–188. 10.1038/nature01964 [DOI] [PubMed] [Google Scholar]
- Epstein RA (2008) Parahippocampal and retrosplenial contributions to human spatial navigation. Trends Cogn Sci 12:388–396. 10.1016/j.tics.2008.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein RA, Patai EZ, Julian JB, Spiers HJ (2017) The cognitive map in humans: spatial navigation and beyond. Nat Neurosci 20:1504–1513. 10.1038/nn.4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves IM, Chang H, Neumann AR, Sun J, Mohajerani MH, McNaughton BL (2021) Spatial information encoding across multiple neocortical regions depends on an intact hippocampus. J Neurosci 41:307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF (2006) Representation of spatial goals in rat orbitofrontal cortex. Neuron 51:495–507. 10.1016/j.neuron.2006.06.032 [DOI] [PubMed] [Google Scholar]
- Flossmann T, Rochefort NL (2021) Spatial navigation signals in rodent visual cortex. Curr Opin Neurobiol 67:163–173. 10.1016/j.conb.2020.11.004 [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA (2007) Hippocampal theta sequences. Hippocampus 17:1093–1099. 10.1002/hipo.20345 [DOI] [PubMed] [Google Scholar]
- Freund MC, Etzel JA, Braver TS (2021) Neural coding of cognitive control: the representational similarity analysis approach. Trends Cogn Sci 25:622–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner MP, Schoenbaum G (2021) The orbitofrontal cartographer. Behav Neurosci 135:267–276. 10.1037/bne0000463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-François P, Rolls ET, Robertson RG (1999) Spatial view cells in the primate hippocampus: allocentric view not head direction or eye position or place. Cereb Cortex 9:197–212. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Burns LD, Cocker ED, Nimmerjahn A, Ziv Y, Gamal AE, Schnitzer MJ (2011) Miniaturized integration of a fluorescence microscope. Nat Methods 8:871–878. 10.1038/nmeth.1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothard KM, Skaggs WE, McNaughton BL (1996) Dynamics of mismatch correction in the hippocampal ensemble code for space: interaction between path integration and environmental cues. J Neurosci 16:8027–8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieves RM, Jeffery KJ (2017) The representation of space in the brain. Behav Process 135:113–131. 10.1016/j.beproc.2016.12.012 [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI (2005) Microstructure of a spatial map in the entorhinal cortex. Nature 436:801–806. 10.1038/nature03721 [DOI] [PubMed] [Google Scholar]
- Hardcastle K, Maheswaranathan N, Ganguli S, Giocomo LM (2017) A multiplexed, heterogeneous, and adaptive code for navigation in medial entorhinal cortex. Neuron 94:375–387.e7. 10.1016/j.neuron.2017.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley T, Trinkler I, Burgess N (2004) Geometric determinants of human spatial memory. Cognition 94:39–75. 10.1016/j.cognition.2003.12.001 [DOI] [PubMed] [Google Scholar]
- Hinman JR, Chapman GW, Hasselmo ME (2019) Neuronal representation of environmental boundaries in egocentric coordinates. Nat Commun 10:2772. 10.1038/s41467-019-10722-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hok V, Save E, Lenck-Santini PP, Poucet B (2005) Coding for spatial goals in the prelimbic/infralimbic area of the rat frontal cortex. Proc Natl Acad Sci USA 102:4602–4607. 10.1073/pnas.0407332102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme OJ, Whiteley L, Shipp S (2010) Spatially distributed encoding of covert attentional shifts in human thalamus. J Neurophysiol 104:3644–3656. 10.1152/jn.00303.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman JM, Zilli EA, Paley AM, Hasselmo ME (2005) Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus 15:739–749. 10.1002/hipo.20106 [DOI] [PubMed] [Google Scholar]
- Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei XX, Suthana N, Sperling MR, Sharan AD, Fried I, Kahana MJ (2013) Direct recordings of grid-like neuronal activity in human spatial navigation. Nat Neurosci 16:1188–1190. 10.1038/nn.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Ronnqvist KC, Tsanov M, Vann SD, Wright NF, Erichsen JT, Aggleton JP, O'Mara SM (2013) The anterior thalamus provides a subcortical circuit supporting memory and spatial navigation. Front Syst Neurosci 7:45. 10.3389/fnsys.2013.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MM, Islam MN, Wright NF, Vann SD, Erichsen JT, Aggleton JP, O'Mara SM (2014) Nucleus reuniens of the thalamus contains head direction cells. Elife 3:e03075. 10.7554/Elife.03075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazayeri M, Afraz A (2017) Navigating the neural space in search of the neural code. Neuron 93:1003–1014. 10.1016/j.neuron.2017.02.019 [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA (2005a) Phase precession of medial prefrontal cortical activity relative to the hippocampal theta rhythm. Hippocampus 15:867–873. 10.1002/hipo.20119 [DOI] [PubMed] [Google Scholar]
