Abstract
The use of alternatives to allogeneic blood continues to rest on the principles that blood transfusions have inherent risks, associated costs, and affect the blood inventory available for health-care delivery. Increasing evidence exists of a fall in the use of blood because of associated costs and adverse outcomes, and suggests that the challenge for the use of alternatives to blood components will similarly be driven by costs and patient outcomes. Additionally, the risk–benefit profiles of alternatives to blood transfusion such as autologous blood procurement, erythropoiesis-stimulating agents, and haemostatic agents are under investigation. Nevertheless, the inherent risks of blood, along with the continued rise in blood costs are likely to favour the continued development and use of alternatives to blood transfusion. We summarise the current roles of alternatives to blood in the management of medical and surgical anaemias.
Introduction
Although the safety of blood has improved substantially since the 1980s, when HIV was discovered to be blood transmissible,1 blood transfusion is an independent risk factor for adverse patient outcomes.2 Blood transfusions have been associated with increased mortality,3 increased length of hospital stay related to infections and sepsis,4 and multi-organ system dysfunction.5 A recent meta-analysis6 of 19 prospective, randomised trials comparing restrictive versus liberal transfusions in more than 6000 patients found that adherence to restrictive blood transfusion decreased hospital mortality and postoperative infections. Erythrocyte damage related to duration of blood storage7 might partly account for these observed adverse patient outcomes. Canine models suggest that old blood has a propensity to haemolyse in vivo, releasing vasoconstrictive cell-free haemoglobin;8 and post transfusion, patients have decreased deformability of the erythrocyte membrane related to duration of blood storage.7 Finally, potential known and unknown risks such as transmission of blood-borne pathogens are still concerns;9 therefore, blood transfusions need to be restricted.
The detection, assessment, and treatment of preoperative anaemia are important in the planning of strategies to minimise allogeneic blood transfusions.10 Anaemia is the most important risk factor for transfusion,10 and 30% of surgical patients present preoperatively with anaemia.3 Additionally, preoperative anaemia (haemoglobin of 100–120 g/L in women and 100–130 g/L in men) has been independently associated with increased mortality and morbidity in patients undergoing non-cardiac surgery.3 The successful management of preoperative anaemia decreases blood transfusions in patients undergoing orthopaedic11 and cardiac surgery.12 The use of algorithm-guided treatment of patients with trauma,13 and in patients undergoing orthopaedic11, 14 and cardiac surgery15, 16 has been associated with decreased transfusion needs and improved patient outcomes such as complications, length of hospital stay, and mortality.
Strategies to decrease perioperative blood losses are also important, including meticulous surgical technique, use of autologous blood salvage, acute normovolaemic haemodilution, and avoidance of coagulopathy and hypothermia.17 In addition to blood and patient safety issues, cost needs to be considered: in the USA, when all the activities involved in blood transfusion are considered, the estimated price of transfusion of one blood unit is between US$700–$1200.18 These costs vary among countries. The administration costs associated with blood transfusion are estimated to be three to five times higher than the purchase costs. We summarise strategies that enable patients to minimise or avoid blood transfusions in the management of surgical and medical anaemias.
Surgical patients
Preoperative management
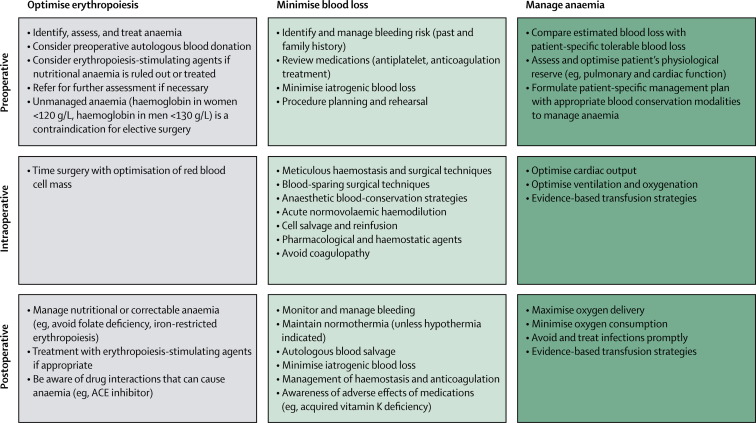
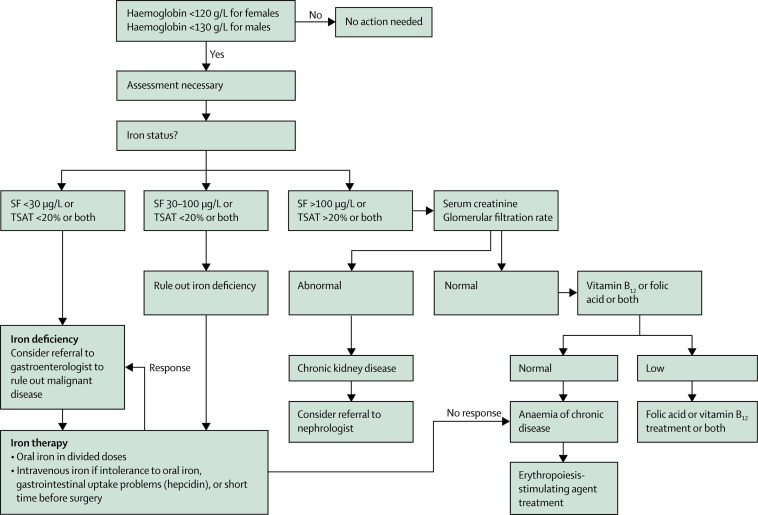
Preoperative planning is essential to reduce or avoid perioperative allogeneic transfusion. A thorough patient history is the best method to discover clinically important disorders in haemostasis—eg, bleeding related to previous surgical and dental procedures, epistaxis, menorrhagia, excessive bleeding with major trauma, and easy bruising or joint or muscle swelling after minor trauma. Figure 1 shows the principles of patient blood management and the use of alternatives to blood. For most non-cardiac surgery procedures, intra-operative tests of the coagulation system are not necessary except in the event of unexpected and substantial bleeding. The most important predictor of need for blood transfusion during surgery is the patient's baseline red blood cell volume. Figure 2 shows how preadmission testing should take place as far in advance as possible (eg, 30 days) of elective surgery to allow time for adequate identification, assessment, and management of anaemia.10, 20 The most frequent, treatable cause of anaemia is iron deficiency.21 The assessment of anaemia should also consider unexpected diagnoses, including chronic kidney disease or occult malignant disease. This approach needs close collaboration among the patients' primary care physicians, surgeons, anaesthesiologists, and the medical director of the institution's pre-admission testing programme, so that investigation of the elective surgical patient takes place far enough in advance (up to 30 days) to allow management of unexpected anaemia.
Figure 1.
Patient blood management
These recommendations apply in the perisurgical period enable treating physicians to have the time and methods to provide patient-centred and evidence-based patient blood management to minimise allogeneic blood transfusions. Modified from Goodnough and Shander,19 by permission of the American Society of Anesthesiologists.
Figure 2.
Algorithm for the detection, assessment, and management of preoperative anaemia
SF=serum ferritin. TSAT=transferrin saturation. Modified from Goodnough and colleagues,10 by permission of Oxford Journals.
