Rational:
Low-grade myofibroblastic sarcoma (LGMS) is an atypical type of tumor composed of myofibroblasts. LGMS in the femoral head neck junction is extremely rare and no case treated by hip arthroscopy was reported.
Patient concerns:
We reported a case of LGMS in the femoral head neck junction treated by hip arthroscopy. A 30-year-old female was admitted to our hospital with discomfort and pain after left hip sprained one year prior. Physical examination revealed swelling of the left hip and magnetic resonance images showed a soft tissue mass in the femoral head neck junction.
Diagnosis:
Via microscopy of pathological specimens, spindle cell proliferative lesions, atypia of some cells, and mitotic figures/pathological mitotic figures of some cells were observed. Immunohistochemistry revealed positive for smooth muscle actin, focally positive for CD34 and CD68, while negative for S-100, desmin, and anaplastic lymphoma kinase. The imaging, histomorphological and immunohistochemical features suggested a final diagnosis of LGMS of the proximal femur.
Interventions:
This patient underwent hip arthroscopy for excision of the soft tissue mass.
Outcomes:
The clinical and imaging follow-up at 6 months postoperatively showed that surgery had achieved good clinical outcomes.
Lessons:
To the best of our knowledge, this is the first case report of LGMS in the femoral head neck junction treated by hip arthroscopy. Beyond the present case, other 120 cases from 58 literatures (1998–2022) are reviewed and discussed. The age of LGMS patients ranged from 11 months to 77 years and the male-to-female ratio was approximately 1.28:1. The location distribution of previously reported LGMS cases and the present case was as follows: Head&neck (45.90%), trunk (30.33%), and extremity (23.77%). Hip arthroscopic excision of LGMS may achieve relatively good clinical outcomes.
Keywords: Low-grade myofibroblastic sarcoma, proximal femur, hip, arthroscopy
1. Introduction
Low-grade myofibroblastic sarcoma (LGMS) was firstly described by Mentzel et al[1], which represents an atypical and extremely rare type of tumor composed of myofibroblasts. It was first classified as a new group of soft tissue and bone tumors by the WHO in 2002, and this classification was maintained in 2020.[2] LGMS has been reported to occur in deep soft tissues with predilection for the head and neck.[3] However, according to previous study by Kim et al,[4] the incidence of LGMS in the extremities or trunk may be higher than expected. Based on the rarity of this condition, this study aims to introduce a case report of LGMS in proximal femur and to perform a review of relevant literature.
2. Case presentation
A 30-year-old female was admitted to our hospital with discomfort and pain after left hip sprained 1 year prior. The pain was progressive in nature. Physical examination revealed swelling of the left hip with tenderness in the greater trochanter, groin area, and posterior buttock. This patient had severe limited hip range of motion and had to walk on crutches. No significant personal or family history was present.
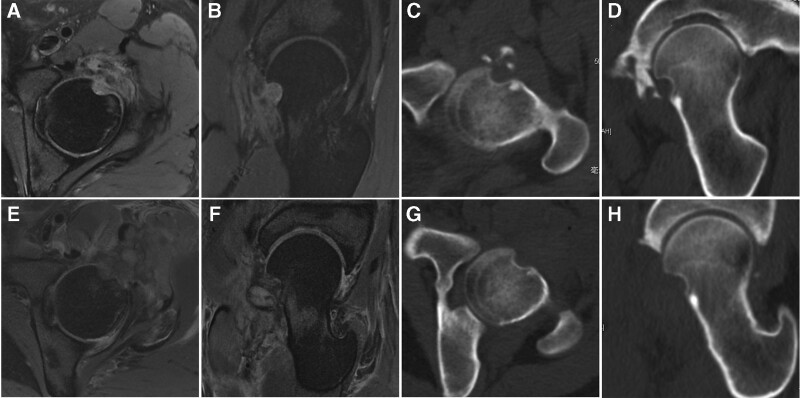
Magnetic resonance images (MRI) showed a soft tissue mass in the anterolateral femoral head neck junction in the left hip (Fig. 1A and B). The mass appeared heterogenously hyperintence in T2-weighted image. Edema of surrounding tissue and adjacent bone defect in femoral head neck junction were observed. Computed tomography (CT) showed ossification in adjacent acetabulum (Fig. 1C and D).
Figure 1.