- Jones MW, Wilson MA (2005b) Theta rhythms coordinate hippocampal–prefrontal interactions in a spatial memory task. PLoS Biol 3:e402. 10.1371/journal.pbio.0030402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian JB, Keinath AT, Frazzetta G, Epstein RA (2018) Human entorhinal cortex represents visual space using a boundary-anchored grid. Nat Neurosci 21:191–194. 10.1038/s41593-017-0049-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JJ, et al. (2017) Fully integrated silicon probes for high-density recording of neural activity. Nature 551:232–236. 10.1038/nature24636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay K, Chung JE, Sosa M, Schor JS, Karlsson MP, Larkin MC, Liu DF, Frank LM (2020) Constant sub-second cycling between representations of possible futures in the hippocampus. Cell 180:552–567.e25. 10.1016/j.cell.2020.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath AT, Epstein RA, Balasubramanian V (2018) Environmental deformations dynamically shift the grid cell spatial metric. Elife 7:e38169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keinath AT, Rechnitz O, Balasubramanian V, Epstein RA (2021) Environmental deformations dynamically shift human spatial memory. Hippocampus 31:89–101. 10.1002/hipo.23265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian NJ, Jutras MJ, Buffalo EA (2012) A map of visual space in the primate entorhinal cortex. Nature 491:761–764. 10.1038/nature11587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Flügge MC, Bongioanni A, Rushworth MF (2022) Medial and orbital frontal cortex in decision-making and flexible behavior. Neuron 110:2743–2770. 10.1016/j.neuron.2022.05.022 [DOI] [PubMed] [Google Scholar]
- Komatsu H, Wurtz RH (1988) Relation of cortical areas MT and MST to pursuit eye movements: I. Localization and visual properties of neurons. J Neurophysiol 60:580–603. 10.1152/jn.1988.60.2.580 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Mur M, Bandettini P (2008) Representational similarity analysis: connecting the branches of systems neuroscience. Front Syst Neurosci 2:4. 10.3389/neuro.06.004.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupic J, Bauza M, Burton S, Barry C, O'Keefe J (2015) Grid cell symmetry is shaped by environmental geometry. Nature 518:232–235. 10.1038/nature14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaChance PA, Todd TP, Taube JS (2019) A sense of space in postrhinal cortex. Science 365:eaax4192. 10.1126/science.aax4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarasimhan KJ, Avila E, Neyhart E, DeAngelis GC, Pitkow X, Angelaki DE (2020) Tracking the mind's eye: primate gaze behavior during virtual visuomotor navigation reflects belief dynamics. Neuron 106:662–674.e5. 10.1016/j.neuron.2020.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long X, Zhang SJ (2021) A novel somatosensory spatial navigation system outside the hippocampal formation. Cell Res 31:649–663. 10.1038/s41422-020-00448-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z, Ku Y (2020) NeuroRA: a Python toolbox of representational analysis from multi-modal neural data. Front Neuroinform 14:563669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Avila E, Caziot B, Laurens J, Dickman JD, Angelaki DE (2021) Spatial modulation of hippocampal activity in freely moving macaques. Neuron 109:3521–3534.e5. 10.1016/j.neuron.2021.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matulewicz P, Ulrich K, Islam M, Mathiasen ML, Aggleton JP, O'Mara SM (2019) Proximal perimeter encoding in the rat rostral thalamus. Sci Rep 9:2865. 10.1038/s41598-019-39396-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaiel AM, Abe ET, Niell CM (2020) Dynamics of gaze control during prey capture in freely moving mice. Elife 9:e57458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser EI, Kropff E, Moser MB (2008) Place cells, grid cells, and the brain's spatial representation system. Annu Rev Neurosci 31:69–89. 10.1146/annurev.neuro.31.061307.090723 [DOI] [PubMed] [Google Scholar]
