Abstract
Both the cerebellum and the basal ganglia are known for their roles in motor control and motivated behavior. These two systems have been classically considered as independent structures that coordinate their contributions to behavior via separate cortico-thalamic loops. However, recent evidence demonstrates the presence of a rich set of direct connections between these two regions. Although there is strong evidence for connections in both directions, for brevity we limit our discussion to the better-characterized connections from the cerebellum to the basal ganglia. We review two sets of such connections: disynaptic projections through the thalamus and direct monosynaptic projections to the midbrain dopaminergic nuclei, the VTA and the SNc. In each case, we review the evidence for these pathways from anatomic tracing and physiological recordings, and discuss their potential functional roles. We present evidence that the disynaptic pathway through the thalamus is involved in motor coordination, and that its dysfunction contributes to motor deficits, such as dystonia. We then discuss how cerebellar projections to the VTA and SNc influence dopamine release in the respective targets of these nuclei: the NAc and the dorsal striatum. We argue that the cerebellar projections to the VTA may play a role in reward-based learning and therefore contribute to addictive behavior, whereas the projection to the SNc may contribute to movement vigor. Finally, we speculate how these projections may explain many of the observations that indicate a role for the cerebellum in mental disorders, such as schizophrenia.
Keywords: Cerebellum, Basal-ganglia, dopamine, motor coordination, reward processing, movement vigor
Introduction
Coordinated movements leading to meaningful behavior require integration of sensory cues that inform the animal of its environment and the state of its body, activating and coordinating a multitude of skeletal muscles, and then ensuring that all movements are timed correctly and take place as intended (Ebbesen and Brecht, 2017). In addition, during goal-directed behavior, the brain computes what actions are optimal, and even what goals are worth pursuing. These are fundamental components of behavior and are thought to be processed by the brain's reward system, which computes motivation: the willingness to perform an action (Salamone and Correa, 2012; Berke, 2018). Even the simplest volitional behavior requires immense neural computation; therefore, the motor systems constitute a major component of the CNS. In mammals, voluntary movements are planned in the cerebral cortex and executed by spinal cord circuits. Two additional major structures of the brain, the basal ganglia and the cerebellum, are also considered to be components of the motor system. By and large, these two systems control movement indirectly by regulating the activity of the upper motor systems of the cerebral cortex and brainstem.
The basal ganglia system ensures that the correct movements are initiated and maintained, while unwanted movements are suppressed (Klaus et al., 2019). In contrast, the cerebellum guarantees that movements take place in a smooth and coordinated way (D'Angelo, 2018). In addition to their motor functions, both the basal ganglia and the cerebellum are involved in processing sensory perception and higher-order cognitive functions (Middleton and Strick, 2000; Wagner and Luo, 2020). Both systems are also essential components of the brain's learning mechanisms (Bostan and Strick, 2018). The basal ganglia include the brain's so-called reward system, which controls both motivation and associative learning primarily as positive-reinforcement and classical conditioning. As with the prominent role of the basal ganglia in associative learning, the cerebellum also plays a central role in learning and memory. Cerebellar learning is primarily understood in the context of error correction in motor learning, for example, in learning to throw or catch a frisbee better and better (Popa et al., 2016). Borrowing terminology from Computer Science, cerebellar learning is often considered a form of error-based supervised learning (Raymond and Medina, 2018) because it uses feedback about the motor system's performance to adjust the system parameters and improve future performance. However, recent studies have indicated that cerebellar signals also process reward information, suggesting a role for the cerebellum in reward-based reinforcement learning (Taylor and Ivry, 2014).
All basal ganglia functions depend crucially on neuromodulation by dopamine (DA). DA release from midbrain nuclei to basal ganglia structures, especially the striatum, is essential for the initiation and vigor of movements (da Silva et al., 2018). Dysfunctions of DA neurons, as in Parkinson's disease, or their targets in the striatum, as in Huntington's disease, result in debilitating deficits of movement and action (Klein et al., 2019). DA in the basal ganglia is also essential for the reinforcement learning system (Lerner et al., 2021), and DA fluctuations are often considered a proxy for motivation and reward processing (Salamone and Correa, 2012).
Both the basal ganglia and the cerebellum systems receive input from the cerebral cortex and sensory systems, and both communicate back to the cortex, via mostly distinct thalamic nuclei (Percheron et al., 1996; Sakai et al., 1996). Thus, either system can be considered a component of a cortical loop, and these two loops have been historically thought to interact at the cortical level. Yet, early functional and anatomic studies (Li and Parker, 1969; Snider et al., 1976; Nieoullon et al., 1978; Nieoullon and Dusticier, 1980) as well as human brain imaging (Kwon and Jang, 2014; Milardi et al., 2016) had suggested the possibility of more direct connections between these structures. The development of modern anatomic techniques allowed for the discovery of interactions between the cerebellum and the basal ganglia independent of the cortical loops (Hoshi et al., 2005; Bostan et al., 2010), leading to a renewed interest in unraveling the nature of interactions between these two important brain structures (Cacciola et al., 2017; Caligiore et al., 2017). In the past decade, a handful of studies used modern techniques in viral-genetic circuit mapping, imaging, optogenetics, and electrophysiology to show that the cerebellum also provides a direct input to the basal ganglia DA centers (Watabe-Uchida et al., 2012; Beier et al., 2015; Carta et al., 2019; Washburn et al., 2022), increasing the dimensions by which the cerebellum can contribute to movement and cognition. Nevertheless, the detailed function of these connections or their mechanism of action remains unclear. In this article, we will review these recent findings and discuss the contribution of the subcortical pathways that connect the cerebellum to the basal ganglia to the processing of (1) movement initiation and coordination and (2) reward-based learning in the basal ganglia. Although there is also strong evidence in support of subcortical connections from the basal ganglia to the cerebellum (Kizer et al., 1976; Ikai et al., 1994; Meissner et al., 2005; Bostan et al., 2010; Sutton et al., 2015; Jwair et al., 2017; Flace et al., 2021), we will not discuss these connections in this review.
Basic cerebellar anatomy and projections to the basal ganglia
Cerebellar processing is performed by the cerebellar cortex, whose anatomic organization is primarily a uniform, repetitive three-layer neuronal circuit. The cellular processing units of the cerebellar cortex are the Purkinje cells, which receive primarily inputs from the pontine nuclei and the inferior olive. The pontine nuclei axons (mossy fibers) synapse onto granule cells, the most abundant cells in the vertebrate brain. Purkinje cells integrate inputs conveyed from the parallel fibers, the granule cell axons, and from climbing fibers, the axons of the inferior olivary neurons. Purkinje cells are tonically active (pacemakers) and have inhibitory GABAergic output. Their axons mainly project to the neurons in the deep cerebellar nuclei (DCN), the cerebellar output nuclei whose efferent projections are sent to other brain regions. As with the Purkinje cells, the DCN neurons are also tonically active. Inhibition by the Purkinje cells results in changes in the pattern and frequency of firing of DCN neurons (Cerminara et al., 2015; Hull and Regehr, 2022).
The DCNs are bilateral and, on each side, are composed of three primary nuclei: the dentate, the interposed, and the fastigial nucleus, each of which somatotopically receives inputs from their overlaying cerebellar cortex. Classical studies suggested that the dentate and interposed nucleus neurons project mainly to the thalamus and the midbrain, while the brainstem and spinal tracts are targeted mainly by the fastigial nuclei (Ito, 1984; Voogd, 2014). While in broad strokes these observations remain valid, recent data show heterogeneity in DCN neurons and broad projections from each nucleus to similar but mostly nonoverlapping target regions (Kebschull et al., 2020), suggesting that each nucleus may have a potentially different functional influence on the target regions. Moreover, historically it is thought that the cerebellar nuclei may serve distinct functions, with the fastigial and interposed nuclei primarily contributing to motor execution, and the dentate to motor planning (Manto and Oulad Ben Taib, 2010; Gao et al., 2018).
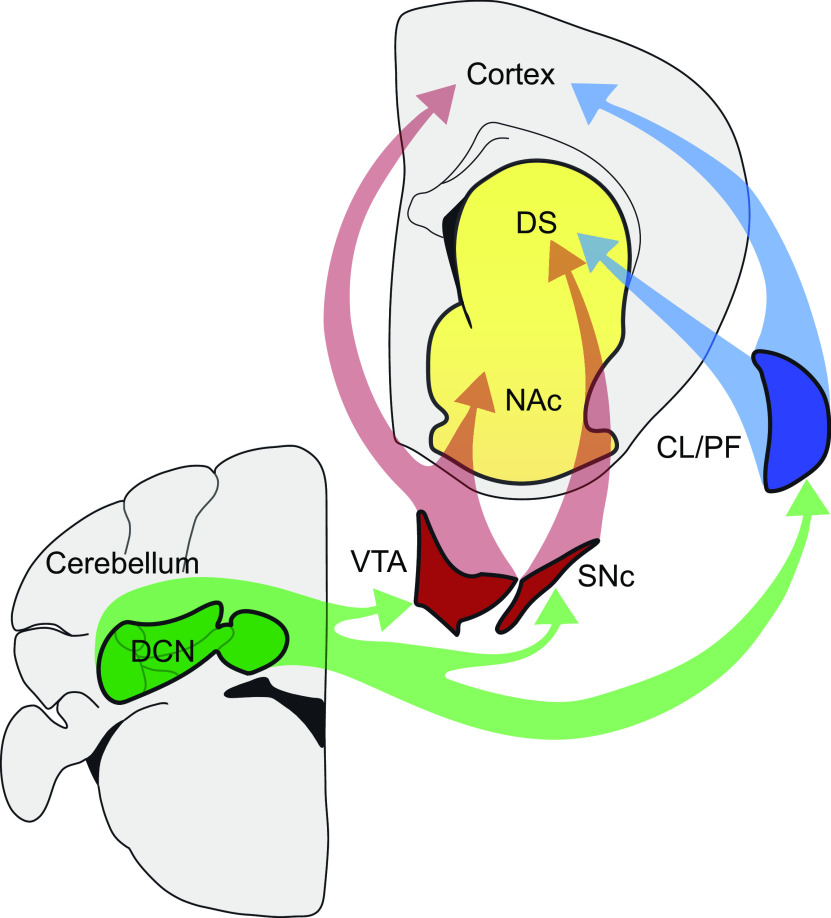
Although early anatomic studies (discussed below) linked the cerebellum with some components of the basal ganglia, recent advances on tracing techniques combined with cutting-edge approaches, such as optogenetics and fiber photometry, have allowed a more rigorous characterization of the details and possible functions of the pathways involved in the cerebellum-basal ganglia connections. Below, we will discuss the evidence linking the cerebellum with the basal ganglia, specifically the pathways through the thalamus and the midbrain DA centers (Fig. 1), and then delineate the possible roles of the cerebellum in these different circuits in terms of motor control, motivation, and reward-related behaviors.
