Abstract
Esophageal leiomyomas and granular cell tumors (GCTs) are the 2 most common subepithelial tumors found in the esophagus. We attempted to differentiate the 2 tumors using endoscopic findings and endoscopic ultrasound (EUS) features. Between December 2008 and June 2021, a total of 38 esophageal GCTs and 11 esophageal leiomyomas originating from the muscularis mucosa were selected. Clinical characteristics and endoscopic features were retrospectively reviewed. Although esophageal GCTs are mainly located in the lower third of the esophagus (81.6%), esophageal leiomyomas are mainly located in the upper third of the esophagus (45.5%). Broad-based (84.2%, P = .002) and whitish-to-yellowish color changes (97.4%, P < .001) are significant endoscopic features of esophageal GCTs. The echogenicity of esophageal leiomyoma was similar to that of proper muscle echogenicity. However, the echogenicity of esophageal GCTs was hyperechoic compared to that of the proper muscle layer (90.0% vs 9.1%, respectively, P < .001). EUS revealed a clearer hyperechoic epithelial lining in the esophageal leiomyoma than in esophageal GCTs (100% vs 26.7%, respectively, P < .001). The 5 endoscopic factors (location of the lower third, broad base, whitish-to-yellowish color, hyper-echogenic, and unclear demarcated hyperechoic epithelial line) were counted to differentiate esophageal GCTs from esophageal leiomyomas. Tumors with 3 or more endoscopic factors were all esophageal GCTs. The characteristic endoscopic and EUS features of esophageal GCTs were broad-based, whitish-to-yellowish colored subepithelial tumors located in the lower third of the esophagus and hyperechoic tumor with an unclear demarcated hyperechoic epithelial line. A combination of these features can predict esophageal GCTs before endoscopic resection.
Keywords: endoscopic ultrasound, esophagus, granular cell tumor, leiomyoma
1. Introduction
Esophageal granular cell tumors (GCTs), previously known as Abrikossoff tumors or granular cell myoblastoma, are rare soft-tissue masses likely derived from Schwann cells.[1] Esophageal GCTs are known as the 2nd most common esophageal subepithelial tumors (SETs) following esophageal leiomyomas, which are benign soft tissue masses arising from the smooth muscle of the muscularis mucosa or muscle propria.[2,3] Therefore, if esophageal leiomyomas do not cause complications or symptoms such as dysphagia, tumor excision is not recommended. However, although most esophageal GCTs are indolent and show a benign course, less than 2% of GCTs found in the body are determined malignant.[4] Thus, resection of all esophageal GCTs should be considered, irrespective of the symptoms. Consequently, differential diagnosis between esophageal GCTs and leiomyomas is important for determining appropriate management strategies for esophageal SETs.
Because SETs are located beneath the epithelial layer, differential diagnosis by conventional endoscopy is difficult, especially for SETs without overlying mucosal changes. In addition, endoscopic forceps biopsy for esophageal SET is inadequate for definitive diagnosis of tumors because the SETs are located beneath the epithelium and the bite-on-bite technique to obtain a large amount of tissue may result in hemorrhage requiring endoscopic hemostatic procedure.[5] In recent years, endoscopic ultrasound (EUS) has been widely used for the diagnosis of gastrointestinal SETs based on the layer of origin and internal echogenicity. However, EUS findings of both esophageal GCTs and leiomyomas have been reported as hypoechoic masses in the muscularis mucosa or submucosa layer.[6] In general, the accuracy of diagnostic EUS without tissue acquisition is reportedly 45.5% to 66.3%.[7,8] Therefore, SETs that have high-risk endoscopic features such as increasing tumor size and surface ulceration need definitive tissue acquisition using various endoscopic techniques such as EUS-Fine needle aspiration and biopsy or direct endoscopic forceps biopsy assisted with unroofing technique.[9–11] However, these endoscopic techniques could be performed safely in highly selected institutions.