Pharmacological alternatives to blood components are important in patient blood management in the surgical setting (figure 2) and in patients with medical anaemias such as anaemia associated with chronic inflammatory disease, chemotherapy-induced anaemias in patients with cancer, and anaemia associated with end-stage chronic kidney disease. The use of erythropoiesis-stimulating agents was first approved to increase the concentration of haemoglobin in patients with chronic kidney disease.22 Erythropoiesis-stimulating agents were then approved for use in patients undergoing elective surgery on the basis of prospective randomised trials that showed reduced allogeneic blood transfusions in patients undergoing orthopaedic surgery. Management of anaemia with erythropoiesis-stimulating agents or iron in patients undergoing orthopaedic10, 11 or cardiac12, 23 surgery is recommended as a blood-conservation strategy. However, the safety of erythropoiesis-stimulating agents in surgical settings has been reasessed after a post-approval study24 showed that patients scheduled for elective spine surgery who received preoperative erythropoiesis-stimulating agents had higher rates of thrombosis (assessed with Doppler evaluation) than patients who received placebo.
Search strategy and selection criteria.
We assessed recent progress to provide a better understanding of best transfusion practices on the basis of evidence-based clinical trials, published clinical practice guidelines, and emerging pathways for improving blood use and clinical patient outcomes. We searched the Cochrane Library, Medline, and Embase for articles published between Jan 1, 2008, and Dec 31, 2012. We used the search terms “blood alternatives”, “pharmacologic alternatives to blood”, “erythropoiesis-stimulating agents”, “recombinant clotting factors”; “haemostasis agents”, and “antifibrinolytic agents”. We largely selected publications in the past 5 years, but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. Review articles and book chapters are cited to provide readers with more details and more references than this article has room for. Our reference list was modified on the basis of comments from peer reviewers. Further information on blood transfusions in specific clinical settings such as stem cell and organ transplantation are available from cited references.
The role and the safety of erythropoiesis-stimulating agents in the management of preoperative patients with anaemia who are undergoing cardiovascular surgery are unresolved issues. Two European trials25, 26 with 76 and 320 patients showed no differences in mortality, thrombotic events, or serious adverse events between patients given erythropoiesis-stimulating agents and patients given placebo; a substantial reduction in blood transfusions was evident. A US study27 also concluded that erythropoietic stimulation was well tolerated despite some late deaths in patients given erythropoietic stimulating agent. However, data from this study led the US Food and Drug Administration to restrict the use of erythropoiesis-stimulating agents in the USA in the elective surgical setting to non-vascular, non-cardiac patients undergoing major elective surgery. Similarly, approval for perisurgical use of erythropoiesis-stimulating agents in the European Union has been restricted to elective orthopaedic surgical patients. Erythropoiesis-stimulating agents remain a valuable means for patients with special requirements, such as Jehovah's Witness patients, for whom blood transfusion is not an option.28 However, until additional safety data are available, the off-label use of erythropoiesis-stimulating agents in patients undergoing cardiac or vascular surgery cannot be supported.
Since the 1980s, preoperative autologous donation has been a common practice in elective surgical settings such as total joint replacement. In 1992, more than 6% of the blood transfused in the USA was autologous.29 Subsequent improvements in blood safety have been accompanied by a decreased interest in preoperative autologous donation.30 Nevertheless, preoperative autologous donation remains a potential strategy for patients undergoing elective surgery because of its conservation of allogeneic blood inventory, its value in patients with red blood cell alloantibodies, and the potential to limit exposure to emerging blood pathogens. The most recent example of an emerging blood pathogen is the West Nile virus, in which the Centers for Disease Control and Prevention recommended deferral of elective surgery or use of preoperative autologous donation pending implementation of a screening test for the pathogen. However, preoperative autologous donation is not recommended in procedures that are unlikely to need transfusion (eg, vaginal hysterectomy, transurethral resection of the prostate, and rhinoplasty)—ie, the less than 10% of cases, for which cross-matched blood would not be ordered.
The increased costs associated with preoperative autologous donation, the reduced risk of infection associated with allogeneic blood transfusions, and advances in surgical techniques to reduce blood loss have made preoperative autologous donation poorly cost effective. In a controlled trial of non-anaemic patients who pre-donated two autologous blood units before total hip replacement surgery, none of the patients, including the control cohort, received allogeneic blood; however, two-thirds of the patients in the preoperative autologous donation cohort received their autologous blood, for an additional cost of $758 per patient.31 Preoperative autologous donation might be appropriate for substantial procedures (eg, total hip revision or scoliosis repair) and for patients with serologic alloantibodies to blood.30
Management of blood loss anaemia
Perioperative autologous blood procurement
Acute normovolaemic haemodilution is the removal of whole blood from a patient while the circulating blood volume is restored with an acellular fluid shortly before substantial surgical blood loss. Blood units are reinfused in the reverse order of collection because the first unit collected and the last unit transfused have the highest concentration of red blood cells, coagulation factors, and platelets. The benefit of acute normovolaemic haemodilution is the reduction of blood loss when whole blood is shed perioperatively at lower haematocrit values achieved with acute normovolaemic haemodilution. Because blood collected by acute normovolaemic haemodilution is stored at room temperature in the operating room and is returned to the patient within 8 h of collection, platelets and coagulation factors remain functional. Additionally, unit testing is not essential. Acute normovolemic haemodilution is cheaper but equivalent to preoperative autologous donation in selected clinical settings (eg, patients with a high preoperative haemoglobin concentration undergoing a surgical procedure with a high expected blood loss such as a revision hip replacement), for reduction of allogeneic blood transfusions.29 Other outcomes including anaesthesia and surgery times, intraoperative haemodynamic values, and length of hospital stay were also similar for preoperative autologous donation and acute normovolaemic haemodilution. Low-volume acute normovolaemic haemodilution did not reduce allogeneic blood for patients undergoing cardiac value replacement surgery.32 Except for patients undergoing open heart coronary artery bypass surgery and Jehovah's Witnesses, there is no presently defined role for acute normovolaemic haemodilution.
As with preoperative autologous donation and acute normovolaemic haemodilution, the safety and efficacy of autologous blood salvage has undergone scrutiny. A controlled study in patients undergoing cardiothoracic surgery showed little efficacy when transfusion requirements and clinical outcomes were followed.33 Collection of the equivalent of one blood unit is possible for less expensive methods with unwashed blood, but at least two blood units need to be recovered with a cell-saver instrument with washed blood in order to achieve cost-effectiveness. Therefore, this technology is useful in cost savings and blood-inventory conservation for patients with substantial blood loss.34, 35 However, the above cost calculations only took into account the product cost and not the costs related to administration of blood.17 If full costs of blood administration are considered, autologous blood procurement becomes more cost effective.18
Cardiac surgery
In cardiac surgery, perioperative assays for platelet count, haemoglobin concentration, and coagulation abnormalities are obtained, and treatment should be guided by results. Point-of-care devices to monitor coagulation, coupled with treatment algorithms for pharmacological and transfusion treatment, have roles in liver transplantation, cardiac,15, 16 and trauma13 surgery. In a prospective randomised study in patients undergoing aortic surgery with clinically relevant coagulopathic bleeding after cardiopulmonary bypass, the administration of fibrinogen guided by thrombelastometry decreased overall transfusion needs from 13 to 2 units of allogeneic blood products (p<0·001) and increased the percentage of patients who avoided all allogeneic blood products from 0–45% (p<0·001).16
For patients with qualitative platelet function abnormalities, desmopressin promotes platelet aggregation through the release of von Willebrand factor from the endothelium and was initially approved for the treatment of von Willebrand disease. Meta-analyses of clinical trials in patients without von Willebrand disease undergoing surgery have shown a trend in reducing the need for allogeneic blood transfusions36 with only a small reduction in blood transfusions37 with desmopressin treatment. In particular, patients with impaired platelet function38 or those on platelet inhibitors39 might benefit from treatment with desmopressin. The drug also improves platelet function in the presence of hypothermia and acidosis.40 Initial concerns regarding increased thrombotic complications were not confirmed in more recent meta-analyses.37 The routine use of desmopressin as a prophylactic agent is still debated in cardiac surgeries, and is not presently recommended.23
Point-of-care testing-based transfusion algorithms show promise in the identification of real-time coagulopathies that can be given targeted treatment.41 A retrospective cohort study done at University Hospital Essen, Germany, reviewed 3865 patients undergoing cardiac surgery and the incidence of intraoperative allogeneic blood transfusion before and after implementation of point-of-care testing assays including activated clotting time, thromboelastometry, and whole-blood impedance aggregometry (also called multiple electrode aggregometry). Algorithm implementation after point-of-care testing allowed for the transfusion of plasma, platelets, fibrinogen concentrate, and prothrombin complex concentrate only after abnormal point-of-care testing values were obtained. Findings showed a significant decrease in blood and plasma transfusions, a significant increase in platelet, fibrinogen concentrate, and prothrombin complex concentrate administration, and reduction by 50% of rates of re-operation for bleeding and for thrombotic complications.
