Abstract
Prefrontal cortex (PFC) inhibitory microcircuits regulate the gain and timing of pyramidal neuron firing, coordinate neural ensemble interactions, and gate local and long-range neural communication to support adaptive cognition and contextually tuned behavior. Accordingly, perturbations of PFC inhibitory microcircuits are thought to underlie dysregulated cognition and behavior in numerous psychiatric diseases and relevant animal models. This review, based on a Mini-Symposium presented at the 2022 Society for Neuroscience Meeting, highlights recent studies providing novel insights into: (1) discrete medial PFC (mPFC) interneuron populations in the mouse brain; (2) mPFC interneuron connections with, and regulation of, long-range mPFC afferents; and (3) circuit-specific plasticity of mPFC interneurons. The contributions of such populations, pathways, and plasticity to rodent cognition are discussed in the context of stress, reward, motivational conflict, and genetic mutations relevant to psychiatric disease.
Keywords: inhibitory neurons, prefrontal cortex, plasticity, microcircuits, cognition
Introduction
The PFC coordinates neural communication across expansive brain networks to facilitate high-order cognitive functions. Complex inhibitory microcircuits within PFC, comprised of its many GABAergic interneurons and their interconnections, dynamically gate and integrate neural input from distal brain regions to support contextually tuned behaviors (Miller and Cohen, 2001). Unsurprisingly, disruptions to these microcircuits have been implicated in a wide array of neuropsychiatric disorders, including schizophrenia, autism spectrum disorder, and depression (Dienel and Lewis, 2019; Fogaça and Duman, 2019; Yan and Rein, 2022). Therefore, characterization of the rich diversity of the interneurons that comprise PFC microcircuits, their synaptic connectivity, and the plastic nature of this connectivity stand to inform the neural basis of typical and disordered cognition. Here, we briefly review recent advances in the parsing of rodent mPFC interneurons into molecularly, anatomically, and functionally defined subpopulations. We also describe newly uncovered complexity in the synaptic connections between mPFC interneurons and their distal inputs, and novel mechanisms of plasticity and neuromodulation that regulate this long-range synaptic connectivity. How these distinct cell types, circuits, and circuit adaptations guide rodent cognitive functions is discussed in relation to stressful and rewarding experiences, motivational conflict, and disease-relevant genetic insults.
Prefrontal interneuron populations
The rodent mPFC harbors a network of inhibitory interneurons interspersed among glutamatergic pyramidal neurons. Despite their vast heterogeneity stemming from differences in developmental origin, genetic profile, morphology, connectivity, and functional properties (DeFelipe et al., 2013; Kepecs and Fishell, 2014), nearly all mPFC interneurons can be broadly (albeit imperfectly) classified based on their expression of parvalbumin (PV), somatostatin (SST), or the 5HT3a serotonin receptor (Rudy et al., 2011; Tremblay et al., 2016). PV-INs, the most abundant of these interneuron subtypes, exert potent inhibitory control over pyramidal neurons by synapsing onto their somatic and perisomatic compartments (Kepecs and Fishell, 2014). SST-INs are less abundant and tend to innervate the distal dendrites of pyramidal neurons (Kepecs and Fishell, 2014), positioning them to gate the influence of local and long-range inputs. Recent work indicates that mPFC SST-INs can also disinhibit pyramidal neurons through their monosynaptic inhibition of neighboring PV-INs (Xu et al., 2019; Cummings and Clem, 2020; Jiang et al., 2021). Interneurons that express vasoactive intestinal polypeptide (VIP-INs) represent the largest group of 5HT3a-positive interneurons (Rudy et al., 2011; Tremblay et al., 2016). VIP-INs are the canonical “disinhibitors”: in mPFC and neocortex broadly, VIP-INs inhibit SST-INs and to a lesser extent PV-INs to promote pyramidal neuron activity (Pi et al., 2013). Importantly, the foundational studies examining interneuron specializations were conducted in sensory cortices (Rudy et al., 2011; Tremblay et al., 2016). While recent work indicates that interneuron classes are broadly conserved across neocortex (e.g., Tasic et al., 2018), specific classifications and properties of mPFC interneurons are likely to differ from those of sensory regions (e.g., Whissell et al., 2015; Y. Kim et al., 2017).
Novel molecularly and functionally defined prefrontal interneuron subpopulations
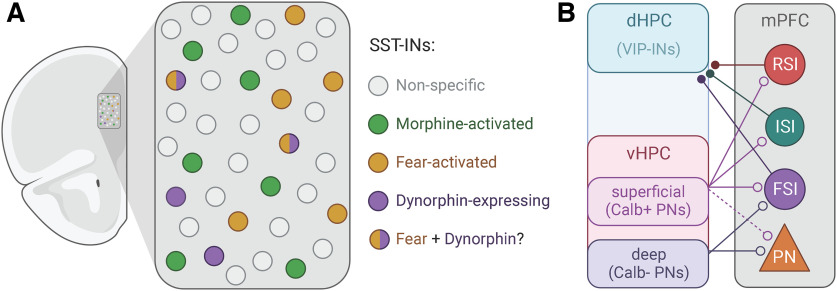
Within these heterogeneous populations are interneuron subclasses, each with unique anatomical connectivity, physiological properties, and behavioral contributions, that are defined by expression of additional proteins and neuropeptides, such as, calretinin, (Saffari et al., 2019), cholecystokinin (CCK) (Nguyen et al., 2020), and corticotropin-releasing factor (P. Chen et al., 2020; see also Tasic et al., 2018; Yao et al., 2021). Among these is a novel subclass of interneurons expressing prodynorphin (PDyn)-derived peptides, including dynorphins (Dyn). In mPFC, PDyn is expressed in pyramidal neurons and a subset of SST-INs (Sohn et al., 2014; ACNP 60th Annual Meeting, 2021). Tejeda and colleagues have recently demonstrated that Dyn+ SST-INs, which comprise ∼10% of SST-INs, are localized to deeper mPFC layers relative to Dyn-lacking SST-INs (Fig. 1A) (ACNP 60th Annual Meeting, 2021). Using intersectional viral and genetic approaches, Tejeda and colleagues further demonstrated that Dyn+ SST-INs are heavily activated by footshocks and footshock-predictive cues (ACNP 60th Annual Meeting, 2021). Interestingly, Dyn+ SST-INs immediately adapted their activity on the first omission of the shock during threat extinction procedures by switching to inhibitory responses during the shock-predictive cue. In contrast, Dyn-lacking SST-INs were activated by footshocks but showed little response to footshock-predictive cues during associative learning or inhibitory responses during threat extinction, consistent with the notion that Dyn+ SST-INs represent a distinct subtype of SST-IN.
Figure 1.
A, Schematic represents distinct and overlapping populations of SST-INs in mPFC (ACNP 60th Annual Meeting, 2021; Cummings et al., 2022). B, Schematic represents simplified connectivity motifs between superficial (Calb+) and deep (Calb-lacking) pyramidal neurons in vHPC with downstream mPFC neuron types (Sánchez-Bellot et al., 2022), and between long-range GABAergic neurons in mPFC with VIP-INs in dHPC (Malik et al., 2022). SST-INs, somatostatin-positive interneurons; dHPC, dorsal hippocampus; mPFC, medial prefrontal cortex; PN, pyramidal neurons; FSI, fast-spiking interneurons; ISI, irregular-spiking interneurons; RSI, regular-spiking interneurons. Created with www.BioRender.com.
Supplementing these and other genetic approaches to dissecting novel interneuron subclasses and their behavioral contributions (Ma et al., 2006; He et al., 2016) is new work that seeks to characterize experientially and behaviorally defined interneuron populations (Cummings et al., 2021). In a recent study, Cummings et al. (2022) developed a novel intersectional viral, transgenic, and activity-dependent tagging strategy to gain genetic access to mPFC SST-INs that are activated in response to an experimental manipulation. With this approach, the authors were able to tag, manipulate, and functionally interrogate populations of mPFC SST-INs activated by auditory fear conditioning or morphine administration (Fig. 1A). Doing so, they found that fear-activated SST-INs, which represented ∼30% of all prelimbic mPFC SST-INs, were selectively reactivated during memory retrieval and were necessary and sufficient for the expression of cued fear. Fear-tagged SST-INs also exhibited unique circuit properties, including greater inhibitory drive onto PV-INs and non–fear-tagged pyramidal neurons. Moreover, morphine treatment recruited a non-overlapping population of SST-INs that promoted motivational reward behaviors and opposed fear memory expression. Furthermore, optogenetic activation of fear- and morphine-responsive SST-INs recruited distributed brain networks related to fear and reward processing, respectively (Cummings et al., 2022).
