Background:
This study aimed to determine the potential advantages of midazolam co-induction with general anesthesia (GA) over the use of propofol alone.
Methods:
We conducted a randomized, placebo-controlled, single-blinded clinical trial of 102 patients, aged 18 to 65, American Society of Anesthesiologists II and III, who underwent elective laparoscopic gallbladder surgery. Patients were randomly divided into 3 groups: the placebo group (C) received 1 mL of 0.9% saline intravenously and the test groups received intravenous midazolam at doses of 0.03 mg/kg (M1) or 0.06 mg/kg (M2) before induction of GA. We assessed effects of midazolam co-induction on arterial pressure and heart rate (HR) in the early stage of GA prior to surgical incision and effects on perioperative and postoperative glycemia and cortisol levels. Systolic/mean/diastolic (SAP/MAP/DAP) arterial pressure and HR were measured 4 times (preoperative, on the third, sixth and ninth minute after atracurium administration). Cortisol was measured on 3 occasions (preoperatively, 60 minutes after surgical incision, and the following morning) and glucose on 4 occasions (preoperatively, 15 and 60 minutes after incision, and the following morning). We also assessed the incidence of postoperative anxiety, postoperative nausea and vomiting (PONV), and propofol requirement for induction.
Results:
SAP/MAP/DAP were significantly higher in M2 immediately after induction compared to the other study groups (P = .002/.004/.013). Midazolam co-induction led to a significant reduction in postoperative anxiety (P = .03), reduced cortisol concentration 60 minutes after surgical incision (P < .001) and propofol requirements (P < .001).
Conclusion subsections:
Midazolam co-induction prevented a marked decline in SAP/MAP/DAP immediately after induction of GA, led to reduced postoperative anxiety and cortisol response to surgery, and reduced propofol requirements for induction.
Keywords: cortisol, general anesthesia, glucose, hemodynamics, midazolam co-induction, PONV, postoperative anxiety
1. Introduction
Midazolam is a short-acting benzodiazepine with anxiolytic, hypnotic, anticonvulsant, muscle relaxant, and anterograde amnestic effects.[1] It has been widely used for premedication, induction and maintenance of anesthesia, and intravenous sedation.[1] Reports have further suggested it has effects on the incidence of postoperative nausea and vomiting (PONV).[2]
The term co-induction of anesthesia has been used to describe the practice of administering a small dose of a sedative or other anesthetic agent to reduce the required dose of the induction agent.[3] Midazolam, when used as co-induction agent for general anesthesia (GA), has been shown to reduce the dose of propofol required for induction by up to 50% without affecting the recovery profile.[4] In addition, other potentially beneficial properties with often conflicting results have been reported when midazolam is used as a co-induction agent.
We hypothesized that midazolam co-induction would decrease the propofol dose required to induce GA, leading to less arterial pressure decline in the early stage of GA prior to surgical incision, and lower glycemia and cortisol levels. The secondary objective was to assess the effects of midazolam co-induction on PONV and postoperative anxiety.
2. Methods
2.1. Ethical considerations
This study was approved by the University Clinical Hospital Mostar Ethics Committee (2062/14; 03.04.2014). All patients were informed of the nature of the trial, that their data would be collected, and were blinded to the treatment allocation. Written informed consent was obtained from all the patients.
2.2. Patient selection
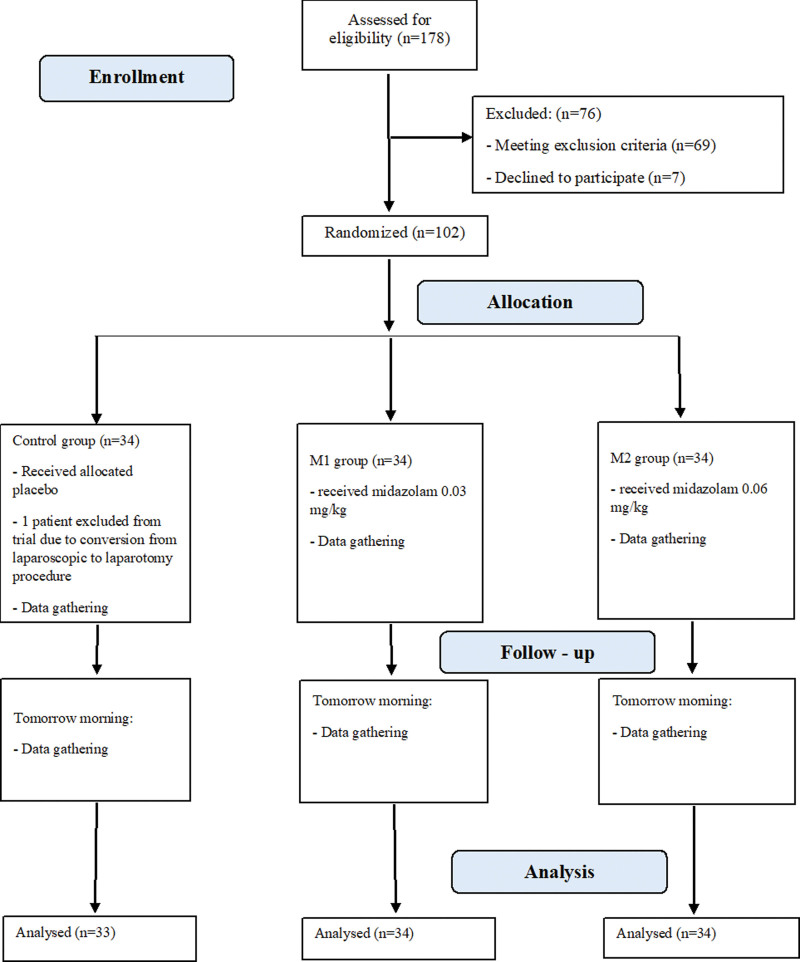
We conducted a randomized, single-center, placebo-controlled, single-blinded clinical trial from July 1, 2018 to June 26, 2020 of patients aged 18 to 65, with American Society of Anesthesiologists physical status classification II and III, scheduled for elective laparoscopic cholecystectomy. A history of diabetes mellitus, chronic benzodiazepine therapy, or chronic cardiac arrhythmias were the basic exclusion criteria. Patients with an initial arterial pressure >180/105 mm Hg or <100/60 mm Hg were also excluded as patients who were subjected to conversion from laparoscopic to laparotomy procedures. All surgical procedures were performed between 8:30 AM and 11:00 AM. The Consolidated Standards of Reporting Trials (CONSORT) flow diagram was used for patient enrollment (Fig. 1).