Therefore, in the present study, we attempted to differentiate esophageal GCTs and leiomyomas using conventional endoscopic findings and high-frequency EUS-probe examinations.
2. Methods
2.1. Patients
Medical records of patients diagnosed with either esophageal GCTs or leiomyomas between December 2008 and June 2021 in the Pusan National University Yangsan Hospital were considered for this study. Those that were confirmed by histopathology after endoscopic mucosal resection or endoscopic forceps biopsy were included in the study. Finally, 45 patients had been enrolled in the study (34 with GCTs, 11 with leiomyomas).
2.2. Histopathology
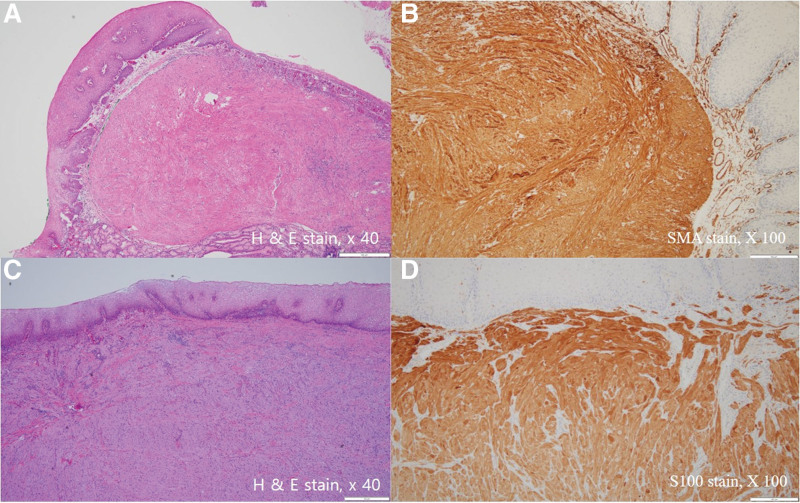
The resected or biopsied specimens were fixed in 10% formalin, embedded in paraffin wax, and sliced into sections of 2 mm thickness. The tissue sections were stained with hematoxylin and eosin, anti-S-100 antibody, anti-smooth muscle actin antibody, and c-kit (CD117). GCTs were defined as S-100 positive tumors, and leiomyomas were defined as smooth muscle actin-positive and c-kit-negative tumors[12,13] (Fig. 1). All tissue slides were blindly reviewed by 2 pathologists. Discordant cases were reevaluated under a multi-headed microscope to reach agreement.
Figure 1.
Histologic findings of endoscopically resected esophageal leiomyoma and granular cell tumor. (a) and (b) show relatively well-demarcated subepithelial tumor mass composed of spindle cells positive for smooth muscle actin (SMA). (c) and (d) show relatively unclear demarcated subepithelial tumor composed of polygonal cells positive for S100.
2.3. Conventional endoscopic and endoscopic ultrasound examinations
After the detection of esophageal SETs, several endoscopic photographs were obtained. All enrolled esophageal SETs were firm. The locations of the lesions were classified as upper, middle, and lower third according to the distance from the incisor teeth: upper third (15–24 cm), middle third (24–32 cm), and lower third (32–40 cm). Lesion size was estimated from pathologic specimens or EUS findings. Erosive esophagitis was defined as definite esophageal mucosal erosion. Gross type tumors were described as narrow-based or broad-based tumors. Narrow-based tumors were defined as elevated lesions with a clear notched base or peduncle, and broad-based tumors were defined as elevated lesions without a notch or peduncle. The color of the overlying mucosa was recorded as whitish-to-yellowish or reddish color compared to the surrounding normal esophageal mucosa. The surface appearance of tumors is classified as round, flat, or cobblestone (or molar tooth appearance) (Fig. 2).