Long-range GABAergic prefrontal projection neurons
The vast majority of mPFC GABAergic neurons project locally and play crucial roles in local microcircuit computations and input-output transformations. In contrast, a small fraction of mPFC GABAergic neurons send axons to remote cortical and subcortical brain regions, thus forming long-range GABAergic projections (Lee et al., 2014; Tomioka et al., 2015; Malik et al., 2022). Although the existence of sparse long-range GABAergic projection neurons in hippocampus (Sik et al., 1994; Jinno, 2009) and sensorimotor cortices (Tamamaki and Tomioka, 2010; Melzer and Monyer, 2020) has been known for over a decade, we are only now beginning to understand the organization and function of prefrontal long-range GABAergic projections.
The first evidence of long-range GABAergic projection neurons in the mPFC came from Lee et al. (2014), who described the properties and function of mPFC GABAergic projections to the nucleus accumbens, a key subcortical node for reward and aversion circuits in the brain. The authors demonstrated that optogenetic activation of this molecularly heterogeneous population of accumbens-projecting long-range GABAergic neurons enhanced aversion behavior. This and subsequent studies have revealed axon collaterals of mPFC long-range GABAergic neurons in multiple subcortical structures, such as the BLA, claustrum, striatum, and VTA (Tomioka et al., 2015).
The recent discovery of long-range GABAergic neurons from the mPFC to the dorsal hippocampus (dHPC) by Malik et al. (2022) has disrupted long-held assumptions about the neural pathways supporting mPFC-HPC communication and the nature of this top-down information flow. Indeed, it was widely assumed that the mPFC transmits information to the dHPC near exclusively via indirect excitatory connections with the thalamic nucleus reuniens (Vertes et al., 2007). Enriching this understanding, Malik et al. (2022) demonstrated that monosynaptic projections from a molecularly and physiologically mixed population of GABAergic neurons in the mPFC preferentially inhibit dHPC VIP-INs (Fig. 1B). As in mPFC and cortex generally, VIP-INs disinhibit hippocampal microcircuits (Acsády et al., 1996); thus, stimulating mPFC-dHPC long-range GABAergic projections increased dHPC feedforward inhibition (a mechanism capable of enhancing the “signal-to-noise ratio” of select excitatory neural pathways/ensembles) (Buzsáki, 1984) and enhanced object-related dHPC spatial encoding of objects in the environment (Malik et al., 2022). Accordingly, activating or inhibiting long-range GABAergic projections enhanced or suppressed object exploration, respectively.
Projection-specific targeting of prefrontal interneurons
mPFC receives long-range excitatory inputs from across the brain, including contralateral mPFC, thalamus, BLA, and ventral hippocampus (vHPC) (Anastasiades and Carter, 2021). Each of these projections is proposed to support unique functional roles during behavior (Sierra-Mercado et al., 2011). For example, BLA inputs are thought to support the learning, expression, and updating of affective associations (e.g., aversive threat memory) (Sotres-Bayon et al., 2012; Janak and Tye, 2015), whereas vHPC inputs are thought to convey context, or state information on which associations can be formed (Gershman et al., 2010; Maren et al., 2013; Marek et al., 2018).
Differential targeting of prefrontal interneurons by inputs from widespread brain regions
Although much evidence for the function of long-range inputs into mPFC has focused on their interactions with pyramidal neurons, a key means by which these long-range inputs influence mPFC circuitry is via dense and specific connectivity with local interneurons (Anastasiades and Carter, 2021). Anatomical studies using anterograde and retrograde tracing techniques suggest that all interneuron types in mPFC receive glutamatergic inputs by long-range sources (Ährlund-Richter et al., 2019; Q. Sun et al., 2019). Accordingly, electrophysiological recordings have shown that long-range inputs form excitatory synaptic connections with mPFC PV-, SST-, and VIP-INs, albeit in different layers that correspond to the biased laminar positioning of each interneuron type (Delevich et al., 2015; McGarry and Carter, 2016; Marek et al., 2018; Lee et al., 2019; Anastasiades et al., 2021). However, researchers have identified notable preferential targeting of interneuron types by different long-range inputs. For example, contralateral mPFC inputs preferentially target PV+ chandelier cells in layer 2/3 (Lu et al., 2017), whereas vHPC inputs show robust targeting of layer 5 CCK-INs (Liu et al., 2020) and layer 2/3 VIP-INs (Lee et al., 2019). Similarly, inputs from mediodorsal thalamus target a specific class of layer 1 VIP-INs, whereas inputs from ventromedial thalamus show biased targeting of apical tuft-targeting, neuron-derived neurotrophic factor-expressing interneurons (Collins et al., 2018; Anastasiades et al., 2021). This network of generalized and specific targeting of different interneuron types provides the foundation needed for contextually tuned mPFC computations.
Differential targeting of prefrontal interneurons by intermingled long-range inputs
The complex innervation of mPFC interneuron types is accompanied by marked heterogeneity of afferent projection neurons. Indeed, regions that provide mPFC afferents, such as the hippocampus (Cembrowski et al., 2016, 2018a, 2018b; Gergues et al., 2020), thalamus (C. Gao et al., 2020), contralateral mPFC (Murugan et al., 2017), and BLA (J. Kim et al., 2016), are composed of intermingled populations of genetically distinct neurons that often have opposing function during behavior.
Recent work characterized important structural and functional heterogeneity within vHPC inputs to the mPFC (Sánchez-Bellot et al., 2022). Consistent with a previous study showing at least two molecularly distinct populations of mPFC-projecting vHPC neurons (Cembrowski et al., 2018a), Sánchez-Bellot et al. (2022) found two populations of mPFC-projecting neurons in vHPC that were differentiated by their expression of Calbindin1 (Calb1), their position along the radial axis of the vHPC pyramidal neuron layer (deep and superficial), and their biased targeting of mPFC cell types. Calb1+ vHPC input preferentially targeted adapting mPFC interneurons (corresponding to CCK-INs and SST-INs), whereas Calb1-lacking vHPC input preferentially targeted pyramidal neurons and fast-spiking interneurons (corresponding to PV+ basket cells; Fig. 1B). Thus, these parallel vHPC-mPFC pathways are well placed to control the balance of feedforward inhibition onto pyramidal neuron dendrites and somas. Sánchez-Bellot et al. (2022) further showed that the parallel inputs from vHPC had distinct activity during, and control over, exploratory behavior. Calb1+ input was preferentially active on entry to the open arms of the elevated plus maze, whereas Calb1-lacking input was active on entry to the closed arms. Consistent with this opposing activity in the two pathways and known behavioral effects of directly manipulating the mPFC populations they target (Soumier and Sibille, 2014; Canetta et al., 2016; Berg et al., 2019), activation of Calb1+ input promoted exploration of the open arms, whereas activation of Calb1-lacking input reduced open arm exploration.
Prefrontal interneuron plasticity
Plastic changes of interneuron structure and function, whether through disease-relevant genetic insult, exogenous neuronal activation, receptor modulation, or experience-induced alterations, are poised to remodel computations within mPFC, and the routing of circuit-specific information through mPFC, to sculpt behavior. Numerous interneuron adaptations have been reported following each of these types of manipulations, manifesting as changes to mesoscopic and microscopic interneuron structure (e.g., Boksa et al., 2016; Al-Absi et al., 2020; Gildawie et al., 2020; Bueno-Fernandez et al., 2021), protein expression (e.g., Stedehouder et al., 2018; Mukherjee et al., 2019; Reichelt et al., 2021), and intrinsic physiology (e.g., Campanac and Hoffman, 2013; Dao et al., 2020; Zorrilla de San Martin et al., 2020). Here, we highlight some recent advances in our understanding of how plasticity in the synaptic and circuit connectivity of mPFC interneurons can reshape typical and disordered mPFC function and associated behavior (see also Yang et al., 2021).
