Abstract
Acalabrutinib is a Bruton tyrosine kinase inhibitor approved for patients with chronic lymphocytic leukemia (CLL). ASCEND is the pivotal phase 3 study of acalabrutinib versus investigator’s choice of idelalisib plus rituximab (IdR) or bendamustine plus rituximab (BR) in patients with relapsed/refractory (R/R) CLL. In the primary ASCEND analysis (median 16.1-month follow-up), acalabrutinib showed superior efficacy with an acceptable tolerability profile versus IdR/BR; here, we report final ~4 year follow-up results. Patients with R/R CLL received oral acalabrutinib 100 mg twice daily until progression or unacceptable toxicity, or investigator’s choice of IdR or BR. A total of 310 patients (acalabrutinib, n = 155; IdR, n = 119; BR, n = 36) were enrolled. At median follow-up of 46.5 months (acalabrutinib) and 45.3 months (IdR/BR), acalabrutinib significantly prolonged investigator-assessed progression-free survival (PFS) versus IdR/BR (median, not reached [NR] vs 16.8 months; P < 0.001); 42-month PFS rates were 62% (acalabrutinib) versus 19% (IdR/BR). Median overall survival (OS) was NR (both arms); 42-month OS rates were 78% (acalabrutinib) versus 65% (IdR/BR). Adverse events led to drug discontinuation in 23%, 67%, and 17% of patients in the acalabrutinib, IdR, and BR arms, respectively. Events of clinical interest (acalabrutinib vs IdR/BR) included all-grade atrial fibrillation/flutter (8% vs 3%), all-grade hypertension (8% vs 5%), all-grade major hemorrhage (3% vs 3%), grade ≥3 infections (29% vs 29%), and second primary malignancies excluding nonmelanoma skin cancer (7% vs 2%). At ~4 years follow-up, acalabrutinib maintained favorable efficacy versus standard-of-care regimens and a consistent tolerability profile in patients with R/R CLL.
INTRODUCTION
Bruton tyrosine kinase (BTK) inhibitors have improved patient outcomes in chronic lymphocytic leukemia (CLL), including in the relapsed/refractory (R/R) setting.1 The BTK inhibitors acalabrutinib and ibrutinib are approved treatment options for patients with R/R CLL. Ibrutinib, a covalent BTK inhibitor, was first approved by the US Food and Drug Administration (FDA) in 20132 and has demonstrated long-term efficacy as a single agent compared with ofatumumab, an anti-CD20 antibody, in patients with R/R CLL in the RESONATE study.3 However, increased rates of cardiovascular toxicity (eg, hypertension, atrial fibrillation, bleeding) are associated with continuous ibrutinib use, with treatment-emergent atrial fibrillation leading to dose reductions in approximately 50% of patients with atrial fibrillation events,4–6 likely due to off-target binding to non-BTK kinases with analogous cysteine residues.7,8
Acalabrutinib is a next-generation covalent BTK inhibitor with greater selectivity for BTK compared with ibrutinib7 and an improved cardiovascular tolerability profile as demonstrated in the ELEVATE-RR study.9 Acalabrutinib was approved by the FDA for the treatment of CLL in 2019.10 ASCEND is the pivotal phase 3 study of acalabrutinib versus investigator’s choice of idelalisib plus rituximab (IdR) or bendamustine plus rituximab (BR) in patients with R/R CLL. In the primary analysis of ASCEND, acalabrutinib monotherapy demonstrated significantly improved progression-free survival (PFS) with an acceptable tolerability profile compared with investigator’s choice of IdR/BR at a median follow-up of 16.1 months.11
The objective of the current analysis is to report the final efficacy and safety results from the ASCEND study at a median study follow-up of approximately 4 years.
METHODS
Study design and treatment
ASCEND (clinicaltrials.gov identifier: NCT02970318; ACE-CL-309) is a phase 3, randomized, multicenter, open-label study. The full study design has been previously described and reported.11 Patients were randomly assigned via a centralized procedure in a 1:1 ratio to receive acalabrutinib monotherapy or investigator’s choice of treatment (IdR or BR). Randomization was stratified by del(17p) status (yes vs no), Eastern Cooperative Oncology Group (ECOG) performance status (0−1 vs 2), and lines of prior therapy received (1−3 vs ≥4). Acalabrutinib (100 mg) was administered orally twice daily until progressive disease (PD) or unacceptable toxicity. In the investigator’s choice arm, idelalisib (150 mg) was administered orally twice daily until PD or unacceptable toxicity in combination with rituximab (375 mg/m2 intravenously [IV] on day 1 of the first cycle, followed by 500 mg/m2 IV every 2 weeks for 4 doses and then every 4 weeks for 3 doses for a total of 8 infusions); alternatively, bendamustine (70 mg/m2 IV on days 1 and 2 of cycles 1 through 6) was administered in combination with rituximab (375 mg/m2 IV on day 1 of the first cycle and 500 mg/m2 IV thereafter on day 1 of cycles 2 through 6). Patients receiving investigator’s choice of therapy who had confirmed disease progression were permitted to cross over to receive acalabrutinib monotherapy. Dose modifications were allowed for management of adverse events (AEs). Patients were withdrawn from the study due to withdrawal of consent, loss to follow-up, or death.
The institutional review board or independent ethics committee at each site approved the protocol. The study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice. All patients provided written informed consent.
Patient population
Eligible patients were adults aged ≥18 years with CLL who had previously received at least 1 systemic therapy. Patients were required to have an ECOG performance status of 2 or less and adequate hematologic, hepatic, and renal function. Patients with significant cardiovascular disease requiring concomitant warfarin or equivalent vitamin K antagonist treatment, or who had received prior treatment with BTK, phosphoinositide 3-kinases, tyrosine-protein kinase, or B-cell lymphoma 2 inhibitors were excluded.
Study end points and assessments
The previously reported primary study end point was Independent Review Committee–assessed PFS.11 After the primary end point was met, PFS was assessed only by the investigator. Investigator-assessed PFS was defined as the time from randomization until disease progression, assessed based on International Workshop on Chronic Lymphocytic Leukemia 2008 criteria,12 or death from any cause, whichever occurred first. Other efficacy end points included overall survival (OS), overall response rate (ORR), duration of response (DOR), and time to next CLL treatment (TTNT). OS was defined as the time from randomization until death due to any cause. ORR was defined as the proportion of patients who achieved a complete response (CR), CR with an incomplete blood count recovery (CRi), nodular partial response (nPR), or partial response (PR) over the course of the study. DOR was defined as the time from first documentation of objective response (CR, CRi, nPR, or PR) to the earliest time of disease progression or death from any cause. TTNT was defined as the time from randomization to the start of non–protocol-specified treatment for CLL.
Safety was assessed based on treatment-emergent AEs and clinical laboratory tests. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03 and reported up to 30 days after the last dose of study drug, or at documented disease progression, whichever was longer.
Statistical analysis
Efficacy analyses were performed in the intent-to-treat population (all randomized patients). OS was analyzed using the data throughout the study follow-up, regardless of treatment crossover. Safety analyses were performed in the safety population, which included patients who received at least 1 dose of any study drug.
