Background:
The aim of this meta-analysis is to investigate the association between Angiotensin II type 1 receptor (AT1R)-1166A/C, Angiotensin II type 2 receptor (AT2R)-1675A/G polymorphisms and susceptibility to preeclampsia (PE).
Methods:
Online databases, including Web of Science, PubMed, EMBASE, CINAHL, CENTRAL, Scopus, Lilacs/SciELO, and Chinese National Knowledge Infrastructure, China Wan Fang, China Science and Technology Journal Database, were used to perform the literature search up to April 2022. The odds ratio (OR) and 95% confidence interval (CI) were used as effect size. The data was analyzed by Stata 15.0 software.
Results:
According to the inclusion and exclusion criteria, a total of 22 case-control studies were identified, including 3524 cases and 6308 controls. Our meta-analysis showed that the AT1R -1166 A/C allele was significantly associated with susceptibility to PE (A vs C: OR = 0.82, 95% CI: 0.69-0.96, P = .013), and there was significant difference in recessive gene model (AA vs AC + CC: OR = 0.81, 95% CI: 0.67-0.97, P = .021). However, no association was found between AT2R-1675A/G polymorphism and susceptibility to PE.
Conclusion:
our meta-analysis suggested that AT1R-1166A/C polymorphism had an association with susceptibility to PE, but AT2R-1675A/G polymorphism had no association with susceptibility to PE.
Keywords: AT1R, AT2R, meta-analysis, polymorphism, preeclampsia, susceptibility
1. Introduction
Preeclampsia (PE) is a pregnancy-specific syndrome, a type of hypertensive disorder in pregnancy, characterized by new-onset hypertension and proteinuria after 20 weeks of gestation.[1] Seriously, it will cause renal dysfunction, liver injury, pulmonary edema, heart failure and multisystem dysfunction, endangering the life and health of pregnant women and perinatal children.[2] Approximately 2% to 8% of pregnancies worldwide suffer from PE, a leading cause of maternal and perinatal morbidity and mortality.[3] However, the etiology and pathogenic mechanisms of PE have yet been poorly elucidated. Thereby, there are still no effective measures for prevention and treatment.[4] It is considered that PE is an accessory of suitable interactions among immunity, inflammation, diet, and genetic factors, leading to a decrease in trophoblast invasiveness and abnormal remodeling of uterine spiral artery.[5,6] Increasing evidences suggest that genetic factors, including gene polymorphisms, contribute to the etiology, development and complexity of PE.[7] Copy number variations have also been reported to be associated with the susceptibility to PE.[8–10] In addition, more and more studies have identified that single nucleotide polymorphisms (SNP) are closely related to susceptibility to PE.[11–13]
The circulating renin-angiotensin system (RAS), composed of a series of peptide hormones and corresponding enzymes, is an important humoral regulation system, which is involved in regulating the balance of blood pressure, water and electrolyte, and maintaining the relative stability of the human body environments.[14–16] Angiotensin (ANG) II is a major bioactive peptide produced by the hydrolysis of ANG I by ANG-converting enzyme. The ANG II is involved in regulating blood pressure, vascular growth promotion, aldosterone synthesis, and release by binding ANG receptors, mainly found in vascular smooth cells, glomerular zone cells, and some parts of the brain, heart and kidney organs.[17,18]
ANG receptors, a member of the G protein-coupled receptor family mainly, consist of ANG II type 1 receptor (AT1R) and ANG II type 2 receptor (AT2R).[19,20] Studies have reported that the expression of AT1R significantly increased in the placenta of PE pregnant women than normal pregnant women, which suggested that AT1R may participate in the pathogenesis of PE and restricted fetal growth.[21–23] Besides, previous studies showed that the abnormal expression of AT2R in the placenta during pregnancy would lead to shallow placenta implantation and participate in the pathophysiology of pregnancy-induced hypertension.[24,25] However, the exact mechanism of AT1R and AT2R on the pathogenesis of PE has still not been elucidated. A growing numbers of evidence suggest that the different polymorphisms in the AT1R gene, located on chromosome 3q21-q25, and the AT2R gene, located on X chromosome q22-23, have closely related to the susceptibility of PE. In particular, it has been defined that a single nucleotide polymorphism (SNP) in the 3’ untranslated region of the AT1R gene (-1166A/C; rs5186) and an SNP of the AT2R gene (-1675A/G; rs5194) have a significant association with susceptibility to PE. However, it is controversial for different ethnic groups about the association between the SNPs and the susceptibility to PE.[26–29] In order to attenuate the limitations of distinct epidemic genetic characteristics and insufficient sample size of individual study, we performed a systemic review and a meta-analysis of all eligible studies to discuss whether the AT1R -1166A/C, AT2R-1675A/G polymorphisms are correlated to susceptibility to PE.
2. Methods
2.1. Search strategy
Online databases, including Web of Science, PubMed, EMBASE, CINAHL, CENTRAL, Scopus, Lilacs/SciELO, Chinese National Knowledge Infrastructure, China Wan Fang, China Science and Technology Journal Database, were comprehensively searched for literature about AT1R -1166A/C and AT2R-1675A/G polymorphisms associated with Susceptibility to PE until April 2022. The searching keywords were used as follows: (“preeclampsia” OR “pre-eclampsia” OR “pregnancy hypertension”) AND (“polymorphism” OR “single nucleotide polymorphism” OR “SNP” OR “variant” OR “gene polymorphism” OR “genetic polymorphism”) AND (“angiotensin II receptor type 1” OR “AT1R” OR “angiotensin II type 1 receptor” OR “Ang II receptor type 1” OR “Ang II type 1 receptor” OR “angiotensin II receptor type 2” OR “AT2R” OR “angiotensin II type 2 receptor” OR “Ang II receptor type 2” OR “Ang II type 2 receptor”). Each database was searched independently by 2 researchers and finally cross-checked. There were no restrictions on language.
2.2. Inclusion and exclusion criteria
All studies were included in this meta-analysis according to the following criteria: Case-control study or cohort study focused on associations between AT1R -1166A/C and AT2R-1675A/G polymorphisms and susceptibility to PE; The studies provided adequate original and complete data in the case and control groups for genotype frequencies; The study participants were pregnant women with PE.
Criteria for inclusion were as follows: Meeting abstract or patent application; Editorial, review, abstract, etc; Repeated publication; the defective and incomplete data reported in the study.
2.3. Risk assessment of bias
Two researchers separately evaluated the bias risk of the included literature according to the Newcastle-Ottawa Scale (NOS),[30] and finally cross-checked. The total NOS scores were 9, with 7 to 9 as high-quality research, 5 to 6 as medium-quality research, and less than 5 as low-quality research.
2.4. Data extraction
The relevant data extraction was carefully performed from all the eligible publications by 2 independent researchers. Any divergences between the 2 researchers were resolved by discussion. The information extracted from each included study was as follows: first author, publication year, country, age of PE and controls, genotyping methods, case inclusion criteria, source of controls, total number of cases and controls group, number of cases and controls of each studied genotype frequencies.
2.5. Statistical analysis
The odds ratio (OR) and its 95% confidence interval (CI) were calculated to estimate the association between AT1R -1166A/C, AT2R-1675A/G polymorphisms and susceptibility to PE. In our meta-analysis, allele gene model, homozygous model, heterozygous, dominant, and recessive gene model were used to evaluate the association between AT1R -1166A/C, AT2R-1675A/G polymorphisms and susceptibility to PE, respectively. If P value <.05 of the heterogeneity test or I2 > 50%, the random-effects model was applied to calculate the pooled ORs; otherwise, the fixed-effects model was used. Whether the meta-analysis was significant was determined by Z test, in which P < .05 was considered that there was a statistically significant correlation between each SNP and susceptibility to PE. Begg’ s funnel plot was performed to assess potential publication bias. Stata 15.0 software (Stata Corporation) was used to perform all statistical data analysis.
