Abstract
During gram-negative sepsis, human monocytes are triggered to produce large quantities of proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) in response to endotoxin (lipopolysaccharide [LPS]). Several studies have identified signal transduction pathways that are activated by LPS, including activation of nuclear factor-κB (NF-κB) and activation of mitogen-activated protein kinases (MAPKs), including ERK1 and ERK2, c-Jun N-terminal kinase, and p38. In this study, the relevance of ERK1 and ERK2 activation for LPS-induced TNF-α production by primary human monocytes has been addressed with PD-098059, which specifically blocks activation of MAPK kinase (MEK) by Raf-1. TNF-α levels in the monocyte culture supernatant, induced by 10 ng of LPS/ml, were reduced by PD-098059 (50 μM). In addition, PD-098059 also reduced TNF-α mRNA expression when cells were stimulated for 1 h with LPS. On the other hand, LPS-induced interleukin-10 (IL-10) levels in the monocyte supernatant were only slightly inhibited by PD-098059. Ro 09-2210, a recently identified MEK inhibitor, completely abrogated TNF-α levels at nanomolar concentrations. IL-10 levels also were strongly reduced. To show the efficacy of PD-098059 and Ro 09-2210, ERK1 and -2 activation was monitored by Western blotting with an antiserum that recognizes the phosphorylated (i.e., activated) forms of ERK1 and ERK2. Addition of LPS to human monocytes resulted in activation of both ERK1 and ERK2 in a time- and concentration (50% effective concentration between 1 and 10 ng of LPS/ml)-dependent manner. Activation of ERK2 was blocked by PD-098059 (50 μM), whereas ERK1 seemed to be less affected. Ro 09-2210 completely prevented LPS-induced ERK1 and ERK2 activation. LPS-induced p38 activation also was prevented by Ro 09-2210. These data further support the view that the ERK signal transduction pathway is causally involved in the synthesis of TNF-α by human monocytes stimulated with LPS.
One of the most potent stimuli for monocytes is bacterial endotoxin (lipopolysaccharide [LPS]), which is derived from the outer cell wall of gram-negative bacteria. In response to LPS, monocytes produce large quantities of proinflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and IL-6, followed by the production of IL-10, which has anti-inflammatory properties (6, 22). Many studies have shown that these cytokines play a pivotal role in the pathogenesis of bacterial sepsis, although the precise function of each cytokine individually remains to be elucidated (8, 11, 31).
At low LPS concentrations (≤100 ng/ml), LPS binding to monocytes is mediated by CD14 and LPS-binding protein, a serum-derived protein which facilitates binding to CD14 (28). Although CD14 is indispensable for LPS signaling, membrane-bound CD14 is a nontransmembrane molecule linked to the plasma membrane by a glycosylphosphatidyl inositol anchor. LPS signaling is therefore possibly mediated by a putative second transmembrane molecule, some candidates for which have been described previously (29). Alternatively, LPS is internalized (2) and may act as a second messenger itself, since it has remarkable similarity to ceramide (32).
As demonstrated in several studies, LPS induces many intracellular responses, including activation of nuclear factor-κB (NF-κB) and activation of members of the mitogen-activated protein kinase (MAPK) family (25). As tested in various cell types, LPS can induce activation of extracellular-signal-regulated kinase-1 (ERK1) and ERK2 (3, 18), c-Jun N-terminal kinases (JNKs) (13, 23), and p38 (14, 23). Activation of p38 is necessary for production of TNF-α, as demonstrated by use of the specific p38 inhibitor SB 203580 (16). More recently, it was demonstrated that JNK is involved in TNF-α translation (24). Studies with the murine macrophage cell line RAW 264.7, in which Raf-1 is either activated as an estrogen receptor–Raf-1 hybrid or blocked by dominant negative Raf-1 or Ras, suggest that the Raf-1/MEK1-MEK2/ERK1-ERK2 pathway is involved in TNF-α production (10, 12).
In the present study, we have tested the effect of PD-098059 (which specifically blocks MAPK kinase [MEK] activation by Raf-1 [1]) on LPS-induced TNF-α production by primary human monocytes, since such experiments provide information concerning the role of the ERK pathway in a system which is relevant to human sepsis. PD-098059 reduced TNF-α protein levels and TNF-α mRNA expression in a dose-dependent manner. On the other hand, IL-10 protein levels were slightly affected. The inhibitory effect of PD-098059 on LPS-induced activation of ERK1 and ERK2 was also seen. Ro 09-2210, a MEK inhibitor which blocks activation of ERK1 and -2, but also JNK and p38, was even more potent and completely abrogated TNF-α levels. In addition, this compound blocked IL-10. These data indicate that in human monocytes TNF-α production requires activation of the ERK pathway and suggest that IL-10 synthesis is under control of JNK and/or p38.
MATERIALS AND METHODS
Reagents.
