Abstract
Infected Rathke's cleft cysts (RCC) are extremely rare with only a few published cases. We report the case of a 31-year-old man who presented with headaches, visual disturbance, and hypopituitarism secondary to an infected RCC with extension of abscesses along the optic tract. Magnetic resonance imaging showed ring enhancing cystic lesions within an expanded sella with suprasellar and intraparenchymal extension. The radiological appearance suggested a high-grade optic glioma, but an endoscopic transsphenoidal biopsy revealed frank pus in the pituitary fossa, which subsequently grew Staphylococcus aureus . Pathological examination of the cyst wall showed an inflamed RCC. Following a prolonged course of intravenous antibiotics, the infection resolved and vision improved. RCC abscesses are rare and the intracranial extension of the infection in our case makes it unique.
Keywords: Rathke's cleft cyst, pituitary abscess, transphenoidal surgery
Introduction
Pituitary abscesses are rare, accounting for 0.27 to 0.6% of pituitary lesions. 1 2 Pituitary abscess associated with a Rathke's cleft cyst (RCC) is even rarer and only around 60 cases have been published to date. 3 4 5 6 7 8 9 10 11 12 13
Primary pituitary abscess occurs in normal pituitary tissue, without an underlying structural cause. Pituitary abscesses developing on the background of a preexisting sellar lesion or following pituitary surgery are named secondary pituitary abscesses, such as in the case we describe. 12 While radiological findings of a pituitary abscess may be nonspecific, intracranial extension is most unusual; here, we describe the course of such an abscess associated with an RCC that gave rise to peculiar radiological findings leading to diagnostic challenges.
Case Report
A previously fit and well 31-year-old man presented to his local hospital with 1-week history of generalized severe headache. Brain computed tomography (CT) was reported as negative for acute intracranial pathology, notably without any evidence of sinus infection.
He represented 5 weeks later with worsening headache and blurring of vision. He also reported recent onset of erectile dysfunction and reduced libido. He had no polyuria or polydipsia. Neuroophthalmic assessment revealed asymmetric bitemporal field defects, the left worse than the right, with slightly reduced left best corrected visual acuity (BCVA) of 6 of 9. This was consistent with an asymmetric compression of the optic chiasm. The left visual acuity continued to deteriorate over the subsequent days to 6 of 18. Systemically, he was well without pyrexia or hemodynamic instability. Pituitary function tests showed hypogonadotropic hypogonadism and mild hyperprolactinemia (538mU/L, 73–407). His other pituitary function was intact. His C-reactive protein (CRP) was normal (2mmol/L) and erythrocyte sedimentation rate (ESR) was mildly elevated (29mm/h). Further brain CT revealed a large area of hypodensity centered on the left thalamus/basal ganglia, and subsequent magnetic resonance imaging (MRI) with contrast showed a medium size pituitary cyst with suprasellar extension and two contiguous tandem cysts with thick enhancing walls along the left optic tract ( Fig. 1 ). The sellar/suprasellar cyst was compressing the optic chiasm and the lateral cysts along the left optic tract were causing perilesional edema of the adjacent brain parenchyma. These imaging characteristics were consistent with high-grade optic pathway glioma with extension into the thalamus.
Fig. 1.

Preoperative imaging. ( A ) CT plain imaging, axial view showing hypodensity/edema in left basal ganglia region, tracking along visual radiation ( white arrow ). ( B ) Gadolinium-enhanced T1-weighted imaging, sagittal view, showing multi-septate edge enhancing lesion arising from the sella ( white arrow ) and a second smaller contiguous cyst at the superior/posterior aspect. ( C ) Gadolinium-enhanced T1-weighted imaging, axial view showing multi-septate edge enhancing lesion extending along the left optic tract ( white arrow ) in contact with the suprasellar component of the pituitary cyst. ( D ) T2-weighted imaging, axial view showing edema around the abscess ( white arrow ). CT, computed tomography.
