Abstract
Background
Despite the universal use of the two-dose trivalent measles-mumps-rubella (MMR) vaccine in the past two decades, outbreaks of these diseases still occur in countries with high vaccine uptake, giving rise to concerns about primary and secondary failure of MMR vaccine components. We aimed to provide seroconversion and waning rate estimates for the measles, mumps, and rubella components of MMR vaccines.
Methods
In this systematic review and meta-analysis we searched PubMed (including MEDLINE), Web of Science, and Embase for randomised controlled trials, cohort studies, or longitudinal studies reporting the immunogenicity and persistence of MMR vaccines, published in English from database inception to Dec 31, 2019. Studies were included if they investigated vaccine-induced immunity in healthy individuals who received a trivalent MMR vaccine, including different dosages and timepoints of vaccine administration. Studies featuring coadministration of MMR with other vaccines, maternal immunity to the MMR vaccine, or non-trivalent formulations of the vaccine were excluded. Pooled seroconversion and waning rates were estimated by random-effects meta-analyses. This study is registered with PROSPERO, CRD42019116705.
Findings
We identified 3615 unique studies, 62 (1·7%) of which were eligible for analysis. Estimated overall seroconversion rates were 96·0% (95% CI 94·5–97·4; I2=91·1%) for measles, 93·3% (91·1–95·2; I2=94·9%) for mumps when excluding the Rubini strain, 91·1% (87·4–94·1; I2=96·6%) for mumps when including the Rubini strain, and 98·3% (97·3–99·2; I2=93·0%) for rubella. Estimated overall annual waning rates were 0·009 (95% CI 0·005–0·016; I2=85·2%) for measles, 0·024 (0·016–0·039; I2=94·7%) for mumps, and 0·012 (0·010–0·014; I2=93·3%) for rubella.
Interpretation
Our meta-analysis provides estimates of primary and secondary vaccine failure, which are essential to improve the accuracy of mathematical and statistical modelling to understand and predict the occurrence of future measles, mumps, and rubella outbreaks in countries with high vaccine uptake.
Funding
European Research Council.
Introduction
Vaccines offer direct protection against infection and, by decreasing the pool of susceptible hosts in a population, provide indirect protection of unvaccinated individuals through herd immunity.1 Despite high vaccine uptake, vaccine-preventable disease outbreaks still occur in unvaccinated clusters within highly vaccinated populations. A relatively small proportion of the cases might be due to primary vaccine failure—the individual lack of seroconversion or adaptive immune response after vaccination; or secondary vaccine failure—the gradual loss of immunity over time after vaccination (ie, waning immunity).2 Vaccine efficacy and effectiveness, influenced by primary and secondary vaccine failure, are essential inputs for immunisation policy.3
Globally, most countries immunise against measles using one of the many available measles-mumps-rubella (MMR) vaccines. Numerous measles outbreaks have been reported during the past two decades in various parts of the world, including Europe.4, 5, 6 In the Americas, measles elimination was declared in 2016, but recent outbreaks have raised concerns about the sustainability of elimination.7 Since the 1990s, regional elimination targets have been reset for various reasons, but the past decade has seen a general increase in vaccine hesitancy among the general public, particularly towards MMR vaccination. In 1997, the WHO Regional Office for Europe proposed a European-wide phased measles elimination, distinguishing groups of countries depending on their progress towards elimination.8 This strategy was based on achieving and maintaining immunity to measles at levels that mathematical modelling estimated to be necessary for European-wide elimination of the disease by 2007. It assumed high vaccination coverage, particularly for the first dose, leading to potentially cost-saving elimination strategies.9 Dwindling population-level immunity globally, especially in children aged 5–9 years, needs to be raised together with improved immunity in older age groups.10 The current WHO 2020 measles and rubella elimination target is now generally considered to be unattainable and must be revised, as vaccine-induced immunity levels decline below the target values in various age groups.11 Numerous mumps outbreaks have also been reported,12, 13, 14 but no large-scale rubella outbreaks have been documented in countries with high MMR vaccination coverage and a catch-up vaccination programme.15
Research in context.
Evidence before this study
We searched PubMed (including MEDLINE), Web of Science, and Embase with the terms “measles”, “mumps”, “rubella”, “MMR”, “immunogenicity”, “seroconversion”, “primary vaccine failure”, “persistence”, “waning”, or “secondary vaccine failure”, to pinpoint all articles published in English from database inception to Dec 31, 2019. Studies were included if they investigated the vaccine-induced immunity in healthy individuals who received at least one dose of trivalent measles-mumps-rubella (MMR) vaccine. The primary aim of this systematic review was to provide a comprehensive overview of the existing body of evidence and consequently to estimate seroconversion and waning rates, relying on random-effects meta-analysis estimates using the DerSimonian-Laird estimator for the between-study variability. Several authors have reviewed the existing literature on immunogenicity and persistence of MMR vaccines; nevertheless overall estimates of seroconversion and vaccine failure are not readily available in the literature.
Added value of this study
In this systematic review, we identified 3615 unique studies across 24 countries to quantify primary and secondary vaccine failure for the three components in combined MMR vaccines. Our meta-analysis results provide accurate and up-to-date information on seroconversion and waning immunity after MMR vaccination.
Implications of all the available evidence
Our paper considers the combined scientific evidence to estimate both the level of MMR vaccine-induced humoral immunity, and how this wanes over time, after a single dose or two doses of MMR vaccine at a given age. This estimation will directly improve the parametrisation of mathematical models, and therefore also their accuracy, as well as the relevance and certainty of policies based on those models. This work contributes to identifying how vaccination schedules could be adapted and what level of vaccination coverage should be reached, to prevent the resurgence of measles, mumps, and rubella worldwide.