3. Results
3.1. The characteristics of studies included in the meta-analysis
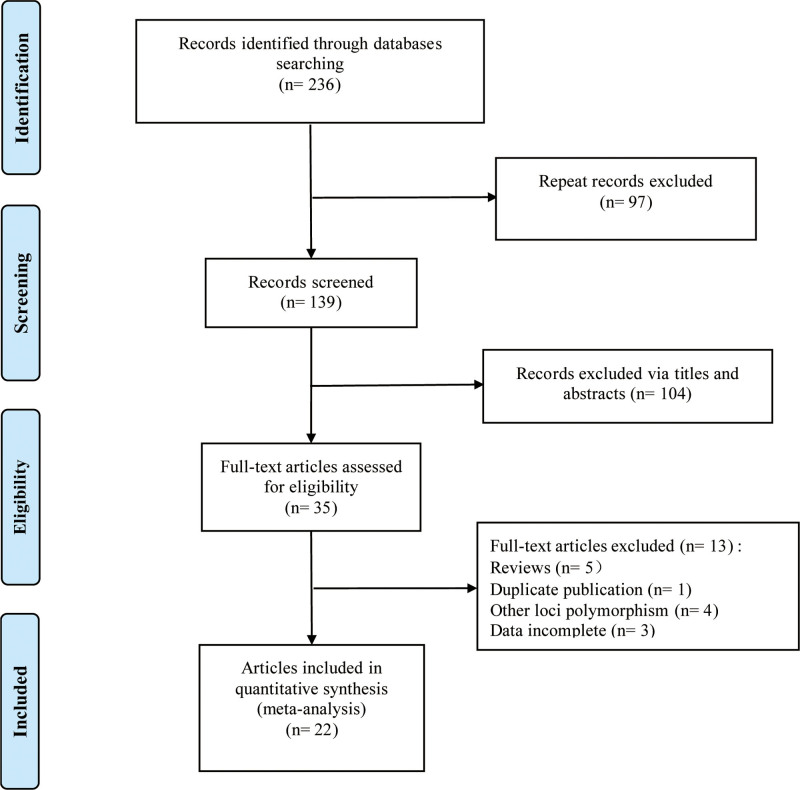
According to inclusion and exclusion criteria, a total of 22 case-control studies were included in our meta-analysis, in which the correlation research of AT1R -1166A/C and AT2R-1675A/G polymorphisms and susceptibility to PE were both involved in 4 studies.[26–29,31–48] The flow diagram of the included studies selection is shown in Figure 1. At first, a total of 81 articles were retrieved. Then, 35 duplicate studies among databases were excluded. By reviewing the title and abstract, 11 irrelevant articles were deleted. A total of 3524 cases and 6308 controls, composed of Caucasians, Asians and Africans, were included. Besides, Akbar et al’ study[29] included 3 populations. The general characteristics and NOS scores of 22 eligible studies in the meta-analysis are summarized in Table 1.
Figure 1.
Flow diagram of the selection of included studies.
Table 1.
The baseline characteristics of the eligible studies included in this meta-analysis.
| Study | Yr | Country | Methods | Ages (yrs) (Case/Control) | Case selection | Source of control | Sample size case/control | Polymorphisms studied | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Nalogowska[26] | 2000 | Poland | PCR-RFLP | 20-48/18-42 | SBP/DBP >140/90 mm Hg after 20 wks of pregnancy in a previously normotensive woman | Consecutive healthy normotensive pregnant women with median age 27 yrs recruited from the same centers | 122/144 | AT1R -1166A/C | 8 |
| Bouba[27] | 2003 | Greece | PCR-ASO | 21-45/17-48 | 1.SBP/DBP >140/90 mm Hg | Normotensive pregnant women had undergone at least 2 pregnancies | 41/102 | AT1R -1166A/C | 8 |
| 2.Proteinuria >300 mg/L or 300 mg/24 h | |||||||||
| Plummer[28] | 2004 | UK | PCR-ASO | 28.4 ± 5.76/27.6 ± 5.14 | SBP/DBP >140/90 mm Hg after 20 wks of pregnancy in a previously normotensive woman | Normotensive pregnant women | 98/118 | AT1R -1166A/C | 8 |
| Roberts[31] | 2004 | South Africa | PCR-ASO | Mean:26.3/25.0 | SBP/DBP ≥140/90 mm Hg | Healthy pregnant normotensive participants who had delivered normally | 204/338 | AT1R -1166A/C | 7 |
| Seremak-Mrozikiewicz[34] | 2005 | Poland | PCR-RFLP | 29.3 ± 5.6/27.6 ± 5.6 | The American College of Obstetricians and Gynecologists | Healthy pregnant women | 47/113 | AT1R -1166A/C | 8 |
| Benedetto[32] | 2007 | Italy | PCR-RFLP | 30 ± 4/30 ± 4 | 1.SBP/DBP >140/90 mm Hg | Normotensive pregnant women | 120/103 | AT1R -1166A/C | 8 |
| 2.Proteinuria >300 mg/24 h | |||||||||
| Li H[33] | 2007 | China | PCR-RFLP | 22-40/23-38 | 1.SBP/DBP >140/90 mm Hg | Eligible subjects who were not affected by preeclampsia in the pregnancy progressing to >20 wks gestation | 133/105 | AT1R -1166A/C | 7 |
| 2.Proteinuria > 300 mg/L | |||||||||
| Huang Y[48] | 2007 | China | PCR-RFLP | 29 ± 4; 29 ± 3 | 1.SBP/DBP ≥140/90 mm Hg | Normal pregnant women in hospital during the same period | 58/102 | AT1R -1166A/C | 7 |
| 2.Proteinuria >300 mg/24 h | |||||||||
| Jiang MQ[47] | 2008 | China | PCR-RFLP | 26.42 ± 4.10/NR | 1.SBP/DBP ≥140/90 mm Hg | Normal delivery in hospital at the same time | 55/70 | AT1R -1166A/C | 7 |
| 2.Proteinuria >300 mg/24 h | |||||||||
| Akbar[29] | 2009 | Afro-Caribbean | PCR-RFLP | 31.88 ± 5.72/28.92 ± 6.51;27.26 ± 4.91/26.45 ± 4.37; 31.85 ± 5.79; 29.72 ± 5.61 | International society for study of hypertension in pregnancy | Normal pregnancy | 67/119 | AT1R -1166A/C, AT2R-1675A/G | 8 |
| Asian-Pakistani | 122/189 | ||||||||
| Caucasian | 47/118 | ||||||||
| Deng J[45] | 2010 | China | PCR-RFLP | 30.82 ± 5.16/29.64 ± 3.25 | 1. SBP/DBP ≥140/90 mm Hg | Normal delivery women | 50/100 | AT1R -1166A/C | 7 |
| 2. Proteinuria >300 mg/24 h | |||||||||
| Procopciuc[36] | 2011 | Romania | PCR-RFLP | 28.19 ± 4.6/28.55 ± 5.09 | 1. SBP/DBP ≥140/90 mm Hg | Normal pregnant women | 21/71 | AT1R -1166A/C | 8 |
| 2. Proteinuria >300 mg/24 h | |||||||||
| Salimi[35] | 2011 | Iran | PCR-RFLP | 27.2 ± 7.8/26.2 ± 6.2 | 1.SBP/DBP ≥140/90 mm Hg | Healthy pregnant women | 125/132 | AT1R -1166A/C | 8 |