The Pituitary Multidisciplinary Team (MDT) discussion recommended biopsy of the presumed high-grade tumor. Surprisingly, the intraoperative findings during the endoscopic transsphenoidal biopsy were in keeping with an abscess, rather than a tumor. Frank pus was drained from the pituitary fossa, without evidence of solid tissue consistent with tumor ( Fig. 2 ). The pus was evacuated and the cyst wall was sampled for biopsy. Empiric antibiotics were started intraoperatively and intraoperative histological examination showed epithelium with reactive atypia ruling out a malignant tumor. Initial culture grew a highly sensitive Staphylococcus aureus .
Fig. 2.

Intraoperative photographs. ( A ) Pus filled the sphenoid sinus following dural sellotomy. ( B ) Following aspiration of the pus, the cyst wall was dissected of the dura.
The formal histological examination revealed a heavily inflamed cyst lined by cuboidal epithelium in the vicinity of anterior pituitary gland tissue ( Fig. 3A ), with focally prominent cilia typical for RCC ( Fig. 3B ). There were regions of suppuration in the lumen of the cyst ( Fig. 3C ). Squamous metaplasia and mild reactive atypia were seen in places, reflective of secondary changes to the inflammation ( Fig. 3D ). The overall appearances were consistent with an inflamed RCC with abscess formation ( Fig. 3 ).
Fig. 3.
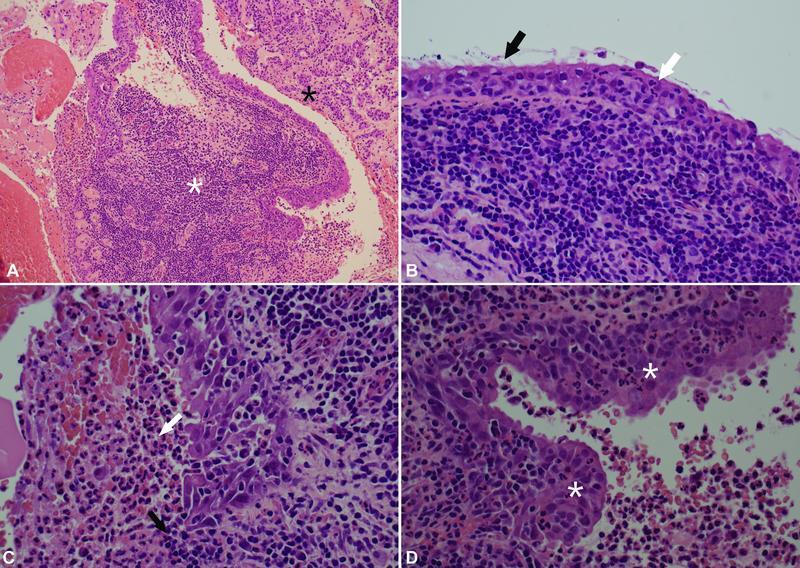
Histology of the specimen. ( A ) Histology illustrates a benign cyst with acute-on-chronic inflammation ( white asterisk ) in the vicinity of atrophic/fibrotic anterior pituitary gland tissue ( black asterisk ). ( B ) The cyst epithelium is focally ciliated ( black arrow ) and acutely inflamed by infiltrating neutrophils ( white arrow ). Underneath, there is a dense chronic inflammatory infiltrate. ( C ) Focally, the epithelium is eroded by inflammation ( black arrow ), and purulent material is seen in the cyst lumen ( white arrow ). ( D ) The epithelium shows squamous metaplasia and mild reactive atypia in places ( white asterisk ), as secondary changes to the inflammation.
Septic screen that included skin examination, blood cultures, cardiac ultrasound, CT chest/abdomen/pelvis, and orthopentogram revealed no source of infection. The postoperative period was uncomplicated with good clinical improvement and normalization of vision. A standard course of 6 weeks of intravenous (IV) antibiotics (ceftriaxone) was administered. Pituitary function at follow-up showed ongoing hypopituitarism (adrenocorticotrophic hormone [ACTH], thyroid stimulating hormone [TSH], and gonadotrophin deficiency). Repeat imaging 6 weeks after surgery showed no recurrence of the sellar cyst and significant reduction in the size of the two presumed abscesses along the left optic tract. The 3-month postoperative imaging demonstrated almost complete resolution of the abscess.