LPS (from Salmonella typhimurium) was obtained from Sigma, St. Louis, Mo. Enzyme-linked immunosorbent assays (ELISAs) for human TNF-α and IL-10 were from the Central Laboratory for Blood Transfusion, Amsterdam, The Netherlands. Trizol, reverse transcriptase (Superscript), and RPMI 1640 were purchased from Life Technologies (Gaithersburg, Md.). Deoxynucleoside triphosphates (dNTPs), biotinylated primers, and digoxigenin-labeled probes for TNF-α and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) were supplied by Pharmacia (Uppsala, Sweden). Random hexamer primers, DNase I (RNase free), and the digoxigenin detection fluorescence ELISA kit were obtained from Boehringer (Mannheim, Germany). RNase inhibitor and Taq DNA polymerase were from Promega (Madison, Wis.), whereas PD-098059 (2′-amino-3′-methoxyflavone) was obtained from Biomol Research Laboratories (Plymouth Meeting, Pa.). The ERK1-ERK2 or p38 phosphospecific antibodies purified from rabbit antiserum were purchased from New England BioLabs (Beverly, Mass.). Polypropylene materials were manufactured by Costar, Cambridge, Mass. (96-well plates) or Becton Dickinson Labware, Lincoln Park, N.J. (14-ml round-bottom tubes). Ro 09-2210 was kindly donated by T. Murray, Roche Products Limited, Hertfordshire, United Kingdom.
Isolation of monocytes.
Human monocytes were isolated from the buffy coat obtained from informed healthy volunteers at the Bloodbank Utrecht. First, peripheral blood mononuclear cells were separated from the polymorphonuclear leukocyte fraction by centrifugation (1,000 × g, 20 min, room temperature) of the buffy coat (containing 6.5 mM sodium citrate) layered on Ficoll (1.078 g/ml) in Leuco Sep tubes (Greiner). Next, monocytes were purified from peripheral blood mononuclear cells by countercurrent elutriation as described before (7). For our experiments, the >80% pure monocyte cell fractions (viability, >95%) were used.
Cell culture conditions.
Monocytes were cultured in RPMI 1640 supplemented with 4% A+B+ serum, or with 10% fetal calf serum (for PD-098059 experiments followed by ELISA), and gentamicin (10 μg/ml). Cells were warmed for 15 min at 37°C in a 5% CO2 incubator before treatment with PD-098059 or Ro 09-2210 for 30 min at 37°C. After preincubation, monocytes were stimulated with 10 ng of LPS per ml for different time periods in 96-well polypropylene plates (200 μl/well; 1.5 × 106 cells/ml). The supernatants were collected and stored at −20°C until TNF-α- or IL-10-specific ELISAs were performed. Cells used for Western blotting were incubated in polypropylene tubes (1 ml/tube; 2 × 106 cells/ml).
Reverse transcriptase PCR.
Cells (1.5 × 106/ml) were stimulated with 10 ng of LPS per ml in the presence or absence of PD-098059 for 1 h at 37°C in 14-ml polypropylene tubes. Total RNA was isolated from 107 monocytes/sample with Trizol. In brief, cell pellets were lysed with 1 ml of Trizol for 10 min at room temperature. After treatment with 0.2 ml of chloroform for 2 min, the samples were centrifuged (10,000 × g) for 15 min at 4°C. RNA was precipitated from the aqueous phase with 0.5 ml of isopropanol (10 min, room temperature) and pelleted by centrifugation (10,000 × g) at 4°C for 10 min. The pellet was washed with 70% ethanol and dissolved in 100 μl of diethylpyrocarbonate (DEPC)-treated water. Possible contamination of the total RNA with genomic DNA was excluded by DNase treatment for 15 min at 37°C. RNA was repurified by phenol-chloroform-isoamyl alcohol extraction, precipitated with 70% ethanol, and dissolved in DEPC-treated water (50 μl). Before cDNA synthesis, RNA samples were used for a 40-cycle PCR with GAPDH primers to confirm the absence of contaminating DNA. cDNA was prepared by adding 1 μg of total RNA to a mixture (final volume, 25 μl) containing 200 U of reverse transcriptase, 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 8 mM dithiothreitol, 0.5 mM dNTPs, 80 nmol of random hexamer primers, and 20 U of RNase inhibitor at 37°C for 1 h. After the reaction, the volume was adjusted with DEPC-treated water to 200 μl/μg of RNA initially used. PCR was performed with primer pairs for human TNF-α or GAPDH (Table 1). The primer pairs contained one biotinylated primer to enable further quantification of the PCR products with a digoxigenin detection ELISA. For each PCR, 5 μl of cDNA was added to a mixture (final volume, 25 μl) containing 0.5 U of Taq DNA polymerase, 0.4 mM dNTPs, 10 mM Tris-HCl (pH 9.0), 50 mM KCl, 0.1% Triton X-100, 2 mM MgCl2, and 0.2 μM primers. PCR was carried out on a thermal cycler (Perkin-Elmer, Norwalk, Conn.) for 25 cycles (GAPDH) or 29 cycles (TNF-α), with a single cycle consisting of 1 min at 94°C (denaturation), 1 min at 60°C (annealing), and 2 min at 72°C (extension). The final cycle was completed with an additional extension of 10 min at 72°C. Biotinylated PCR products were quantified with a digoxigenin detection fluorescence ELISA. For each sample, 5 μl of PCR products containing the biotinylated strands was incubated with 1.25 μl of denaturation buffer (200 mM NaOH, 40 mM EDTA [final concentrations]) for 5 min at room temperature. Subsequently, denaturation was stopped with 6.25 μl of neutralization buffer (75 mM Na2HPO4 [final concentration], pH 6.0) before 200 μl of hybridization buffer (62.5 mM Na2HPO4, 0.94 M NaCl, 94 mM citric acid, 10 mM MgCl2, 0.125% Tween 20, and 0.0625% bovine serum albumin [final concentrations], pH 6.5) containing 20 ng of digoxigenin-labeled probes (Table 1) per ml was added. Samples were then transferred to streptavidin-coated eight-well strips, and the assay was carried out according to the instructions of the manufacturer. Fluorescence was measured on a Cytofluor II meter (PerSeptive Biosystems, Framington, Mass.) at excitation and emission wavelengths of 450 and 550 nm, respectively. Signal intensities of TNF-α fluorescence were corrected for background (PCR product omitted, probe present) and subsequently normalized with GAPDH values corrected for the background.