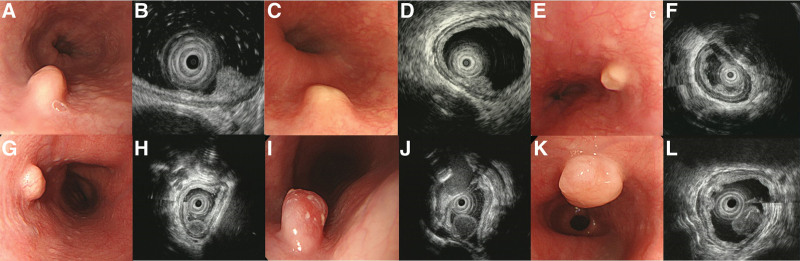
Figure 2.
Endoscopic and EUS findings of esophageal granular cell tumors and leiomyoma originated from muscularis mucosa. (a) Shows esophageal GCT with normal overlying mucosa with narrow base, (c) shows broad-based yellowish surface GCT, (e) shows broad based yellowish molar tooth appearance GCT. (b), (d), (f) show hyperechoic with unclear overlying hyperechoic epithelial line during EUS. (g) Shows leiomyoma with normal overlying mucosa with narrow base, (i) showed leiomyoma with reddish surface change with narrow base. (k) Shows leiomyoma with whitish-to-yellowish surface change with narrow base. (h), (j), (l) show hypoechoic mass similar to proper muscle layer with clear hyperechoic epithelial line. EUS = endoscopic ultrasonography, GCT = granular cell tumor.
EUS was performed in 30 GCTs and 11 leiomyomas with a high-frequency EUS catheter probe (20 MHz; Olympus, Tokyo, Japan) following the water-filled method. All endoscopic examinations were performed under intravenous conscious sedation (midazolam 2.5–8 mg). Approximately 5 to 10 endosonograms were recorded for each lesion, and these images were reviewed by 3 experienced endosonographers (DG Ryu, SJ Kim, and Choi CW), who had previously performed more than 1000 examinations and were blinded to the final diagnosis. Discordant cases were reevaluated to reach an agreement. The following endoscopic and EUS features were recorded for all the tumors: maximal diameter; echogenicity in comparison with the normal proper muscle layer (hyperechoic or not); homogeneity (homogenous or heterogeneous), and; clarity of the circular hyperechoic epithelial line.
2.4. Statistical analysis
Statistical analyses were performed for each lesion. The differences in the conventional endoscopic and EUS features between GCTs and leiomyomas were assessed using the chi-square test, and patient age and tumor size were assessed using the Student’s t-test. Statistical significance was set at P < .05. The sensitivity and specificity of endoscopic features for diagnosing GCTs were evaluated through receiver operating characteristic (ROC) analysis. Statistical calculations were performed using PASW Statistics for Windows, Version 21.0, (SPSS Inc., Chicago, IL).
2.5. Ethical statement
The present study was approved by the Ethics Committee of Pusan National University Yangsan Hospital, where this study was performed (Institutional Review Board no. 05-2021-259). Informed consent was waived by the ethics committee (Institutional Review Board of Pusan National University Yangsan Hospital) because the subject’s medical records were anonymized prior to analysis. The study was conducted in accordance with the principles of the Declaration of Helsinki.
3. Results
3.1. Baseline characteristics
During the study period, a total of 38 esophageal GCTs (from 35 patients) and 11 esophageal leiomyomas originating from the muscularis mucosa were enrolled. Among patients with GCTs, 2 (5.7%, 2/35) had multiple esophageal GCTs. The patients’ mean ages for esophageal GCTs and esophageal leiomyoma were 56.0 ± 8.4 and 63.9 ± 13.1 years, respectively. Esophageal GCTs were slightly male predominant (55.3%), but esophageal leiomyomas were predominantly female (62.7%). Most tumors were found during screening endoscopic examination (77.6%). Endoscopic biopsies were performed for 32 GCTs and seven leiomyomas. In all patients, only one time of biopsy was performed. Twenty-two GCTs (68.8%, 22/32) and none of the esophageal leiomyomas were confirmed by endoscopic forceps biopsy (Table 1).