Analysis of investigator-assessed PFS was performed using a stratified log-rank test. The hazard ratio (HR) for acalabrutinib versus investigator’s choice was based on a stratified Cox proportional-hazards model, and P values were based on stratified log-rank test (stratified by randomization stratification factors as recorded in an interactive voice/web response system); HRs for acalabrutinib versus IdR or BR separately were based on an unstratified Cox proportional-hazards model, and P values were based on unstratified log-rank test. Analyses of investigator-assessed PFS for acalabrutinib versus IdR/BR were also performed in prespecified subgroups based on prognostic variables, using similar methodology as for the analysis of acalabrutinib versus IdR or BR. Patients not meeting the criteria for PFS and alive by the analysis date were censored.
Analyses of OS, DOR, and TTNT for acalabrutinib versus IdR/BR were performed using the same methods as described for investigator-assessed PFS for acalabrutinib versus IdR/BR. In post hoc analyses of OS by high-risk genomic subgroup, HRs were based on an unstratified Cox proportional-hazards model, and P values were based on an unstratified log-rank test. Analysis of ORR was based on a Cochran-Mantel-Haenszel test adjusted for randomization stratification factors; the 95% confidence interval (CI) was based on normal approximation using Wilson’s score.
RESULTS
Between February 21, 2017, and January 17, 2018, 398 patients were assessed for eligibility; 310 patients were randomized (acalabrutinib, n = 155; investigator’s choice, n = 155 [IdR, n = 119; BR, n = 36]); of the patients randomized to the investigator’s choice treatment arm, 118 patients received IdR and 35 patients received BR. Overall, the median age was 67 years; 228 patients (74%) had unmutated immunoglobulin heavy chain variable region (IGHV) genes, 86 (28%) had chromosome 17p deletion [del(17p)] and/or TP53 mutations, 35 (11%) had both del(17p) and TP53 mutations, 13 (4%) had del(17p) without TP53 mutation, 37 (12%) had TP53 mutations without del(17p), 6 (2%) had complex karyotype (CK), and 129 (42%) were Rai stage 3−4 (Table 1). Patients in the acalabrutinib arm had a median of 1 (range, 1−8) prior line of therapy and patients in the investigator’s choice arm had a median of 2 (range, 1−10) prior lines of therapy.
Table 1.
Baseline Characteristics
| Characteristic | Acalabrutinib Monotherapy (n = 155) | Investigator’s Choice (IdR/BR) (n = 155) | Total (N = 310) |
|---|---|---|---|
| Age, median (range), y | 68 (32–89) | 67 (34–90) | 67 (32–90) |
| ≥65 y, n (%) | 97 (63) | 98 (63) | 195 (63) |
| Men, n (%) | 108 (70) | 100 (65) | 208 (67) |
| Baseline Rai stage, n (%) | |||
| 0–II | 90 (58) | 90 (58) | 180 (58) |
| III–IV | 65 (42) | 64 (41) | 129 (42) |
| Unknown | 0 | 1 (0.6) | 1 (0.3) |
| ECOG PS, n (%) | |||
| 0 | 58 (37) | 55 (35) | 113 (36) |
| 1 | 78 (50) | 79 (51) | 157 (51) |
| 2 | 19 (12) | 21 (14) | 40 (13) |
| Cytopenia(s), n (%) | |||
| Absolute neutrophil count ≤1.5 × 109/L | 14 (9) | 9 (6) | 23 (7) |
| Hemoglobin ≤11 g/dL | 49 (32) | 46 (30) | 95 (31) |
| Platelets ≤100 × 109/L | 57 (37) | 57 (37) | 114 (37) |
| β2-microglobulin >3.5 mg/L, n (%) | 120 (77) | 126 (81) | 246 (79) |
| Bulky disease, n (%) | |||
| <5 cm | 79 (51) | 80 (52) | 159 (51) |
| ≥5 cm | 76 (49) | 75 (48) | 151 (49) |
| Genomic status, n (%) | |||
| del(11q) | 39 (25) | 44 (28) | 83 (27) |
| del(17p)a | 27 (17) | 21 (14) | 48 (16) |
| TP53 mutation | 39 (25) | 34 (22) | 73 (24) |
| del(17p)a and/or TP53 mutation | 44 (28) | 42 (27) | 86 (28) |
| del(17p)a and TP53 mutation | 22 (14) | 13 (8) | 35 (11) |
| del(17p)a without TP53 mutation | 5 (3) | 8 (5) | 13 (4) |
| TP53 mutation without del(17p) | 17 (11) | 20 (13) | 37 (12) |
| Unmutated IGHV | 109 (70) | 119 (77) | 228 (74) |
| Complex karyotypeb | 3 (2) | 3 (2) | 6 (2) |
aMutation status for patients with del(17p) were based on those recorded in the clinical database.
bComplex karyotype was defined as .3 aberrations.
BR = bendamustine plus rituximab; ECOG PS = Eastern Cooperative Oncology Group performance status; IdR = idelalisib plus rituximab; IGHV = immunoglobulin heavy chain variable region genes; TP53 = tumor protein p53.
At the time of data cutoff (September 3, 2021) for the final analysis, 112 of 154 patients (73%) in the acalabrutinib monotherapy arm had completed ≥24 months of treatment (Table 2). In the IdR arm, 92 of 118 (78%) patients had completed ≥6 months of rituximab treatment, and 27 of 118 patients (23%) had completed ≥24 months of idelalisib treatment. In the BR arm, 28 of 35 patients (80%) had completed ≥6 cycles of rituximab treatment, and 29 of 35 patients (83%) had completed ≥6 cycles of bendamustine treatment. Of 155 patients assigned to the investigator’s choice arm, 80 (52%) crossed over to receive subsequent acalabrutinib monotherapy.
Table 2.
Patient Disposition and Treatment Exposure
| Parameter | Acalabrutinib (n = 155) | IdR (n = 119) | BR (n = 36) |
|---|---|---|---|
| Time on study, median (range), mo | 46.5 (0.53–54.2) | 45.7 (0.03–53.0) | 44.5 (0.53–52.6) |
| Patients who discontinued treatment, n (%) | 154 (100) | 118 (100) | 7 (20)a |
| Reasons for treatment discontinuation, n (%) | |||
| Planned study termination by sponsor | 74 (48) | 10 (8) | 0 |
| Adverse eventb | 35 (23) | 73 (62) | 6 (17) |
| Progressive disease | 34 (22) | 22 (19) | 1 (3) |
| Death | 6 (4) | 2 (2) | 0 |
| Investigator discretion | 1 (1) | 6 (5) | 0 |
| Withdrawal of consent | 1 (1) | 1 (1) | 0 |
| Lost to follow-up | 1 (1) | 0 | 0 |
| Other | 2 (1) | 4 (3) | 0 |
| Duration of treatment exposure, median (range),c mo | 44.2 (1.1–54.2) | 11.5 (0.1–52.3)d | 5.6 (1.0–7.1)e |
| Relative dose intensity, median (range),c % | 99.1 (48.3–100.0) | 88.4 (46.6–100.0)d | 96.4 (14.5–102.5)e |
| Received ≥6 IV treatment cycles,c n (%) | NA | 92 (78)f | 29 (83)e |
a80% of patients completed BR treatment.
bData for treatment discontinuations due to adverse events were captured from the treatment termination case report form.
cThree patients were randomized but not treated (acalabrutinib, n = 1; IdR, n = 1; BR, n = 1) and are not included in the treatment exposure calculations.
dIdelalisib only.
eBendamustine only.
fRituximab only.