Figure 1.
Flow diagram showing patient enrollment.
2.3. Randomization and masking
The patients were randomly divided into 3 groups using sealed envelopes prepared by an unbiased healthcare professional. First group of patients (C) received 1 mL of 0.9% saline intravenously (iv), the second group (M1) received 0.03 mg/kg iv of midazolam and third group (M2) received midazolam at a dose of 0.06 mg/kg iv before GA was induced. Upon inclusion, patients were assigned successive numbers. Healthcare professionals who performed anesthesiologic and surgical procedures are not listed as the authors of this trial. The healthcare professionals who administered questionnaires and collected blood samples for analysis on the postoperative day were blinded to the treatment allocation and are not listed as authors.
2.4. Methods and measurements
All patients fasted overnight before the surgery. After entering the operating room, iv access was secured and monitoring was performed including lead II electrocardiography, pulse oximetry, arterial line for invasive blood pressure measurements, and bispectral (BIS) index monitoring (Aspect Medical Systems, Inc., Newton, MA).
Induction was initiated 60 seconds after midazolam/placebo administration with fentanyl 0.001 mg/kg, propofol until the appropriate depth of anesthesia was reached (BIS ≤ 60), and atracurium 0.5 mg/kg. Orotracheal intubation was performed 3 min after atracurium administration. After checking for and securing adequate tube position, surgeons approached and disinfected the patient, and covered the surgical field. The first surgical incision began after the t9 measurement point.
Immediately after intubation, a mixture of inhalation anesthetics (O2 40%, N2O 60%, sevoflurane 2%) was started on Dräger Primus (Drägerwerk AG & Co. KGaA, Lübeck, Germany) to achieve minimum alveolar concentration (MAC) = 1, corrected for age,[5] with a flow rate of 6 L/min. Upon reaching MAC = 1, the dial setting of sevoflurane was titrated to equalize it with the expiratory concentration of sevoflurane. After equalization of the inspiratory and expiratory concentrations of sevoflurane, the fresh gas flow was reduced to 3 L/min. If the BIS (40–60) or MAC (MAC = 1) values went out of the set parameters for more than 30 seconds, inspiratory sevoflurane concentration was corrected by ±10%. Infusion of 0.9% NaCl started after midazolam/placebo administration at a rate of 20 mL/min. Following the final surgical stitch, inhalational anesthetics were turned off, and patients were ventilated with 100% O2 with fresh gas flow 6 L/min. The patients were extubated in the operating room upon reaching an adequate level of consciousness and muscle strength. After reaching a modified Aldrete score of ≥9, the patients were discharged to the ward, as is standard practice at our institution. No PONV prophylaxis was administered. In cases of vomiting, metoclopramide 10 mg iv was used as a rescue antiemetic. Non-steroidal anti-inflammatory drugs were used for postoperative analgesia.
Systolic/mean/diastolic arterial pressure (SAP/MAP/DAP) and heart rate (HR) were recorded on 4 occasions: preoperatively (baseline) values (t0), 3 minutes after atracurium administration, that is, before orotracheal intubation (t3), 6 (t6), and 9 minutes (t9) after atracurium administration. We investigated the occurrence of hypotension/hypertension at measurement points defined as MAP lower/higher than 20% of the baseline values. Glycemia (GLU) was monitored on 4 occasions: preoperatively (T0), 15 (T1), and 60 (T2) minutes after surgical incision and the next morning (T4) at 8 AM. Concentrations of the hormone cortisol (COR) in the blood were monitored on 3 occasions: preoperatively (T0), 60 minutes after the surgical incision (T1), and tomorrow morning (T2) at 8 AM. The incidence of PONV in all 3 groups was investigated using a simple questionnaire. The levels of postoperative anxiety were assessed using the Zung Self-Rating Anxiety Scale (SAS) (raw score).
Enhanced recovery after the surgery pathway is not implemented at our institution.
2.5. Statistical data analysis
To calculate the required sample size, an a priori analysis was performed using G * Power program (version 3.1.9.2; University of Kiel, Germany). ANOVA tests for repeated measurements were performed with the following parameters: significance level α = 0.05, test power β = 80%, Cohen’s d factor = 0.25, correction factor for repeated measurements = 0.5, and number of repeated measurements = 4.
Statistical analysis of the collected data was performed using IBM SPSS Statistics, version 25.0. (IBM Corp., Armonk, NY, IBM Corp.), and Microsoft Excel (version 2016, Microsoft Corporation, Redmond, WA). The normality of the distribution of continuous variables was tested using the Shapiro–Wilk test. The results of the categorical variables are presented as numbers and percentages, and the χ2 or Fisher’s exact test was used to test the significance of the differences. The results of the numerical variables are presented as the mean and standard deviation, and 1-way ANOVA for independent samples was used to test the significance of the differences. P values < .05 indicated statistical significance. P values that could not be expressed in the 3 decimal places were expressed as P < .001.
3. Results
We assessed 178 patients for eligibility (Fig. 1), and 102 were ultimately included in the trial. There were 69 patients who met one or more of the exclusion criteria, and 7 patients who refused to participate. In the placebo group, 1 patient was subsequently excluded to meet the exclusion criteria. Thus, in the final analysis, the midazolam groups comprised 34 participants, with 33 participants in the control group.
Table 1 provides the baseline patient characteristics and duration of anesthesia and surgery. Overall, there were no significant differences in general patient characteristics between the study arms.
Table 1.