Declining immunity in parts of the world is thought to have three main causes: declining vaccination uptake or staying below target uptake level in subpopulations; waning vaccine-induced protection, reinforced by the decline in natural boosting of immunity by exposure; and historically low vaccine coverage in individuals older than 20 years of age. Vaccine hesitancy flares up when incidence of the disease declines through so-called prevalence-elastic vaccine demand,16 with scarcity of the disease influencing the perception of the trade-off between vaccine-preventable disease and risks of adverse events caused by the vaccination.17
The level (immunogenicity) and duration (persistence) of MMR vaccine-induced immunity through IgG antibodies is documented in many studies, but overall estimates of MMR seroconversion and vaccine failure are scarce in the literature. Therefore, we systematically reviewed primary and secondary vaccine failure estimates for MMR vaccines, and did meta-analyses to estimate the overall seroconversion and waning rates as proxies for primary and secondary vaccine failures for measles, mumps, and rubella immunity. We considered the presence of antibodies against these three diseases as a proxy of immunity, although an absence of antibodies does not equate no protection, as cellular immunity also provides some degree of protection.18
Methods
Search strategy and selection criteria
We followed the PRISMA guidelines (appendix pp 2–3) and preregistered our protocol.19, 20 Four researchers (JS, SA, PB, and NH) predefined inclusion and exclusion criteria (appendix p 10). Studies eligible for inclusion were those that: investigated vaccine-induced immunity in healthy individuals on administration of a trivalent MMR vaccine; investigated various timings of vaccine administration, different timepoints after vaccination at which immunity against the target pathogen was studied, including both single-dose MMR vaccination as well as second-dose administration of vaccine; and were randomised controlled trials, cohort studies, or longitudinal studies. Ineligible studies were those that investigated: maternal immunity related to the trivalent MMR vaccine; MMR vaccination in pregnant or non-healthy or immunocompromised individuals; a tetravalent measles-mumps-rubella-varicella (MMRV) vaccine only; the effect of simultaneous vaccination with MMR vaccine and other vaccines (coadministration); and monovalent or bivalent vaccines of measles, mumps, or rubella. Studies were also excluded if they were conference proceedings, letters to the editor, or review papers (except if they were concerning primary or secondary MMR vaccine failure); or were cross-sectional serological surveys, outbreak and case-report studies, studies with unclear description of methods or with no usable data, or in-vitro experiment or animal studies.
We searched PubMed (including MEDLINE), Web of Science, and Embase for eligible articles published in English from database inception to Dec 31, 2019, using the search terms “measles”, “mumps”, “rubella”, “MMR”, “immunogenicity”, “seroconversion”, “primary vaccine failure”, “persistence”, “waning”, or “secondary vaccine failure” (appendix pp 5–9). Two researchers (JS and SA) independently screened the search results by title, abstract, keywords, and full text if necessary (if there was any doubt after reviewing the title and abstract). Publications considered eligible were retained for review of the full text obtained through online downloads, interlibrary loans, or email communication with the corresponding author. Eligibility was confirmed after reading the full text. In addition, all references in the Cochrane review concerning MMR vaccination21 that met the inclusion criteria were reviewed. Additional articles were identified for analysis by screening reference lists of articles already selected for review. All full texts considered eligible by both reviewers were included in the final review. In five cases of disagreement, a third reviewer (NH) was consulted to make a final decision regarding article eligibility.
Data analysis
Duplicate publications were identified using Endnote X8 before two reviewers (JS and SA) extracted data including study and patient characteristics, vaccine information (no restriction based on vaccines or strains, administration route, or detection method used), seroconversion rates, and persistence of immunity. Quality of the eligible studies was evaluated according to the Cochrane risk-of-bias tool22 by both reviewers independently. In two cases a third reviewer (NH) was consulted to make a final decision regarding the quality of the data. Publication bias was investigated by constructing a funnel plot and formally tested using the test by Peters and colleagues23 for seroconversion studies, and Egger's test24 for persistence studies.
For each eligible immunogenicity study, we estimated seroconversion rates as the proportion of individuals who were seropositive for anti-measles, anti-mumps, and anti-rubella IgG after MMR vaccination in the total number of vaccinated participants, together with 95% Clopper-Pearson exact confidence limits.25 Seroconversion after the second MMR dose was measured in the seronegative individuals.
For waning vaccine-induced immunity, we derived the exponential waning rates using three different methods according to the data from each study. First, if only the study duration together with the number of seropositive individuals at the end of the study was given, and starting from a documented 100% seroconverted population, we calculated an empirical estimate of the waning rate. Second, if persistence was reported over time in different individuals, we used a generalised linear model for a binary outcome to estimate an exponential waning rate. Third, repeated measurements over time in the same individuals were analysed using a generalised estimating equations26 approach. The annual exponential waning rate represents the decay in seropositivity. For example, 10 years after vaccination, the expected loss of seropositivity equals 8·6% (=[1 – e (−0·009 × 10)]× 100), with 0·009 taken as the annual waning rate (appendix p 11).
Study-specific rates were combined per component, taking information from vaccine strains and both MMR vaccine doses together, to obtain overall seroconversion and waning rate estimates using a meta-analysis random-effects model with the DerSimonian-Laird estimator for the between-study variability.27, 28 The Freeman-Tukey double arcsine variance-stabilising transformation,29 which allows calculation of an overall proportion from a set of proportions, was used for the seroconversion rates; waning rates were log-transformed before pooling, after which, pooled estimates were back-transformed. Inverse-variance weighting was used and CIs for the combined effects were constructed using the Knapp and Hartung method,30 which provides an adjustment to the SEs to account for uncertainty in τ2, the (total) amount of heterogeneity or variability among the different studies.