- Nili H, Wingfield C, Walther A, Su L, Marslen-Wilson W, Kriegeskorte N (2014) A toolbox for representational similarity analysis. PLoS Comput Biol 10:e1003553. 10.1371/journal.pcbi.1003553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel JP, Balzani E, Avila E, Lakshminarasimhan K, Bruni S, Alefantis P, Savin C, Angelaki DE (2022) Coding of latent variables in sensory, parietal, and frontal cortices during virtual closed-loop navigation. bioRxiv 465526. 10.1101/2021.10.22.465526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J (1976) Place units in the hippocampus of the freely moving rat. Exp Neurol 51:78–109. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Burgess N (1996) Geometric determinants of the place fields of hippocampal neurons. Nature 381:425–428. 10.1038/381425a0 [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J (1971) The hippocampus as a spatial map: preliminary evidence from unit activity in the freely-moving rat. Brain Res 34:171–175. 10.1016/0006-8993(71)90358-1 [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Krupic J (2021) Do hippocampal pyramidal cells respond to nonspatial stimuli? Physiol Rev 101:1427–1456. 10.1152/physrev.00014.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L (1978) The hippocampus as a cognitive map. Oxford: Oxford UP. [Google Scholar]
- Padoa-Schioppa C, Assad JA (2006) Neurons in the orbitofrontal cortex encode economic value. Nature 441:223–226. 10.1038/nature04676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piray P, Daw ND (2021) Linear reinforcement learning in planning, grid fields, and cognitive control. Nat Commun 12:4942. 10.1038/s41467-021-25123-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polti I, Nau M, Kaplan R, van Wassenhove V, Doeller CF (2022) Rapid encoding of task regularities in the human hippocampus guides sensorimotor timing. BioRxiv 454928. 10.1101/2021.08.03.454928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poo C, Agarwal G, Bonacchi N, Mainen ZF (2022) Spatial maps in piriform cortex during olfactory navigation. Nature 601:595–599. 10.1038/s41586-021-04242-3 [DOI] [PubMed] [Google Scholar]
- Popal H, Wang Y, Olson IR (2019) A guide to representational similarity analysis for social neuroscience. Soc Cogn Affect Neurosci 14:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riceberg JS, Srinivasan A, Guise KG, Shapiro ML (2022) Hippocampal signals modify orbitofrontal representations to learn new paths. Curr Biol 32:3407–3413.e6. 10.1016/j.cub.2022.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch MR, Taylor AR, Schoenbaum G (2006) Encoding of time-discounted rewards in orbitofrontal cortex is independent of value representation. Neuron 51:509–520. 10.1016/j.neuron.2006.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondi-Reig L, Paradis AL, Lefort JM, Babayan BM, Tobin C (2014) How the cerebellum may monitor sensory information for spatial representation. Front Syst Neurosci 8:205. 10.3389/fnsys.2014.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem AB, Diamanti EM, Fournier J, Harris KD, Carandini M (2018) Coherent encoding of subjective spatial position in visual cortex and hippocampus. Nature 562:124–127. 10.1038/s41586-018-0516-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer JF, Folschweiller S, Bartos M (2022) Topographically organized representation of space and context in the medial prefrontal cortex. Proc Natl Acad Sci USA 119:569–571. 10.1073/pnas.2117300119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli F, Yoganarasimha D, Knierim JJ (2008) Influence of boundary removal on the spatial representations of the medial entorhinal cortex. Hippocampus 18:1270–1282. 10.1002/hipo.20511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitzer-Torbert NC, Redish AD (2008) Task-dependent encoding of space and events by striatal neurons is dependent on neural subtype. Neuroscience 153:349–360. 10.1016/j.neuroscience.2008.01.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrimpf M, Kubilius J, Lee MJ, Murty NA, Ajemian R, DiCarlo JJ (2020) Integrative benchmarking to advance neurally mechanistic models of human intelligence. Neuron 108:413–423. 10.1016/j.neuron.2020.07.040 [DOI] [PubMed] [Google Scholar]
- Shadlen MN, Shohamy D (2016) Decision making and sequential sampling from memory. Neuron 90:927–939. 10.1016/j.neuron.2016.04.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ML, Riceberg JS, Seip-Cammack K, Guise KG (2014) Functional interactions of prefrontal cortex and the hippocampus in learning and memory. In: Space, time and memory in the hippocampal formation, pp 517–560. Vienna: Springer. [Google Scholar]
- Sharp PE, Blair HT, Cho J (2001) The anatomical and computational basis of the rat head-direction cell signal. Trends Neurosci 24:289–294. 10.1016/S0166-2236(00)01797-5 [DOI] [PubMed] [Google Scholar]