TABLE 1.
Primer sequences used for PCR to determine human TNF-α or GAPDH
| Type of sequence | Cytokine | Orientationa | Sequence (5′→3′) | Size (bp) |
|---|---|---|---|---|
| Primer | TNF-α | Sense (B) | GAC CTC TCT CTA ATC AGC CC | 510 |
| Antisense | GCA ATG ATC CCA AAG TAG ACC TGC CC | |||
| GAPDH | Sense | CCA TGG AGA AGG CTG GGG | 195 | |
| Antisense (B) | CAA AGT TGT CAT GGA TGA CC | |||
| Probeb | TNF-α | ATC TCT CAG CTC CAC GCC ATT GGC CAG GA | ||
| GAPDH | CTG CAC CAC CAA CTG CTT AGC |
B indicates a 5′-biotinylated primer.
Probes were labeled with digoxigenin at the 5′ site in order to quantify biotinylated PCR products in a digoxigenin ELISA (see Materials and Methods).
Measurement of ERK1 and -2 and p38 activation.
To monitor ERK1 and -2 activation, sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis was performed, followed by Western blotting with a purified rabbit antiserum containing antibodies directed against Thr202- and Tyr204-phosphorylated (i.e., active) ERK1 (p44) and ERK2 (p42) or Thr180- and Tyr182-phosphorylated p38.
First, monocytes (1.5 × 106 cells/ml) were preincubated for 30 min with PD-098059 (50 μM) or Ro 09-2210 (10 to 1,000 nM) at 37°C before stimulation with LPS (10 ng/ml or the indicated concentrations) at 37°C for the indicated times. After incubation, the cells (2 × 106/sample) were washed with ice-cold phosphate-buffered saline and resuspended in 80 μl of lysis buffer (1% Triton X-100, 50 mM Tris-HCl [pH 8.0], 100 mM NaCl) containing several inhibitors (1 μg of antipain per ml, 2 μg of benzamidine per ml, 1 μg of leupeptin per ml, 1 μg of chymostatin per ml, 1 μg of pepstatin A per ml, and 1 mM phenylmethylsulfonyl fluoride). After addition of 20 μl of 5× sample buffer (final concentrations of 2% SDS, 2% β-mercaptoethanol, and 10% glycerol in 300 mM Tris-Cl, pH 6.8), the lysates were boiled for 2 min. Samples were run on a 10% polyacrylamide minigel for 1 h at 150 V and blotted for 2 h (50 V) on a 0.45- μm-pore-size polyvinylidene difluoride (PVDF) membrane at 4°C. The PVDF membrane was blocked with 0.6% bovine serum albumin (in Tris-buffered saline–Tween 20 [TBST] plus 1 mM EDTA) for 1 h at room temperature and subsequently incubated for 1 h with the ERK1 and -2 or p38 phosphospecific rabbit antibodies diluted (1:2,000 in blocking buffer). After being washed six times with TBST for 3 min, the PVDF membrane was incubated at room temperature for 2 h with goat anti-rabbit–peroxidase conjugate (1:8,000 in blocking buffer). The PVDF filter was again washed six times with TBST and then twice with phosphate-buffered saline for 5 min. Thereafter, proteins were visualized with enhanced chemiluminescence on film (Kodak X-OMAT LS).
RESULTS
Effect of PD-098059 and Ro 09-2210 on LPS-induced TNF-α production.