| 2.Proteinuria ≥0.3g/24 h or + 1 on a urine dipstick | |||||||||
| Zhou A[38] | 2013 | Australia and New Zealand | Mass | 26.8 ± 5.4/28.2 ± 5.6 | SBP/DBP ≥140/90 mm Hg before the onset of labor or postpartum | Normotensive pregnancies with delivery of a healthy and appropriately grown infant at 37 wks’ gestation | 115/1068 | AT1R -1166A/C, AT2R-1675A/G | 8 |
| Rahimi[37] | 2013 | Iran | PCR-RFLP | 29.3 ± 6.4/27.4 ± 6.4 | 1.SBP/DBP ≥140/90 mm Hg | Age- and parity-matched controls | 181/92 | AT1R -1166A/C | 8 |
| 2.Proteinuria ≥0.3 g/24 h | |||||||||
| Kvehaugen[39] | 2013 | Norway | Taqman | 26.6 ± 5.57/29.6 ± 5.07 | The American College of Obstetricians and Gynecologist criteria | Any woman with a DNA sample in HUNT2 registered in MBRN without a diagnosis of preeclampsia in any of their pregnancies | 1142/2309 | AT1R -1166A/C | 8 |
| Alkanli[46] | 2014 | Turkey | PCR-RFLP | 27.87 ± 6.44/27.39 ± 6.87 | The World Health Organization Detecting Pre-eclampsia | Eligible subjects who were not affected by PE | 75/75 | AT1R -1166A/C | 8 |
| Zhang H[40] | 2017 | China | Taqman | 28.77 ± 5.39/28.33 ± 4.86 | SBP/DBP ≥140/90 mm Hg, with or without convulsions or seizures | Healthy normotensive pregnant women delivering at the same hospital | 235/347 | AT1R -1166A/C | 7 |
| Andrea[41] | 2018 | Slovakia | TaqMan | 28.6 ± 4.4/28.8 ± 6.5 | 1.SBP/DBP ≥140/90 mm Hg | Healthy pregnant women delivering at term | 50/42 | AT1R -1166A/C | 8 |
| 2.Proteinuria ≥300 mg/24 h | |||||||||
| Soltani-Zangbar[42] | 2018 | Iran | PCR-RFLP | 28.3 ± 4.1/27.3 ± 3.7 | 1.SBP/DBP ≥140/90 mm Hg | Volunteer pregnant women without any history of autoimmunity, malignancy, hypertension or family history for PE | 212/218 | AT1R -1166A/C, AT2R-1675A/G | 8 |
| 2.Proteinuria ≥0.3 g/24 h or + 1 on a urine dipstick | |||||||||
| Procopciuc[43] | 2019 | Romania | PCR-RFLP | 28.7 ± 5.1/28.4 ± 4.7 | International Society for the Study of Hypertension in Pregnancy | Normal pregnant women | 87/130 | AT1R -1166A/C | 8 |
| Azimi-Nezhad[44] | 2020 | Iran | ASO | 20-37/NR | 1.SBP/DBP >140/90 mm Hg | Normotensive women with at least one normal pregnancy without history of PE | 117/103 | AT1R -1166A/C, AT2R-1675A/G | 8 |
| 2.Proteinuria >300 mg/24 h |
ASO = allele-specific oligonucleotide hybridization, AT1R = Angiotensin II type 1 receptor, AT2R = Angiotensin II type 2 receptor, DBP = diastolic blood pressure, HUNT2 = phase 2 of the Nord-Trondelag Health Study, MBRN = medical birth registry of Norway, NOS = Newcastle-Ottawa Scale, NR = not reported, PCR = polymerase chain reaction, PE = preeclampsia, RFLP = restriction fragment length polymorphism, SBP = systolic blood pressure.
3.2. Association between AT1R -1166 A/C and AT2R-1675 A/G polymorphisms and susceptibility to PE
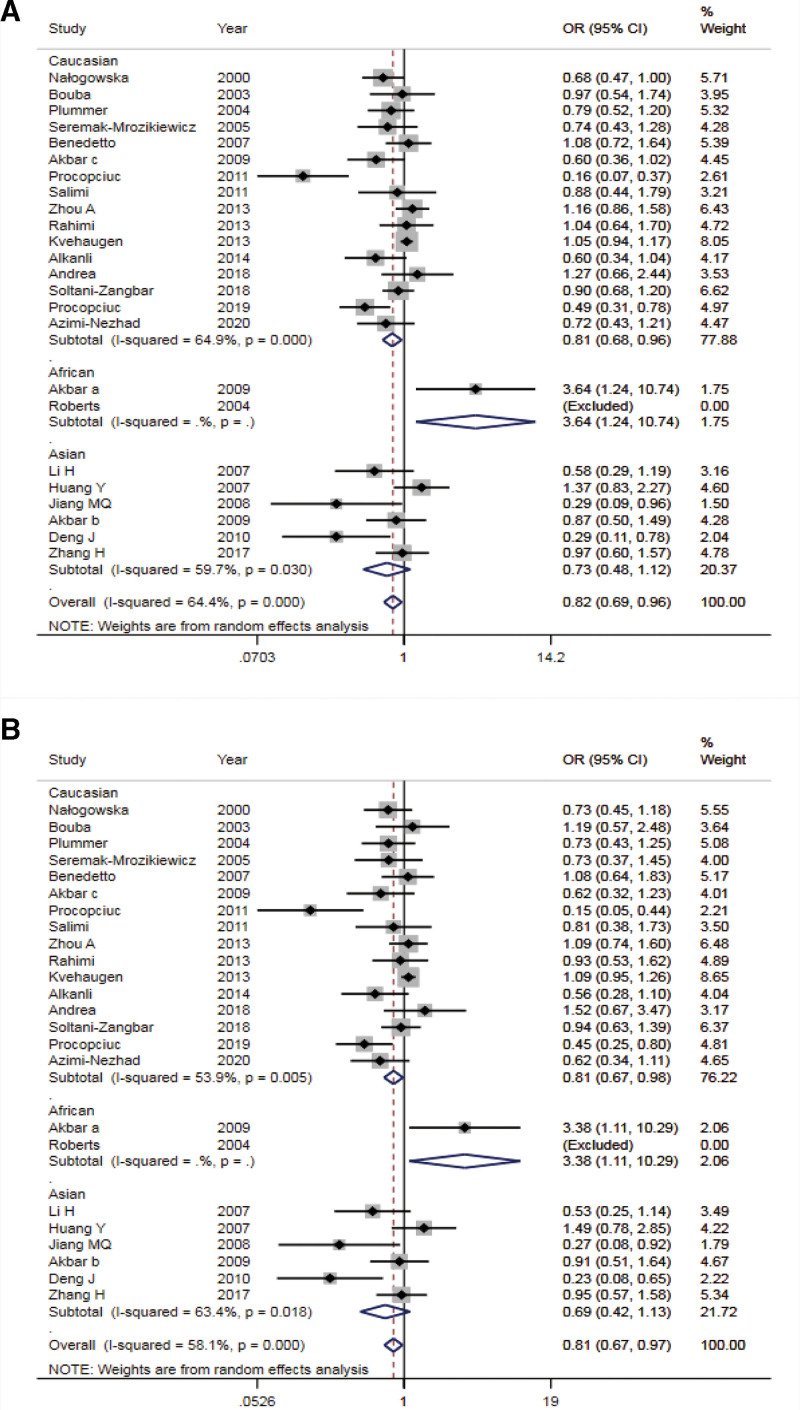
In this meta-analysis, we evaluated the association between 2 polymorphisms of AT1R -1166 A/C and AT2R-1675 A/G and susceptibility to PE. A summary of the detailed results of the meta-analysis was provided in Table 2. The pooled analysis of AT1R -1166A/C polymorphism revealed that AT1R -1166 A/C polymorphism was significantly associated with PE under allele model analysis (OR = 0.82, 95% CI: 0.69-0.96, P = .013) (Fig. 2A). Stratification analysis by ethnicity showed that there was a significant correlation between AT1R -1166 C allele and PE in Caucasians (OR = 0.81, 95% CI: 0.68-0.96, P = .014). Nevertheless, the results of AT1R -1166 A/C locus homozygous, dominant, and heterozygous models illustrated no significant association with susceptibility to PE (AA vs CC: OR = 0.89, 95% CI: 0.75-1.06, P = .131; AA + AC vs CC: OR = 0.89, 95% CI: 0.75-1.05, P = .166; AC vs CC: OR = 0.91, 95%CI: 0.75-1.10, P = .322). Similarly, no significant differences were observed by using stratification analysis based on ethnicity in homozygote and dominant models. Interestingly, there was an association between AT1R -1166 A/C polymorphism and susceptibility to PE in the analysis of the recessive gene model (AA vs AC + CC: OR = 0.81, 95% CI: 0.67-0.97, P = .021) (Fig. 2B).