Patients demographics, Apfel and Modified Aldrete score, duration of anesthesia and surgery.
| Control (n = 33) | M1 (n = 34) |
M2 (n = 34) |
P | |
|---|---|---|---|---|
| Gender—n (%) | .59† | |||
| Male | 12 (36.4) | 16 (47.1) | 16 (47.1) | |
| Female | 21 (63.6) | 18 (52.9) | 18 (52.9) | |
| Age (yr)—M (SD) | 51.7 (9.2) | 49.41 (11.5) | 51.85 (11.9) | .58* |
| BMI—M (SD) | 27.0 (3.9) | 26.8 (3.9) | 27.9 (3.7) | .46* |
| ASA classification—M (SD) | 2.4 (0.5) | 2.4 (0.5) | 2.4 (0.5) | .87* |
| Apfel score—M (SD) | 1.2 (0.7) | 1.2 (0.8) | 1.1 (0.8) | .93* |
| Modified Aldrete score—M (SD) | 9.1 (0.3) | 9.1 (0.4) | 9.1 (0.4) | .94* |
| Duration of anesthesia—min (SD) | 60.3 (11.1) | 60.6 (11.7) | 61.4 (12.8) | .93* |
| MAC–hours—h (SD) | 0.8 (0.2) | 0.8 (0.2) | 0.8 (0.2) | .93* |
| Duration of surgery—min (SD) | 47.3 (10.3) | 46.6 (10.9) | 47.4 (11.6) | .94* |
| Comorbidities—n (%) | ||||
| Hypertension | 21 (63.6) | 19 (55.9) | 22 (64.7) | .71† |
| Cardiopathy | 0 | 2 (5.9) | 0 | .32‡ |
| Asthma/KOPB | 3 (9.1) | 2 (5.9) | 1 (2.9) | .52‡ |
| Obesity (BMI > 30) | 10 (30.3) | 5 (14.7) | 9 (26.5) | .29† |
| Hypothyreosis | 0 | 1 (2.9) | 2 (5.9) | .77‡ |
| Hyperthyreosis | 1 (3.0) | 2 (5.9) | 0 | .54‡ |
| Hyperlipidemia | 2 (6.1) | 2 (5.9) | 4 (11.8) | .72‡ |
| Gastritis | 1 (3.0) | 3 (8.8) | 1 (2.9) | .61‡ |
| Smoking | 15 (45.5) | 15 (41.2) | 16 (47.1) | .88† |
| Other | 2 (6.1) | 3 (8.8) | 3 (8.58) | >.99‡ |
| Medications—n (%) | ||||
| ACE inhibitors | 14 (42.4) | 18 (52.9) | 19 (55.9) | .51† |
| Beta-adrenergic blockers | 5 (15.2) | 6 (17.6) | 5 (14.7) | .93† |
| Calcium channel blockers | 2 (6.1) | 2 (5.9) | 2 (5.9) | >.99‡ |
| Alpha—adrenergic blockers | 1 (3.0) | 1 (2.9) | 2 (5.9) | >.99† |
| Statins | 4 (12.1) | 4 (11.8) | 6 (17.6) | .81‡ |
| Thyreostatics | 1 (3.0) | 2 (5.9) | 0 | .54‡ |
| Levothyroxine | 0 | 1 (2.9) | 2 (5.9) | .77‡ |
| Bronchodilators | 3 (9.1) | 2 (5.9) | 1 (2.9) | .52‡ |
| Proton pump inhibitors | 1 (3.0) | 2 (5.9) | 1 (2.9) | >.99‡ |
| Other | 0 | 1 (2.9) | 0 | >.99‡ |
ASA = American Society of Anesthesiologists, BMI = body mass index, h = hour, M (SD) = mean (standard deviation), MAC = minimum alveolar concentration; min = minutes, n (%) = number (percentage).
1-way ANOVA.
χ2 test.
Fisher’s exact test.
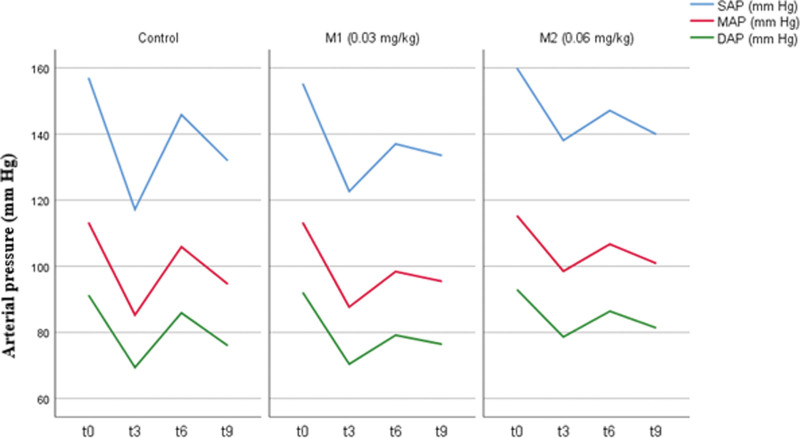
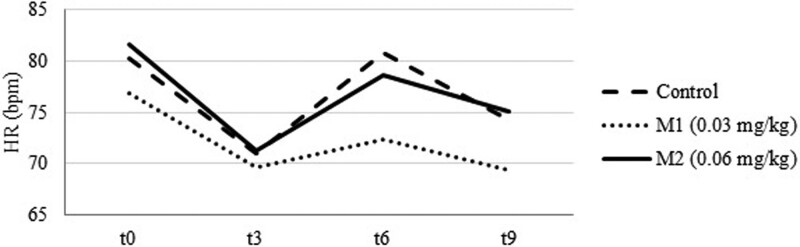
Table 2 shows values of SAP/MAP/DAP, HR, GLU and COR blood levels at measurement points. After an initial drop at t3 (Fig. 2) SAP/MAP/DAP and HR (Fig. 3) were elevated at t6 and dropped again at t9 in all tested groups. Patients in M2 group had significantly higher SAP [138.1 ± 26.4 (M2) vs 122.6 ± 21.4 (M1) vs 117.2 ± 23.7 mm Hg (C); P = .009 and P = .001 respectively, CI 95%], MAP [98.5 ± 18.6 (M2) vs 87.7 ± 15.5 (M1) vs 85.3 ± 16.5 mm Hg (C); P = .008 and P = .03 respectively, CI 95%] and DAP [78.6 ± 14.0 (M2) vs 70.4 ± 13.4 (M1) vs 69.4 ± 13.9 mm Hg (C); P = .016 and P = .007, respectively, CI 95%] at t3 than patients in M1 and C. HR was significantly higher in C than M1 [80.8 ± 12.4 (C) vs 72.3 ± 10.2 bpm (M1); P = .019, CI 95%] at the t6 measurement point. No other significant differences were found.