BR = bendamustine plus rituximab; IdR = idelalisib plus rituximab; IV = intravenous; NA = not applicable.
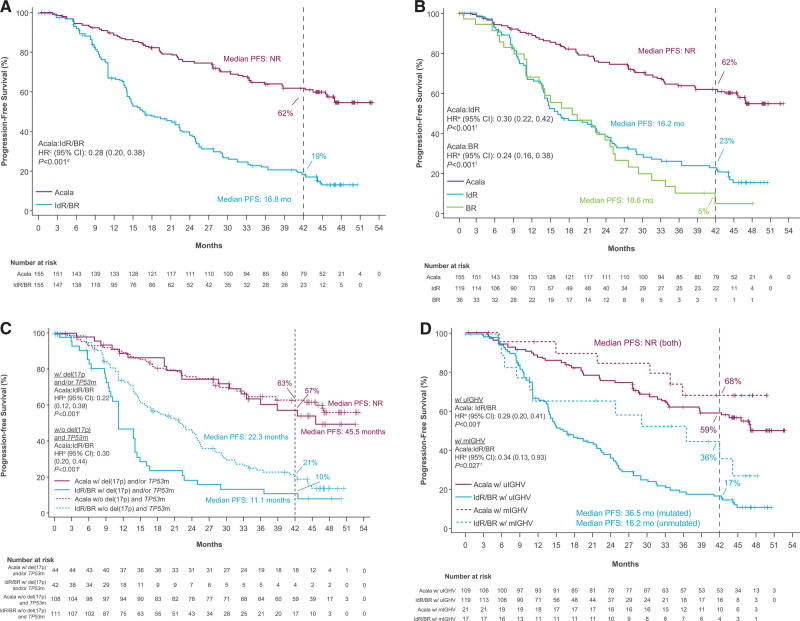
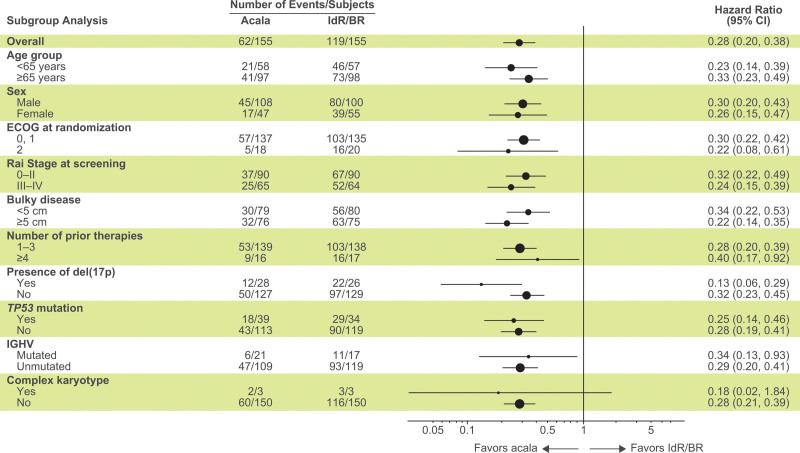
At a median study follow-up of 46.5 months (acalabrutinib) and 45.3 months (IdR/BR), 93 of 155 patients (60%) in the acalabrutinib monotherapy arm were disease progression–free and alive compared with 36 of 155 patients (23%) in the investigator’s choice arm. Acalabrutinib monotherapy significantly prolonged investigator-assessed PFS versus investigator’s choice IdR/BR (median, not reached [NR] vs 16.8 months; HR, 0.28; 95% CI, 0.20–0.38; P < 0.001) and compared with both IdR (median, NR vs 16.2 months; HR, 0.30; 95% CI, 0.22–0.42; P < 0.001) and BR (median, NR vs 18.6 months; HR, 0.24; 95% CI, 0.16−0.38; P < 0.001; Figure 1A, B). The 42-month PFS rates were 62% for acalabrutinib monotherapy versus 23% for IdR and 5% for BR. In patients with del(17p) and/or TP53 mutation, median PFS was 45.5 months for acalabrutinib versus 11.1 months for IdR/BR (HR, 0.22; 95% CI, 0.12–0.39; P < 0.001); in patients without del(17p) and TP53 mutation, median PFS was NR for acalabrutinib versus 22.3 months for IdR/BR (HR, 0.30; 95% CI, 0.20–0.44; P < 0.001) (Figure 1C). In patients with unmutated IGHV, median PFS was NR for acalabrutinib versus 16.2 months for IdR/BR (HR, 0.29; 95% CI, 0.20–0.41; P < 0.001) (Figure 1D). The PFS rates with acalabrutinib monotherapy were consistently numerically higher compared with IdR/BR across prespecified patient subgroups, including those with high-risk genomic features (Figure 2).
Figure 1.
Investigator-assessed progression-free survival. Outcomes are shown for (A) acalabrutinib vs IdR/BR, (B) acalabrutinib vs IdR or BR, (C) acalabrutinib vs IdR/BR by del(17p) and/or TP53 mutation status,a,b and (D) acalabrutinib vs IdR/BR by IGHV mutation status (ITT population). aMutation status for patients with del(17p) were based on values entered manually into IXRS. bBecause there were no IdR/BR–treated patients at risk by 42 mo in the del(17p) subgroup based on IXRS data, 42-mo PFS rates were not available for that analysis. cHR was based on stratified Cox proportional-hazards model, stratified by randomization stratification factors as recorded in an interactive voice/web response system. dP value was based on stratified log-rank test, stratified by randomization stratification factors as recorded in an interactive voice/web response system. eHRs were based on unstratified Cox proportional-hazards model. fP values were based on unstratified log-rank test. BR = bendamustine plus rituximab; CI = confidence interval; IdR = idelalisib plus rituximab; IGHV = immunoglobulin heavy chain variable region genes; HR = hazard ratio; ITT = intent-to-treat; IXRS = interactive voice/web response system; NR = not reached; mo, months; PFS = progression-free survival; mIGHV = mutated IGHV; TP53m, mutated tumor protein p53; TP53, tumor protein p53; uIGHV = unmutated IGHV.
Figure 2.
Subgroup analysis of investigator-assessed progression-free survival. Forest plot showing progression-free survival analyzed by prespecified subgroups according to baseline demographic and clinical characteristics. Hazard ratios were based on stratified Cox proportional-hazards model, stratified by randomization stratification factors as recorded in interactive voice/web response system. Data for the del(17p) subgroup analysis are based on those recorded in IXRS. BR = bendamustine plus rituximab; CI = confidence interval; ECOG PS = Eastern Cooperative Oncology Group performance status; IdR = idelalisib plus rituximab; IGHV = immunoglobulin heavy chain variable region genes; IXRS = interactive voice/web response system; NE = not estimable; TP53 = tumor protein p53.
