Abstract
The study aimed to test the hypothesis that homeostatic microbiome (HM) disorders lead to the increased indirect influence of certain microorganisms (MO) in the gastrointestinal tract, causing a disorder of insulin secretion, insulin resistance, and diabetes. We highlighted Candida and certain types of bacteria since previous in vitro research showed they significantly affect insulin secretion and can cause insulin resistance in obese patients with metabolic syndrome. After determining the type of MO present in the throat swab and the stool, the oral glucose tolerance test (OGTT) test, and analysis of glucose and insulin secretion were performed in patients (n = 38) who were positive for certain types of MO compared to negative patients. Finally, all patients were divided into two groups: overweight patients (body mass index [BMI] < 30) and obese patients (BMI > 30). These two groups were compared for the percentage of certain types of MO to determine which MO can affect an increase in obesity and BMI. The presence of Diphtheroids in the throat (60.5%) reduces insulin secretion in patients compared with the negative group (194.5: 332.4) and the difference was statistically significant (P = .030). The presence of Candida in the throat (10%) increases insulin secretion, but the difference was statistically insignificant. The presence of Candida in the stool (28.9%) also increases insulin secretion and the difference was statistically significant (P = .038). Cumulative results (throat + stool) were similar (180: 332, P = .022). Analysis of BMI showed that the percentage of Diphtheroids in the throat decreases with increased body weight (53.8: 75%) while the percentage of Candida (38.5: 8.3%) and Enterobacter (61.5: 25%) increases, but these differences were statistically insignificant (P > .05). Diphtheroids in the throat can reduce insulin secretion by synthesizing their metabolites. Candida albicans is a conditional pathogen and as a significant indirect factor induces increased insulin secretion and insulin resistance. There are indications that elevated levels of Candida in the intestinal system can cause increased body weight of patients. C albicans should be considered a new factor in the pathogenesis of diabetes.
Keywords: Candida albicans, Diphtheroids, Enterobacter sp, homeostatic microbiome, insulin secretion, pathogenesis of diabetes, Pseudomonas aeruginosa, Staphylococcus aureus
1. Introduction
Type 2 diabetes is a persistent and in the initial phase almost imperceptible disease that causes serious clinical manifestations over the years. It is a result of two interrelated problems – insulin resistance and insulin secretion disorder.[1] Several factors are involved in the etiopathogenesis of this disease: physical inactivity, overweight,[2] genetic factors, malnutrition in the fetal and prenatal period, and some drugs (e.g., diuretics, anti-hypertensives, steroids). The risk of type 2 diabetes increases with increased adipose tissue storage in obese people. Adipose tissue produces adiponectin, tumor necrosis factor (TNF), alpha, leptin, resistin, and IL-6, thereby affecting insulin resistance and possibly pancreatic β-cell dysfunction.[3–5] New factors in the etiopathogenesis of diabetes mellitus include microbiological agents, due to direct infection of the pancreas, or saprophytic flora disorder.[8] Homeostatic microbiome (HM) is a set of all microorganisms (bacteria, fungi, and viruses) which by their mutual relationship and action maintain the normal homeostasis of the organism.[6]
Our in vitro studies conducted in pancreatic islet culture,[7,8] have shown that certain types of bacteria and fungi can directly cause increased or decreased insulin secretion of the pancreas leading to insulin resistance and the development of diabetes mellitus. Based on these results, the following hypothesis was presented – the presence of these microorganisms in the human pancreas can lead to insulin resistance, thus increasing the chances of development of type 2 diabetes mellitus, or they can cause β-cell destruction due to activation of the immune system in response to infection, thus inducing type 1 diabetes mellitus.[6]
Patients with metabolic syndrome were followed in this study. Metabolic syndrome includes central (abdominal) obesity, some disorders of glucoregulation diabetes mellitus type 2, impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), hypertension, and hyperlipoproteinemia.[9] Metabolic syndrome was determined according to the recommendations of the National Cholesterol Education Program (NCEP).[10] In particular, the most important risk factors for metabolic syndrome are abdominal obesity and insulin resistance.[11] In obese individuals, proinflammatory cytokines and hormones are released from adipose tissue and participate in the development of insulin resistance.[12] Insulin resistance is characterized by reduced sensitivity of peripheral tissues to insulin, which leads to the development of hyperinsulinemia.[13] Lipotoxicity is also present in obese people, due to the accumulation of lipid precursors which are toxic to cells.[14]
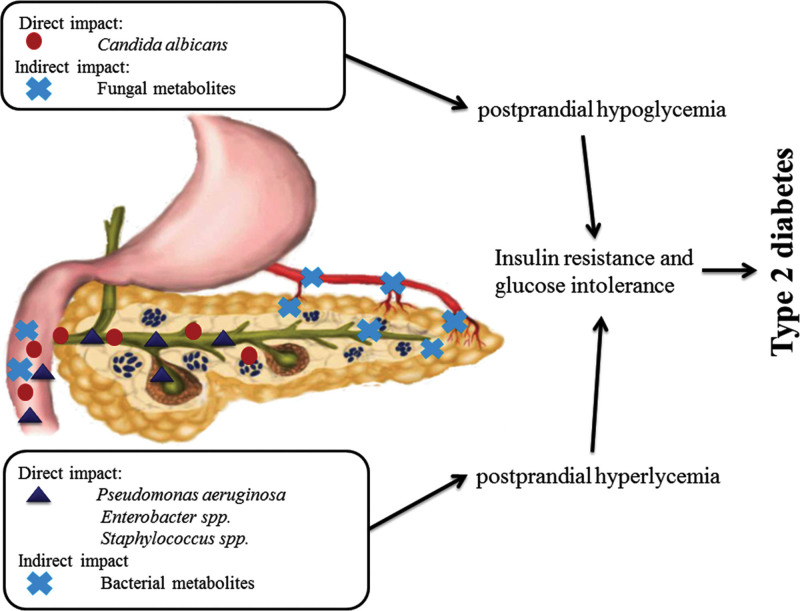
My previous in vitro studies have shown that certain strains of microorganisms can provoke increased or decreased insulin secretion. Candida in pancreatic islet culture induces increased insulin secretion.[8] while certain strains of bacteria, such as Pseudomonas, Staphylococcus, and Enterobacter reduce insulin secretion.[7] Based on these results, the hypothesis was presented that microorganisms by their action can cause insulin resistance, a prerequisite for the development of diabetes (Fig. 1).
Figure 1.
The direct and indirect influence of Candida and bacteria on the development of diabetes mellitus type 2. (Nikolic DM. Military-medical and pharmaceutical review 2018;75(11):1110–1117).