(A, B) Preoperative axial and oblique sagittal MRI showed soft tissue mass in the femoral head neck junction. (C, D) Preoperative axial and oblique sagittal CT showed adjacent bone defect and ossification. (E, F) Postoperative axial and oblique sagittal MRI showed total excision of the mass. (G, H) Postoperative axial and oblique sagittal CT showed total excision of the mass. CT = computed tomography, MRI = magnetic resonance images.
This patient underwent hip arthroscopy for excision of the mass. Outside-in approach was used because of anterior ossification. The soft tissue mass, adjacent bone tissue, hyperplastic synovium, and ossification in the acetabulum were debrided. The range of motion returned normal after surgery, just like the contralateral hip. Two pathological specimens were taken during the operation, which were acetabulum ossification and soft tissue mass. The bony tissue of the left acetabulum contained part of dense connective tissue, in which a few proliferative spindle cells could be seen and the shape was mild. In the left hip soft tissue mass, spindle cell proliferative lesions were observed. Some cells showed atypia. Some cells had mitotic figures, and occasionally pathological mitotic figures. Tumor borders were difficult to identify. The soft tissue mass was sent for immunohistochemistry, which revealed positive for smooth muscle actin (SMA), focally positive for CD34 and CD68, while negative for S-100, Desmin, P53, nuclear β-catenin, ALK1, SOX10, MUC4, and STAT6.
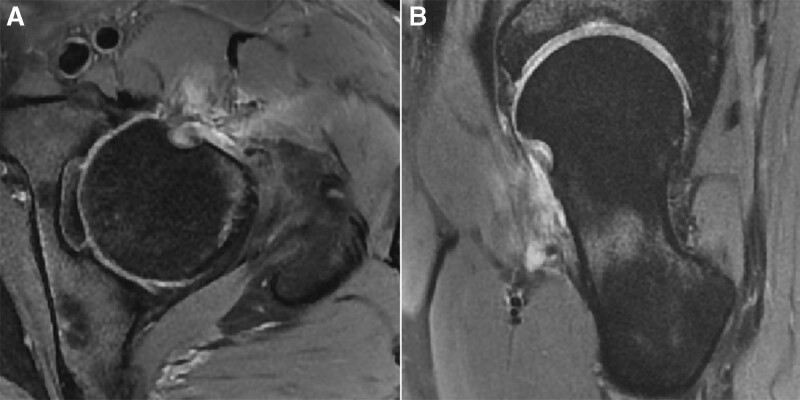
The imaging, histomorphological, and immunohistochemical features suggested a final diagnosis of LGMS of the proximal femur. Postoperative CT and MRI were conducted 1 day after surgery and revealed that the tumor was completely removed (Fig. 1E–H). Adjuvant chemotherapy or radiotherapy were not used for this patient. The patient underwent MRI follow-up 6 months after surgery and MRI showed no local recurrence (Fig. 2). Modified Harris Hip Score and visual analog scale for pain improved from 60 and 5 preoperatively to 78 and 1 postoperatively 6 months after surgery.
Figure 2.
(A, B) Axial and oblique sagittal MRI 6 months after surgery showed no local recurrence. MRI = magnetic resonance image.
The patient had provided informed consent for publication of the case, and the study protocol was approved by Medical Ethics Committee of Peking University Third Hospital.
3. Discussion
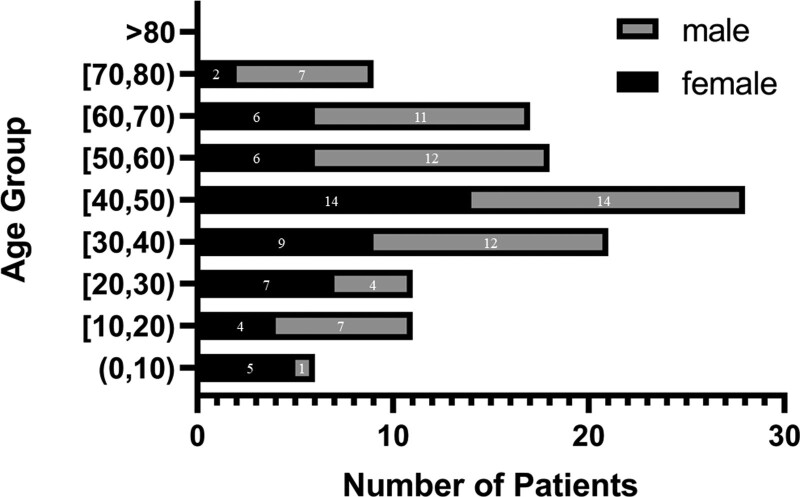
Two electronic databases were searched: PubMed (1966-), Web of Science (1900-), with the key words “low-grade myofibroblastic sarcoma” and its synonyms. Non-English literature and inaccessible literature were excluded. We analyzed 120 cases from 58 literatures. With this present case, we have a basic understanding for the information and characteristics of the LGMS patient population (Table 1). Among the 121 reported LGMS patients, the age of them ranged from 11 months to 77 years (mean, 42.45; median, 44). The male-to-female ratio was approximately 1.28:1 (68 males versus 53 females). The detailed age and gender distribution are shown in Figure 3.