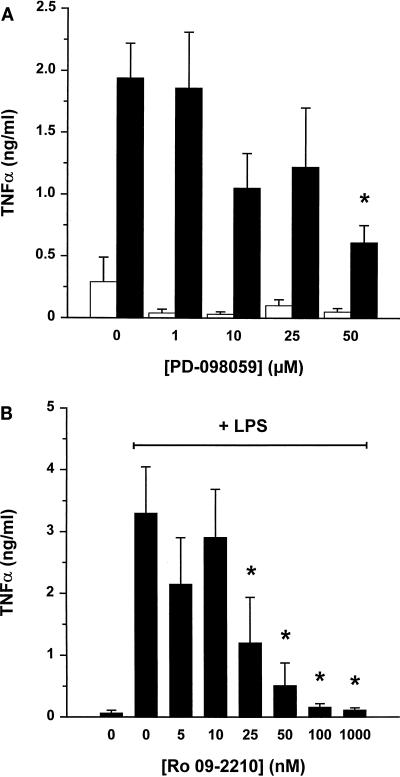
To activate TNF-α production, human monocytes were incubated with LPS (10 ng/ml). Addition of various concentrations of PD-098059 (a specific inhibitor of the ERK1-ERK2 pathway) reduced TNF-α levels in a dose-dependent manner (Fig. 1A). Also, when measured at different incubation intervals (i.e., 1, 2, 4, and 20 h), LPS-induced TNF-α levels were reduced by 50 μM PD-098059 (data not shown).
FIG. 1.
Effect of PD-098059 and Ro 09-2210 on LPS-induced TNF-α levels in human monocyte culture supernatant. (A) Human monocytes (1.5 × 106 cells/ml) were preincubated with the indicated concentrations of PD-098059 for 30 min and subsequently treated with buffer (white bars) or 10 ng of LPS per ml (black bars) for 20 h at 37°C. (B) Cells were incubated with the indicated concentrations of Ro 09-2210 for 30 min before treatment with 10 ng of LPS/ml for 4 h. Supernatants were collected for a TNF-α ELISA. Results are mean values from five independent experiments ± standard errors. ∗, significant difference (P < 0.05) by repeated-measures analysis of variance followed by the Newmans-Keuls test.
We next tested the effect of a different MEK inhibitor, Ro 09-2210. This fungal compound is an inhibitor of several MEKs, including MKK1, -4, -6, and -7 and SEK, but downstream kinases such as JNK, p38, and MAPKAP1 and -2 are not inhibited directly (18a). However, MKK1 seems to be the most sensitive kinase for inhibition by Ro 09-2210 (fourfold lower 50% inhibitory concentrations). Interestingly, LPS-induced TNF-α levels were completely eliminated by nanomolar concentrations of Ro 09-2210 (Fig. 1B). In the absence of LPS stimulation, the effect of Ro 09-2210 was minimal (data not shown).
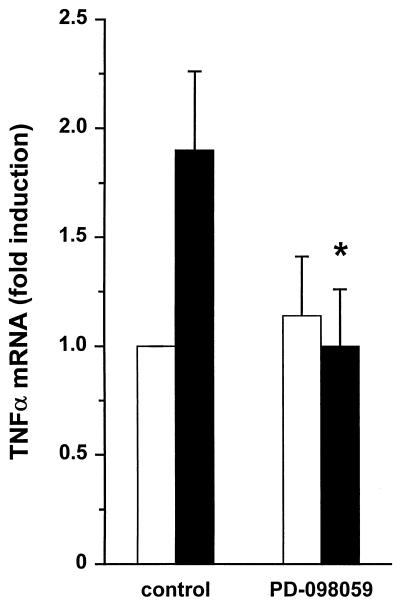
We then evaluated the effect on LPS-induced TNF-α mRNA expression, to examine at which level PD-098059 interferes with TNF-α synthesis. Reverse transcriptase PCR fragments for TNF-α were quantified with a fluorescence ELISA by using a digoxigenin-labeled TNF-α probe. LPS-induced TNF-α mRNA levels were reduced to background expression (unstimulated cells) by 50 μM PD-098059 (Fig. 2), showing that the inhibitory effect of PD-098059 is found at the level of transcription and/or mRNA stabilization.
FIG. 2.
Effect of PD-098059 on LPS-induced TNF-α mRNA expression in human monocytes. Human monocytes were pretreated with PD-098059 (50 μM, 30 min) and subsequently incubated with buffer (white bars) or 10 ng of LPS/ml (black bars) for 1 h. Thereafter, reverse transcriptase PCR was performed on RNA isolates, and PCR fragments were quantified with a digoxigenin-labeled probe as described in Materials and Methods. Results are expressed as fold induction [(TNF-αx/GAPDHx)/(TNF-αcontrol/GAPDHcontrol)]. Results are expressed as the means from four independent experiments ± standard errors. ∗, significant difference (P < 0.05) by Student’s t test for paired samples.