Table 1.
Baseline characteristics of enrolled patients with esophageal granular cell tumors and leiomyomas.
| Granular cell tumor (n = 38) | Leiomyoma (n = 11) | Total (n = 49) | |
|---|---|---|---|
| Age, years, median (range) | 53 (39–80) | 64 (39–78) | 56 (39–80) |
| Male, Sex, n (%) | 21 (55.3) | 3 (27.3) | 24 (49.0) |
| Follow up days, median (range) | 265 (6–2965) | 1019 (8–4131) | 371 (6–4131) |
| Diagnostic method, n (%) | |||
| Biopsy only | 4 (10.5) | 0 (0) | 4 (8.2) |
| Cap-EMR | 2 (5.3) | 0 (0) | 2 (4.1) |
| EMR | 4 (10.5) | 9 (81.8) | 13 (26.5) |
| Ligation-EMR | 26 (68.4) | 2 (18.2) | 28 (57.1) |
| Underwater-EMR | 2 (5.3) | 0 (0) | 2 (4.1) |
| Symptoms, n (%) | |||
| Dyspepsia | 1 (2.6) | 2 (18.2) | 3 (6.1) |
| Epigastric pain | 1 (2.6) | 0 (0) | 1 (2.0) |
| Globus | 1 (2.6) | 1 (9.1) | 2 (4.1) |
| Without symptoms | 31 (81.6) | 7 (63.6) | 38 (77.6) |
| Reflux | 4 (10.5) | 1 (9.1) | 5 (10.2) |
EMR = endoscopic mucosal resection.
3.2. Conventional endoscopic features
The mean tumor size between esophageal GCTs and leiomyomas was not significantly different (6.8 ± 2.5 vs 8.0 ± 2.7 mm, respectively). Although esophageal GCTs are mainly located in the lower third of the esophagus (81.6%), esophageal leiomyoma was mainly located in the upper third of the esophagus (45.5%) (P = .010). Only 13.2% of esophageal GCTs were combined with erosive esophagitis. Broad-based (84.2%, P = .002) and whitish-to-yellowish color changes (97.4%, P < .001) were significant endoscopic features of esophageal GCTs (Table 2).
Table 2.
Endoscopic features of esophageal granular cell tumors and leiomyomas.
| Granular cell tumor (n = 38) | Leiomyoma (n = 11) | Total (n = 49) | P value | |
|---|---|---|---|---|
| Tumor location, n (%) | .010 | |||
| Lower third | 31 (81.6) | 4 (36.4) | 35 (71.4) | |
| Middle third | 1 (2.6) | 2 (18.2) | 3 (6.1) | |
| Upper third | 6 (15.8) | 5 (45.5) | 11 (22.4) | |
| Lesion size (mm, mean ± SD) | 6.8 ± 2.5 | 8.0 ± 2.7 | 7.1 ± 2.6 | .173 |
| Erosive esophagitis, n (%) | 5 (13.2) | 0 (0) | 5 (10.2) | .204 |
| Gross type, n (%) | .002 | |||
| Narrowed-base | 6 (15.8) | 7 (63.6) | 13 (26.5) | |
| Broaded-base | 32 (84.2) | 4 (36.4) | 36 (73.5) | |
| Surface color, n (%) | <.001 | |||
| Normal | 1 (2.6) | 8 (72.7) | 9 (18.4) | |
| Whitish to yellowish | 37 (97.4) | 1 (9.1) | 38 (77.6) | |
| Reddish | 0 (0) | 2 (18.2) | 2 (4.1) | |
| Surface appearance, n (%) | .597 | |||
| Flat | 11 (28.9) | 4 (36.4) | 15 (30.6) | |
| Cobble stone | 13 (34.2) | 2 (18.2) | 15 (30.6) | |
| Round | 14 (36.8) | 5 (45.5) | 19 (38.8) |
3.3. Endoscopic ultrasound features
All tumors showed homogeneous hypoechoic SETs originating from the muscularis mucosa or submucosa layers. There was no evidence of tumor invasion into adjacent organs in all patients. Two different EUS features were different between esophageal GCTs and leiomyomas. The echogenicity of esophageal leiomyoma was similar to that of proper muscle echogenicity (hypoechoic). However, the echogenicity of esophageal GCTs was hyperechoic compared to that of the proper muscle layer (90.0% vs 9.1%, respectively, P < .001). Hyperechoic epithelial lining was clearer in esophageal leiomyoma than esophageal GCTs (100% vs 26.7%, respectively, P < .001) (Table 3).