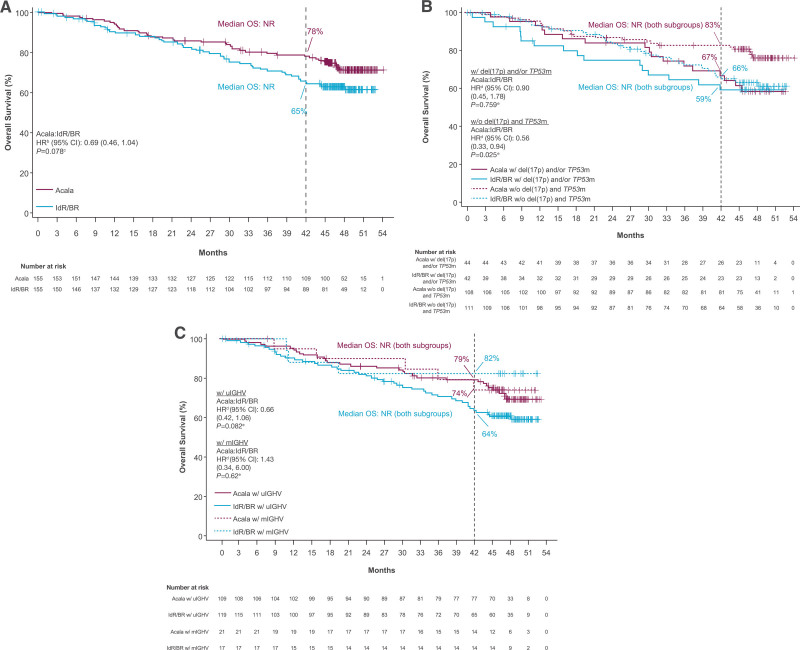
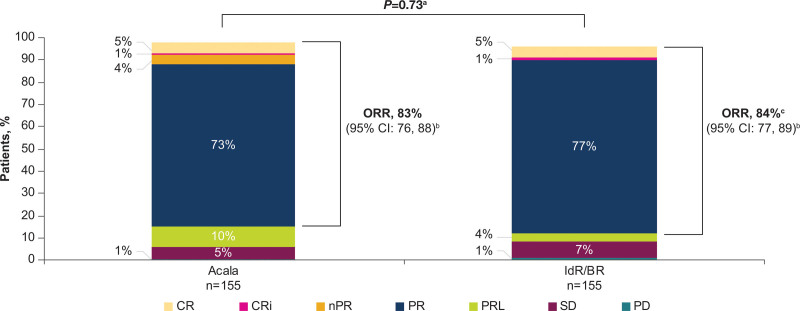
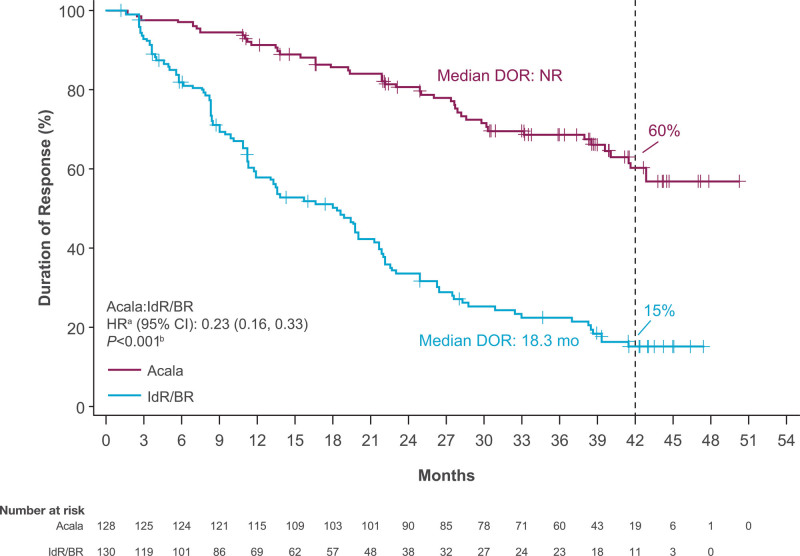
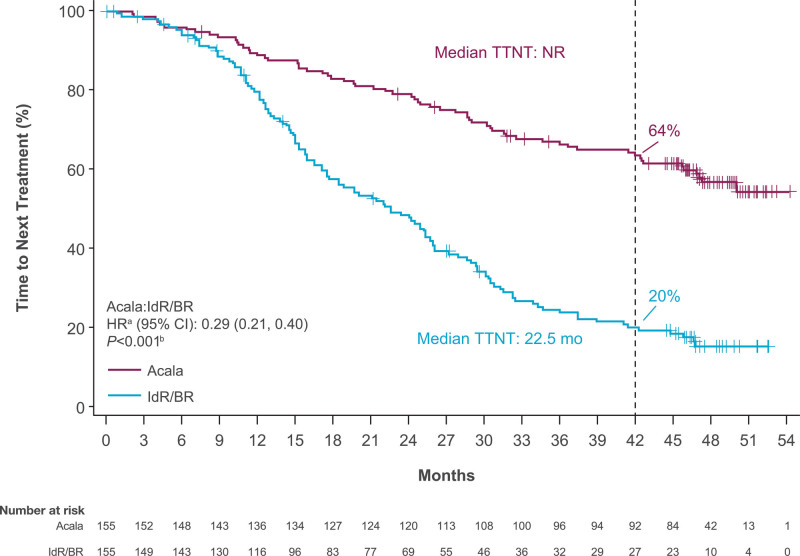
At the time of data cutoff for this analysis, 26% of patients (n = 41) in the acalabrutinib-monotherapy arm and 35% of patients (n = 54 [IdR, n = 41; BR, n = 13]) in the investigator’s choice arm had died. Median OS was NR in either arm (HR, 0.69; 95% CI, 0.46−1.04; P = 0.078); the 42-month OS rate was 78% in the acalabrutinib monotherapy therapy arm versus 65% in the IdR/BR arm (Figure 3A). Similar OS outcomes were seen in high-risk subgroups, including patients with del(17p) and/or TP53 mutation (HR, 0.90; 95% CI, 0.45−1.78; P = 0.759) and patients with unmutated IGHV (HR, 0.66; 95% CI, 0.42−1.06; P = 0.082) (Figure 3B, C). The ORR was comparable between acalabrutinib monotherapy (n = 128; 83%) and IdR/BR (n = 130; 84%); ORR including partial response with lymphocytosis was 92% (n = 143) versus 88% (n = 136), respectively (Figure 4). CR rate was comparable in the acalabrutinib and investigator’s choice arms (both n = 8; 5%). Five of the 6 patients with CK (acalabrutinib, n = 2; IdR/BR, n = 3) experienced PD and died; 1 patient in the acalabrutinib arm achieved a PR and is still alive. Median DOR was NR with acalabrutinib monotherapy and 18.3 months (95% CI, 11.9−21.7) with IdR/BR (HR, 0.23; 95% CI, 0.16−0.33; P < 0.001) (Figure 5). Median TTNT was NR with acalabrutinib monotherapy and 22.5 months (95% CI, 17.5−25.9) with IdR/BR (HR, 0.29; 95% CI, 0.21−0.40; P < 0.001) (Figure 6). Patients in the acalabrutinib monotherapy and IdR/BR groups received a median of 1 subsequent therapy, and the most common subsequent therapy in both treatment arms was venetoclax (acalabrutinib, 10%; IdR/BR, 9%) (Suppl. Table S1).
