Abstract
Classical models have traditionally focused on the left posterior inferior frontal gyrus (Broca's area) as a key region for motor planning of speech production. However, converging evidence suggests that it is not critical for either speech motor planning or execution. Alternative cortical areas supporting high-level speech motor planning have yet to be defined. In this review, we focus on the precentral gyrus, whose role in speech production is often thought to be limited to lower-level articulatory muscle control. In particular, we highlight neurosurgical investigations that have shed light on a cortical region anatomically located near the midpoint of the precentral gyrus, hence called the middle precentral gyrus (midPrCG). The midPrCG is functionally located between dorsal hand and ventral orofacial cortical representations and exhibits unique sensorimotor and multisensory functions relevant for speech processing. This includes motor control of the larynx, auditory processing, as well as a role in reading and writing. Furthermore, direct electrical stimulation of midPrCG can evoke complex movements, such as vocalization, and selective injury can cause deficits in verbal fluency, such as pure apraxia of speech. Based on these findings, we propose that midPrCG is essential to phonological-motoric aspects of speech production, especially syllabic-level speech sequencing, a role traditionally ascribed to Broca's area. The midPrCG is a cortical brain area that should be included in contemporary models of speech production with a unique role in speech motor planning and execution.
Introduction
Speech production requires motor planning and control of vocal tract muscles to speak fluently. The generation of a proper sequence of motor commands before articulation poses a unique neurocomputational challenge for the human brain given how quickly and precisely vocal tract movements must be made.
The classic language model posits that a single frontal region, Broca's area in the left posterior inferior frontal gyrus (IFG), is responsible for speech motor planning (Broca, 1861; Geschwind, 1970). Broca's area is thought to transform phonological representations (the auditory speech sounds that comprise utterances) into sequences of motor commands able to be executed by the motor cortex in the ventral precentral gyrus (vPrCG), which projects to vocal tract muscles (Geschwind, 1970) (Fig. 1A).
Figure 1.
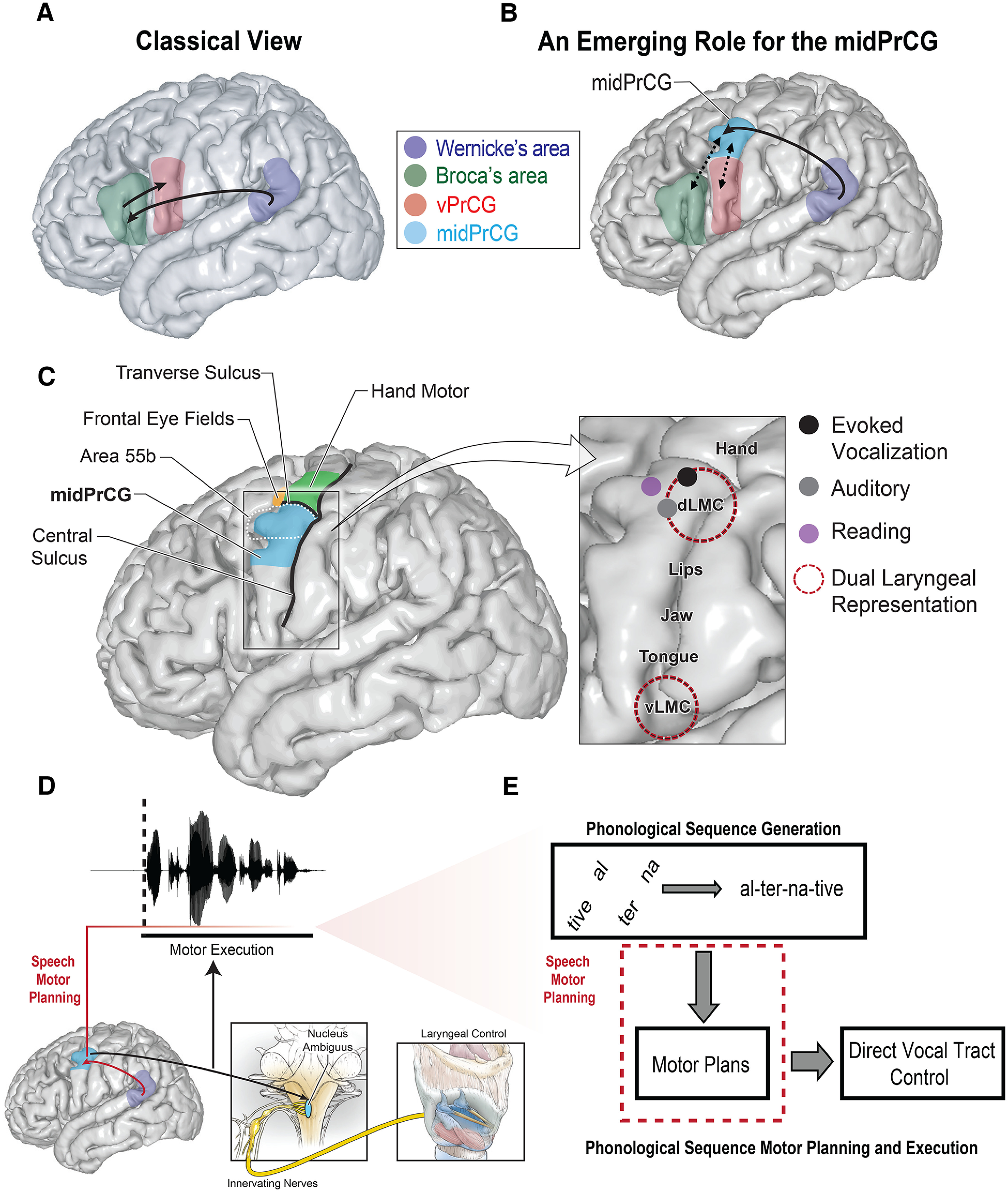
A, Classical view of speech production in the brain, which is the foundation for many contemporary models. Arrows indicate left hemisphere information transfer from Wernicke's area (posterior temporoparietal) to Broca's area (left posterior IFG) to the vPrCG for motor execution. B, Proposed model with an emerging role for the midPrCG in speech production. We propose that the midPrCG receives inputs from Wernicke's region (posterior temporoparietal, solid arrow) and communicates with other key frontal regions (dashed arrows) during speech production. C, Left, The anatomic location of the midPrCG and neighboring functional regions. Right, Examples of speech involvements seen in the region. Colored points in the zoom-in view on the right indicate observed functions, via ECoG or stimulation. The location of the points is approximate and does not reflect precise localization. Text labels and dotted outlines indicate the relative location of vocal tract motor representations (dLMC: dorsal laryngeal motor cortex; vLMC: ventral laryngeal motor cortex). D, Illustration of proposed midPrCG functions, occurring both before and during speech production: namely, speech motor planning of phonological sequences and laryngeal control. Projections from the midPrCG are sent to the nucleus ambiguous in the brainstem where motor neurons send innervations to the larynx. Dotted black line indicates the onset of production. Red line with fading color indicates time span of the midPrCG's role in speech motor planning, which starts before the speech onset and may continue throughout production. Red line with arrow indicates proposed communications between midPrCG and posterior temporoparietal regions to support speech motor planning. E, Neural processes that give rise to speech output. Speech motor planning specifically refers to transforming generated phonological sequences (e.g., syllable sequences) into aspects of a motor representation to properly shape the vocal tract through execution of direct motor control.
The role of Broca's area, however, is highly controversial. Accumulating evidence strongly challenges its involvement in articulatory control, as patients with focal stroke or surgical resection to Broca's area in the left IFG regularly fail to exhibit Broca's aphasia (Mohr et al., 1978; Benzagmout et al., 2007; Rolston et al., 2015; Gajardo-Vidal et al., 2021; Mandonnet and Duffau, 2021; Andrews et al., 2022; Wilson et al., 2022). In neurosurgical clinical settings, this debate has direct implications for patient care and decision-making. For example, a brain tumor that is newly discovered in the anatomic boundaries of Broca's area is often presumed to be inoperable. This influences the decision on whether to biopsy alone or resect the tumor, which will significantly affect long-term prognosis and quality of life for the patient (Chang et al., 2011).
This paper is not meant to be a general review of speech production research, but rather to describe the specific perspectives and experiences gained through neurosurgery in “eloquent” brain regions. Neurosurgery offers a unique opportunity to directly observe language operations in the human brain from multiple angles. First, careful behavioral study of patients before and after surgery can reveal new associations between lesions and speech symptoms. Data from surgical patients can complement data from stroke or neurodegenerative patients because surgical lesions and resection can occur in virtually any cortical region unlike vascular-defined patterns in stroke or degenerative atrophy patterns which can be confounding. Second, direct access to the cortex facilitates higher spatial and temporal resolution investigations from neural recording techniques, such as electrocorticography (ECoG) and single-neuron recordings. Third, access to the cortex during brain mapping procedures also allows for direct electrocortical stimulation which can evoke transient speech behavioral movements or deficits from precise locations.
In this review, we focus on how these neurosurgical techniques have provided new insights into the mechanisms of speech production. We draw on historical and contemporary advancements to propose that a novel region on the precentral gyrus, which we refer to as the middle precentral gyrus (midPrCG), is a critical, yet often-overlooked node in the speech production network (Fig. 1B). The midPrCG includes both primary motor cortex and premotor cortex and has important functions for speech production, including motor planning and execution. We begin by defining the key anatomic landmarks of the region. Next, we review the numerous, but disparate speech-related findings implicated to this region and propose a potential shared role in internal generative speech production, which supports speech motor planning. Finally, we speculate how the midPrCG fits into the broader language network and enumerate key future directions in better understanding its role.