Table 1.
Details of included articles: information and study sample characteristics.
| Authors | No. of cases | Age mean | Sex (M/F) | Site | Treatment | Clinical outcome |
|---|---|---|---|---|---|---|
| Mentzel et al.[1] | 18 | 42 | 11/7 | Head&neck 5 | S 12 | NR 8 |
| Trunk 6 | S, RT 2 | R 3 | ||||
| Extremity 7 | S, CTX 1 | NA 7 | ||||
| S, RT, CTX 1 | ||||||
| NA 2 | ||||||
| Montgomery et al.[5] | 10 | 54.4 | 8/2 | Head&neck 4 | S 7 | R 5 |
| Trunk 3 | S, RT 2 | NR 4 | ||||
| Extremity 3 | NA 1 | NA 1 | ||||
| Chang et al.[6] | 1 | 28 | 0/1 | Head&neck 1 | S 1 | R 1 |
| Roth et al.[7] | 1 | 46 | 0/1 | Trunk 1 | S 1 | NR 1 |
| San Miguel et al.[8] | 1 | 51 | 0/1 | Extremity 1 | S 1 | NR 1 |
| Morgan et al.[9] | 1 | 46 | 0/1 | Trunk 1 | S 1 | NR 1 |
| Tajima et al.[10] | 1 | 64 | 0/1 | Trunk 1 | S 1 | NA 1 |
| Laco et al.[11] | 1 | 24 | 0/1 | Head&neck 1 | S 1 | NR 1 |
| Takahama et al.[12] | 1 | 42 | 1/0 | Head&neck 1 | RT, CTX 1 | DOD 1 |
| Meng et al.[13] | 14 | 30.5 | 9/5 | Head&neck 6 | S 8 | NR 8 |
| Trunk 5 | S, CTX 4 | R 5 | ||||
| Extremity 3 | S, RT 2 | NA 1 | ||||
| Coyne et al.[14] | 1 | 44 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Meng et al.[15] | 3 | 34 | 3/0 | Head&neck 3 | S, RT 3 | R 3 |
| Jay et al.[16] | 1 | 41 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Eisenstat et al.[17] | 1 | 5 | 0/1 | Trunk 1 | sudden death | NA 1 |
| Agaimy et al.[18] | 2 | 60-70 | 0/2 | Trunk 2 | S 2 | R 2 |
| Nagata et al.[19] | 1 | 36 | 1/0 | Extremity 1 | S 1 | NR 1 |
| Morii et al.[20] | 1 | 46 | 0/1 | Trunk 1 | S 1 | NR 1 |
| Demarosi et al.[21] | 2 | 56 | 1/1 | Head&neck 2 | S 2 | NR 2 |
| Niedzielska et al.[22] | 1 | 54 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Arora et al.[23] | 1 | 38 | 0/1 | Extremity 1 | S 1 | NR 1 |
| Humphries et al.[24] | 1 | 15 | 0/1 | Trunk 1 | S 1 | NA 1 |
| Montebugnoli et al.[25] | 1 | 37 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Miyazawa et al.[26] | 1 | 65 | 1/0 | Trunk 1 | S 1 | NR 1 |
| Ni et al.[27] | 1 | 41 | 0/1 | Head&neck 1 | S 1 | NR 1 |
| Yamada et al.[28] | 1 | 73 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Khosla et al.[29] | 1 | 55 | 1/0 | Head&neck 1 | S, RT 1 | NR 1 |
| Murakami et al.[30] | 1 | 24 | 0/1 | Trunk 1 | S 1 | NR 1 |
| Saito et al.[31] | 1 | 50 | 0/1 | Extremity 1 | S 1 | NR 1 |
| Cai et al (2013)[32] | 2 | 30.5 | 2/0 | Head&neck 2 | S 2 | NR 2 |
| Oylumlu et al.[33] | 1 | 36 | 1/0 | Trunk 1 | S, CTX 1 | Cardiac metastasis |
| Kordač et al.[34] | 1 | 40 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Qiu et al.[35] | 2 | 37 | 1/1 | Head&neck 2 | S 2 | NR 1 R 1 |
| Han et al.[36] | 1 | 26 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Myong et al.[37] | 1 | 61 | 0/1 | Trunk 1 | S 1 | NR 1 |
| Maruyama et al.[38] | 1 | 43 | 0/1 | Head&neck 1 | S 1 | NR 1 |
| Hadjigeorgiou et al.[39] | 1 | 55 | 1/0 | Trunk 1 | S 1 | NR 1 |
| Zhang et al.[40] | 1 | 2 | 0/1 | Head&neck 1 | S 1 | NR 1 |