Table 3.
Characteristics of endoscopic ultrasound between esophageal granular cell tumors and leiomyomas.
| Granular cell tumor (n = 30) | Leiomyoma (n = 11) | Total (n = 41) | P value | |
|---|---|---|---|---|
| Echogenicity compared with proper muscle, n (%) | <.001 | |||
| Hyperechoic | 27 (90.0) | 1 (9.1) | 28 (68.3) | |
| Isoechoic | 3 (10.0) | 10 (90.9) | 13 (31.7) | |
| Well demarcated hyperechoic epithelial line, n (%) | 8 (26.7) | 11 (100) | 19 (46.3) | <.001 |
3.4. Combination of 5 endoscopic factors associated with esophageal GCTs
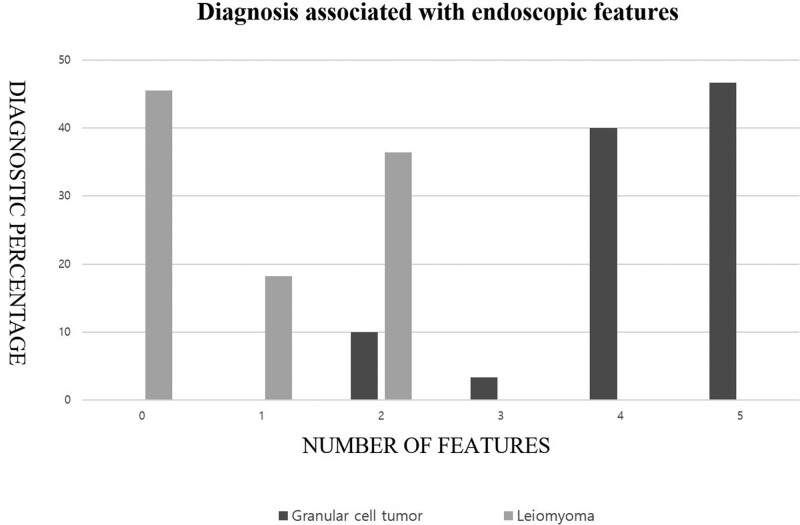
The 5 significant endoscopic factors from univariate analysis (location of lower third, broad-based, whitish-to-yellowish color, hyper-echogenic, and unclear demarcated hyperechoic epithelial line) were combined to differentiate esophageal GCTs from esophageal leiomyoma. Tumors with 3 or more endoscopic factors were all esophageal GCTs (sensitivity of 90%, specificity 100%). Tumors with 1 or 0 endoscopic feature were all esophageal leiomyomas (Fig. 3 and Table 4).
Figure 3.
Combination conventional endoscopy and endoscopic ultrasound can differentiate esophageal granular cell tumor and leiomyoma.
Table 4.
Diagnostic sensitivity and specificity of cumulative endoscopic features*.
| Numbers | Granular cell tumor (n = 30) | Leiomyoma (n = 11) | Sensitivity | Specificity |
|---|---|---|---|---|
| 5, n (%) | 14 (46.7) | 0 (0) | 0.467 | 1.000 |
| 4, n (%) | 12 (40.0) | 0 (0) | 0.867 | 1.000 |
| 3, n (%) | 1 (3.3) | 0 (0) | 0.900 | 1.000 |
| 2, n (%) | 3 (10.0) | 4 (36.4) | 1.000 | 0.636 |
| 1, n (%) | 0 (0) | 2 (18.2) | 1.000 | 0.455 |
| 0, n (%) | 0 (0) | 5 (45.5) | 1.000 | 0.000 |
The 5 endoscopic factors (location of lower third, broad base, whitish-to-yellowish color, hyper-echogenic, and unclear demarcated hyperechoic epithelial line).