The second part of the hypothesis refers to the indirect action of microorganisms: Due to HM disorder, overgrowth of these microorganisms in the intestinal system and mucous surfaces can indirectly affect insulin secretion. They release certain metabolites that through blood can reach the pancreas.[6] This hypothesis was tested in a pilot program with patients who have metabolic syndrome and insulin resistance. The aim was to determine the strains of microorganisms that cause these effects in vivo, in patients.
2. Material and Methods
Study design: The research was designed to determine first the type of microorganisms (MO) present in stool and throat swabs of all patients. After that, the oral glucose tolerance test (OGTT) test and the analysis of glucose and insulin secretion were performed in patients who were positive for certain types of MO compared to negative patients. Finally, all patients were divided into two groups. The first group is overweight patients (body mass index [BMI] < 30) and the second group is obese patients (BMI > 30). These two groups were compared for the percentage of certain types of MO to determine MO that can affect the increase in obesity and BMI.
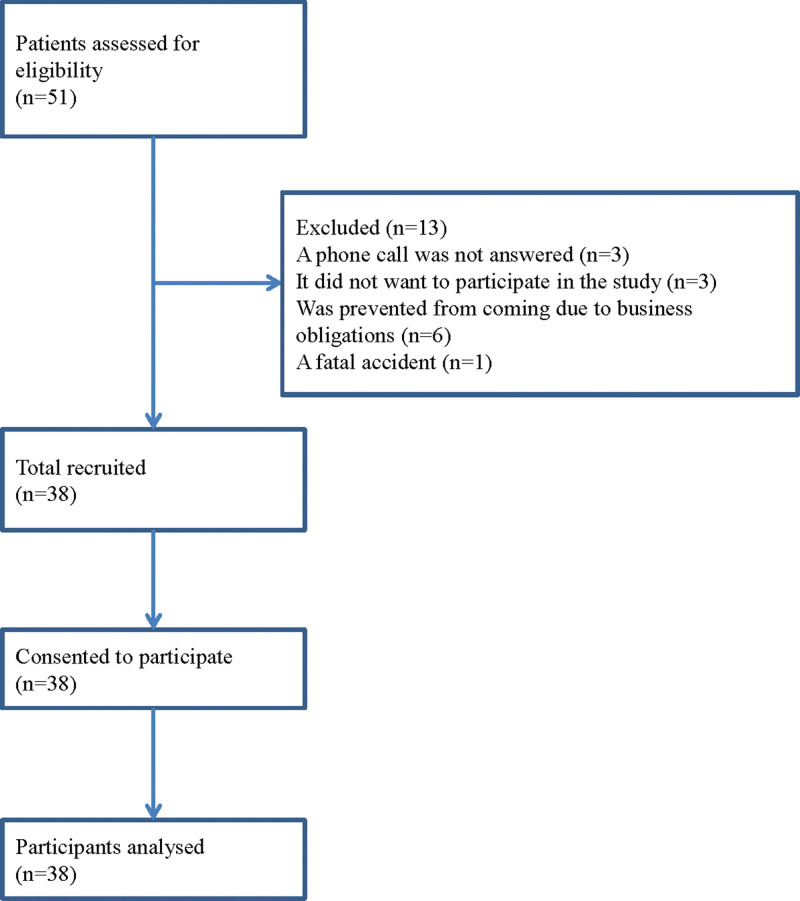
Throat swabs and stool specimens were taken from patients (36 women and 2 men, mean age 60.6 ± 10.4, min = 34, max = 72) who came to the Cabinet for Nutrition and Prevention of Metabolic Disorders at the Clinic for Endocrinology, Diabetes, and Metabolic Diseases, University Clinical Center of Serbia in Belgrade, due to problems with overweight, in the period from 2017 to 2020 (Fig. 2).
Figure 2.
Flow diagram of study participation.
Participants were informed about the purpose of this research and gave their written consent for voluntary participation in this study, so it is conducted following the decision of the Ethics Committee of the Medical Faculty in Belgrade (No. 29/XII-10). This study follows the principles of the Declaration of Helsinki.
All patients who participated in this study were obese Caucasians from the territory of Belgrade and had different professions. All parameters to determine the BMI of patients were obtained. Anthropometric measurements: body weight, body height, body mass index, waist circumference, hip circumference, and waist to hip circumference ratio, were performed. Body mass index was calculated as body weight in kilograms divided by the square of the body height in meters. Glucose regulation was tested by OGTT which was performed on fasting patients by drinking 75 gr of glucose dissolved in 300 mL of water. Then the values of glycemia and insulin were measured at 0, 30, and 120 minutes. Insulin levels were determined by radioimmunoassay microbiological analyses of samples (stool and throat swabs) were performed by standard procedure at the Institute for Microbiology, Clinical Center in Serbia. Microbiological isolates were identified based on microscopic, cultural, and biochemical properties. Microscopic slides were prepared directly from patient samples, and also from colonies on culture plates, and then Gram stained. We used Columbia blood agar plates (7% sheep blood), MacConkey agar and XLD agar plates, and Sabourand dextrose agar. Depending on the cultural and morphological characteristics of isolated microorganisms, we have prepared a small series of biochemical tests.
In this cross-sectional study, results are presented as count (%), means ± standard deviation, or median (25th–75th Percentile) depending on data type and distribution. Groups are compared using parametric (t-test) and nonparametric (Pearson Chi-square test, Fisher exact test, Mann–Whitney U test) tests. All P values less than .05 were considered significant. All data were analyzed using SPSS 20.0 (IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.).
3. Results
Microbiological analysis of throat swab samples detected seven different types of microorganisms in different percentages (Table 1). Most abundant is Staphylococcus aureus (97.4%), followed by Streptococcus alfa haemolyticus (92%), Diphtheroids (60%), and Neisseria sp (44.7%). The least represented are Moraxela cathoralis and Candida albicans with 15.8% and 10.5% respectively.
Table 1.