General functional and anatomic characteristics
Human sensorimotor cortex is composed of the precentral and postcentral gyri. The precentral gyrus can be further subdivided into premotor and primary motor cortex. In speech production models, the precentral gyrus' role is often primarily limited to execution of motor plans for control of the vocal tract muscles (Hickok, 2012; Guenther, 2016). The postcentral gyrus is conceptualized as the primary region responsible for somatosensory input and feedback. Abundant evidence, across comparative domains of neurophysiology, has challenged these assumptions. Premotor cortex has been found to show activations that are correlated with higher-order characteristics of a motor task, such as planning and sensory monitoring (Pearce and Moran, 2012; Fang et al., 2019; Fornia et al., 2020; Takei et al., 2021), in addition to having direct projections to the vocal tract muscles (Cerkevich et al., 2022). Primary motor cortex has a capacity to encode functional task states and integrate sensory inputs into motor plans, functions beyond direct activation of musculature (Salinas and Romo, 1998; Pruszynski et al., 2011; Fuertinger et al., 2015; Griffin et al., 2015; McCrimmon et al., 2018). Thus, there is strong evidence that the precentral gyrus need not be limited to execution of muscle motor commands and instead may also play a role in generating the proper motor plans.
The midPrCG, as presently defined, encompasses both traditionally defined primary motor cortex (Brodmann area 4) and premotor cortex (Brodmann area 6) (Brodmann, 1909, 1910). It is bound caudally by the central sulcus and rostrally by the precentral sulcus (Fig. 1C). We refer to it as “middle” because it is anatomically positioned posterior to the middle frontal gyrus, and anatomically near the midpoint of the lateral precentral gyrus. We also use “middle” to make distinction from “dorsal” and “ventral” precentral cortical regions which have distinct functions. Functionally, the midPrCG is bound by a hand motor representation dorsally (“hand knob”), and a motor representation of oral and facial muscles ventrally. The frontal eye fields are located rostrally, in addition to language-related areas in the posterior middle frontal gyrus (to be discussed below). Of note, we have observed that separation of the midPrCG from the hand motor area is often marked by an anatomic landmark of a small transverse sulcus that extends from the precentral sulcus and traverses across the precentral gyrus.
Many studies have implicated midPrCG across a variety of language or speech tasks. We will begin by discussing the most direct motor role observed in the midPrCG, control of the larynx.
Motor control of the larynx
The larynx is an essential vocal tract structure for generating voiced speech sounds and modulating pitch in speech for intonation. Early electrical stimulation studies found orofacial motor representations on the vPrCG with a single cortical representation per body part organized in a somatotopic body map layout (Leyton and Sherrington, 1917; Penfield and Boldrey, 1937). That is, adjacent cortical areas generally represent adjacent body locations. In one of the earliest such studies in humans, neurosurgeon Harvey Cushing described a laryngeal representation in the ventral most portion of the precentral gyrus, directly adjacent to the Sylvian fissure (Cushing, 1909; Pendleton et al., 2012). Later, in his original descriptions of the motor cortex, Penfield was noncommittal about a laryngeal localization possibly because the movements of the larynx were not readily visible during his intraoperative explorations with awake patients. Penfield did, however, describe two speech-related phenomena, speech arrest and vocalization, during electrical cortical stimulation (Penfield and Rasmussen, 1949). Speech arrest and vocalization were reportedly observed at multiple locations and partially overlapped with induced orofacial movements from the precentral and postcentral gyrus. The topic was revisited in humans over 50 years later with the use of functional magnetic resonance imaging (fMRI). Studies in rhesus monkeys and humans, with fMRI, provided conflicting accounts of cortical localization of the larynx (Jürgens, 1974; Simonyan and Jürgens, 2003; Terumitsu et al., 2006; Brown et al., 2008; Grabski et al., 2012).
In Bouchard et al. (2013), we studied the dynamic cortical representations of speech syllables and related the underlying millimeter and millisecond resolution of ECoG neurophysiology with articulator representations. Functional representations for the lips, jaw, tongue, and larynx could be identified. However, a result that we did not anticipate was the observation of two laryngeal representations in the precentral gyrus. One was observed in the ventral most aspect near the subcentral gyrus, a U-shaped gyrus where the central sulcus terminates. The other was found to be more dorsal, between the hand and lip functional representations. This was unexpected because it did not fit the known somatotopic layout of the body parts, that progresses from the front of the face/mouth toward the throat as you move from dorsal to ventral. We called the more dorsal area “dorsal laryngeal motor cortex” (dLMC) and the more ventral area “ventral LMC” (vLMC). The dLMC was the dorsal-most vocal tract speech articulator representation found and falls within the region we now define as the midPrCG. Notably, the functional localization of the dLMC and vLMC has been confirmed by a recent fMRI study that used novel analysis techniques to account for individual variation and breathing that may have complicated earlier imaging attempts (Eichert et al., 2020).
To better understand and confirm this localization, we performed a set of ECoG experiments where it was found that dLMC activity was strongly correlated with pitch control during the prosodic intonation of speech (Dichter et al., 2018). Pitch is modulated by tensing and relaxing the vocal folds through contraction of the cricothyroid and thyroarytenoid muscles in the larynx, respectively. Of note, dLMC activity was correlated with pitch during both speaking and singing with bilateral representations. dLMC auditory responses, tuned to vocal pitch changes, were also observed when microphone recordings of the participant's spoken utterances were played back through audio speakers. Voicing, which is created by a different laryngeal movement of adduction of the vocal folds, was found to be localized to both dLMC and vLMC.
The existence of auditory pitch responses in the dLMC could suggest that the observed cortical responses were primarily from feedback, and not actual motor efferent signals to the larynx. We therefore applied electrocortical stimulation to both the dLMC and vLMC and documented evoked contractions of laryngeal musculature, as measured by electromyography during asleep brain mapping surgeries (Dichter et al., 2018). Additionally, during awake surgery brain mapping, we discovered that electrical stimulation of the dLMC selectively evoked involuntary vocalization. This observation contrasts with Penfield's description that vocalization could be elicited throughout the precentral gyrus. Vocalization is a more complex movement that requires the coordination between laryngeal movement and respiration (Jürgens, 2009). This suggests that dLMC may involve more than direct laryngeal motor control. The region has premotor and primary motor populations that may be capable of coordinating and integrating across multiple muscle groups to generate sound. No clear evidence for stimulation-evoked somatosensory perception or proprioception of laryngeal movements has been described for the postcentral gyrus.
A common brain stimulation effect observed during language mapping is speech arrest, usually performed as a patient is counting numbers or naming pictures. Speech arrest sites are often interpreted as the functional equivalent of Broca's area (Quiñones-Hinojosa et al., 2003) or language altogether. Some have speculated that the speech arrest phenomenon is responsible for speech planning or the “final motor output” (Tate et al., 2014) before command signals are sent to the articulators. While this interpretation is very common, direct evidence is lacking and several reports have challenged this view. First, Penfield described speech arrest primarily localized to the precentral gyrus (throughout midPrCG and vPrCG) and occasionally from the pars opercularis (Penfield and Rasmussen, 1949). He observed these sites bilaterally while also describing how the right precentral gyrus could be removed without major effects on speech. Recent studies have corroborated some of his early findings, with possible speech arrest cluster centroids in the vPrCG and midPrCG, rather than Broca's area (Tate et al., 2014; Wu et al., 2015; Lu et al., 2021). Speech arrest is found in either the ventral or middle region, but rarely both dorsal and ventral location in the same individual, so there is considerable variability from person to person. Second, speech arrest can be observed in other areas, such as the supplementary motor area (SMA), where resections may lead to transient but not longstanding difficulties with speech (Lu et al., 2021; Zhou et al., 2021; Pinson et al., 2022). Third, at a behavioral level, speech arrest is complete cessation of speech, whereas one would expect additional effects, such as distortions or sequencing errors, if the cortical sites were critical for speech planning. Fourth, recent stimulation studies have suggested an alternative interpretation for speech arrest, namely, that stimulation is activating a general inhibitory process to suppress speech output, rather than disrupting motor planning itself.
A recent case series from our group documented how stimulation at speech arrest sites can also arrest nonspeech behaviors, such as music production (Leonard et al., 2019b) and manual gestures (Breshears et al., 2019). That is, we proposed a new interpretation that speech arrest responses are part of a broader stopping circuit, which involves other brain areas, such as the pre-SMA and the basal ganglia (Breshears et al., 2019; Leonard et al., 2019b). Our proposal of a stopping mechanism has important clinical implications. Speech arrest sites are often considered the gold standard method of localizing speech motor control and are intentionally preserved, even if doing so is suboptimal for surgical treatment. The consequences of removing speech arrest sites have never been shown, but there is a possibility it could be tolerated if its function is part of a distributed inhibitory circuit and not a critical speech planning area. Certainly, patients typically recover speech functions after SMA resection. These inhibitory effects may be an important part of premotor functionality (Filevich et al., 2012); as such, the concentration of speech arrest sites to the precentral gyrus further supports a role beyond lower level articulatory muscle control.
Auditory representations
Decades worth of research has demonstrated that the superior temporal gyrus (STG) is critical for our comprehension of speech sounds and encodes auditory features during perception (Mesgarani et al., 2014; Yi et al., 2019; Bhaya-Grossman and Chang, 2022). Surprisingly, the precentral gyrus is also activated by simple speech perception tasks across many imaging studies (Price et al., 1996; Wilson et al., 2004). Early fMRI studies suggested that the precentral gyrus was somatotopically activated by speech sounds that differentially involved key speech articulators, such as the lips and tongue (i.e., a labial sound activated the lip motor representation) (Pulvermüller et al., 2006). At the time, this result was interpreted as evidence for the “motor theory” hypothesis of speech comprehension, which argued that speech sounds are mapped to corresponding articulatory motor representations during perception (Liberman et al., 1967; Liberman and Mattingly, 1985; Pulvermüller et al., 2006; Pulvermüller and Fadiga, 2010).