Table 2.
Analysis of systolic/mean/diastolic arterial pressure, heart rate, glucose and cortisol levels between groups by individual measurements.
| Control group (C) |
M1 group (0.03 mg/kg) |
M2 group (0.06 mg/kg) |
P * | |
|---|---|---|---|---|
| SAP (mm Hg) | ||||
| t0 | 157.0 (18.3) | 155.3 (18.3) | 159.9 (18.2) | .56 |
| t3 | 117.2 (23.7) | 122.6 (21.4) | 138.1 (26.4) †,‡ | .002 |
| t6 | 145.8 (32.7) | 137.0 (28.2) | 147.1 (26.3) | .30 |
| t9 | 131.9 (29.7) | 133.5 (23.7) | 139.9 (24.4) | .41 |
| MAP (mm Hg) | ||||
| t0 | 113.3 (12.9) | 113.2 (14.2) | 115.3 (13.6) | .76 |
| t3 | 85.3 (16.5) | 87.7 (15.5) | 98.5 (18.6)†,‡ | .004 |
| t6 | 105.9 (24.2) | 98.4 (19.7) | 106.7 (18.2) | .20 |
| t9 | 94.6 (20.5) | 95.4 (16.4) | 100.9 (17.5) | .30 |
| DAP (mm Hg) | ||||
| t0 | 91.3 (10.0) | 92.1 (9.0) | 92.9 (7.6) | .75 |
| t3 | 69.4 (13.9) | 70.4 (13.4) | 78.6 (14.0) †,‡ | .01 |
| t6 | 85.9 (20.7) | 79.1 (16.2) | 86.4 (14.4) | .16 |
| t9 | 76.0 (16.5) | 76.4 (13.5) | 81.4 (14.4) | .26 |
| HR (bpm) | ||||
| t0 | 80.2 (10.0) | 76.9 (11.8) | 81.7 (10.9) | .18 |
| t3 | 71.1 (11.9) | 69.6 (11.2) | 71.2 (11.0) | .81 |
| t6 | 80.8 (12.4)‡ | 72.3 (10.2) | 78.6 (13.5) | .01 |
| t9 | 74.3 (12.8) | 69.3 (12.5) | 75.1 (13.9) | .14 |
| GLU (mmol/L) | ||||
| T0 | 5.0 (0.6) | 5.1 (0.6) | 5.1 (0.7) | .68 |
| T1 | 5.7 (0.7) | 5.6 (0.8) | 5.6 (0.9) | .97 |
| T2 | 6.4 (0.7) | 6.1 (0.8) | 6.0 (0.8) | .12 |
| T3 | 4.8 (0.6) | 4.7 (0.5) | 4.8 (0.5) | .81 |
| COR (nmol/L) | ||||
| T0 | 419.5 (134.5) | 397.7 (113.1) | 408.5 (110.3) | .76 |
| T1 | 694.5 (149.9) ‡,§ | 589.6 (122.6) | 545.9 (127.9) | <.001 |
| T2 | 396.4 (128.3) | 371.5 (157.3) | 340.0 (115.2) | .24 |
COR = cortisol, DAP = diastolic arterial pressure, GLU = glucose, HR = heart rate, MAP = mean arterial pressure, SAP = systolic arterial pressure.
1-way ANOVA.
P < .05 compared to control group.
P < .05 compared to M1 group.
§P < .05 compared to M2 group.
Figure 2.
Systolic/mean/diastolic arterial pressure (SAP/MAP/DAP) values at measurement times.
Figure 3.
Heart rate (HR) values at measurement times.
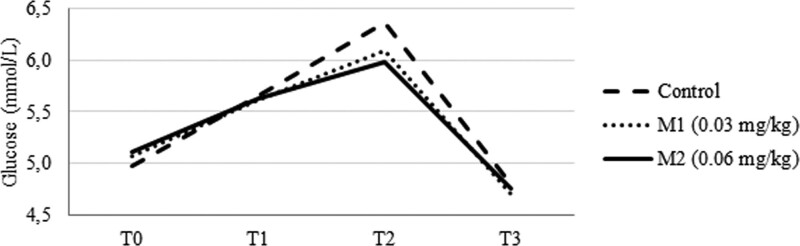
GLU levels increased at T2 and T3 (Fig. 4) in all groups and then declined at T4. The C group showed slightly higher GLU levels at all measurement points compared to M1 and M2, but this difference was not significant.
Figure 4.
Glucose levels at measurement times.
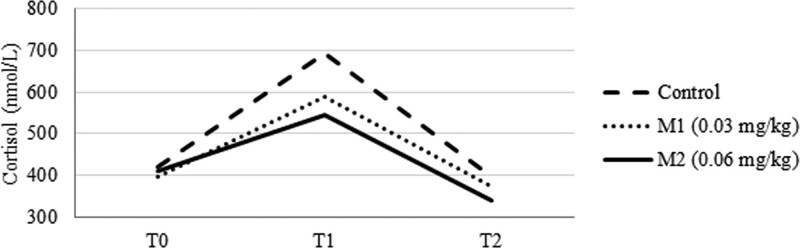
The C group had significantly higher COR levels at T1 (Fig. 5) than the intervention groups [694.5 ± 149.9 nmol/L (C) vs 589.6 ± 122.6 nmol/L (M1) vs 545.9 ± 127.9 nmol/L (M2); P = .009 and P < .001 respectively, CI 95%]. The C group also had higher COR levels the following morning than M1 and M2, but this difference was not significant.
Figure 5.