Figure 1.
Anatomical pathways connecting the cerebellum with the basal ganglia. The figure refers only to the noncanonical pathways described in this review. The traditional cerebello-thalamo-cortical pathways are omitted. The DCN of the cerebellum connect with the DS via the thalamic nuclei (CL and PF), and with the NAc and DS via the VTA and the SNc, respectively. The thalamic pathway conveys fast glutamatergic signals to the striatum, contributing to motor coordination. Projections via the VTA and SNc allow the cerebellum to directly contribute to DA release in the DS and the NAc. The VTA, as well as the thalamus, also projects to the cerebral cortex. VTA cortical projections suggest that the cerebellum can directly contribute to cortical DA levels via direct projections to the VTA (this connection is not discussed in this review).
The thalamic pathway and its influence on motor coordination
The cerebellum and basal ganglia have been traditionally considered to be interconnected via the cerebral cortex (Haber and Gdowski, 2004; Nieuwenhuys et al., 2007; Voogd and Ruigrok, 2012). However, both the basal ganglia and the cerebellum communicate to the cortex through the thalamus, albeit through different thalamic nuclei (Percheron et al., 1996; Sakai et al., 1996), although some degree of convergence exists (Hintzen et al., 2018). The thalamus, in turn, has a myriad of connections to subcortical structures, so the idea that the cerebellum and the basal ganglia may have connections through the thalamus has been present for a long time. Interestingly, such connections have been examined in nonmammalian vertebrates. Both the cerebellum and the basal ganglia are brain structures that have been conserved throughout the vertebrate evolutionary history (Nieuwenhuys, 1967; Grillner and Robertson, 2016), and both by far predate the evolution of the neocortex (Van Essen et al., 2018). Many studies have examined the role of these structures in a variety of behaviors in nonmammalian vertebrates (Reiner et al., 1998; Gómez et al., 2010; Brown et al., 2011; Montgomery and Perks, 2019). In songbirds that learn song by imitation, both structures have been implicated in song learning (Ziegler and Ackermann, 2017; Daou and Margoliash, 2021). Connections from the cerebellum to the song-related components of the basal ganglia had been suggested by anatomic studies (Vates et al., 1997; Person et al., 2008; Nicholson et al., 2018), but the functional significance of these connections to sensorimotor learning in songbirds was unknown. A recent study confirmed the presence of a disynaptic connection between the cerebellum and Area X, a striatal nucleus of the songbird basal ganglia, through the thalamus. This pathway is mediated by the dorsal thalamic zone, which is the bird homolog of the mammalian intralaminar, midline, and mediodorsal nuclei (Veenman et al., 1997). Electrical stimulation of the DCN in anesthetized zebra finches provoked a strong increase in the firing rate of Area X neurons, indicating that cerebellar activity can drive these striatal neurons. In addition, DCN lesions resulted in lower imitation scores in song learning, suggesting that the cerebellar projection to the basal ganglia contributes to song learning (Pidoux et al., 2018).
Does a similar pathway exist in mammals? An early anatomic study in the rat used double labeling with traditional tracers and electron microscopy to show an overlapping distribution, in the central lateral nucleus (CL) of the thalamus, of retrograde-labeled neurons of the dorsolateral striatum and anterograde-labeled cerebellar dentate nucleus axon terminals (Ichinohe et al., 2000). The presence of a similar connection was shown using modern anatomic retrograde rabies virus tracing methods in the macaque monkey. This study showed that both the motor and the nonmotor domains of the dentate nucleus are linked to the striatum via a disynaptic connection (Hoshi et al., 2005), and suggested that the intralaminar thalamic nuclei (Smith et al., 2004; Xiao et al., 2018) (which include the CL and the parafascicular nucleus [PF]) may be the intermediary connection between the two structures. The physiological properties of such a connection were explored more recently, in behaving rodents, in a study that specifically examined the connections from the dentate nucleus to the dorsal striatum (DS)-projecting CL thalamic nucleus neurons (Chen et al., 2014). Optogenetic stimulation of the cerebellar axons in the thalamus evoked rapid (∼10 ms) excitation of dorsal striatal neuronal activity, which was abolished by the inactivation of the thalamic CL, but not of the cerebral cortex. This was the first physiological proof that, in mammals, the cerebellum communicates with the basal ganglia independent of the classical cortical route. This study further showed that the cerebello-thalamostriatal pathway can adjust long-term plasticity of cortical projections to the striatum by switching LTP of the corticostriatal synapse to LTD, thus pointing to a potential function of simultaneous activity in these two pathways. Although the basal ganglia connections cover the anterior-medial parts of the thalamus, the cerebellum mainly projects to the posterior-lateral regions of the thalamus. Nevertheless, the existence of overlapping zones, mainly through medial thalamic and intralaminar nuclei, supports the presence of a similar thalamic interaction between the two systems in humans.
What potential function may the cerebello-thalamostriatal (Cb-CL/PF-DS) pathway serve? Cerebellar activity correlates with movement kinematics and is necessary for the real-time adjustment and optimization of the muscle activity (Ito, 1984), while it is thought that the basal ganglia are primarily concerned with the selection of optimal motor commands (Mink, 1996; Doya, 1999; Redgrave et al., 1999; Doya, 2000). It is possible that the real-time muscle kinematics conveyed by the Cb-CL/PF to the basal ganglia would be of value to its selection of the optimal motor commands for complex motor tasks, particularly for movements that require high temporal precision and thus synchronization between the two structures. In support of this hypothesis, Diaz-Hernandez et al. (2018) reported that thalamostriatal projections were activated for several milliseconds before a mouse initiated movement sequences in an operant task, and that inhibition of their activity led to a delay in movement initiation, suggesting that the basal ganglia relies on this information on the millisecond timescale for movement initiation. Studies of dystonia also supported this idea. In a mouse model of cerebellar-induced dystonia, it was shown that the Cb-CL projections erroneously transmit irregular high-frequency activity to the striatum, causing the dorsal striatal neurons to fire in high-frequency bursts. Interrupting the Cb-CL activity rescued the dystonia in these mice (Chen et al., 2014). Together, these studies indicate that the Cb-CL-DS signaling is essential for intact motor coordination, and inappropriately timed or excessive signaling from the cerebellum to the striatum via the thalamus can aggravate or cause abnormal movement disorders, such as dystonia.
Interactions through the midbrain dopaminergic nuclei
Midbrain DA nuclei are a key brain region in charge of providing DA to the basal ganglia and the cerebral cortex. DA release in the basal ganglia is necessary for reward processing and movement initiation, and impairment in DA release dynamics could produce severe behavioral consequences in motor and nonmotor domains. Recent evidence showing that the cerebellum directly contributes to midbrain dopaminergic activity in behaving animals suggests that cerebellar input plays a direct role in basal ganglia DA levels, and proposes that this circuit might play a central role in linking the cerebellum to motor and nonmotor dysfunctions. Below, we review the recent work supporting this idea.
Projections to the VTA and their potential influence on motivation and reward evaluation
The ventral tegmental area (VTA) is a ventromedial midbrain nucleus, and is one of the major sources of DA in the brain together with the substantia nigra. The VTA plays a role in a variety of brain functions, such as positive and negative reinforcement, decision-making, salience, and aversion (Ungless et al., 2004; Berridge, 2007; Matsumoto and Hikosaka, 2009; Bromberg-Martin et al., 2010; Lammel et al., 2012; Fry et al., 2021). The VTA receives inputs from different brain regions, including the PFC, NAc, lateral habenula, and the ventral pallidum (Geisler et al., 2007; Watabe-Uchida et al., 2012; Ogawa et al., 2014; Beier et al., 2015; Faget et al., 2016) and sends dopaminergic projections to several brain regions, primarily through two major pathways: the mesolimbic pathway to the NAc (Fallon and Moore, 1978; Mogenson et al., 1980; Phillipson and Griffiths, 1985) and the mesocortical pathway to the PFC (Swanson, 1982) (see Fig. 1). The mesolimbic pathway has been involved in reward-related behaviors, motivation, and aversion (Salamone, 1994; Cardinal et al., 2002; Carelli, 2002; Yun et al., 2004; Goto and Grace, 2005). The VTA is mainly composed of DA neurons, while the rest of the neurons are GABAergic or (a smaller fraction) glutamatergic. The GABAergic and glutamatergic subgroups include both local interneurons and projection neurons, and some fraction of these corelease DA as well (Morales and Margolis, 2017).
Almost 50 years ago, some anatomic studies pointed out cerebellar projections to the VTA, but there were no follow-up explorations of this pathway until the recent development of modern tracing methods. The early studies performed tracing experiments using post-lesion staining of degenerating projections, and anterograde or retrograde tracing in cats and rats, and showed that all three DCNs contribute to VTA projections (Snider et al., 1976; Phillipson, 1979; Teune et al., 2000). The development of transsynaptic anterograde and retrograde viruses allowed researchers to corroborate these results. Using transgenic mice lines combined with retrograde viruses to selectively target specific cell types, it was demonstrated that the DCN send direct projections to VTA DA, GABAergic, and glutamatergic neurons (Watabe-Uchida et al., 2012; Beier et al., 2015; An et al., 2021). There are, however, some disagreement in these studies on the DCN origin and identity of these projections. Baek et al. (2022) have observed projections in the dorsolateral VTA mainly from the dentate nucleus and to a lesser extent from the interposed nucleus, but not from the fastigial nucleus. However, by using a transsynaptic anterograde virus to label postsynaptic target cells, another study has shown that the posterior fastigial nucleus also contributes to these projections (Fujita et al., 2020). Results from our group have also corroborated this pathway. Similarly, we have observed cerebellar inputs coming from the three cerebellar nuclei, targeting DA and GABAergic VTA neurons. Projections from each DCN nuclei target different, but overlapping regions in the VTA, suggesting potentially specific contributions of each DCN to the VTA pathway (Oñate and Khodakhah, unpublished observation). Physiologic characterization of the DCN projections that used optogenetic stimulation to demonstrate the presence of functional monosynaptic cerebellar projections to the VTA only found excitatory connections (Carta et al., 2019; Baek et al., 2022).