However, it remained unclear whether the representations reflected auditory or articulatory information. We recorded ECoG responses from the sensorimotor cortex while participants listened to consonant-vowel syllables (Cheung et al., 2016). As a control, we also recorded ECoG while participants spoke aloud the same syllables.
Our results showed two pieces of evidence that are distinct from what “motor theory” would predict. First, listening responses mainly occurred in a dorsal and a ventral region along the precentral gyrus, rather than covering the entire region where articulators are represented. This dorsal region overlaps with the midPrCG, where high magnitude responses to speech sounds are observed. Second, listening responses to speech sounds do not directly map to their corresponding place of articulation; that is, a bilabial sound such as /b/ does not map to lip motor regions, and an alveolar tongue sound such as /d/ does not activate tongue motor representations.
Rather, auditory responses in the midPrCG displayed a similar pattern of tuning to auditory responses in the STG (Mesgarani et al., 2014; Cheung et al., 2016), following the manner of articulation which is driven by the acoustic distinctions of speech sounds. Both the STG and midPrCG were found to encode acoustic-phonetic features of the speech sounds (e.g., being selective to voicing and distinguishing fricative vs plosive consonants). Indeed, this paper demonstrated that auditory spectrotemporal receptive fields could be derived from the motor cortex. This provides strong evidence that, during listening, midPrCG represents auditory rather than motor information. It further suggests the possibility that a phonological (speech sound) representation exists in the midPrCG.
Subsequent fMRI studies confirmed these results and showed detailed spectrotemporal tuning in the region and robust functional connectivity to the STG (Venezia et al., 2019, 2021). Our work also separately demonstrated that the midPrCG tracks other features about the perceived sound, such as changes in pitch (Dichter et al., 2018). Additional literature has corroborated this finding by showing that the region tracks perceived pitch and loudness (envelope) (Ding et al., 2016; Keitel et al., 2018; Berezutskaya et al., 2020). In a study where participants were played a time-lagged version of their speech while they continued to produce a given word or phrase, the midPrCG increased activity, suggesting a potential role in auditory-motor integration (Ozker et al., 2022).
Despite the existence of auditory representations in the midPrCG, lesions in the precentral gyrus do not reliably lead to speech perception or comprehension deficits. We proposed that, instead, auditory representations in this region may support speech production (Cheung et al., 2016). Instead of evoking articulatory representations to facilitate speech perception (as motor theory suggests), the direct integration of acoustic phonological and motor articulatory processing may be important for production (Fig. 1D). There is extensive evidence that these representations may aid in speech perception under noisy or acoustically degraded conditions (Callan et al., 2004; Du et al., 2014). One interpretation is that precentral activations may be particularly important for perceptually challenging situations where internally generated motor representations of speech may help with sound discrimination (Leonard et al., 2016).
A region that similarly may integrate sensory (visual and auditory) and motor responses has been identified in macaques with similar anatomical landmarks to the midPrCG (Graziano and Gandhi, 2000; Cooke and Graziano, 2004). It is located ventral to the spur of the arcuate sulcus, a potential analog of the transverse sulcus in humans, and dorsal to primary orofacial motor representations. When this region is stimulated, macaques perform complex, coordinated muscle movements that resemble self-defense maneuvers. Presumably, such functionality in the precentral gyrus is important for the generation of ethologically relevant behaviors and serves species-specific functions. In humans, the unique sensorimotor and multisensory properties of the midPrCG may have evolved to support speech production. Stimulation in the human midPrCG does not cause limb movements, but instead evokes vocalization, a coordinated gesture important for speech production. Multisensory and multimodal representations, in non-human primates, have been argued to reflect a capacity for motor planning by forming appropriate motor commands according to relevant sensory information (Andersen et al. 1997; Andersen and Buneo 2002). Multimodal representations also allow motor commands to be generated from multiple input modalities and be coordinated for multiple motor output modalities, creating a flexible interface for planning movements. In human speech, representations of this nature could serve to bridge phonological auditory targets (through externally presented stimuli, such as listening or reading, or self-generated targets) with sequences of motor commands for production (such as overtly produced speech or written text). In the next section, we further discuss multisensory and multimodal phonological representations in the midPrCG.
Additional phonological representations
The midPrCG is also involved in silent reading and phonological working memory. During silent reading, a transformation from graphemes (letters) to speech sound representations, such as phonemes, is theorized to occur (Taylor et al., 2013; Carreiras et al., 2014). Traditionally, research on grapheme to phoneme transformation has focused on lateral temporoparietal regions (Geschwind, 1970; Booth et al., 2002; Binder et al., 2005). However, there is growing evidence that the precentral gyrus, including the midPrCG, may play a role in this process (Dehaene et al., 2001; Yen et al., 2019; Kaestner et al., 2021, 2022). ECoG has demonstrated that responses in the midPrCG are strongest when reading real words rather than nonsensical strings of letters or characters (Kaestner et al., 2021). Additionally, the midPrCG overlaps with a region referred to as Exner's area, often implicated in studies of reading and writing (Matsuo et al., 2003; Roux et al., 2009). Although there is debate on the relevance of Exner's area in modern models of language or specifically reading (Roux et al., 2010), many lesion studies have associated areas near the proposed midPrCG region with deficits in reading and writing tasks (Rapcsak et al., 1988; Cloutman et al., 2009; Roux et al., 2009; Ishihara et al., 2010). Focal seizures that are triggered by reading (reflex epilepsy) have seizure onset zones in middle regions of the precentral gyrus (Salek-Haddadi et al., 2009; De Beeck et al., 2011; Safi et al., 2016).
In addition to reading, the midPrCG is activated by another internal, nondirect motor representation of speech, phonological working memory. Phonological working memory is the capacity for temporarily maintaining and manipulating speech sounds for short-term retrieval. Recent neuroimaging work has shown that the midPrCG is reliably recruited during phonological working memory and that this recruitment does not depend on whether participants overtly spoke the words (Scott and Perrachione, 2019). Interestingly, phonological working memory has been conceptualized as relying on a periodic “refreshing” of the representations through silent rehearsal (Baddeley and Hitch, 1974; Baddeley, 1986, 2003), suggesting the involvement of brain areas responsible for speech motor planning, although it is not clear the representation is articulatory in nature.
ECoG has allowed us to precisely describe the language network supporting word repetition. Many sites on the precentral gyrus, including midPrCG, show significant responses to listening and repeating, as well as during a silent delay period during which the word is maintained and potentially planned before production (Leonard et al., 2019a). Our results are confirmed by other ECoG studies showing robust pre-speech activations in areas overlapping the midPrCG (Forseth et al., 2021; Woolnough et al., 2022). The nature of these representations should be explored in future studies. Interestingly, integrity of midPrCG gray matter is also related to phonological abilities (i.e., ability to manipulate phonemes as in phoneme deletion, blending, and replacement) in individuals with primary progressive aphasia (Henry et al., 2016).
Together, these results demonstrate that the midPrCG exhibits extensive multimodal functions. A potential linking hypothesis is that the midPrCG processes internal generative phonological representations of speech. This capacity may be used during speech production to interface with phonological information to plan motor sequences. Indeed, across other domains of neuroscience, premotor populations are known to be active during planning of an upcoming action (Nakayama et al., 2008; Pearce and Moran, 2012; Li et al., 2016; Xu et al., 2022). Internal generative phonological processing may also assist during language comprehension, either to speech and reading stimuli, especially when the sensory stimuli are not clear.
Speech motor planning
Many have hypothesized that speech production requires moving from a phonological representation of an utterance to sequences of articulatory motor commands (Levelt and Wheeldon, 1994; Cholin et al., 2011; Guenther, 2016). Per this view, after the appropriate conceptual item has been retrieved from the lexicon, the proper low-level phonological units (i.e., syllables or phonemes) that compromise the auditory concept must be selected and sequenced into the correct order. This phonological representation is then transformed into a motor code corresponding to the proper sequence of motor commands to be executed by shaping the vocal tract for articulation (Van Der Merwe, 2021). This stage, the conversion from a phonological to a motor representation, is what we here refer to as “speech motor planning” (Fig. 1E).
Support for speech motor planning as its own stage of speech production is evidenced by the existence of apraxia of speech (AOS), an acquired motor speech disorder characterized by difficulty initiating speech, slow rate, articulatory groping, and trouble making transitions between syllables, among other characteristics (Basilakos et al., 2015; Duffy, 2019; Code, 2021). AOS is distinct from aphasia, as word finding, syntactic construction, and comprehension can be unimpaired, and distinct from dysarthria, as articulatory muscle strength can be entirely preserved despite the inability to produce fluent speech. AOS often occurs with lesions to the language dominant left hemisphere (Berthier, 2005). AOS, aphasia, and/or dysarthria often co-occur (Haley et al., 2012; Clark et al., 2014; Graff-Radford et al., 2014; Code, 2021), making their unique neural substrates difficult to study in the context of defined stroke or neurodegeneration patterns. However, two recent large voxel-based lesion symptom mapping studies in a stroke population revealed strong associations between AOS and damage to the precentral gyrus, extending into the midPrCG (Basilakos et al., 2015; Itabashi et al., 2016). These results are in line with other stroke lesion overlay and case studies (Fox et al., 2001; Terao et al., 2007; Graff-Radford et al., 2014; Moser et al., 2016). Neurodegeneration studies have also shown that midPrCG neural volume loss is associated with a primary progressive form of AOS (Josephs et al., 2013; Duffy et al., 2021).
Neurosurgical resection offers insights into language disorders that are not bound by the spatial patterns of stroke or neurodegeneration. Indeed, a rare case of pure AOS was observed recently by our group following resection of the midPrCG and posterior middle frontal gyrus (pMFG). In Chang et al. (2020), a right-handed man experienced lasting pure AOS after resection of the left pMFG and midPrCG (Chang et al., 2020). A battery of speech and language assessments were administered, revealing severely nonfluent speech despite near-normal performance on language tasks along with normal articulatory strength. This AOS was not present after an initial resection to a smaller, more anterior portion of this region (centered in pMFG), but emerged only following extension of the resection into more medial and posterior regions, including the midPrCG.