Cortisol levels at measurement times.
Table 3 shows the incidence of hypotension/hypertension, PONV, postoperative anxiety score and propofol requirements. Incidence of hypotension at t3 was higher in C and M1 compared to M2 group [18 (54.5 %) vs 18 (52.9 %) vs 9 (27.3 %); P = .033, CI 95%]. C also had a higher incidence of postoperative nausea and vomiting than the intervention groups but this difference was not statistically significant. Postoperative anxiety was statistically higher in placebo than M1 and particularly M2 group [43.4 ± 7.3 (C) vs 40.3 ± 5.1 (M1) vs 39.9 ± 4.9 (M2); P = .03 and P = .01 respectively, CI 95%]. The lower dose of midazolam significantly reduced the propofol required to induce GA and further reduction was shown at the higher dose [2.1 ± 0.2 mg/kg (C) vs 1.9 ± 0.2 mg/kg (M1) vs 1.8 ± 0.2 mg/kg (M2); P = .001 and P < .001 respectively, CI 95%).
Table 3.
Incidence of hypotension/hypertension, postoperative nausea and vomiting, postoperative anxiety score and propofol requirements.
| Control group (C) |
M1 group (0.03 mg/kg) |
M2 group (0.06 mg/kg) |
P * | |
|---|---|---|---|---|
| t3 | .03† | |||
| Hypotension—n (%) | 18 (54.5) | 18 (52.9) | 9 (27.3) | |
| Hypertension—n (%) | 1 (3.0) | 0 | 0 | |
| t6 | .85† | |||
| Hypotension—n (%) | 8 (24.2) | 8 (23.5) | 8 (24.2) | |
| Hypertension—n (%) | 3 (9.1) | 1 (2.9) | 1 (3.0) | |
| t9 | .34† | |||
| Hypotension—n (%) | 11 (33.3) | 15 (44.1) | 9 (27.3) | |
| Hypertension—n (%) | 0 | 0 | 0 | |
| Anxiety (Raw score)—M (SD) | 43.4 (7.3) | 40.3 (5.1) | 39.9 (4.9) | .03* |
| Propofol (mg kg−1)—M (SD) | 2.1 (0.2) | 1.9 (0.2) | 1.8 (0.2) | <.001* |
| Nausea—n (%) | 19 (57.6) | 17 (50.0) | 13 (38.2) | .29† |
| Vomiting—n (%) | 12 (36.4) | 8 (23.5) | 6 (17.6) | .21† |
M (SD) = mean (standard deviation), n (%) = number (percentage).
1-way ANOVA.
χ2 test.
4. Discussion
The development of hemodynamic changes is one of the most significant adverse effects associated with GA. Evidence suggests that the occurrence of hypotension/hypertension during GA is an independent factor that influences the treatment outcomes of surgical patients.[6] Hemodynamic changes (mostly hypotension) can often occur after induction of GA and before surgical incision.[6] Even short periods of hypotension can lead to tissue hypoperfusion and the development of complications that can increase postoperative morbidity and mortality.[7]
Trials investigating the effects of midazolam-propofol co-induction on hemodynamic stability have yielded conflicting results. A study in the pediatric population showed that co-induction with midazolam and ketamine reduced the decline in systolic blood pressure compared to the propofol-only group.[8] A comparison of the co-inductive effects of midazolam with propofol to propofol alone in patients older than 65 years of age showed a significantly greater decrease in MAP in the propofol group.[9]
A study comparing midazolam co-induction, propofol preconditioning, and a propofol-only control groups found no significant differences in blood pressure and HR between these groups.[4] A similar study of patients older than 60 years showed almost identical results.[10] Another study of 2 age groups reported that co-induction with midazolam in 2 different doses led to lower systolic blood pressure compared to the control group, but this was not statistically significant.[11]
In our trial, patients in the C and M1 groups had significantly lower SAP/MAP/DAP values immediately after induction than those in the M2 group (Table 2 and Fig. 2). This shows that midazolam co-induction has a protective effect against the decline in arterial pressure and possible hypotension in the first minutes of GA compared to propofol alone. Other measurement points showed no significant differences, but the trend shown in Figure 2 suggests that midazolam co-induction leads to smaller variations in SAP/MAP/DAP during the first minutes of GA compared to propofol alone, particularly with higher doses of midazolam.
HR analysis (Fig. 3) showed statistically higher values in the C group than in the M1 group at t6. There were no other statistically significant differences, which is in line with the results of similar studies.[4,10,11] However, Win et al found that co-induction with midazolam led to a significant increase in HR compared to that in patients who received only propofol.[12]
One of the basic goals of co-induction is the need for a lower dose of the main anesthetic.[3] Our data confirmed this hypothesis (Table 3) showing that patients receiving midazolam required significantly lower doses of propofol to induce GA. Similar results have been reported in the literature[4,10–12] and this trend is particularly significant in elderly patients.[11]
Surgical stress is defined as the impact exerted on the human body during a surgical procedure.[13] It is characterized by the activation of the sympathetic nervous system and increased secretion of pituitary hormones, which have secondary effects on hormone secretion from target organs.[14] Surgical trauma leads to increased secretion of adrenocorticotrophic hormones from the pituitary gland, which in turn stimulates cortisol secretion from the adrenal cortex. As a result, the levels of cortisol in the blood begin to rise very quickly after the start of surgery, and from initial values of about 400 nmol/L it can increase up to 1500 nmol/L or more after 4 to 6 hours, depending on the severity of trauma.[14] Recent studies have shown that elevated cortisol levels can be recorded up to 7 days after surgery and are correlated with the invasiveness of the surgical or anesthetic technique, age, and sex of the patient.[15]
Nearly 30 years ago, Desborough described the effects of midazolam induction on GA on the suppression of cortisol and insulin secretion compared with thiopental-induced anesthesia.[16] Similar results were reported in other studies,[17] while others achieved cortisol suppression using midazolam as an oral premedication in children.[18] Adams et al published 2 studies at the turn of the century that investigated the effects of midazolam on vascular stability and the stress response of patients during anesthesia. The first study explored the effects of midazolam co-induction on vascular, sympatho-adrenergic, and stress responses in elderly patients undergoing major abdominal surgery under total intravenous anesthesia (TIVA).[19] In subsequent research, midazolam was used during co-induction, but also during the maintenance of TIVA in combination with propofol and fentanyl.[20] In both cases, no significant effects of midazolam on the patient’s vascular stability and stress response to surgery were observed.