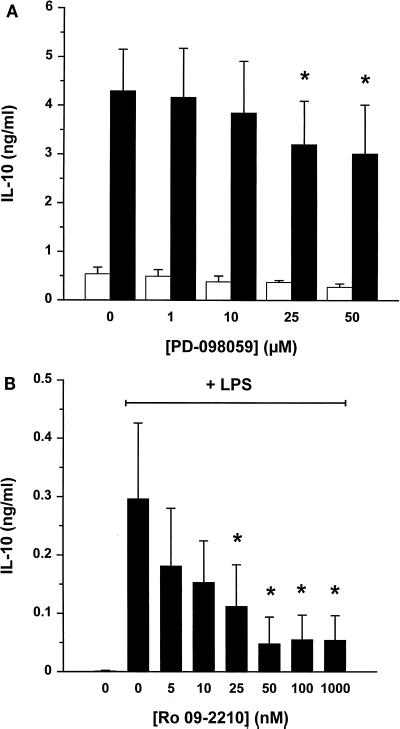
Effect of PD-098059 and Ro 09-2210 on LPS-induced IL-10 production.
LPS stimulation of human monocytes results in the release of several cytokines that interact in complex autocrine and paracrine regulatory mechanisms. To test whether the inhibitory effect of PD-098059 or Ro 09-2210 could be shown for other cytokines, the effect of IL-10 synthesis was monitored. IL-10 levels in monocyte supernatants taken after 20 h of incubation with LPS (10 ng/ml) were measured by ELISA. IL-10 levels were inhibited by PD-098059 (Fig. 3A), but the effect was small compared to the decrease in TNF-α levels (Fig. 1A). We conclude, therefore, that the inhibitory effect of PD-098059 on TNF-α synthesis cannot be extrapolated in a quantitative way to cytokine production in general. On the other hand, IL-10 levels were considerably reduced by Ro 09-2210 (Fig. 3B).
FIG. 3.
Effect of PD-098059 and Ro 09-2210 on LPS-induced IL-10 levels in human monocyte culture supernatant. Elutriated human monocytes (1.5 × 106 cells/ml) were preincubated with various concentrations of PD-098059 (A) or Ro 09-2210 (B) for 30 min at 37°C, followed by a 20-h incubation with buffer (white bars) or 10 ng of LPS/ml (black bars). IL-10 ELISA was performed with culture supernatants, and data are expressed as means from five (A) or three (B) independent experiments ± standard errors. ∗, significant difference (P < 0.05) by repeated-measures analysis of variance followed by the Newmans-Keuls test.
Effect of PD-098059 and Ro 09-2210 on LPS-induced ERK1 and ERK2 activation.
In several experimental systems it has been demonstrated that LPS induces activation of ERK2 (3, 18, 25). However, most experiments are conducted with the murine macrophage cell line RAW 264.7, which responds to high concentrations of LPS. In this study, we show activation of both ERK1 and ERK2 after addition of LPS to human monocytes (by Western blotting with a phosphospecific ERK1-ERK2 antiserum). Activation by 10 ng of LPS per ml was transient, with an apparent maximal effect at approximately 20 min (Fig. 4). Importantly, the activation was modest compared to the effect of phorbol myristate acetate (PMA) (80 ng/ml, 5 min) or N-formyl-methionyl-leucyl-phenyalanine (1 μM, 2 min). Moreover, the fMLP-induced ERK2 activation much more rapid (maximum at 2 min) than the activation induced by LPS (Fig. 4). The effect of LPS was concentration dependent (50% effective concentration of between 1 and 10 ng/ml) (Fig. 5A), and pretreatment with PD-098059 (50 μM) almost completely blocked activation of ERK2 when monocytes were incubated with 1 or 10 ng of LPS/ml (Fig. 5A). Interestingly, LPS-induced ERK1 activation appeared to be less affected by PD-098059. When very high LPS concentrations (100 and 1,000 ng of LPS/ml) were used, no inhibitory effect of PD-098059 was observed. Ro 09-2210 also prevented activation of both ERK1 and ERK2 in a dose-dependent manner when monocytes were stimulated with 10 ng of LPS/ml for 15 min (Fig. 5B).
FIG. 4.
Effect of LPS on ERK1 and ERK2 activation in human monocytes. Cells (2 × 106 cells/sample) were incubated with 10 ng of LPS/ml, 1 μM fMLP, or 80 ng of PMA/ml for the indicated times. Samples were analyzed by SDS-polyacrylamide gel electrophoresis and Western blotting with an ERK1-ERK2 phosphospecific antibody which recognizes only the activated forms of ERK1 and ERK2. ERK1 appears as the upper band (44 kDa); ERK2 appears as the lower band (42 kDa). The depicted data are typical of those from three independent experiments.
FIG. 5.
Effect of PD-098059 on LPS-induced activation of ERK1 and ERK2 in human monocytes. (A) Human monocytes were preincubated for 30 min with buffer or 50 μM PD-098059, followed by a 15-min incubation with the indicated concentrations of LPS. (B) To test the effect of Ro 09-2210, cells were preincubated with the indicated concentrations of Ro 09-2210 for 30 min at 37°C before activation with 10 ng of LPS/ml for 15 min. ERK1 and -2 activation was determined by Western blotting with an ERK1-ERK2 phosphospecific antibody. ERK1 appears as the upper band (44 kDa); ERK2 appears as the lower band (42 kDa). Results are representative of those from two independent experiments.