We investigated heterogeneity in the estimated overall seroconversion and waning rates using the I 2 statistic.31 As a secondary analysis, we subdivided studies by vaccine strain for measles and mumps to estimate strain-specific seroconversion rates, but not for rubella as there is only one dominant rubella strain. A subdivision based on the method of detection (ELISA or other test) was done to determine any influence on the estimated MMR seroconversion rates. As there are suspected differences in amounts of heterogeneity within the different measles and mumps strains, and the method of detection, we fitted a single mixed-effects meta-regression model with strain as fixed effect for measles and mumps and used a two-sided omnibus test (appendix pp 12–13).32
All statistical analyses were done at a 5% significance level, using the statistical software packages metafor (for primary vaccine failures) and meta (for secondary vaccine failures) in R version 3.4.3.32, 33 This study is registered with PROSPERO, CRD42019116705.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
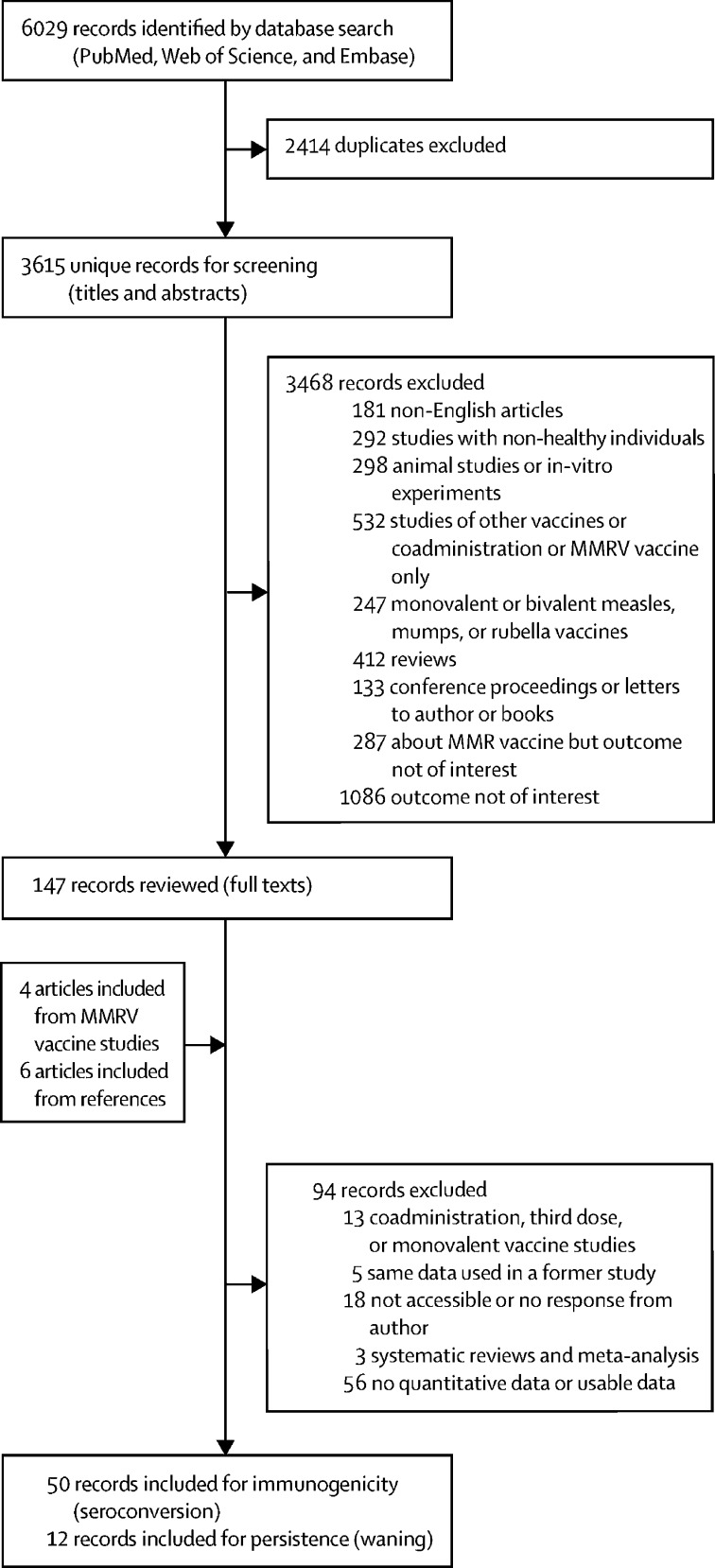
Of 6029 records identified, 3615 were screened after removing duplicate records, and 147 were selected for full-text review (figure 1 ). Four studies concerning MMRV vaccination with an MMR vaccine group as the control group, and six articles identified via reference checking were added. In total, 62 studies were identified (table ), 50 for MMR immunogenicity and 12 for MMR persistence; 23 studies (37%) were done in Europe, 17 (27%) in Asia, 13 (21%) in North America, five (8%) in central America or South America, and one (2%) in Australia. Three studies (5%) were done in more than one country.
Figure 1.
Study selection
Some of the 62 records included had data on more than one MMR vaccine. MMRV=measles-mumps-rubella-varicella. MMR=measles-mumps-rubella.
Table.
Study characteristics
| Total (n=62) | Seroconversion studies (n=50) | Waning studies (n=12) | |
|---|---|---|---|
| Region | |||
| Europe | 23 (37%) | 15 (30%) | 8 (67%) |
| Asia | 17 (27%) | 17 (34%) | 0 |
| North America | 13 (21%) | 9 (18%) | 4 (33%) |
| Central America | 2 (3%) | 2 (4%) | 0 |
| South America | 3 (5%) | 3 (6%) | 0 |
| >1 region | 3 (5%) | 3 (6%) | 0 |
| Australia | 1 (2%) | 1 (2%) | 0 |
| Study design | |||
| Clinical trial | 14 (22%) | 14 (28%) | 0 |
| Follow-up study | 13 (21%) | 2 (4%) | 11 (92%) |
| Randomised trial | 13 (21%) | 12 (24%) | 1 (8%) |
| Prospective study | 11 (18%) | 11 (22%) | 0 |
| Randomised controlled trial | 11 (18%) | 11 (22%) | 0 |
| Administration route | |||
| Subcutaneous | 29 (47%) | 28 (56%) | 1 (8%) |
| Intramuscular | 1 (2%) | 1 (2%) | 0 |
| Other* | 3 (4%) | 3 (6%) | 0 |
| Not available | 29 (47%) | 18 (36%) | 11 (92%) |
| Vaccine (n=87) | |||
| MMR-II (Merck) | 30 (34%) | 20 (28%) | 10 (62%) |
| Priorix | 16 (18%) | 15 (21%) | 1 (6%) |
| Not available | 12 (14%) | 11 (16%) | 1 (6%) |
| Triviraten | 5 (6%) | 5 (7%) | 0 |
| Trimovax | 5 (6%) | 5 (7%) | 0 |
| MMR-Vax | 3 (4%) | 3 (4%) | 0 |
| Serum MMR | 3 (4%) | 3 (4%) | 0 |
| Pluserix | 2 (2%) | 0 | 2 (13%) |
| Tresivax | 2 (2%) | 2 (3%) | 0 |
| MMR (Razi Institute) | 2 (2%) | 2 (3%) | 0 |
| Cadila MMR | 2 (2%) | 2 (3%) | 0 |
| Other† | 5 (6%) | 3 (4%) | 2 (13%) |
87 vaccine combinations were used in the 62 studies identified. MMR=measles-mumps-rubella.
Aerosol versus subcutaneous, subcutaneous versus intramuscular, or disposable syringe jet injector versus needle-syringe.
MMR (SPIIPL), Berna MMR, Immravax, JCV-001, or Trivirix.