Antifibrinolytic agents have been used to reduce blood loss in patients undergoing complex open heart procedures. Antifibrinolytics inhibit the physiological fibrinolytic pathway, which is responsible for limiting and dissolving clots. Aminocaproic acid reduces blood loss and blood transfusions in patients undergoing cardiac surgery.42 Aprotinin was removed from the market in 2007, but a re-investgation of the Blood Conservation Using Antifibrinolytics in a Randomized Trial (BART) study43 and other data have led to a reversal of this decision and plans exist for the reintroduction of aprotinin in Canada and the European Union for patients undergoing cardiac surgery.44 A Cochrane review concluded that antifibrinolytics provide worthwhile reductions in blood loss and allogeneic blood transfusions; and that although aprotinin seems to be slightly more effective, the lysine analogues seem to have no serious adverse events.45 A meta-analysis of studies including more than 10 000 patients has shown that the lysine analogue tranexamic acid reduces the need for transfusion in patients undergoing surgery.46 In a clinical trial (CRASH 2) of 20 000 patients with trauma,47 early administration of 1 g tranexamic acid before an 8-h infusion of another gram resulted in a decrease in mortality and thrombotic complications, if treatment was started within the first 3 h after trauma.
Fibrinogen has a central role in the formation of the platelet plug.48 Recent recommendations call for a minimum fibrinogen concentration of 1·5–2·0 g/L in surgical patients49, 50 and for a fibrinogen concentration of more than 2·0 g/L in postpartum bleeding.51 Treatment with fibrinogen concentrates is more effective than conventional treatment with plasma in the repletion of fibrinogen concentrations in patients with haemorrhage.52 In a prospective randomised study in patients undergoing coronary artery bypass surgery 2 g of fibrinogen decreased blood loss and the decrease in haemoglobin postoperatively.53 Finally, in a prospective randomised study in patients undergoing aortic surgery with clinically relevant coagulopathic bleeding after cardiopulmonary bypass, administration of fibrinogen guided by thrombelastometry decreased overall transfusion needs from 13 to 2 units of allogeneic blood products (p<0·001).16 Although reports have been published on the use of fibrinogen concentrate as a haemostatic agent, evidence for the usefulness of this treatment is not yet conclusive in all surgery specialties.
Trauma
Patients with blunt or penetrating trauma and massive haemorrhage need complex resuscitation to address dilutional and consumptive coagulopathies that are often accompanied by hypothermia and acidosis.54 Point-of-care testing has enabled analysis of these coagulation abnormalities in the setting of major trauma. Rugeri and colleagues55 did a prospective observational study in which 90 patients with trauma underwent standard coagulation assays along with thrombelastometry. Their data showed the ability of point-of-care testing technology to rapidly detect in-vivo coagulopathic changes in these patients.
Rapid thrombelastography was shown to be better than conventional coagulation tests in the prediction of transfusion needs in nearly 2000 trauma patients.50 A meta-analysis concluded that point-of-care testing had a substantial effect on the amount of bleeding in massively transfused patients, but did not show improvement in morbidity or mortality.56 More studies are needed to further investigate the role of point-of-care testing in the diagnosis and treatment of coagulopathy in patients with trauma. Further studies are also needed to assess the appropriateness and optimisation of early goal-directed treatment in patients with coagulopathic trauma, and to find out if component-specific treatment could improve present transfusion protocols, reduce transfusion-associated complications, and improve coagulopathy-related morbidity and mortality.
Prothrombin complex concentrates are increasingly used off-label in trauma and surgical settings associated with coagulopathy.49 Targeted treatment with prothrombin complex concentrates with rotation thrombelastometry-based algorithms has shown significant reductions in transfused blood components.57 Nevertheless, the role of prothrombin complex concentrates in perioperative-related bleeding remains uncertain.58 To avoid thrombotic complications, care should be taken to avoid excessive treatment via accurate monitoring of patients' coagulation status.
Inherited and acquired coagulopathies
A history of bleeding episodes or physical signs of bleeding mandate a full bleeding workup and consultation with a haematologist to assess platelet numbers and function and the presence of inherited or acquired coagulation factor deficiencies. The patient's medication profile can reveal inhibitory drugs, such as aspirin or aspirin-containing compounds, non-steroidal anti-inflammatory drugs, warfarin, chronic steroid use, platelet inhibitors, oral anti-thrombin or Xa anticoagulants, or low molecular weight heparin. The history might also reveal acquired coagulation disorders associated with parenchymal liver disease, renal failure, or myeloproliferative syndromes.
Prothrombin complex concentrates, one-factor concentrates, and recombinant coagulation factors are approved for use in patients with inherited coagulation factor deficiencies. Some of these products have been approved as haemostatic agents in patients with acquired coagulopathies, such as the management of acute reversal of warfarin coagulopathy, alongside vitamin K treatment.59 Prothrombin complex concentrates that contain all four (II, IX, X, and VII) of the vitamin K-dependent clotting factors are approved internationally, including the USA. Three-factor prothrombin complex concentrates are approved only for the replacement of factor IX; therefore, the use of these three-factor prothrombin complex concentrates for reversal of warfarin is controversial.59
The safety of prothrombin complex concentrates compared with plasma is a subject of debate.59 In the setting of emergency reversal of warfarin coagulopathy, a review of eight clinical studies60 identified a thromboembolic event rate of 0·9% associated with treatment with prothrombin complex concentrates. These thrombotic events arose during emergency reversal of anticoagulation in patients with a high thrombosis risk attributable to underlying disease. Guidelines from several medical societies on the use of prothrombin complex concentrates for acute warfarin reversal have been published59 and have recommended that prothrombin complex concentrates be given as an alternative to fresh frozen plasma. The most recently updated guidelines from the American College of Chest Physicians61 recommend treatment with prothrombin complex concentrates instead of plasma for acute reversal of warfarin coagulopathy. The uncertain role of prothrombin complex concentrates in comparison with treatment with plasma for acute warfarin reversal is partly attributable to the variability in the contents and amount of clotting factors in these preparations;59 regulatory approval status for different countries; scarcity among hospital formularies, particularly in small community hospitals; and potential risks of thrombogenicity in patients with an underlying disease who have a high thrombotic potential.
