Abstract
The existence of an accessory middle cerebral artery (AMCA) usually has no pathological significance. Three patients developed cerebral infarction due to thromboembolic occlusion of the main trunk of the middle cerebral artery (MCA). In these patients, AMCA originating from the anterior cerebral artery was intact, and ran to the lateral side along the main MCA. Emergency endovascular treatment to remove the thrombus in the main MCA was performed, and MCA was recanalized. In one patient, the main MCA re-occluded and cerebral infarction developed on the next day. The diameter of AMCA is commonly smaller than that of the main MCA. Therefore, volume of ischemic region depends on the collateral blood flow to the left MCA territory by AMCA. Once an anomalous MCA is detected in a patient with cerebral infarction involving the MCA territory, close examinations to assess the anatomy of both the main and anomalous MCA are mandatory.
Keywords: anomaly, accessory, middle cerebral artery, cerebral infarction, thromboembolism
Introduction
Three types of middle cerebral artery (MCA) anomalies are known: accessory MCA (AMCA), duplicated MCA (DMCA), and fenestration. 1 2 3 Among them, AMCA and DMCA supplementarily supply blood to the MCA territory.
However, there have been some reports describing cerebral infarction or ischemic stroke due to thromboembolic events in an anomalous MCA. 4 5 6 7 8 9 10 11 12 13 In such cases, diagnosis and treatment are compromised. If the existence of an anomalous MCA is not recognized, it may be difficult to understand the discrepancy between the symptoms and radiological findings. Furthermore, in an emergency situation necessitating the recovery of blood flow, prompt and precise recognition of an anomalous MCA is essential.
Recently, we treated three patients with AMCA who developed cerebral infarction in the MCA territory. The cerebral infarction was due to the occlusion of the main MCA, not AMCA. In this report, we describe patients associated with AMCA who developed cerebral infarction due to the occlusion of the main MCA. We discuss the radiological findings and management of these rare cases.
Case Presentation
Case 1
An 81-year-old woman with a medical history of myocardial infarction, hyperlipidemia, chronic kidney disease, and chronic heart failure presented with aphasia and right hemiparesis. Computed tomography (CT) on admission demonstrated a hyperdense artery sign in the left MCA, but no low-density area in the cerebrum ( Fig. 1A ). Diffusion-weighted images (DWI) of magnetic resonance imaging (MRI) revealed a hyperintense region in the posterior part of the MCA territory of the left temporal lobe ( Fig. 1B ). However, the region did not show a high intensity on fluid-attenuated inversion recovery (FLAIR) images ( Fig. 1B ). MR angiography (MRA) demonstrated faint opacification of the left internal carotid artery (ICA), and a small artery originating from the left ACA and running toward the left ( Fig. 1C ). Due to the poor quality of MRA images, the artery could not be diagnosed as AMCA. Based on the findings of DWI and FLAIR MRI, emergency endovascular treatment to remove the thrombus in MCA was conducted.
Fig. 1.
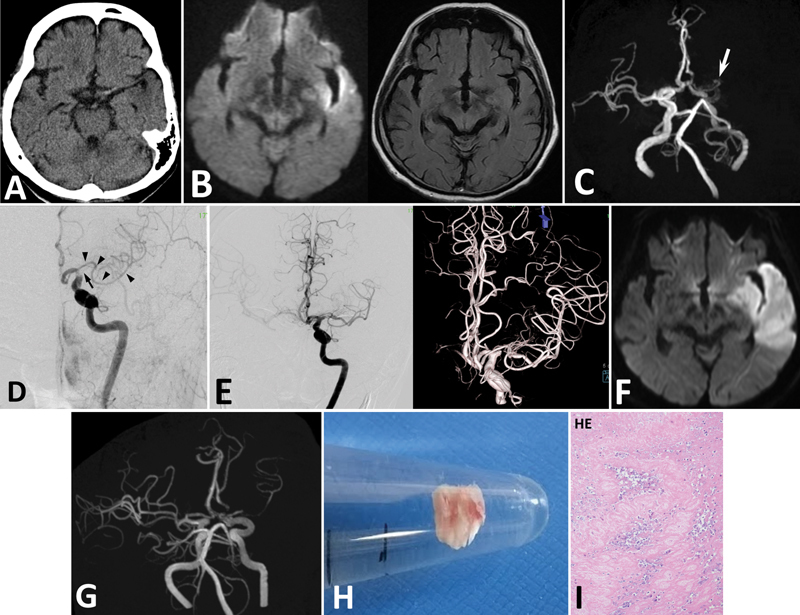
Case 1. ( A ) CT demonstrating a high density in the left MCA, but no low-density area in the left cerebrum. ( B ) DWI of MRI demonstrating a high-intensity region in the left cerebrum ( left panel ). FLAIR images showing no high-intensity region in the left cerebrum ( right ). ( C ) MRA faintly demonstrating the left ICA, and a small artery originating from ACA ( arrow ). However, opacification of this AMCA is not clear. ( D ) Angiography showing defect of contrast medium in the left ICA and MCA indicating thromboembolism. The main trunk of MCA is occluded at its origin ( arrow ). AMCA originates from ACA ( arrowheads ). ( E ) After endovascular treatment, the left MCA trunk is recanalized with residual stenosis in M1 of MCA. AMCA is patent. MRI ( F ) and MRA ( G ) on the next day showing cerebral infarction in the MCA territory, and re-occlusion of the main MCA. ( H ) The removed thrombus is a white thrombus. ( I ) Pathological examination of the thrombus revealing that it is composed of fibrous tissue and white blood cells (100×). HE; hematoxylin and eosin stain.