In order to understand the possible functional roles of this pathway, it is important to identify the targets of the VTA neurons that receive cerebellar input. A major output of the VTA is through the mesolimbic projections to the NAc. Anatomical tracing experiments to trace the input of cell type-specific neurons in the VTA based on the output suggested the existence of a disynaptic pathway from the cerebellum to the accumbens through this pathway that involves GABAergic, glutamatergic, and dopaminergic neurons (Beier et al., 2015, 2019). Corroborating this circuit, a recent study (D'Ambra et al., 2021) showed an overlap in the VTA neurons that receive input from the DCN (tagged using an anterograde viral tracer) and those that project to NAc (tagged using a retrograde tracer). In addition, intersectional tracing using a transsynaptic virus combined with an anterograde virus showed cerebellar VTA projections to accumbens (D'Ambra et al., 2021). Data from our group have also confirmed these results. Using a combination of transsynaptic anterograde and retrograde virus tracing, we mapped and identified the cells in the DCN and in the VTA that form this disynaptic pathway. We found that neurons from all three DCN nuclei project to DA and non-DA neurons in the VTA (the Cb-VTA pathway) that then send extensive projections to all regions of the NAc (Oñate and Khodakhah, unpublished observation).
Previous data from our group have also shown that the Cb-VTA pathway is functionally active. The DCN sends monosynaptic excitatory projections to both dopaminergic and nondopaminergic neurons of the VTA (Carta et al., 2019). Given that VTA sends major projections to the NAc, this suggests that cerebellar activity could trigger excitation of the VTA neurons and consequently modify the mesolimbic neuronal activity. A similar study used amperometry measurements of DA to show that electrical stimulation of the dentate nuclei evokes DA release in the NAc (Holloway et al., 2019). Using fiber photometry to measure DA activity with the fluorescent sensor dLight, we also recently found that optogenetic stimulation of the Cb-VTA pathway can drive DA release in the NAc (Vera and Khodakhah, unpublished observation). Interestingly, stimulation of this pathway evoked not only DA release but also fast excitation of NAc single units (D'Ambra et al., 2021; Vera and Khodakhah, unpublished observation), suggesting that cerebellar activity can have strong influence in the activity of the NAc.
Although the cerebellum has been traditionally thought to process mainly motor-related information, it is well known that it also contributes to a variety of other brain functions, such as sensory and cognitive processing (Rapoport et al., 2000; Noroozian, 2014; Bostan and Strick, 2018; Schmahmann, 2019). Pertaining to our topic, several recent studies have reported that the cerebellum encodes and processes reward-related signals (Ohmae and Medina, 2015; Wagner et al., 2017; Heffley et al., 2018; Heffley and Hull, 2019; Kostadinov et al., 2019; Larry et al., 2019; Kostadinov and Hausser, 2022), functions that are typically attributed to the limbic system and are also the purview of the basal ganglia. But is this reward-related information conveyed to the basal ganglia?
Some clues from earlier studies suggest an answer to this question. Impairment in Purkinje cells, granule cells, or the DCNs could cause a reduction in motivated behaviors (Berntson and Schumacher, 1980; Caston et al., 1998; Bauer et al., 2011). As we described above, the cerebellum has direct projections to the VTA dopaminergic neurons. It is well known that VTA dopaminergic neurons, in particular the projections to the NAc, play a central role in reward-driven motivated behaviors and in reward-based reinforcement learning (Saunders et al., 2018; Mohebi et al., 2019). Interestingly, time-restricted optogenetic inactivation of the VTA-accumbens projection does not affect ongoing movements (Lee et al., 2020) but influences upcoming future behaviors (Chang et al., 2016; Lee et al., 2020). These findings suggest that the Cb-VTA-NAc pathway may contribute to the computation of motivation and influence learning processes. Indeed, fiber photometry measurement of the fluorescent calcium indicator GCaMP in behaving mice showed that the activity of the Cb-VTA projection was increased during social interaction, compared with exploration of an empty chamber (Carta et al., 2019), and scaled with reward value in a Pavlovian task (Yoshida and Khodakhah, unpublished observation). In addition, optogenetic stimulation of the Cb-VTA projection produced conditioned place preference, and its optogenetic inhibition impaired social interactions in a three chamber test (Carta et al., 2019).
The mesolimbic DA pathway is known to be an essential component of brain processes underlying addiction (Nestler, 2001; Koob and Volkow, 2010). The fact that the Cb-VTA projection is involved in motivational processing therefore indicates that this projection may also contribute to the pathologic mechanisms underlying addiction. Although the cerebellum is not generally considered as part of the neural circuitry responsible for drug abuse and addiction, studies of drug-dependent humans have consistently yielded tantalizing evidence, suggesting cerebellar involvement in reward processing and in addiction (Volkow et al., 2006; Thomas et al., 2008; Miquel et al., 2009, 2016, 2019, 2020; Moulton et al., 2014; Wagner et al., 2017; Guarque-Chabrera et al., 2022). For example, cerebellar gray matter volume is reduced in long-term addicted individuals compared with controls (Moulton et al., 2014; Miquel et al., 2016). Even more compellingly, functional imaging studies show that the cerebellum is activated in human subjects receiving methylphenidate, cocaine, or nicotine (Volkow et al., 1997, 2006; Domino et al., 2000; Risinger et al., 2005; Zubieta et al., 2005). Moreover, in drug abusers, cues predictive of drugs activate the cerebellum (Grant et al., 1996; Schneider et al., 2001; Bonson et al., 2002; Anderson et al., 2006), and the strength of these activations correlates with subjective ratings of craving (Grant et al., 1996; G. J. Wang et al., 1999; Sell et al., 2000; Kilts et al., 2001; Schneider et al., 2001; Bonson et al., 2002; Risinger et al., 2005; Anderson et al., 2006; Lou et al., 2012). These striking and consistent correlations seen in human subjects suggest that the cerebellum may participate in craving, relapse, and even primary drug reinforcement. Recent studies in rodents find that chronic cocaine treatment causes structural plasticity in cerebellar Purkinje cells (Vazquez-Sanroman et al., 2015), and that cerebellar granule neurons exhibit c-Fos activation in mice expressing a cocaine-conditioned place preference (Carbo-Gas et al., 2014), leading to strong evidence for cerebellar involvement in cocaine-induced habit formation (Domino et al., 2000; Miquel et al., 2019, 2020; Gil-Miravet et al., 2021; Sanchez-Hernandez et al., 2021). These studies strongly indicate that the cerebellum is one of the brain regions involved in the pathologic mechanisms of addiction, possibly by its modulation of the mesolimbic pathway.
The cerebello-nigrostriatal pathway and its possible role in movement vigor
Another dopaminergic center in the midbrain is the substantia nigra pars compacta (SNc), located in the ventrolateral region, next to the VTA. Like the VTA, the SNc mainly contains DA neurons; and although it also includes GABAergic and glutamatergic neurons, DA neurons dominate by far (Nair-Roberts et al., 2008). The major output of the SNc is through its (nigrostriatal) projections to the DS, where the release of DA modulates the properties of target neurons and their synapses. DA release in the DS has been associated with motor control, primarily because degeneration of DA neurons in the nigrostriatal system, a primary hallmark of Parkinson's disease, is linked to difficulty in initiating movements (hypokinesia) and rigidity, and its hyperactivity could favor hyperkinetic movements (Wang et al., 2013). Notably, studies using MRI for fiber tracking have suggested the presence of cerebellar connections with the substantia nigra and the VTA in healthy humans (Kwon and Jang, 2014; Milardi et al., 2016), and that the relative connectivity with these two regions is modified in patients with Parkinson's disease (O'Shea et al., 2022), suggesting that cerebellar activity is a required input for these dopaminergic centers in humans.
As discussed for the VTA in the previous section, older anatomic tracing studies had implicated the presence of direct cerebellar projections to the SNc. Lesions in each DCN nucleus, followed by a staining method to detect neuronal degenerated terminals revealed that the DCN projects to the substantia nigra, targeting medial and lateral regions of the SNc (Snider et al., 1976). This work was not followed up for several decades, until the development of modern neuronal tracing techniques (as discussed above) allowed for a more rigorous reevaluation of these connections. Using a retrograde viral approach to target specific neuronal cell types shows inputs in the cerebellum projecting to DA, GABAergic, and glutamatergic neurons in the SNc (Watabe-Uchida et al., 2012; An et al., 2021). More recently, this result was corroborated by multiple groups. Using a transsynaptic anterograde virus to label all target cells, Fujita et al. (2020) showed that, specifically, the caudoventral region of the fastigial nucleus sends projections to SNc DA and non-DA neurons. Our own anatomic work using the same approach, but targeting the entire DCN, confirmed these projections to both DA and non-DA neurons of the SNc. By mapping the target cells in detail, we found cells throughout the entire SNc. Additionally, injection of a retrograde virus in the SNc to map all originating neurons demonstrated that cerebellar inputs come from all three DCN (Washburn et al., 2022). In a similar study, to label the input of SNc-DA neurons based on their output, particularly to the striatum, input cells in the cerebellum were observed, providing anatomic data for the existence of this disynaptic circuit (Menegas et al., 2015). In addition to anatomic studies, both older and recent studies have explored the functionality of this pathway. Electric stimulation of the DCN was shown early on to alter DA levels in the striatum (Nieoullon et al., 1978; Nieoullon and Dusticier, 1980). More recently, our group showed that the projections from DCN neurons to the SNc (the Cb-SNc pathway) form monosynaptic glutamatergic synapses, targeting AMPARs and NMDARs, with both DA and non-DA neurons. Optogenetic stimulation of this pathway in slice preparations produced fast excitatory synaptic currents in SNc neurons. Additionally, in vivo optogenetic stimulation of this pathway increased SNc single-unit activity and evoked DA release in the DS, as measured with the fluorescent DA sensor dLight (Washburn et al., 2022). Finally, in vivo optogenetic activation of the Cb-SNc pathway increased the probability of locomotion in head-fixed animals ambulating on a treadmill. Together, these data show that cerebellar projections to the SNc can play a prominent role in the DA modulation of the basal ganglia and may constitute an additional pathway by which the cerebellum contributes to movement coordination.