Discussion
RCCs are benign lesions developing between the anterior and posterior pituitary gland from remnants of the embryological Rathke's pouch. 14
A preexisting pituitary lesion, such as adenoma, RCC, or craniopharyngioma, is considered a risk factor for developing a pituitary abscess. 6 However, they only make up a small percentage of all pituitary abscesses. 10 15 There have been around 60 reported cases of RCC associated abscess. Only one-third of them are single case reports of patients presenting acutely without prior knowledge of pituitary disease. 1 3 4 6 7 8 9 11 12 13 15 16 17 18 19 20 21 One case involved a patient with previously known RCC for which they had not yet had surgery. 5 Several other cases were presentations of secondary pituitary abscess in patients previously operated on for their RCC. 2 3 Interestingly, in a review of 6,832 patients who had undergone transsphenoidal surgery for pituitary disease, 23 later presented with pituitary abscess (0.3%), and 8 of them had RCC removal as the primary operation. 2
The clinical and radiological presentations of pituitary abscess associated with RCC have been widely variable in the literature to date, making preoperative diagnosis challenging. 12 16 17 Most have been described in females, with a median age within the fourth decade of life, 3 and only a handful of cases involving pediatric populations. 7 8 11 17 The clinical presentation in our case of headache, visual disturbance with associated manifestations of hypopituitarism is not uncommon. In a recent literature review of pituitary abscess associated with RCC, headache occurred in 70% of patients, visual disturbance in 35%, and hypopituitarism in 80%. 3 Septic manifestations, such as fever with raised CRP and white cell count, were reported in only 30%; therefore, the lack of these in our case report is not unexpected. 3 12
Risk factors associated with an increased risk of abscess development within an RCC are previous surgical or irradiation treatment for the RCC, as well as immunosuppression. 2 3 12 14 18 The majority, however, occurs spontaneously, as with our patient. 3 12 Furthermore, other sites of infection predisposing to abscess formation in an RCC have been linked. Such cases include local spread from the sphenoid sinus, direct translocation of infection locally from meningitis or thrombophlebitis, 6 11 19 or hematogenous spread from a distant nidus of infection. 3 20 However, most cases do not have evidence of preceding infection elsewhere, and the abscesses are cryptogenic. 11 12
It has been hypothesized that RCC abscesses develop from direct contact with the sphenoid sinuses, due to proximity to the sella and from similar pathogens causing chronic sinusitis. 3 22 Given that most cases do not have radiological evidence of sinusitis or anatomical sinusal defect, spread via the venous drainage system that supplies both the sphenoid sinus and the pituitary gland has been suggested. 14
A unique finding of our case is the extension of the abscess along the optic pathway and the associated radiological appearances. We speculate that the abscess originated in the sella and extended to the left optic tract by contiguous spread. Most pituitary MRI protocols do not include sequences specific to infection, such as diffusion-weighted imaging (DWI), and their sensitivity in identifying pituitary abscess may be limited. 12 However, a review 23 of MRIs of 51 patients with a pituitary abscess, suggested that 96% of cases had MR evidence of local invasion to at least one adjacent structure. Despite this, there was no case demonstrating such extensive spread into the optic pathway. Moreover, a correct diagnosis of pituitary abscess was made in two-thirds of cases preoperatively due to what they found to be more “typical” MRI findings within this group. These included iso- or hypointensity on T1-weighted imaging (59% of cases), iso- or hyperintensity on T2-weighted imaging (76.5% of cases), disappearance of the posterior pituitary bright spot (86% of cases), and ring enhancement postgadolinium injection in 82%.
