Abstract
Adherence of Entamoeba histolytica trophozoites to colonic mucin, epithelium, and other target cells is mediated by the amebic Gal/GalNAc lectin. We constructed in vitro expression vectors containing full-length (residues 1 to 1280), cysteine-poor (1 to 353 and 1 to 480), and cysteine-rich (356 to 1143 and 480 to 900) fragments of the gene encoding the heavy subunit of the adherence lectin, hgl2. In vitro transcription followed by translation using a nuclease-treated rabbit reticulocyte lysate system was carried out. Immunoreactivity of in vitro-translated Hgl2 was confirmed by immunoprecipitation with lectin-specific monoclonal antibodies (MAbs) 1G7 and 8A3, which recognize linear epitopes. Protein disulfide isomerase (PDI) refolding of Hgl2 enhanced immunoreactivity (P < 0.05) with the conformationally dependent MAb 3F4. Binding of PDI-refolded full-length (P < 0.001) and cysteine-rich (P = 0.005) Hgl2 to CHO cells was galactose dependent and competitively inhibited by native hololectin (50% inhibitory concentration of 39.6 ng/ml). The cysteine-poor region (1 to 353) did not bind CHO cells. Both full-length (1 to 1280) and cysteine-rich (356 to 1143) Hgl2 bound the glyconeoconjugate GalNAc19BSA in a GalNAc-specific manner. The smaller cysteine-rich fragment (480 to 900) also exhibited GalNAc-specific binding but to a lesser extent (P < 0.05) than residues 1 to 1280 and 356 to 1143. Neither the cysteine-poor fragment (1 to 480), luciferase (protein control), nor control translation reactions (without hgl2 lectin mRNA) bound GalNAc19BSA. Binding to GalNAc19BSA was shown to be dependent on the concentration of GalNAc19BSA coated in each well or 35S-lectin added (KD = 0.85 ± 0.37 pM). Binding was competitively inhibited by the terminal GalNAc-containing glycoprotein asialofetuin (P < 0.005). Taken together, these data provide direct evidence that the cysteine-rich region of the Gal/GalNAc lectin heavy subunit contains one or more carbohydrate-binding domains.
Entamoeba histolytica is the causative agent of amebiasis. Infection leads to an estimated 40 million to 50 million cases of amebic colitis or liver abscess annually. Amebiasis is surpassed only by malaria and schistosomiasis as a leading cause of death caused by parasitic disease (32). The pathogenesis of E. histolytica infection involves adherence to colonic mucin (5), cytolysis of host epithelial and immune effector cells (8, 9, 22), and modulation of host immune functions including proteolysis of secretory immunoglobulin A (IgA) (24, 31), complement evasion (26), and inhibition of macrophage defense mechanisms (3). Adherence to several cell types is mediated by the Gal/GalNAc-specific lectin, which is composed of a single membrane-spanning 170-kDa heavy subunit (13, 30) linked by disulfide bonds to either a 31- or 35-kDa light subunit (14, 29). The 31-kDa isomer is thought to be glycosylphosphatidylinositol anchored, the significance of which is unclear (15). Both the heavy and light subunits are encoded by multiple genes (15, 21). Interestingly, a homologous Gal/GalNAc lectin is also present and expressed in the morphologically identical but genetically distinct nonpathogenic ameba Entamoeba dispar (20).
The heavy subunit is an immunodominant amebic surface protein and is recognized by antisera from patients with invasive disease (16). Monoclonal antibodies (MAbs) generated against the heavy subunit have been reported to both inhibit and enhance adherence, possibly owing to conformational regulation of ligand interaction (19, 28). Epitopes recognized by adherence-inhibitory MAbs map to the cysteine-rich segment (residues 596 to 1082) of the heavy subunit, suggesting indirectly that the carbohydrate-binding domain(s) lies within this region (11). Others have suggested that a sugar-binding domain lies within the pseudorepeat region (436 to 624) (10). Binding studies using amebic membranes with glyconeoconjugates show that the Gal/GalNAc lectin probably relies on subsite and subunit multivalency in order to achieve avid adherence (1).
In this study, we used the cDNA encoding hgl2 in order to construct in vitro expression vectors. Full-length Hgl2 (residues 1 to 1280 [FL Hgl2]) and cysteine-rich Hgl2 (residues 356 to 1143 [CR1 Hgl2] and 480 to 900 [CR2 Hgl2]) were translated in a cell-free system, shown to be immunoreactive with lectin heavy subunit-specific MAbs and protein disulfide isomerase (PDI) refolded into a more native conformation. Using this approach, we directly demonstrate Gal/GalNAc-inhibitable binding by the cysteine-rich region of the heavy subunit.
MATERIALS AND METHODS
Strains and culture condition.