All movements underlying natural behavior are initiated and executed with a specific delay, speed, frequency, and with appropriate force, attributes that are collectively referred to as movement vigor. All components of movement vigor are flexible and subject to change depending on behavioral need. A large body of research in primates, including humans, and rodents shows that the basal ganglia play a central role in controlling movement vigor (Berardelli et al., 2001; Boecker et al., 2008; Palmiter, 2008; Howe and Dombeck, 2016; da Silva et al., 2018; Shah et al., 2020) and that nigrostriatal DA plays a critical role in adjusting movement vigor (Panigrahi et al., 2015; Mendonça et al., 2021). It is also known that the DCN activity precedes movement onset (Thach, 1970, 1975; Armstrong et al., 1979; Fortier et al., 1989) similar to what has been observed for the SNc DA neurons (Boecker et al., 2008; Howe and Dombeck, 2016; da Silva et al., 2018), and that inhibiting the activity of the cerebellar nuclei slows reaction times in motor tasks (Meyer-Lohmann et al., 1977; Trouche and Beaubaton, 1980; Miller and Brooks, 1982; Tsujimotoet al., 1993). Although the cerebellar thalamocortical pathway is known to contribute to movement initiation (Dacre et al., 2021), as discussed above, recent work from our laboratory has also shown that the Cb-SNc projection directly influences the activity of the SNc DA neurons and their DA release in the DS. Interestingly, in a simple Pavlovian task where animals were given a liquid reward following a sensory cue, the Cb-SNc projections showed activation to water reward and a much higher activation to sweet water reward, indicating that this pathway conveys information on the reward value (Washburn et al., 2022). The cerebellum is commonly thought as a “learning machine” that uses cortical and sensory input to learn to predict the neural activity needed to coordinate posture and movement (Ito, 1984, 2006). Knowing that the cerebellum computes reward value information, it is plausible that the cerebellum uses sensory and cortical signals to compute reward likelihood and communicate this information to the SNc. The SNc then can use this information, together with those arriving from other brain regions, to determine the appropriate movement vigor.
Given the evidence showing the strong activation of the SNc DA neurons by the Cb-SNc activity, it is logical to think of this pathway in the context of Parkinson's disease. Parkinson's disease is caused by the degeneration of SNc DA neurons (Damier et al., 1999; Dauer and Przedborski, 2003; Michel et al., 2016). A key feature of Parkinson's disease is the difficulty in initiating movements and the movements of Parkinsonian patients, once initiated, tend to be slower (Benecke et al., 1987; Jahanshahi et al., 1992; Pascual-Leone et al., 1994; Chen et al., 2001), both indicative of a deficit in movement vigor. Therapeutic approaches that increase DA receptor activation, such as increasing DA release by administering levodopa, are effective in alleviating these vigor deficits early in the disease (Ehringer and Hornykiewicz, 1960; Hornykiewicz, 1975; Birkmayer and Hornykiewicz, 1998; Hornykiewicz, 2006), indicating that reduced DA is the major cause of the motor symptoms. Recent studies have shown a rapid increase in the activity of SNc DA neurons immediately preceding movement onset (Boecker et al., 2008; Howe and Dombeck, 2016; da Silva et al., 2018) and have demonstrated that SNc DA plays a central role in the control of movement vigor (Berardelli et al., 2001; Mazzoni et al., 2007; Palmiter, 2008; Turner and Desmurget, 2010; Panigrahi et al., 2015; Mendonça et al., 2021). As described above, the Cb-SNc projection can convey movement vigor information to the basal ganglia by increasing the activity of SNc DA neurons and striatal DA levels. Although the Cb-SNc pathway is not the sole projection that contributes to regulation of SNc DA neurons, it is nonetheless plausible that increasing the activity of the Cb-SNc pathway may alleviate the Parkinson's-like motor symptoms if the DA neurons are only partially lost. This hypothesis could be readily examined in animal models of Parkinson's disease with optogenetic excitation of the Cb-SNc pathway. If excitation of the Cb-SNc pathway can reduce Parkinson's-like vigor deficits, activation of this pathway provides a potential therapeutic substrate by deep brain stimulation or transcranial magnetic stimulation of DCNs that can be readily targeted in Parkinsonian patients.
Beyond reward and movement: thoughts on the potential role of the cerebellum and basal ganglia interactions in schizophrenia
Both the cerebellum and basal ganglia are implicated in diverse functions, such as motor, cognitive, reward, and emotional processing. As discussed, recent findings have revealed an extensive set of direct, cortex-independent, pathways by which the cerebellum communicates with the basal ganglia. These pathways allow the cerebellum to transmit information at multiple timescales, with neurotransmitters, such as glutamate, enabling fast, short-latency communication with millisecond time resolution, and neuromodulators, such as DA, supporting slower modulation of the basal ganglia in the 100 ms to seconds timescale. As discussed above, a cardinal feature of the cerebellar circuitry is its ability to provide predictive outputs based on learned associations between a wide array of sensory and cortical inputs. It is plausible, therefore, that the cerebellum constantly samples the environment and one's intent using its extensive sensory and cortical inputs, and via its direct projections provides the basal ganglia with predictions that help formulate diverse sets of behaviors, ranging from movement to reward processing, motivated behavior, and cognition.
Further scrutiny of such potential functional frameworks might allow for formulation and test of hypotheses to better understand the brain, and also to account for the diversity of nonmotor disorders in which the cerebellum is implicated. As an example of the latter, it might be interesting to briefly consider the role of the cerebellum in schizophrenia. In patients, a substantial decrease in cerebellar size (Laidi et al., 2015; Moberget et al., 2018, 2019) changes in cerebellar connectivity with the cortex and basal ganglia (Cao et al., 2018; Moberget et al., 2018; Anteraper et al., 2021), and cerebellar, cortical, and striatal hypoactivity (Lungu et al., 2013) are some of the most common and consistent findings. A major debilitating feature of schizophrenia is the presence of negative symptoms, cardinal among which are apathy and amotivation (Millan et al., 2014; Mosolov and Yaltonskaya, 2021). Given that motivated behavior requires reward processing by the midbrain DA centers that target both the PFC and the ventral striatum, the negative symptoms of schizophrenia might be the consequence of the reduced interactions between the cerebellum and the basal ganglia DA network as described here. Such a failure in communication between the cerebellum and basal ganglia might prevent the cerebellum from conveying to the basal ganglia its predictions related to desirability of upcoming behaviors, leading to apathy and amotivation. Indeed, in support of such a possibility, preliminary reports suggest that transcranial magnetic stimulation of the cerebellum can partially restore its functional connectivity with the cortex and the basal ganglia, and concurrently lessen the severity of the negative symptoms in patients (Demirtas-Tatlidede, et al., 2010; Tikka et al., 2015; Garg et al., 2016; Brady et al., 2019; Basavaraju et al., 2021; Zhu et al., 2021; Bègue et al., 2022).
The recent advances in our understanding of the nature of the interactions between the cerebellum and basal ganglia, and the potential contributions that these interactions may make in motor and nonmotor functions of the brain, have generated significant excitement in the field and have forced reevaluation of many long-held beliefs. No doubt, the fertile ground thus generated will motivate a large number of exciting new hypotheses that need to be rigorously tested in the coming years.
Footnotes
F.N. was supported by National Institutes of Health Grant MH060605. K.K. was supported by National Institutes of Health Grants MH115604, NS079750, and DA044761. We thank members of the K.K. laboratory for comments.
The authors declare no competing financial interests.