4. Discussion
In the present study, we discovered significant conventional endoscopic and EUS features that help differentiate esophageal GCTs from esophageal leiomyomas originating from the muscularis mucosa. The combination of conventional endoscopic findings such as tumor location (upper third), broad-based appearance, and whitish-to-yellowish surface color are important endoscopic findings during initial endoscopic examinations. After conventional endoscopic examinations, EUS is a valuable tool for characterizing SETs. More significant EUS findings of GCTs were hyperechoic than proper muscle and an unclear demarcated hyperechoic epithelial line from GCT. Considering both conventional endoscopic and EUS findings, all esophageal GCTs had 3 or more endoscopic characteristic findings.
In South Korea, free-of-charge biennial endoscopic examinations for adults aged over 40 years is recommended by the National Cancer Gastric Screening Program. Increasing screening endoscopic examination leads to increased detection of asymptomatic upper gastrointestinal SETs. The most common location of gastrointestinal SETs is the stomach; and esophageal SETs account for 19.2% of upper gastrointestinal SETs.[14] Reportedly, the 2 most common esophageal SETs are leiomyomas and GCTs.[2,3] However, the management plans for these 2 SETs are different. Esophageal leiomyoma is a benign tumor in which endoscopic resection is not mandatory. Although esophageal GCTs are rare and definite treatment guidelines are not established, less than 2% of GCTs have been reported as malignant tumors.[4] Therefore, in curative resection of esophageal GCTs, if possible, endoscopic resection should be considered. Moreover, in our study, resection was recommended for patients with GCT even if the size was small. In contrast, in cases of leiomyoma, we recommended follow up examinations. Four patients with GCT diagnosed by forceps biopsy were followed up because they did not want to undergo resection. Fortunately, these patients did not show any significant changes during the follow-up period.
However, definite endoscopic differentiation using conventional endoscopy and EUS findings is difficult. Endoscopic forceps biopsy is a valuable confirmatory diagnostic tool for gastrointestinal epithelial tumors such as dysplasia, squamous cell carcinoma or adenocarcinoma. However, the diagnostic rate of endoscopic forceps biopsy may be inadequate because the main tumors are located beneath the epithelium. In the present study, endoscopic forceps biopsy did not reveal leiomyoma, and 68.8% of esophageal GCTs were confirmed by endoscopic forceps biopsy. This finding may be associated with the EUS features with a hyperechoic definite line in leiomyoma and unclear demarcation in GCTs. Pathologic findings of the resected esophageal GCT showed that the GCTs were located closer to the epithelium than the leiomyoma. Therefore, endoscopic forceps biopsy could be an efficient diagnostic tissue for GCT; if highly suspicious esophageal GCTs are present, endoscopic forceps biopsy may be diagnostic in some cases.