Genetic insult-induced prefrontal interneuron plasticity
Rodent models bearing genetic mutations relevant to psychiatric diseases, such as schizophrenia and autism spectrum disorder, have deepened our understanding of how specific disease-related genes regulate prefrontal interneuron structure and function. Indeed, a wealth of data now links the pathophysiology of these neurodevelopmental disorders with impaired local and long-range synaptic connections with mPFC inhibitory microcircuits (e.g., Cho et al., 2015; Vogt et al., 2015; Selimbeyoglu et al., 2017; Delevich et al., 2020).
Animal models for the study of autism spectrum disorder present particularly robust links between specific gene disruptions and mPFC interneuron synaptic dysfunction. For example, a recent study showed that mice lacking one copy of the Pogz gene, which is involved in chromatin regulation and strongly linked with autism (Stessman et al., 2016), exhibit abnormal anxiety-related avoidance, impaired oscillatory synchrony between the vHPC and mPFC, and deficits in hippocampal excitatory input to fast-spiking (putative PV+) mPFC interneurons (Cunniff et al., 2020). Further support for autism-related microcircuit adaptations come from mice modeling the 16p11.2 duplication syndrome (Weiss et al., 2008), which show deficient GABAergic synaptic transmission and concurrent hyperexcitability in mPFC pyramidal neurons, as well as social and cognitive deficits. All of these phenotypes were rescued by restoring expression of the GABA synapse regulator, Npas4 (Rein et al., 2021). Likewise, a loss-of-function mutation in the autism-associated Shank3 gene (Durand et al., 2007) was recently shown to reduce dendritic inhibition onto mPFC pyramidal neurons via decreased NMDAR currents in, and reduced firing of, SST-INs (Ali et al., 2021). Strikingly, selective deletion of Shank3 from only BLA-projecting mPFC pyramidal neurons resulted in reduced inhibitory transmission onto these cells and reduced sociability (S. Kim et al., 2022). Together, these and many other studies are helping to inform the causal links between disease-relevant genetic insults, microcircuit connectivity, and behavior.
Activity-induced interneuron plasticity
Numerous studies have described long-term synaptic plasticity at long-range connections with the rodent mPFC following brain stimulation (e.g., Laroche et al., 1990; Takita et al., 1999; Maroun and Richter-Levin, 2003). In particular, connectivity between vHPC and mPFC has been demonstrated to be highly plastic, and this plasticity has been linked with cognitive function and disease-relevant dysfunction (e.g., Jay et al., 2004; Baudin et al., 2012; Tripathi et al., 2020; Park et al., 2021). Although mPFC interneurons have been implicated in gating some forms of activity-induced vHPC-mPFC plasticity (Caballero et al., 2014; Alvarez et al., 2020), plastic changes at vHPC and other long-range inputs to mPFC interneurons themselves are vastly unexplored (Lu et al., 2007; Sarihi et al., 2008; H. X. Chen et al., 2009). Further, given roles for mPFC interneurons in cognition-relevant functional connectivity between vHPC and mPFC (Abbas et al., 2018; Lee et al., 2019), and vHPC-mPFC dysconnectivity in models relevant to psychiatric disease (Mukai et al., 2015; Tamura et al., 2016; Song et al., 2022), it is important to understand how vHPC inputs interact with mPFC inhibitory microcircuits, whether these interactions are disrupted in disease-relevant models, and whether these interactions are plastic, offering a potential path to correcting circuit dysconnectivity (Kupferschmidt and Gordon, 2022).
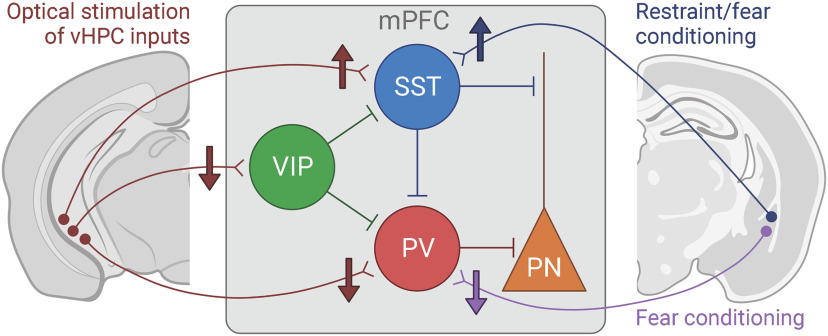
To these ends, Clarity and colleagues used an all-optical approach to characterize in vivo dynamics and activity-induced plasticity of discrete mPFC interneuron population responses to vHPC input stimulation (Kupferschmidt et al., 2022). In wildtype and Df(16)A+/– mice that model the schizophrenia-predisposing 22q11.2 deletion syndrome, vHPC inputs were optogenetically stimulated and postsynaptic Ca2+ responses in mPFC SST-, VIP-, and PV-INs were monitored with fiber photometry. SST-IN responses to vHPC terminal stimulation were weak at baseline in wildtype and Df(16)A+/– mice but progressively increased over 50 d of minimal, periodic optogenetic stimulation (Fig. 2). The potentiation of SST-IN Ca2+ responses was blunted in Df(16)A+/– relative to wildtype mice, and partially recovered with additional high-frequency optical vHPC input stimulation. In contrast, VIP- and PV-IN responses to vHPC input stimulation were initially strong but rapidly suppressed in wildtype and Df(16)A+/– mice that received additional high-frequency optical stimulation. By reshaping the recruitment of mPFC interneurons by long-range inputs, these forms of plasticity and others like them stand to bias the routing of pathway-specific information through mPFC and may be leveraged to influence cognition-relevant circuit function and dysfunction.
Figure 2.
Schematic represents simplified long-range connectivity changes following activity-induced plasticity or stress/fear-associated experience. Left, Cell type-specific changes in in vivo functional connectivity between vHPC inputs and mPFC interneuron population types following repeated optical stimulation of vHPC inputs to mPFC (Kupferschmidt et al., 2022). Right, Bidirectional changes to synaptic connectivity between BLA inputs and mPFC SST-INs versus PV-INs following exposure to restraint stress (Joffe et al., 2022) or fear conditioning (Cummings and Clem, 2020). vHPC, ventral hippocampus; mPFC. medial prefrontal cortex; SST, somatostatin; VIP, vasoactive intestinal polypeptide; PV, parvalbumin; PN, pyramidal neuron. Created with www.BioRender.com.
Modulation-induced interneuron plasticity
The mPFC is rich with neuromodulators capable of regulating the synaptic input, output, and intrinsic excitability of mPFC interneurons. Dopamine (e.g., W. J. Gao and Goldman-Rakic, 2003; Floresco and Tse, 2007; Tierney et al., 2008; Anastasiades et al., 2019), acetylcholine (e.g., Komal et al., 2015; Tikhonova et al., 2018; Maksymetz et al., 2019), serotonin (e.g., Puig et al., 2010; Zhong and Yan, 2011), norepinephrine (e.g., Wang et al., 2013; Luo et al., 2015), endocannabinoids (e.g., Younts and Castillo, 2014; Liu et al., 2020), and glutamate (e.g., H. Sun and Neugebauer, 2011; Maksymetz et al., 2021), as well as various neuropeptides (e.g., Nakajima et al., 2014; Aracri et al., 2015; Vollmer et al., 2016; Birdsong et al., 2019; Casello et al., 2022) are among the many agents that exert complex and interacting receptor-, cell-, and circuit-specific modulation of mPFC interneurons.