- Shinder ME, Taube JS (2011) Active and passive movement are encoded equally by head direction cells in the anterodorsal thalamus. J Neurophysiol 106:788–800. 10.1152/jn.01098.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Wilson MA, Barnes CA (1996) Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus 6:149–172. [DOI] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI (2008) Representation of geometric borders in the entorhinal cortex. Science 322:1865–1868. 10.1126/science.1166466 [DOI] [PubMed] [Google Scholar]
- Sosa M, Giocomo LM (2021) Navigating for reward. Nat Rev Neurosci 22:472–487. 10.1038/s41583-021-00479-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachenfeld KL, Botvinick MM, Gershman SJ (2017) The hippocampus as a predictive map. Nat Neurosci 20:1643–1653. 10.1038/nn.4650 [DOI] [PubMed] [Google Scholar]
- Stackman RW, Taube JS (1997) Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J Neurosci 17:4349–4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensola H, Stensola T, Solstad T, Frøland K, Moser MB, Moser EI (2012) The entorhinal grid map is discretized. Nature 492:72–78. 10.1038/nature11649 [DOI] [PubMed] [Google Scholar]
- Strait CE, Sleezer BJ, Blanchard TC, Azab H, Castagno MD, Hayden BY (2016) Neuronal selectivity for spatial positions of offers and choices in five reward regions. J Neurophysiol 115:1098–1111. 10.1152/jn.00325.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Shin JD, Jadhav SP (2021) Multiple time-scales of decision-making in the hippocampus and prefrontal cortex. Elife 10:e66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS (1995) Head direction cells recorded in the anterior thalamic nuclei of freely moving rats. J Neurosci 15:70–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB (1990) Head-direction cells recorded from the postsubiculum in freely moving rats: I. Description and quantitative analysis. J Neurosci 10:420–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingley D, Buzsáki G (2018) Transformation of a spatial map across the hippocampal-lateral septal circuit. Neuron 98:1229–1242.e5. 10.1016/j.neuron.2018.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es DM, van der Zwaag W, Knapen T (2019) Topographic maps of visual space in the human cerebellum. Curr Biol 29:1689–1694.e3. 10.1016/j.cub.2019.04.012 [DOI] [PubMed] [Google Scholar]
- Wallis JD (2007) Orbitofrontal cortex and its contribution to decision-making. Annu Rev Neurosci 30:31–56. 10.1146/annurev.neuro.30.051606.094334 [DOI] [PubMed] [Google Scholar]
- Wang C, Chen X, Lee H, Deshmukh SS, Yoganarasimha D, Savelli F, Knierim JJ (2018) Egocentric coding of external items in the lateral entorhinal cortex. Science 362:945–949. 10.1126/science.aau4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington JC, Muller TH, Mark S, Chen G, Barry C, Burgess N, Behrens TE (2020) The Tolman-Eichenbaum Machine: unifying space and relational memory through generalization in the hippocampal formation. Cell 183:1249–1263.e23. 10.1016/j.cell.2020.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener SI (1993) Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. J Neurosci 13:3802–3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Redish AD (2015) Decoding the cognitive map: ensemble hippocampal sequences and decision making. Curr Opin Neurobiol 32:8–15. 10.1016/j.conb.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Schoenbaum G (2016) Over the river, through the woods: cognitive maps in the hippocampus and orbitofrontal cortex. Nat Rev Neurosci 17:513–523. 10.1038/nrn.2016.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Marrero-Garcia Y, Schoenbaum G (2017) Suppression of ventral hippocampal output impairs integrated orbitofrontal encoding of task structure. Neuron 95:1197–1207.e3. 10.1016/j.neuron.2017.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikenheiser AM, Gardner MP, Mueller LE, Schoenbaum G (2021) Spatial representations in rat orbitofrontal cortex. J Neurosci 41:6933–6945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SB, Sleezer BJ, Hayden BY (2018) Robust encoding of spatial information in orbitofrontal cortex and striatum. J Cogn Neurosci 30:898–913. 10.1162/jocn_a_01259 [DOI] [PubMed] [Google Scholar]
- Zhu S, Lakshminarasimhan KJ, Arfaei N, Angelaki DE (2022) Eye movements reveal spatiotemporal dynamics of visually-informed planning in navigation. Elife 11:e73097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski MC, Shin JD, Jadhav SP (2019) Coherent coding of spatial position mediated by theta oscillations in the hippocampus and prefrontal cortex. J Neurosci 39:4550–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]