Types of microorganisms detected in the throat and stool swabs of the patients. Example: Out of a total number of examined patients, Candida was detected in stool samples of 11 subjects, which makes up about 28.9%.
| N (%) | Mean percentage representation of positive samples | |
|---|---|---|
| Throat | ||
| Diphtheroids | 23 (60.5%) | 12.6 |
| Staphylococcus aureus | 37 (97.4%) | 28.7 |
| Moraxella catarrhalis | 6 (15.8%) | 30.0 |
| Streptococcus pneumonie | 9 (23.7%) | 31.1 |
| Streptococcus viridans | 35 (92.1%) | 37.7 |
| Neisseria sp. | 17 (44.7%) | 22.9 |
| Candida albicans | 4 (10.5%) | 17.5 |
| Stool | ||
| Escherichia coli | 33 (86.8%) | 53.2 |
| Enterococcus sp. | 16 (42.1%) | 16.2 |
| Citrobacter sp. | 9 (23.7%) | 29.4 |
| Candida albicans | 11 (28.9%) | 31.8 |
| Morganella morganii | 6 (15.8%) | 23.3 |
| Proteus sp. | 10 (26.3%) | 30.5 |
| Pseudomonas aeruginosa | 6 (15.8%) | 15.0 |
| Klepsiella sp. | 4 (10.5%) | 20.0 |
| Alcaligenes faecalis | 1 (2.6%) | 20.0 |
| Enterobacter sp. | 19 (50%) | 29.7 |
| Providencia stuartii | 1 (2.6%) | 30.0 |
| Diphtheroids | 1 (2.6%) | 20.0 |
| Streptococcus sp. | 2 (5.3%) | 15.0 |
| Serratia sp. | 2 (5.3%) | 15.0 |
| Bacillus sp. | 1 (2.6%) | 10.0 |
| Providencia rettgeri | 1 (2.6%) | 30.0 |
| Throat + stool | ||
| Candida albicans | 13 (34.2%) | 32.3 |
N (%) = count (percent).
Microbiological analysis of stool specimens detected 16 microorganisms. The most abundant are Escherichia coli and Enterococcus sp. 86.8% and 42.1%, respectively. Klepsiela, Pseudomonas aeryginosa, Morganela morganini, Citrobacter, Proteus sp, C albicans are present in 10% to 30%. Other microorganisms are present in less than 10% (Table 1). Mean values of detected microorganisms are shown in the right column of Table 1.
The dependence of glucose AUC (area under curve) on the type of MO present in the sample is shown in Table 2. The results show that certain MO does not influence glucose AUC. The values obtained for a positive and negative group of patients are very similar and there is no statistical significance between them. This applies to both types of samples, throat swabs and stool specimens (Table 2).
Table 2.
Glucose AUC in patients positive or negative for a specific type of MO (No and Yes groups). No statistically significant differences were detected (P > .05).
| NO | YES | P value | |||
|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | ||
| Throat | |||||
| Diphtheroids | 15 | 30.9 ± 5.6 | 23 | 29.6 ± 5.8 | .751 |
| Staphylococcus aureus | 1 | 27.7 | 37 | 30.2 ± 5.8 | |
| Moraxella catarrhalis | 32 | 30.1 ± 6.1 | 6 | 30.7 ± 2.5 | .958 |
| Streptococcus pneumonie | 29 | 30.4 ± 5.3 | 9 | 29.5 ± 7.2 | .539 |
| Streptococcus viridans | 3 | 24.7 ± 6.4 | 35 | 30.6 ± 5.5 | |
| Neisseriasp. | 21 | 30.8 ± 5.4 | 17 | 29.5 ± 6.1 | .596 |
| Candida albicans | 34 | 29.8 ± 5.5 | 4 | 33.4 ± 7.3 | .391 |
| Stool | |||||
| Escherichia coli | 5 | 32.8 ± 6.1 | 33 | 29.8 ± 5.6 | .641 |
| Enterococcus sp. | 22 | 30.2 ± 6.1 | 16 | 30.1 ± 5.2 | .971 |
| Citrobacter sp. | 29 | 29.7 ± 6.2 | 9 | 31.6 ± 3.3 | .641 |
| Candida albicans | 27 | 31.1 ± 4.8 | 11 | 27.8 ± 7.2 | .114 |
| Morganella morganii | 32 | 30.1 ± 5.7 | 6 | 30.9 ± 6.0 | .744 |
| Proteus sp. | 28 | 29.6 ± 6.2 | 10 | 31.8 ± 4.0 | .354 |
| Pseudomonas aeruginosa | 32 | 30.4 ± 5.2 | 6 | 29.1 ± 8.5 | .541 |
| Klepsiella sp. | 34 | 30.4 ± 5.7 | 4 | 28.1 ± 6.4 | .569 |
| Alcaligenes faecalis | 37 | 30.1 ± 5.8 | 1 | 34.4 | |
| Enterobacter sp. | 19 | 30.3 ± 5.5 | 19 | 30.1 ± 6.1 | .846 |
| Providencia stuartii | 37 | 30.3 ± 5.7 | 1 | 24.7 | |
| Diphtheroids | 37 | 29.9 ± 5.6 | 1 | 38.7 | |
| Streptococcus sp. | 37 | 30.1 ± 5.8 | 1 | 33.2 | |
| Serratia sp. | 36 | 30.2 ± 5.8 | 2 | 29.8 ± 3.8 | |
| Bacillus sp. | 36 | 30.5 ± 5.6 | 2 | 23.7 ± 5.8 | |
| Providencia rettgeri | 37 | 30.3 ± 5.7 | 1 | 24.6 | |
| Throat + stool | |||||
| Candida albicans | 25 | 31.4 ± 4.8 | 13 | 27.8 ± 6.6 | .069 |
AUC = area under curve, Mean = arithmetic mean, MO = microorganisms, N = count, SD = standard deviation.
Results for the influence of MO on insulin AUC are given in Table 3. A comparison of positive and negative groups of patients (presence or absence of certain types of MO, respectively) showed that patients with Diphtheroids in the throat had a lower total insulin secretion (194.5: 332.4) and the difference was statistically significant (P ≥ .030). Patients with Candida in the stool had increased insulin secretion compared to negative patients (374.1: 199) and an increase was statistically significant (P = .038).
Table 3.