Most of the included articles were clinical trials, observational studies, or longitudinal follow-up studies (table). ELISA was the most frequently used method to detect IgG antibodies against measles, mumps, and rubella (appendix pp 30–35). Immunogenicity and persistence was evaluated for both the first and second MMR vaccination doses in healthy individuals aged from 8 months to 44 years (appendix pp 27–29). Characteristics of the included publications concerning MMR vaccine persistence are presented in the appendix (pp 36–40). Different forms of the MMR vaccine were used in the eligible studies (table), and MMR-II (Merck) was the most frequently used (30 [34%] of 87 vaccination groups).
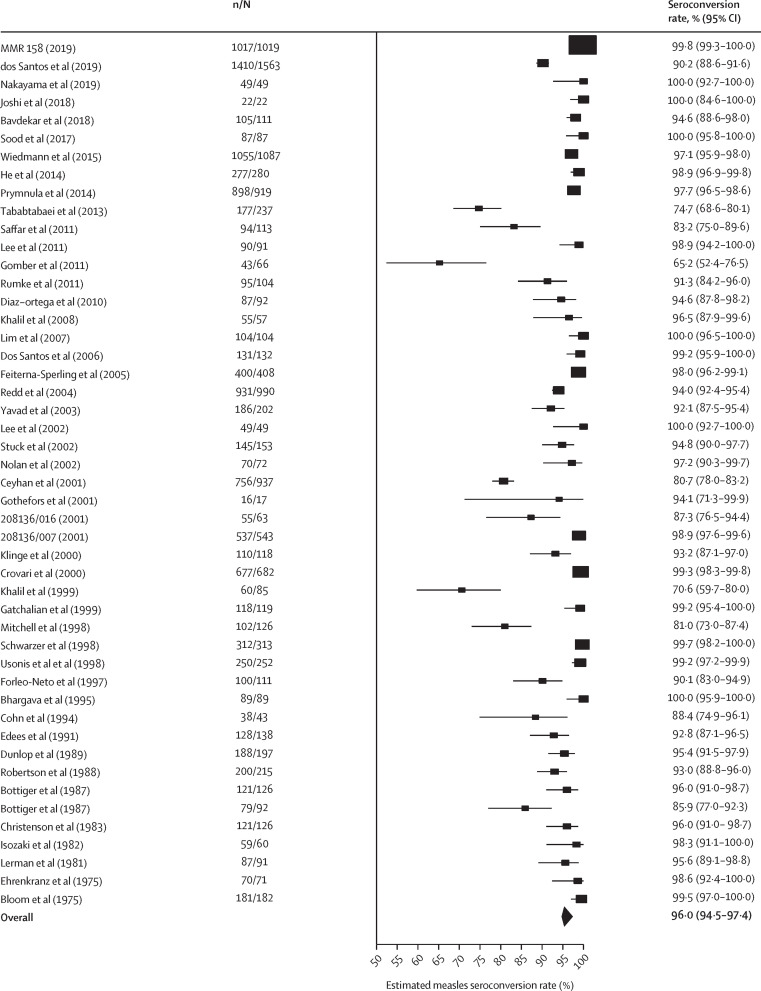
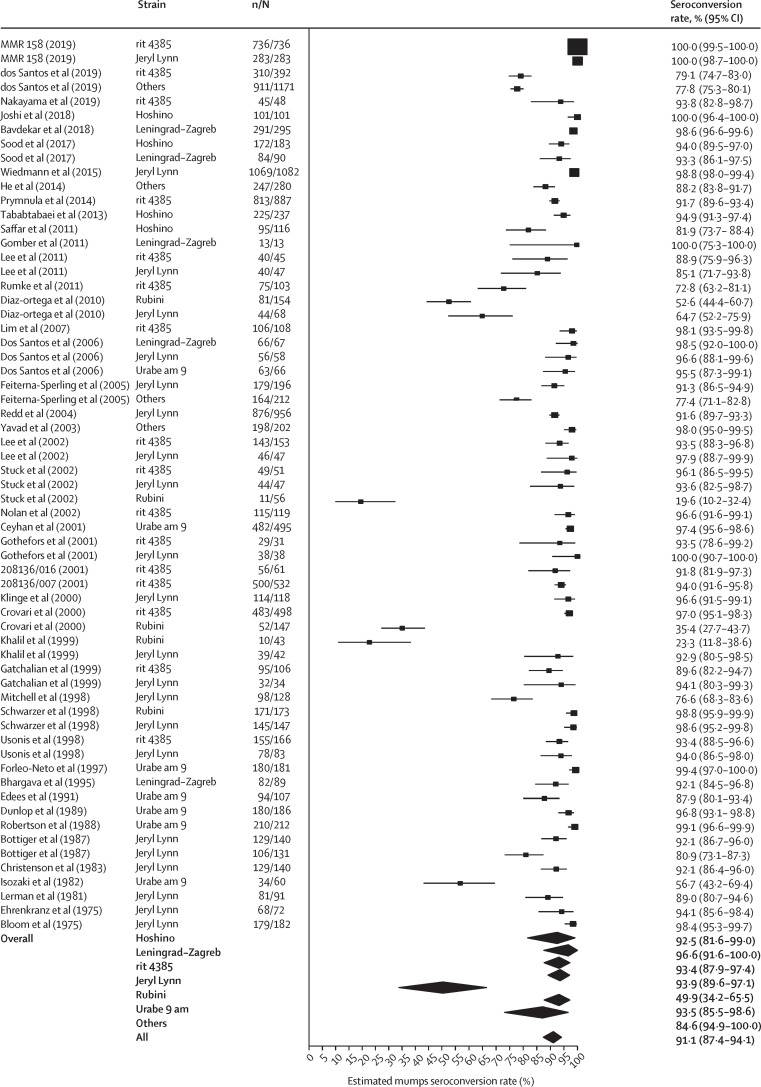
Overall estimated seroconversion rates were 96·0% (95% CI 94·5–97·4) for measles (figure 2 ), 91·1% (87·4–94·1) for mumps (figure 3 ), and 98·3% (97·3–99·2) for rubella (appendix p 41). Excluding the Rubini strain, the overall estimated seroconversion rate for mumps was 93·3% (91·1–95·2; I 2=94·9%). There was no significant difference in seroconversion rates after the first and the second MMR doses (p=0·77 for measles, p=0·50 for mumps, and p=0·11 for rubella), and the rates are considered independent of age at vaccination (appendix p 17). We found no regional differences in the overall seroconversion rates (appendix p 21).
Figure 2.