Figure 3.
Overall survival. (A) acalabrutinib vs IdR/BR,a (B) acalabrutinib vs IdR/BR by del(17p) and/or TP53 mutation status, and (C) acalabrutinib vs IdR/BR by IGHV mutation status. aEighty patients (52%) in the IdR/BR arm crossed over to the acalabrutinib arm. bBased on stratified Cox proportional-hazards model, stratified by randomization stratification factors as recorded in interactive voice/web response system. cBased on stratified log-rank test, stratified by randomization stratification factors as recorded in interactive voice/web response system. dBased on unstratified Cox proportional-hazards model. eBased on unstratified log-rank test. fBased on IXRS data. BR = bendamustine plus rituximab; CI = confidence interval; HR = hazard ratio; IdR = idelalisib plus rituximab; IGHV = immunoglobulin heavy chain variable region genes; IXRS = interactive voice/web response system; mIGHV = mutated IGHV; NR = not reached; OS = overall survival; TP53 = tumor protein p53; TP53m = mutated tumor protein p53; uIGHV = unmutated IGHV.
Figure 4.
Investigator-assessed overall response rate with acalabrutinib and IdR/BR. Response assessments were missing in 3 (2%) patients in the acalabrutinib arm and in 6 (4%) patients in the IdR/BR arm. Response rates were based on the International Workshop on Chronic Lymphocytic Leukemia 2008 criteria.12 aBased on Cochran-Mantel-Haenszel test with adjustment for randomization stratification factors as recorded in an interactive voice/web response system. b95% confidence interval based on normal approximation (with use of Wilson’s score). cThe sum of the CR, CRi, and PR rates differs slightly from the ORR due to rounding. BR = bendamustine plus rituximab; CI = confidence interval; CR = complete response; CRi = complete response with incomplete blood count recovery; IdR = idelalisib plus rituximab; nPR = nodular partial response; ORR = overall response rate; PD = progressive disease; PR = partial response; PRL = partial response with lymphocytosis; SD = stable disease.
Figure 5.
Duration of response with acalabrutinib and IdR/BR. aBased on stratified Cox proportional hazards model, stratified by randomization stratification factors as recorded in interactive voice/web response system. bBased on stratified log-rank test, stratified by randomization stratification factors as recorded in interactive voice/web response system. BR = bendamustine plus rituximab; CI = confidence interval; DOR = duration of response; HR = hazard ratio; IdR = idelalisib plus rituximab; NR = not reached.
Figure 6.
Time to next treatment with acalabrutinib and IdR/BR. aBased on stratified Cox proportional hazards model, stratified by randomization stratification factors as recorded in interactive voice/web response system. bBased on stratified log-rank test, stratified by randomization stratification factors as recorded in interactive voice/web response system. BR = bendamustine plus rituximab; CI = confidence interval; HR = hazard ratio; IdR = idelalisib plus rituximab; NR = not reached; TTNT = time to next treatment.
Median duration of exposure was 44.2 months (range, 1.1−54.2) with acalabrutinib monotherapy, 11.5 months (range, 0.1−52.3) for idelalisib in the IdR arm, and 5.6 months (range, 1.0−7.1) for bendamustine in the BR arm. Overall, 149 of 154 patients (97%) receiving acalabrutinib monotherapy, 117 of 118 patients (99%) receiving IdR, and 28 of 35 patients (80%) receiving BR experienced at least 1 AE. AEs of any grade occurring in at least 20% of patients were neutropenia, headache, diarrhea, and upper respiratory infection in the acalabrutinib monotherapy arm; neutropenia and diarrhea in the IdR arm; and neutropenia, fatigue, infusion-related reactions, and nausea in the BR arm.
Grade ≥3 AEs occurred more often with IdR (n = 108; 92%) than with acalabrutinib monotherapy (n = 104; 68%) or BR (n = 17; 49%). The most common grade ≥3 AEs (≥10% incidence) in the 154 patients receiving acalabrutinib monotherapy were neutropenia (n = 29; 19%), anemia (n = 20; 13%), and pneumonia (n = 15; 10%). The most common grade ≥3 AEs in the 118 patients receiving IdR were neutropenia (n = 47; 40%), diarrhea (n = 31; 26%), and pneumonia (n = 12; 10%). In the 35 patients receiving BR, the most common grade ≥3 AE was neutropenia (n = 11; 31%) (Table 3).
Table 3.
Most Common AEs in ≥10% (Any Grade) or ≥5% (Grade ≥3) of Patients in Any Cohort
| Common AEsa, n (%) | Acalabrutinib (n = 154) | IdR (n = 118) | BR (n = 35) | |||
|---|---|---|---|---|---|---|
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| Neutropenia | 37 (24) | 29 (19) | 55 (47) | 47 (40) | 12 (34) | 11 (31) |
| Headache | 36 (23) | 1 (1) | 7 (6) | 0 | 0 | 0 |
| Diarrhea | 33 (21) | 3 (2) | 62 (53) | 31 (26) | 5 (14) | 0 |
| Upper respiratory tract infection | 31 (20) | 3 (2) | 20 (17) | 4 (3) | 4 (11) | 1 (3) |
| Pneumonia | 30 (19) | 15 (10) | 17 (14) | 12 (10) | 2 (6) | 1 (3) |
| Anemia | 27 (18) | 20 (13) | 13 (11) | 8 (7) | 4 (11) | 3 (9) |
| Cough | 27 (18) | 0 | 18 (15) | 1 (1) | 2 (6) | 0 |
| Pyrexia | 25 (16) | 5 (3) | 23 (19) | 8 (7) | 6 (17) | 1 (3) |
| Arthralgia | 20 (13) | 2 (1) | 7 (6) | 0 | 1 (3) | 0 |
| Thrombocytopenia | 20 (13) | 6 (4) | 19 (16) | 10 (8) | 5 (14) | 1 (3) |
| Bronchitis | 19 (12) | 2 (1) | 9 (8) | 1 (1) | 3 (9) | 1 (3) |
| Fatigue | 19 (12) | 2 (1) | 10 (8) | 1 (1) | 8 (23) | 1 (3) |
| Respiratory tract infection | 18 (12) | 2 (1) | 8 (7) | 2 (2) | 0 | 0 |
| Nausea | 13 (8) | 0 | 17 (14) | 1 (1) | 7 (20) | 0 |
| Infusion-related reaction | 1 (1) | 0 | 9 (8) | 2 (2) | 8 (23) | 1 (3) |
| Rash | 15 (10) | 0 | 17 (14) | 4 (3) | 2 (6) | 0 |
| Nasopharyngitis | 13 (8) | 0 | 13 (11) | 0 | 1 (3) | 0 |
| Constipation | 14 (9) | 0 | 9 (8) | 0 | 5 (14) | 2 (6) |
aAEs were graded according to the Common Toxicity Criteria of the National Cancer Institute, version 4.03 and reported until 30 d after the last dose of study drug or at documented disease progression, whichever was longer.