References
- An S, Li X, Deng L, Zhao P, Ding Z, Han Y, Luo Y, Liu X, Li A, Luo Q, Feng Z, Gong H (2021) A whole-brain connectivity map of VTA and SNc glutamatergic and GABAergic neurons in mice. Front Neuroanat 15:818242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Maas LC, Frederick BD, Bendor JT, Spencer TJ, Livni E, Lukas SE, Fischman AJ, Madras BK, Renshaw PF, Kaufman MJ (2006) Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology 31:1318–1326. 10.1038/sj.npp.1300937 [DOI] [PubMed] [Google Scholar]
- Anteraper SA, Guell X, Collin G, Qi Z, Ren J, Nair A, Seidman LJ, Keshavan MS, Zhang T, Tang Y, Li H, McCarley RW, Niznikiewicz MA, Shenton ME, Stone WS, Wang J, Whitfield-Gabrieli S (2021) Abnormal function in dentate nuclei precedes the onset of psychosis: a resting-state fMRI study in high-risk individuals. Schizophr Bull 47:1421–1430. 10.1093/schbul/sbab038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DM, Cogdell B, Harvey RJ (1979) Discharge patterns of Purkinje cells in cats anaesthetized with alpha-chloralose. J Physiol 291:351–366. 10.1113/jphysiol.1979.sp012818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek SJ, Park JS, Kim J, Yamamoto Y, Tanaka-Yamamoto K (2022) VTA-projecting cerebellar neurons mediate stress-dependent depression-like behaviors. Elife 11:e72981. 10.7554/eLife.72981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavaraju R, Ithal D, Thanki MV, Ramalingaiah AH, Thirthalli J, Reddy RP, Brady RO Jr, Halko MA, Bolo NR, Keshavan MS, Pascual-Leone A, Mehta UM, Kesavan M (2021) Intermittent theta burst stimulation of cerebellar vermis enhances fronto-cerebellar resting state functional connectivity in schizophrenia with predominant negative symptoms: a randomized controlled trial. Schizophr Res 238:108–120. 10.1016/j.schres.2021.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer DJ, Kerr AL, Swain RA (2011) Cerebellar dentate nuclei lesions reduce motivation in appetitive operant conditioning and open field exploration. Neurobiol Learn Mem 95:166–175. 10.1016/j.nlm.2010.12.009 [DOI] [PubMed] [Google Scholar]
- Bègue I, Brakowski J, Seifritz E, Dagher A, Tobler PN, Kirschner M, Kaiser S (2022) Cerebellar and cortico-striatal-midbrain contributions to reward-cognition processes and apathy within the psychosis continuum. Schizophr Res 246:85–94. 10.1016/j.schres.2022.06.010 [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L (2015) Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162:622–634. 10.1016/j.cell.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beier KT, Gao XJ, Xie S, DeLoach KE, Malenka RC, Luo L (2019) Topological organization of ventral tegmental area connectivity revealed by viral-genetic dissection of input-output relations. Cell Rep 26:159–167.e6. 10.1016/j.celrep.2018.12.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benecke R, Rothwell JC, Dick JP, Day BL, Marsden CD (1987) Disturbance of sequential movements in patients with Parkinson's disease. Brain 110: 361–379. 10.1093/brain/110.2.361 [DOI] [PubMed] [Google Scholar]
- Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson's disease. Brain 124:2131–2146. 10.1093/brain/124.11.2131 [DOI] [PubMed] [Google Scholar]
- Berke JD (2018) What does dopamine mean? Nat Neurosci 21:787–793. 10.1038/s41593-018-0152-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Schumacher KM (1980) Effects of cerebellar lesions on activity, social interactions, and other motivated behaviors in the rat. J Comp Physiol Psychol 94:706–717. 10.1037/h0077702 [DOI] [PubMed] [Google Scholar]
- Berridge KC (2007) The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 191:391–431. 10.1007/s00213-006-0578-x [DOI] [PubMed] [Google Scholar]
- Birkmayer W, Hornykiewicz O (1998) The effect of l-3,4-dihydroxyphenylalanine (=DOPA) on akinesia in parkinsonism. Parkinsonism Relat Disord 4:59–60. 10.1016/S1353-8020(98)00013-3 [DOI] [PubMed] [Google Scholar]
- Boecker H, Jankowski J, Ditter P, Scheef L (2008) A role of the basal ganglia and midbrain nuclei for initiation of motor sequences. Neuroimage 39:1356–1369. 10.1016/j.neuroimage.2007.09.069 [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant SJ, Contoreggi CS, Links JM, Metcalfe J, Weyl HL, Kurian V, Ernst M, London ED (2002) Neural systems and cue-induced cocaine craving. Neuropsychopharmacology 26:376–386. 10.1016/S0893-133X(01)00371-2 [DOI] [PubMed] [Google Scholar]
- Bostan AC, Dum RP, Strick PL (2010) The basal ganglia communicate with the cerebellum. Proc Natl Acad Sci USA 107:8452–8456. 10.1073/pnas.1000496107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Strick PL (2018) The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 19:338–350. 10.1038/s41583-018-0002-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady RO Jr, Gonsalvez I, Lee I, Öngür D, Seidman LJ, Schmahmann JD, Eack SM, Keshavan MS, Pascual-Leone A, Halko MA (2019) Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry 176:512–520. 10.1176/appi.ajp.2018.18040429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68:815–834. 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ME, Martin JR, Rosenbluth J, Ariel M (2011) A novel path for rapid transverse communication of vestibular signals in turtle cerebellum. J Neurophysiol 105:1071–1088. 10.1152/jn.00986.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciola A, Milardi D, Livrea P, Flace P, Anastasi G, Quartarone A (2017) The known and missing links between the cerebellum, basal ganglia, and cerebral cortex. Cerebellum 16:753–755. 10.1007/s12311-017-0850-0 [DOI] [PubMed] [Google Scholar]
- Caligiore D, Pezzulo G, Baldassarre G, Bostan AC, Strick PL, Doya K, Helmich RC, Dirkx M, Houk J, Jorntell H, Lago-Rodriguez A, Galea JM, Miall RC, Popa T, Kishore A, Verschure PF, Zucca R, Herreros I (2017) Consensus paper. Towards a systems-level view of cerebellar function: the interplay between cerebellum, basal ganglia, and cortex. Cerebellum 16:203–229. 10.1007/s12311-016-0763-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, et al. (2018) Cerebello-thalamo-cortical hyperconnectivity as a state-independent functional neural signature for psychosis prediction and characterization. Nat Commun 9:3836. 10.1038/s41467-018-06350-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbo-Gas M, Vazquez-Sanroman D, Aguirre-Manzo L, Coria-Avila GA, Manzo J, Sanchis-Segura C, Miquel M (2014) Involving the cerebellum in cocaine-induced memory: pattern of cFos expression in mice trained to acquire conditioned preference for cocaine. Addict Biol 19:61–76. 10.1111/adb.12042 [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ (2002) Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 26:321–352. 10.1016/S0149-7634(02)00007-6 [DOI] [PubMed] [Google Scholar]
- Carelli RM (2002) The nucleus accumbens and reward: neurophysiological investigations in behaving animals. Behav Cogn Neurosci Rev 1:281–296. 10.1177/1534582302238338 [DOI] [PubMed] [Google Scholar]
- Carta F, Chen CH, Schott AL, Dorizan S, Khodakhah K (2019) Cerebellar modulation of the reward circuitry and social behavior. Science 363:248. 10.1126/science.aav0581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caston J, Chianale C, Delhaye-Bouchaud N, Mariani J (1998) Role of the cerebellum in exploration behavior. Brain Res 808:232–237. 10.1016/S0006-8993(98)00847-6 [DOI] [PubMed] [Google Scholar]
- Cerminara NL, Lang EJ, Sillitoe RV, Apps R (2015) Redefining the cerebellar cortex as an assembly of non-uniform Purkinje cell microcircuits. Nat Rev Neurosci 16:79–93. 10.1038/nrn3886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CY, Esber GR, Marrero-Garcia Y, Yau HJ, Bonci A, Schoenbaum G (2016) Brief optogenetic inhibition of dopamine neurons mimics endogenous negative reward prediction errors. Nat Neurosci 19:111–116. 10.1038/nn.4191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CH, Fremont R, Arteaga-Bracho EE, Khodakhah K (2014) Short latency cerebellar modulation of the basal ganglia. Nat Neurosci 17:1767–1775. 10.1038/nn.3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Kumar S, Garg RR, Lang AE (2001) Impairment of motor cortex activation and deactivation in Parkinson's disease. Clin Neurophysiol 112:600–607. 10.1016/S1388-2457(01)00466-7 [DOI] [PubMed] [Google Scholar]
- D'Angelo E (2018) Physiology of the cerebellum. Handb Clin Neurol 154:85–108. [DOI] [PubMed] [Google Scholar]
- D'Ambra AF, Jung SJ, Ganesan S, Antzoulatos EG, Fioravante D (2021) Cerebellar activation bidirectionally regulates nucleus accumbens medial shell and core. bioRxiv 283952. 10.1101/2020.09.28.283952. [DOI] [Google Scholar]
- da Silva JA, Tecuapetla F, Paixao V, Costa RM (2018) Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554:244–248. 10.1038/nature25457 [DOI] [PubMed] [Google Scholar]
- Dacre J, Colligan M, Clarke T, Ammer JJ, Schiemann J, Chamosa-Pino V, Claudi F, Harston JA, Eleftheriou C, Pakan JM, Huang CC, Hantman AW, Rochefort NL, Duguid I (2021) A cerebellar-thalamocortical pathway drives behavioral context-dependent movement initiation. Neuron 109:2326–2338.e8. 10.1016/j.neuron.2021.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 122:1437–1448. 10.1093/brain/122.8.1437 [DOI] [PubMed] [Google Scholar]
- Daou A, Margoliash D (2021) Intrinsic plasticity and birdsong learning. Neurobiol Learn Mem 180:107407. 10.1016/j.nlm.2021.107407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S (2003) Parkinson's disease: mechanisms and models. Neuron 39:889–909. 10.1016/S0896-6273(03)00568-3 [DOI] [PubMed] [Google Scholar]
- Demirtas-Tatlidede A, Freitas C, Cromer JR, Safar L, Ongur D, Stone WS, Seidman LJ, Schmahmann JD, Pascual-Leone A (2010) Safety and proof of principle study of cerebellar vermal theta burst stimulation in refractory schizophrenia. Schizophr Res 124:91–100. 10.1016/j.schres.2010.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Hernandez E, Contreras-Lopez R, Sanchez-Fuentes A, Rodriguez-Sibrian L, Ramirez-Jarquin JO, Tecuapetla F (2018) The thalamostriatal projections contribute to the initiation and execution of a sequence of movements. Neuron 100:739–752.e5. 10.1016/j.neuron.2018.09.052 [DOI] [PubMed] [Google Scholar]