Recombinant activated factor VII complexes directly with tissue factor released from the subendothelium at sites of vascular disruption. Recombinant activated factor VII also binds to activated platelets, which concentrates factor X activation to sites of tissue injury. Approved indications of recombinant activated factor VII are treatment of bleeding episodes (or prevention of bleeding from invasive procedures) in patients with congenital haemophilia A or haemophilia B who have inhibitors to factors VIII or IX; patients with congenital factor VII deficiency; and in patients with acquired haemophilia. Additionally, in the European Union, recombinant activated factor VII is approved for patients with inherited qualitative platelet defects. However, these approved indications accounted for only 3121 (4·2%) of 73 747 cases reported to use recombinant activated factor VII in the USA from 2000–08.62 A systematic literature review found little evidence of efficacy for five off-label clinical settings (intracranial haemorrhage, cardiac surgery, trauma, liver transplantation, and prostatectomy), with no mortality reduction associated with use of recombinant activated factor VII in these settings.63
The safety profile of recombinant activated factor VII suggests an increased risk of thrombotic arterial events that might be under-reported by treating physicians. Levi and colleagues64 analysed 35 randomised trials with 4468 patients including patients with haemophilia with inhibitors to factors VIII or IX, and off-label settings such as trauma, liver transplantation, and patients with gastrointestinal bleeding caused by cirrhosis. Investigators found that 11·1% of patients had thromboembolic events. Rates of venous thromboembolic events were similar for patients who received recombinant activated factor VII (5·3%) compared with patients given placebo (5·7%); however, arterial events were significantly higher (5·5% vs 3·2%, p<0·003) in patients older than 75 years of age who were receiving recombinant activated factor VII than in patients given placebo.
New alternatives in development
Factor XIII is activated by thrombin and crosslinks soluble fibrin monomers into insoluble fibrin strands. Additionally, activated factor XIII protects the developing clot from fibrinolysis and has important roles in wound healing.65 Low concentrations of factor XIII (<60% in neurosurgery and <70% in cardiac surgery) are associated with postoperative haemorrhage, and replacement treatment has been shown to reduce postoperative bleeding.66, 67 Factor XIII also decreased transfusion needs in patients at high risk of intraoperative bleeding.68 Therefore, the perioperative monitoring and targeted replacement of factor XIII concentrations is a promising approach to decrease transfusion needs and postoperative bleeding. Factor XIII has been recommended as a haemostatic agent to stabilise clots in patients bleeding after cardiac surgery, when other treatments have not yielded satisfactory results.23 The source of factor XIII varies according to local availability: factor XIII concentrate, cryoprecipitate, or plasma. Human recombinant factor XIII is approved and has been successfully used in the treatment of congenital factor XIII deficiency,69 but is not yet approved for patients with acquired factor XIII deficiency.65 More data are needed to justify the routine use of human recombinant factor XIII as a haemostatic agent.
The use of topical haemostatics continues to evolve and enter clinical practice at a rapid rate.70 Although reports exist of antibody formation against bovine coagulation factors with cross-reactivity to human coagulation factors resulting in coagulopathy, this has not been seen with products containing (recombinant) human thrombin.71 These agents can be especially useful when access to the bleeding site is difficult.72 A controlled study in patients undergoing cardiac surgery has shown that use of topical haemostatics can result in less blood loss and fewer transfusions.73
Medical anaemias
Anaemia of chronic disease
The Circular of Information for the Use of Human Blood and Blood Components identifies four haematinics that should be given instead of a blood transfusion, when appropriate: iron, folate, vitamin B12, and erythropoietin.22 With molecular biology, our understanding of the pathophysiology of anaemia of chronic disease has improved substantially. While sequestration of iron has long been known to be central to the pathogenesis of the anaemia of chronic disease, it is now known that the regulation of iron homoeostasis is through hepcidin, as the central regulator of gastrointestinal iron absorption, iron storage in macrophages, and resultant plasma iron concentration.74 Iron sequestration mediated by hepcidin is a common cause of iron-restricted erythropoiesis in patients with inflammation. Hepcidin acts to sequester iron by inhibiting the exit of storage iron from hepatocytes and macrophages into plasma, and also absorption of dietary iron from duodenal enterocytes. Hepcidin binds and degrades the iron exporter ferroportin, and thereby reduces the iron available for erythropoiesis.
Hepcidin production is regulated under normal conditions by iron stores and erythropoietic activity. Increased plasma and storage iron stimulate hepcidin production, which in turn inhibits dietary iron absorption. Conversely, iron deficiency and increased erythropoietic activity (eg, myelodysplastic syndromes or haemolytic anaemias) suppress hepcidin to very low concentrations, which allows increased absorption of dietary iron and release of iron storage and increased haemoglobin synthesis.75 The mechanisms by which erythropoiesis affects hepcidin production are not well understood, but both direct and indirect effects of anaemia and erythropoiesis might play a part. Candidate mediators include soluble factors released by erythroid precursors, and decreased circulating or stored iron.76 Hypoxia can change hepcidin production directly through hypoxia-inducible factor or indirectly via increased erythropoietin production and erythropoiesis.77 Hepcidin concentrations are high in several inflammatory diseases including rheumatological diseases, inflammatory bowel disease, infections, critical illness, and malignant diseases.75 Increased hepcidin concentrations cause the retention of iron in macrophages and enterocytes, leading to hypoferraemia, iron-restricted erythropoiesis, and decreased responsiveness to treatment with erythropoiesis-stimulating agents. The development and commercial availability of an accurate and reproducible immunoassay for human hepcidin has improved our understanding of the pathogenic role of hepcidin in a range of iron disorders, and in the future, might be useful in the identification of patients with iron-restricted erythropoiesis who are intolerant or unresponsive to oral iron, and for whom treatment with IV iron might be needed to correct the anaemia.78, 79
Several clinical trials were undertaken in an attempt to show improved clinical outcomes in patients given erythropoiesis-stimulating agents for anaemia associated with inflammation, such as chronic kidney disease or cancer (table ).24, 43, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91 Aggressive management of anaemia in the cohorts given erythropoiesis-stimulating agents was associated with increased morbidity (thrombosis or cardiovascular events) and increased mortality. Literature reviews and meta-analyses of trials of erythropoiesis-stimulating agents for both approved and off-label oncology settings have analysed survival and other safety outcomes.92 The mechanisms behind the association between increased morbidity and mortality outcomes and erythropoiesis-stimulating agents are unclear. One hypothesis is that erythropoiesis-stimulating agents stimulate disease progression or thrombosis or both by activation of erythropoietin receptors present on tumour cells or associated vascular endothelium.93 However, other investigators have found no evidence that erythropoietin receptors are functionally expressed on tumours94 or are present on non-haemopoietic cells.95
Table.
Post-approval clinical trials of erythropoiesis-stimulating agents
| Target control (haemoglobin g/L) | Reference, year | Clinical outcomes | ||
|---|---|---|---|---|
| Surgery | ||||
| SPINE | <130 | Stowell, 200923 | Increased thrombosis | |
| Chronic kidney disease | ||||
| Dialysis vs predialysis | ≥130 vs 100 | Besarab, 199880 | Decreased OS | |
| CHOIR | 135 vs 113 | Singh, 200681 | Decreased OS | |
| CREATE | 130–150 vs 105–115 | Drueke, 200682 | Decreased OS | |
| TREAT | 130 vs 90 | Pfeffer, 200683 | Decreased OS | |
| Oncology | ||||
| Chemotherapy | ||||
| Lymphoma | ≥140–150 (F,M) | Hedenus, 200384 | Decreased OS | |
| Breast (BEST) | >140 | Leyland-Jones, 200585 | Decreased OS | |
| Breast (PREPARE) | ≥130 | Untch, 201186 | Increased thrombosis | |
| Radiotherapy | ||||
| Head and neck (ENHANCE) | ≥140–150 (F,M) | Henke, 200387 | Decreased OS | |
| Head and neck (DAHANCA) | >155 | Overgaard, 200788 | Decreased disease-free survival | |
| Chemotherapy and radiotherapy | ||||
| (Gynecologic Oncology) | >140 | Thomas, 200889 | Decreased OS | |
| No or palliative treatment | ||||
| Non-small cell lung | ≥140 | Wright, 200790 | Decreased OS | |
| Non-myeloid cancer | ≥130 | Smith, 200891 | Decreased OS | |
OS=overall survival. F=female. M=male. Reproduced from Goodnough and Shander,44 by permission of the International Anesthesia Research Society.