| Niu et al. [41] | 1 | 51 | 0/1 | Trunk 1 | S 1 | DOD 1 |
| Mikami et al.[42] | 1 | 38 | 0/1 | Head&neck 1 | S 1 | NR 1 |
| Peng et al.[43] | 1 | 44 | 0/1 | Trunk 1 | S, CTX 1 | NR 1 |
| Taweevisit et al.[44] | 1 | 66 | 1/0 | Head&neck 1 | S, RT 1 | NR 1 |
| Ghosh et al[45] | 2 | 36.5 | 1/1 | Head&neck 2 | S 2 | R 2 |
| Wu et al[46] | 1 | 30 | 1/0 | Head&neck and Trunk | supportive treatment | NA 1 |
| Hou et al[47] | 1 | 24 | 0/1 | Extremity 1 | S 1 | NA 1 |
| Bai et al[48] | 1 | 74 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Scardina et al[49] | 1 | 62 | 0/1 | Trunk 1 | S 1 | R 1 |
| Nair et al.[50] | 1 | 63 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Mulay et al. [51] | 1 | 48 | 1/0 | Head&neck 1 | S, RT 1 | NR 1 |
| Tang et al.[52] | 1 | 11 month | 0/1 | Head&neck 1 | S 1 | NR 1 |
| Kuo et al.[53] | 1 | 77 | 1/0 | Trunk 1 | S 1 | NR 1 |
| Yonezawa et al.[3] | 1 | 69 | 0/1 | Head&neck 1 | S 1 | NR 1 |
| Jayasooriya et al.[54] | 3 | 10.7 | 1/2 | Head&neck 3 | S 3 | NR 2 |
| NA 1 | ||||||
| Padmawar et al.[55] | 1 | 13 | 0/1 | Head&neck 1 | S 1 | NR 1 |
| Kim et al.[4] | 15 | 50.8 | 8/7 | Head&neck 1 | S 15 | NR 13 |
| Trunk 4 | R 2 | |||||
| Extremity 10 | ||||||
| Emanuelli et al.[56] | 1 | 11 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Gonçalves et al.[57] | 1 | 27 | 1/0 | Head&neck 1 | S 1 | NR 1 |
| Concepcion-Torio et al.[58] | 1 | 38 | 1/0 | Head&neck 1 | RT 1 | R 1 |
| Lin et al.[59] | 1 | 45 | 1/0 | Trunk 1 | S, CTX 1 | DOD 1 |
| Present case | 1 | 30 | 0/1 | Extremity 1 | S 1 | NR for now |
CTX = chemotherapy, DOD = death of disease, NA = not available, NR = no recurrence, R = recurrence, S = surgery, RT = radiotherapy.
Figure 3.
Age and gender distribution of patient populations. According to the reported cases, LGMS patients were mostly among 40- to 49-year-olds. LGMS = low-grade myofibroblastic sarcoma.
We classified locations of LGMS into 3 groups: Head&neck (56 cases, 45.90%), Trunk (37 cases, 30.33%), and Extremity (29 cases, 23.77%). It should be noted that one of the patients had LGMS which occurred in multiple organs, including the diaphragmatic pleura and head and neck region.[46] The case was counted into Head&neck group and Trunk group, respectively. In addition, several new primary sites of LGMS have been reported in recent years, including limbus,[51] orbit,[52,58] and multiorgan.[46]
LGMS was reported to be prone to local recurrence rather than distant metastasis.[4] And the data we collected support this conclusion. Notably, 2 cases of cardiac metastasis of LGMS were reported, both of which resulted in the eventual death of the patient.[17,33] Among the 121 patients with LGMS, the number of No Recurrence cases during the follow-up was 76 (62.81%), which suggests that LGMS patients after proper treatment generally have good prognosis. And Chan et al[60] reported that the 5-year overall survival rate of LGMS was 71.6% and the disease-specific survival rate was 76.3%, which is consistent with our conclusion.