Table 2.
Meta-analysis of the relationship between AT1R -1166 A/C and AT2R-1675 A/G polymorphisms and susceptibility to PE.
| Number | Study of association | Heterogeneity of study design | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Polymorphism studied | Ethnicity | Cases | Controls | OR | 95%CI | Z (P value) | P | I2 (%) | Model | |
| AT1R -1166 A/C | A vs C | Overall | 3524 | 6308 | 0.82 | 0.69-0.96 | 2.48 (.013) | <.001 | 64.4 | REM |
| Caucasian | 2600 | 4938 | 0.81 | 0.68-0.96 | 2.26(.014) | <.001 | 64.9 | REM | ||
| Asian | 653 | 913 | 0.73 | 0.48-1.13 | 1.42(.155) | .030 | 59.7 | REM | ||
| AA + AC vs CC | Overall | 3524 | 6308 | 0.89 | 0.75-1.05 | 1.38(.166) | .191 | 20.5 | FEM | |
| Caucasian | 2600 | 4938 | 0.87 | 0.73-1.03 | 1.58(.113) | .078 | 35.6 | FEM | ||
| Asian | 653 | 913 | 1.25 | 0.47-3.30 | 0.45(.652) | .804 | 0.0 | FEM | ||
| AA vs AC + CC | Overall | 3524 | 6308 | 0.81 | 0.67-0.97 | 2.30(.021) | <.001 | 58.1 | REM | |
| Caucasian | 2600 | 4938 | 0.81 | 0.67-0.98 | 2.18(.029) | .005 | 53.9 | REM | ||
| Asian | 653 | 913 | 0.69 | 0.42-1.13 | 1.49(.136) | .018 | 63.4 | REM | ||
| AA vs CC | Overall | 3524 | 6308 | 0.89 | 0.75-1.06 | 1.51(.131) | .062 | 33.8 | FEM | |
| Caucasian | 2600 | 4938 | 0.87 | 0.73-1.04 | 0.52(.604) | .021 | 46.6 | REM | ||
| Asian | 653 | 913 | 1.29 | 0.49-3.43 | 1.29(.199) | .752 | 0.0 | FEM | ||
| AC vs CC | Overall | 3524 | 6308 | 0.91 | 0.75-1.10 | 0.99(.322) | .614 | 0.0 | FEM | |
| Caucasian | 2600 | 4938 | 0.89 | 0.73-1.08 | 1.19(.232) | .435 | 1.5 | FEM | ||
| Asian | 653 | 913 | 1.45 | 0.54-3.93 | 0.74(.461) | .685 | 0.0 | FEM | ||
| AT2R-1675 A/G | A vs G | Overall | 684 | 1831 | 0.96 | 0.68-1.35 | 0.26(.798) | <.001 | 83.20 | REM |
| AA vs AG + GG | Overall | 684 | 1831 | 1.10 | 0.78-1.55 | 0.54(.592) | .073 | 50.40 | REM | |
| AA + AG vs GG | Overall | 684 | 1831 | 0.83 | 0.44-1.59 | 0.55(.580) | <.001 | 86.90 | REM | |
| AA vs GG | Overall | 684 | 1831 | 0.95 | 0.48-1.86 | 0.16(.874) | <.001 | 80.80 | REM | |
| AG vs GG | Overall | 684 | 1831 | 0.80 | 0.42-1.51 | 0.70(.484) | <.001 | 85.2 | REM | |
AT1R = angiotensin II type 1 receptor, AT2R = angiotensin II type 2 receptor, FEM = fixed-effects model, OR = odds ratio, PE = preeclampsia, REM = random-effects model.
Figure 2.
Forest plots of the AT1R -1166 A/C polymorphisms associated with susceptibility to preeclampsia. A: allele gene model; B: recessive model. AT1R = angiotensin II type 1 receptor.
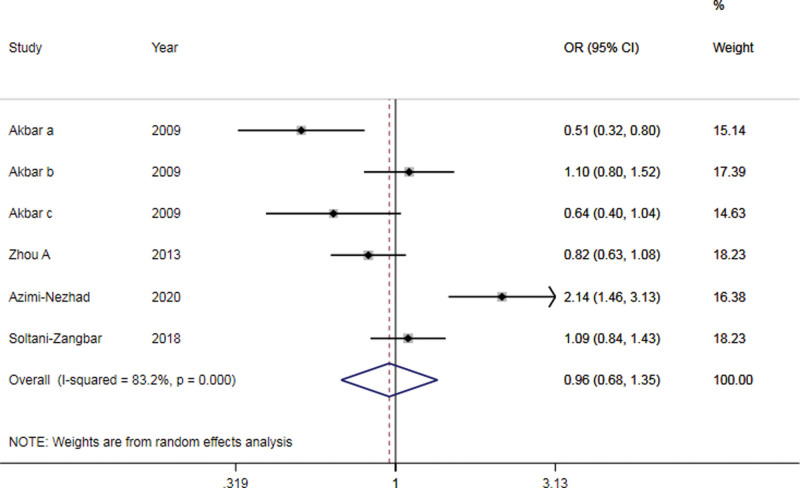
According to overall genetic model analysis, no significant associations were discovered between AT2R-1675 A/G polymorphisms and susceptibility to PE (A vs G: OR = 0.96, 95% CI: 0.68-1.439, P = .798; AA vs GG: OR = 0.95, 95% CI: 0.48-1.86, P = .87; AA + AG vs GG: OR = 1.10, 95% CI: 0.78-1.55, P = .592; AA vs AG + GG: OR = 0.83, 95% CI: 0.44-1.59, P = .580; AG vs GG: OR = 0.80, 95%CI: 0.42-1.51, P = .484) (Fig. 3 and Table 2). Owing to few cases and control groups, stratified Analysis was not carried out.
Figure 3.
Forest plots of the AT2R-1675 A/G allele gene model associated with susceptibility to preeclampsia.