Dyn, through its actions on κ opioid receptors (KORs), is emerging as a potent modulator of mPFC interneuron circuit function and stress-related motivated behavior. Recent work by Tejeda and colleagues revealed that Dyn inhibits glutamate release from various KOR-expressing mPFC afferents (e.g., BLA, paraventricular nucleus of the thalamus, contralateral mPFC) onto pyramidal neurons (ACNP 60th Annual Meeting, 2021). Further, Dyn/KOR signaling differentially regulates BLA inputs onto mPFC interneurons (ACNP 60th Annual Meeting, 2021). Specifically, direct excitation of SST-INs by BLA inputs to the mPFC was inhibited by Dyn, an effect that was absent at BLA synapses innervating mPFC PV interneurons. These results suggest that Dyn/KOR signaling can filter excitatory inputs onto mPFC interneurons in a synapse-specific manner and reduce SST-IN-mediated feedforward inhibition of pyramidal neurons. Moreover, by inhibiting local GABA release from KOR-expressing SST- and PV-IN terminals, Dyn potently inhibited polysynaptic inhibition driven by incoming glutamatergic inputs, regardless of whether the inputs themselves express KORs. Together, these findings demonstrate that Dyn/KOR signaling is poised to directly suppress KOR-expressing excitatory inputs while concurrently amplifying mPFC engagement by KOR-lacking inputs via disinhibition (ACNP 60th Annual Meeting, 2021). This complex synaptic modulation by Dyn appears to have implications for behavior under threatening environmental conditions, as evidenced by in vivo Dyn release within mPFC in response to environmental threat, and impaired toggling between active and passive defense strategies following PDyn knockdown in the mPFC (ACNP 60th Annual Meeting, 2021).
Experience-induced interneuron plasticity
By engaging mechanisms of activity- and modulation-induced neural plasticity, salient experiences can trigger robust and persistent synaptic changes in mPFC. Although experience-induced plastic changes in synaptic physiology of mPFC pyramidal cells have been the subject of significant research, we are just beginning to appreciate how salient events and environmental factors shape synaptic function in mPFC interneurons (e.g., Canetta et al., 2016; Skorput and Yeh, 2016; Slaker et al., 2018).
Recent findings reveal that stressful experiences readily facilitate synaptic adaptations in mPFC interneurons. In separate studies, restraint stress (Joffe et al., 2022) and footshock conditioning (Cummings and Clem, 2020; ACNP 60th Annual Meeting, 2021) were shown to each enhance Ca2+ responses within SST-INs and potentiate excitatory transmission from the BLA onto mPFC SST-INs (Fig. 2). The potentiation induced by these aversive experiences appears selective to SST-INs, as excitatory transmission in PV-INs was unaltered by restraint stress (Joffe et al., 2022) and seemingly reduced following footshock conditioning (Perova et al., 2015; Cummings and Clem, 2020) (Fig. 2). Initial efforts to parse the mechanisms mediating this stress-induced potentiation of excitatory drive onto SST-INs have implicated postsynaptic mGlu5 metabotropic glutamate receptor signaling. Indeed, mice lacking mGlu5 receptors in SST-INs showed no mGlu1/5 agonist-induced LTP, no stress-induced increases in excitatory drive onto SST-INs (or corresponding increases in pyramidal cell inhibition), resilience to stress-induced deficits in spatial working memory task performance, and impaired cue-associated fear learning (Joffe et al., 2022).
Although stressful experiences can alter both synaptic transmission in mPFC inhibitory microcircuits and behavior, whether these synaptic alterations promote the encoding of experience-induced learning and behavioral adaptations is less clear. Early support for this more causal role comes from Cummings and Clem (2020), who showed that excitatory drive onto SST-INs was potentiated in mice that formed associative fear memories through paired footshock-tone presentations, but not in mice that received unpaired footshocks and tones. These data provide compelling evidence that persistent synaptic changes in mPFC SST-INs are not an unavoidable consequence of a stressful experience; rather, interneuron plasticity appears to instruct the formation of persistent memories (i.e., CS-US association) and future behaviors (i.e., conditioned freezing).
Further evidence for a causal link between experience-induced synaptic and behavioral adaptations comes from studies of PV-IN plasticity following stress and drug exposure. Perova et al. (2015) showed that male mice displaying a phenotype of “helplessness” following repeated footshocks (i.e., fewer escapes and longer escape latencies) showed reduced excitatory synaptic strength in mPFC PV-INs. In contrast, PV-IN synaptic strength was unaltered in “resilient” mice, despite undergoing an identical shock experience. A similar link was established by Ferranti et al. (2022) in a study of the neural adaptations encoding alcohol reward. By manipulating the timing of the same intoxicating dose of alcohol, the researchers conditioned mice to express either a place preference or aversion to the drug. Despite both groups of mice receiving identical alcohol exposure, only those that formed a rewarding alcohol association exhibited enhanced excitatory drive in PV-INs. Thus, bidirectional synaptic adaptations in mPFC interneurons may help encode specific behavioral adaptations to salient experience, rather than simply reflect a history of such experience.
Conclusions
In conclusion, we have highlighted some recent advances in our understanding of the rich diversity of mPFC interneuron subpopulations, the neural pathways they are embedded within and regulate, and the dynamic changes they undergo to remodel mPFC computations and distal network interactions to shape cognitive functions. That these interneurons and their connections are engaged and dysregulated by stress and psychoactive drug exposure, fear learning, motivational conflict, and disease-relevant genetic insults suggests their privileged contributions to various forms of disordered cognition. Importantly, while this mini-review did not segregate findings based on mPFC subregions, considerable regional variations in the anatomical, molecular, and functional properties of mPFC interneurons and their embedded circuits further complicate the study of interneuron contributions to typical and disordered cognition (Heidbreder and Groenewegen, 2003; Euston et al., 2012; Laubach et al., 2018; Anastasiades and Carter, 2021). Through continued innovation and democratization of transcriptomic sequencing, elaboration of intersectional genetic strategies, and expansion of the toolset to tag populations based on dynamic cellular processes, the next decade of neuroscience research will see remarkable advances in the parsing of these important cells into increasingly refined subclasses (Bugeon et al., 2022; Zeng, 2022). By pairing these advances with more sophisticated tools to monitor and manipulate interneuron populations, we stand to build a detailed guide to their interconnections, plasticity, and behavioral contributions. As tools emerge that enable synapse-specific manipulations (e.g., altered connectivity between defined presynaptic and postsynaptic elements) (Ransey et al., 2021; Prakash et al., 2022), we will similarly need innovation in behavioral analyses sensitive to the subtle effects these targeted manipulations may yield. Last, alongside efforts to identify and target molecules and synaptic processes unique to select cell types and circuits in the rodent brain, we must advance similar efforts in nonhuman primates with due consideration of the anatomical, functional, and behavioral homology across species.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Intramural Research Program ZIANS003168 to D.A.K.; National Institute of Mental Health R00 MH122228 to K.A.C.; National Institute on Alcohol Abuse and Alcoholism K99/R00 AA027806 to M.E.J.; National Institute of Mental Health R01 MH062646 to M.E.J.; National Institute of Neurological Disorders and Stroke R37 NS031373 to M.E.J.; Wellcome Trust Sir Henry Dale Fellowship 109360/Z/15/Z to A.M.; Brain and Behavior Research Foundation National Alliance for Research on Schizophrenia and Depression Young Investigator Grant to R.M. and H.A.T.; Department of Defense TSCRP 127574A to R.M.; Wellcome Trust 206074/Z/17/Z to C.S.-B.; National Institute of Mental Health Intramural Research Program ZIAMH002970 to H.A.T.; and NIH Center for Compulsive Behaviors Fellowship to H.Y.C. We thank all those in our laboratories who have contributed to the studies described.
The authors declare no competing financial interests.