Insulin AUC in patients positive or negative for a specific type of MO (No and Yes groups). Statistically significant differences were detected in patients positive for Diphtheroids in the throat, Candida albicans, and Proteus sp in the stool, and in patients positive for Candida albicans in both (throat + stool) samples (P ≤ .05).
| NO | YES | P value | |||
|---|---|---|---|---|---|
| N | Med (25–75th perc.) | N | Med (25–75th perc.) | ||
| Throat | |||||
| Diphtheroids | 15 | 332.4 (179.8–514.3) | 23 | 194.5 (142.8–361.7) | .030 |
| Staphylococcus aureus | 1 | 374.1 | 37 | 199.8 (153.9–390.4) | – |
| Moraxella catarrhalis | 32 | 214.475 (164.05–384.8) | 6 | 182.575 (145.9–390.4) | .598 |
| Streptococcus pneumonie | 29 | 199.4 (145.9–366.5) | 9 | 305.5 (157.7–395.5) | .379 |
| Streptococcus viridans | 3 | 194.5 (153.95–305.5) | 35 | 211.2 (145.9–395.5) | .644 |
| Neisseria sp. | 21 | 211.2 (153.95–366.5) | 17 | 199.4 (170.4–437.4) | .908 |
| Candida albicans | 34 | 199.4 (145.9–390.4) | 4 | 327.2 (260.7–494.8) | .216 |
| Stool | |||||
| Escherichia coli | 5 | 374.1 (153.95–657.1) | 33 | 199.85 (157.7–366.5) | .331 |
| Enterococcus sp. | 22 | 267.3 (170.4–390.4) | 16 | 196.95 (128–363.975) | .271 |
| Citrobacter sp. | 29 | 211.2 (170.4–395.5) | 9 | 177.9 (142.9–374.1) | .543 |
| Candida albicans | 27 | 199.0 (142.9–361.7) | 11 | 374.1 (194.5–555.4) | .038 |
| Morganella morganii | 32 | 205.525 (155.825–382.25) | 6 | 246.175 (142.9–437.4) | .953 |
| Proteus sp. | 28 | 290 (174.15–404.7) | 10 | 166.9 (122.55–211.2) | .037 |
| Pseudomonas aeruginosa | 32 | 242.85 (155.825–392.95) | 6 | 180.075 (142.8–229.1) | .493 |
| Klepsiella sp. | 34 | 220.15 (170.4–395.5) | 4 | 149.925 (129.95–257.825) | .167 |
| Alcaligenes faecalis | 37 | 211.2 (157.7–390.4) | 1 | 142.9 (142.9–142.9) | – |
| Enterobacter sp. | 19 | 199.85 (142.9–332.45) | 19 | 321.95 (153.95472.2) | .311 |
| Providencia stuartii | 37 | 211.2 (157.7–390.4) | 1 | 142.8 (142.8–142.8) | – |
| Diphtheroids | 37 | 199.85 (153.95–374.1) | 1 | 437.4 (437.4–437.4) | – |
| Streptococcus sp. | 37 | 199.85 (153.95–374.1) | 1 | 413.9 (413.9–413.9) | – |
| Serratia sp. | 36 | 220.15 (155.825–392.95) | 2 | 172.875 (145.9–199.85) | .518 |
| Bacillus sp. | 36 | 205.525 (155.825–392.95) | 2 | 244.05 (114–374.1) | .640 |
| Providencia rettgeri | 37 | 199.85 (153.95–374.1) | 1 | 472.2 (472.2–472.2) | – |
| Throat + stool | |||||
| Candida albicans | 25 | 180.3 (142.90–361.70) | 13 | 332.45 (199.40–514.35) | .022 |
AUC = area under curve, Med = median, MO = microorganisms, N = count, Perc = percentile.
Analysis of cumulative results (both throat + stool samples) showed that patients with Candida in both samples had an increased AUC compared to the negative group (332: 180.3) and the difference was statistically significant P = .022. For other detected MO, the differences between the two groups were not statistically significant (P ≥ .05).
This study included the influence of MO on BMI (Table 4). Patients were divided into two groups. The first group consists of 12 subjects with a BMI < 30 (overweight patients) and the second group of 26 subjects with a BMI > 30 (obese patients). A comparison of both groups regarding the type of MO present in the throat or stool, showed no statistically significant differences, P > .05.
Table 4.
Comparison of the percentage of certain microorganisms in overweight patients (BMI < 30) and obese patients (BMI > 30). No statistical difference present (P > .05).
| BMI | P value | ||
|---|---|---|---|
| <30 [N (%)] | 30 + [N (%)] | ||
| Throat | |||
| Diphtheroids | 9 (75.0%) | 14 (53.8%) | .294 |
| Staphylococcus aureus | 12 (100%) | 25 (96.2%) | 1.000 |
| Moraxella catarrhalis | 0 | 6 (23.1%) | .179 |
| Streptococcus pneumonie | 2 (16.7%) | 7 (26.9%) | .689 |
| Streptococcus viridans | 12 (100%) | 23 (88.5%) | .538 |
| Neisseriasp. | 5 (41.7%) | 12 (46.2%) | 1.000 |
| Candida albicans | 2 (16.7%) | 2 (7.7%) | .577 |
| Stool | |||
| Escherichia coli | 11 (91.7%) | 22 (84.6%) | 1.000 |
| Enterococcussp. | 5 (41.7%) | 11 (42.3%) | 1.000 |
| Citrobactersp. | 2 (16.7%) | 7 (26.9%) | .689 |
| Candida albicans | 1 (8.3%) | 10 (38.5%) | .121 |
| Morganella morganii | 2 (16.7%) | 4 (15.4%) | 1.000 |
| Proteussp. | 4 (33.3%) | 6 (23.1%) | .694 |
| Pseudomonas aeruginosa | 3 (25.0%) | 3 (11.5%) | .357 |
| Klepsiellasp. | 1 (8.3%) | 3 (11.5%) | 1.000 |
| Alcaligenes faecalis | 1 (8.3%) | 0 | .316 |
| Enterobactersp. | 3 (25.0%) | 16 (61.5%) | .079 |
| Providencia stuartii | 0 | 1 (3.8%) | 1.000 |
| Diphtheroids | 0 | 1 (3.8%) | 1.000 |
| Streptococcussp. | 0 | 1 (3.8%) | 1.000 |
| Serratiasp. | 1 (8.3%) | 1 (3.8%) | .538 |
| Bacillussp. | 0 | 2 (7.7%) | 1.000 |
| Providencia rettgeri | 0 | 1 (3.8%) | 1.000 |
| Throat + stool | |||
| Candida albicans | 2 (16.7%) | 11 (42.3%) | .158 |
BMI = body mass index.
However, Candida was present in a higher percentage in the stool samples of obese than in overweight patients, 38.5% and 8.3% respectively. Similarly, Enterobacter was more present in the second (61.5%) than in the first group (25%). No statistically significant difference was likely detected due to the small number of participants (P = .121, P = .079).
4. Discussion
Previous in vitro studies in pancreatic islet culture have shown that Pseudomonas aeruginosa, Enterobacter sp, and S aureus decrease insulin secretion while C albicans increases insulin secretion, so this section will pay particular attention to these microorganisms and their effects in the studied patients. A new microorganism, Diphtheroids has also appeared in the throat that has not been detected in the pancreas and pancreatic islets culture.