Axenic E. histolytica HM1:IMSS (ATCC 30459; American Type Culture Collection, Rockville, Md.) was grown in TYI-S-33 medium supplemented with penicillin (100 U/ml) and streptomycin sulfate (100 mg/ml) (Life Technologies, Gaithersburg, Md.) as defined by Diamond et al. (6). E. histolytica Gal/GalNAc lectin and antilectin MAbs were obtained as previously described (18, 25). CHO cells were grown in Dulbecco’s minimal essential medium supplemented with 10% fetal calf serum and 100 mg of gentamicin per ml (all obtained from Life Technologies, Gaithersburg, Md.) in 75-cm2 plastic tissue culture flasks (Corning Costar, Cambridge, Mass.). Cells were harvested with 0.25% trypsin in Dulbecco’s phosphate-buffered saline (DPBS) without Ca2+ and Mg2+ (Life Technologies).
In vitro expression vectors and constructs.
Oligonucleotides KK79 (5′-ACGT TCT AGA TTA AAT ATC TTA TTA TTA TGT-3′) and KK80 (5′-ACGT GTC GAC TTA TCC ATT GTA AGT AGC TGC-3′) were synthesized so as to correspond to the 5′ and 3′ ends of hgl2 (Genbank accession no. L00636) in order to generate the full-length (residues 1 to 1280) heavy subunit which contains a portion of the signal peptide. The sense and antisense oligonucleotides were designed with restriction sites for XbaI and SalI, respectively. A 3.8-kb fragment was amplified from HM1:IMSS genomic DNA by using high-fidelity Taqplus DNA polymerase (Stratagene, La Jolla, Calif.). The amplified product was digested with XbaI and SalI, cloned into modified pGEM-4Z (Promega Corp., Madison, Wis.) containing the avian myeloblastosis virus UTL and SP6 promoter upstream of the XbaI site (pGEM-4Z-FL). In similar fashion, the following plasmid constructs were generated: pGEM-4Z-CP (1–353) [expressing cysteine-poor Hgl2 (residues 1 to 353), designated CP (1–353) Hgl2], using PCR sense primer KK79 and antisense KK114 (5′-ATG TGG TCC AAG TGA AAC AAG-3′); pDISP-CP (1–480), using sense primer DP50 (5′-CGC CCG GGA TGA AAT TAT TAT TAT TAA ATA TG) and antisense primer DP51 (5′-GAC CTG CAG ATT AGC TTT TTG TTT ACA TAC); pDISP-CR2 (480–900), using sense primer DP48 (5′-CGC CCG GGT GTA AAC AGA AAG CTA ATT G) and antisense primer DP49 (5′-GAC CTG CAG ATA TGC TTC TTT GTA TGC CTC); and pcDNA3 CR1 (356–1143), generated by restriction digestion of in-frame EcoRI sites at 1070 and 3428 in hgl2. Firefly luciferase mRNA (Promega) was used as a control for translation experiments. Plasmid DNA was purified from transformed Escherichia coli DH5α, using QIAfilter columns (Qiagen, Chatsworth, Calif.). Plasmid constructs were linearized with XbaI (pcDNA3) or SalI (pGEM-4Z and pDISP) for in vitro transcription.
In vitro transcription and translation.
SalI- or XbaI-linearized full-length and cysteine-rich constructs were phenol-chloroform extracted and ethanol precipitated, followed by resuspension in diethyl pyrocarbonate-treated water. Linearized plasmid DNA (100 to 800 ng) was added to in vitro transcription reagents as recommended by the manufacturer (Promega). T7 or SP6 RNA polymerase transcription was carried out at 37°C for 1 h. RNA concentration was approximated by 1% agarose gel electrophoresis. RNA transcript was added directly to the rabbit reticulocyte lysate system reagents as outlined by the manufacturer (20a).
Translation was carried out in the presence of l-[35S]cysteine or [35S]methionine (ICN Pharmaceuticals, Costa Mesa, Calif.) for 1.5 h at 30°C. Translation products were analyzed by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) on a 7.5% gel. Gels were fixed for 30 min in 20% methanol–10% acetic acid followed by the organic scintillant En3Hance (NEN Research Products, Boston, Mass.) for 45 min, rinsed for 1 min in distilled water, dried, and exposed to Hyperfilm (Amersham Life Science).
TCA precipitation of in vitro-translated proteins.