Angiography demonstrated that the thrombus existed at the tip of the left ICA extending to the left MCA; however, the left ICA was not completely occluded ( Fig. 1D ). The left anterior cerebral artery (ACA) was opacified. The main trunk of MCA was obstructed at its origin, and a small artery was found originating from the A1/A2 junction of the left ACA ( Fig. 1D ). The artery ran laterally and could easily be confused with a branch from the main MCA. The artery was diagnosed as AMCA. The thrombus in the left ICA and MCA was removed using an aspiration catheter (Penumbra, Penumbra Inc., CA, USA) and stent retriever (Trevo, Stryker, Tokyo, Japan). Finally, the left MCA was recanalized with residual stenosis of MCA and partial defect of contrast medium in M1 ( Fig. 1E ). CT and MRI on the next day revealed cerebral infarction in the left MCA territory ( Fig. 1F ). On MRA, the left MCA main trunk was re-occluded and the AMCA was intact ( Fig. 1G ).
The removed thrombus was whitish ( Fig. 1H ), and pathological examination revealed that the specimen was composed of fibrous tissue with a small amount of white blood cells ( Fig. 1I ). These findings implied that there existed stenosis in the MCA and thrombus was locally formed and extended in the atherosclerotic artery.
She was transferred to another hospital for rehabilitation with right hemiparesis and aphasia on the 61st day.
Case 2
An 87-year-old man with a history of hypertension presented with aphasia and right hemiparesis. He visited a local hospital, and his symptoms fluctuated. He was transferred to our hospital. After 5 hours from onset, he was somnolent and showed right paralysis. MRI and MRA showed cerebral infarction in the left corona radiata, and occlusion of the left MCA ( Fig. 2A ). On MRA, anomalous MCA branch was not opacified. Angiography showed occlusion of the left MCA at M1 and AMCA originating from the A1/2 junction of the left ACA ( Fig. 2B ). AMCA was thin and ran laterally along A1 and MCA. The AMCA was easily distinguished from the main trunk of MCA, because of its thin diameter. The MCA was recanalized by aspiration using aspiration catheter (Penumbra) ( Fig. 2C ). Postoperative MRI showed no enlargement of cerebral infarction and patent MCA main trunk. AMCA was not demonstrated on MRA postoperatively ( Fig. 2D ). His postoperative course was uneventful.
Fig. 2.