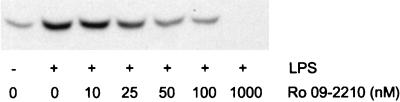
Effect of Ro 09-2210 on LPS-induced p38 activation.
As mentioned before, Ro 09-2210 inhibits multiple MEKs. To test if the multiple inhibitory effect of Ro 09-2210 can be extended to human monocytes, we investigated LPS-induced p38 activation in the presence of Ro 09-2210. To monitor p38 activation, Western blotting was carried out with phosphospecific p38 antibodies that interact only with activated p38. As shown in Fig. 6, Ro 09-2210 blocked activation of p38 in a dose-dependent manner. At concentrations of 50 nM and higher, a strong reduction of p38 activation is observed, which is in the same range as the concentrations necessary to decrease TNF-α and IL-10 levels (Fig. 1B and 3B).
FIG. 6.
Effect of Ro 09-2210 on LPS-induced p38 activation in human monocytes. Human monocytes (2 × 106 cells/sample) were preincubated with various concentrations of Ro 09-2210 for 30 min at 37°C. After stimulation with 10 ng of LPS/ml for 15 min, samples were lysed and used for Western blotting with p38 phosphospecific polyclonal antibodies that recognize the activated form of this MAPK.
DISCUSSION
In this study, we show that the ERK pathway is involved in the production of TNF-α by LPS-stimulated human monocytes. Addition of LPS resulted in the transient (maximum at ∼20 min) and dose-dependent (50% effective concentration of between 1 and 10 ng/ml) activation of ERK1 and ERK2. Although LPS is a very potent stimulus for human monocytes, activation of ERK1 and ERK2 was modest compared to effects of fMLP and PMA. This suggests that only a small fraction of total ERK1 and ERK2 is utilized by LPS to activate human monocytes. Activation of ERK2 and, to a lesser extent, ERK1 was reduced by PD-098059, an inhibitor of MEK-1 activation by Raf-1 (1). Previous studies had demonstrated the importance of the ERK pathway for TNF-α synthesis in the murine macrophage cell line RAW 264.7 by manipulation of Ras or Raf-1. Moreover, LPS-induced TNF-α synthesis in rat primary astrocytes is also blocked by PD-098059 (4). The need for activation of the ERK pathway is not restricted to LPS, since Fcγ receptor-induced TNF-α mRNA accumulation is also inhibited by PD-098059 in THP-1 and NK cells (27). Theoretically, the ERK pathway is coupled to TNF-α production, because activated ERK phosphorylates the ternary complex factor Elk-1, resulting in increased transcription of c-Fos and hence enhanced formation of AP-1 (15). The TNF-α promoter contains several AP-1-binding regions (tetradecanoyl phorbol acetate response elements) (26) and is therefore potentially regulated by AP-1.
JNK and p38 also are activated in response to LPS (13, 14, 23). These additional MAPK signaling pathways are reported to regulate TNF-α mRNA translation (16, 24). Interestingly, Ro 09-2210 inhibits the ERK1 and -2, JNK, and p38 pathways simultaneously (18a). In our hands, LPS-induced activation of p38 was also blocked by Ro 09-2210 in human monocytes. This suggests that Ro 09-2210 prevents both TNF-α transcription and translation. This could explain the complete inhibitory effect of Ro 09-2210 on TNF-α levels, whereas PD-098059 was partially effective.
Combined with the well-established role of NF-κB, it appears that TNF-α production is regulated in a complex manner, involving several signal transduction pathways working together. In addition, in one study it was reported that PD-098059 inhibits LPS-induced activation of NF-κB (20), suggesting cross-talk between the signaling pathways. Similar to findings reported by Foey et al. (9), IL-10 production was only slightly affected by the ERK pathway inhibitor. This suggests that expression of TNF-α is regulated differently from that of IL-10, although some caution is necessary, since the difference between the TNF-α and IL-10 reductions was found only with 50 μM PD-098059. The TNF-α and IL-10 promoters differ in many aspects, including the absence of an NF-κB binding site in the human IL-10 promoter and the presence of a cyclic AMP response element (21, 26). The IL-10 promoter contains a putative AP-1 recognition site (21). However, the minor sensitivity of LPS-induced IL-10 production to PD-098059 suggests that ERK1- and -2-induced AP-1 synthesis is not essential under these experimental conditions. On the other hand, IL-10 production is inhibited by the specific p38 inhibitor SB203580, indicating that p38 activation is involved (5, 9). This could explain the inhibitory effect of Ro 09-2210 on IL-10 levels, since Ro 09-2210 also blocks p38. It is possible that inhibition of JNK by Ro 09-2210 is linked to down regulation of TNF-α and IL-10. Unfortunately, to our knowledge no specific JNK pathway inhibitor which could further help to delineate the role of JNK has been described.