3.3. Publication bias
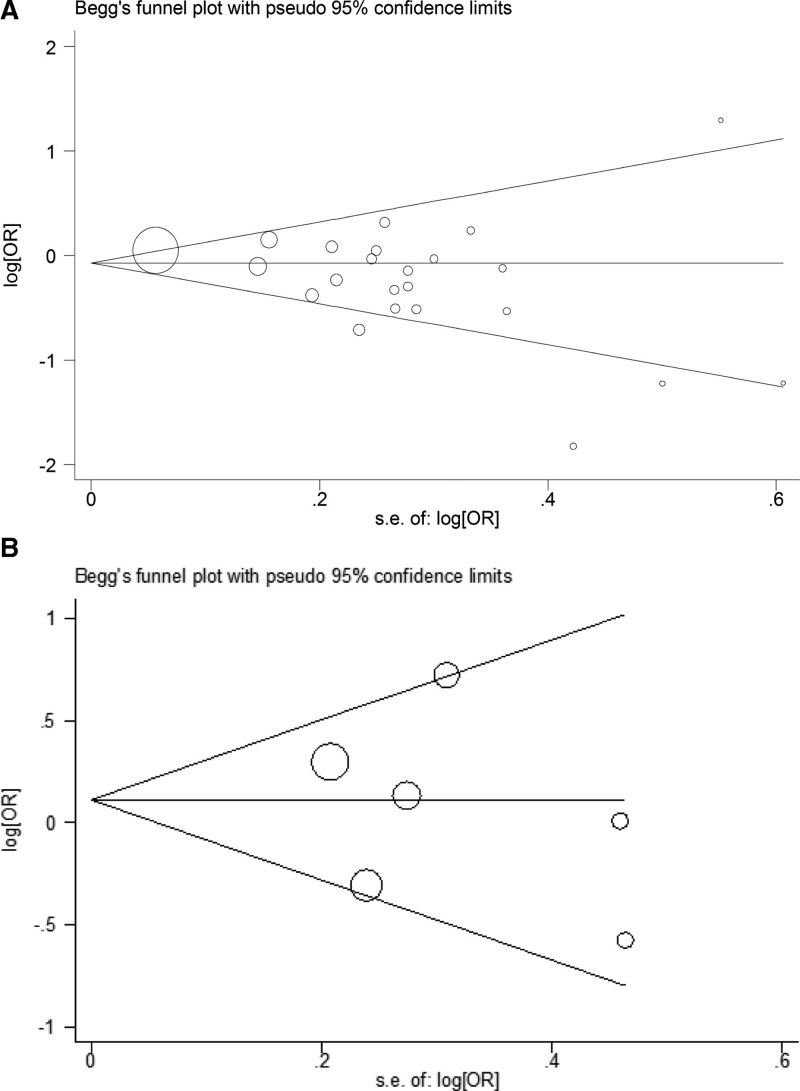
Begg’ s funnel plot was applied to assess potential publication bias, revealing no significant asymmetry in AT1R -1166 A/C (P = .113, Fig. 4A) and AT2R-1675 A/G (P = .707, Fig. 4B) polymorphisms in the allele contrast.
Figure 4.
Begg’s funnel plot for assessing publication bias of allele gene model in (A) AT1R -1166 A/C locus and (B) AT2R-1675 A/G locus related to susceptibility to preeclampsia. AT1R = angiotensin II type 1 receptor, AT2R = angiotensin II type 2 receptor.
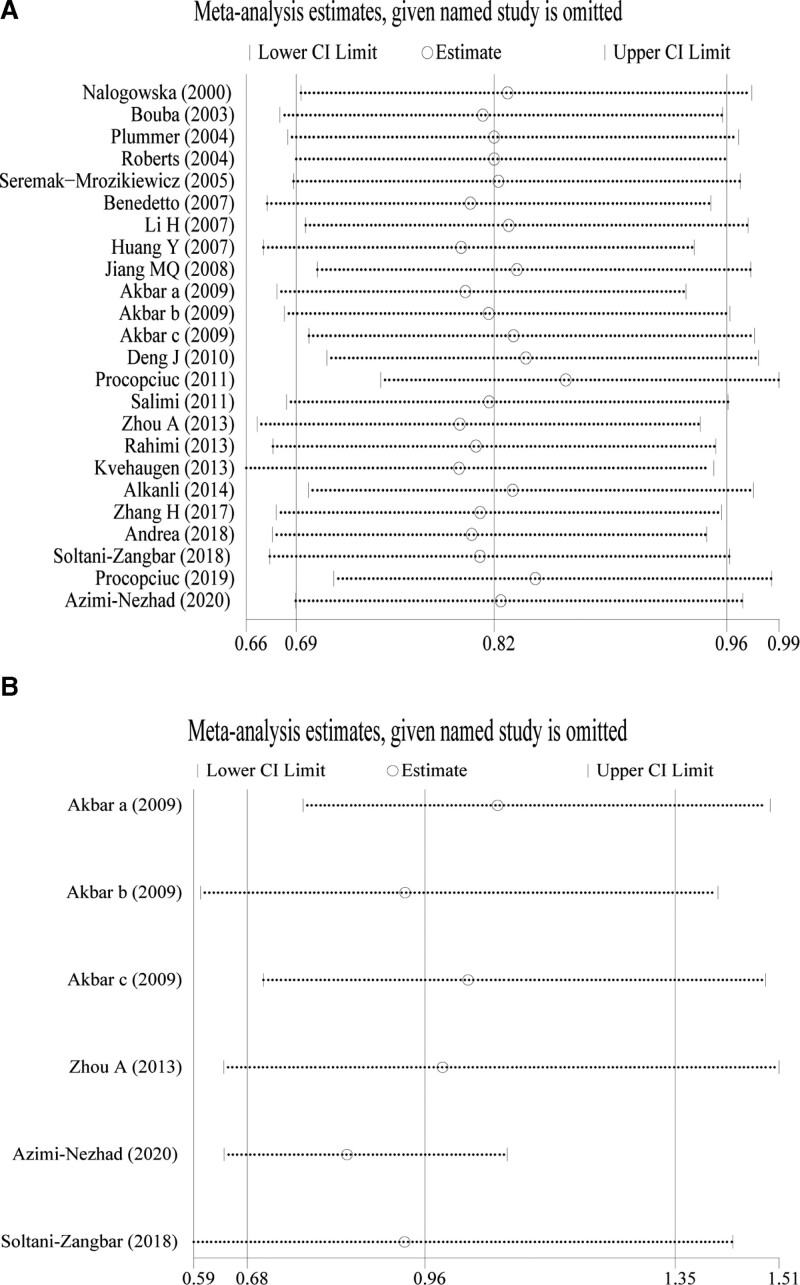
3.4. Sensitivity analysis
The robustness of the conclusions obtained was verified by excluding each study and then performing a meta-analysis again. In the analyses of the AT1R -1166 A/C (Fig. 5A) and AT2R-1675 A/G (Fig. 5B) locus allele models, the original conclusions were not significantly altered by omitting any study. In AT1R -1166A/A locus recessive gene model, when 1 study was removed,[43] the original conclusion was significantly altered (Fig. S1B, http://links.lww.com/MD/H549). When omitting any of the studies, AT1R -1166 A/C (Dominant, homozygous, and heterozygous model) and AT2R-1675 A/G (Dominant, recessive, homozygous, and heterozygous model) other gene models had no significant changes in their original findings (Fig. S1A, C, D, http://links.lww.com/MD/H549; Supplemental Fig. S2A-D, http://links.lww.com/MD/H550). This indicates that the findings of this meta-analysis are robust.
Figure 5.
Sensitivity analysis of allele gene model in (A) AT1R -1166 A/C locus and (B) AT2R-1675 A/G locus related to susceptibility to preeclampsia. AT1R = angiotensin II type 1 receptor, AT2R = angiotensin II type 2 receptor.