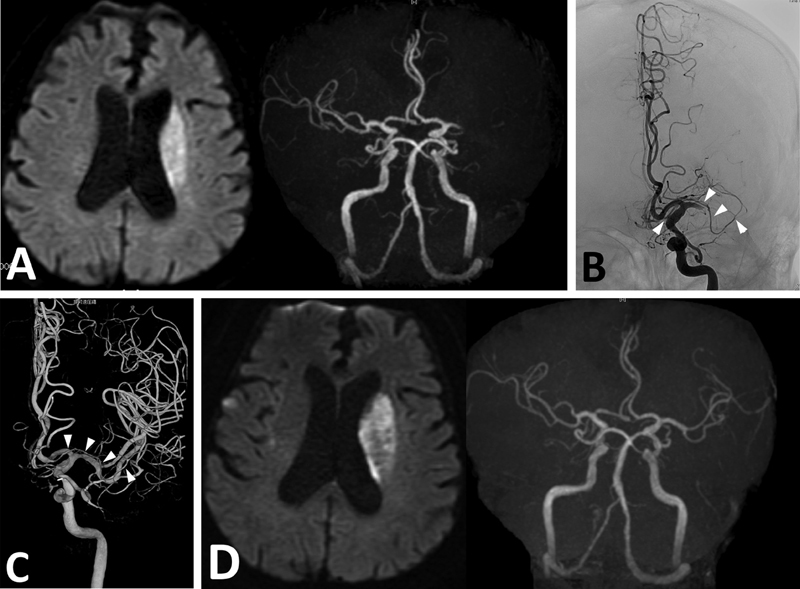
Case 2. ( A ) DWI of MRI demonstrating cerebral infarction in the left corona radiata ( left ). MRA demonstrating occlusion of the left MCA. No anomalous MCA branch is demonstrated ( right panel ). ( B ) Angiography showing occlusion of the left MCA trunk, and AMCA originating from the left ACA ( arrowheads ). ( C ) The occluded MCA is recanalized by aspiration. The AMCA is intact ( arrowheads ). ( D ) Postoperative MRI showing no change in cerebral infarction ( left ). AMCA is not demonstrated postoperatively on MRA ( right panel ).
He underwent carotid artery stenting for the contralateral internal carotid artery stenosis on the 15th day. He was transferred to another hospital for rehabilitation with left hemiparesis on the 23rd day.
Case 3
A 62-year-old man with a history of hypertension and hyperlipidemia presented with left hemiparesis and agnosia of right side. DWI of MRI revealed a hyperintense region in the right MCA territory. MRA demonstrated occlusion of right intracranial ICA and MCA, and faint opacification of right cervical ICA ( Fig. 3A ).
Fig. 3.
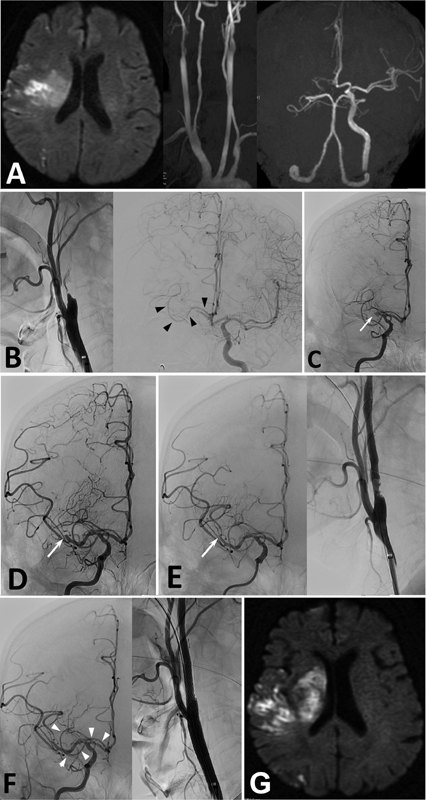
Case 3. ( A ) DWI of MRI showing cerebral infarctions in the right MCA territory. MRA showing occlusion of the right ICA. ( B ) Angiography showing occlusion of the right ICA ( left panel ). Left carotid angiography showing filling of contrast medium to the right A1 of ACA via AComA, and AMCA originating from the right A1/2 of ACA ( right panel, arrowheads ). ( C ) After aspiration of the thrombus from right ICA, angiography showing MCA occlusion ( arrow ). ( D ) After aspiration of the thrombus from the right MCA, angiography demonstrating occlusion at M2 ( arrow ). ( E ) After additional aspiration, right MCA is occluded at M3 portion ( arrow ). Residual stenosis of the right ICA origin is noted. ( F ) Postoperative angiography demonstrating occlusion of an M3 branch of MCA, and patent AMCA ( arrowheads ). Right ICA origin is well dilated. ( G ) Postoperative DWI of MRI showing slight enlargement of cerebral infarction.
Angiography demonstrated severe stenosis of the right cervical ICA and occlusion of distal ICA ( Fig. 3B ). Left carotid angiography showed collateral blood flow to the right A1 of ACA via anterior communicating artery (AComA). In addition, an artery originating from right A1/2 junction of ACA was demonstrated and ran along the right A1 and M1 to the right MCA territory ( Fig. 3B ). The main trunk of MCA was not demonstrated. The condition was diagnosed as right ICA and MCA occlusion due to thromboembolism from preexisting stenosis of cervical ICA.