Forest plot of the seroconversion rates for measles
The overall seroconversion rate estimate was obtained from a meta-analysis random-effects model with the DerSimonian-Laird estimator. I2=91·1% (95% CI 88·6–94·8). 95% CIs for the individual studies were Clopper-Pearson exact confidence limits. References for included studies are given in the appendix (pp 46–54). n=number of seropositive patients. N=total number of individuals tested.
Figure 3.
Forest plot of the seroconversion rate for mumps per strain and overall
The overall seroconversion rate estimate per strain was obtained from a single mixed model, strain as a fixed effect, with the DerSimonian-Laird estimator. I2=96·6% (95% CI 96·5–98·4). 95% CIs for the individual studies were Clopper-Pearson exact confidence limits. References for included studies are given in the appendix (pp 46–54). n=number of seropositive patients. N=total number of individuals tested.
Certain measles, mumps, and rubella strains are less immunogenic than others, especially the mumps component. Therefore, we did a meta-analysis subgrouping the studies on the basis of measles and mumps strains, but not rubella, as most studies used the RA 27/3 rubella strain (appendix pp 18, 20). Differences between measles strains were not significant (p=0·60), but a significant difference was found between mumps strains (p<0·0001). After excluding the Rubini strain, this difference did not persist (p=0·66). Additionally, when subdivided on the basis of serological test (ELISA or other tests), seroconversion rates were generally higher when ELISA was used. However, this difference between tests was only significant for measles (p=0·043), with no significant differences found for mumps (p=0·14) or rubella (p=0·74).
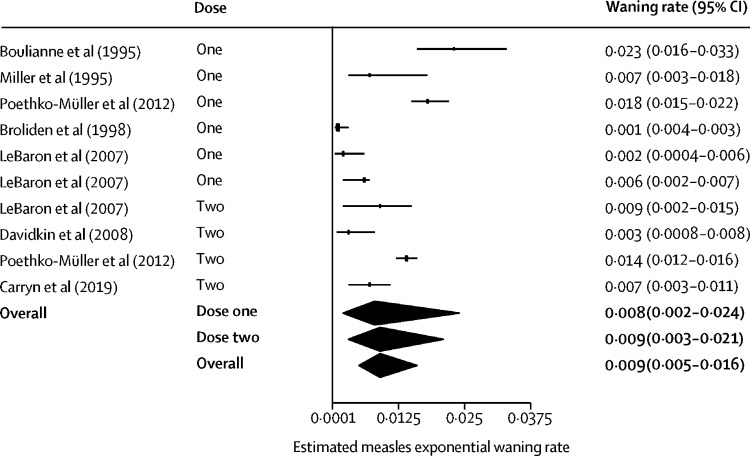
Combining all MMR vaccine types, annual exponential waning rates after the first dose were 0·008 (95% CI 0·002–0·024) for measles, 0·039 (0·028–0·056) for mumps, and 0·014 (0·012–0·017) for rubella, and after the second dose were 0·009 (0·003–0·021) for measles, 0·016 (0·008–0·031) for mumps, and 0·010 (0·009–0·012) for rubella. Overall annual exponential waning rates for doses 1 and 2 combined are estimated to be 0·009 (0·005–0·016; I 2=85·2%) for measles, 0·024 (0·016–0·039; I 2=94·7%) for mumps, and 0·012 (0·010–0·014; I 2=93·3%) for rubella (figure 4 , appendix pp 43–44). The seroconversion and waning rates with different estimators for the between-study variance are displayed in the appendix (p 19).
Figure 4.
Forest plot of the waning rate for measles
The waning rate estimates for dose one, dose two, and both doses combined for measles was obtained from a meta-analysis random-effects model, with the DerSimonian-Laird estimator. I2=85·2% (95% CI 74·4–91·4) for both doses combined. References for included studies are given in the appendix (pp 46–54).
A simple hypothetical projection based on the overall meta-analysis results, to display how the proportion seroconverted in a group of 100 vaccinated individuals would evolve over a time span of 50 years, is shown in the appendix (p 45). This projection assumes that every MMR vaccine recipient received a single dose of MMR vaccine and that they are not exposed to wild-type viruses at any time after vaccination. After 35 years, for instance, the proportion of individuals who became seronegative was about 30% (95% CI 14·2–46·1) for measles, 65% (36·8–80·1) for mumps, and 35% (31·3–43·3) for rubella.
The results of the risk-of-bias assessment, summarising the proportions of outcomes categorised as high, low, and unclear risk of bias, from all included articles together, are shown in the appendix (p 22). Because not all questions were applicable for all studies, a not applicable category was added. The main source of risk of bias was lack of blinding of all outcomes, which in some studies was not possible or not applicable. Studies that involved a comparison between two or more vaccines considered blinding of outcomes, whereas some studies only investigated one MMR vaccine and thus blinding was not performed. Only three (5%) of the 62 studies had a high risk of measurement bias. In some cases there was insufficient information to determine the risk of allocation concealment or how missing data were handled. In addition, separate funnel plots were constructed for measles, mumps, and rubella for seroconversion and waning studies (appendix pp 25–26). No publication bias was found for seroconversion of the measles (p=0·58), mumps (p=0·27), and rubella (p=0·86) components when stratifying by strain. There was no publication bias present in the persistence studies concerning the mumps (p=0·19) and rubella (p=0·056) components, but evidence of publication bias was found for the measles component (p=0·013), even when excluding studies with large sample sizes (p=0·033).
Discussion
Eliminating measles, mumps, and rubella infections requires achieving and maintaining high age-stratified threshold levels of population immunity, while accommodating vaccine failure. Using systematic review and meta-analyses we estimated overall seroconversion and waning rates for measles, mumps, and rubella components of combined MMR vaccines. Several authors have reviewed the immunogenicity and persistence of MMR vaccines,21, 34, 35, 36 but there are no specific estimates of primary and secondary MMR vaccine failure rates for the three individual components available in the literature, and such information is very much fragmented. It is essential to account for vaccine failure in mathematical and statistical models to adjust available coverage estimates with respect to vaccine uptake, and consequently determine existing population susceptibility to those infections.12, 37, 38, 39
We have documented studies on primary and secondary vaccine failure for the three MMR components and synthesised available information through meta-analytic approaches. Assessing whether infection is due to primary vaccine failure is easier than attributing it to secondary vaccine failure as the latter requires information about the presence of an immune response following primary vaccination, which is often lacking.40 Our findings provide more accurate, up-to-date information on seroconversion and waning immunity, to improve modelling projections, which are used to formulate public health policy. These insights will expand our understanding of the immunological within-host dynamics after MMR vaccination for predictions of measles, mumps, or rubella outbreaks within highly vaccinated populations, to improve control and prevention of these diseases. Our systematic review provides evidence for the decline of IgG antibody levels for measles, mumps, and rubella over time and our meta-analysis provides overall yearly waning rate estimates for the three components of the MMR vaccine.