4. Discussion
PE, a pregnancy-specific hypertension disease, seriously endangers the life and health of pregnancies and perinatal infants. However, the etiology and pathogenesis of PE have not yet been interpreted clearly. A growing numbers of evidence show that the occurrence and development of PE are caused by the interaction of multiple variables including heredity, among which genetic polymorphism has been paid more and more attention. RAS is an important humoral regulatory system composed of a series of peptide hormones and corresponding enzymes, involved in regulating the balance of blood pressure, water and electrolyte to maintain the stability of the human body environment. Under the pathological conditions, The RAS is an important mechanism involved in the pathogenesis of hypertension.[14] Meanwhile, it has been demonstrated that almost all components of the RAS are up-regulated in normal pregnancy, but renin activity, ANG II, and aldosterone in PE decrease.[49] The underlying mechanisms remain unexplored. AT1R, a major ANG II- 1 receptor, has been reported to be involved in the occurrence of PE, which activates the RAS through the stimulation of autoantibody AT1-AA.[49,50] Several studies have demonstrated that AT1R gene polymorphism, mainly focusing on 1166 A/C polymorphism, is associated with PE.[29,51] In addition, another ANG receptor AT2R gene polymorphism has also been reported to be associated with the susceptibility to PE.[24,51] However, the conclusions of the association between AT1R and AT2R gene polymorphisms and susceptibility to PE are contradictory. Therefore, in the present study, we synthesized the data based on previous studies to investigate the correlation between AT1R and AT2R gene polymorphisms and susceptibility to PE.
AT1R is a heterologous G protein-coupled receptor, which plays a role by activating phospholipase C, tyrosine kinase, or non-receptor tyrosine kinase.[52,53] In addition, the genetic variation in the AT1R gene can promote its structural abnormalities and expression changes, thus affecting its regulatory response. The AT1R gene is located at position 24 on the long arm of chromosome 3. It has been reported that the SNPs of AT1R gene are associated with an increased risk of hypertension,[54,55] heart disease,[56,57] or diabetic nephropathy.[58] Notably, a substantial number of correlative researches have elucidated that a variation in 3’ untranslated region of the AT1R gene at 1166 position that AT1R -1166 A/C was closely related to essential hypertension and can be used as a predictor of screening hypertension susceptible families.[59] Furthermore, a growing number of studies have explored whether AT1R -1166 A/C was also associated with PE, a special hypertension disease. Nałogowska Głośnicka et al showed that AT1R -1166 A/C polymorphism was associated with the increased risk of pregnancy-induced hypertension.[26] It was also reported that the interaction between AT1R 1166 C allele and AT2R 1332 G allele was relevant to the risk of mild PE.[60] Interestingly, AT1R 1166 C allele was revealed to play a significant role in PE development among Chinese pregnant women.[40] On the contrary, in some studies, there were no correlation between AT1R -1166 A/C polymorphism and susceptibility to PE.[29,35,51] In 2015, a systematic review and meta-analysis by Li et al showed the AT1R -1166 A/C polymorphism was not associated with PIH or PE among a pooled analysis of overall genetic models,[61] which was consistent with previous results of Zhao et al’ s study.[62] Five years have passed, we updated the systematic analysis because of the new progress in studying the association between PE and AT1R -1166 A/C polymorphism. The present study shows that AT1R 1166 C allele can increase susceptibility to PE and promote the occurrence of PE in comprehensive population, as well as in Caucasians, which is inconsistent with the results of previous meta-analysis, owing to the larger sample size and changing proportion of ethnic groups. Surprisingly, no significant connection between AT1R -1166 A/C polymorphism and susceptibility to PE was found by homozygous model, dominant and heterozygous model analysis in our meta-analysis. More likely, the random-effects model analysis was performed to evaluate the correlation between AT1R -1166 A/C polymorphism and PE under allele control model and recessive contrast model.
AT2R gene is located on the X chromosome and a single nucleotide gene polymorphism (+1675G/A; rs5194) within the coding region at position + 1675 has been defined that is related to gene transcription and translation start point. AT2R gene, which consists of 3 exons and 2 introns, is located on the X chromosome and a single nucleotide polymorphism related to the transcription and translation starting point was found at + 1675 in the coding region, which has also been defined to be associated with hypertensive diseases.[63–65] Meanwhile, it was reported that AT2R-1675 A/G polymorphism was involved in PE or eclampsia in Afro-Caribbean pregnant women.[29] Mohammad et al demonstrated that AT2R-1675 A/G polymorphism was associated with PE alone but was related to susceptibility to PE in Iranian women when combined with AT1R polymorphism.[42] In 2015, a meta-analysis by Li et al[61] showed that AT2R-1675 A/G polymorphism was associated with PE. In the present study, we updated the meta-analysis including 2 new studies. The results illustrated that AT2R-1675 A/G polymorphism was not associated with susceptibility to PE. On the one hand, the previous sample size is too small to attenuate the test efficiency. On the other hand, genetic differences caused by different ethnic groups caused inconsistent results. Moreover, we found that the previous meta-analysis included the wrong literature, 2 of which studied the association between AT2R–1332A/G gene polymorphism and PE.[28,60]
Begg’s Test and funnel plot showed no significant publication bias in the analyses of AT1R -1166 A/C and AT2R -1332A/G polymorphisms associated with susceptibility to PE. Moreover, sensitivity analysis showed good robustness of the findings in our meta-analysis. Therefore, the conclusions obtained in this study are reliable.
However, there were some limitations in this meta-analysis. Firstly, the literature and sample size included in this study were limited, which might have a certain impact on the robustness of the conclusions. Secondly, Asian and Caucasian populations were mainly enrolled in our meta-analysis, while there were few studies on African populations. Among the analysis of AT1R-1166A/C locus polymorphism, the results, only including 1 study[29] of African population, showed that allele A and genotype AA significantly increased susceptibility to PE, which was inconsistent with the conclusions of Caucasian population. Limited by the number of studies, no firm conclusions can be drawn at present. Thirdly, in the study of AT2R-1675 A/G polymorphism, the 5 gene models analyzed all have significant heterogeneity. Due to the limited number of included literature, as well as the limited information provided by the original literature, it was difficult to determine the source of heterogeneity further.
In conclusion, our meta-analysis suggested that AT1R 1166 C allele had a significant association with susceptibility to PE, but AT2R-1675 A/G polymorphism had no effect on susceptibility to PE. Considering the limitations of this study, such as a limited sample size, more studies with more rigorous design and larger scope are needed to verify the findings of this meta-analysis in the future.
Author contributions
Conceptualization: Yi Quan, Long Zhang, Junliang Guo.
Data curation: Ping Liu.
Formal analysis: Ping Liu.
Investigation: Junliang Guo.
Methodology: Ping Liu, Junliang Guo.
Project administration: Junliang Guo.
Resources: Yi Quan, Ping Liu, Long Zhang.
Software: Long Zhang.
Supervision: Long Zhang.
Validation: Long Zhang.
Visualization: Long Zhang.
Writing – original draft: Yi Quan, Junliang Guo.
Writing – review & editing: Yi Quan.
Supplementary Material
Abbreviations:
- ANG =
- angiotensin
- AT1R =
- angiotensin II type 1 receptor
- CI =
- confidence interval
- NOS =
- Newcastle-Ottawa scale
- OR =
- odds ratio
- PE =
- preeclampsia
- RAS =
- renin-angiotensin system
- SNP =
- single nucleotide polymorphisms
This work was supported by Natural Science Foundation of Sichuan Province (Young Scholars Project, 2022NSFSC1532).
Supplemental Digital Content is available for this article.