On the basis of this evidence, use of erythropoiesis-stimulating agents in chronic kidney disease and cancer has been affected by updates in guidelines96, 97 and revised labels that show safety concerns98 for target haemoglobin concentrations. In these patients, treatment with erythropoiesis-stimulating agents should only be initiated when concentrations of haemoglobin are less than 100 g/L or in patients with symptomatic anaemia. In this setting, a low dose of erythropoiesis-stimulating agent is given to produce red cell responses sufficient for patients to avoid allogeneic blood transfusions. In clinical practice, the increased risks of death and thromboembolic events should be balanced against the benefits of reduced exposure to blood transfusions with long-term treatment with erythropoiesis-stimulating agents, and needs to take into account each patient's clinical circumstances and preferences.99 Strategies to extend the labelled indications for erythropoiesis-stimulating agents into other patient populations such as in congestive heart failure have been unsuccessful; in a recent study, treatment with erythropoiesis-stimulating agents did not improve clinical outcomes in patients with systolic heart failure and mild to moderate anaemia.100
Iron-restricted erythropoiesis
The most common cause of inappropriate blood transfusion on medical services is the transfusion of patients for iron-deficiency anaemia. Absolute deficiency of storage iron is the most common cause of iron-restricted erythropoiesis, especially in young children, pregnant women, premenopausal women, and elderly people.101 11% of men and 10·2% of women aged 65 years and older are anaemic, with an overall rate of more than 20% at age 85 years and older. Of older people with anaemia, a third are nutritionally deficient, with absolute iron deficiency being the most common aetiology of these.102 Ageing is a pro-inflammatory state, in which iron absorption in the elderly might be impaired because of a hepcidin-mediated effect.
Blood loss (eg, in females with menses, gastrointestinal lesions, or community blood donors) is the main cause of iron deficiency and is important, not only because of its prevalence, but because proper diagnosis and management of the bleeding lesion is important.103 Therapeutic management, after any pathological cause of blood loss has been considered, is focused mainly on repletion of iron stores. Most patients with iron deficiencies respond well to oral iron treatment, but intravenous iron might be necessary when hepcidin-mediated mechanisms inhibit oral iron absorption in the presence of inflammation.21
Iron-restricted erythropoiesis can happen with absolute iron deficiency, functional iron deficiency, or, as described above, under anaemia of chronic disease and iron sequestration.21 Most individuals with an absolute iron deficiency without inflammation respond well to oral iron treatment, but intravenous iron might be necessary in patients with inflammation-mediated hepcidin effects.20 Functional iron deficiency can arise in patients with substantial erythropoietin-mediated erythropoiesis or upon treatment with erythropoiesis-stimulating agents, seen by a reduction in transferrin saturation in patients with cancer and in patients with chronic kidney disease undergoing dialysis treatment. Functional iron deficiency can be ameliorated with IV iron treatment.21 An algorithm approach for the assessment and management of anaemia based on initial assessment of iron status has been recommended by a consortium of European societies for patients scheduled for elective surgery (figure 2).10
IV iron has been recommended for the management of anaemia in patients with chronic kidney disease who are unresponsive to erythropoiesis-stimulating agents.97 Studies in patients with cancer with chemotherapy-induced anaemia who are given erythropoiesis-stimulating agents found significant improvements in haemoglobin concentrations and hematopoietic responses in patients given IV iron compared with those receiving oral iron or no iron.104 The low but defined risks of IV iron, along with current labelled indications that restrict use in patients with end-stage renal disease for various preparations, have ensured that the role of IV iron outside the setting of renal dialysis patients remains in evolution.
Conclusions
Since the recognition that non-A, non-B hepatitis, and HIV were transmissible by blood transfusion, the benefit/risk profile and use of alternatives to blood have been driven by risks related to blood transfusion. The role of the alternatives to blood need to be better defined by patient-centred treatment plans based on anticipated perioperative blood loss and likelihood of blood transfusion The presence of preoperative anaemia, the type of surgical procedure, the likelihood of coagulopathy, and patient comorbidities will further affect the need for blood transfusion or its alternatives. The successful use of point-of-care testing suggests that more targeted treatment can improve patient outcomes such as multi-organ system failure associated with trauma and blood transfusions.105 In this context, randomised prospective trials are needed. The success of the CRASH 2 study47 that used early antifibrinolytic treatment in patients with trauma, suggests that more targeted treatments to address associated coagulopathies such as prothrombin concentrates (II, VII, IX, and X), fibrinogen concentrates, recombinant factor VIIa, or recombinant XIII might be of value in specific patient settings. Potential future areas of research include point-of-care testing coupled with therapeutic algorithms. Quality indicators measuring patient outcomes (ie, hospital stay and mortality) need to be used so that the alternatives to blood transfusion can be more effectively assessed and incorporated into clinical practice.
This is the second in a Series of three papers about blood transfusion
Acknowledgments
Acknowledgments
Jason Calcagno provided invaluable assistance in preparation of this manuscript.
Contributors
DRS and LTG researched, drafted, and wrote the review jointly.
Conflicts of interest
DRS's academic department is receiving grant support from the Swiss National Science Foundation (Bern, Switzerland), the Swiss Society of Anaesthesiology and Reanimation (Bern, Switzerland), the Swiss Foundation for Anesthesia Research (Zurich, Switzerland), Bundesprogramm Chancengleichheit (Bern, Switzerland), CSL Behring (Bern, Switzerland), and Vifor SA (Villars-sur-Glâne, Switzerland). In the past 5 years, DRS has received honoraria or travel support for consulting or lecturing from the following companies: AMGEN GmbH (Munich, Germany), Baxter AG (Volketswil, Switzerland), Baxter S.p.A. (Rome, Italy), CSL Behring GmbH (Hattersheim am Main, Germany and Bern, Switzerland), Ethicon Biosurgery (Sommerville, NJ, USA), Janssen-Cilag AG (Baar, Switzerland), Janssen-Cilag EMEA (Beerse, Belgium), Novo Nordisk A/S, (Bagsvärd, Denmark), Octapharma AG (Lachen, Switzerland), Organon AG (Pfäffikon, Switzerland), Oxygen Biotherapeutics (Costa Mesa, CA, USA), Pentapharm GmbH (now tem Innovations GmbH; Munich, Germany), ratiopharm Arzneimittel Vertriebs-GmbH (Vienna, Austria), Roche Pharma (Switzerland) AG (Reinach, Switzerland), Vifor Pharma Deutschland GmbH (Munich, Germany), Vifor Pharma Österreich GmbH (Vienna, Austria), Vifor (International) AG (St Gallen, Switzerland). LTG is a consultant for Amgen (Thousand Oaks, CA, USA), Luitpold (Shirley, NY, USA), Vifor (Glattbrugg, Switzerland), CSL Behring (King of Prussia, PA, USA), Hemocue (Cypress, CA, USA), and Octapharma (Hoboken, NJ, USA).