The most common treatment for LGMS was surgical excision/wide excision. Some clinicians used chemotherapy or radiotherapy as adjuvant treatment strategies, while most were more concerned about whether the tumor is completely removed. Based on the data we collected, once diagnosed properly and excised wide enough, LGMS is less likely to recur. A quantitative study by Xu et al[61] supports our observations. They reported that age greater than 60 years, positive nodal status, and no surgical treatment were independent prognostic factors for patients with LGMS, whereas chemotherapy and radiation treatment were not. However, some authors reported that LGMS occurring in the Head&neck may not obtain adequate resection margins due to surgical limitations.[21,32] Recently, Lin et al[59] reported that Apatinib functioned as an effective treatment of LGMS via potential VEGFR-PI3K/Akt signaling pathway.
LGMS is an atypical type of tumor composed of myofibroblasts, which results in the strong expression of Vimentin.[62] Therefore, immunohistochemistry can be used for diagnosis and differential identification. Gonçalves et al[57] reported that in their literature review consisting of 30 studies, 26 and 10 of them used α-SMA (alpha-SMA) and muscle-specific actin as myogenic marker, respectively. In our present case, immunohistochemistry revealed positive for SMA, suggesting the tumor cells were derived from myofibroblasts. Also, it’s well known that S-100 protein is a specific biomarker for schwannoma and malignant peripheral nerve sheath tumor.[11] S-100 should be negative in LGMS, which is consistent with our present case. In addition, immunohistochemistry revealed negative for anaplastic lymphoma kinase, helping to differentiate LGMS from inflammatory myofibroblastic tumor.[63] Inflammatory myofibroblastic tumor is a type of tumor similar to LGMS because of their morphologic similarity and the overlapping immunophenotype.[64]
4. Conclusion
To the best of our knowledge, this is the first case report of LGMS in the femoral head neck junction treated by hip arthroscopy. Beyond the present case, other 120 cases from 58 literatures (1998–2022) are reviewed and discussed. The age of LGMS patients ranged from 11 months to 77 years and the male-to-female ratio was approximately 1.28:1. The location distribution of previously reported LGMS cases and the present case was as follows: head&neck (45.90%), trunk (30.33%), and extremity (23.77%). Wide excision with clear margins may achieve relatively good clinical outcomes.
Author contributions
Conceptualization: Yan Xu, Jianquan Wang, Yingfang Ao, Guanying Gao.
Data curation: Guanying Gao, Yuhao Liu.
Writing—original draft: Guanying Gao, Yuhao Liu.
Writing—review and editing: Yan Xu, Jianquan Wang, Yingfang Ao, Guanying Gao.
Abbreviations:
- CT =
- computed tomography
- LGMS =
- low-grade myofibroblastic sarcoma
- MRI =
- magnetic resonance images,
- SMA
- = smooth muscle actin.
GG and YL contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Informed consent was obtained from the patient for publication of this case report.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Gao G, Liu Y, Ao Y, Wang J, Xu Y. Low-grade myofibroblastic sarcoma of the proximal femur: A case report and literature review. Medicine 2022;101:45(e31715).
Contributor Information
Guanying Gao, Email: gao-guan@126.com.
Yuhao Liu, Email: 453239915@qq.com.
Yingfang Ao, Email: yingfang.ao@vip.sina.com.
Jianquan Wang, Email: kenyl417@vip.sina.com.