Diphtheroids found in the patient’s throat probably represent a milder form of infection causing a mild inflammatory reaction. After S aureus (97.4%) and Streptococcus alpha hemolyticus (92%), they are the third most abundant bacteria by 60% (Table 1). Subjects positive for Diphtheroids showed no signs of severe infections and were not treated with any antibiotic therapy. Diphtheroids belong to the phylum actinobacteria. They live in commensal relationships with both humans and animals. Corynebacterium is part of the human saliva microbiome.[15] Corynebacterium species is widespread, it is found in soil, water, plants, and what is important for us in food products. Non-diphtheroid species can occur in the mucous membranes and skin flora of humans and animals.[16,17] In clinical practice, attention is generally paid to infectious strains. Some species of Corynebacterium are used for industrial production, for instance, Corynebacterium glutamicum is used for the production of amino acids, glutamic acid, and lysine, which are used in food and pharmaceutical products. The most significant pathogen of Coryneform bacteria is Corynebacterium diphtheriae, the primary cause of diphtheria. It is an acute and contagious infection characterized by pseudomembranes of dead epithelial cells, white blood cells, red blood cells, and fibrin formed around the tonsils and the back of the throat.[18] The literature often states that this phenomenon is caused by debilitated host immunity, which is not true. Shifting from commensal to a pathogen form can be a consequence of the disrupted relationship between the microorganisms and the increased number of some MOs at the expense of others. Or it is a dislocation of certain strains to places where they express their pathogenicity. In this study, only the genus (Corynobacterium), but no particular species were determined. Nonpathogenic species of Corynebacteria such as Corynebacteria glutamycym produce glutamic acid, which is, as monosodium-glutamate, used in the production of yogurt and soy sauce.[19] How complex the relationship between bacteria and their struggle for dominance in the mucosa is shown by the fact that some species produce metabolites similar to antibiotics: bacteriocins such as corynecin-linocins antitumor agents.[20,21] Glutamate as a derivative of glutamic acid has significant participation in the body’s metabolism and is also known as a neurotransmitter. In animal studies, the addition of glutamic acid dimethyl ester mainly enhances insulin release at an intermediate glucose concentration in the rat pancreas.[22] L-glutamine alone failed to stimulate proinsulin biosynthesis or insulin release in rat pancreatic islets.[23] Subjects positive for Diphtheroids in the throat had a statistically significant, lower total insulin secretion, 194.5: 332.4 (Table 3). However, in the literature, there is no evidence that diphtheroid’s metabolites may affect the reduction of insulin secretion in human research models. No diphtheroid infections have been reported in patients with diabetes.[24] It was also observed that with increasing body weight, the percentage of diphtheroid in the throat decreases (75:53%) (Table 4). The glucose AUC analysis showed no differences between Diphtheroid positive and negative groups (Table 2).
In our study, Candida was detected in the throat and stool of 10% and 30% of the patients, respectively (Table 1). In Table 3 there are two statistically significant results: patients who had stool candidiasis had increased insulin secretion compared to negative patients (374.1: 199). Regarding cumulative results (stool + throat samples), patients positive for Candida in both samples had increased insulin AUC compared to the negative group (332: 180.3) and the difference was statistically significant P = .022. For other detected MOs, the differences were not statistically significant. In our previously published studies, it was established that C albicans increases insulin secretion up to seven times in human pancreatic islet culture.[8] In the literature, there are data on the possible influence of Candida on the host’s glucose homeostasis in systemic infections. Studies on mice have shown that Candida uses pharmacological or genetic agents to affect glucose metabolism and disrupt the host’s glucose homeostasis. Candida depletes glucose leading to the rapid death of macrophages.[25] In this particular manuscript, there is an interesting fact. Metformin was administered to healthy mice, but they didn’t have low blood glucose even after long-term administration. However, in the case of infection with small doses of C albicans, the same animals had severe hypoglycemia even on the first day of infection. Our research has confirmed that Candida increases insulin secretion provoking decreasing in blood glucose levels. Low glucose level enables Candida the transition from yeast to hyphal cells, one of the key factors in the virulence and spreading of the disease.[26] However, increased insulin secretion leads to hypoglycemia, which causes hunger and forces the body to take food and sugar. This maintains a nutrient medium that allows the reproduction of Candida and its superiority over other MOs in competition for the same ecological niche, intestinal mucosa. The genus Candida includes more than 350 species present in humans and other mammals, birds, fish, insects, arthropods, animal waste, plants, and substrates naturally rich in sugars (e.g. honey, nectar, grapes, fermentation, and dairy products), fresh and sea water and airborne particles.[27] C albicans is a commensal and a constituent of the normal microflora in 80% of the human population, and predominately colonizes the mucosal surface of the gastrointestinal tract, genitourinary tract, and, to a lesser extent, the skin.[28,29] However, especially in immunocompromised patients (e.g., cancer chemotherapy, AIDS, organ transplantation, or neonates) or when the competing flora are eliminated (e.g., after antibiotic treatment), C albicans becomes an opportunistic pathogen that can cause superficial as well as systemic and potentially life-threatening infections.[30,31] During the mucosal invasion, cells of C albicans induce glycolytic pathways, tricarboxylic acid cycle, and beta oxidative fatty acid gene. C albicans is highly adapted to different environmental conditions thanks to the SAP gene family, with 10 members encoding aspartic proteinases in response to the available nitrogen and carbon source.[32–34] The proposed mechanism of infection includes digestion of host proteins for nutrient supply and degradation of antibodies and complement components/[35] Candida has developed several mechanisms that enable successful colonization and evasion of host immune response. This leads to the question of whether the presence of Candida in the food is desirable or not. Consumption of sugar-rich food means intake of Candida too. Does the presence of Candida help degrade sugar, thus contributing to metabolism or it is considered just a pathogenic agent? Why it is advisable to eat raw fruits and tubers during the diet? By consuming organically grown food, microbiomes are also introduced which help the breakdown of that food in the gastrointestinal system. A similar process occurs with the natural rot of fruits and vegetables in nature.[36] In our study, the influence of MO on BMI was examined. Patients were divided into two groups. The first group consisted of patients with BMI < 30 (12 overweight patients), and the second group was patients with BMI > 30 (26 obese patients). A comparison of both groups regarding the presence of certain MO in the throat or the stool, showed no statistically significant differences, P > .05. Analysis of results showed that obese patients had a higher percentage of Candida in stool samples compared to overweight patients, 38.5% and 8.3% respectively. This indicates that the percentage of Candida in an intestinal system increases with increased BMI.