The results of our study showed that 1 hour after surgical incision, the control group had significantly higher levels of cortisol than the intervention groups (Table 2). The morning after surgery, the control group still had higher cortisol levels, but this difference was not statistically significant.
These results are similar to those obtained in studies using midazolam as an oral premedication[18] but differ from those reported by Adams et al.[19,20] Although similar in design, there are 2 major differences between our study and those conducted by Adams, which could have influenced the results. The surgical procedures in our study were less invasive and of shorter duration, additionally, different techniques of anesthesia were used (balanced anesthesia compared to TIVA), which could explain the different results.
Hyperglycemia often occurs during surgery, and the occurrence of previously unrecognized insulin resistance is becoming more common.[21] According to some authors, the magnitude of hyperglycemia primarily depends on the severity of the surgical procedure, that is surgical tissue trauma.[22] During elective intraperitoneal procedures, blood glucose often rises to 7 to 10 mmol/L and during cardiac surgery, to >10 mmol/L in non-diabetic patients. Laparoscopic surgery is thought to have less of an impact on the development of hyperglycemia than classical open procedures.[22]
Conflicting results have been reported regarding the effects of individual anesthetics used in GA on perioperative and postoperative glycemia. Some authors have shown better glycemic control with isoflurane compared to TIVA with midazolam in cardiac surgery,[23] while other studies did not find significant differences between TIVA with midazolam and balanced anesthesia,[24] or between TIVA with propofol or midazolam.[25] Our study did not find significant glycemic differences between the study groups.
The incidence of PONV after GA without prophylactic use of antiemetic drugs is about 20% to 30%.[26] However, it can reach up to 80% in patients with risk factors, such as female sex, history of motion sickness or PONV after previous GA, nonsmokers, and the use of postoperative opioids.[26]
PONV rarely leads to serious medical complications but significantly affects the quality of postoperative recovery and treatment costs.[27] Patients cite PONV as one of the most inconvenient complications of surgery and are willing to pay up to 68 euros to avoid it, while those who had previous experience with it would pay up to 99 euros.[28]
Several studies have explored the potential antiemetic effects of midazolam. Benzodiazepines are thought to act by inhibit the synthesis, release, and postsynaptic effects of dopamine.[29] It is also possible that the antidopaminergic effect is achieved by binding to γ-aminobutyric acid receptors: thus, anxiolysis as a secondary effect may also contribute to antiemesis.[29]
Premedication with midazolam (0.075 mg/kg) has been shown to have a significant prophylactic effect on PONV during cholecystectomy under GA.[30] A comparison of the antiemetic effects of low-dose midazolam administered as a premedication or near the end of surgery showed that better PONV prophylaxis was achieved near the end.[31] Finally, a meta-analysis showed that preoperative or perioperative intravenous administration of midazolam significantly affected the incidence of PONV and can be used as part of a multimodal approach to prevent this complication.[2]
In our study, the incidence of PONV was lower in the M1 and M2 group, compared to C, although this difference was not statistically significant. Comparing our results with those where higher doses of midazolam were used,[30] it can be concluded that midazolam co-induction may have an appropriate antiemetic effect at higher doses than those used in our research.
Anxiety is a human reaction to any unknown situation and is defined as a state of uneasiness and apprehension.[32] The occurrence of anxiety may depend on the current situation or the tendency of the individual to express it, that is, the character trait. People with high anxiety traits are likely to respond to new situations (such as surgery) with anxiety. Situational anxiety is a current condition stemming from a specific situation in which a person finds himself or herself and is most often transient in nature.[32]
Almost every patient in the perioperative period experiences a certain level of anxiety, which can lead to increased pain levels in the perioperative period, increased intraoperative use of anesthetics, and decreased overall treatment satisfaction.[32] High levels of postoperative anxiety are shown to be correlated with the occurrence of atrial fibrillation after heart surgery[33] as well as the intensity of postoperative pain.[32]
There are several risk factors associated with the development of postoperative anxiety in adults after elective surgery, such as American Society of Anesthesiologists III patient status, moderate to severe postoperative pain, severe preoperative anxiety, previous psychiatric disorders, history of smoking, and a negative self-perception of the future. However, techniques of neuraxial anesthesia/analgesia and the use of systemic multimodal analgesia may have a protective effect against postoperative anxiety.[34]
SAS is a norm-referenced scale filled in by patients, which is widely used as a screening tool for anxiety disorders.[35,36] Although it was developed in 1971, SAS continues to be extensively used in research, particularly in medical disciplines.[37] However, certain problems are associated with the use of this scale. Zung established 2 different methods for determining the results: raw score (20–80) and index score (25–100)[35,36] and subsequently lowered the scale for a clinically significant score from the original sum of 40 (index 50) to 36 (index 45).
Benzodiazepines have long been the main pharmacotherapy for postoperative anxiety; however, in some cases, their use has been associated with the development of postoperative delirium, especially in elderly patients.[32] Some studies have indicated that intramuscular administration of midazolam 30 minutes before surgery results in lower postoperative anxiety compared with the placebo group.[38] Oral premedication with midazolam has been reported to have similar effects as clonidine and dexmedetomidine on postoperative anxiety levels in the pediatric population.[39] Continuous infusion of low-dose midazolam has a better effect on perioperative and early postoperative anxiety scores than dexmedetomidine infusion in patients undergoing surgery under regional anesthesia.[40]
Our results showed that midazolam co-induction has a beneficial effect on postoperative anxiety, which is visible at lower doses and more pronounced when using higher co-induction doses.
The findings of this trial should be interpreted in the context of the study methodology and its limitations, such as the single-center setting and one-blinded design. Similar studies used higher doses of midazolam (0.075 mg/kg). Since the surgical procedures in this trial were relatively short (<1 hour), we focused on lower doses to avoid delayed recovery.