Other findings from resective neurosurgery further support the notion that the midPrCG is critical in speech motor planning. Transient fluency deficits, in part characterized by speech motor deficits, following resective neurosurgery were localized to middle regions of the precentral gyrus using voxel-based lesion symptom mapping (Wilson et al., 2015). Finally, the somewhat mysterious acquired foreign accent syndrome, a motor speech disorder that results in speakers being perceived as having a foreign accent (Jonkers et al., 2017), has also been associated with lesions near this region of the precentral gyrus (Takayama et al., 1993; Tokida et al., 2017; Higashiyama et al., 2021).
Relationship between speech motor planning and other speech functions in the midPrCG
With premotor, motor, and sensory functions identified in the midPrCG, a natural question is whether they are supported by the same neural populations. One hypothesis is that different functions reside in different spatial locations within the midPrCG, in line with subregions defined by cytoarchitecture. The midPrCG is anatomically diverse, overlapping both cytoarchitecture-defined premotor cortex (BA6) and primary motor cortex (BA4). Neural populations that are on the rostral aspect of the midPrCG in BA6 may differentially support higher-level functions, such as speech motor planning and phonological processing. In contrast, neural populations closer to the central sulcus in BA4, known to play a direct role in muscular control, could be more singularly involved in laryngeal motor control. Further probing the spatial organization or overlap of functionality in the midPrCG should be the topic of future studies in neurosurgical patients and may reveal anatomic delineations to the region we defined as the midPrCG.
A compelling alternative, based on evidence from studies of laryngeal control, is that neural populations tuned to each function may be distributed throughout the region. Premotor areas may have both direct projections to innervating laryngeal nerves as evidenced in nonhuman primates (Cerkevich et al., 2022), and a role in higher-level speech motor planning. Additionally, while primary motor cortex may be enriched in projections to innervating nerves, there is evidence to suggest higher-order encodings related to task goals and feedback (Pruszynski et al., 2011; Fuertinger et al., 2015; McCrimmon et al., 2018). A distributed organization throughout the region is also supported by stimulation studies. Stimulation at sites throughout the midPreCG can evoke vocalization, a function that requires both coordination of the larynx and control of respiratory exhalation (Breshears et al., 2015; Dichter et al., 2018). Computations to support laryngeal control and speech motor planning may be performed at the microcircuit level, and only revealed by single-unit neuronal recordings in humans.
Because of our specific interest in speech motor control for language production and the clinical context where our data originate, the majority of the work described herein refers to the midPrCG in the left hemisphere, the dominant hemisphere for language in the vast majority of individuals (Springer et al., 1999; Wilson et al., 2018). However, it is worth noting that functions, such as laryngeal motor control, auditory processing, and reading, engage both the left and right midPrCG. The lateralization of functions in the midPrCG, specifically speech motor planning, should be the focus of future task-based experiments.
Comparison with other key frontal language regions
What additional regions may communicate with the midPrCG to coordinate speech production? A first candidate region is Broca's area in the left posterior IFG, classically proposed to be involved in speech production. Several ECoG studies have suggested that Broca's area is involved in speech preparation, reporting neural activity primarily before the start of speech production (Flinker et al., 2015; Conner et al., 2019; Wang et al., 2021; Castellucci et al., 2022). Despite this, multiple lines of evidence have strongly challenged whether Broca's area plays a critical role in speech motor planning. Such evidence dates as early as Penfield's seminal studies, showing that resection of Broca's area was associated with only transient aphasias (Penfield and Roberts, 1959; Mohr et al., 1978). Similarly, recent resection studies have shown minimal appreciable effects on fluent speech articulation with resections to Broca's area (Rolston et al., 2015; Wilson et al., 2015; Korkar et al., 2021); rather, such effects have been seen with resection of the precentral gyrus (Wilson et al., 2015; Chang et al., 2020; Andrews et al., 2022). Direct electrocortical stimulation to Broca's area can cause deficits in naming (anomia) (Penfield and Roberts, 1959; Chang et al., 2017; Herbet et al., 2018; Lu et al., 2021), and the region has been implicated in syntactic processing (Chang et al., 2018; Matchin and Hickok, 2020). Given this, Broca's area may have a higher-order language role for sentence-level production, but neurosurgical evidence suggests that it is not essential for speech motor planning specifically or the production of fluently articulated speech.
Another frontal region is the pMFG, which is directly adjacent to the midPrCG. In a study of over 440 young adults, the Human Connectome Project identified key language regions, through a combination of fMRI activations to a story listening task, functional connectivity, and myelin architecture (Glasser et al., 2016). Interestingly, the project identified area 55b, strongly overlapping with the midPrCG and pMFG, as one key language region (Fig. 1C, mapped onto the cvs avg35 pial surface in MNI152 template space). Evidence for the pMFG's importance in language is corroborated by transient speech hesitancy and dysfluency following surgical resection (Morshed et al., 2021). However, transient effects were shown to fully resolve in all patients after 90 days. In addition to Broca's area in the left posterior IFG, pMFG activations peaked while planning, but not during or close to articulating, responses in conversational speech (Castellucci et al., 2022). Thus, while growing evidence suggests a role of the pMFG in language, it is unlikely that the pMFG alone is responsible for speech motor planning or articulatory coordination.
To what extent are the midPrCG and vPrCG functionally separable? A potential difference between the vPrCG and midPrCG is the level of interface with phonological representations. Internal phonological representations, such as reading, phonological working memory, and auditory responses, appear more concentrated to the midPrCG (Cheung et al., 2016; Scott and Perrachione, 2019; Kaestner et al., 2021, 2022). Indeed, Human Connectome Project area 55b, overlapping the midPrCG, was partially defined by its very strong responses to story listening, and has strong functional connectivity with known language areas (posterior STG and IFG) (Glasser et al., 2016). Additionally, this area is regularly implicated in fMRI studies attempting to map aspects of the language network (Yen et al., 2019; Lipkin et al., 2022). Area 55b need not be its own distinct region as the isolating task was story listening, a nonspecific language localizer. Instead, this provides further evidence for a region largely within the midPrCG that is capable of holding phonological representations and communicating with higher-order language regions. Notably, the project did not find a corresponding region on the vPrCG.
A midPrCG phonological-motor interface is also supported by the lesion literature. Lesions to the midPrCG more commonly result in pure AOS, a speech motor planning disorder, whereas lesions to the vPrCG more often result in dysarthria or Broca's aphasia (Duffau et al., 2008; Basilakos et al., 2015; Duffy, 2019; Andrews et al., 2022). Neural populations primarily in the posterior bank of the vPrCG (along the central sulcus) and ventral postcentral gyrus encode articulatory kinematic trajectories, coordinated movements across muscle groups that lead to specialized configurations of the vocal tract during speech production (Chartier et al., 2018). These representations include supralaryngeal articulators as well as the vLMC. The premotor functions are less defined currently.
How does the midPrCG implement speech motor planning?
For any region with a key role within a network, one would expect strong connectivity to the rest of the network. Correspondingly, we expect the midPrCG to have connectivity to other regions implicated in speech. Indeed, there is strong evidence of functional connectivity between the midPrCG, IFG, and posterior temporoparietal regions, including the posterior STG, a region known to be important for speech production (Fuertinger et al., 2015; Glasser et al., 2016; Genon et al., 2017, 2018; Baboyan et al., 2021). The midPrCG is also a termination point for key white matter tracts in the speech network, in particular, the superior longitudinal fasciculus (SLF) and arcuate fasciculus (Fernández-Miranda et al., 2015). The SLF connects posterior temporoparietal areas to prefrontal and precentral areas (Bernal and Altman, 2010). Of its major branches, SLF II and SLF III were shown to connect the supramarginal gyrus and angular gyrus to the premotor and prefrontal regions, including areas within the midPrCG (Makris et al., 2005; Martino et al., 2013; Chang et al., 2015; Nakajima et al., 2020). Stimulation of the SLF underlying the supramarginal gyrus caused impairment of phonological processing (Maldonado et al., 2011). These connections establish that the midPrCG is structurally linked to the rest of the core language network.
As a framework, we conceptualize speech planning to involve two broad steps in generative speech production: the phonological sequencing of speech units and the execution and coordination of these phonological sequences (Fig. 1E). There is a strong body of evidence to suggest that posterior temporoparietal regions, including the angular gyrus and posterior STG, are important for phonological sequence generation in speech production (Binder, 2015, 2017). Lesions or stimulation to these regions often induce paraphasias, involving phoneme substitutions or rearrangements within an utterance (Wernicke, 1874; Quigg and Fountain, 1999; Rohrer et al., 2010; Roux et al., 2012; Leyton et al., 2014; Leonard et al., 2019a; Wilson et al., 2022). In such cases, motor implementation appears conserved with no AOS or dysarthria.
In contrast, lesions to the midPrCG seem to have a greater impact on motor production, primarily resulting in the distortions and syllable segregations characteristic of AOS along with mild impacts on writing. A distinctive feature of AOS is the increased difficulty in producing complex sequences of sounds (i.e., repeating multisyllabic words, including consonant clusters such as “catastrophe”) (Haley and Overton, 2001; Duffy et al., 2021). The midPrCG may play a critical role in the process of coordinating complex phonological sequences into motor plans, such that, when it is lesioned, the result is AOS. Indeed, an fMRI study implicated several areas that may be involved in syllable sequencing, which included the midPrCG among other regions (Bohland and Guenther, 2006), although the nature of the representation remains unclear. Interestingly, prior work has also made associations between this midPrCG region and reading and writing (Rapcsak et al., 1988; Roux et al., 2009; Kaestner et al., 2021, 2022). Under this paradigm, the midPrCG may be multimodal in terms of motor output, given that lesions can lead to deficits in both spoken (orofacial motor) and written (hand motor) production. A linking hypothesis is that the midPrCG is recruited to bridge generated phonological sequences from posterior temporoparietal regions into the motor domain. This hypothesis is supported by the strong functional and structural connectivity between the midPrCG and these posterior temporoparietal regions (Fernández-Miranda et al., 2015; Glasser et al., 2016; Genon et al., 2017, 2018; Baboyan et al., 2021). Such communication, however, is a hypothesis and must be tested by future studies that probe the production process with high temporal resolution.