Other previous studies as well as the present study showed that most esophageal GCTs were <10 mm.[6,15,16] All esophageal GCTs found in the present study were benign. Most esophageal GCTs are found without symptoms during endoscopic screening for gastric cancer. One previous study reported that chronic inflammation, such as reflux esophagitis, might be the cause of esophageal granular cell tumors. However, the present study showed no significant endoscopic or symptomatic differences between esophageal GCTs and esophageal leiomyomas.[16] In contrast to esophageal leiomyoma, most of the esophageal GCTs were located in the lower third of the esophagus. This finding is comparable with those of previous reports.[6,15,16]
On endoscopic examination, GCTs are known as hard, smooth-surfaced SETs with whitish-to-yellowish surface color change. Sometimes it is referred to as “molar tooth appearance” or “sweet corn appearance.[16,17]” In the present study, most of the esophageal GCTs showed broad-based tumors with whitish-to-yellowish color changes. The closer location of GCTs to the epithelial layer in the pathologic findings may be associated with the detection of surface color changes during endoscopic examination. However, the molar tooth appearance and cobble stone surface appearance were not significantly different in the present study. In addition to endoscopic findings, we evaluated the different EUS patterns between GCTs and leiomyomas originating from the muscularis mucosa. In general, EUS findings of GCTs are homogenous hypoechoic submucosal tumors with clearly demarcated lines.[18] However, some previous studies have reported that GCTs have hyperechoic echogenicity than proper muscle layers and unclear borders.[15,17] Our study showed similar EUS findings. The echogenicity of GCTs was hyperechoic in 90% of cases compared to the proper muscle. In addition, we focused on the unclear demarcated border of the hyperechoic epithelial line on the surface of the tumor. The leiomyoma is a well-circumscribed tumor; however, GCT is known to be a variably (well to poorly) circumscribed SET.[15] In the present study, a well-demarcated hyperechoic epithelial line was less frequent in GCTs than in leiomyomas (26.7% vs 100%, respectively). Generally, GCTs show lower cellularity than leiomyoma on pathologic examination, and lower cellular density is associated with higher echogenicity compared with muscle echogenicity. In addition, GCTs are located closer to the hyperechoic epithelium than the leiomyoma. Therefore, during EUS examination, differentiation between the hyperechoic epithelial line and hyperechoic GCTs may be difficult. The unique findings of the present study, combined with endoscopic and EUS findings, may be helpful for the differential diagnosis between GCTs and leiomyomas. In the present study, more than 3 features of EUS or endoscopic findings were related to esophageal GCTs. None of the features were associated with esophageal leiomyoma.
This study had some limitations. First, a retrospective medical record review comparing the endoscopic and EUS features of esophageal GCTs and leiomyomas originating from the muscularis mucosa has a selective bias. Second, a single center study with a small number of cases such as this may not generalize the study results. However, considering the rare incidence of esophageal GCTs, the additive effect of information from the present study to previous studies may be useful for managing esophageal GCTs.
In conclusion, most GCTs showed broad-based, whitish-to-yellowish-colored SETs located in the lower third of the esophagus. Additional EUS findings of hyperechoic submucosal tumors with unclear demarcated hyperechoic epithelial linings were features of esophageal GCTs. A combination of these features would be helpful for diagnosing esophageal GCTs to determine the need for endoscopic resection.
Acknowledgments
Dae Gon Ryu, Su Jin Kim and Cheol Woong Choi share the first author ship.
Author contributions
Dae Gon Ryu, Cheol Woong Choi and Su Jin Kim designed the study and wrote the main manuscript text. Cheol Woong Choi supervised the study. Hyung Wook Kim and Su Bum Park collected the data. Chung Su Hwang and Bong Soo Son gave technical support. All authors reviewed the manuscript.
Abbreviations:
- EUS =
- endoscopic ultrasonography
- GCT =
- granular cell tumor
- ROC =
- receiver operating characteristic
- SET =
- subepithelial tumor
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Ryu DG, Kim SJ, Choi CW, Hwang CS, Kim HW, Park SB, Son BS. Combination conventional endoscopy and endoscopic ultrasound can differentiate between esophageal granular cell tumors and leiomyomas. Medicine 2022;101:45(e31435).
This study was supported by a 2022 research grant from Pusan National University Yangsan Hospital.
Contributor Information
Dae Gon Ryu, Email: gon22gon@naver.com.
Su Jin Kim, Email: mdkhwook@gmail.com.
Chung Su Hwang, Email: chungsu1982@gmail.com.
Hyung Wook Kim, Email: mdkhwook@gmail.com.
Su Bum Park, Email: psubumi@hanmail.net.
Bong Soo Son, Email: wtknight98@gmail.com.