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
How to cite this article: Quan Y, Liu P, Zhang L, Guo J. The effect of AT1R-1166A/C and AT2R-1675A/G polymorphisms on susceptibility to preeclampsia: A systematic review and meta-analysis. Medicine 2022;101:45(e31008).
Contributor Information
Yi Quan, Email: quanyimomo@163.com.
Ping Liu, Email: cd1202013@163.com.
Long Zhang, Email: ZhangLongSCU@163.com.
References:
- [1].Socha M, Malinowski B, Puk O, et al. The NLRP3 inflammasome role in the pathogenesis of pregnancy induced hypertension and preeclampsia. Cells-Basel. 2020;9:1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jena MK, Sharma NR, Petitt M, et al. Pathogenesis of preeclampsia and therapeutic approaches targeting the placenta. Biomolecules. 2020;10:953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gyselaers W. Preeclampsia is a syndrome with a cascade of pathophysiologic events. J Clin Med. 2020;9:2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].He A, Zhou Y, Wei Y, et al. Potential protein biomarkers for preeclampsia. Cureus. 2020;12:e8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia-eclampsia. Am J Obstet Gynecol. 2009;200:481. [DOI] [PubMed] [Google Scholar]
- [6].Mutze S, Rudnik-Schoneborn S, Zerres K, et al. Genes and the preeclampsia syndrome. J Perinat Med. 2008;36:38–58. [DOI] [PubMed] [Google Scholar]
- [7].Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40:213–20. [DOI] [PubMed] [Google Scholar]
- [8].Qiu C, Hevner K, Enquobahrie DA, et al. A case-control study of maternal blood mitochondrial DNA copy number and preeclampsia risk. Int J Mol Epidemiol Genet. 2012;3:237–44. [PMC free article] [PubMed] [Google Scholar]
- [9].Cotter AM, Martin CM, O’Leary JJ, et al. Increased fetal RhD gene in the maternal circulation in early pregnancy is associated with an increased risk of pre-eclampsia. BJOG. 2005;112:584–7. [DOI] [PubMed] [Google Scholar]
- [10].Zhao L, Triche EW, Walsh KM, et al. Genome-wide association study identifies a maternal copy-number deletion in PSG11 enriched among preeclampsia patients. BMC Pregnancy Childbirth. 2012;12:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rokni M, Salimi S, Sohrabi T, et al. Association between miRNA-152 polymorphism and risk of preeclampsia susceptibility. Arch Gynecol Obstet. 2019;299:475–80. [DOI] [PubMed] [Google Scholar]
- [12].Haram K, Mortensen JH, Nagy B. Genetic aspects of preeclampsia and the HELLP syndrome. J Pregnancy. 2014;2014:910751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Giannakou K, Evangelou E, Papatheodorou SI. Genetic and non-genetic risk factors for pre-eclampsia: umbrella review of systematic reviews and meta-analyses of observational studies. Ultrasound Obstet Gynecol. 2018;51:720–30. [DOI] [PubMed] [Google Scholar]
- [14].Te RL, van Esch JH, Roks AJ, et al. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res. 2015;116:960–75. [DOI] [PubMed] [Google Scholar]
- [15].Pahlavani M, Kalupahana NS, Ramalingam L, et al. Regulation and functions of the renin-angiotensin system in white and brown adipose tissue. Compr Physiol. 2017;7:1137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ames MK, Atkins CE, Pitt B. The renin-angiotensin-aldosterone system and its suppression. J Vet Intern Med. 2019;33:363–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Forrester SJ, Booz GW, Sigmund CD, et al. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev. 2018;98:1627–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Antonucci E, Gleeson PJ, Annoni F, et al. Angiotensin II in refractory septic shock. Shock. 2017;47:560–6. [DOI] [PubMed] [Google Scholar]
- [19].Ardaillou R. Angiotensin II receptors. J Am Soc Nephrol. 1999;10(Suppl 11):S30–9. [PubMed] [Google Scholar]
- [20].Thomas WG, Mendelsohn FA. Angiotensin receptors: form and function and distribution. Int J Biochem Cell Biol. 2003;35:774–9. [DOI] [PubMed] [Google Scholar]
- [21].Laskowska M, Laskowska K, Vinson G, et al. Evaluation of placental angiotensin type 1 receptors in women with hypertension during pregnancy. J Matern Fetal Neonatal Med. 2004;16:223–29. [DOI] [PubMed] [Google Scholar]
- [22].Mistry HD, Kurlak LO, Broughton PF. The placental renin-angiotensin system and oxidative stress in pre-eclampsia. Placenta. 2013;34:182–86. [DOI] [PubMed] [Google Scholar]
- [23].Leung PS, Tsai SJ, Wallukat G, et al. The upregulation of angiotensin II receptor AT(1) in human preeclamptic placenta. Mol Cell Endocrinol. 2001;184:95–102. [DOI] [PubMed] [Google Scholar]
- [24].Li C, Peng W, Zhang H, et al. Association of angiotensin receptor 2 gene polymorphisms with pregnancy induced hypertension risk. Hypertens Pregnancy. 2018;37:87–92. [DOI] [PubMed] [Google Scholar]
- [25].Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376:631–44. [DOI] [PubMed] [Google Scholar]
- [26].Nalogowska-Glosnicka K, Lacka BI, Zychma MJ, et al. Angiotensin II type 1 receptor gene A1166C polymorphism is associated with the increased risk of pregnancy-induced hypertension. Med Sci Monit. 2000;6:523–29. [PubMed] [Google Scholar]
- [27].Bouba I, Makrydimas G, Kalaitzidis R, et al. Interaction between the polymorphisms of the renin-angiotensin system in preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2003;110:8–11. [DOI] [PubMed] [Google Scholar]
- [28].Plummer S, Tower C, Alonso P, et al. Haplotypes of the angiotensin II receptor genes AGTR1 and AGTR2 in women with normotensive pregnancy and women with preeclampsia. Hum Mutat. 2004;24:14–20. [DOI] [PubMed] [Google Scholar]
- [29].Akbar SA, Khawaja NP, Brown PR, et al. Angiotensin II type 1 and 2 receptors gene polymorphisms in pre-eclampsia and normal pregnancy in three different populations. Acta Obstet Gynecol Scand. 2009;88:606–11. [DOI] [PubMed] [Google Scholar]
- [30].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [31].Roberts CB, Rom L, Moodley J, et al. Hypertension-related gene polymorphisms in pre-eclampsia, eclampsia and gestational hypertension in Black South African women. J Hypertens. 2004;22:945–8. [DOI] [PubMed] [Google Scholar]
- [32].Benedetto C, Marozio L, Ciccone G, et al. Synergistic effect of renin-angiotensin system and nitric oxide synthase genes polymorphisms in pre-eclampsia. Acta Obstet Gynecol Scand. 2007;86:678–82. [DOI] [PubMed] [Google Scholar]
- [33].Li H, Ma Y, Fu Q, et al. Angiotensin-converting enzyme insertion/deletion (ACE I/D) and angiotensin II type 1 receptor (AT1R) gene polymorphism and its association with preeclampsia in Chinese women. Hypertens Pregnancy. 2007;26:293–301. [DOI] [PubMed] [Google Scholar]
- [34].Seremak-Mrozikiewicz A, Dubiel M, Drews K, et al. 1166C mutation of angiotensin II type 1 receptor gene is correlated with umbilical blood flow velocimetry in women with preeclampsia. J Matern Fetal Neonatal Med. 2005;17:117–21. [DOI] [PubMed] [Google Scholar]