5. Conclusion
Our study showed that midazolam co-induction led to a reduction in postoperative anxiety and cortisol levels 60 minutes after surgical incision. SAP/MAP/DAP were significantly higher immediately after anesthesia induction in the M2 group than in the other study groups. The propofol dose required for anesthesia induction was lower when midazolam was used as a co-induction agent.
Individual participant data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) will be available beginning 3 months and ending 5 years following article publication to achieve aims in the approved proposal or for individual participant data for meta-analysis.
Proposals for data access should be directed to corresponding author email.
Author contributions
Mirko Mihalj and Vesna Golubović substantially contributed to the conception and design of the methodology of the manuscript. Iva Mikulić and Vinka Mikulić contributed to data acquisition. Dajana Vladić and Boris Matić wrote the original draft of this manuscript. Zoran Karlović critically revised the manuscript. Analysis and interpretation of data: Outsourced. All authors have read and approved the final version of the manuscript. All authors contributed equally to the manuscript and have read and approved the final version.
Conceptualization: Mirko Mihalj, Vesna Golubović.
Data curation: Iva Mikulić, Vinka Mikulić.
Methodology: Mirko Mihalj.
Project administration: Mirko Mihalj, Vesna Golubović.
Supervision: Mirko Mihalj.
Writing – original draft: Dajana Vladić-Spaić, Boris Matić.
Writing – review & editing: Zoran Karlović.
Abbreviations:
- BIS =
- bispectral index monitoring
- C =
- placebo/control group of patients
- COR =
- cortisol
- GA =
- general anesthesia
- GLU =
- glucose
- HR =
- heart rate
- M1 =
- group of patients that received intravenous midazolam at dose of 0.03 mg/kg
- M2 =
- group of patients that received intravenous midazolam at dose of 0.06 mg/kg
- MAC =
- minimum alveolar concentration
- PONV =
- postoperative nausea and vomiting
- SAP/MAP/DAP =
- systolic/mean/diastolic blood pressure
- SAS =
- Zung’s Self-rating Anxiety Scale
- TIVA =
- total intravenous anesthesia
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
The authors have no funding and conflicts of interest to disclose.
This study was approved by University Clinical Hospital Mostar ethics committee (2062/ 14; 03.04.2014).
This study is retrospectively registered in Deutsches Register Klinischer Studien (DRKS00024564).
How to cite this article: Mihalj M, Karlović Z, Vladić-Spaić D, Matić B, Mikulić I, Mikulić V, Golubović V. Effects of midazolam co-induction to general anesthesia: A randomized clinical trial. Medicine 2022;101:45(e31400).
Contributor Information
Zoran Karlović, Email: zoran.karlovic@hotmail.com.
Dajana Vladić-Spaić, Email: daki.vladic@hotmail.com.
Boris Matić, Email: boris.matic30@gmail.com.
Iva Mikulić, Email: barac.vinka@gmail.com.
Vinka Mikulić, Email: barac.vinka@gmail.com.
Vesna Golubović, Email: vesna.golubovic1208@gmail.com.
References
- [1].Reves JG, Fragen RJ, Vinik HR, et al. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310–24. [PubMed] [Google Scholar]
- [2].Grant MC, Kim J, Page AJ, et al. The effect of intravenous midazolam on postoperative nausea and vomiting: a meta-analysis. Anesth Analg. 2016;122:656–63. [DOI] [PubMed] [Google Scholar]
- [3].Whitwam JG. Co-induction of anaesthesia: day-case surgery. Eur J Anaesthesiol Suppl. 1995;12:25–34. [PubMed] [Google Scholar]
- [4].Amatya A, Marhatta MN, Shrestha GS, et al. A comparison of midazolam co-induction with propofol priming in propofol induced anesthesia. J Nepal Health Res Counc. 2014;12:44–8. [PubMed] [Google Scholar]
- [5].Mapleson WW. Effect of age on MAC in humans: a meta-analysis. Br J Anaesth. 1996;76:179–85. [DOI] [PubMed] [Google Scholar]
- [6].Reich DL, Hossain S, Krol M, et al. Predictors of hypotension after induction of general anesthesia. Anesth Analg. 2005;101:622–8. [DOI] [PubMed] [Google Scholar]
- [7].Jor O, Maca J, Koutna J, et al. Hypotension after induction of general anesthesia: occurrence, risk factors, and therapy. A prospective multicentre observational study. J Anesth. 2018;32:673–80. [DOI] [PubMed] [Google Scholar]
- [8].Goel S, Bhardwaj N, Jain K. Efficacy of ketamine and midazolam as co-induction agents with propofol for laryngeal mask insertion in children. Paediatr Anaesth. 2008;18:628–34. [DOI] [PubMed] [Google Scholar]
- [9].Lim YS, Kang DH, Kim SH, et al. The cardiovascular effects of midazolam co-induction to propofol for induction in aged patients. Korean J Anesthesiol. 2012;62:536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jones NA, Elliott S, Knight J. A comparison between midazolam co-induction and propofol predosing for the induction of anaesthesia in the elderly. Anaesthesia. 2002;57:649–53. [DOI] [PubMed] [Google Scholar]
- [11].Cressey DM, Claydon P, Bhaskaran NC, et al. Effect of midazolam pretreatment on induction dose requirements of propofol in combination with fentanyl in younger and older adults. Anaesthesia. 2001;56:108–13. [DOI] [PubMed] [Google Scholar]
- [12].Win NN, Kohase H, Yoshikawa F, et al. Haemodynamic changes and heart rate variability during midazolam-propofol co-induction. Anaesthesia. 2007;62:561–8. [DOI] [PubMed] [Google Scholar]
- [13].Giannoudis PV, Dinopoulos H, Chalidis B, et al. Surgical stress response. Injury. 2006;37(Suppl 5):S3–9. [DOI] [PubMed] [Google Scholar]