AE = adverse event; BR = bendamustine plus rituximab; IdR = idelalisib plus rituximab.
Serious AEs (SAEs) were also reported more frequently with IdR treatment (n = 77; 65%) than with acalabrutinib monotherapy (n = 70; 45%) or BR treatment (n = 9; 26%). SAEs of any grade occurring in 5 or more patients receiving acalabrutinib monotherapy were pneumonia (n = 14; 9%), COVID-19 pneumonia (n = 6; 4%), and pyrexia (n = 5; 3%). SAEs of any grade occurring in 5 or more patients receiving IdR were diarrhea (n = 19; 16%), pneumonia (n = 12; 10%), and pyrexia (n = 8; 7%). In the BR arm, there were no SAEs of any grade occurring in 5 or more patients.
AEs led to dose reduction in 10 of 154 patients (6%) receiving acalabrutinib monotherapy, 14 of 118 patients (12%) receiving IdR, and 5 of 35 patients (14%) receiving BR. AEs led to drug discontinuation (captured from AE case report form) in 36 of 154 patients (23%) who received acalabrutinib monotherapy, 79 of 118 patients (67%) who received IdR, and 6 of 35 patients (17%) who received BR. All AEs leading to treatment discontinuation are presented in Suppl. Table S2, and the outcomes of patients who discontinued due to AEs are presented in Suppl. Table S3.
Regarding events of clinical interest, atrial fibrillation/flutter events of any grade occurred in 12 of 154 patients (8%) in the acalabrutinib monotherapy arm versus 4 of 118 (3%) and 1 of 35 (3%) patients in the IdR and BR arms, respectively (Table 4). Two of the 12 patients in the acalabrutinib monotherapy arm who developed atrial fibrillation had a history of atrial fibrillation. Major hemorrhage events, defined as any serious or grade ≥3 hemorrhage or central nervous system hemorrhage of any grade excluding immune thrombocytopenic purpura, occurred at a similar incidence in the acalabrutinib monotherapy (n = 5; 3%), IdR (n = 3; 3%), and BR (n = 1; 3%) arms. All 5 patients in the acalabrutinib monotherapy arm who experienced grade ≥3 hemorrhage had 1 or more major risk factors for bleeding (concomitant use of anticoagulants or antiplatelets, n = 3; medical history of autoimmune thrombocytopenia, n = 1; prior trauma or surgical procedures, n = 2; predisposing pathological conditions, n = 2). Any-grade hypertension occurred in 12 (8%) patients in the acalabrutinib monotherapy arm, 7 (6%) patients in the IdR arm, and no patients in the BR arm. Infections of any grade occurred in 105 (68%) patients in the acalabrutinib monotherapy arm, 86 (73%) patients in the IdR arm, and 17 (49%) patients in the BR arm. Grade ≥3 fungal infections occurred in 2 patients in the acalabrutinib monotherapy arm (pneumonia fungal, n = 1; pneumocystis jirovecii pneumonia, n = 1) and 1 patient in the IdR arm (pneumocystis jirovecii pneumonia, n = 1) (Suppl. Table S4). Secondary primary malignancies excluding nonmelanoma skin cancers occurred in 11 (7%) patients in the acalabrutinib monotherapy arm versus 2 (2%) patients in the IdR arm and 1 (3%) patient in the BR arm; the median time from first dose to onset of these malignancies was 340.0 days (range, 29.0−1137.0) with acalabrutinib monotherapy, 454.5 days (range, 92.0−817.0) with IdR, and 29.0 days (range, 29.0−29.0) with BR. There were no cases of ventricular tachyarrhythmias. There was 1 case of sudden death in the acalabrutinib arm, which was reported as “sudden death, not otherwise specified” and considered not related to acalabrutinib by the investigator.
Table 4.
Events of Clinical Interesta
| ECI, n (%) | Acalabrutinib (n = 154) | IdR (n = 118) | BR (n = 35) | |||
|---|---|---|---|---|---|---|
| All Grades | Grade ≥3 | All Grades | Grade ≥3 | All Grades | Grade ≥3 | |
| Cardiac events | 24 (16) | 8 (5) | 12 (10) | 6 (5) | 3 (9) | 3 (9) |
| Atrial fibrillation/flutter | 12 (8) | 2 (1) | 4 (3) | 1 (1) | 1 (3) | 1 (3) |
| Hemorrhage | 47 (31) | 4 (3) | 10 (8) | 3 (3) | 2 (6) | 1 (3) |
| Major hemorrhageb | 5 (3) | 4 (3)c | 3 (3) | 3 (3)d | 1 (3) | 1 (3)e |
| Hypertension | 12 (8) | 7 (5) | 7 (6) | 1 (1) | 0 | 0 |
| Infections | 105 (68) | 45 (29) | 86 (73) | 40 (34) | 17 (49) | 4 (11) |
| Second primary malignancies | 28 (18) | 13 (8) | 5 (4) | 1 (1) | 2 (6) | 2 (6) |
| Second primary malignancies excluding non-melanoma skin | 11 (7) | 10 (6) | 2 (2) | 1 (1) | 1 (3) | 1 (3) |
| Tumor lysis syndrome | 1 (1) | 1 (1) | 1 (1) | 1 (1) | 0 | 0 |
aECIs were based on combined AE terms for infections, bleeding events, hypertension, and second primary malignancies excluding non-melanoma skin and on a single AE term for atrial fibrillation or flutter.
AEs were graded according to the Common Toxicity Criteria of the National Cancer Institute, version 4.03 and reported until 30 d after the last dose of study drug or at documented disease progression, whichever was longer.
bMajor hemorrhage was defined as any serious or grade .3 hemorrhage or central nervous system hemorrhage of any grade, excluding immune thrombocytopenic purpura.
cIncludes events of grade 4 gastrointestinal hemorrhage (n = 1), grade 3 gastrointestinal hemorrhage (n = 1), grade 4 immune thrombocytopenic purpura (n = 1), and grade 3 intestinal hemorrhage (n = 1).
dIncludes events of grade 3 gastrointestinal hemorrhage (n = 1), grade 3 and 4 immune thrombocytopenic purpura (n = 1), and grade 3 hematuria (n = 1).
eGrade 3 hemorrhagic anemia and grade 3 tumor hemorrhage (n = 1).
AE = adverse event; BR = bendamustine plus rituximab; ECI = event of clinical interest; IdR = idelalisib plus rituximab.
In the acalabrutinib monotherapy arm, 16 of 154 patients (10%) experienced at least 1 fatal (grade 5) AE versus 9 of 118 patients (8%) in the IdR arm and 2 of 35 patients (7%) in the BR arm (Suppl. Table S5).