References
- [1].Mentzel T, Dry S, Katenkamp D, et al. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol. 1998;22:1228–38. [DOI] [PubMed] [Google Scholar]
- [2].Board WCoTE. WHO Classification of Tumours: Soft Tissue and Bone Tumours. International Agency for Research on Cancer. Lyon, France: IARD Press, 2020. [Google Scholar]
- [3].Yonezawa H, Yamamoto N, Hayashi K, et al. Low-grade myofibroblastic sarcoma of the levator scapulae muscle: a case report and literature review. BMC Musculoskelet Disord. 2020;21:836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kim JH, Choi W, Cho HS, et al. Surgical treatment and long-term outcomes of low-grade myofibroblastic sarcoma: a single-center case series of 15 patients. World J Surg Oncol. 2021;19:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Montgomery E, Goldblum JR, Myofibrosarcoma FC. a clinicopathologic study. Am J Surg Pathol. 2001;25:219–28. [DOI] [PubMed] [Google Scholar]
- [6].Chang SE, Choi JH, Sung KJ, et al. A case of cutaneous low-grade myofibroblastic sarcoma. J Dermatol. 2001;28:383–7. [DOI] [PubMed] [Google Scholar]
- [7].Roth TM, Fratkin J, Woodring TC, et al. Low-grade myofibroblastic sarcoma of the vulva. Gynecol Oncol. 2004;92:361–4. [DOI] [PubMed] [Google Scholar]
- [8].San Miguel P, Fernández G, Ortiz-Rey JA, et al. Low-grade myofibroblastic sarcoma of the distal phalanx. J Hand Surg Am. 2004;29:1160–3. [DOI] [PubMed] [Google Scholar]
- [9].Morgan PB, Chundru S, Hatch SS, et al. Uncommon malignancies: case 1. Low-grade myofibroblastic sarcoma of the breast. J Clin Oncol. 2005;23:6249–51. [DOI] [PubMed] [Google Scholar]
- [10].Tajima Y, Sudoh K, Matsumoto A, et al. Femoral neuropathy induced by a low-grade myofibroblastic sarcoma of the groin. J Neurol. 2005;252:1416–7. [DOI] [PubMed] [Google Scholar]
- [11].Laco J, Simáková E, Slezák R, et al. Low grade myofibroblastic sarcoma of tongue: a case report. Cesk Patol. 2006;42:150–3. [PubMed] [Google Scholar]
- [12].Takahama A, Jr., Nascimento AG, Brum MC, et al. Low-grade myofibroblastic sarcoma of the parapharyngeal space. Int J Oral Maxillofac Surg. 2006;35:965–8. [DOI] [PubMed] [Google Scholar]
- [13].Meng GZ, Zhang HY, Bu H, et al. Myofibroblastic sarcomas: a clinicopathological study of 20 cases. Chin Med J (Engl). 2007;120:363–9. [PubMed] [Google Scholar]
- [14].Coyne JD. Low-grade myofibroblastic sarcoma of the piriform fossa: a case report with a literature review of a tumour with a predilection for the head and neck. Br J Oral Maxillofac Surg. 2007;45:335–7. [DOI] [PubMed] [Google Scholar]
- [15].Meng GZ, Zhang HY, Bu H, et al. Myofibroblastic sarcoma of the nasal cavity and paranasal sinus: a clinicopathologic study of 6 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:530–9. [DOI] [PubMed] [Google Scholar]
- [16].Jay A, Piper K, Farthing PM, et al. Low-grade myofibroblastic sarcoma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:e52–8. [DOI] [PubMed] [Google Scholar]
- [17].Eisenstat J, Gilson T, Reimann J, et al. Low-grade myofibroblastic sarcoma of the heart causing sudden death. Cardiovasc Pathol. 2008;17:55–9. [DOI] [PubMed] [Google Scholar]
- [18].Agaimy A, Wünsch PH, Schroeder J, et al. Low-grade abdominopelvic sarcoma with myofibroblastic features (low-grade myofibroblastic sarcoma): clinicopathological, immunohistochemical, molecular genetic and ultrastructural study of two cases with literature review. J Clin Pathol. 2008;61:301–6. [DOI] [PubMed] [Google Scholar]
- [19].Nagata Y, Matsuno T, Hamada N, et al. Low-grade myofibroblastic sarcoma of the palm. Scand J Plast Reconstr Surg Hand Surg. 2008;42:164–7. [DOI] [PubMed] [Google Scholar]
- [20].Morii T, Mochizuki K, Sano H, et al. Occult myofibroblastic sarcoma detected on FDG-PET performed for cancer screening. Ann Nucl Med. 2008;22:811–5. [DOI] [PubMed] [Google Scholar]
- [21].Demarosi F, Bay A, Moneghini L, et al. Low-grade myofibroblastic sarcoma of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:248–54. [DOI] [PubMed] [Google Scholar]