References
- 1.Busch MP, Kleinman SH, Nemo GJ. Current and emerging infectious risks of blood transfusions. JAMA. 2003;289:959–962. doi: 10.1001/jama.289.8.959. [DOI] [PubMed] [Google Scholar]
- 2.Isbister JP, Shander A, Spahn DR, Erhard J, Farmer SL, Hofmann A. Adverse blood transfusion outcomes: establishing causation. Transfus Med Rev. 2011;25:89–101. doi: 10.1016/j.tmrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–1407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 4.Bernard AC, Davenport DL, Chang PK, Vaughan TB, Zwischenberger JB. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg. 2009;208:931–937. doi: 10.1016/j.jamcollsurg.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Vincent JL, Baron JF, Reinhart K, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288:1499–1507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 6.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4 doi: 10.1002/14651858.CD002042.pub3. CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank SM, Abazyan B, Ono M, et al. Decreased erythrocyte deformability after transfusion and the effects of erythrocyte storage duration. Anesth Analg. 2013;116:975–981. doi: 10.1213/ANE.0b013e31828843e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solomon SB, Wang D, Sun J, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121:1663–1672. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vamvakas EC, Blajchman MA. Transfusion-related mortality: the ongoing risks of allogeneic blood transfusion and the available strategies for their prevention. Blood. 2009;113:3406–3417. doi: 10.1182/blood-2008-10-167643. [DOI] [PubMed] [Google Scholar]
- 10.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Na HS, Shin SY, Hwang JY, Jeon YT, Kim CS, Do SH. Effects of intravenous iron combined with low-dose recombinant human erythropoietin on transfusion requirements in iron-deficient patients undergoing bilateral total knee replacement arthroplasty. Transfusion. 2011;51:118–124. doi: 10.1111/j.1537-2995.2010.02783.x. [DOI] [PubMed] [Google Scholar]
- 12.Yoo YC, Shim JK, Kim JC, Jo YY, Lee JH, Kwak YL. Effect of single recombinant human erythropoietin injection on transfusion requirements in preoperatively anemic patients undergoing valvular heart surgery. Anesthesiology. 2011;115:929–937. doi: 10.1097/ALN.0b013e318232004b. [DOI] [PubMed] [Google Scholar]
- 13.Schochl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15:R83. doi: 10.1186/cc10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kotze A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108:943–952. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 15.Weber CF, Gorlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–547. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 16.Rahe-Meyer N, Solomon C, Hanke A, et al. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebo-controlled trial. Anesthesiology. 2013;118:40–50. doi: 10.1097/ALN.0b013e3182715d4d. [DOI] [PubMed] [Google Scholar]
- 17.Shander A, Van Aken H, Colomina MJ, et al. Patient blood management in Europe. Br J Anaesth. 2012;109:55–68. doi: 10.1093/bja/aes139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–765. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 19.Goodnough LT, Shander A. Patient blood management. Anesthesiology. 2012;116:1367–1376. doi: 10.1097/ALN.0b013e318254d1a3. [DOI] [PubMed] [Google Scholar]
- 20.Goodnough LT, Nemeth E, Ganz T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood. 2010;116:4754–4761. doi: 10.1182/blood-2010-05-286260. [DOI] [PubMed] [Google Scholar]
- 21.Goodnough LT. Iron deficiency syndromes and iron-restricted erythropoiesis. Transfusion. 2012;52:1584–1592. doi: 10.1111/j.1537-2995.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 22.Goodnough LT, Monk TG, Andriole GL. Erythropoietin therapy. N Engl J Med. 1997;336:933–938. doi: 10.1056/NEJM199703273361307. [DOI] [PubMed] [Google Scholar]
- 23.Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the society of thoracic surgeons and the society of cardiovascular anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 24.Stowell CP, Jones SC, Enny C, Langholff W, Leitz G. An open-label, randomized, parallel-group study of perioperative epoetin alfa versus standard of care for blood conservation in major elective spinal surgery: safety analysis. Spine. 2009;34:2479–2485. doi: 10.1097/BRS.0b013e3181bd163f. [DOI] [PubMed] [Google Scholar]
- 25.Sowade O, Warnke H, Scigalla P, et al. Avoidance of allogeneic blood transfusions by treatment with epoetin beta (recombinant human erythropoietin) in patients undergoing open-heart surgery. Blood. 1997;89:411–418. [PubMed] [Google Scholar]
- 26.Weltert L, D'Alessandro S, Nardella S, et al. Preoperative very short-term, high-dose erythropoietin administration diminishes blood transfusion rate in off-pump coronary artery bypass: a randomized blind controlled study. J Thorac Cardiovasc Surg. 2010;139:621–626. doi: 10.1016/j.jtcvs.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 27.D'Ambra MN, Gray RJ, Hillman R, et al. Effect of recombinant human erythropoietin on transfusion risk in coronary bypass patients. Ann Thorac Surg. 1997;64:1686–1693. doi: 10.1016/s0003-4975(97)00839-4. [DOI] [PubMed] [Google Scholar]
- 28.Goodnough LT, Shander A, Spence R. Bloodless medicine: clinical care without allogeneic blood transfusion. Transfusion. 2003;43:668–676. doi: 10.1046/j.1537-2995.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- 29.Goodnough LT, Brecher ME, Kanter MH, AuBuchon JP. Transfusion medicine. Second of two parts: blood conservation. N Engl J Med. 1999;340:525–533. doi: 10.1056/NEJM199902183400706. [DOI] [PubMed] [Google Scholar]
- 30.Brecher ME, Goodnough LT. The rise and fall of preoperative autologous blood donation. Transfusion. 2001;41:1459–1462. doi: 10.1046/j.1537-2995.2001.41121459.x. [DOI] [PubMed] [Google Scholar]
- 31.Billote DB, Glisson SN, Green D, Wixson RL. A prospective, randomized study of preoperative autologous donation for hip replacement surgery. J Bone Joint Surg Am. 2002;84:1299–1304. doi: 10.2106/00004623-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Virmani S, Tempe DK, Pandey BC, et al. Acute normovolemic hemodilution is not beneficial in patients undergoing primary elective valve surgery. Ann Card Anaesth. 2010;13:34–38. doi: 10.4103/0971-9784.58832. [DOI] [PubMed] [Google Scholar]
- 33.Bell K, Stott K, Sinclair CJ, Walker WS, Gillon J. A controlled trial of intra-operative autologous transfusion in cardiothoracic surgery measuring effect on transfusion requirements and clinical outcome. Transfus Med. 1992;2:295–300. doi: 10.1111/j.1365-3148.1992.tb00173.x. [DOI] [PubMed] [Google Scholar]
- 34.Weltert L, Nardella S, Rondinelli MB, Pierelli L, De Paulis R. Reduction of allogeneic red blood cell usage during cardiac surgery by an integrated intra- and postoperative blood salvage strategy: results of a randomized comparison. Transfusion. 2013;53:790–797. doi: 10.1111/j.1537-2995.2012.03836.x. [DOI] [PubMed] [Google Scholar]
- 35.Carless PA, Henry DA, Moxey AJ, O'Connell DL, Brown T, Fergusson DA. Cell salvage for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2006;4 doi: 10.1002/14651858.CD001888.pub2. CD001888. [DOI] [PubMed] [Google Scholar]