- [14].Desborough JP. The stress response to trauma and surgery. Br J Anaesth. 2000;85:109–17. [DOI] [PubMed] [Google Scholar]
- [15].Prete A, Yan Q, Al-Tarrah K, et al. The cortisol stress response induced by surgery: a systematic review and meta-analysis. Clin Endocrinol (Oxf.). 2018;89:554–67. [DOI] [PubMed] [Google Scholar]
- [16].Desborough JP, Hall GM, Hart GR, et al. Midazolam modifies pancreatic and anterior pituitary hormone secretion during upper abdominal surgery. Br J Anaesth. 1991;67:390–6. [DOI] [PubMed] [Google Scholar]
- [17].Misiolek H, Wojcieszek E, Dyaczynska-Herman A. Comparison of influence of thiopentone, propofol and midazolam on blood serum concentration of noradrenaline and cortisol in patients undergoing non-toxic struma operation. Med Sci Monit. 2000;6:319–24. [PubMed] [Google Scholar]
- [18].Gomes HS, Corrêa-Faria P, Silva TA, et al. Oral midazolam reduces cortisol levels during local anaesthesia in children: a randomised controlled trial. Braz Oral Res. 2015;29:1–9. [DOI] [PubMed] [Google Scholar]
- [19].Adams HA, Vonderheit G, Schmitz CS, et al. Sympathoadrenergic, hemodynamic and stress response during coinduction with propofol and midazolam. Anasthesiol Intensivmed Notfallmed Schmerzther. 2000;35:293–9. [DOI] [PubMed] [Google Scholar]
- [20].Adams HA, Hermsen M, Kirchhoff K, et al. Co-maintenance with propofol and midazolam: sympathoadrenergic reactions, hemodynamic effects, stress response, EEG and recovery. Anasthesiol Intensivmed Notfallmed Schmerzther. 2002;37:333–40. [DOI] [PubMed] [Google Scholar]
- [21].Kwon S, Thompson R, Dellinger P, et al. Importance of perioperative glycemic control in general surgery: a report from the surgical care and outcomes assessment program. Ann Surg. 2013;257:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Schricker T, Lattermann R. Perioperative catabolism. Can J Anaesth. 2015;62:182–93. [DOI] [PubMed] [Google Scholar]
- [23].Hsu CH, Hsu YC, Huang GS, et al. Isoflurane compared with fentanyl-midazolam-based anesthesia in patients undergoing heart transplantation: a retrospective cohort study. Medicine (Baltim). 2016;95:e4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sourgiadaki E, Konstantopoulos K, Raggou D, et al. A comparative study of conventional inhalation anaesthesia and total intravenous anaesthesia (TIVA) performed with midazolam and alfentanil. Minerva Anestesiol. 1994;60:715–8. [PubMed] [Google Scholar]
- [25].Oztekin I, Gökdoğan S, Oztekin DS, et al. Effects of propofol and midazolam on lipids, glucose, and plasma osmolality during and in the early postoperative period following coronary artery bypass graft surgery: a randomized trial. Yakugaku Zasshi. 2007;127:173–82. [DOI] [PubMed] [Google Scholar]
- [26].Apfel CC, Läärä E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology. 1999;91:693–700. [DOI] [PubMed] [Google Scholar]
- [27].Dzwonczyk R, Weaver TE, Puente EG, et al. Postoperative nausea and vomiting prophylaxis from an economic point of view. Am J Ther. 2012;19:11–5. [DOI] [PubMed] [Google Scholar]
- [28].Kerger H, Turan A, Kredel M, et al. Patients’ willingness to pay for anti-emetic treatment. Acta Anaesthesiol Scand. 2007;51:38–43. [DOI] [PubMed] [Google Scholar]
- [29].Rodolà F. Midazolam as an anti-emetic. Eur Rev Med Pharmacol Sci. 2006;10:121–6. [PubMed] [Google Scholar]
- [30].Heidari SM, Saryazdi H, Saghaei M. Effect of intravenous midazolam premedication on postoperative nausea and vomiting after cholecystectomy. Acta Anaesthesiol Taiwan. 2004;42:77–80. [PubMed] [Google Scholar]
- [31].Safavi MR, Honarmand A. Low dose intravenous midazolam for prevention of PONV, in lower abdominal surgery-preoperative vs intraoperative administration. Middle East J Anaesthesiol. 2009;20:75–81. [PubMed] [Google Scholar]
- [32].Jellish WS, O’Rourke M. Anxiolytic use in the postoperative care unit. Anesthesiol Clin. 2012;30:467–80. [DOI] [PubMed] [Google Scholar]
- [33].Tully PJ, Bennetts JS, Baker RA, et al. Anxiety, depression, and stress as risk factors for atrial fibrillation after cardiac surgery. Heart Lung. 2011;40:4–11. [DOI] [PubMed] [Google Scholar]
- [34].Caumo W, Schmidt AP, Schneider CN, et al. Risk factors for postoperative anxiety in adults. Anaesthesia. 2001;56:720–8. [DOI] [PubMed] [Google Scholar]
- [35].Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371–9. [DOI] [PubMed] [Google Scholar]
- [36].Zung WW. The measurement of affects: depression and anxiety. Mod Probl Pharmacopsychiatry. 1974;7:170–88. [DOI] [PubMed] [Google Scholar]
- [37].Dunstan DA, Scott N. Norms for Zung’s self-rating anxiety scale. BMC Psychiatry. 2020;20:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kain ZN, Sevarino F, Pincus S, et al. Attenuation of the preoperative stress response with midazolam: effects on postoperative outcomes. Anesthesiology. 2000;93:141–7. [DOI] [PubMed] [Google Scholar]
- [39].Schmidt AP, Valinetti EA, Bandeira D, et al. Effects of preanesthetic administration of midazolam, clonidine, or dexmedetomidine on postoperative pain and anxiety in children. Paediatr Anaesth. 2007;17:667–74. [DOI] [PubMed] [Google Scholar]
- [40].Senses E, Apan A, Köse EA, et al. The effects of midazolam and dexmedetomidine infusion on peri-operative anxiety in regional anesthesia. Middle East J Anaesthesiol. 2013;22:35–40. [PubMed] [Google Scholar]