Emergency endovascular treatment was performed. A guiding catheter (OPTIMO, 9Fr, Tokai Medical Products, Aichi, Japan) was placed in the right common carotid artery. A filter wire (Boston Scientific, Tokyo, Japan) was advanced to the right ICA and the stenotic cervical ICA was dilated with balloon catheter (Shiden, 5.5 × 30 mm, Kaneka Medical Products, Osaka, Japan). However, no recanalization of the right ICA was achieved. An aspiration catheter (Thrombuster, 7Fr, Kaneka Medical Products) was advanced to the occluded portion of right ICA, and thrombus was aspirated. Angiography showed recanalization of right ICA and occlusion of right MCA at M1 ( Fig. 3C ). Another aspiration catheter (Embovac, Johnson & Johnson, Tokyo) was advanced to the occluded portion of right M1 and aspirated. Significant amount of thrombus was aspirated by manual aspiration. Angiography at this moment showed occlusion of MCA at M2 ( Fig. 3D ). After repeated aspiration, angiography showed M3 occlusion, patent AMCA, and a residual stenosis with irregular wall of right ICA origin ( Fig. 3E ). To prevent thrombus formation and embolization of distal arteries, carotid artery stenting was performed. After a filter wire was advanced to the petrous portion, a stent (Carotid Wallstent, 10 × 30 mm, Boston Scientific) was advanced to the stenotic portion and deployed. Dilation of the lesion with balloon catheter (Shiden, 5.5 × 30 mm) was added. Postoperative angiography demonstrated dilation of the ICA, occlusion of M3 of MCA, and patent AMCA ( Fig. 3F ). Postoperative DWI of MRI showed slight enlargement of cerebral infarction ( Fig. 3G ).
His postoperative course was uneventful. He was transferred to another hospital for rehabilitation with left hemiparesis on the 14th day.
Discussion
The incidences of AMCA and DMCA have been reported as 0.3 to 0.4 and 0.2 to 2.9%, respectively. 14 Each anomalous artery shows a different distribution. DMCA runs to the temporal region, whereas AMCA follows a course parallel to the main MCA trunk and ends at the territory of the orbitofrontal and prefrontal arteries. 14 AMCA and DMCA are usually smaller than the main MCA trunk and show perforating branches. 14 In our cases, the diameters of AMCAs were smaller than those of the occluded main MCA trunks, and the origin was ACA. Therefore, AMCA was not confused as the main trunk of MCA on angiography. However, it took time to understand the anatomy of this anomalous artery during emergency endovascular procedures. If the diameter of AMCA is not smaller than the main MCA, it is not easy to recognize the exact affected artery. 9 10 11 13
Patients with anomalous MCA occasionally show cerebral infarction due to occlusion of one of the MCA branches, a main trunk or an anomalous branch. In such a situation, accurate diagnosis is sometimes difficult. 4 5 6 7 8 9 10 11 12 13 In our cases, based on MRA findings, AMCAs were not clearly demonstrated. In Case 1, the artery was not initially considered as AMCA and was not paid sufficient attention. Subsequent angiography demonstrated occlusion of the main MCA and intact AMCA. Therefore, it was not difficult to recognize the occluded artery. In contrast, if a small anomalous MCA is occluded with an intact main MCA, the main MCA is visualized by radiological examination. Recognition of thromboembolism in AMCA, not the main MCA, might be difficult. Commonly, AMCA is smaller than the main trunk, thrombus tends to flow into and occlude the bigger main MCA. Therefore, an occluded smaller AMCA might be overlooked. 4 5 6 7 8 9 10 11 12 13
There is a report describing a patient with AMCA who developed cerebral infarction due to occlusion of the main MCA. 8 In that case, the AMCA supplied sufficient blood flow to the MCA territory. Therefore, the infarction was small, even though the main MCA was occluded. In a patient with an anomalous MCA, the anomalous artery serves as a supplementary artery that supplies blood to the MCA territory. The area of blood flow from the supplementary AMCA might vary in each case. In Cases 1 and 2, AMCAs were small and collateral blood flow supplied by AMCA was not sufficient. Therefore, in Case 1, after re-occlusion of the main MCA, cerebral infarction developed to a significant size. In Case 3, although AMCA was smaller than the main MCA, AMCA supplied a moderate area of temporal lobe.