- [22].Niedzielska I, Janic T, Mrowiec B. Low-grade myofibroblastic sarcoma of the mandible: a case report. J Med Case Rep. 2009;3:8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Arora R, Gupta R, Sharma A, et al. A rare case of low-grade myofibroblastic sarcoma of the femur in a 38-year-old woman: a case report. J Med Case Rep. 2010;4:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Humphries WE, 3rd, Satyan KB, Relyea K, et al. Low-grade myofibroblastic sarcoma of the sacrum. J Neurosurg Pediatr. 2010;6:286–90. [DOI] [PubMed] [Google Scholar]
- [25].Montebugnoli L, Venturi M, Gissi DB, et al. Low-grade myofibroblastic sarcoma of the gingiva. BMJ Case Rep. 2010;2010:bcr0720103166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Miyazawa M, Naritaka Y, Miyaki A, et al. A low-grade myofibroblastic sarcoma in the abdominal cavity. Anticancer Res. 2011;31:2989–94. [PubMed] [Google Scholar]
- [27].Ni C, Xu YY, Zhou SH, et al. Differential diagnosis of inflammatory myofibroblastic tumour and low-grade myofibroblastic sarcoma: two case reports with a literature review. J Int Med Res. 2011;39:311–20. [DOI] [PubMed] [Google Scholar]
- [28].Yamada T, Yoshimura T, Kitamura N, et al. Low-grade myofibroblastic sarcoma of the palate. Int J Oral Sci. 2012;4:170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Khosla D, Yadav BS, Kumar R, et al. Low-grade myofibroblastic sarcoma of the larynx: a rare entity with review of literature. J Cancer Res Ther. 2013;9:284–6. [DOI] [PubMed] [Google Scholar]
- [30].Murakami Y, Tsubamoto H, Hao H, et al. Long-term disease-free survival after radical local excision of low-grade myofibroblastic sarcoma of the vulva. Gynecol Oncol Case Rep. 2013;5:34–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saito T, Mitomi H, Kurisaki A, et al. Low-grade myofibroblastic sarcoma of the distal femur. Int J Surg Case Rep. 2013;4:195–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cai C, Dehner LP, El-Mofty SK. In myofibroblastic sarcomas of the head and neck, mitotic activity and necrosis define grade: a case study and literature review. Virchows Arch. 2013;463:827–36. [DOI] [PubMed] [Google Scholar]
- [33].Oylumlu M, Yildiz A, Ercan S, et al. Cardiac metastasis of a low-grade myofibroblastic sarcoma. Echocardiogr. 2014;31:E1–4. [DOI] [PubMed] [Google Scholar]
- [34].Kordač P, Nikolov DH, Smatanová K, et al. Low-grade myofibroblastic sarcoma of the larynx: case report and review of literature. Acta Medica. 2015;57:162–4. [DOI] [PubMed] [Google Scholar]
- [35].Qiu JY, Liu P, Shi C, et al. Low-grade myofibroblastic sarcomas of the maxilla. Oncol Lett. 2015;9:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Han SR, Yee GT. Low grade myofibroblastic sarcoma occurred in the scalp. J Korean Neurosurg Soc. 2015;58:385–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Myong NH, Min JW. Low-grade myofibroblastic sarcoma arising in fibroadenoma of the breast-A case report. Diagn Pathol. 2016;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Maruyama T, Nakasone T, Nimura F, et al. Indolent growth of low-grade myofibroblastic sarcoma of the cheek mimics benign lesions: A case report and literature review. Oncol Lett. 2017;13:4307–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hadjigeorgiou GF, Samaras V, Varsos V. Low-grade myofibroblastic sarcoma of the thoracic spine: report of an extreme rare case. Br J Neurosurg. 2017;31:731–3. [DOI] [PubMed] [Google Scholar]
- [40].Zhang S, Ma Y, Ma T, et al. Low-grade myofibroblastic sarcoma of the orbit: a case report and literature review. Medicine (Baltim). 2017;96:e9172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Niu R, Wang JF, Zhang DC, et al. Low-grade myofibroblastic sarcoma of gastric cardia on 18F-FDG positron emission tomography/computed tomography: An extremely rare case report. Medicine (Baltim). 2018;97:e9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mikami Y, Fujii S, Kohashi KI, et al. Low-grade myofibroblastic sarcoma arising in the tip of the tongue with intravascular invasion: A case report. Oncol Lett. 2018;16:3889–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Peng L, Tu Y, Li Y, et al. Low-grade myofibroblastic sarcoma of the pancreas: A case report and literature review. J Cancer Res Ther. 2018;14(Supplement):S796–9. [DOI] [PubMed] [Google Scholar]
- [44].Taweevisit M, Thorner PS. Distinctive features of low-grade myofibroblastic sarcoma on aspiration cytology: a case report. Cytopathology. 2018;29:578–81. [DOI] [PubMed] [Google Scholar]