Clinical implications
The described research has strong implications for better treating and preventing language-related disorders. A key aspect of many neurosurgical procedures is mapping language functions to areas on the cortex to prevent postoperative complications (Sagar et al., 2019). Better understanding the role of the midPrCG may improve outcomes in language following resective neurosurgery, as it provides a greater understanding of which regions may be causally relevant for speech.
Conclusion
In this review, we have provided strong evidence for the inclusion of the midPrCG as a novel and specific node in models of speech production. We argue that the region has multiple critical roles, including direct laryngeal motor control and higher-level speech motor planning. Future studies should directly test this functionality with an emphasis on better characterizing the nature of speech motor representations in the region. This review highlights the need to look beyond the classical language network to fully describe the richness of human speech.
Footnotes
A.B.S. was supported by the National Institute of General Medical Sciences Medical Scientist Training Program Grant T32GM007618. T.L.S. was supported by National Institute on Deafness and Other Communication Disorders Grant F32DC019531. D.F.L. was supported by National Institute on Deafness and Other Communication Disorders Grant F32DC020096. We thank Laura Gwilliams and Matthew K. Leonard for critical feedback in drafting the manuscript.
The authors declare no competing financial interests.
References
- Andersen RA, Buneo CA (2002) Intentional Maps in Posterior Parietal Cortex. Annual Review of Neuroscience 25:189–220. 10.1146/annurev.neuro.25.112701.142922 [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J (1997) Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annual Review of Neuroscience 20:303–330. 10.1146/annurev.neuro.20.1.303 [DOI] [PubMed] [Google Scholar]
- Andrews JP, Cahn N, Speidel BA, Chung JE, Levy DF, Wilson SM, Berger MS, Chang EF (2022) Dissociation of Broca's area from Broca's aphasia in patients undergoing neurosurgical resections. J Neurosurg. Advance online publication. Retrieved Aug 5, 2022. 10.3171/2022.6.JNS2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baboyan V, Basilakos A, Yourganov G, Rorden C, Bonilha L, Fridriksson J, Hickok G (2021) Isolating the white matter circuitry of the dorsal language stream: connectome-symptom mapping in stroke induced aphasia. Hum Brain Mapp 42: 5689–5702. 10.1002/hbm.25647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A (1986) Working memory. New York: Clarendon/Oxford UP. [Google Scholar]
- Baddeley A (2003) Working memory: looking back and looking forward. Nat Rev Neurosci 4:829–839. 10.1038/nrn1201 [DOI] [PubMed] [Google Scholar]
- Baddeley AD, Hitch G (1974) Working memory. In: Psychology of learning and motivation (Bower GH, ed), pp 47–89. San Diego: Academic. [Google Scholar]
- Basilakos A, Rorden C, Bonilha L, Moser D, Fridriksson J (2015) Patterns of poststroke brain damage that predict speech production errors in apraxia of speech and aphasia dissociate. Stroke 46:1561–1566. 10.1161/STROKEAHA.115.009211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzagmout M, Gatignol P, Duffau H (2007) Resection of World Health Organization Grade II gliomas involving Broca's area: methodological and functional considerations. Neurosurgery 61:741. 10.1227/01.NEU.0000298902.69473.77 [DOI] [PubMed] [Google Scholar]
- Berezutskaya J, Baratin C, Freudenburg ZV, Ramsey NF (2020) High-density intracranial recordings reveal a distinct site in anterior dorsal precentral cortex that tracks perceived speech. Hum Brain Mapp 41:4587–4609. 10.1002/hbm.25144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal B, Altman N (2010) The connectivity of the superior longitudinal fasciculus: a tractography DTI study. Magn Reson Imaging 28:217–225. 10.1016/j.mri.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Berthier ML (2005) Poststroke aphasia: epidemiology, pathophysiology and treatment. Drugs Aging 22:163–182. 10.2165/00002512-200522020-00006 [DOI] [PubMed] [Google Scholar]
- Bhaya-Grossman I, Chang EF (2022) Speech computations of the human superior temporal gyrus. Annu Rev Psychol 73:79–102. 10.1146/annurev-psych-022321-035256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Medler DA, Desai R, Conant LL, Liebenthal E (2005) Some neurophysiological constraints on models of word naming. Neuroimage 27:677–693. 10.1016/j.neuroimage.2005.04.029 [DOI] [PubMed] [Google Scholar]
- Binder JR (2015) The Wernicke area. Neurology 85:2170–2175. 10.1212/WNL.0000000000002219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR (2017) Current controversies on Wernicke's area and its role in language. Curr Neurol Neurosci Rep 17:58. 10.1007/s11910-017-0764-8 [DOI] [PubMed] [Google Scholar]
- Bohland JW, Guenther FH (2006) An fMRI investigation of syllable sequence production. Neuroimage 32:821–841. 10.1016/j.neuroimage.2006.04.173 [DOI] [PubMed] [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Gitelman DR, Parrish TB, Marsel Mesulam M (2002) Functional anatomy of intra- and cross-modal lexical tasks. Neuroimage 16:7–22. 10.1006/nimg.2002.1081 [DOI] [PubMed] [Google Scholar]
- Bouchard KE, Mesgarani N, Johnson K, Chang EF (2013) Functional organization of human sensorimotor cortex for speech articulation. Nature 495:327–332. 10.1038/nature11911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breshears JD, Molinaro AM, Chang EF (2015) A probabilistic map of the human ventral sensorimotor cortex using electrical stimulation. J Neurosurg 123:340–349. 10.3171/2014.11.JNS14889 [DOI] [PubMed] [Google Scholar]
- Breshears JD, Southwell DG, Chang EF (2019) Inhibition of manual movements at speech arrest sites in the posterior inferior frontal lobe. Neurosurgery 85:E496–E501. 10.1093/neuros/nyy592 [DOI] [PubMed] [Google Scholar]
- Broca P (1861) Remarques Sur Le Siège de La Faculté Du Langage Articulé, Suivies d'une Observation d'aphémie (Perte de La Parole). Bull Mem Soc Anatomique Paris 6:330–357. [Google Scholar]
- Brodmann K (1909) Vergleichende Lokalisationslehre Der Grosshirnrinde in Ihren Prinzipien Dargestellt Auf Grund Des Zellenbaues. Leipzig: J. A. Barth. [Google Scholar]
- Brodmann K, et al. (1910) Feinere Anatomie des Großhirns. In: Handbuch der Neurologie: Erster Band: Allgemeine Neurologie (Abelsdorff G, et al., eds), pp 206–307. Monographien aus dem Gesamtgebiete der Neurologie und Psychiatrie. Berlin: Springer. [Google Scholar]
- Brown S, Ngan E, Liotti M (2008) A larynx area in the human motor cortex. Cereb Cortex 18:837–845. 10.1093/cercor/bhm131 [DOI] [PubMed] [Google Scholar]
- Callan DE, Jones JA, Callan AM, Akahane-Yamada R (2004) Phonetic perceptual identification by native- and second-language speakers differentially activates brain regions involved with acoustic phonetic processing and those involved with articulatory–auditory/orosensory internal models. Neuroimage 22:1182–1194. 10.1016/j.neuroimage.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Carreiras M, Armstrong BC, Perea M, Frost R (2014) The what, when, where, and how of visual word recognition. Trends Cogn Sci 18:90–98. 10.1016/j.tics.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Castellucci GA, Kovach CK, Howard MA, Greenlee JD, Long MA (2022) A speech planning network for interactive language use. Nature 602:117–122. 10.1038/s41586-021-04270-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerkevich CM, Rathelot JA, Strick PL (2022) Cortical basis for skilled vocalization. Proc Natl Acad Sci USA 119:e2122345119. 10.1073/pnas.2122345119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Clark A, Smith JS, Polley MY, Chang SM, Barbaro NM, Parsa AT, McDermott MW, Berger MS (2011) Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long-term survival: clinical article. J Neurosurg 114:566–573. 10.3171/2010.6.JNS091246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Raygor KP, Berger MS (2015) Contemporary model of language organization: an overview for neurosurgeons. J Neurosurg 122:250–261. 10.3171/2014.10.JNS132647 [DOI] [PubMed] [Google Scholar]
- Chang EF, Breshears JD, Raygor KP, Lau D, Molinaro AM, Berger MS (2017) Stereotactic probability and variability of speech arrest and anomia sites during stimulation mapping of the language dominant hemisphere. J Neurosurg 126:114–121. 10.3171/2015.10.JNS151087 [DOI] [PubMed] [Google Scholar]
- Chang EF, Kurteff G, Wilson SM (2018) Selective interference with syntactic encoding during sentence production by direct electrocortical stimulation of the inferior frontal gyrus. J Cogn Neurosci 30:411–420. 10.1162/jocn_a_01215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Kurteff G, Andrews JP, Briggs RG, Conner AK, Battiste JD, Sughrue ME (2020) Pure apraxia of speech after resection based in the posterior middle frontal gyrus. Neurosurgery 87:E383–E389. 10.1093/neuros/nyaa002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier J, Anumanchipalli GK, Johnson K, Chang EF (2018) Encoding of articulatory kinematic trajectories in human speech sensorimotor cortex. Neuron 98:1042–1054.e4. 10.1016/j.neuron.2018.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung C, Hamilton LS, Johnson K, Chang EF (2016) The auditory representation of speech sounds in human motor cortex. Elife 5:e12577. 10.7554/eLife.12577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholin J, Dell GS, Levelt WJM (2011) Planning and articulation in incremental word production: syllable-frequency effects in English. J Exp Psychol Learn Mem Cogn 37:109–122. 10.1037/a0021322 [DOI] [PubMed] [Google Scholar]