References
- Abbas AI, Sundiang MJ, Henoch B, Morton MP, Bolkan SS, Park AJ, Harris AZ, Kellendonk C, Gordon JA (2018) Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron 100:926–939.e3. 10.1016/j.neuron.2018.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACNP 60th Annual Meeting (2021) Panels, mini-panels and study groups. Neuropsychopharmacology 46:1–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acsády L, Görcs TJ, Freund TF (1996) Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience 73:317–334. 10.1016/0306-4522(95)00609-5 [DOI] [PubMed] [Google Scholar]
- Ährlund-Richter S, Xuan Y, van Lunteren JA, Kim H, Ortiz C, Pollak Dorocic I, Meletis K, Carlén M (2019) A whole-brain atlas of monosynaptic input targeting four different cell types in the medial prefrontal cortex of the mouse. Nat Neurosci 22:657–668. 10.1038/s41593-019-0354-y [DOI] [PubMed] [Google Scholar]
- Al-Absi AR, Qvist P, Okujeni S, Khan AR, Glerup S, Sanchez C, Nyengaard JR (2020) Layers II/III of prefrontal cortex in Df(h22q11)/+ mouse model of the 22q11.2 deletion display loss of parvalbumin interneurons and modulation of neuronal morphology and excitability. Mol Neurobiol 57:4978–4988. 10.1007/s12035-020-02067-1 [DOI] [PubMed] [Google Scholar]
- Ali F, Shao LX, Gerhard DM, Sweasy K, Pothula S, Pittenger C, Duman RS, Kwan AC (2021) Inhibitory regulation of calcium transients in prefrontal dendritic spines is compromised by a nonsense Shank3 mutation. Mol Psychiatry 26:1945–1966. 10.1038/s41380-020-0708-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RJ, Pafundo DE, Zold CL, Belforte JE (2020) Interneuron NMDA receptor ablation induces hippocampus-prefrontal cortex functional hypoconnectivity after adolescence in a mouse model of schizophrenia. J Neurosci 40:3304–3317. 10.1523/JNEUROSCI.1897-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiades PG, Carter AG (2021) Circuit organization of the rodent medial prefrontal cortex. Trends Neurosci 44:550–563. 10.1016/j.tins.2021.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiades PG, Boada C, Carter AG (2019) Cell-type-specific D1 dopamine receptor modulation of projection neurons and interneurons in the prefrontal cortex. Cereb Cortex 29:3224–3242. 10.1093/cercor/bhy299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiades PG, Collins DP, Carter AG (2021) Mediodorsal and ventromedial thalamus engage distinct L1 circuits in the prefrontal cortex. Neuron 109:314–330.e4. 10.1016/j.neuron.2020.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aracri P, Banfi D, Pasini ME, Amadeo A, Becchetti A (2015) Hypocretin (orexin) regulates glutamate input to fast-spiking interneurons in layer V of the Fr2 region of the murine prefrontal cortex. Cereb Cortex 25:1330–1347. 10.1093/cercor/bht326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin A, Blot K, Verney C, Estevez L, Santamaria J, Gressens P, Giros B, Otani S, Daugé V, Naudon L (2012) Maternal deprivation induces deficits in temporal memory and cognitive flexibility and exaggerates synaptic plasticity in the rat medial prefrontal cortex. Neurobiol Learn Mem 98:207–214. 10.1016/j.nlm.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Berg L, Eckardt J, Masseck OA (2019) Enhanced activity of pyramidal neurons in the infralimbic cortex drives anxiety behavior. PLoS One 14:e0210949. 10.1371/journal.pone.0210949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birdsong WT, Jongbloets BC, Engeln KA, Wang D, Scherrer G, Mao T (2019) Synapse-specific opioid modulation of thalamo-cortico-striatal circuits. Elife 8:e45146. 10.7554/eLife.45146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P, Zhang Y, Nouel D, Wong A, Wong TP (2016) Early development of parvalbumin-, somatostatin-, and cholecystokinin-expressing neurons in rat brain following prenatal immune activation and maternal iron deficiency. Dev Neurosci 38:342–353. 10.1159/000454677 [DOI] [PubMed] [Google Scholar]
- Bueno-Fernandez C, Perez-Rando M, Alcaide J, Coviello S, Sandi C, Castillo-Gómez E, Nacher J (2021) Long term effects of peripubertal stress on excitatory and inhibitory circuits in the prefrontal cortex of male and female mice. Neurobiol Stress 14:100322. 10.1016/j.ynstr.2021.100322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugeon S, Duffield J, Dipoppa M, Ritoux A, Prankerd I, Nicoloutsopoulos D, Orme D, Shinn M, Peng H, Forrest H, Viduolyte A, Reddy CB, Isogai Y, Carandini M, Harris KD (2022) A transcriptomic axis predicts state modulation of cortical interneurons. Nature 607:330–338. 10.1038/s41586-022-04915-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G (1984) Feed-forward inhibition in the hippocampal formation. Prog Neurobiol 22:131–153. [DOI] [PubMed] [Google Scholar]
- Caballero A, Thomases DR, Flores-Barrera E, Cass DK, Tseng KY (2014) Emergence of GABAergic-dependent regulation of input-specific plasticity in the adult rat prefrontal cortex during adolescence. Psychopharmacology (Berl) 231:1789–1796. 10.1007/s00213-013-3216-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanac E, Hoffman DA (2013) Repeated cocaine exposure increases fast-spiking interneuron excitability in the rat medial prefrontal cortex. J Neurophysiol 109:2781–2792. 10.1152/jn.00596.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canetta S, Bolkan S, Padilla-Coreano N, Song LJ, Sahn R, Harrison NL, Gordon JA, Brown A, Kellendonk C (2016) Maternal immune activation leads to selective functional deficits in offspring parvalbumin interneurons. Mol Psychiatry 21:956–968. 10.1038/mp.2015.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casello SM, Flores RJ, Yarur HE, Wang H, Awanyai M, Arenivar MA, Jaime-Lara RB, Bravo-Rivera H, Tejeda HA (2022) Neuropeptide system regulation of prefrontal cortex circuitry: implications for neuropsychiatric disorders. Front Neural Circuits 16:796443. 10.3389/fncir.2022.796443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cembrowski MS, Bachman JL, Wang L, Sugino K, Shields BC, Spruston N (2016) Spatial gene-expression gradients underlie prominent heterogeneity of CA1 pyramidal neurons. Neuron 89:351–368. 10.1016/j.neuron.2015.12.013 [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Phillips MG, DiLisio SF, Shields BC, Winnubst J, Chandrashekar J, Bas E, Spruston N (2018a) Dissociable structural and functional hippocampal outputs via distinct subiculum cell classes. Cell 173:1280–1292.e18. 10.1016/j.cell.2018.03.031 [DOI] [PubMed] [Google Scholar]
- Cembrowski MS, Wang L, Lemire AL, Copeland M, DiLisio SF, Clements J, Spruston N (2018b) The subiculum is a patchwork of discrete subregions. Elife 7:e37701. 10.7554/eLife.37701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HX, Jiang M, Akakin D, Roper SN (2009) Long-term potentiation of excitatory synapses on neocortical somatostatin-expressing interneurons. J Neurophysiol 102:3251–3259. 10.1152/jn.00641.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Lou S, Huang ZH, Wang Z, Shan QH, Wang Y, Yang Y, Li X, Gong H, Jin Y, Zhang Z, Zhou JN (2020) Prefrontal cortex corticotropin-releasing factor neurons control behavioral style selection under challenging situations. Neuron 106:301–315.e7. 10.1016/j.neuron.2020.01.033 [DOI] [PubMed] [Google Scholar]