S aureus was present in 97% of throat swab samples. S aureus is a Gram-positive, anaerobe bacterium, member of Firmicutes. This bacteria is usually a commensal of human microbiota often present in the upper respiratory tract and on the skin. It can also become an extracellular opportunistic pathogen, causing localized infections like soft tissue abscesses, but also life-threatening systemic diseases such as infective endocarditis, and meningitis. Pathogenic strains produce exotoxins that damage tissue and protect bacteria from the host’s immune response.[37,38] Previous studies showed that Staphylococcus reduces insulin secretion in cell culture. However, in the body, the relationship between bacteria and hosts is much more complex. It is interesting that S aureus, exhibits polymorphism in the glucocorticoid receptor gene, resulting in increased corticosteroid production.[39] Corticosteroids have a major effect on the metabolism of the human body. They promote lipolysis and protein catabolism. Glucocorticoids cause a decrease in peripheral glucose uptake and utilization and at the same time stimulate the process of gluconeogenesis in the liver, that is the process of glucose synthesis from non-carbohydrate components, glycerol, and amino acids formed during lipolysis and protein catabolism. Due to this effect of glucocorticoids on carbohydrate metabolism, there is an increase in blood glucose levels. Hyperglycemia leads to increased insulin secretion. Table 3 showed that patients positive for Staphylococcus in stool samples (199: 374) had reduced insulin secretion compared to negative patients. These results match the results of the glucose stimulation test, the counterpart for OGTT, in pancreatic islet cell culture,[7] where these bacteria also caused reduced insulin secretion. Cholesterol-lowering therapy may reduce the pathogenicity of Staphylococcus, due to similarities in the pathways for staphyloxanthin and human cholesterol biosynthesis.[40] These results are obtained from studies on mice and we don’t know how this will reflect in humans. This type of therapy was not monitored in the examined patients and taken into account.
Enterobacter sp was detected in 50% of stool samples (Table 1). A comparison of patients positive or negative for Enterobacter (Table 3), showed that bacteria increases insulin secretion (321: 199), while it decreases in cell culture.[7] There are two reasons why. First, there was a small number of examined patients. Second, there are other bacteria present in the microflora that, by their synergistic action, can also change insulin secretion, while in cell culture is present only one type of infection. If Enterobacter penetrates and infects the pancreas, it causes a decreased insulin secretion. However, if it overgrows in the intestinal system, it causes increased insulin secretion. In both cases, Enterobacter contributes to the development of insulin resistance. Enterobacter is a genus of gram-negative, facultatively anaerobic, rod-shaped bacteria that are widely distributed in the environment and is a part of the normal flora of the gastrointestinal tract in 40% to 80% of people. Like most members of the Enterobacteriaceae, these organisms are capable of causing opportunistic infection in hospitalized or weakened patients.[41] The urinary and respiratory tract are frequent targets of infection. The genus Enterobacter is a member of the coliform group of bacteria. A recent study suggests that bacteria can contribute to the development of obesity in humans via endotoxin and inflammation. The authors demonstrated that bacteria isolated from morbidly obese patients induce obesity and insulin resistance in germ-free mice.[42] When the abundance of bacteria decreases from 35% in the patient’s gut to non-detectable, weight loss occurs. Strain Enterobacter cloacae B29, isolated from the patients, induced obesity and insulin resistance in C57BL/6J-free mice fed a high-fat diet. These data are consistent with our results since with weight gain the percentage of bacteria in the intestinal system increases (Table 4). Enterobacter was more prevalent in obese patients than in the overweight group, 61.5% and 25% respectively. Due to the small number of analyzed patients, the difference was not statistically significant (P = .079).
P aeruginosa was found in 15.8% of stool samples. P aeruginosa is a species of great medical importance, it is widespread and is found in soil, water, and on the surface of the skin in humans as well as in residential areas. It can cause sepsis in people with reduced immunity. Lung, kidney, and urinary tract infections can be potentially life-threatening.[43] Due to the lack of oxygen from the substrate, P aeruginosa uses nitrates and nitrites, and if there are none, it is capable of fermenting arginine and pyruvate by phosphorylation.[44] P aeruginosa creates membrane vesicles (MV) that are released into the culture medium during normal growth. Their release increases approximately threefold after exposure to four times the minimum inhibitory concentration (MIC) of gentamicin. In addition to LPS, several other enzymes (e.g. phospholipase C, protease, hemolysin, and alkaline phosphatase) contribute to the pathogenicity of pseudomonas infections. These vesicles could play an important role in genetic transformation by serving as a transport vehicle for DNA and virulence factors and are likely to be involved in septic shock.[45] Type of diet influence the development of certain microorganisms and their transition from commensal to pathogen form (instant disruption of the homeostatic microbiome). Low levels of phosphate in the human intestinal system activate the transition from benign symbiont to a pathogen and deadly toxins are released in the intestine that can be fatal for the host. This can be alleviated by providing excess phosphate instead of antibiotics.[46] Patients positive for Pseudomonas had a lower insulin secretion than the negative group, 180 and 242, 8 respectively (Table 3). These results confirm previously published data,[7] Pseudomonas in pancreatic islet cell culture reduces insulin secretion. BMI analysis shows when body weight increases, the percentage of Pseudomonas in the intestinal tract of patients decreases (25: 11.5%).
5. Conclusion
We examined the direct influence of microorganisms on insulin secretion in cell culture (in vitro) which is parallel to the direct infection of the pancreas in vivo and we concluded the following:
There is an increased number of microorganisms on intestinal mucosa due to the disrupted HM. They secrete their metabolites into the blood, thus affecting the host’s overall metabolism and causing insulin secretion disorder, insulin resistance, and obesity, prerequisites for the development of diabetes.
Diphtheroids were present in 60% of throat swab samples. Our study showed that patients having Diphtheroids in the throat had lower total insulin secretion. It was also found that with increasing body weight, the percentage of diphtheroid in the throat decreases.
The analysis of throat swabs revealed a high percentage of S aureus as much as 97%. Staphylococcus reduces insulin secretion, but its percentage in the throat does not change with the change in the patient’s body weight.
Enterobacter was detected in 50% of stool samples. A comparison of groups of patients positive and negative for Enterobacter revealed that bacteria cause increased insulin secretion, while in cell culture they cause decreased insulin secretion.[7] The main reasons are a small number of examined patients and other bacteria present in the microflora that, by their synergistic action, can also change insulin secretion, while in cell culture was only one type of infection. If Enterobacter penetrates and infects the pancreas it will cause a decrease in insulin secretion. However, if it overgrows in the intestinal system it causes an increase in insulin secretion. In both cases, Enterobacter contributes to the development of insulin resistance.