- [45].Ghosh A, Bandopadhyay A, Sarkar R. Low-grade myofibroblastic sarcoma of maxillary sinus and buccal mucosa: two rare cases and review of the literature. Indian J Pathol Microbiol. 2019;62:119–21. [DOI] [PubMed] [Google Scholar]
- [46].Wu X, Guo L, Li S, et al. Low-grade myofibroblastic sarcoma with abdominal pain, a stuffy nose, hearing loss, and multiple cavity effusion: a case report and literature review. J Int Med Res. 2020;48:300060519895661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hou W, Su M, Li Q, et al. Low-grade myofibroblastic sarcoma demonstrated on 99mTc-MDP bone scan and 18F-FDG PET/CT . Clin Nucl Med. 2020;45:549–51. [DOI] [PubMed] [Google Scholar]
- [48].Bai Y, Li X, Yin Z. Management of low-grade myofibroblastic sarcoma of the larynx. Ear Nose Throat J. 2020;99:Np82–np83. [DOI] [PubMed] [Google Scholar]
- [49].Scardina L, Franceschini G, Di Leone A, et al. Low-grade myofibroblastic sarcoma of the breast. Breast J. 2020;26:2077–8. [DOI] [PubMed] [Google Scholar]
- [50].Nair NP, Kaushal D, Rao M, et al. Evaluation and management of an uncommon tumor of the larynx: a case report and literature review of laryngeal low-grade myofibroblastic sarcoma. Cureus. 2020;12:e11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mulay K, Sen M, Honavar SG. Limbal, low-grade myofibroblastic sarcoma: case report and literature review. Indian J Ophthalmol. 2020;68:2538–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Tang L, Xu H, Gao H, et al. Primary low-grade myofibroblastic sarcoma: a rare case report of this tumor in the orbit and literature review. Eur J Ophthalmol. 2020;et al. :1120672120970392. [DOI] [PubMed] [Google Scholar]
- [53].Kuo YR, Yang CK, Chen A, et al. Low-grade myofibroblastic sarcoma arising from keloid scar on the chest wall after thoracic surgery. Ann Thorac Surg. 2020;110:e469–71. [DOI] [PubMed] [Google Scholar]
- [54].Jayasooriya PR, Athukorala C, Attygalla M, et al. Low-grade myofibroblastic sarcoma of the oral cavity: a report of three cases illustrating an emerging disease in children. Dermatopathology (Basel). 2021;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Padmawar NS, Bhadange S, Mustilwar RG, et al. Aberrant location of low-grade myofibroblastic sarcoma of the gingiva in posterior Maxilla. Int J Clin Pediatr Dent. 2021;14:816–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Emanuelli E, O’Connor M, Garg RK. Management of a highly vascular low-grade myofibroblastic sarcoma of the mandible. Plast Reconstr Surg Glob Open. 2022;10:e4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Gonçalves JM, Marola LHG, Vieira DSC, et al. The challenging diagnosis of low-grade myofibroblastic sarcoma: a case report and literature update. Oral Oncol. 2022;126:105762. [DOI] [PubMed] [Google Scholar]
- [58].Concepcion-Torio K, Mandal P, Woo KI, et al. Primary low-grade myofibroblastic sarcoma of the orbit. Ophthalmic Plast Reconstr Surg. 2022;38:212–3. [DOI] [PubMed] [Google Scholar]
- [59].Lin Y, Gao X, Liu Z, et al. Effective treatment of low-grade myofibroblastic sarcoma with apatinib: a case report and literature review. Pharmgenomics Pers Med. 2022;15:573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chan JY, Gooi Z, Wong EW, et al. Low-grade myofibroblastic sarcoma: a population-based study. Laryngoscope. 2017;127:116–21. [DOI] [PubMed] [Google Scholar]
- [61].Xu Y, Xu G, Wang X, et al. Is there a role for chemotherapy and radiation in the treatment of patients with low-grade myofibroblastic sarcoma? Clin Transl Oncol. 2021;23:344–52. [DOI] [PubMed] [Google Scholar]
- [62].Ross R, Everett NB, Tyler R; Wound healing and collagen formation. VI. The origin of the wound fibroblast studied in parabiosis. J Cell Biol. 1970;44:645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cessna MH, Zhou H, Sanger WG, et al. Expression of ALK1 and p80 in inflammatory myofibroblastic tumor and its mesenchymal mimics: a study of 135 cases. Mod Pathol. 2002;15:931–8. [DOI] [PubMed] [Google Scholar]
- [64].Meng GZ, Zhang HY, Zhang Z, et al. Myofibroblastic sarcoma vs nodular fasciitis: a comparative study of chromosomal imbalances. Am J Clin Pathol. 2009;131:701–9. [DOI] [PubMed] [Google Scholar]