References
- [1].Rejas RA, Campos MS, Cortes AR, et al. The neural histogenetic origin of the oral granular cell tumor: an immunohistochemical evidence. Med Oral Patol Oral Cir Bucal. 2011;16:e6–10. [DOI] [PubMed] [Google Scholar]
- [2].Lee LS, Singhal S, Brinster CJ, et al. Current management of esophageal leiomyoma. J Am Coll Surg. 2004;198:136–46. [DOI] [PubMed] [Google Scholar]
- [3].Thumallapally N, Ibrahim U, Kesavan M, et al. Esophageal granular cell tumor: a case report and review of literature. Cureus. 2016;8:e782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marolleau F, Baert F, Mertens V, et al. Abrikossoff cell tumor of the oesophagus: a case report and review of the literature. Acta Clin Belg. 2008;63:273–6. [DOI] [PubMed] [Google Scholar]
- [5].Ji JS, Lee BI, Choi KY, et al. Diagnostic yield of tissue sampling using a bite-on-bite technique for incidental subepithelial lesions. Korean J Intern Med. 2009;24:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wang HQ, Liu AJ. Esophageal granular cell tumors: case report and literature review. World J Gastrointest Oncol. 2015;7:123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Karaca C, Turner BG, Cizginer S, et al. Accuracy of EUS in the evaluation of small gastric subepithelial lesions. Gastrointest Endosc. 2010;71:722–7. [DOI] [PubMed] [Google Scholar]
- [8].Lim TW, Choi CW, Kang DH, et al. Endoscopic ultrasound without tissue acquisition has poor accuracy for diagnosing gastric subepithelial tumors. Medicine (Baltim). 2016;95:e5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Choi CW, Kang DH, Kim HW, et al. Direct endoscopic biopsy for subepithelial tumor larger than 20 mm after removal of overlying mucosa. Scand J Gastroenterol. 2017;52:779–83. [DOI] [PubMed] [Google Scholar]
- [10].Lee JH, Choi KD, Kim MY, et al. Clinical impact of EUS-guided trucut biopsy results on decision making for patients with gastric subepithelial tumors ≥2 cm in diameter. Gastrointest Endosc. 2011;74:1010–8. [DOI] [PubMed] [Google Scholar]
- [11].Standards of PC, Faulx AL, Kothari S, et al. The role of endoscopy in subepithelial lesions of the GI tract. Gastrointest Endosc. 2017;85:1117–32. [DOI] [PubMed] [Google Scholar]
- [12].Ordonez NG, Mackay B. Granular cell tumor: a review of the pathology and histogenesis. Ultrastruct Pathol. 1999;23:207–22. [DOI] [PubMed] [Google Scholar]
- [13].Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000;13:1134–42. [DOI] [PubMed] [Google Scholar]
- [14].Song JH, Kim SG, Chung SJ, et al. Risk of progression for incidental small subepithelial tumors in the upper gastrointestinal tract. Endoscopy. 2015;47:675–9. [DOI] [PubMed] [Google Scholar]
- [15].Kim DU, Kim GH, Ryu DY, et al. Endosonographic features of esophageal granular cell tumors using a high-frequency catheter probe. Scand J Gastroenterol. 2011;46:142–7. [DOI] [PubMed] [Google Scholar]
- [16].Shi Y, Chai N, Zhong L, et al. Experience with esophageal granular cell tumors: clinical and endoscopic analysis of 22 cases. Dig Dis Sci. 2021;66:1233–9. [DOI] [PubMed] [Google Scholar]
- [17].Iwamuroe M, Tanaka T, Kanzaki H, et al. Esophageal granular cell tumors can be differentiated from leiomyomas using endoscopic ultrasonography. Intern Med. 2018;57:1509–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Palazzo L, Landi B, Cellier C, et al. Endosonographic features of esophageal granular cell tumors. Endoscopy. 1997;29:850–3. [DOI] [PubMed] [Google Scholar]