- 36.Carless PA, Henry DA, Moxey AJ, et al. Desmopressin for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2004;1 doi: 10.1002/14651858.CD001884.pub2. CD001884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crescenzi G, Landoni G, Biondi-Zoccai G, et al. Desmopressin reduces transfusion needs after surgery: a meta-analysis of randomized clinical trials. Anesthesiology. 2008;109:1063–1076. doi: 10.1097/ALN.0b013e31818db18b. [DOI] [PubMed] [Google Scholar]
- 38.Steinlechner B, Zeidler P, Base E, et al. Patients with severe aortic valve stenosis and impaired platelet function benefit from preoperative desmopressin infusion. Ann Thorac Surg. 2011;91:1420–1426. doi: 10.1016/j.athoracsur.2011.01.052. [DOI] [PubMed] [Google Scholar]
- 39.Reiter RA, Mayr F, Blazicek H, et al. Desmopressin antagonizes the in vitro platelet dysfunction induced by GPIIb/IIIa inhibitors and aspirin. Blood. 2003;102:4594–4599. doi: 10.1182/blood-2002-11-3566. [DOI] [PubMed] [Google Scholar]
- 40.Hanke AA, Dellweg C, Kienbaum P, Weber CF, Gorlinger K, Rahe-Meyer N. Effects of desmopressin on platelet function under conditions of hypothermia and acidosis: an in vitro study using multiple electrode aggregometry*. Anaesthesia. 2010;65:688–691. doi: 10.1111/j.1365-2044.2010.06367.x. [DOI] [PubMed] [Google Scholar]
- 41.Gorlinger K, Dirkmann D, Hanke AA, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115:1179–1191. doi: 10.1097/ALN.0b013e31823497dd. [DOI] [PubMed] [Google Scholar]
- 42.Hutton B, Joseph L, Fergusson D, Mazer CD, Shapiro S, Tinmouth A. Risks of harms using antifibrinolytics in cardiac surgery: systematic review and network meta-analysis of randomised and observational studies. BMJ. 2012;345:e5798. doi: 10.1136/bmj.e5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fergusson DA, Hebert PC, Mazer CD, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358:2319–2331. doi: 10.1056/NEJMoa0802395. [DOI] [PubMed] [Google Scholar]
- 44.Goodnough LT, Shander A. Current status of pharmacologic therapies in patient blood management. Anesth Analg. 2013;116:15–34. doi: 10.1213/ANE.0b013e318273f4ae. [DOI] [PubMed] [Google Scholar]
- 45.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;3 doi: 10.1002/14651858.CD001886.pub2. CD001886. [DOI] [PubMed] [Google Scholar]
- 46.Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ. 2012;344:e3054. doi: 10.1136/bmj.e3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts I, Shakur H, Afolabi A, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–1101. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 48.Furie B, Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008;359:938–949. doi: 10.1056/NEJMra0801082. [DOI] [PubMed] [Google Scholar]
- 49.Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17:R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holcomb JB, Minei KM, Scerbo ML, et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann Surg. 2012;256:476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 51.Cortet M, Deneux-Tharaux C, Dupont C, et al. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108:984–989. doi: 10.1093/bja/aes096. [DOI] [PubMed] [Google Scholar]
- 52.Kozek-Langenecker S, Sorensen B, Hess JR, Spahn DR. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care. 2011;15:R239. doi: 10.1186/cc10488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karlsson M, Ternstrom L, Hyllner M, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost. 2009;102:137–144. doi: 10.1160/TH08-09-0587. [DOI] [PubMed] [Google Scholar]
- 54.Goodnough LT, Spain DA, Maggio P. Logistics of transfusion support for patients with massive hemorrhage. Curr Opin Anaesthesiol. 2013;26:208–214. doi: 10.1097/ACO.0b013e32835d6f8f. [DOI] [PubMed] [Google Scholar]
- 55.Rugeri L, Levrat A, David JS, et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J Thromb Haemost. 2007;5:289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 56.Afshari A, Wikkelso A, Brok J, Moller AM, Wetterslev J. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011;3 doi: 10.1002/14651858.CD007871.pub2. CD007871. [DOI] [PubMed] [Google Scholar]
- 57.Innerhofer P, Westermann I, Tauber H, et al. The exclusive use of coagulation factor concentrates enables reversal of coagulopathy and decreases transfusion rates in patients with major blunt trauma. Injury. 2013;44:209–216. doi: 10.1016/j.injury.2012.08.047. [DOI] [PubMed] [Google Scholar]
- 58.Sorensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint R. Clinical review: prothrombin complex concentrates—evaluation of safety and thrombogenicity. Crit Care. 2011;15:R201. doi: 10.1186/cc9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodnough LT, Shander A. How I treat warfarin-associated coagulopathy in patients with intracerebral hemorrhage. Blood. 2011;117:6091–6099. doi: 10.1182/blood-2010-11-316075. [DOI] [PubMed] [Google Scholar]
- 60.Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus. 2010;8:149–154. doi: 10.2450/2010.0149-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th edn: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(suppl 2):7–47. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Logan AC, Yank V, Stafford RS. Off-Label Use of Recombinant Factor VIIa in U.S. Hospitals: Analysis of Hospital Records. Ann Intern Med. 2011;154:516–522. doi: 10.7326/0003-4819-154-8-201104190-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yank V, Tuohy CV, Logan AC, et al. Systematic review: benefits and harms of in-hospital use of recombinant factor VIIa for off-label indications. Ann Intern Med. 2011;154:529–540. doi: 10.7326/0003-4819-154-8-201104190-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levi M, Levy JH, Andersen HF, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–1800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 65.Levy JH, Greenberg C. Biology of Factor XIII and clinical manifestations of Factor XIII deficiency. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03865.x. published online Aug 28. [DOI] [PubMed] [Google Scholar]
- 66.Godje O, Gallmeier U, Schelian M, Grunewald M, Mair H. Coagulation factor XIII reduces postoperative bleeding after coronary surgery with extracorporeal circulation. Thorac Cardiovasc Surg. 2006;54:26–33. doi: 10.1055/s-2005-872853. [DOI] [PubMed] [Google Scholar]
- 67.Gerlach R, Tolle F, Raabe A, Zimmermann M, Siegemund A, Seifert V. Increased risk for postoperative hemorrhage after intracranial surgery in patients with decreased factor XIII activity: implications of a prospective study. Stroke. 2002;33:1618–1623. doi: 10.1161/01.str.0000017219.83330.ff. [DOI] [PubMed] [Google Scholar]
- 68.Korte WC, Szadkowski C, Gahler A, et al. Factor XIII substitution in surgical cancer patients at high risk for intraoperative bleeding. Anesthesiology. 2009;110:239–245. doi: 10.1097/ALN.0b013e318194b21e. [DOI] [PubMed] [Google Scholar]
- 69.Inbal A, Oldenburg J, Carcao M, Rosholm A, Tehranchi R, Nugent D. Recombinant factor XIII: a safe and novel treatment for congenital factor XIII deficiency. Blood. 2012;119:5111–5117. doi: 10.1182/blood-2011-10-386045. [DOI] [PubMed] [Google Scholar]
- 70.Spotnitz WD, Burks S. Hemostats, sealants, and adhesives III: a new update as well as cost and regulatory considerations for components of the surgical toolbox. Transfusion. 2012;52:2243–2255. doi: 10.1111/j.1537-2995.2012.03707.x. [DOI] [PubMed] [Google Scholar]