Thromboembolic event in one of the MCA branches in a patient with an anomalous MCA is extremely rare. However, in the presence of an anomalous MCA, there is a possibility that the main or anomalous MCA may be affected. In a case with relatively thick AMCA, it may be difficult to recognize the exact occluded artery. Therefore, care should be taken to correctly diagnose the MCA branch that is affected. After correct diagnosis and recognition of the anatomy, prompt treatment is necessary in emergency situations.
Conclusion
In patients with cerebral infarction and AMCA, close examination to identify the affected MCA branch is necessary if the infarction involves the MCA territory.
Footnotes
Conflict of Interest None declared.
References
- 1.Nomura M, Yamashima T, Kita D, Kida S, Kajinami K, Yamashita J. Duplication of the middle cerebral artery associated with an unruptured aneurysm. Acta Neurochir (Wien) 2000;142(02):221–222. doi: 10.1007/s007010050029. [DOI] [PubMed] [Google Scholar]
- 2.Nomura M, Tamase A, Kamide T, Mori K, Seki S, Iida Y.Accessory middle cerebral artery associated with an unruptured aneurysm at its origin Surg Neurol Int 20156(Suppl 16):S421–S423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iida Y, Tamase A, Kamide T, Mori K, Seki S, Nomura M.Aneurysm at origin of duplicated middle cerebral artery associated with another aneurysm Surg Neurol Int 20156(Suppl 21):S549–S552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komiyama M, Nishikawa M, Yasui T. The accessory middle cerebral artery as a collateral blood supply. Am J Neuroradiol. 1997;18(03):587–590. [PMC free article] [PubMed] [Google Scholar]
- 5.Menon B K, Aaron S, Bal S, Hill M D, Demchuk A M, Goyal M. Acute embolic occlusion of the accessory middle cerebral artery mimicking an internal carotid artery terminus aneurysm. Neurol India. 2011;59(06):908–909. doi: 10.4103/0028-3886.91378. [DOI] [PubMed] [Google Scholar]
- 6.Kuwahara H, Matsumura K, Watanabe M, Fujigasaki H. Intravenous t-PA for the occlusion of an accessory MCA. Intern Med. 2013;52(01):163. doi: 10.2169/internalmedicine.52.8999. [DOI] [PubMed] [Google Scholar]
- 7.Hiramatsu Y, Wakita M, Matsuoka H, Kasuya J, Hamada R, Takashima H. Cerebral infarction associated with accessory middle cerebral arteries: two case reports. Intern Med. 2014;53(12):1381–1384. doi: 10.2169/internalmedicine.53.1760. [DOI] [PubMed] [Google Scholar]
- 8.Liu Z S, Zhou L J, Sun Y, Kuang X W, Wang W, Li C. Sufficient collateral blood supply from accessory middle cerebral artery in a patient with acute ischemic stroke. Interv Neuroradiol. 2015;21(02):215–217. doi: 10.1177/1591019915583230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayer-Karpinska A, Lutz J, Birnbaum T, Dieterich M, Wollenweber F A. Severe MCA stroke without MCA occlusion? Thrombectomy uncovers accessory middle cerebral artery. Neurology. 2015;85(09):831–832. doi: 10.1212/WNL.0000000000001894. [DOI] [PubMed] [Google Scholar]
- 10.Matsunaga Y, Hayashi K, Okamura K.A case of embolic infarction associated with accessory middle cerebral artery and treated using mechanical thrombectomy No Shinkei Geka 201947111165–1171.in Japanese [DOI] [PubMed] [Google Scholar]
- 11.Deguchi I, Osada T, Kimura H. A case of acute cerebral infarction associated with an accessory middle cerebral artery in a patient who underwent thrombectomy. Acute Med Surg. 2019;7(01):e459. doi: 10.1002/ams2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke J, Maingard J, Chandra R V, Slater L A, Brooks M, Asadi H. Acute middle cerebral artery stroke in a patient with a patent middle cerebral artery. Neurol Clin Pract. 2019;9(03):250–255. doi: 10.1212/CPJ.0000000000000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ray N, Dhanasekaran J, Joseph S, Jella L. Tandem occlusion involving accessory middle cerebral artery in acute ischaemic stroke: management strategies. BMJ Case Rep. 2020;13(02):e233287. doi: 10.1136/bcr-2019-233287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komiyama M, Nakajima H, Nishikawa M, Yasui T. Middle cerebral artery variations: duplicatred and accessory arteries. Am J Neuroradiol. 1998;19(01):45–49. [PMC free article] [PubMed] [Google Scholar]