Similarly, of all the reported cases of RCC-associated abscess to date, none describes such as an extensive course of abscess spread, nor mentions of optic pathway or basal ganglia involvement, making our case unique. The MRI appearance and the spread of the lesion in our patient led to the initial radiological diagnosis of high-grade optic glioma. Interestingly, there was evidence of restricted diffusion on DWI on the MRI head which has been previously thought to be present in cases of pituitary abscess. 14
The provisional diagnosis of a pituitary abscess was made intraoperatively when purulent material was found in the pituitary fossa and empiric antibiotic treatment commenced immediately. The histological examination confirmed findings typical for RCC that had undergone inflammatory changes, without evidence of neoplasia. Almost all previously reported cases showed histological appearances of RCC with or without evidence of inflammation. 1 7 18 19 20 Of those who had more nonspecific histology, the diagnosis was made with the combination of radiological, intraoperative, histological, and microbiological findings. 3 12
Surgical drainage of the pituitary abscess is the treatment of choice aiming to treat the infection and to obtain samples for microbiological and histological examination. 3 14 Empiric antibiotics should be administered once pus is confirmed intraoperatively, followed by a prolonged targeted antibiotic course, if the responsible organism is identified. The transsphenoidal route is the approach of choice for the sellar abscess 15 but cannot be used for drainage of the cysts along the optic tract. In our case, we elected to treat the intraparenchymal abscesses medically with antibiotics because of their relatively small size; the risks associated with a needle aspiration through a bur hole in this deep-seated location, the fact that we had already obtained pus sample and equally importantly, and the fact that the vision improved following aspiration of the sellar abscess. Needle aspiration of the contiguous abscesses would have been considered in this case, only if medical management had failed. Despite treatment, the rate of recurrence of abscesses among these patients is high in reported cases with at least 18 patients of the 60 reported cases developing reaccumulation of the abscess after initial surgical drainage. 1 4 9 11 14 17 19 21 Most of these reaccumulated within 6 months after the operation and some relapsed multiple times, one patient requiring five surgeries to drain the abscess. 11
Postoperative improvement of endocrine and visual deficits following treatment of infected RCC has been reported at 66 and 75%, respectively. 3 Our patient had rapid visual improvement but did not recover any pituitary function. Close follow-up will be needed, given the rate of recurrence.
Conclusion
Abscess in an RCC with extension along the optic pathway has not been previously described. This case is unique in that respect and the atypical findings on imaging posed diagnostic challenges. The intraoperative findings and pathological review led to the correct diagnosis and management.
Patient Consent
Written informed consent for publication of their details was obtained from the patient.
Funding Statement
Funding None.
Footnotes
Conflict of Interest None declared.
References
- 1.Jain K C, Varma A, Mahapatra A K. Pituitary abscess: a series of six cases. Br J Neurosurg. 1997;11(02):139–143. doi: 10.1080/02688699746492. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Yang C, Bao X. Clinical features and treatment of secondary pituitary abscess after transsphenoidal surgery: a retrospective study of 23 cases. World Neurosurg. 2018;113:e138–e145. doi: 10.1016/j.wneu.2018.01.197. [DOI] [PubMed] [Google Scholar]
- 3.Aranda F, García R, Guarda F J. Rathke's cleft cyst infections and pituitary abscesses: case series and review of the literature. Pituitary. 2021;24(03):374–383. doi: 10.1007/s11102-020-01115-2. [DOI] [PubMed] [Google Scholar]