Endogenously produced TNF-α is important for the subsequent synthesis of IL-10 by human monocytes through autocrine and paracrine mechanisms (30). Therefore, reduction of TNF-α levels by PD-098059 should theoretically result in decreased IL-10 synthesis. However, as mentioned before, PD-098059 had little inhibitory effect on IL-10 levels. An explanation for this discrepancy is that TNF-α levels are not totally reduced by PD-098059. The remaining TNF-α production could be sufficient for autocrine stimulation of monocytes to produce IL-10.
Based on the data presented in this and other studies, the ERK pathway is a potential therapeutic target for treatment and prevention of bacterial sepsis in which excessive TNF-α production occurs. First, inhibition of the ERK pathway results in reduced TNF-α levels. Second, our results and those of others (9) suggest that the ERK signal transduction route is selective, because IL-10 is less sensitive to inhibition of this pathway. This could be favorable, since IL-10 promotes LPS hyporesponsiveness of human monocytes (22), which may contribute to dampen the hyperactivated immune system during sepsis.
In addition, blocking the ERK pathway could be potentially interesting for the treatment of other diseases, such as psoriasis, atherosclerosis, and cancer (17). Pretreatment of mice with the tyrosine kinase inhibitor AG 126 protected against a lethal dose of LPS (19). In this study, protection correlated with reduction of serum TNF-α levels and in vitro with inhibition of ERK2. The use of PD-098059 is even more attractive because of the high specificity of this compound for the ERK pathway. However, our preliminary experiments indicated that PD-098059 did not inhibit LPS-stimulated TNF-α production in whole blood, probably due to neutralization by plasma (data not shown). Due to the maximal solubility limit, it was not possible to raise the dose of PD-098059 in order to overcome the neutralizing effect. In this respect, Ro 09-2210 seems to be more promising, since this compound reduced LPS-induced TNF-α production in whole blood, although 100-fold-higher concentrations were needed compared to those in experiments with isolated cells (data not shown). However, Ro 09-2210 also inhibits IL-10 levels, which could be a disadvantage.
In conclusion, the complex regulation of LPS-induced TNF-α production by human monocytes involves activation of ERK1, ERK2, and parallel cascades leading to activation of JNK and p38. Improved knowledge of the mechanisms that control TNF-α production could expand the therapeutic possibilities for interference with inflammatory responses in a more specific way.
ACKNOWLEDGMENTS
We are grateful to Machiel de Vos and Jeena Middel for isolation of human monocytes and to Leonie Boven for establishing the TNF-α reverse transcriptase PCR. Karel Zuur and Ian Leistikow are gratefully acknowledged for performing whole-blood experiments with PD-098059, and T. Murray is gratefully acknowledged for donating Ro 09-2210.
REFERENCES
- 1.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 2.Antal P. Quantitation of CD14 dependent LPS-binding, -internalization and -activation of human monocytes. Thesis. Utrecht, The Netherlands: University Medical Center Utrecht; 1997. [Google Scholar]
- 3.Arditi M, Zhou J, Torres M, Durden D L, Stins M, Kim K S. Lipopolysaccharide stimulates the tyrosine phosphorylation of mitogen-activated protein kinases p44, p42, and p41 in vascular endothelial cells in a soluble CD14-dependent manner. Role of protein tyrosine phosphorylation in lipopolysaccharide-induced stimulation of endothelial cells. J Immunol. 1995;155:3994–4003. [PubMed] [Google Scholar]
- 4.Bhat N R, Zhang P, Lee J C, Hogan E L. Extracellular signal-regulated kinase and p38 subgroups of mitogen-activated protein kinases regulate inducible nitric oxide synthase and tumor necrosis factor-alpha gene expression in endotoxin-stimulated primary glial cultures. J Neurosci. 1998;18:1633–1641. doi: 10.1523/JNEUROSCI.18-05-01633.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 6.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figdor C G, Bont W S, de Vries J E, van Es W L. Isolation of large numbers of highly purified lymphocytes and monocytes with a modified centrifugal elutriation technique. J Immunol Methods. 1981;40:275–288. doi: 10.1016/0022-1759(81)90359-8. [DOI] [PubMed] [Google Scholar]
- 8.Fisher C J, Agosti J M, Opal S M, Lowry S F, Balk R A, Sadoff J C, Abraham E, Schein R M H, Benjamin E. Treatment of septic shock with the tumor necrosis factor receptor:Fc fusion protein. N Engl J Med. 1996;334:1697–1702. doi: 10.1056/NEJM199606273342603. [DOI] [PubMed] [Google Scholar]
- 9.Foey A D, Parry S L, Williams L M, Feldmann M, Foxwell B M, Brennan F M. Regulation of monocyte IL-10 synthesis by endogenous IL-1 and TNF-alpha: role of the p38 and p42/44 mitogen-activated protein kinases. J Immunol. 1998;160:920–928. [PubMed] [Google Scholar]
- 10.Geppert T D, Whitehurst C E, Thompson P, Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994;1:93–103. [PMC free article] [PubMed] [Google Scholar]
- 11.Glausner M P, Zanetti G, Baumgartner J D, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 12.Hambleton J, McMahon M, DeFranco A L. Activation of Raf-1 and mitogen-activated protein kinase in murine macrophages partially mimics lipopolysaccharide-induced signaling events. J Exp Med. 1995;182:147–154. doi: 10.1084/jem.182.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hambleton J, Weinstein S L, Lem L, DeFranco A L. Activation of c-Jun N-terminal kinase in bacterial lipopolysaccharide-stimulated macrophages. Proc Natl Acad Sci USA. 1996;93:2774–2778. doi: 10.1073/pnas.93.7.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han J, Lee J D, Bibbs L, Ulevitch R J. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 15.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 16.Lee J C, Young P R. Role of CSB/p38/RK stress response kinase in LPS and cytokine signaling mechanisms. J Leukoc Biol. 1996;59:152–157. doi: 10.1002/jlb.59.2.152. [DOI] [PubMed] [Google Scholar]
- 17.Levitzki A, Gazit A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- 18.Liu M K, Herrera Velit P, Brownsey R W, Reiner N E. CD14-dependent activation of protein kinase C and mitogen-activated protein kinases (p42 and p44) in human monocytes treated with bacterial lipopolysaccharide. J Immunol. 1994;153:2642–2652. [PubMed] [Google Scholar]
- 18a.Murray, T. Personal communication.