- Clark HM, Duffy JR, Whitwell JL, Ahlskog JE, Sorenson J, Josephs KA (2014) Clinical and imaging characterization of progressive spastic dysarthria. Eur J Neurol 21:368–376. 10.1111/ene.12271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloutman L, Gingis L, Newhart M, Davis C, Heidler-Gary J, Crinion J, Hillis AE (2009) A neural network critical for spelling. Ann Neurol 66:249–253. 10.1002/ana.21693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Code C (2021) Contemporary issues in apraxia of speech. Aphasiology 35:391–396. 10.1080/02687038.2021.1896108 [DOI] [Google Scholar]
- Conner CR, Kadipasaoglu CM, Shouval HZ, Hickok G, Tandon N (2019) Network dynamics of Broca's area during word selection. PLoS One 14:e0225756. 10.1371/journal.pone.0225756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DF, Graziano MSA (2004) Sensorimotor integration in the precentral gyrus: polysensory neurons and defensive movements. J Neurophysiol 91:1648–1660. 10.1152/jn.00955.2003 [DOI] [PubMed] [Google Scholar]
- Cushing H (1909) A note upon the faradic stimulation of the postcentral gyrus in conscious patients. Brain 32:44–53. 10.1093/brain/32.1.44 [DOI] [Google Scholar]
- De Beeck MO, Legros B, Gaspard N, Bourguignon M, Jurysta F, Van Bogaert P, Goldman S, Jousmäki V, De Tiège X (2011) Supplementary motor cortex involvement in reading epilepsy revealed by magnetic source imaging. Epilepsia 52:e31–e34. 10.1111/j.1528-1167.2011.03050.x [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L, Cohen L, Bihan DL, Mangin JF, Poline JB, Rivière D (2001) Cerebral mechanisms of word masking and unconscious repetition priming. Nat Neurosci 4:752–758. 10.1038/89551 [DOI] [PubMed] [Google Scholar]
- Dichter BK, Breshears JD, Leonard MK, Chang EF (2018) The control of vocal pitch in human laryngeal motor cortex. Cell 174:21–31.e9. 10.1016/j.cell.2018.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Melloni L, Zhang H, Tian X, Poeppel D (2016) Cortical tracking of hierarchical linguistic structures in connected speech. Nat Neurosci 19:158–164. 10.1038/nn.4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Buchsbaum BR, Grady CL, Alain C (2014) Noise differentially impacts phoneme representations in the auditory and speech motor systems. Proc Natl Acad Sci USA 111:7126–7131. 10.1073/pnas.1318738111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffau H, Gatignol ST, Mandonnet E, Capelle L, Taillandier L (2008) Intraoperative subcortical stimulation mapping of language pathways in a consecutive series of 115 patients with grade II glioma in the left dominant hemisphere. J Neurosurg 109:461–471. 10.3171/JNS/2008/109/9/0461 [DOI] [PubMed] [Google Scholar]
- Duffy JR (2019) Motor speech disorders: substrates. In: Differential diagnosis, and management. Amsterdam: Elsevier. [Google Scholar]
- Duffy JR, Utianski RL, Josephs KA (2021) Primary progressive apraxia of speech: from recognition to diagnosis and care. Aphasiology 35:560–591. 10.1080/02687038.2020.1787732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichert N, Papp D, Mars RB, Watkins KE (2020) Mapping human laryngeal motor cortex during vocalization. Cereb Cortex 30:6254–6269. 10.1093/cercor/bhaa182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W, Li J, Qi G, Li S, Sigman M, Wang L (2019) Statistical inference of body representation in the macaque brain. Proc Natl Acad Sci USA 116:20151–20157. 10.1073/pnas.1902334116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Miranda JC, Wang Y, Pathak S, Stefaneau L, Verstynen T, Yeh FC (2015) Asymmetry, connectivity, and segmentation of the arcuate fascicle in the human brain. Brain Struct Funct 220:1665–1680. 10.1007/s00429-014-0751-7 [DOI] [PubMed] [Google Scholar]
- Filevich E, Kühn S, Haggard P (2012) Negative motor phenomena in cortical stimulation: implications for inhibitory control of human action. Cortex 48:1251–1261. 10.1016/j.cortex.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Flinker A, Korzeniewska A, Shestyuk AY, Franaszczuk PJ, Dronkers NF, Knight RT, Crone NE (2015) Redefining the role of Broca's area in speech. Proc Natl Acad Sci USA 112:2871–2875. 10.1073/pnas.1414491112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornia L, Puglisi G, Leonetti A, Bello L, Berti A, Cerri G, Garbarini F (2020) Direct electrical stimulation of the premotor cortex shuts down awareness of voluntary actions. Nat Commun 11:705. 10.1038/s41467-020-14517-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forseth K, Pitkow X, Fischer-Baum S, Tandon N (2021) What the brain does as we speak. bioRxiv 429841. 10.1101/2021.02.05.429841. [DOI] [Google Scholar]
- Fox RJ, Kasner SE, Chatterjee A, Chalela JA (2001) Aphemia: an isolated disorder of articulation. Clin Neurol Neurosurg 103:123–126. 10.1016/S0303-8467(01)00126-3 [DOI] [PubMed] [Google Scholar]
- Fuertinger S, Horwitz B, Simonyan K (2015) The functional connectome of speech control. PLoS Biol 13:e1002209. 10.1371/journal.pbio.1002209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajardo-Vidal A, Lorca-Puls DL, Team P, Warner H, Pshdary B, Crinion JT, Leff AP, Hope TM, Geva S, Seghier ML, Green DW, Bowman H, Price CJ (2021) Damage to Broca's area does not contribute to long-term speech production outcome after stroke. Brain 144:817–832. 10.1093/brain/awaa460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon S, Li H, Fan L, Müller VI, Cieslik EC, Hoffstaedter F, Reid AT, Langner R, Grefkes C, Fox PT, Moebus S, Caspers S, Amunts K, Jiang T, Eickhoff SB (2017) The right dorsal premotor mosaic: organization, functions, and connectivity. Cereb Cortex 27:2095–2110. 10.1093/cercor/bhw065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genon S, Reid A, Li H, Fan L, Müller VI, Cieslik EC, Hoffstaedter F, Langner R, Grefkes C, Laird AR, Fox PT, Jiang T, Amunts K, Eickhoff SB (2018) The heterogeneity of the left dorsal premotor cortex evidenced by multimodal connectivity-based parcellation and functional characterization. Neuroimage 170:400–411. 10.1016/j.neuroimage.2017.02.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N (1970) The organization of language and the brain. Science 170:940–944. 10.1126/science.170.3961.940 [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M, Smith SM, Van Essen DC (2016) A multi-modal parcellation of human cerebral cortex. Nature 536:171–178. 10.1038/nature18933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabski K, Lamalle L, Vilain C, Schwartz JL, Vallée N, Tropres I, Baciu M, Le Bas JF, Sato M (2012) Functional MRI assessment of orofacial articulators: neural correlates of lip, jaw, larynx, and tongue movements. Hum Brain Mapp 33:2306–2321. 10.1002/hbm.21363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Jones DT, Strand EA, Rabinstein AA, Duffy JR, Josephs KA (2014) The neuroanatomy of pure apraxia of speech in stroke. Brain Lang 129:43–46. 10.1016/j.bandl.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano MS, Gandhi S (2000) Location of the polysensory zone in the precentral gyrus of anesthetized monkeys. Exp Brain Res 135:259–266. 10.1007/s002210000518 [DOI] [PubMed] [Google Scholar]
- Griffin DM, Hoffman DS, Strick PL (2015) Corticomotoneuronal cells are 'functionally tuned.' Science 350:667–670. 10.1126/science.aaa8035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther FH (2016) Neural control of speech. Cambridge, MA: Massachusetts Institute of Technology. [Google Scholar]
- Haley KL, Jacks A, de Riesthal M, Abou-Khalil R, Roth HL (2012) Toward a quantitative basis for assessment and diagnosis of apraxia of speech. J Speech Lang Hear Res 55:S1502–S1517. 10.1044/1092-4388(2012/11-0318) [DOI] [PubMed] [Google Scholar]
- Haley KL, Overton HB (2001) Word length and vowel duration in apraxia of speech: the use of relative measures. Brain Lang 79:397–406. 10.1006/brln.2001.2494 [DOI] [PubMed] [Google Scholar]
- Henry ML, Wilson SM, Babiak MC, Mandelli ML, Beeson PM, Miller ZA, Gorno-Tempini ML (2016) Phonological processing in primary progressive aphasia. J Cogn Neurosci 28:210–222. 10.1162/jocn_a_00901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbet G, Moritz-Gasser S, Duffau H (2018) Electrical stimulation of the dorsolateral prefrontal cortex impairs semantic cognition. Neurology 90:e1077–e1084. 10.1212/WNL.0000000000005174 [DOI] [PubMed] [Google Scholar]
- Hickok G (2012) Computational neuroanatomy of speech production. Nat Rev Neurosci 13:135–145. 10.1038/nrn3158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashiyama Y, Hamada T, Saito A, Morihara K, Okamoto M, Kimura K, Joki H, Kishida H, Doi H, Ueda N, Takeuchi H, Tanaka F (2021) Neural mechanisms of foreign accent syndrome: lesion and network analysis. Neuroimage Clin 31:102760. 10.1016/j.nicl.2021.102760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara K, Ichikawa H, Suzuki Y, Shiota J, Nakano I, Kawamura M (2010) Is lesion of Exner's area linked to progressive agraphia in amyotrophic lateral sclerosis with dementia? An autopsy case report. Behav Neurol 23:153–158. 10.1155/2010/146912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itabashi R, Nishio Y, Kataoka Y, Yazawa Y, Furui E, Matsuda M, Mori E (2016) Damage to the left precentral gyrus is associated with apraxia of speech in acute stroke. Stroke 47:31–36. 10.1161/STROKEAHA.115.010402 [DOI] [PubMed] [Google Scholar]