- Domino EF, Minoshima S, Guthrie S, Ohl L, Ni L, Koeppe RA, Zubieta JK (2000) Nicotine effects on regional cerebral blood flow in awake, resting tobacco smokers. Synapse 38:313–321. [DOI] [PubMed] [Google Scholar]
- Doya K (1999) What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw 12:961–974. 10.1016/S0893-6080(99)00046-5 [DOI] [PubMed] [Google Scholar]
- Doya K (2000) Complementary roles of basal ganglia and cerebellum in learning and motor control. Curr Opin Neurobiol 10:732–739. 10.1016/S0959-4388(00)00153-7 [DOI] [PubMed] [Google Scholar]
- Ebbesen CL, Brecht M (2017) Motor cortex: to act or not to act? Nat Rev Neurosci 18:694–705. 10.1038/nrn.2017.119 [DOI] [PubMed] [Google Scholar]
- Ehringer H, Hornykiewicz O (1960) Distribution of noradrenaline and dopamine (3-hydroxytyramine) in the human brain and their behavior in diseases of the extrapyramidal system. Klin Wochenschr 38:1236–1239. [DOI] [PubMed] [Google Scholar]
- Faget L, Osakada F, Duan J, Ressler R, Johnson AB, Proudfoot JA, Yoo JH, Callaway EM, Hnasko TS (2016) Afferent inputs to neurotransmitter-defined cell types in the ventral tegmental area. Cell Rep 15:2796–2808. 10.1016/j.celrep.2016.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallon JH, Moore RY (1978) Catecholamine innervation of the basal forebrain: IV. Topography of the dopamine projection to the basal forebrain and neostriatum. J Comp Neurol 180:545–580. 10.1002/cne.901800310 [DOI] [PubMed] [Google Scholar]
- Flace P, Livrea P, Basile GA, Galletta D, Bizzoca A, Gennarini G, Bertino S, Branca JJ, Gulisano M, Bianconi S, Bramanti A, Anastasi G (2021) The cerebellar dopaminergic system. Front Syst Neurosci 15:650614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier PA, Kalaska JF, Smith AM (1989) Cerebellar neuronal-activity related to whole-arm reaching movements in the monkey. J Neurophysiol 62:198–211. 10.1152/jn.1989.62.1.198 [DOI] [PubMed] [Google Scholar]
- Fry BR, Pence NT, McLocklin A, Johnson AW (2021) Disruptions in effort-based decision-making following acute optogenetic stimulation of ventral tegmental area dopamine cells. Learn Mem 28:104–108. 10.1101/lm.053082.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H, Kodama T, du Lac S (2020) Modular output circuits of the fastigial nucleus for diverse motor and nonmotor functions of the cerebellar vermis. Elife 9:e58613. 10.7554/eLife.58613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Davis C, Thomas AM, Economo MN, Abrego AM, Svoboda K, De Zeeuw CI, Li N (2018) A cortico-cerebellar loop for motor planning. Nature 563:113–116. 10.1038/s41586-018-0633-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S, Sinha VK, Tikka SK, Mishra P, Goyal N (2016) The efficacy of cerebellar vermal deep high frequency (theta range) repetitive transcranial magnetic stimulation (rTMS) in schizophrenia: a randomized rater blind-sham controlled study. Psychiatry Res 243:413–420. 10.1016/j.psychres.2016.07.023 [DOI] [PubMed] [Google Scholar]
- Geisler S, Derst C, Veh RW, Zahm DS (2007) Glutamatergic afferents of the ventral tegmental area in the rat. J Neurosci 27:5730–5743. 10.1523/JNEUROSCI.0012-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Miravet I, Melchor-Eixea I, Arias-Sandoval E, Vasquez-Celaya L, Guarque-Chabrera J, Olucha-Bordonau F, Miquel M (2021) From back to front: a functional model for the cerebellar modulation in the establishment of conditioned preferences for cocaine-related cues. Addict Biol 26:e12834. [DOI] [PubMed] [Google Scholar]
- Gómez A, Durán E, Salas C, Rodríguez F (2010) Cerebellum lesion impairs eyeblink-like classical conditioning in goldfish. Neuroscience 166:49–60. 10.1016/j.neuroscience.2009.12.018 [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA (2005) Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci 8:805–812. 10.1038/nn1471 [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A (1996) Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 93:12040–12045. 10.1073/pnas.93.21.12040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Robertson B (2016) The basal ganglia over 500 million years. Curr Biol 26:R1088–R1100. 10.1016/j.cub.2016.06.041 [DOI] [PubMed] [Google Scholar]
- Guarque-Chabrera J, Gil-Miravet I, Olucha-Bordonau F, Melchor-Eixea I, Miquel M (2022) When the front fails, the rear wins: cerebellar correlates of prefrontal dysfunction in cocaine-induced memory in male rats. Prog Neuropsychopharmacol Biol Psychiatry 112:110429. 10.1016/j.pnpbp.2021.110429 [DOI] [PubMed] [Google Scholar]
- Haber SN, Gdowski MJ (2004) The basal ganglia. In: The human nervous system (Paxinos G, Mai JK eds), pp 677–738. San Diego: Elsevier. [Google Scholar]
- Heffley W, Hull C (2019) Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum. Elife 8:e46764. 10.7554/eLife.46764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffley W, Song EY, Xu Z, Taylor BN, Hughes MA, McKinney A, Joshua M, Hull C (2018) Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions. Nat Neurosci 21:1431–1441. 10.1038/s41593-018-0228-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintzen A, Pelzer EA, Tittgemeyer M (2018) Thalamic interactions of cerebellum and basal ganglia. Brain Struct Funct 223:569–587. 10.1007/s00429-017-1584-y [DOI] [PubMed] [Google Scholar]
- Holloway ZR, Paige NB, Comstock JF, Nolen HG, Sable HJ, Lester DB (2019) Cerebellar modulation of mesolimbic dopamine transmission is functionally asymmetrical. Cerebellum 18:922–931. 10.1007/s12311-019-01074-w [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O (1975) Parkinsonism induced by dopaminergic antagonists. Adv Neurol 9:155–164. [PubMed] [Google Scholar]
- Hornykiewicz O (2006) The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm Suppl 9–15. [DOI] [PubMed] [Google Scholar]
- Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL (2005) The cerebellum communicates with the basal ganglia. Nat Neurosci 8:1491–1493. 10.1038/nn1544 [DOI] [PubMed] [Google Scholar]
- Howe MW, Dombeck DA (2016) Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535:505–510. 10.1038/nature18942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull C, Regehr WG (2022) The cerebellar cortex. Annu Rev Neurosci 45:151–175. 10.1146/annurev-neuro-091421-125115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe N, Mori F, Shoumura K (2000) A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res 880:191–197. 10.1016/S0006-8993(00)02744-X [DOI] [PubMed] [Google Scholar]
- Ikai Y, Takada M, Mizuno N (1994) Single neurons in the ventral tegmental area that project to both the cerebral and cerebellar cortical areas by way of axon collaterals. Neuroscience 61:925–934. 10.1016/0306-4522(94)90413-8 [DOI] [PubMed] [Google Scholar]
- Ito M (1984) The cerebellum and neural control. New York: Raven. [Google Scholar]
- Ito M (2006) Cerebellar circuitry as a neuronal machine. Prog Neurobiol 78:272–303. [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Brown RG, Marsden CD (1992) Simple and choice reaction time and the use of advance information for motor preparation in Parkinson's disease. Brain 115:539–564. 10.1093/brain/115.2.539 [DOI] [PubMed] [Google Scholar]
- Jwair S, Coulon P, Ruigrok TJ (2017) Disynaptic subthalamic input to the posterior cerebellum in rat. Front Neuroanat 11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull JM, Richman EB, Ringach N, Friedmann D, Albarran E, Kolluru SS, Jones RC, Allen WE, Wang Y, Cho SW, Zhou H, Ding JB, Chang HY, Deisseroth K, Quake SR, Luo L (2020) Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 370:eabd5059. 10.1126/science.abd5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP (2001) Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 58:334–341. 10.1001/archpsyc.58.4.334 [DOI] [PubMed] [Google Scholar]
- Kizer JS, Palkovits M, Brownstein MJ (1976) The projections of the A8, A9 and A10 dopaminergic cell bodies: evidence for a nigral-hypothalamic-median eminence dopaminergic pathway. Brain Res 108:363–370. 10.1016/0006-8993(76)90192-X [DOI] [PubMed] [Google Scholar]
- Klaus A, Alves da Silva J, Costa RM (2019) What, if, and when to move: basal ganglia circuits and self-paced action initiation. Annu Rev Neurosci 42:459–483. 10.1146/annurev-neuro-072116-031033 [DOI] [PubMed] [Google Scholar]
- Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG (2019) Dopamine: functions, signaling, and association with neurological diseases. Cell Mol Neurobiol 39:31–59. 10.1007/s10571-018-0632-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238. 10.1038/npp.2009.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostadinov D, Hausser M (2022) Reward signals in the cerebellum: origins, targets, and functional implications. Neuron 110:1290–1303. 10.1016/j.neuron.2022.02.015 [DOI] [PubMed] [Google Scholar]
- Kostadinov D, Beau M, Blanco-Pozo M, Hausser M (2019) Predictive and reactive reward signals conveyed by climbing fiber inputs to cerebellar Purkinje cells. Nat Neurosci 22:950–962. 10.1038/s41593-019-0381-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HG, Jang SH (2014) Differences in neural connectivity between the substantia nigra and ventral tegmental area in the human brain. Front Hum Neurosci 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidi C, d'Albis MA, Wessa M, Linke J, Phillips ML, Delavest M, Bellivier F, Versace A, Almeida J, Sarrazin S, Poupon C, Dudal KL, Daban C, Hamdani N, Leboyer M, Houenou J (2015) Cerebellar volume in schizophrenia and bipolar I disorder with and without psychotic features. Acta Psychiatr Scand 131:223–233. 10.1111/acps.12363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC (2012) Input-specific control of reward and aversion in the ventral tegmental area. Nature 491:212–217. 10.1038/nature11527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larry N, Yarkoni M, Lixenberg A, Joshua M (2019) Cerebellar climbing fibers encode expected reward size. Elife 8:e46870. 10.7554/eLife.46870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Claar LD, Hachisuka A, Bakhurin KI, Nguyen J, Trott JM, Gill JL, Masmanidis SC (2020) Temporally restricted dopaminergic control of reward-conditioned movements. Nat Neurosci 23:209–216. 10.1038/s41593-019-0567-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner TN, Holloway AL, Seiler JL (2021) Dopamine, updated: reward prediction error and beyond. Curr Opin Neurobiol 67:123–130. 10.1016/j.conb.2020.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CL, Parker LO (1969) Effect of dentate stimulation on neuronal activity in the globus pallidus. Exp Neurol 24:298–309. 10.1016/0014-4886(69)90023-5 [DOI] [PubMed] [Google Scholar]