We found considerable heterogeneity in published seroconversion rates for MMR components, which persisted in subgroup analyses for different measles and mumps vaccine strains. Substantial heterogeneity also exists in the retrieved waning rates for all three components, even when discriminating between waning rates after administration of the first or second MMR dose. Because of the low number of publications within each dosing category and per strain, one should interpret the results with caution. We did not do a subgroup analysis to study differences in waning rates across strains as most vaccines included the same measles and mumps vaccine strains. Heterogeneity is presumably induced by many factors, including diverging clinical and methodological aspects. Moreover, the evaluation of seropositivity was not identical in all the eligible studies in terms of timing of vaccination or evaluation, test used, or threshold values applied to discriminate between seropositive and seronegative individuals. Each potential source of variability could be evaluated individually, but not collectively. Given the presence and extent of variability between studies identified in our meta-analysis and of publication bias concerning the persistence of the measles component, our findings should be interpreted with caution.
In general, MMR vaccines have proven to be highly effective in diminishing the overall incidence and prevalence of measles, mumps, and rubella infection. The results of this meta-analysis for measles, mumps, and rubella, taking all strain types together, are in line with the general consensus that vaccine-induced mumps immunity is less persistent over time than that of measles and rubella. More specifically, IgG antibodies seem to decline slowly, with most individuals remaining seropositive for many years, and vaccine-induced immunity seems to be long term, at least for measles and rubella. Primary vaccine failure levels are crucial to determine vaccine coverage levels required to achieve virus elimination through herd immunity. Secondary vaccine failure levels have a less clear role but are also important, mainly to maintain elimination. Both primary and secondary vaccine failures are pathogen-specific.
The absence of measles herd immunity is mostly due to missing vaccination goals, as the high infectivity of measles requires very high and homogeneous immunity levels. In the USA, most individuals with measles had incomplete or unknown MMR vaccination status.41 Outbreaks of measles in highly vaccinated populations mainly occur in groups of susceptible individuals—ie, adult cohorts who were not infected and not vaccinated as young children, children who are too young to be vaccinated or whose parents refuse vaccination, or those who did not respond to vaccination (primary vaccine failure).42, 43, 44 Waning of vaccine-induced immunity for measles has been identified in the literature and shown through our meta-analysis, but there is no evidence that waning has a major role in the observed measles resurgence in many countries worldwide.45 Nevertheless, cases of (modified) measles have been reported as secondary vaccine failures in vaccinated individuals that were exposed to measles during outbreaks. However, the capacity of these individuals to transmit the disease might be diminished, and they might not have a role in sustaining transmission.40, 46
Over the past two decades, there has been a simultaneous escalation in the number of mumps cases in different vaccinated populations,15, 16, 39 raising concerns over the immunogenicity of the mumps strains in current vaccines, and whether these vaccines are sufficiently effective to prevent outbreaks. We found significant differences in seroconversion rate across the mumps strains, but not when the Rubini strain was excluded, which fits with the findings of Dayan and colleagues,47 that the highest proportion of cases in outbreaks of mumps were among those individuals immunised using the Rubini strain. Our analysis supports the WHO recommendation not to use vaccines containing Rubini strains. Moreover, several studies found a higher attack-rate with increasing time since vaccination,12, 14 indicating that waning most likely has a role in the current mumps outbreaks. In a systematic review of prospective and retrospective cohort studies estimating the effectiveness of the Jeryl Lynn mumps strain, exponential waning rates were derived by minimising the sum of squared errors between the proportion initially protected and the estimates of the ratio of vaccine effectiveness, resulting in an average waning rate of 0·036 (95% CI 0·020–0·059) for mumps.36 We derived the exponential waning rates directly from each study and estimated a similar overall waning rate of 0·024 (0·016–0·039).
Rubella is probably better controlled than mumps or measles because of its relatively low transmissibility, together with a high seroconversion rate. Neither primary nor secondary vaccine failure seem to be an issue with rubella given the scarcity of outbreaks, with the exception of a recent outbreak in Poland due to its historically low vaccination coverage.48 The annual waning rate for rubella we obtained from our meta-analysis is considerably higher than a model-based estimate obtained from an Australian serological survey.49 As we did in a previous study,38 overall rubella waning rates can also be estimated on the basis of country-specific serological data, selecting individuals vaccinated twice with the MMR vaccine. In the current study the overall waning rate for rubella was retrieved by a meta-analysis fixed-effects model rather than a mixed-effects model because of the small number of eligible studies we found.
The second dose is given to decrease the proportion of people with no response after the first MMR dose,45 and we found no significant difference in seroconversion rates for all components after first and second MMR doses.
High-quality cross-sectional serological surveys require high levels of quality control and assurance of serological assays. Despite updated WHO guidelines,50 important barriers to high-quality serological surverys remain, such as the difficulty in standardising different laboratory assays (especially in the past) and the determination of appropriate cutoffs for seropositivity, especially in the absence of a correlate of humoral protection.51, 52 We generally observed higher seroconversion rates when an ELISA was used, although this finding was only significant for the measles component. Different studies using the same assay used different cutoffs, leading to underestimation or overestimation of seroconversion rates. Despite these drawbacks, seroprevalence studies are important as they provide a direct measure of a population's immunological profile, especially as the prevalence of disease burden declines after the introduction of a vaccine. Accumulating years of widespread vaccination causes a shift in the relative contribution of natural and vaccine-derived immunity, creating generations who have not been exposed to wild-type measles, mumps, and rubella viruses, so waning immune-memory is unlikely to be boosted by natural wild-type infection.53, 54
We did not consider coadministration of the MMR vaccine with other vaccines, such as varicella. Nevertheless, previous studies have shown that coadministration of varicella and MMR vaccines,55 either as a combination,56 or as concomitant injections,57 did not significantly affect the immunogenicity of the MMR components. We also note that seropositivity for anti-measles, anti-mumps, and anti-rubella IgG was used as a proxy for both natural infection or vaccine-induced protection, and seronegativity as a proxy for susceptibility to these infections. Some people without detectable antibodies might have a degree of protection via cellular immunity, but this proportion remains unknown,18 so could not be taken into account in our model. Therefore, it is important to note that our results are based on humoral immunity only. There are few studies concerning persistence of MMR-induced immunity within dosing, so our results should not be overinterpreted.