Pseudomonas aeruginosis was detected in 15.8% of stool samples. Patients positive for Pseudomonas had lower insulin secretion compared to the negative group. These results confirm previously published data where Pseudomonas reduces insulin secretion in cell culture.[7] Analysis of BMI data revealed that an increase in body weight reduces the percentage of Pseudomonas in the intestinal tract.
The hypothesis that C albicans can disrupt insulin secretion, thus leading to diabetes,[6,8] has been confirmed. Namely, in the group of examined subjects, Candida was present in 10% of the throat samples and 30% of stool samples. If Candida overgrows in the human intestinal system, these patients will undoubtedly have increased insulin secretion during OGTT. This condition undoubtedly leads to insulin resistance. Cumulative results (throat + stool samples) confirm the synergistic effect of Candida. Multivariate statistical analyzes of the influence of Candida on insulin secretion (insulin AUC), glucose status (glucose AUC), and the percentage of Candida concerning the patient’s body weight showed that:
Patients with Candida in the stool had increased insulin secretion (insulin AUC) compared to negative patients (374.1: 199) and an increase was statistically significant (P = .038).
Cumulative results (throat + stool samples) showed that patients with Candida in both samples had higher insulin AUC compared to the negative group (332: 180.3) and the difference was statistically significant P = .022.
Obese patients had a higher percentage of Candida in stool samples compared to overweight patients, 38.5% and 8.3% respectively. This indicates that the percentage of Candida present in the intestinal system significantly increases with increasing BMI.
It was determined that C albicans has direct and indirect effects on insulin secretion and should be seriously taken into account as one of the possible factors involved in the pathogenesis of diabetes.
Author contributions
DN, II, VDS, and LR collected clinical data. IS, MS, and DG performed the statistical analysis. All authors interpreted the data. DN drafted the first version of the manuscript. VDS, II, LR, IS, MS, and DG critically reviewed the manuscript. All authors have read and approved the final version of the manuscript.
Conceptualization: Dragan M. Nikolic.
Data curation: Dragan M. Nikolic, Vesna Dimitrijevic-Sreckovic, Lazar T. Ranin, Iva D. Ilic, Ivan A. Soldatovic.
Formal analysis: Lazar T. Ranin, Iva D. Ilic.
Investigation: Dragan M. Nikolic, Drasko M. Gostiljac.
Methodology: Dragan M. Nikolic.
Software: Milos M. Stojanovic, Ivan A. Soldatovic.
Validation: Dragan M. Nikolic, Vesna Dimitrijevic-Sreckovic, Milos M. Stojanovic, Iva D. Ilic, Drasko M. Gostiljac, Ivan A. Soldatovic.
Writing – original draft: Dragan M. Nikolic.
Abbreviations:
- AUC =
- area under curve
- BMI =
- body mass index
- HM =
- homeostatic microbiome
- MO =
- microorganisms
- OGTT =
- oral glucose tolerance test
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (grant No. 200110). There is no conflict of interest between the mentioned institution that financed this research and the author.
The authors have no conflicts of interest to disclose.
How to cite this article: Nikolic DM, Dimitrijevic-Sreckovic V, Ranin LT, Stojanovic MM, Ilic ID, Gostiljac DM, Soldatovic IA. Homeostatic microbiome disruption as a cause of insulin secretion disorders. Candida albicans, a new factor in pathogenesis of diabetes: A STROBE compliant cross-sectional study. Medicine 2022;101:45(e31291).
Contributor Information
Vesna Dimitrijevic-Sreckovic, Email: vesnadsendo@gmail.com.
Lazar T. Ranin, Email: lazarr@verat.net.
Milos M. Stojanovic, Email: specmedico@gmail.com.
Iva D. Ilic, Email: ivailic1996@gmail.com.
Drasko M. Gostiljac, Email: doctor@med.bg.ac.rs.
Ivan A. Soldatovic, Email: soldatovic.ivan@gmail.com.
References
- [1].Bloomgarden T. Highlights from The First World Congress on the insulin resistance syndrome. Diab Mellit Care. 2004;27:602–9. [DOI] [PubMed] [Google Scholar]
- [2].Fletcher B, Gulanick M, Lamendola C. Risk factors for type 2 diabetes mellitus. J Cardiovasc Nurs. 2002;16:17–23. [DOI] [PubMed] [Google Scholar]
- [3].Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. [DOI] [PubMed] [Google Scholar]
- [4].Nesto RW. The relation of insulin resistance syndromes to risk of cardiovascular disease. Rev Cardiovasc Med. 2003;4:11–8. [PubMed] [Google Scholar]
- [5].Scheen AJ. Pathophysiology of type 2 diabetes mellitus. Acta Clin Belg. 2003;58:335–41. [DOI] [PubMed] [Google Scholar]
- [6].Nikolic DM. Diabetes mellitus and obesity as a result of a disrupted homeostatic microbiome. New data on aetiopathogenesis of diabetes mellitus. Milit Med Pharm Rev. 2018;75:1110–7. [Google Scholar]
- [7].Nikolic DM. Effects of bacterial infection on insulin secretory capacity of human adult pancreatic islets. Br J Biomed Sci. 2011;68:181–4. [DOI] [PubMed] [Google Scholar]
- [8].Nikolic DM. Effects of Candida on insulin secretion of human adult pancreatic islets and possible onset of diabetes mellitus. Br J Biomed Sci. 2014;71:73–8. [DOI] [PubMed] [Google Scholar]
- [9].Mindikoglu AL, Abdulsada MM, Jain A, et al. Intermittent fasting from dawn to sunset for four consecutive weeks induces anticancer serum proteome response and improves metabolic syndrome. Sci Rep. 2020;10:18341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].National Cholesterol Education Program (NCEP). Adult treatment panel (ATP) III. JAMA. 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- [11].American Diabetes Association. Relationship between CVD risk and metabolic syndrome. Diabetes Care. 2005;28:2289–304. [DOI] [PubMed] [Google Scholar]
- [12].Wondmkun Y. Obesity, insulin resistance, and type 2 diabetes: associations and therapeutic implications. Diabetes Metab Syndr Obes. 2020;13:3611–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].González-Salazar LE, Pichardo-Ontiveros E, Palacios-Gonzáález B, et al. Effect of the intake of dietary protein on insulin resistance in subject with obesity: a randomized controlled clinical trial. Eur J Nutr. 2020. [DOI] [PubMed] [Google Scholar]
- [14].Da Silva RC, Nayak N, Caymo AM, et al. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol Rep. 2020;8:e14607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wang K, Lu W, Tu Q, et al. “Preliminary analysis of salivary microbiome and their potential roles in oral lichen planus” Sci Rep. 2016;6:22943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Collins MD. “Corynebacterium caspium sp. nov., from a Caspian seal (Phoca caspica). Int J Syst Evol Microbiol. 2004;54:925–8. [DOI] [PubMed] [Google Scholar]
- [17].Yassin AF. “Corynebacterium glaucum sp. nov” Int J Syst Evol Microbiol. 2003;53:705–9. [DOI] [PubMed] [Google Scholar]
- [18].“Difteria: MedlinePlus enciclopedia médica.” Available at: www.nlm.nih.gov.