- 19.Novogrodsky A, Vanichkin A, Patya M, Gazit A, Osherov N, Levitzki A. Prevention of lipopolysaccharide-induced lethal toxicity by tyrosine kinase inhibitors. Science. 1994;264:1319–1322. doi: 10.1126/science.8191285. [DOI] [PubMed] [Google Scholar]
- 20.Pahan K, Sheikh F G, Khan M, Namboodiri A M, Singh I. Sphingomyelinase and ceramide stimulate the expression of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1998;273:2591–2600. doi: 10.1074/jbc.273.5.2591. [DOI] [PubMed] [Google Scholar]
- 21.Platzer C, Meisel C, Vogt K, Platzer M, Volk H D. Up-regulation of monocytic IL-10 by tumor necrosis factor-α and cAMP elevating drugs. Int Immunol. 1995;7:517–523. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- 22.Randow F, Syrbe U, Meisel C, Krausch D, Zuckermann H, Platzer C, Volk H D. Mechanism of endotoxin desensitization: involvement of interleukin 10 and transforming growth factor β. J Exp Med. 1995;181:1887–1892. doi: 10.1084/jem.181.5.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanghera J S, Weinstein S L, Aluwalia M, Girn J, Pelech S L. Activation of multiple proline-directed kinases by bacterial lipopolysaccharide in murine macrophages. J Immunol. 1996;156:4457–4465. [PubMed] [Google Scholar]
- 24.Swantek J L, Cobb M H, Geppert T D. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-α translation by blocking JNK/SAPK. Mol Cell Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sweet M J, Hume D A. Endotoxin signal transduction in macrophages. J Leukoc Biol. 1996;60:8–26. doi: 10.1002/jlb.60.1.8. [DOI] [PubMed] [Google Scholar]
- 26.Takashiba S, Shapira L, Amar S, Van Dyke T E. Cloning and characterization of human TNF alpha promoter region. Gene. 1993;131:307–308. doi: 10.1016/0378-1119(93)90314-s. [DOI] [PubMed] [Google Scholar]
- 27.Trotta R, Kanakaraj P, Perussia B. Fc gamma R-dependent mitogen-activated protein kinase activation in leukocytes: a common signal transduction event necessary for expression of TNF-alpha and early activation genes. J Exp Med. 1996;184:1027–1035. doi: 10.1084/jem.184.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulevitch R J, Tobias P S. Recognition of endotoxin by cells leading to transmembrane signaling. Curr Opin Immunol. 1994;6:125–130. doi: 10.1016/0952-7915(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 29.Vita N, Lefort S, Sozzani P, Reeb R, Richards S, Borysiewicz L K, Ferrara P, Labeta M O. Detection and biochemical characteristics of the receptor for complexes of soluble CD14 and bacterial lipopolysaccharide. J Immunol. 1997;158:3457–3462. [PubMed] [Google Scholar]
- 30.Wanidworanun C, Strober W. Predominant role of tumor necrosis factor-α in human monocyte IL-10 synthesis. J Immunol. 1993;151:6853–6861. [PubMed] [Google Scholar]
- 31.Westendorp R G J, Langermans J A M, Huizinga T W J, Elouali A H, Verweij C L, Boomsma D I, Vandenbrouke J P. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997;349:170–173. doi: 10.1016/s0140-6736(96)06413-6. [DOI] [PubMed] [Google Scholar]
- 32.Wright S D, Kolesnick R N. Does endotoxin stimulate cells by mimicking ceramide? Immunol Today. 1995;16:297–302. doi: 10.1016/0167-5699(95)80185-5. [DOI] [PubMed] [Google Scholar]