DISCUSSION
Similar to the findings of the previously reported primary analysis of ASCEND at a median follow-up of 16.1 months,11 this final analysis of ASCEND demonstrates continued and significant improvement in PFS with acalabrutinib monotherapy versus investigator’s choice of treatment (IdR or BR) at a median follow-up of 46.5 months in patients with R/R CLL. The PFS rates with acalabrutinib monotherapy were also consistently numerically higher compared with IdR/BR in patients with high-risk genomic features, including those with del(17p) and/or TP53 mutation and those with unmutated IGHV. The favorable tolerability profile of acalabrutinib was generally maintained in this final analysis relative to the primary report, with no new safety findings identified.11
Compared with the primary analysis of ASCEND,11 the present analysis demonstrated favorable efficacy and consistent safety results with approximately 30 months of additional follow-up. At approximately 4 years of follow-up in the final analysis of ASCEND, the reduction in the risk of progression or death (ie, PFS) with acalabrutinib versus investigator’s choice of therapy (IdR/BR) was 72%, slightly higher than the 69% risk reduction reported in the primary analysis at a median of 16.1 months of follow-up.11 Median OS remains NR in either treatment arm at the final analysis; however, the reduction in the risk of death increased from 16% in the primary analysis (95% CI, 0.42–1.66; P = 0.61)11 to 31% (95% CI, 0.46–1.04; P = 0.078) in the present analysis. Median OS also remains NR in acalabrutinib- and IdR/BR-treated patients with or without del(17p) and/or TP53 mutation; OS was significantly prolonged with acalabrutinib in patients without del(17p) and TP53 mutation (HR, 0.56; P = 0.025), but not in patients with del(17p) and/or TP53 mutation (HR, 0.90; P = 0.759). The investigator-assessed ORR and CR rates (CR + CR with incomplete blood count recovery) for acalabrutinib also increased from 79% and 2%, respectively, in the primary analysis11 to 83% and 6% in the final analysis. The tolerable safety profile of acalabrutinib was maintained, with a low incidence of any-grade cardiac events of clinical interest reported in both the final and primary11 analyses (16% and 13%, respectively).
The results from the final analysis of ASCEND are in line with outcomes reported from studies of other BTK inhibitors, including ibrutinib and zanubrutinib, in R/R CLL. In the RESONATE study at a median follow-up of 44 months in patients with R/R CLL who received a median 3 prior lines of therapy, ibrutinib also demonstrated a median PFS and median OS of NR; 54% of patients had discontinued ibrutinib (27% due to disease progression and 12% due to AEs).13 In the phase 1/2 AU-003 study at a median follow-up of 43.7 months in patients with R/R CLL who received a median 2 prior lines of therapy, zanubrutinib demonstrated a median PFS of 61.4 months, and 22% of patients had discontinued zanubrutinib treatment due to disease progression.14
The combination of venetoclax plus an anti-CD20 antibody therapy is another effective targeted treatment option in the R/R CLL setting. In the MURANO study, which evaluated the combination of venetoclax plus rituximab in patients with R/R CLL who had received a median 1 prior line of therapy,15 the 4-year PFS rate was 57% and the OS rate was 85%16; 18% had discontinued treatment due to AEs.15 The results from the MURANO study were similar to the results seen with acalabrutinib monotherapy in the present analysis, where the 42-month PFS rate was 62% and the 42-month OS rate was 78%, with 23% of patients having discontinued treatment due to AEs. While recent reports of long-term follow-up data in patients with R/R CLL apply mostly to patients previously treated with chemoimmunotherapy, the future treatment landscape for R/R CLL will be different, as most patients will receive BTK inhibitors and other targeted therapies in the first-line setting.11
In the final analysis of ASCEND, the incidences of grade ≥3 AEs, SAEs, and AEs leading to treatment discontinuation remained higher in the IdR arm versus the acalabrutinib and BR arms, consistent with the primary report.11 The safety profile of acalabrutinib in this final analysis of the ASCEND study was consistent with reports from other long-term phase 2 and 3 studies of acalabrutinib monotherapy in patients with previously treated CLL.9,17 In the first phase 3 head-to-head study (ELEVATE-RR) of 2 BTK inhibitors (acalabrutinib vs ibrutinib) in patients with previously treated CLL, the incidence of any-grade atrial fibrillation/flutter was lower with acalabrutinib (9.4% vs 16.0%, respectively; P = 0.02), as was the incidence of hypertension (9.4% vs 23.2%; P < 0.001), at a median follow-up of 40.9 months.9 These incidences were somewhat lower in the final analysis of the ASCEND study for acalabrutinib monotherapy (8% for both atrial fibrillation/flutter and hypertension) at a similar median study follow-up.
One limitation of this final analysis of the ASCEND study is the high percentage of patients (52%) who crossed over from the IdR/BR arm to the acalabrutinib monotherapy arm at progression. Because patients were not censored at progression/crossover for the OS analysis, the ability to detect differences in OS between the treatment arms was limited. In addition, the 6-cycle treatment duration for rituximab and bendamustine in the IdR/BR comparator arm relative to the treat-to-progression regimens for acalabrutinib monotherapy and idelalisib should be considered when interpreting both the efficacy and safety data in the ASCEND study. Finally, while the IdR/BR comparator regimen was considered a standard-of-care treatment at the time of study initiation, these regimens, particularly idelalisib, are not considered a preferred therapy for patients with R/R CLL in the current treatment paradigm.18
In conclusion, at ~4 years follow-up, the final analysis of the phase 3 ASCEND study demonstrated a significant and clinically meaningful improvement in PFS with acalabrutinib monotherapy versus IdR or BR treatment regimens, regardless of high-risk genomic subgroup in this population of patients with R/R CLL. Acalabrutinib also demonstrated a tolerability profile consistent with the known safety profile of acalabrutinib. These findings support the use of acalabrutinib monotherapy as an effective treatment for patients with R/R CLL, including those with high-risk disease characteristics.
ACKNOWLEDGMENTS
We thank all the patients, their families, and the investigators and their study teams who participated in this study. The study was sponsored by AstraZeneca. Medical writing assistance, funded by AstraZeneca, was provided by Claire Jarvis, PhD, and Cindy Gobbel, PhD, of Peloton Advantage, LLC, an OPEN Health company, under the direction of the authors.
AUTHOR CONTRIBUTIONS
PG, AP, MW, DL, MS, IK, AI, JdS, SD, PC, GM, AJ, EA, GU, and WJ were the study investigators. PG, AP, MW, DL, MS, IK, AI, JdS, SD, PC, GM, AJ, EA, JL, WJ provided patients or study materials. PG, AP, MW, DL, MS, IK, AI, JdS, SD, PC, JL, WJ collected and assembled the data. AP, MS, MW analyzed the data. PG, MS, MW, TY, WJ did data interpretation. PG, MW, MS, IK, AJ, EA, JL, WJ prepared the manuscript. All authors participated in the critical review and revision of this manuscript and provided approval of the manuscript for submission.