- Lou M, Wang E, Shen Y, Wang J (2012) Cue-elicited craving in heroin addicts at different abstinent time: an fMRI pilot study. Subst Use Misuse 47:631–639. 10.3109/10826084.2011.646381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lungu O, Barakat M, Laventure S, Debas K, Proulx S, Luck D, Stip E (2013) The incidence and nature of cerebellar findings in schizophrenia: a quantitative review of fMRI literature. Schizophr Bull 39:797–806. 10.1093/schbul/sbr193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manto M, Oulad Ben Taib N (2010) Cerebellar nuclei: key roles for strategically located structures. Cerebellum 9:17–21. 10.1007/s12311-010-0159-8 [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O (2009) Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459:837–841. 10.1038/nature08028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni P, Hristova A, Krakauer JW (2007) Why don't we move faster? Parkinson's disease, movement vigor, and implicit motivation. J Neurosci 27:7105–7116. 10.1523/JNEUROSCI.0264-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner W, Leblois A, Hansel D, Bioulac B, Gross CE, Benazzouz A, Boraud T (2005) Subthalamic high frequency stimulation resets subthalamic firing and reduces abnormal oscillations. Brain 128:2372–2382. 10.1093/brain/awh616 [DOI] [PubMed] [Google Scholar]
- Mendonça MD, da Silva JA, Hernandez LF, Castela I, Obeso J, Costa RM (2021) Transient dopamine neuron activity precedes and encodes the vigor of contralateral movements. bioRxiv 440527. 10.1101/2021.04.20.440527. [DOI] [Google Scholar]
- Menegas W, Bergan JF, Ogawa SK, Isogai Y, Umadevi Venkataraju K, Osten P, Uchida N, Watabe-Uchida M (2015) Dopamine neurons projecting to the posterior striatum form an anatomically distinct subclass. Elife 4:e10032. 10.7554/eLife.10032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lohmann J, Hore J, Brooks VB (1977) Cerebellar participation in generation of prompt arm movements. J Neurophysiol 40:1038–1050. 10.1152/jn.1977.40.5.1038 [DOI] [PubMed] [Google Scholar]
- Michel PP, Hirsch EC, Hunot S (2016) Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 90:675–691. 10.1016/j.neuron.2016.03.038 [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL (2000) Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn 42:183–200. 10.1006/brcg.1999.1099 [DOI] [PubMed] [Google Scholar]
- Milardi D, Arrigo A, Anastasi G, Cacciola A, Marino S, Mormina E, Calamuneri A, Bruschetta D, Cutroneo G, Trimarchi F, Quartarone A (2016) Extensive direct subcortical cerebellum-basal ganglia connections in human brain as revealed by constrained spherical deconvolution tractography. Front Neuroanat 10:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Fone K, Steckler T, Horan WP (2014) Negative symptoms of schizophrenia: clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol 24:645–692. 10.1016/j.euroneuro.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Miller AD, Brooks VB (1982) Parallel pathways for movement initiation of monkeys. Exp Brain Res 45:328–332. 10.1007/BF01208592 [DOI] [PubMed] [Google Scholar]
- Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50:381–425. 10.1016/S0301-0082(96)00042-1 [DOI] [PubMed] [Google Scholar]
- Miquel M, Toledo R, Garcia LI, Coria-Avila GA, Manzo J (2009) Why should we keep the cerebellum in mind when thinking about addiction? Curr Drug Abuse Rev 2:26–40. 10.2174/1874473710902010026 [DOI] [PubMed] [Google Scholar]
- Miquel M, Vazquez-Sanroman D, Carbo-Gas M, Gil-Miravet I, Sanchis-Segura C, Carulli D, Manzo J, Coria-Avila GA (2016) Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci Biobehav Rev 60:1–11. 10.1016/j.neubiorev.2015.11.005 [DOI] [PubMed] [Google Scholar]
- Miquel M, Nicola SM, Gil-Miravet I, Guarque-Chabrera J, Sanchez-Hernandez A (2019) A working hypothesis for the role of the cerebellum in impulsivity and compulsivity. Front Behav Neurosci 13:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquel M, Gil-Miravet I, Guarque-Chabrera J (2020) The cerebellum on cocaine. Front Syst Neurosci 14:586574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moberget T, et al. (2018) Cerebellar volume and cerebellocerebral structural covariance in schizophrenia: a multisite mega-analysis of 983 patients and 1349 healthy controls. Mol Psychiatry 23:1512–1520. 10.1038/mp.2017.106 [DOI] [PubMed] [Google Scholar]
- Moberget T, Alnæs D, Kaufmann T, Doan NT, Córdova-Palomera A, Norbom LB, Rokicki J, van der Meer D, Andreassen OA, Westlye LT (2019) Cerebellar gray matter volume is associated with cognitive function and psychopathology in adolescence. Biol Psychiatry 86:65–75. 10.1016/j.biopsych.2019.01.019 [DOI] [PubMed] [Google Scholar]
- Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97. 10.1016/0301-0082(80)90018-0 [DOI] [PubMed] [Google Scholar]
- Mohebi A, Pettibone JR, Hamid AA, Wong JM, Vinson LT, Patriarchi T, Tian L, Kennedy RT, Berke JD (2019) Dissociable dopamine dynamics for learning and motivation. Nature 570:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery J, Perks K (2019) Understanding cerebellum in vertebrate neuroethology: from sensing in sharks and electric fish to motor sequences in movement and birdsong. Behav Neurosci 133:267–281. 10.1037/bne0000317 [DOI] [PubMed] [Google Scholar]
- Morales M, Margolis EB (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18:73–85. 10.1038/nrn.2016.165 [DOI] [PubMed] [Google Scholar]
- Mosolov SN, Yaltonskaya PA (2021) Primary and secondary negative symptoms in schizophrenia. Front Psychiatry 12:766692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton E, Becerra L, Johnson A, Burstein R, Borsook D (2014) Altered hypothalamic functional connectivity with autonomic circuits and the locus coeruleus in migraine. PLoS One 9:e95508. 10.1371/journal.pone.0095508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA (2008) Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience 152:1024–1031. 10.1016/j.neuroscience.2008.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ (2001) Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci 2:119–128. 10.1038/35053570 [DOI] [PubMed] [Google Scholar]
- Nicholson DA, Roberts TF, Sober SJ (2018) Thalamostriatal and cerebellothalamic pathways in a songbird, the Bengalese finch. J Comp Neurol 526:1550–1570. 10.1002/cne.24428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A, Dusticier N (1980) Changes in dopamine release in caudate nuclei and substantia nigrae after electrical stimulation of the posterior interposate nucleus of cat cerebellum. Neurosci Lett 17:167–172. 10.1016/0304-3940(80)90079-8 [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Cheramy A, Glowinski J (1978) Release of dopamine in both caudate nuclei and both substantia nigrae in response to unilateral stimulation of cerebellar nuclei in the cat. Brain Res 148:143–152. 10.1016/0006-8993(78)90384-0 [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R (1967) Comparative anatomy of the cerebellum. Prog Brain Res 25:1–93. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, Voogd J, Huijzen C (2007) The human central nervous system. Berlin: Springer. [Google Scholar]
- Noroozian M (2014) The role of the cerebellum in cognition: beyond coordination in the central nervous system. Neurol Clin 32:1081–1104. 10.1016/j.ncl.2014.07.005 [DOI] [PubMed] [Google Scholar]
- O'Shea IM, Popal HS, Olson IR, Murty VP, Smith DV (2022) Distinct alterations in cerebellar connectivity with substantia nigra and ventral tegmental area in Parkinson's disease. Sci Rep 12:3289. 10.1038/s41598-022-07020-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa SK, Cohen JY, Hwang D, Uchida N, Watabe-Uchida M (2014) Organization of monosynaptic inputs to the serotonin and dopamine neuromodulatory systems. Cell Rep 8:1105–1118. 10.1016/j.celrep.2014.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmae S, Medina JF (2015) Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci 18:1798–1803. 10.1038/nn.4167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter RD (2008) Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann NY Acad Sci 1129:35–46. 10.1196/annals.1417.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigrahi B, Martin KA, Li Y, Graves AR, Vollmer A, Olson L, Mensh BD, Karpova AY, Dudman JT (2015) Dopamine is required for the neural representation and control of movement vigor. Cell 162:1418–1430. 10.1016/j.cell.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Sole J, Brasil-Neto JP, Cohen LG, Hallett M (1994) Akinesia in Parkinson's disease: I. Shortening of simple reaction time with focal, single-pulse transcranial magnetic stimulation. Neurology 44:884–891. 10.1212/wnl.44.5.884 [DOI] [PubMed] [Google Scholar]
- Percheron G, Francois C, Talbi B, Yelnik J, Fenelon G (1996) The primate motor thalamus. Brain Res Brain Res Rev 22:93–181. 10.1016/0165-0173(96)00003-3 [DOI] [PubMed] [Google Scholar]
- Person AL, Gale SD, Farries MA, Perkel DJ (2008) Organization of the songbird basal ganglia, including Area X. J Comp Neurol 508:840–866. 10.1002/cne.21699 [DOI] [PubMed] [Google Scholar]
- Phillipson OT (1979) Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol 187:117–143. 10.1002/cne.901870108 [DOI] [PubMed] [Google Scholar]
- Phillipson OT, Griffiths AC (1985) The topographic order of inputs to nucleus accumbens in the rat. Neuroscience 16:275–296. 10.1016/0306-4522(85)90002-8 [DOI] [PubMed] [Google Scholar]
- Pidoux L, Blanc PL, Levenes C, Leblois A (2018) A subcortical circuit linking the cerebellum to the basal ganglia engaged in vocal learning. Elife 7:e32167. 10.7554/eLife.32167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa LS, Streng ML, Hewitt AL, Ebner TJ (2016) The errors of our ways: understanding error representations in cerebellar-dependent motor learning. Cerebellum 15:93–103. 10.1007/s12311-015-0685-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, van Reekum R, Mayberg H (2000) The role of the cerebellum in cognition and behavior: a selective review. J Neuropsychiatry Clin Neurosci 12:193–198. 10.1176/jnp.12.2.193 [DOI] [PubMed] [Google Scholar]