- Cho KK, Hoch R, Lee AT, Patel T, Rubenstein JLR, Sohal VS (2015) Gamma rhythms link prefrontal interneuron dysfunction with cognitive inflexibility in Dlx5/6(+/-) mice. Neuron 85:1332–1343. 10.1016/j.neuron.2015.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DP, Anastasiades PG, Marlin JJ, Carter AG (2018) Reciprocal circuits linking the prefrontal cortex with dorsal and ventral thalamic nuclei. Neuron 98:366–379.e4. 10.1016/j.neuron.2018.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KA, Clem RL (2020) Prefrontal somatostatin interneurons encode fear memory. Nat Neurosci 23:61–74. 10.1038/s41593-019-0552-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KA, Lacagnina AF, Clem RL (2021) GABAergic microcircuitry of fear memory encoding. Neurobiol Learn Mem 184:107504. 10.1016/j.nlm.2021.107504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings KA, Bayshtok S, Dong TN, Kenny PJ, Clem RL (2022) Control of fear by discrete prefrontal GABAergic populations encoding valence-specific information. Neuron. 110:3036–3052.e5. 10.1016/j.neuron.2022.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunniff MM, Markenscoff-Papadimitriou E, Ostrowski J, Rubenstein JL, Sohal VS (2020) Altered hippocampal-prefrontal communication during anxiety-related avoidance in mice deficient for the autism-associated gene Pogz. Elife 9:e54835. 10.7554/eLife.54835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao NC, Suresh Nair M, Magee SN, Moyer JB, Sendao V, Brockway DF, Crowley NA (2020) Forced abstinence from alcohol induces sex-specific depression-like behavioral and neural adaptations in somatostatin neurons in cortical and amygdalar regions. Front Behav Neurosci 14:86. 10.3389/fnbeh.2020.00086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, et al. (2013) New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci 14:202–216. 10.1038/nrn3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevich K, Jaaro-Peled H, Penzo M, Sawa A, Li B (2020) Parvalbumin interneuron dysfunction in a thalamo-prefrontal cortical circuit in disc1 locus impairment mice. eNeuro 7:ENEURO.0496-19.2020. 10.1523/ENEURO.0496-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevich K, Tucciarone J, Huang ZJ, Li B (2015) The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. J Neurosci 35:5743–5753. 10.1523/JNEUROSCI.4565-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dienel SJ, Lewis DA (2019) Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiol Dis 131:104208. 10.1016/j.nbd.2018.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand CM, et al. (2007) Mutations in the gene encoding the synaptic scaffolding protein SHANK3 are associated with autism spectrum disorders. Nat Genet 39:25–27. 10.1038/ng1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euston DR, Gruber AJ, McNaughton BL (2012) The role of medial prefrontal cortex in memory and decision making. Neuron 76:1057–1070. 10.1016/j.neuron.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferranti AS, Johnson KA, Winder DG, Conn PJ, Joffe ME (2022) Prefrontal cortex parvalbumin interneurons exhibit decreased excitability and potentiated synaptic strength after ethanol reward learning. Alcohol 101:17–26. 10.1016/j.alcohol.2022.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Tse MT (2007) Dopaminergic regulation of inhibitory and excitatory transmission in the basolateral amygdala-prefrontal cortical pathway. J Neurosci 27:2045–2057. 10.1523/JNEUROSCI.5474-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça MV, Duman RS (2019) Cortical GABAergic dysfunction in stress and depression: new insights for therapeutic interventions. Front Cell Neurosci 13:87. 10.3389/fncel.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Leng Y, Ma J, Rooke V, Rodriguez-Gonzalez S, Ramakrishnan C, Deisseroth K, Penzo MA (2020) Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat Neurosci 23:217–228. 10.1038/s41593-019-0572-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao WJ, Goldman-Rakic PS (2003) Selective modulation of excitatory and inhibitory microcircuits by dopamine. Proc Natl Acad Sci USA 100:2836–2841. 10.1073/pnas.262796399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergues MM, Han KJ, Choi HS, Brown B, Clausing KJ, Turner VS, Vainchtein ID, Molofsky AV, Kheirbek MA (2020) Circuit and molecular architecture of a ventral hippocampal network. Nat Neurosci 23:1444–1452. 10.1038/s41593-020-0705-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershman SJ, Blei DM, Niv Y (2010) Context, learning, and extinction. Psychol Rev 117:197–209. 10.1037/a0017808 [DOI] [PubMed] [Google Scholar]
- Gildawie KR, Honeycutt JA, Brenhouse HC (2020) Region-specific effects of maternal separation on perineuronal net and parvalbumin-expressing interneuron formation in male and female rats. Neuroscience 428:23–37. 10.1016/j.neuroscience.2019.12.010 [DOI] [PubMed] [Google Scholar]
- He M, Tucciarone J, Lee S, Nigro MJ, Kim Y, Levine JM, Kelly SM, Krugikov I, Wu P, Chen Y, Gong L, Hou Y, Osten P, Rudy B, Huang ZJ (2016) Strategies and tools for combinatorial targeting of GABAergic neurons in mouse cerebral cortex. Neuron 91:1228–1243. 10.1016/j.neuron.2016.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidbreder CA, Groenewegen HJ (2003) The medial prefrontal cortex in the rat: evidence for a dorso-ventral distinction based upon functional and anatomical characteristics. Neurosci Biobehav Rev 27:555–579. 10.1016/j.neubiorev.2003.09.003 [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292. 10.1038/nature14188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Spedding M (2004) Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases. Neurotox Res 6:233–244. 10.1007/BF03033225 [DOI] [PubMed] [Google Scholar]
- Jiang C, Wang X, Le Q, Liu P, Liu C, Wang Z, He G, Zheng P, Wang F, Ma L (2021) Morphine coordinates SST and PV interneurons in the prelimbic cortex to disinhibit pyramidal neurons and enhance reward. Mol Psychiatry 26:1178–1193. 10.1038/s41380-019-0480-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S (2009) Structural organization of long-range GABAergic projection system of the hippocampus. Front Neuroanat 3:13. 10.3389/neuro.05.013.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe ME, Maksymetz J, Luschinger JR, Dogra S, Ferranti AS, Luessen DJ, Gallinger IM, Xiang Z, Branthwaite H, Melugin PR, Williford KM, Centanni SW, Shields BC, Lindsley CW, Calipari ES, Siciliano CA, Niswender CM, Tadross MR, Winder DG, Conn PJ (2022) Acute restraint stress redirects prefrontal cortex circuit function through mGlu5 receptor plasticity on somatostatin-expressing interneurons. Neuron 110:1068–1083.e5. 10.1016/j.neuron.2021.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G (2014) Interneuron cell types are fit to function. Nature 505:318–326. 10.1038/nature12983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Pignatelli M, Xu S, Itohara S, Tonegawa S (2016) Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci 19:1636–1646. 10.1038/nn.4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Kim YE, Song I, Ujihara Y, Kim N, Jiang YH, Yin HH, Lee TH, Kim IH (2022) Neural circuit pathology driven by Shank3 mutation disrupts social behaviors. Cell Rep 39:110906. 10.1016/j.celrep.2022.110906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Yang GR, Pradhan K, Venkataraju KU, Bota M, García Del Molino LC, Fitzgerald G, Ram K, He M, Levine JM, Mitra P, Huang ZJ, Wang XJ, Osten P (2017) Brain-wide maps reveal stereotyped cell-type-based cortical architecture and subcortical sexual dimorphism. Cell 171:456–469.e22. 10.1016/j.cell.2017.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komal P, Estakhr J, Kamran M, Renda A, Nashmi R (2015) cAMP-dependent protein kinase inhibits α7 nicotinic receptor activity in layer 1 cortical interneurons through activation of D1/D5 dopamine receptors. J Physiol 593:3513–3532. 10.1113/JP270469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Gordon JA (2022) Shaping long-range functional connectivity through prefrontal interneuron plasticity. Neuropsychopharmacology Advance online publication. Retrieved Aug 1, 2022. 10.1038/s41386-022-01395-1. 10.1038/s41386-022-01395-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Clarity T, Mikofsky R, Myroshnychenko M, Bowen-Kauth MS, Gilchrist K, Gordon J, Gordon J (2022) Divergent forms of in vivo plasticity between ventral hippocampal inputs and medial prefrontal cortex microcircuits in a mouse model of 22q11.2 deletion syndrome. Biol Psychiatry 91:S285–S286. 10.1016/j.biopsych.2022.02.723 [DOI] [Google Scholar]
- Laroche S, Jay TM, Thierry AM (1990) Long-term potentiation in the prefrontal cortex following stimulation of the hippocampal CA1/subicular region. Neurosci Lett 114:184–190. 10.1016/0304-3940(90)90069-L [DOI] [PubMed] [Google Scholar]