- Jonkers R, van der Scheer F, Gilbers D (2017) The common denominator in the perception of accents in cases with foreign accent syndrome. Aphasiology 31:1021–1043. 10.1080/02687038.2016.1232362 [DOI] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, Jack CR, Whitwell JL (2013) Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology 81:337–345. 10.1212/WNL.0b013e31829c5ed5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens U (1974) On the elicitability of vocalization from the cortical larynx area. Brain Res 81:564–566. [DOI] [PubMed] [Google Scholar]
- Jürgens U (2009) The neural control of vocalization in mammals: a review. J Voice 23:1–10. 10.1016/j.jvoice.2007.07.005 [DOI] [PubMed] [Google Scholar]
- Kaestner E, Thesen T, Devinsky O, Doyle W, Carlson C, Halgren E (2021) An intracranial electrophysiology study of visual language encoding: the contribution of the precentral gyrus to silent reading. J Cogn Neurosci 33:2197–2214. 10.1162/jocn_a_01764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner E, Wu X, Friedman D, Dugan P, Devinsky O, Carlson C, Doyle W, Thesen T, Halgren E (2022) The precentral gyrus contributions to the early time-course of grapheme-to-phoneme conversion. Neurobiol Lang 3:18–45. 10.1162/nol_a_00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitel A, Gross J, Kayser C (2018) Perceptually relevant speech tracking in auditory and motor cortex reflects distinct linguistic features. PLoS Biol 16:e2004473. 10.1371/journal.pbio.2004473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkar GH, Isnard J, Montavont A, Catenoix H, Rheims S, Guénot M (2021) Awake craniotomy for epilepsy surgery on eloquent speech areas: a single-centre experience. Epileptic Disord 23:347–356. 10.1684/epd.2021.1275 [DOI] [PubMed] [Google Scholar]
- Leonard MK, Baud MO, Sjerps MJ, Chang EF (2016) Perceptual restoration of masked speech in human cortex. Nat Commun 7:13619. 10.1038/ncomms13619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MK, Cai R, Babiak MC, Ren A, Chang EF (2019a) The peri-Sylvian cortical network underlying single word repetition revealed by electrocortical stimulation and direct neural recordings. Brain Lang 193:58–72. 10.1016/j.bandl.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MK, Desai M, Hungate D, Cai R, Singhal NS, Knowlton RC, Chang EF (2019b) Direct cortical stimulation of inferior frontal cortex disrupts both speech and music production in highly trained musicians. Cogn Neuropsychol 36:158–166. 10.1080/02643294.2018.1472559 [DOI] [PubMed] [Google Scholar]
- Levelt WJ, Wheeldon L (1994) Do speakers have access to a mental syllabary? Cognition 50:239–269. 10.1016/0010-0277(94)90030-2 [DOI] [PubMed] [Google Scholar]
- Leyton AS, Sherrington CS (1917) Observations on the excitable cortex of the chimpanzee, orangutan, and gorilla. Exp Physiol 11:135–222. 10.1113/expphysiol.1917.sp000240 [DOI] [Google Scholar]
- Leyton CE, Ballard KJ, Piguet O, Hodges JR (2014) Phonologic errors as a clinical marker of the logopenic variant of PPA. Neurology 82:1620–1627. 10.1212/WNL.0000000000000387 [DOI] [PubMed] [Google Scholar]
- Li N, Daie K, Svoboda K, Druckmann S (2016) Robust neuronal dynamics in premotor cortex during motor planning. Nature 532:459–464. 10.1038/nature17643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman AM, Cooper FS, Shankweiler DP, Studdert-Kennedy M (1967) Perception of the speech code. Psychol Rev 74:431–461. 10.1037/h0020279 [DOI] [PubMed] [Google Scholar]
- Liberman AM, Mattingly IG (1985) The motor theory of speech perception revised. Cognition 21:1–36. 10.1016/0010-0277(85)90021-6 [DOI] [PubMed] [Google Scholar]
- Lipkin B, Tuckute G, Affourtit J, Small H, Mineroff Z, Kean H, Jouravlev O, Rakocevic L, Pritchett B, Siegelman M, Hoeflin C, Pongos A, Blank IA, Struhl MK, Ivanova A, Shannon S, Sathe A, Hoffmann M, Nieto-Castañón A, Fedorenko E (2022) Probabilistic atlas for the language network based on precision fMRI data from >800 individuals. Sci Data 9:1–10. 10.1038/s41597-022-01645-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Zhao Z, Zhang J, Wu B, Zhu Y, Chang EF, Wu J, Duffau H, Berger MS (2021) Functional maps of direct electrical stimulation-induced speech arrest and anomia: a multicentre retrospective study. Brain 144:2541–2553. 10.1093/brain/awab125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN (2005) Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15:854–869. 10.1093/cercor/bhh186 [DOI] [PubMed] [Google Scholar]
- Maldonado IL, Moritz-Gasser S, Duffau H (2011) Does the left superior longitudinal fascicle subserve language semantics? A brain electrostimulation study. Brain Struct Funct 216:263–274. 10.1007/s00429-011-0309-x [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Duffau H (2021) Broca's area: why was neurosurgery neglected for so long when seeking to re-establish the scientific truth? Brain 144:e60. 10.1093/brain/awab195 [DOI] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Berger MS, Lawton MT, Arnold CM, Marco de Lucas E, Duffau H (2013) Analysis of the subcomponents and cortical terminations of the Perisylvian superior longitudinal fasciculus: a fiber dissection and DTI tractography study. Brain Struct Funct 218:105–121. 10.1007/s00429-012-0386-5 [DOI] [PubMed] [Google Scholar]
- Matchin W, Hickok G (2020) The cortical organization of syntax. Cereb Cortex 30:1481–1498. 10.1093/cercor/bhz180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Kato C, Sumiyoshi C, Toma K, Duy Thuy DH, Moriya T, Fukuyama H, Nakai T (2003) Discrimination of Exner's area and the frontal eye field in humans: functional magnetic resonance imaging during language and saccade tasks. Neurosci Lett 340:13–16. 10.1016/S0304-3940(03)00050-8 [DOI] [PubMed] [Google Scholar]
- McCrimmon CM, Wang PT, Heydari P, Nguyen A, Shaw SJ, Gong H, Chui LA, Liu CY, Nenadic Z, Do AH (2018) Electrocorticographic encoding of human gait in the leg primary motor cortex. Cereb Cortex 28:2752–2762. 10.1093/cercor/bhx155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesgarani N, Cheung C, Johnson K, Chang EF (2014) Phonetic feature encoding in human superior temporal gyrus. Science 343:1006–1010. 10.1126/science.1245994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR (1978) Broca aphasia: pathologic and clinical. Neurology 28:311–324. 10.1212/wnl.28.4.311 [DOI] [PubMed] [Google Scholar]
- Morshed RA, Lee AT, Wang EJ, Young JS, Cha S, Hervey-Jumper SL, Berger MS (2021) Functional outcomes after resection of middle frontal gyrus diffuse gliomas. J Neurosurg 137:1–8. 10.3171/2021.8.JNS211624 [DOI] [PubMed] [Google Scholar]
- Moser D, Basilakos A, Fillmore P, Fridriksson J (2016) Brain damage associated with apraxia of speech: evidence from case studies. Neurocase 22:346–356. 10.1080/13554794.2016.1172645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R, Kinoshita M, Shinohara H, Nakada M (2020) The superior longitudinal fascicle: reconsidering the fronto-parietal neural network based on anatomy and function. Brain Imaging Behav 14:2817–2830. 10.1007/s11682-019-00187-4 [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Yamagata T, Tanji J, Hoshi E (2008) Transformation of a virtual action plan into a motor plan in the premotor cortex. J Neurosci 28:10287–10297. 10.1523/JNEUROSCI.2372-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozker M, Doyle W, Devinsky O, Flinker A (2022) A cortical network processes auditory error signals during human speech production to maintain fluency. PLoS Biol 20:e3001493. 10.1371/journal.pbio.3001493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce TM, Moran DW (2012) Strategy-dependent encoding of planned arm movements in the dorsal premotor cortex. Science 337:984–988. 10.1126/science.1220642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton C, Zaidi HA, Chaichana KL, Raza SM, Carson BS, Cohen-Gadol AA, Quinones-Hinojosa A (2012) Harvey Cushing's contributions to motor mapping: 1902–1912. Cortex 48:7–14. 10.1016/j.cortex.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Penfield W, Rasmussen T (1949) Vocalization and arrest of speech. Arch Neurol Psychiatry 61:21–27. 10.1001/archneurpsyc.1949.02310070027002 [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E (1937) Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60:389–443. 10.1093/brain/60.4.389 [DOI] [Google Scholar]
- Penfield W, Roberts L (1959) Speech and brain-mechanisms. Princeton, NJ: Princeton UP. [Google Scholar]
- Pinson H, Van Lerbeirghe J, Vanhauwaert D, Van Damme O, Hallaert G, Kalala JP (2022) The supplementary motor area syndrome: a neurosurgical review. Neurosurg Rev 45:81–90. 10.1007/s10143-021-01566-6 [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJ, Warburton EA, Moore CJ, Howard D, Patterson K, Frackowiak RS, Friston KJ (1996) Hearing and saying: the functional neuro-anatomy of auditory word processing. Brain 119:919–931. 10.1093/brain/119.3.919 [DOI] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH (2011) Primary motor cortex underlies multi-joint integration for fast feedback control. Nature 478:387–390. 10.1038/nature10436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvermüller F, Fadiga L (2010) Active perception: sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci 11:351–360. 10.1038/nrn2811 [DOI] [PubMed] [Google Scholar]