- [19].Date M, Itaya H, Matsui H, et al. “Secretion of human epidermal growth factor by Corynebacterium glutamicum” Lett Appl Microbiol. 2006;42:66–70. [DOI] [PubMed] [Google Scholar]
- [20].Suzuki T, Honda H, Katsumata. “Production of antibacterial compounds analogous to chloramphenicol by a n-Paraffin-grown Bacterium” Agric Biol Chem. 1972;36:2223–8. [Google Scholar]
- [21].Milas L, Scott MT. “Antitumor activity of Corynebacterium Parvum.” In Ford, Marvella E.; Watson, Dennis K. (eds.). Adv Cancer Res. 1978;26:257–306. [DOI] [PubMed] [Google Scholar]
- [22].Pierre M, Asllan G, Claes BW. Implication of glutamate in the kinetics of insulin secretion in rat and mouse perfused pancreas. Diabetes. 2002;51:99–102. [DOI] [PubMed] [Google Scholar]
- [23].Sener A, Somers G, Devis G, et al. The stimulus-secretion coupling of amino acid-induced insulin release biosynthetic and secretory responses of rat pancreatic islet to L-Leucine and L-Glutamine. Diabetologia. 1981;21:135–42. [DOI] [PubMed] [Google Scholar]
- [24].Janine C, Cresio A. Infections in patients with diabetes mellitus: a review of pathogenesis. Indian J Endocrinol Metab. 2012;16:27–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Timothy MT, Jiyoti VP, Harison SF, et al. Glucose homeostasis is important for immune cell viability during candida challenge and host survival of systemic fungal infection. Cell Metab. 2018;27:988–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uppuluri P, Chaturved AK, Srinivasan A, et al. Dispersion as an important step in the Candida albicans biofilm developmental cycle. PLoS Pathog. 2010;6:e1000828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nunn MA, Schaefer SM, Petrou MA, et al. Environmentalsource of Candida dubliniensis. Emerg Infect Dis. 2007;13:747–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Staib P, Kretschmar M, Nichterlein T, et al. Differential activation of a Candida albicans virulence gene familyduring infection. Proc Natl Acad Sci USA. 2000;97:6102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Berman J, Sudbery PE. Candida albicans: a molecular revolutionbuilt on lessons from budding yeast. Nat Rev Genet. 2002;3:918–30. [DOI] [PubMed] [Google Scholar]
- [30].Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kaur R, Dhakad MS, Goyal R, et al. Spectrum of opportunistic fungal infections in HIV/AIDS patients in Tertiary Care Hospital in India. Can J Infect Dis Med Microbiol. 2016;2016:2373424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zakikhany K. In vivo transcript profiling of Candida albicans identifies a gene essential for interepithelial dissemination. Cell Microbiol. 2007;9:2938–54. [DOI] [PubMed] [Google Scholar]
- [33].Wilson D. Identifying infection-associated genes of Candida albicans in the postgenomic era. FEMS Yeast Res. 2009;9:688–700. [DOI] [PubMed] [Google Scholar]
- [34].Hube B. Expression of seven members of the gene family encoding secretory aspartyl proteinases in Candida albicans. Mol Microbiol. 1994;14:87–99. [DOI] [PubMed] [Google Scholar]
- [35].Alistar JPB, Gordon DB, Mihai GN, et al. Metabolism impacts upon Candida immunogenicity and pathogenicity at multiple levels. Trends Microbiol. 2014;22:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Nikolic DM. Homeostatic microbiome (microbiota)—formation, maintenance and influence on human health. J Gastroenterol Hepatol Res. 2018;7:2729–33. Available at: http://www.ghrnet.org/index.php/joghr/article/view/2471. [Google Scholar]
- [37].Hoffman B. Williams gynecology (2nd ed.). New York: McGraw-Hill Medical, 2012. p. 65. [Google Scholar]
- [38].Lowy FD. Is Staphylococcus aureus an intracellular pathogen? Trends Microbiol. 2000;8:343–416. [DOI] [PubMed] [Google Scholar]
- [39].Fagerlund A, Granum PE, Håvarstein LS. “Staphylococcus aureus competence genes: mapping of the SigH, ComK1 and ComK2 regulons by transcriptome sequencing.” Mol Microbiol. 2014;94:557–79. [DOI] [PubMed] [Google Scholar]
- [40].Liu CI, Liu GY, Song Y, et al. “A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence” Science. 2008;319:1391–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yeo J, Lee YH, Jeon SM, et al. Supplementation of a novelmicrobial biopolymer, PGB1, from new Enterobactersp. BL-2delays the deterioration of type 2 diabetic mice. J Microbiol Biotechnol. 2007;17:1893–900. [PubMed] [Google Scholar]
- [42].Fei N, Zhao L. “An opportunistic pathogen isolated from the gut of an obese human causes obesity in germ-free mice” ISME J. 2012;7:880–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Balcht A, Smith R. Pseudomonas aeruginosa: infections and treatment. Informa Health Care. 1994:83–4. [Google Scholar]
- [44].Schobert M, Jahn D. “Anaerobic physiology of Pseudomonas aeruginosa in the cystic fibrosis lung”. Int J Med Microbiol. 2010;300:549–56. [DOI] [PubMed] [Google Scholar]
- [45].Kadurugamuwa J, Beveridge T. Virulence factors are released from Pseudomonas aeruginosa in association with membrane vesicles during normal growth and exposure to gentamicin: a novel mechanism of enzyme secretion. J Bacteriol. 1995;177:3998–4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].“Research could lead to new non-antibiotic drugs to counter hospital infections” (Press release). University of Chicago Medical Center. 2009-04-14. Available at: http://www.globalizationandhealth.com/content/1/1/14 [Access date January 18, 2010].