- Laubach M, Amarante LM, Swanson K, White SR (2018) What, if anything, is rodent prefrontal cortex? eNeuro 5:ENEURO.0315-18.2018. 10.1523/ENEURO.0315-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AT, Vogt D, Rubenstein JL, Sohal VS (2014) A class of GABAergic neurons in the prefrontal cortex sends long-range projections to the nucleus accumbens and elicits acute avoidance behavior. J Neurosci 34:11519–11525. 10.1523/JNEUROSCI.1157-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AT, Cunniff MM, See JZ, Wilke SA, Luongo FJ, Ellwood IT, Ponnavolu S, Sohal VS (2019) VIP interneurons contribute to avoidance behavior by regulating information flow across hippocampal-prefrontal networks. Neuron 102:1223–1234.e4. 10.1016/j.neuron.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dimidschstein J, Fishell G, Carter AG (2020) Hippocampal inputs engage CCK+ interneurons to mediate endocannabinoid-modulated feed-forward inhibition in the prefrontal cortex. Elife 9:e55267. 10.7554/eLife.55267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Li C, Zhao JP, Poo M, Zhang X (2007) Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J Neurosci 27:9711–9720. 10.1523/JNEUROSCI.2513-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Tucciarone J, Padilla-Coreano N, He M, Gordon JA, Huang ZJ (2017) Selective inhibitory control of pyramidal neuron ensembles and cortical subnetworks by chandelier cells. Nat Neurosci 20:1377–1383. 10.1038/nn.4624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo F, Tang H, Cheng ZY (2015) Stimulation of α1-adrenoceptors facilitates GABAergic transmission onto pyramidal neurons in the medial prefrontal cortex. Neuroscience 300:63–74. 10.1016/j.neuroscience.2015.04.070 [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A (2006) Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci 26:5069–5082. 10.1523/JNEUROSCI.0661-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymetz J, Joffe ME, Moran SP, Stansley BJ, Li B, Temple K, Engers DW, Lawrence JJ, Lindsley CW, Conn PJ (2019) M1 muscarinic receptors modulate fear-related inputs to the prefrontal cortex: implications for novel treatments of posttraumatic stress disorder. Biol Psychiatry 85:989–1000. 10.1016/j.biopsych.2019.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksymetz J, Byun NE, Luessen DJ, Li B, Barry RL, Gore JC, Niswender CM, Lindsley CW, Joffe ME, Conn PJ (2021) mGlu1 potentiation enhances prelimbic somatostatin interneuron activity to rescue schizophrenia-like physiological and cognitive deficits. Cell Rep 37:109950. 10.1016/j.celrep.2021.109950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Li Y, Schamiloglu S, Sohal VS (2022) Top-down control of hippocampal signal-to-noise by prefrontal long-range inhibition. Cell 185:1602–1617.e17. 10.1016/j.cell.2022.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Jin J, Goode TD, Giustino TF, Wang Q, Acca GM, Holehonnur R, Ploski JE, Fitzgerald PJ, Lynagh T, Lynch JW, Maren S, Sah P (2018) Hippocampus-driven feed-forward inhibition of the prefrontal cortex mediates relapse of extinguished fear. Nat Neurosci 21:384–392. 10.1038/s41593-018-0073-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I (2013) The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 14:417–428. 10.1038/nrn3492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G (2003) Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J Neurosci 23:4406–4409. 10.1523/JNEUROSCI.23-11-04406.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry LM, Carter AG (2016) Inhibitory gating of basolateral amygdala inputs to the prefrontal cortex. J Neurosci 36:9391–9406. 10.1523/JNEUROSCI.0874-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer S, Monyer H (2020) Diversity and function of corticopetal and corticofugal GABAergic projection neurons. Nat Rev Neurosci 21:499–515. 10.1038/s41583-020-0344-9 [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD (2001) An integrative theory of prefrontal cortex function. Annu Rev Neurosci 24:167–202. 10.1146/annurev.neuro.24.1.167 [DOI] [PubMed] [Google Scholar]
- Mukai J, Tamura M, Fénelon K, Rosen AM, Spellman TJ, Kang R, MacDermott AB, Karayiorgou M, Gordon JA, Gogos JA (2015) Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron 86:680–695. 10.1016/j.neuron.2015.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Carvalho F, Eliez S, Caroni P (2019) Long-lasting rescue of network and cognitive dysfunction in a genetic schizophrenia model. Cell 178:1387–1402.e14. 10.1016/j.cell.2019.07.023 [DOI] [PubMed] [Google Scholar]
- Murugan M, Jang HJ, Park M, Miller EM, Cox J, Taliaferro JP, Parker NF, Bhave V, Hur H, Liang Y, Nectow AR, Pillow JW, Witten IB (2017) Combined social and spatial coding in a descending projection from the prefrontal cortex. Cell 171:1663–1677.e16. 10.1016/j.cell.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Görlich A, Heintz N (2014) Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 159:295–305. 10.1016/j.cell.2014.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen R, Venkatesan S, Binko M, Bang JY, Cajanding JD, Briggs C, Sargin D, Imayoshi I, Lambe EK, Kim JC (2020) Cholecystokinin-expressing interneurons of the medial prefrontal cortex mediate working memory retrieval. J Neurosci 40:2314–2331. 10.1523/JNEUROSCI.1919-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park AJ, Harris AZ, Martyniuk KM, Chang CY, Abbas AI, Lowes DC, Kellendonk C, Gogos JA, Gordon JA (2021) Reset of hippocampal–prefrontal circuitry facilitates learning. Nature 591:615–619. 10.1038/s41586-021-03272-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perova Z, Delevich K, Li B (2015) Depression of excitatory synapses onto parvalbumin interneurons in the medial prefrontal cortex in susceptibility to stress. J Neurosci 35:3201–3206. 10.1523/JNEUROSCI.2670-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi HJ, Hangya B, Kvitsiani D, Sanders JI, Huang ZJ, Kepecs A (2013) Cortical interneurons that specialize in disinhibitory control. Nature 503:521–524. 10.1038/nature12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash M, Murphy J, St Laurent R, Friedman N, Crespo EL, Bjorefeldt A, Pal A, Bhagat Y, Kauer JA, Shaner NC, Lipscombe D, Moore CI, Hochgeschwender U (2022) Selective control of synaptically-connected circuit elements by all-optical synapses. Commun Biol 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Watakabe A, Ushimaru M, Yamamori T, Kawaguchi Y (2010) Serotonin modulates fast-spiking interneuron and synchronous activity in the rat prefrontal cortex through 5-HT1A and 5-HT2A receptors. J Neurosci 30:2211–2222. 10.1523/JNEUROSCI.3335-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransey E, Chesnov K, Wisdom E, Bowman R, Rodriguez T, Adamson E, Thomas GE, Almoril-Porras A, Schwennesen H, Colón-Ramos D, Hultman R, Bursac N, Dzirasa K (2021) Long-term precision editing of neural circuits using engineered gap junction hemichannels. bioRxiv. doi: 10.1101/2021.08.24.457429 10.1101/2021.08.24.457429. [DOI] [Google Scholar]
- Reichelt AC, Lemieux CA, Princz-Lebel O, Singh A, Bussey TJ, Saksida LM (2021) Age-dependent and region-specific alteration of parvalbumin neurons, perineuronal nets and microglia in the mouse prefrontal cortex and hippocampus following obesogenic diet consumption. Sci Rep 11:5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein B, Tan T, Yang F, Wang W, Williams J, Zhang F, Mills A, Yan Z (2021) Reversal of synaptic and behavioral deficits in a 16p11.2 duplication mouse model via restoration of the GABA synapse regulator Npas4. Mol Psychiatry 26:1967–1979. 10.1038/s41380-020-0693-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J (2011) Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71:45–61. 10.1002/dneu.20853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saffari R, Grotefeld K, Kravchenko M, Zhang M, Zhang W (2019) Calretinin+-neurons-mediated GABAergic inhibition in mouse prefrontal cortex. Prog Neuropsychopharmacol Biol Psychiatry 94:109658. 10.1016/j.pnpbp.2019.109658 [DOI] [PubMed] [Google Scholar]
- Sánchez-Bellot C, AlSubaie R, Mishchanchuk K, Wee RW, MacAskill AF (2022) Two opposing hippocampus to prefrontal cortex pathways for the control of approach and avoidance behaviour. Nat Commun 13:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarihi A, Jiang B, Komaki A, Sohya K, Yanagawa Y, Tsumoto T (2008) Metabotropic glutamate receptor type 5-dependent long-term potentiation of excitatory synapses on fast-spiking GABAergic neurons in mouse visual cortex. J Neurosci 28:1224–1235. 10.1523/JNEUROSCI.4928-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selimbeyoglu A, Kim CK, Inoue M, Lee SY, Hong AS, Kauvar I, Ramakrishnan C, Fenno LE, Davidson TJ, Wright M, Deisseroth K (2017) Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Sci Transl Med 9:eaah6733. 10.1126/scitranslmed.aah6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ (2011) Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology 36:529–538. 10.1038/npp.2010.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sik A, Ylinen A, Penttonen M, Buzsáki G (1994) Inhibitory CA1-CA3-hilar region feedback in the hippocampus. Science 265:1722–1724. 10.1126/science.8085161 [DOI] [PubMed] [Google Scholar]