- [35].Salimi S, Mokhtari M, Yaghmaei M, et al. Association of angiotensin-converting enzyme intron 16 insertion/deletion and angiotensin II type 1 receptor A1166C gene polymorphisms with preeclampsia in South East of Iran. J Biomed Biotechnol. 2011;2011:941515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Procopciuc LM, Caracostea G, Zaharie G, et al. Maternal/newborn genotype contribution of the renin-angiotensin system (Met235Thr, Thr174Met, I/D-ACE, A2350G-ACE, A1166C-AT2R1, C3123A- AT2R2, 83A/G-REN) to the risk of pre-eclampsia: a Romanian study. J Renin Angiotensin Aldosterone Syst. 2011;12:539–48. [DOI] [PubMed] [Google Scholar]
- [37].Rahimi Z, Rahimi Z, Mozafari H, et al. Preeclampsia and angiotensin converting enzyme (ACE) I/D and angiotensin II type-1 receptor (AT1R) A1166C polymorphisms: association with ACE I/D polymorphism. J Renin Angiotensin Aldosterone Syst. 2013;14:174–80. [DOI] [PubMed] [Google Scholar]
- [38].Zhou A, Dekker GA, Lumbers ER, et al. The association of AGTR2 polymorphisms with preeclampsia and uterine artery bilateral notching is modulated by maternal BMI. Placenta. 2013;34:75–81. [DOI] [PubMed] [Google Scholar]
- [39].Kvehaugen AS, Melien O, Holmen OL, et al. Single nucleotide polymorphisms in G protein signaling pathway genes in preeclampsia. Hypertension. 2013;61:655–61. [DOI] [PubMed] [Google Scholar]
- [40].Zhang H, Li YX, Peng WJ, et al. The gene variants of maternal/fetal renin-angiotensin system in preeclampsia: a hybrid case-parent/mother-control study. Sci Rep. 2017;7:5087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mendelova A, Holubekova V, Grendar M, et al. Association between 3’UTR polymorphisms in genes ACVR2A, AGTR1 and RGS2 and preeclampsia. Gen Physiol Biophys. 2018;37:185–92. [DOI] [PubMed] [Google Scholar]
- [42].Soltani-Zangbar MS, Pahlavani B, Zolghadri J, et al. Angiotensin Type 2 receptor gene polymorphisms and susceptibility to preeclampsia. J Reprod Infertil. 2018;19:95–9. [PMC free article] [PubMed] [Google Scholar]
- [43].Procopciuc LM, Nemeti G, Buzdugan E, et al. Renin-angiotensin system gene variants and risk of early- and late-onset preeclampsia: a single center case-control study. Pregnancy Hypertens. 2019;18:1–8. [DOI] [PubMed] [Google Scholar]
- [44].Azimi-Nezhad M, Teymoori A, Ebrahimzadeh-Vesal R. Association of CYP11B2 gene polymorphism with preeclampsia in north east of Iran (Khorasan province). Gene. 2020;733:144358. [DOI] [PubMed] [Google Scholar]
- [45].Deng J, You FZ, Cheng GM, et al. Correlation between gene polymorphisms of ACE, ATlR, eNoS and severe preeclampsia. Maternal Child Health Care China. 2010;35:5277–79. [Google Scholar]
- [46].Alkanli N. Lack of association between ACE I/D and AGTR1 A1166C gene polymorphisms and preeclampsia in turkish pregnant women of trakya region. J Gynecol Obstetrics. 2014;4:49. [Google Scholar]
- [47].Jiang MQ, Fu F. Relationship between polymorphism of angiotensin system related genes and hypertensive disorder complicating pregnancy. Pract Clin Med. 2008;5:25–8. [Google Scholar]
- [48].Huang Y, Li YX, Shao JC, et al. Relation between genetic polymorphisms of the renin -angiotensin system and preeclampsia in Kunming Area. Yunnan Med J. 2007;1:8–10. [Google Scholar]
- [49].Irani RA, Xia Y. Renin angiotensin signaling in normal pregnancy and preeclampsia. Semin Nephrol. 2011;31:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Haspula D, Clark MA. Heterologous regulation of the cannabinoid type 1 receptor by angiotensin II in astrocytes of spontaneously hypertensive rats. J Neurochem. 2016;139:523–36. [DOI] [PubMed] [Google Scholar]
- [51].Aung M, Konoshita T, Moodley J, et al. Association of gene polymorphisms of four components of renin-angiotensin-aldosterone system and preeclampsia in South African black women. Eur J Obstet Gynecol Reprod Biol. 2017;215:180–7. [DOI] [PubMed] [Google Scholar]
- [52].Jackson L, Eldahshan W, Fagan SC, et al. Within the brain: the renin angiotensin system. Int J Mol Sci . 2018;19:876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Huang MM, Guo AB, Sun JF, et al. Angiotensin II promotes the progression of human gastric cancer. Mol Med Rep. 2014;9:1056–60. [DOI] [PubMed] [Google Scholar]
- [54].Tong J, Wang Y, Yuan J, et al. Effect of interaction between noise and A1166C site of AT1R gene polymorphism on essential hypertension in an iron and steel enterprise workers. J Occup Environ Med. 2017;59:412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Liu DX, Zhang YQ, Hu B, et al. Association of AT1R polymorphism with hypertension risk: an update meta-analysis based on 28,952 subjects. J Renin Angiotensin Aldosterone Syst. 2015;16:898–909. [DOI] [PubMed] [Google Scholar]
- [56].Amir O, Amir RE, Paz H, et al. Relation between AT1R gene polymorphism and long-term outcome in patients with heart failure. Cardiology. 2009;112:151–7. [DOI] [PubMed] [Google Scholar]
- [57].Mettimano M, Romano-Spica V, Ianni A, et al. AGT and AT1R gene polymorphism in hypertensive heart disease. Int J Clin Pract. 2002;56:574–7. [PubMed] [Google Scholar]
- [58].Ali Z, Kusrini I, Shahab A, et al. Association between A1166C polymorphism of the angiotensin II Type-1 receptor gene and type-2 diabetic nephropathy in an indonesian malay population. Acta Med Indones. 2018;50:314–9. [PubMed] [Google Scholar]
- [59].Parchwani DN, Patel DD, Rawtani J, et al. Analysis of association of angiotensin II Type 1 receptor gene A1166C gene polymorphism with essential hypertension. Indian J Clin Biochem. 2018;33:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Rahimi Z, Rahimi Z, Aghaei A, et al. AT2R -1332 G:A polymorphism and its interaction with AT1R 1166 A:C, ACE I/D and MMP-9 -1562 C:T polymorphisms: risk factors for susceptibility to preeclampsia. Gene. 2014;538:176–81. [DOI] [PubMed] [Google Scholar]
- [61].Li Y, Zhu M, Hu R, et al. The effects of gene polymorphisms in angiotensin II receptors on pregnancy-induced hypertension and preeclampsia: a systematic review and meta-analysis. Hypertens Pregnancy. 2015;34:241–60. [DOI] [PubMed] [Google Scholar]
- [62].Zhao L, Dewan AT, Bracken MB. Association of maternal AGTR1 polymorphisms and preeclampsia: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2012;25:2676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Katsuya T, Morishita R. Gene polymorphism of angiotensin II type 1 and type 2 receptors. Curr Pharm Des. 2013;19:2996–3001. [DOI] [PubMed] [Google Scholar]
- [64].Erdmann J, Guse M, Kallisch H, et al. Novel intronic polymorphism (+1675G/A) in the human angiotensin II subtype 2 receptor gene. Hum Mutat. 2000;15:487. [DOI] [PubMed] [Google Scholar]
- [65].Jin JJ, Nakura J, Wu Z, et al. Association of angiotensin II type 2 receptor gene variant with hypertension. Hypertens Res. 2003;26:547–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.