- 71.Singla NK, Gasparis AP, Ballard JL, et al. Immunogenicity and safety of re-exposure to recombinant human thrombin in surgical hemostasis. J Am Coll Surg. 2011;213:722–727. doi: 10.1016/j.jamcollsurg.2011.08.019. [DOI] [PubMed] [Google Scholar]
- 72.Gruen RL, Brohi K, Schreiber M, et al. Haemorrhage control in severely injured patients. Lancet. 2012;380:1099–1108. doi: 10.1016/S0140-6736(12)61224-0. [DOI] [PubMed] [Google Scholar]
- 73.Nasso G, Piancone F, Bonifazi R, et al. Prospective, randomized clinical trial of the FloSeal matrix sealant in cardiac surgery. Ann Thorac Surg. 2009;88:1520–1526. doi: 10.1016/j.athoracsur.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 74.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117:4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nemeth E. Targeting the hepcidin-ferroportin axis in the diagnosis and treatment of anemias. Adv Hematol. 2010;2010:750643. doi: 10.1155/2010/750643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanno T, Miller JL. Iron loading and overloading due to ineffective erythropoiesis. Adv Hematol. 2010;2010:358283. doi: 10.1155/2010/358283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs) J Clin Invest. 2007;117:1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 79.Bregman DB, Morris D, Koch TA, He A, Goodnough LT. Hepcidin levels predict non-responsiveness to oral iron therapy in patients with iron deficiency anemia. Am J Hematol. 2013;88:97–101. doi: 10.1002/ajh.23354. [DOI] [PubMed] [Google Scholar]
- 80.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 81.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 82.Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 83.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 84.Hedenus M, Adriansson M, San Miguel J, et al. Efficacy and safety of darbepoetin alfa in anaemic patients with lymphoproliferative malignancies: a randomized, double-blind, placebo-controlled study. Br J Haematol. 2003;122:394–403. doi: 10.1046/j.1365-2141.2003.04448.x. [DOI] [PubMed] [Google Scholar]
- 85.Leyland-Jones B, Semiglazov V, Pawlicki M, et al. Maintaining normal hemoglobin levels with epoetin alfa in mainly nonanemic patients with metastatic breast cancer receiving first-line chemotherapy: a survival study. J Clin Oncol. 2005;23:5960–5972. doi: 10.1200/JCO.2005.06.150. [DOI] [PubMed] [Google Scholar]
- 86.Untch M, Fasching PA, Konecny GE, et al. PREPARE trial: a randomized phase III trial comparing preoperative, dose-dense, dose-intensified chemotherapy with epirubicin, paclitaxel and CMF versus a standard-dosed epirubicin/cyclophosphamide followed by paclitaxel +/− darbepoetin alfa in primary breast cancer–results at the time of surgery. Ann Oncol. 2011;22:1988–1998. doi: 10.1093/annonc/mdq709. [DOI] [PubMed] [Google Scholar]
- 87.Henke M, Laszig R, Rube C, et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet. 2003;362:1255–1260. doi: 10.1016/S0140-6736(03)14567-9. [DOI] [PubMed] [Google Scholar]
- 88.Overgaard J, Hoff C, San Hansen H. Randomized study of the importance of novel erythropoiesis stimulating protein (Aranesp) for the effect of radiotherapy in patients with primary squamous cell carcinoma of the head and neck (HNSCC): The Danish Head and Neck Cancer Group DAHANCA 10. Eur J Cancer. 2007;7(suppl 5) abstract 6LB. [Google Scholar]
- 89.Thomas G, Ali S, Hoebers FJ, et al. Phase III trial to evaluate the efficacy of maintaining hemoglobin levels above 12·0 g/dL with erythropoietin vs above 10·0 g/dL without erythropoietin in anemic patients receiving concurrent radiation and cisplatin for cervical cancer. Gynecol Oncol. 2008;108:317–325. doi: 10.1016/j.ygyno.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wright JR, Ung YC, Julian JA, et al. Randomized, double-blind, placebo-controlled trial of erythropoietin in non-small-cell lung cancer with disease-related anemia. J Clin Oncol. 2007;25:1027–1032. doi: 10.1200/JCO.2006.07.1514. [DOI] [PubMed] [Google Scholar]
- 91.Smith RE, Jr, Aapro MS, Ludwig H, et al. Darbepoetin alpha for the treatment of anemia in patients with active cancer not receiving chemotherapy or radiotherapy: results of a phase III, multicenter, randomized, double-blind, placebo-controlled study. J Clin Oncol. 2008;26:1040–1050. doi: 10.1200/JCO.2007.14.2885. [DOI] [PubMed] [Google Scholar]
- 92.Bohlius J, Schmidlin K, Brillant C, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373:1532–1542. doi: 10.1016/S0140-6736(09)60502-X. [DOI] [PubMed] [Google Scholar]
- 93.Hadland BK, Longmore GD. Erythroid-stimulating agents in cancer therapy: potential dangers and biologic mechanisms. J Clin Oncol. 2009;27:4217–4226. doi: 10.1200/JCO.2008.21.6945. [DOI] [PubMed] [Google Scholar]
- 94.Swift S, Ellison AR, Kassner P, et al. Absence of functional EpoR expression in human tumor cell lines. Blood. 2010;115:4254–4263. doi: 10.1182/blood-2009-10-248674. [DOI] [PubMed] [Google Scholar]
- 95.Sinclair AM, Coxon A, McCaffery I, et al. Functional erythropoietin receptor is undetectable in endothelial, cardiac, neuronal, and renal cells. Blood. 2010;115:4264–4272. doi: 10.1182/blood-2009-10-248666. [DOI] [PubMed] [Google Scholar]
- 96.Rizzo JD, Brouwers M, Hurley P, et al. American Society of Hematology/American Society of Clinical Oncology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. Blood. 2010;116:4045–4059. doi: 10.1182/blood-2010-08-300541. [DOI] [PubMed] [Google Scholar]
- 97.Kidney Disease: Improving Global Outcomes KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:311–316. [Google Scholar]
- 98.Unger EF, Thompson AM, Blank MJ, Temple R. Erythropoiesis-stimulating agents: time for a reevaluation. N Engl J Med. 2010;362:189–192. doi: 10.1056/NEJMp0912328. [DOI] [PubMed] [Google Scholar]
- 99.Goodnough LT, Shander AS. Erythropoiesis stimulating agents, blood transfusion, and the practice of medicine. Am J Hematol. 2010;85:835–837. doi: 10.1002/ajh.21870. [DOI] [PubMed] [Google Scholar]
- 100.Swedberg K, Young JB, Anand IS, et al. Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013;368:1210–1219. doi: 10.1056/NEJMoa1214865. [DOI] [PubMed] [Google Scholar]
- 101.Guyatt GH, Patterson C, Ali M, et al. Diagnosis of iron-deficiency anemia in the elderly. Am J Med. 1990;88:205–209. doi: 10.1016/0002-9343(90)90143-2. [DOI] [PubMed] [Google Scholar]
- 102.Guralnik JM, Eisenstaedt RS, Ferrucci L, Klein HG, Woodman RC. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–2268. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 103.Raje D, Mukhtar H, Oshowo A, Ingham Clark C. What proportion of patients referred to secondary care with iron deficiency anemia have colon cancer? Dis Colon Rectum. 2007;50:1211–1214. doi: 10.1007/s10350-007-0249-y. [DOI] [PubMed] [Google Scholar]
- 104.Henry DH, Dahl NV, Auerbach M, Tchekmedyian S, Laufman LR. Intravenous ferric gluconate significantly improves response to epoetin alfa versus oral iron or no iron in anemic patients with cancer receiving chemotherapy. Oncologist. 2007;12:231–242. doi: 10.1634/theoncologist.12-2-231. [DOI] [PubMed] [Google Scholar]
- 105.Goodnough LT. Does plasma therapy have a role in clincial medicine? Crit Care Med (in press). [DOI] [PubMed]