In conclusion, this systematic review and meta-analysis provides rigorous evidence that primary and secondary vaccine failures exist for different MMR vaccines and their components. These estimates are crucial for mathematical predictions regarding measles, mumps, and rubella outbreaks, and are sensitive to historical, current, and future MMR vaccine-uptake data as well as vaccine effectiveness over time. As increasingly long periods of time have passed since the average person received MMR vaccination, an important element to improve the accuracy of predictive models is how vaccine-induced immunity wanes over time for each MMR component.
Acknowledgments
Acknowledgments
We thank Keith Veitch (Amsterdam, Netherlands) for editorial advice. This project was funded by the European Research Council under the EU's Horizon 2020 research and innovation programme (grant agreement 682540, TransMID).
Contributors
NH, PB, SA, and JS designed the study and NH conceived the project. JS and SA reviewed the literature, extracted the data, performed the data analysis, data interpretation, and writing of the first draft of the manuscript. JS made the table, panel, and figures. JS, SA, HT, PVD, PB, and NH contributed to, reviewed, and approved the final version of the manuscript.
Declaration of interests
HT reports grants from Pfizer, outside the submitted work. PVD reports grants from GlaxoSmithKline, Pfizer, Sanofi, Merck, Takeda, Baxter, CanSino, Themis, Johnson & Johnson, Abbott, the Bill & Melinda Gates Foundation, the Flemish Government, and the EU, outside the submitted work. NH reports grants from GlaxoSmithKline, Pfizer, Merck, and Johnson & Johnson, outside the submitted work. PB reports grants from Innovative Medicines Initiative of the European Commission, and attended meetings of an advisory board on economic evaluations of vaccines convened by Pfizer in 2019, outside the submitted work. All other authors declare no competing interests.
Supplementary Material
References
- 1.Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. [DOI] [PubMed] [Google Scholar]
- 2.Richard J-L, Zwahlen M, Feuz M, Matter HC. Comparison of the effectiveness of two mumps vaccines during an outbreak in Switzerland in 1999 and 2000: a case-cohort study. Eur J Epidemiol. 2003;18:569–577. doi: 10.1023/a:1024698900332. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Hu Y, Zhang G, Zheng J, Li L, An Z. Meta-analysis of vaccine effectiveness of mumps-containing vaccine under different immunization strategies in China. Vaccine. 2014;32:4806–4812. doi: 10.1016/j.vaccine.2014.05.061. [DOI] [PubMed] [Google Scholar]
- 4.Bernard H, Fischer R, Wild F. Ongoing measles outbreak in southern Bavaria, Germany. Euro Surveill. 2008;13 [PubMed] [Google Scholar]
- 5.Siani A. Measles outbreaks in Italy: a paradigm of the re-emergence of vaccine-preventable diseases in developed countries. Prev Med. 2019;121:99–104. doi: 10.1016/j.ypmed.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Smithson R, Irvine N, Hutton C, Doherty L, Watt A. Spotlight on measles 2010: ongoing measles outbreak in Northern Ireland following an imported case, September–October 2010. Euro Surveill. 2010;15 doi: 10.2807/ese.15.43.19698-en. [DOI] [PubMed] [Google Scholar]
- 7.Pan American Health Organization Region of the Americas is declared free of measles. 2016. https://www.paho.org/hq/index.php?option=com_content&view=article&id=12528:region-americas-declared-free-measles&Itemid=1926&lang=en
- 8.Ramsay M. WHO Regional Office for Europe; Copenhagen: 1999. A strategic framework for the elimination of measles in the European Region. [Google Scholar]
- 9.Beutels P, Gay NJ. Economic evaluation of options for measles vaccination strategy in a hypothetical western European country. Epidemiol Infect. 2003;130:273–283. doi: 10.1017/s0950268802008142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funk S, Knapp JK, Lebo E, et al. Combining serological and contact data to derive target immunity levels for achieving and maintaining measles elimination. BMC Med. 2019;17:180. doi: 10.1186/s12916-019-1413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO Immunisation agenda 2030: a global strategy to leave no one behind. 2020. https://www.who.int/immunization/immunization_agenda_2030/en [DOI] [PubMed]
- 12.Béraud G, Abrams S, Beutels P, Dervaux B, Hens N. Resurgence risk for measles, mumps and rubella in France in 2018 and 2020. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.25.1700796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen C, White JM, Savage EJ, et al. Vaccine effectiveness estimates, 2004-2005 mumps outbreak, England. Emerg Infect Dis. 2007;13:12–17. doi: 10.3201/eid1301.060649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veneti L, Borgen K, Borge KS, et al. Large outbreak of mumps virus genotype G among vaccinated students in Norway, 2015 to 2016. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.38.1700642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panagiotopoulos T, Antoniadou I, Valassi-Adam E, Berger A. Increase in congenital rubella occurrence after immunisation in Greece: retrospective survey and systematic review. BMJ. 1999;319:1462–1467. doi: 10.1136/bmj.319.7223.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philipson T. Private vaccination and public health: an empirical examination for US measles. J Hum Resour. 1996;31:611–630. [Google Scholar]
- 17.Verelst F, Willem L, Kessels R, Beutels P. Individual decisions to vaccinate one's child or oneself: a discrete choice experiment rejecting free-riding motives. Soc Sci Med. 2018;207:106–116. doi: 10.1016/j.socscimed.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Plotkin SA. Complex correlates of protection after vaccination. Clin Infect Dis. 2013;56:1458–1465. doi: 10.1093/cid/cit048. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 20.Schenk J, Abrams S. Immunogenicity and persistence of measles, mumps and rubella vaccines: a systematic review and meta-analysis. 2019. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42019116705 [DOI] [PMC free article] [PubMed]
- 21.Demicheli V, Rivetti A, Debalini MG, Di Pietrantonj C. Vaccines for measles, mumps and rubella in children. Cochrane Database Syst Rev. 2012;2 doi: 10.1002/14651858.CD004407.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT, Altman DG. In: Cochrane handbook for systematic reviews of interventions. Higgins JPT, Green S, editors. Wiley; Chichester: 2008. Assessing risk of bias in included studies. [Google Scholar]