DISCLOSURES
PG: Consulting or Advisory Role: AbbVie, AstraZeneca, BeiGene, BMS, HemaSphere (editor), Janssen, MSD, Lilly/Loxo, Roche, Sanofi; Honoraria: AbbVie, AstraZeneca, BeiGene, BMS, Janssen, MSD, Lilly/Loxo, Roche, Sanofi; Research Funding: AbbVie, AstraZeneca, Janssen Oncology. AP: Speakers’ Bureau: Kedrion Pharma; Honoraria: Celgene, Servier, Takeda, Novartis; Research Funding: Janssen-Cilag, Kartos Therapeutics, Iqvia, Roche, Acerta Pharma, Pharmacyclics, BeiGene, Takeda. DL: Consulting or Advisory Role: AbbVie, AstraZeneca, Novartis, Janssen. MS: Stock and Other Ownership: Lilly, AbbVie, Johnson and Johnson, Merck, Novartis, Gilead Sciences; Honoraria: AbbVie, Roche, Janssen-Cilag, Gilead Sciences, AstraZeneca; Consulting or Advisory Role: AbbVie, Janssen-Cilag, AstraZeneca; Travel, Accommodations, Expenses: AbbVie, AstraZeneca, Janssen-Cilag. IK: Consulting or Advisory Role: Takeda, Roche, Janssen, AbbVie, MSD, Bayer, Pfizer; Speakers’ Bureau: Takeda, Roche, Janssen, AbbVie, MSD, Bayer, Pfizer; Research Funding: Merck, Syneoth, Janssen, Dr. Reddy’s, Bayer, AbbVie, BeiGene; Expert Testimony: Takeda, Roche, Janssen, AbbVie, MSD, Bayer, Pfizer. AI: Consulting or Advisory Role: Janssen, Takeda, Novartis, Pfizer, Roche, Celgene; Travel, Accommodations, Expenses: Novartis, Janssen, Pfizer, Roche; Research Funding: Takeda, Seattle Genetics. JdS: Consulting or Advisory Role: AstraZeneca, AbbVie, Janssen; Speakers’ Bureau: AbbVie, Janssen, AstraZeneca; Travel, Accommodations, Expenses: AbbVie, AstraZeneca. GM: Honoraria: Janssen, Incyte, Roche; Consulting or Advisory Role: Janssen Scientific Affairs, Incyte, Roche. AJ: Stock and Other Ownership Interests: AstraZeneca, GlaxoSmithKline; Honoraria: AbbVie, AstraZeneca; Consulting or Advisory Role: AstraZeneca; Speakers’ Bureau: AstraZeneca; Research Funding: AstraZeneca. EA: Consulting or Advisory Role: AstraZeneca, Janssen Biotech; Travel, Accommodations, Expenses: AstraZeneca; Stock and Other Ownership: Macrogenics, Moderna Therapeutics; Research Funding: Lilly. GU: Honoraria: AbbVie, Pfizer; Consulting or Advisory Role: AbbVie, Bayer, Pfizer, AstraZeneca, Telios, UCB, Karyopharm Therapeutics, Janssen, Takeda, Ascentage Pharma, Oncopeptides, IL-Yang Pharm. MW: Employment and Stock Ownership: AstraZeneca. TY: Employment and Stock Ownership: AstraZeneca. WJ: Consulting or Advisory Role: AstraZeneca, BeiGene, Janssen, Loxo, Sandoz, Roche; Research Funding: AbbVie, AstraZeneca, Bayer, BeiGene, Celltrion, Celgene, Debiopharm, Epizyme, Incyte, Janssen, Loxo, Merck, MEI Pharma, MorphoSys, Novo Nordisk, Roche, Sandoz, Takeda, TG Therapeutics. All the other authors have no conflicts of interest to disclose.
Supplementary Material
Footnotes
Previous Presentation: Presented in part at the Annual Meeting of the American Society of Clinical Oncology, June 3–7, 2022, Chicago, IL, USA.
Clinicaltrial.gov Identifier: NCT02970318.
Supplemental digital content is available for this article.
REFERENCES
- 1.Wen T, Wang J, Shi Y, et al. Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances. Leukemia. 2021;35:312–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imbruvica [package insert]. Sunnyvale, CA, Horsham, PA: Pharmacyclics, Janssen Biotech, Inc.2022. [Google Scholar]
- 3.Munir T, Brown JR, O’Brien S, et al. Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am J Hematol. 2019;94:1353–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Archibald WJ, Rabe KG, Kabat BF, et al. Atrial fibrillation in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib: risk prediction, management, and clinical outcomes. Ann Hematol. 2021;100:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pellegrini L, Novak U, Andres M, et al. Risk of bleeding complications and atrial fibrillation associated with ibrutinib treatment: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;159:103238. [DOI] [PubMed] [Google Scholar]
- 6.Caldeira D, Alves D, Costa J, et al. Ibrutinib increases the risk of hypertension and atrial fibrillation: systematic review and meta-analysis. PLoS One. 2019;14:e0211228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barf T, Covey T, Izumi R, et al. Acalabrutinib (ACP-196): a covalent Bruton tyrosine kinase inhibitor with a differentiated selectivity and in vivo potency profile. J Pharmacol Exp Ther. 2017;363:240–252. [DOI] [PubMed] [Google Scholar]
- 8.Estupiñán HY, Berglöf A, Zain R, et al. Comparative analysis of BTK inhibitors and mechanisms underlying adverse effects. Frontiers Cell Develop Biol. 2021;9:630942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase 3 trial. J Clin Oncol. 2021;39:3441–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calquence [package insert]. Wilmington, DE: AstraZeneca Pharmaceuticals; 2022. [Google Scholar]
- 11.Ghia P, Pluta A, Wach M, et al. ASCEND: phase III, randomized trial of acalabrutinib versus idelalisib plus rituximab or bendamustine plus rituximab in relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol. 2020;38:2849–2861. [DOI] [PubMed] [Google Scholar]
- 12.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Byrd JC, Hillmen P, O’Brien S, et al. Long-term follow-up of the RESONATE phase 3 trial of ibrutinib vs ofatumumab. Blood. 2019;133:2031–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cull G, Burger JA, Opat S, et al. Zanubrutinib for treatment-naïve and relapsed/refractory chronic lymphocytic leukaemia: long-term follow-up of the phase I/II AU-003 study. Br J Haematol. 2022;196:1209–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seymour JF, Kipps TJ, Eichhorst B, et al. Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378:1107–1120. [DOI] [PubMed] [Google Scholar]
- 16.Kater AP, Wu JQ, Kipps T, et al. Venetoclax plus rituximab in relapsed chronic lymphocytic leukemia: 4-year results and evaluation of impact of genomic complexity and gene mutations from the MURANO phase III study. J Clin Oncol. 2020;38:4042–4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrd JC, Wierda WG, Schuh A, et al. Acalabrutinib monotherapy in patients with relapsed/refractory chronic lymphocytic leukemia: updated phase 2 results. Blood. 2020;135:1204–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gentile M, Martino EA, Visentin A, et al. Validation of a survival-risk score (SRS) in relapsed/refractory CLL patients treated with idelalisib-rituximab. Blood Cancer J. 2020;10:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.