- Skorput AGJ, Yeh HH (2016) Chronic gestational exposure to ethanol leads to enduring aberrances in cortical form and function in the medial prefrontal cortex. Alcohol Clin Exp Res 40:1479–1488. 10.1111/acer.13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaker ML, Jorgensen ET, Hegarty DM, Liu X, Kong Y, Zhang F, Linhardt RJ, Brown TE, Aicher SA, Sorg BA (2018) Cocaine exposure modulates perineuronal nets and synaptic excitability of fast-spiking interneurons in the medial prefrontal cortex. eNeuro 5:ENEURO.0221-18.2018. 10.1523/ENEURO.0221-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J, Hioki H, Okamoto S, Kaneko T (2014) Preprodynorphin-expressing neurons constitute a large subgroup of somatostatin-expressing GABAergic interneurons in the mouse neocortex. J Comp Neurol 522:1506–1526. 10.1002/cne.23477 [DOI] [PubMed] [Google Scholar]
- Song L, Xu X, Putthoff P, Fleck D, Spehr M, Hanganu-Opatz IL (2022) Sparser and less efficient hippocampal-prefrontal projections account for developmental network dysfunction in a model of psychiatric risk mediated by gene-environment interaction. J Neurosci 42:601–618. 10.1523/JNEUROSCI.1203-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Pardilla-Delgado E, Quirk GJ (2012) Gating of fear in prelimbic cortex by hippocampal and amygdala inputs. Neuron 76:804–812. 10.1016/j.neuron.2012.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumier A, Sibille E (2014) Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 39:2252–2262. 10.1038/npp.2014.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedehouder J, Brizee D, Shpak G, Kushner SA (2018) Activity-dependent myelination of parvalbumin interneurons mediated by axonal morphological plasticity. J Neurosci 38:3631–3642. 10.1523/JNEUROSCI.0074-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessman HA, et al. (2016) Disruption of POGZ is associated with intellectual disability and autism spectrum disorders. Am J Hum Genet 98:541–552. 10.1016/j.ajhg.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Neugebauer V (2011) MGluR1, but not mGluR5, activates feed-forward inhibition in the medial prefrontal cortex to impair decision making. J Neurophysiol 106:960–973. 10.1152/jn.00762.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Li X, Ren M, Zhao M, Zhong Q, Ren Y, Luo P, Ni H, Zhang X, Zhang C, Yuan J, Li A, Luo M, Gong H, Luo Q (2019) A whole-brain map of long-range inputs to GABAergic interneurons in the mouse medial prefrontal cortex. Nat Neurosci 22:1357–1370. 10.1038/s41593-019-0429-9 [DOI] [PubMed] [Google Scholar]
- Takita M, Izaki Y, Jay TM, Kaneko H, Suzuki SS (1999) Induction of stable long-term depression in vivo in the hippocampal-prefrontal cortex pathway: hippocampal-prefrontal cortex LTD. Eur J Neurosci 11:4145–4148. 10.1046/j.1460-9568.1999.00870.x [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Tomioka R (2010) Long-range GABAergic connections distributed throughout the neocortex and their possible function. Front Neurosci 4:202. 10.3389/fnins.2010.00202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura M, Mukai J, Gordon JA, Gogos JA (2016) Developmental inhibition of Gsk3 rescues behavioral and neurophysiological deficits in a mouse model of schizophrenia predisposition. Neuron 89:1100–1109. 10.1016/j.neuron.2016.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasic B, et al. (2018) Shared and distinct transcriptomic cell types across neocortical areas. Nature 563:72–78. 10.1038/s41586-018-0654-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney PL, Thierry AM, Glowinski J, Deniau JM, Gioanni Y (2008) Dopamine modulates temporal dynamics of feedforward inhibition in rat prefrontal cortex in vivo. Cereb Cortex 18:2251–2262. 10.1093/cercor/bhm252 [DOI] [PubMed] [Google Scholar]
- Tikhonova TB, Miyamae T, Gulchina Y, Lewis DA, Gonzalez-Burgos G (2018) Cell type- and layer-specific muscarinic potentiation of excitatory synaptic drive onto parvalbumin neurons in mouse prefrontal cortex. eNeuro 5:ENEURO.0208-18.2018. 10.1523/ENEURO.0208-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka R, Sakimura K, Yanagawa Y (2015) Corticofugal GABAergic projection neurons in the mouse frontal cortex. Front Neuroanat 9:133. 10.3389/fnana.2015.00133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay R, Lee S, Rudy B (2016) GABAergic interneurons in the neocortex: from cellular properties to circuits. Neuron 91:260–292. 10.1016/j.neuron.2016.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Spedding M, Schenker E, Didriksen M, Cressant A, Jay TM (2020) Cognition- and circuit-based dysfunction in a mouse model of 22q11.2 microdeletion syndrome: effects of stress. Transl Psychiatry 10:41. 10.1038/s41398-020-0687-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertes RP, Hoover WB, Szigeti-Buck K, Leranth C (2007) Nucleus reuniens of the midline thalamus: link between the medial prefrontal cortex and the hippocampus. Brain Res Bull 71:601–609. 10.1016/j.brainresbull.2006.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt D, Cho KK, Lee AT, Sohal VS, Rubenstein JL (2015) The parvalbumin/somatostatin ratio is increased in PTEN mutant mice and by human PTEN ASD alleles. Cell Rep 11:944–956. 10.1016/j.celrep.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmer LL, Schmeltzer S, Schurdak J, Ahlbrand R, Rush J, Dolgas CM, Baccei ML, Sah R (2016) Neuropeptide Y impairs retrieval of extinguished fear and modulates excitability of neurons in the infralimbic prefrontal cortex. J Neurosci 36:1306–1315. 10.1523/JNEUROSCI.4955-13.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HX, Waterhouse BD, Gao WJ (2013) Selective suppression of excitatory synapses on GABAergic interneurons by norepinephrine in juvenile rat prefrontal cortical microcircuitry. Neuroscience 246:312–328. 10.1016/j.neuroscience.2013.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss LA, et al. Autism Consortium (2008) Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med 358:667–675. 10.1056/NEJMoa075974 [DOI] [PubMed] [Google Scholar]
- Whissell PD, Cajanding JD, Fogel N, Kim JC (2015) Comparative density of CCK- and PV-GABA cells within the cortex and hippocampus. Front Neuroanat 9:124. 10.3389/fnana.2015.00124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Liu L, Tian Y, Wang J, Li J, Zheng J, Zhao H, He M, Xu TL, Duan S, Xu H (2019) A disinhibitory microcircuit mediates conditioned social fear in the prefrontal cortex. Neuron 102:668–682.e5. 10.1016/j.neuron.2019.02.026 [DOI] [PubMed] [Google Scholar]
- Yan Z, Rein B (2022) Mechanisms of synaptic transmission dysregulation in the prefrontal cortex: pathophysiological implications. Mol Psychiatry 27:445–465. 10.1038/s41380-021-01092-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SS, Mack NR, Shu Y, Gao WJ (2021) Prefrontal GABAergic interneurons gate long-range afferents to regulate prefrontal cortex-associated complex behaviors. Front Neural Circuits 15:716408. 10.3389/fncir.2021.716408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Z, et al. (2021) A taxonomy of transcriptomic cell types across the isocortex and hippocampal formation. Cell 184:3222–3241.e26. 10.1016/j.cell.2021.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Younts TJ, Castillo PE (2014) Endogenous cannabinoid signaling at inhibitory interneurons. Curr Opin Neurobiol 26:42–50. 10.1016/j.conb.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng H (2022) What is a cell type and how to define it? Cell 185:2739–2755. 10.1016/j.cell.2022.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong P, Yan Z (2011) Differential regulation of the excitability of prefrontal cortical fast-spiking interneurons and pyramidal neurons by serotonin and fluoxetine. PLoS One 6:e16970. 10.1371/journal.pone.0016970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla de San Martin J, Donato C, Peixoto J, Aguirre A, Choudhary V, De Stasi AM, Lourenço J, Potier MC, Bacci A (2020) Alterations of specific cortical GABAergic circuits underlie abnormal network activity in a mouse model of Down syndrome. Elife 9:e58731. 10.7554/eLife.58731 [DOI] [PMC free article] [PubMed] [Google Scholar]