- Pulvermüller F, Huss M, Kherif F, Moscoso del Prado Martin F, Hauk O, Shtyrov Y (2006) Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci USA 103:7865–7870. 10.1073/pnas.0509989103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigg M, Fountain NB (1999) Conduction aphasia elicited by stimulation of the left posterior superior temporal gyrus. J Neurol Neurosurg Psychiatry 66:393–396. 10.1136/jnnp.66.3.393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones-Hinojosa A, Ojemann SG, Sanai N, Dillon WP, Berger MS (2003) Preoperative correlation of intraoperative cortical mapping with magnetic resonance imaging landmarks to predict localization of the Broca area. J Neurosurg 99:311–318. 10.3171/jns.2003.99.2.0311 [DOI] [PubMed] [Google Scholar]
- Rapcsak SZ, Arthur SA, Rubens AB (1988) Lexical agraphia from focal lesion of the left precentral gyrus. Neurology 38:1119–1123. 10.1212/wnl.38.7.1119 [DOI] [PubMed] [Google Scholar]
- Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, Mead S, Beck J, Mummery C, Ourselin S, Warrington EK, Rossor MN, Warren JD (2010) Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage 49:984–993. 10.1016/j.neuroimage.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolston JD, Englot DJ, Benet A, Li J, Cha S, Berger MS (2015) Frontal operculum gliomas: language outcome following resection. J Neurosurg 122:725–734. 10.3171/2014.11.JNS132172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux FE, Dufor O, Giussani C, Wamain Y, Draper L, Longcamp M, Démonet JF (2009) The graphemic/motor frontal area Exner's area revisited. Ann Neurol 66:537–545. 10.1002/ana.21804 [DOI] [PubMed] [Google Scholar]
- Roux FE, Draper L, Köpke B, Démonet JF (2010) Who actually read Exner? Returning to the source of the frontal 'writing centre' hypothesis. Cortex 46:1204–1210. 10.1016/j.cortex.2010.03.001 [DOI] [PubMed] [Google Scholar]
- Roux FE, Durand JB, Jucla M, Réhault E, Reddy M, Démonet JF (2012) Segregation of lexical and sub-lexical reading processes in the left Perisylvian cortex. PLoS One 7:e50665. 10.1371/journal.pone.0050665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi D, Béland R, Nguyen DK, Pouliot P, Mohamed IS, Vannasing P, Tremblay J, Lassonde M, Gallagher A (2016) Recruitment of the left precentral gyrus in reading epilepsy: a multimodal neuroimaging study. Epilepsy Behav Case Rep 5:19–22. 10.1016/j.ebcr.2016.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar S, Rick J, Chandra A, Yagnik G, Aghi MK (2019) Functional brain mapping: overview of techniques and their application to neurosurgery. Neurosurg Rev 42:639–647. 10.1007/s10143-018-1007-4 [DOI] [PubMed] [Google Scholar]
- Salek-Haddadi A, Mayer T, Hamandi K, Symms M, Josephs O, Fluegel D, Woermann F, Richardson MP, Noppeney U, Wolf P, Koepp MJ (2009) Imaging seizure activity: a combined EEG/EMG-fMRI study in reading epilepsy. Epilepsia 50:256–264. 10.1111/j.1528-1167.2008.01737.x [DOI] [PubMed] [Google Scholar]
- Salinas E, Romo R (1998) Conversion of sensory signals into motor commands in primary motor cortex. J Neurosci 18:499–511. 10.1523/JNEUROSCI.18-01-00499.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TL, Perrachione TK (2019) Common cortical architectures for phonological working memory identified in individual brains. Neuroimage 202:116096. 10.1016/j.neuroimage.2019.116096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonyan K, Jürgens U (2003) Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res 974:43–59. 10.1016/S0006-8993(03)02548-4 [DOI] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM (1999) Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain 122:2033–2046. 10.1093/brain/122.11.2033 [DOI] [PubMed] [Google Scholar]
- Takayama Y, Sugishita M, Kido T, Ogawa M, Akiguchi I (1993) A case of foreign accent syndrome without aphasia caused by a lesion of the left precentral gyrus. Neurology 43:1361–1363. 10.1212/wnl.43.7.1361 [DOI] [PubMed] [Google Scholar]
- Takei T, Lomber SG, Cook DJ, Scott SH (2021) Transient deactivation of dorsal premotor cortex or parietal area 5 impairs feedback control of the limb in macaques. Curr Biol 31:1476–1487.e5. 10.1016/j.cub.2021.01.049 [DOI] [PubMed] [Google Scholar]
- Tate MC, Herbet G, Moritz-Gasser S, Tate JE, Duffau H (2014) Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain 137:2773–2782. 10.1093/brain/awu168 [DOI] [PubMed] [Google Scholar]
- Taylor JS, Rastle K, Davis MH (2013) Can cognitive models explain brain activation during word and pseudoword reading? A meta-analysis of 36 neuroimaging studies. Psychol Bull 139:766–791. 10.1037/a0030266 [DOI] [PubMed] [Google Scholar]
- Terao Y, Ugawa Y, Yamamoto T, Sakurai Y, Masumoto T, Abe O, Masutani Y, Aoki S, Tsuji S (2007) Primary face motor area as the motor representation of articulation. J Neurol 254:442–447. 10.1007/s00415-006-0385-7 [DOI] [PubMed] [Google Scholar]
- Terumitsu M, Fujii Y, Suzuki K, Kwee IL, Nakada T (2006) Human primary motor cortex shows hemispheric specialization for speech. Neuroreport 17:1091–1095. 10.1097/01.wnr.0000224778.97399.c4 [DOI] [PubMed] [Google Scholar]
- Tokida H, Shiga Y, Shimoe Y, Yamori S, Tanaka A, Kuriyama M (2017) Foreign accent syndrome caused by the left precentral infarction: a case report. Rinsho Shinkeigaku 57:293–297. 10.5692/clinicalneurol.cn-000988 [DOI] [PubMed] [Google Scholar]
- Van Der Merwe A (2021) New perspectives on speech motor planning and programming in the context of the four-level model and its implications for understanding the pathophysiology underlying apraxia of speech and other motor speech disorders. Aphasiology 35:397–423. 10.1080/02687038.2020.1765306 [DOI] [Google Scholar]
- Venezia JH, Thurman SM, Richards VM, Hickok G (2019) Hierarchy of speech-driven spectrotemporal receptive fields in human auditory cortex. Neuroimage 186:647–666. 10.1016/j.neuroimage.2018.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venezia JH, Richards VM, Hickok G (2021) Speech-driven spectrotemporal receptive fields beyond the auditory cortex. Hear Res 408:108307. 10.1016/j.heares.2021.108307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Korzeniewska A, Usami K, Valenzuela A, Crone NE (2021) The dynamics of language network interactions in lexical selection: an intracranial EEG study. Cereb Cortex 31:2058–2070. 10.1093/cercor/bhaa344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernicke C (1874) Der aphasische Symptomenkomplex. Breslau: Cohn & Weigert. [Google Scholar]
- Wilson SM, Pinar Saygin A, Sereno MI, Iacoboni M (2004) Listening to speech activates motor areas involved in speech production. Nat Neurosci 7:701–702. 10.1038/nn1263 [DOI] [PubMed] [Google Scholar]
- Wilson SM, Lam D, Babiak MC, Perry DW, Shih T, Hess CP, Berger MS, Chang EF (2015) Transient aphasias after left hemisphere resective surgery. J Neurosurg 123:581–593. 10.3171/2015.4.JNS141962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Yen M, Eriksson DK (2018) An adaptive semantic matching paradigm for reliable and valid language mapping in individuals with aphasia. Hum Brain Mapp 39:3285–3307. 10.1002/hbm.24077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SM, Entrup JL, Schneck SM, Onuscheck CF, Levy DF, Rahman M, Willey E, Casilio M, Yen M, Brito A, Kam W, Davis LT, de Riesthal M, Kirshner HS (2022) Recovery from aphasia in the first year after stroke. Brain. Advance online publication. Retrieved Apr 7, 2022. 10.1093/brain/awac129. 10.1093/brain/awac129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolnough O, Donos C, Curtis A, Rollo PS, Roccaforte ZJ, Dehaene S, Fischer-Baum S, Tandon N (2022) A spatiotemporal map of reading aloud. J Neurosci 42:5438–5450. 10.1523/JNEUROSCI.2324-21.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lu J, Zhang H, Zhang J, Yao C, Zhuang D, Qiu T, Guo Q, Hu X, Mao Y, Zhou L (2015) Direct evidence from intraoperative electrocortical stimulation indicates shared and distinct speech production center between Chinese and English languages. Hum Brain Mapp 36:4972–4985. 10.1002/hbm.22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Dong M, Chen Y, Delgado AM, Hughes NC, Zhang L, O'Connor DH (2022) Cortical processing of flexible and context-dependent sensorimotor sequences. Nature 603:464–469. 10.1038/s41586-022-04478-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen M, DeMarco AT, Wilson SM (2019) Adaptive paradigms for mapping phonological regions in individual participants. Neuroimage 189:368–379. 10.1016/j.neuroimage.2019.01.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HG, Leonard MK, Chang EF (2019) The encoding of speech sounds in the superior temporal gyrus. Neuron 102:1096–1110. 10.1016/j.neuron.2019.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhao Z, Zhang J, Farrukh Hameed NU, Zhu F, Feng R, Zhang X, Lu J, Wu J (2021) Electrical stimulation–induced speech–related negative motor responses in the lateral frontal cortex. J Neurosurg 137:496–504. 10.3171/2021.9.JNS211069 [DOI] [PubMed] [Google Scholar]