- 4.Gomez Perun J, Eiras J, Carcavilla L I. [Intrasellar abscess into a cyst of the Rathke's pouch (authors' translation)] Neurochirurgie. 1981;27(03):201–205. [PubMed] [Google Scholar]
- 5.Kimura H, Fukushima T, Matsuda T, Tomonaga M. Abscess formation in a Rathke's cleft cyst [in Japanese] No To Shinkei. 1994;46(04):392–395. [PubMed] [Google Scholar]
- 6.Sato M, Matsushima Y, Taguchi J. A case of pituitary abscess caused by infection of Rathke's cleft cyst [in Japanese] No Shinkei Geka. 1995;23(11):991–995. [PubMed] [Google Scholar]
- 7.Miyamae T, Jingu K, Yagi Y. Secondary hypopituitarism caused by pituitary abscess with Rathke's cleft cyst. Clin Pediatr Endocrinol. 1996;5 08:117–118. [Google Scholar]
- 8.Ono A, Tokoro K, Suzuki N, Kurashima Y, Yamamoto I. Pituitary abscess associated with a rathke's cleft cyst: a case report. Japanese Journal of Neurosurgery. 1997;6:345–349. [Google Scholar]
- 9.Signorelli A, Biroli F, Barbo R.P-4–523 - abscess formation in Rathke's cleft cyst Clin Neurol Neurosurg 199799S1839409433 [Google Scholar]
- 10.Liu F, Li G, Yao Y. Diagnosis and management of pituitary abscess: experiences from 33 cases. Clin Endocrinol (Oxf) 2011;74(01):79–88. doi: 10.1111/j.1365-2265.2010.03890.x. [DOI] [PubMed] [Google Scholar]
- 11.Uchiyama T, Sakai K, Asanuma M, Aoyama T, Hongo K. Pituitary abscess manifesting as meningitis and photophobia associated with Rathke's cleft cyst in a child. Case report. Neurol Med Chir (Tokyo) 2011;51(06):455–459. doi: 10.2176/nmc.51.455. [DOI] [PubMed] [Google Scholar]
- 12.Coulter I C, Mahmood S, Scoones D, Bradey N, Kane P J. Abscess formation within a Rathke's cleft cyst. J Surg Case Rep. 2014;2014(11):rju105. doi: 10.1093/jscr/rju105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naama O, Gazzaz M, Boulahroud O, Elmoustarchid B. Infection of a Rathke cleft cyst: a rare cause of pituitary abscess. Surg Infect (Larchmt) 2014;15(03):358–360. doi: 10.1089/sur.2013.069. [DOI] [PubMed] [Google Scholar]
- 14.Tate M C, Jahangiri A, Blevins L, Kunwar S, Aghi M K.Infected Rathke cleft cysts: distinguishing factors and factors predicting recurrence Neurosurgery 20106703762–769., discussion 769 [DOI] [PubMed] [Google Scholar]
- 15.Vates G E, Berger M S, Wilson C B. Diagnosis and management of pituitary abscess: a review of twenty-four cases. J Neurosurg. 2001;95(02):233–241. doi: 10.3171/jns.2001.95.2.0233. [DOI] [PubMed] [Google Scholar]
- 16.Thomas N, Wittert G A, Scott G, Reilly P L. Infection of a Rathke's cleft cyst: a rare cause of pituitary abscess. Case illustration. J Neurosurg. 1998;89(04):682. doi: 10.3171/jns.1998.89.4.0682. [DOI] [PubMed] [Google Scholar]
- 17.Israel Z H, Yacoub M, Gomori J M. Rathke's cleft cyst abscess. Pediatr Neurosurg. 2000;33(03):159–161. doi: 10.1159/000028997. [DOI] [PubMed] [Google Scholar]
- 18.Obenchain T G, Becker D P. Abscess formation in a Rathke's cleft cyst. Case report. J Neurosurg. 1972;36(03):359–362. doi: 10.3171/jns.1972.36.3.0359. [DOI] [PubMed] [Google Scholar]
- 19.Celikoglu E, Boran B O, Bozbuga M. Abscess formation in Rathke's cleft cyst. Neurol India. 2006;54(02):213–214. [PubMed] [Google Scholar]
- 20.Bognàr L, Szeifert G T, Fedorcsàk I, Pàsztor E.Abscess formation in Rathke's cleft cyst Acta Neurochir (Wien) 1992117(1-2):70–72. [DOI] [PubMed] [Google Scholar]
- 21.Sonntag V KH, Plenge K L, Balis M S. Surgical treatment of an abscess in a Rathke's cleft cyst. Surg Neurol. 1983;20(02):152–156. doi: 10.1016/0090-3019(83)90468-8. [DOI] [PubMed] [Google Scholar]
- 22.Ramadan H H. What is the bacteriology of chronic sinusitis in adults? Am J Otolaryngol. 1995;16(05):303–306. doi: 10.1016/0196-0709(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Gao L, Zhou X. Magnetic resonance imaging characteristics of pituitary abscess: a review of 51 cases. World Neurosurg. 2018;114:e900–e912. doi: 10.1016/j.wneu.2018.03.113. [DOI] [PubMed] [Google Scholar]