- 23.Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–680. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 24.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 26.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 27.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21:607–611. [Google Scholar]
- 30.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 33.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7:40–45. [Google Scholar]
- 34.Lewnard JA, Grad YH. Vaccine waning and mumps re-emergence in the United States. Sci Transl Med. 2018;10 doi: 10.1126/scitranslmed.aao5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nic Lochlainn LM, de Gier B, van der Maas N, et al. Immunogenicity, effectiveness, and safety of measles vaccination in infants younger than 9 months: a systematic review and meta-analysis. Lancet Infect Dis. 2019;19:1235–1245. doi: 10.1016/S1473-3099(19)30395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wellington K, Goa KL. Measles, mumps, rubella vaccine (Priorix; GSK-MMR): a review of its use in the prevention of measles, mumps and rubella. Drugs. 2003;63:2107–2126. doi: 10.2165/00003495-200363190-00012. [DOI] [PubMed] [Google Scholar]
- 37.Abrams S, Beutels P, Hens N. Assessing mumps outbreak risk in highly vaccinated populations using spatial seroprevalence data. Am J Epidemiol. 2014;179:1006–1017. doi: 10.1093/aje/kwu014. [DOI] [PubMed] [Google Scholar]
- 38.Abrams S, Kourkouni E, Sabbe M, Beutels P, Hens N. Inferring rubella outbreak risk from seroprevalence data in Belgium. Vaccine. 2016;34:6187–6192. doi: 10.1016/j.vaccine.2016.10.072. [DOI] [PubMed] [Google Scholar]
- 39.Hens N, Abrams S, Santermans E, et al. Assessing the risk of measles resurgence in a highly vaccinated population: Belgium anno 2013. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.es2015.20.1.20998. [DOI] [PubMed] [Google Scholar]
- 40.Hahné SJM, Nic Lochlainn LM, van Burgel ND, et al. Measles outbreak among previously immunized healthcare workers, the Netherlands, 2014. J Infect Dis. 2016;214:1980–1986. doi: 10.1093/infdis/jiw480. [DOI] [PubMed] [Google Scholar]
- 41.Gastanaduy PA, Redd SB, Clemmons NS, et al. Measles. 2019. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt07-measles.html
- 42.Hyle EP, Rao SR, Bangs AC, et al. Clinical practices for measles-mumps-rubella vaccination among US pediatric international travelers. JAMA Pediatr. 2019;174 doi: 10.1001/jamapediatrics.2019.4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuylen E, Willem L, Broeckhove J, Beutels P, Hens N. Clustering of susceptible individuals within households can drive measles outbreaks: an individual-based model exploration. medRxiv. 2019 doi: 10.1101/2019.12.10.19014282. published online Dec 16. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanyaolu A, Okorie C, Marinkovic A, et al. Measles outbreak in unvaccinated and partially vaccinated children and adults in the United States and Canada (2018–2019): a narrative review of cases. Inquiry. 2019;56 doi: 10.1177/0046958019894098. 46958019894098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strebel PM, Papania MJ, Fiebelkorn AP, Halsey NA. In: Vaccines. 6th edn. Plotkin SA, Orenstein WA, Offit PA, editors. Elsevier; Philadelphia: 2012. Measles vaccine; pp. 352–387. [Google Scholar]
- 46.Rota JS, Hickman CJ, Sowers SB, Rota PA, Mercader S, Bellini WJ. Two case studies of modified measles in vaccinated physicians exposed to primary measles cases: high risk of infection but low risk of transmission. J Infect Dis. 2011;204(suppl 1):S559–S563. doi: 10.1093/infdis/jir098. [DOI] [PubMed] [Google Scholar]
- 47.Dayan GH, Rubin S. Mumps outbreaks in vaccinated populations: are available mumps vaccines effective enough to prevent outbreaks? Clin Infect Dis. 2008;47:1458–1467. doi: 10.1086/591196. [DOI] [PubMed] [Google Scholar]
- 48.Korczyńska MR, Paradowska-Stankiewicz I. Rubella in Poland in 2013. Przegl Epidemiol. 2015;69:213–218. 341–43. [PubMed] [Google Scholar]
- 49.Wood JG, Goeyvaerts N, MacIntyre CR, Menzies RI, McIntyre PB, Hens N. Estimating vaccine coverage from serial trivariate serologic data in the presence of waning immunity. Epidemiology. 2015;26:381–389. doi: 10.1097/EDE.0000000000000278. [DOI] [PubMed] [Google Scholar]
- 50.WHO . Vaccination coverage cluster surveys: reference manual, version 3 drafting working draft. World Health Organization; Geneva: 2015. Immunization, vaccines and biologicals. [Google Scholar]
- 51.Huzly D, Hanselmann I, Neumann-Haefelin D, Panning M. Performance of 14 rubella IgG immunoassays on samples with low positive or negative haemagglutination inhibition results. J Clin Virol. 2016;74:13–18. doi: 10.1016/j.jcv.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 52.Xu W, Zhang Y, Wang H, et al. Global and national laboratory networks support high quality surveillance for measles and rubella. Int Health. 2017;9:184–189. doi: 10.1093/inthealth/ihx017. [DOI] [PubMed] [Google Scholar]
- 53.Heffernan JM, Keeling MJ. Implications of vaccination and waning immunity. Proc Biol Sci. 2009;276:2071–2080. doi: 10.1098/rspb.2009.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winter AK, Martinez ME, Cutts FT, et al. Benefits and challenges in using seroprevalence data to inform models for measles and rubella elimination. J Infect Dis. 2018;218:355–364. doi: 10.1093/infdis/jiy137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma SJL, Li X, Xiong YQ, Yao AL, Chen Q. Combination measles-mumps-rubella-varicella vaccine in healthy children: a systematic review and meta-analysis of immunogenicity and safety. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000001721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nolan T, McIntyre P, Roberton D, Descamps D. Reactogenicity and immunogenicity of a live attenuated tetravalent measles-mumps-rubella-varicella (MMRV) vaccine. Vaccine. 2002;21:281–289. doi: 10.1016/s0264-410x(02)00459-0. [DOI] [PubMed] [Google Scholar]
- 57.Stück B, Stehr K, Bock HL. Concomitant administration of varicella vaccine with combined measles, mumps, and rubella vaccine in healthy children aged 12 to 24 months of age. Asian Pac J Allergy Immunol. 2002;20:113–120. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.