- Raymond JL, Medina JF (2018) Computational principles of supervised learning in the cerebellum. Annu Rev Neurosci 41:233–253. 10.1146/annurev-neuro-080317-061948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redgrave P, Prescott TJ, Gurney K (1999) The basal ganglia: a vertebrate solution to the selection problem? Neuroscience 89:1009–1023. 10.1016/S0306-4522(98)00319-4 [DOI] [PubMed] [Google Scholar]
- Reiner A, Medina L, Veenman CL (1998) Structural and functional evolution of the basal ganglia in vertebrates. Brain Res Brain Res Rev 28:235–285. 10.1016/S0165-0173(98)00016-2 [DOI] [PubMed] [Google Scholar]
- Risinger RC, Salmeron BJ, Ross TJ, Amen SL, Sanfilipo M, Hoffmann RG, Bloom AS, Garavan H, Stein EA (2005) Neural correlates of high and craving during cocaine self-administration using BOLD fMRI. Neuroimage 26:1097–1108. 10.1016/j.neuroimage.2005.03.030 [DOI] [PubMed] [Google Scholar]
- Sakai ST, Inase M, Tanji J (1996) Comparison of cerebellothalamic and pallidothalamic projections in the monkey (Macaca fuscata): a double anterograde labeling study. J Comp Neurol 368:215–228. [DOI] [PubMed] [Google Scholar]
- Salamone JD (1994) The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res 61:117–133. 10.1016/0166-4328(94)90153-8 [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76:470–485. 10.1016/j.neuron.2012.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Hernandez A, Nicolas C, Gil-Miravet I, Guarque-Chabrera J, Solinas M, Miquel M (2021) Time-dependent regulation of perineuronal nets in the cerebellar cortex during abstinence of cocaine-self administration. Psychopharmacology (Berl) 238:1059–1068. 10.1007/s00213-020-05752-0 [DOI] [PubMed] [Google Scholar]
- Saunders BT, Richard JM, Margolis EB, Janak PH (2018) Dopamine neurons create Pavlovian conditioned stimuli with circuit-defined motivational properties. Nat Neurosci 21:1072–1083. 10.1038/s41593-018-0191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD (2019) The cerebellum and cognition. Neurosci Lett 688:62–75. 10.1016/j.neulet.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Wagner M, Franke P, Salloum JB, Shah NJ, Toni I, Sulzbach C, Honig K, Maier W, Gaebel W, Zilles K (2001) Subcortical correlates of craving in recently abstinent alcoholic patients. Am J Psychiatry 158:1075–1083. 10.1176/appi.ajp.158.7.1075 [DOI] [PubMed] [Google Scholar]
- Sell LA, Morris JS, Bearn J, Frackowiak RS, Friston KJ, Dolan RJ (2000) Neural responses associated with cue evoked emotional states and heroin in opiate addicts. Drug Alcohol Depend 60:207–216. 10.1016/S0376-8716(99)00158-1 [DOI] [PubMed] [Google Scholar]
- Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Spain RI, Nutt JG, El-Gohary M, Curtze C, Horak FB (2020) Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson's disease and matched controls during daily living. J Neurol 267:1188–1196. 10.1007/s00415-020-09696-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Raju DV, Pare JF, Sidibe M (2004) The thalamostriatal system: a highly specific network of the basal ganglia circuitry. Trends Neurosci 27:520–527. 10.1016/j.tins.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Snider RS, Maiti A, Snider SR (1976) Cerebellar connections to catecholamine systems: anatomical and biochemical studies. Trans Am Neurol Assoc 101:295–297. [PubMed] [Google Scholar]
- Sutton AC, O'Connor KA, Pilitsis JG, Shin DS (2015) Stimulation of the subthalamic nucleus engages the cerebellum for motor function in parkinsonian rats. Brain Struct Funct 220:3595–3609. 10.1007/s00429-014-0876-8 [DOI] [PubMed] [Google Scholar]
- Swanson LW (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull 9:321–353. 10.1016/0361-9230(82)90145-9 [DOI] [PubMed] [Google Scholar]
- Taylor JA, Ivry RB (2014) Cerebellar and prefrontal cortex contributions to adaptation, strategies, and reinforcement learning. Prog Brain Res 210:217–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teune TM, van der Burg J, van der Moer J, Voogd J, Ruigrok TJ (2000) Topography of cerebellar nuclear projections to the brain stem in the rat. Prog Brain Res 124:141–172. [DOI] [PubMed] [Google Scholar]
- Thach WT (1970) Discharge of cerebellar neurons related to two maintained postures and two prompt movements: II. Purkinje cell output and input. J Neurophysiol 33:537–547. 10.1152/jn.1970.33.4.537 [DOI] [PubMed] [Google Scholar]
- Thach WT (1975) Timing of activity in cerebellar dentate nucleus and cerebral motor cortex during prompt volitional movement. Brain Res 88:233–241. 10.1016/0006-8993(75)90387-X [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y (2008) Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol 154:327–342. 10.1038/bjp.2008.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikka SK, Garg S, Sinha VK, Nizamie SH, Goyal N (2015) Resting state dense array gamma oscillatory activity as a response marker for cerebellar-repetitive transcranial magnetic stimulation (rTMS) in schizophrenia. J ECT 31:258–262. 10.1097/YCT.0000000000000242 [DOI] [PubMed] [Google Scholar]
- Trouche E, Beaubaton D (1980) Initiation of a goal-directed movement in the monkey: role of the cerebellar dentate nucleus. Exp Brain Res 40:311–321. 10.1007/BF00237796 [DOI] [PubMed] [Google Scholar]
- Tsujimoto T, Gemba H, Sasaki K (1993) Effect of cooling the dentate nucleus of the cerebellum on hand movement of the monkey. Brain Res 629:1–9. 10.1016/0006-8993(93)90473-Z [DOI] [PubMed] [Google Scholar]
- Turner RS, Desmurget M (2010) Basal ganglia contributions to motor control: a vigorous tutor. Curr Opin Neurobiol 20:704–716. 10.1016/j.conb.2010.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Magill PJ, Bolam JP (2004) Uniform inhibition of dopamine neurons in the ventral tegmental area by aversive stimuli. Science 303:2040–2042. 10.1126/science.1093360 [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Donahue CJ, Glasser MF (2018) Development and evolution of cerebral and cerebellar cortex. Brain Behav Evol 91:158–169. 10.1159/000489943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F (1997) Reafferent thalamo-'cortical' loops in the song system of oscine songbirds. J Comp Neurol 380:275–290. [DOI] [PubMed] [Google Scholar]
- Vazquez-Sanroman D, Carbo-Gas M, Leto K, Cerezo-Garcia M, Gil-Miravet I, Sanchis-Segura C, Carulli D, Rossi F, Miquel M (2015) Cocaine-induced plasticity in the cerebellum of sensitised mice. Psychopharmacology (Berl) 232:4455–4467. 10.1007/s00213-015-4072-1 [DOI] [PubMed] [Google Scholar]
- Veenman CL, Medina L, Reiner A (1997) Avian homologues of mammalian intralaminar, mediodorsal and midline thalamic nuclei: immunohistochemical and hodological evidence. Brain Behav Evol 49:78–98. 10.1159/000112983 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Angrist B, Hitzemann R, Lieberman J, Pappas N (1997) Effects of methylphenidate on regional brain glucose metabolism in humans: relationship to dopamine D2 receptors. Am J Psychiatry 154:50–55. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Jayne M, Telang F, Swanson JM (2006) Effects of expectation on the brain metabolic responses to methylphenidate and to its placebo in non-drug abusing subjects. Neuroimage 32:1782–1792. 10.1016/j.neuroimage.2006.04.192 [DOI] [PubMed] [Google Scholar]
- Voogd J (2014) What we do not know about cerebellar systems neuroscience. Front Syst Neurosci 8:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voogd J, Ruigrok TJH (2012) Cerebellum and Precerebellar Nuclei. In: The Human Nervous System (Mai JK, Paxinos G eds), pp 471–545. Academic Press. [Google Scholar]
- Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L (2017) Cerebellar granule cells encode the expectation of reward. Nature 544:96–100. 10.1038/nature21726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner MJ, Luo L (2020) Neocortex-cerebellum circuits for cognitive processing. Trends Neurosci 43:42–54. 10.1016/j.tins.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C (1999) Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 64:775–784. 10.1016/S0024-3205(98)00619-5 [DOI] [PubMed] [Google Scholar]
- Wang S, Tan Y, Zhang JE, Luo M (2013) Pharmacogenetic activation of midbrain dopaminergic neurons induces hyperactivity. Neurosci Bull 29:517–524. 10.1007/s12264-013-1327-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn S, Oñate M, Yoshida J, Vera J, Khatami L, Nadim F, Khodakhah K (2022) Cerebellum directly modulates the substantia nigra dopaminergic activity. bioRxiv 492532. 10.1101/2022.05.20.492532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe-Uchida M, Zhu LS, Ogawa SK, Vamanrao A, Uchida N (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74:858–873. 10.1016/j.neuron.2012.03.017 [DOI] [PubMed] [Google Scholar]
- Xiao L, Bornmann C, Hatstatt-Burkle L, Scheiffele P (2018) Regulation of striatal cells and goal-directed behavior by cerebellar outputs. Nat Commun 9:3133. 10.1038/s41467-018-05565-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun IA, Nicola SM, Fields HL (2004) Contrasting effects of dopamine and glutamate receptor antagonist injection in the nucleus accumbens suggest a neural mechanism underlying cue-evoked goal-directed behavior. Eur J Neurosci 20:249–263. 10.1111/j.1460-9568.2004.03476.x [DOI] [PubMed] [Google Scholar]
- Zhu L, Zhang W, Zhu Y, Mu X, Zhang Q, Wang Y, Cai J, Xie B (2021) Cerebellar theta burst stimulation for the treatment of negative symptoms of schizophrenia: a multicenter, double-blind, randomized controlled trial. Psychiatry Res 305:114204. 10.1016/j.psychres.2021.114204 [DOI] [PubMed] [Google Scholar]
- Ziegler W, Ackermann H (2017) Subcortical contributions to motor speech: phylogenetic, developmental, clinical. Trends Neurosci 40:458–468. 10.1016/j.tins.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Xu Y, Koeppe RA, Ni L, Guthrie S, Domino EF (2005) Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry 162:567–577. 10.1176/appi.ajp.162.3.567 [DOI] [PubMed] [Google Scholar]