To determine the efficiency of translation for each transcript, in vitro-synthesized proteins (2 μl) were added to GF/A glass fiber filter circles (Fisherbrand, Pittsburgh, Pa.), dried, placed in 5 ml of scintillation fluid (Cytoscint; ICN), and counted (LS1701; Beckman Instruments Inc., Mississauga, Ontario, Canada) in order to determine the total radioactivity per reaction. Similarly, 2 μl of translated protein was added to 1 N NaOH–2% H2O2 (248 μl) for 10 min at 37°C in order to hydrolyze aminoacyl-tRNAs present in the lysate. After 10 min of incubation, 1 ml of ice-cold 25% trichloroacetic acid (TCA)–2% Casamino Acids was added (30 min, on ice) to precipitate the synthesized protein. The sample was then added to a GF/A glass fiber filter circle (prewetted with ice-cold 5% TCA), washed three times with 3 ml of ice-cold 5% TCA, and rinsed once with 1 ml of acetone. Filter circles were dried at 75°C for 10 min, and added to scintillation fluid vials, and counted (total incorporated radioactivity per reaction). Translation efficiency for each protein was determined with the following formula: % incorporation = (total incorporated radioactivity per reaction/total radioactivity per reaction) × 100. These data were used to standardize equivalent counts of incorporated radioactivity for the various synthesized proteins used in the binding experiments. The amount of 35S-lectin synthesized was determined based on the specific activity of the l-[35S]cysteine (1,075 Ci/mmol) or [35S]methionine (1,175 Ci/mmol) incorporated during in vitro synthesis. The number of cysteine or methionine residues present in FL Hgl2 (98 cysteines and 19 methionines), CR1 Hgl2 (84 cysteines and 10 methionines), CR2 Hgl2 (46 cysteines and 6 methionines), CP Hgl2 (25 cysteines and 10 methionines), and firefly luciferase (4 cysteines and 14 methionines) were used to determine amounts of protein translated for experiments performed with either l-[35S]cysteine or [35S]methionine labeling. The procedure outlined here is described in reference 20a.
Treatment with PDI.
PDI was purchased from Panvera Corp. (Madison, Wis.). Typically, a 40-μl aliquot of the in vitro-translated protein was treated with 20 μl of 0.2 mM oxidized glutathione, 20 μl of 2 mM reduced glutathione, and 20 μl of 20 mg of PDI per ml in 200 mM sodium phosphate buffer (pH 7.5) at room temperature for 15 min. For a negative control, additional aliquots of translated protein were treated with reduced glutathione, oxidized glutathione, and buffer (no PDI).
Immunoprecipitation of in vitro translation products.
A panel of MAbs directed against the lectin heavy subunit (25) was used to immunoprecipitate both full-length and cysteine-rich translation products. Recombinant protein G-Sepharose beads (Zymed, South San Francisco, Calif.) were washed three times in 150 mM NaCl–1 mM EDTA–0.5% Triton X-100–0.5% bovine serum albumin (BSA)–10 mM Tris buffer (pH 8.0) (NETT-BSA). The beads were then incubated with 5 μg of 1G7 (IgG2b), 3F4 (IgG1), 5B8 (IgG1), 7F4 (IgG2b), 8A3 (IgG1), 8C12 (IgG1), CLB (IgG1), or VM58 (IgG1) for 30 min. Equivalent counts of in vitro-translated [35S]Cys-labeled full-length and cysteine-rich Hgl2 were added to the various protein G-Sepharose-coupled MAbs, incubated for 4 to 6 h at room temperature, and rotated end over end. The beads were centrifuged (16,000 × g, 1 min); the supernatant discarded and then washed in the following sequence: NETT–0.5% BSA, NETT, NETT–0.5% NaCl, NETT. The pelleted beads were added to an equal volume of 1× SDS sample buffer and boiled for 10 min with vortex mixing at 5 min. Following centrifugation (16,000 × g, 1 min), the supernatant was loaded on to a prepoured 7.5% polyacrylamide gel for SDS-PAGE and analyzed by fluorography as above.
Immunoprecipitation of native lectin heavy subunit.
Trophozoites (109) at stationary phase were harvested following 3 days of growth in YI-S medium. Trophozoites were washed three times with PBS and resuspended in 500 μg of Sulfo-NHS-Biotin (Pierce, Rockford, Ill.) per ml for 30 min at room temperature in order to biotinylate all surface antigens. Cells were then washed three times with PBS to remove unbound biotin, and crude plasma membranes were prepared according to the method of Aley et al. (2). Extracted membranes were solubilized in trophozoite solubilization buffer as described by Petri and Schnaar (17), with the exception that 2 mM phenylmethylsulfonyl fluoride (PMSF; Sigma Chemical Co., St. Louis, Mo.) and 50 μg of trans-epoxysuccinyl-l-leucylamide(4-guanidino)-butane (E-64; Sigma) per ml were used for protease inhibitors in order to reduce degradation of the 170-kDa heavy subunit. Solubilized membranes (100 μg) were added to 20 μg of MAb 1G7, 3F4, or CLB coupled to protein G-Sepharose beads as described earlier. Immunoprecipitation buffer used was NETT–0.5% BSA containing 50 μg of E-64 per ml and 0.4 mM PMSF. Antibody-coupled beads and solubilized membrane fraction were rotated end over end overnight at 4°C in immunoprecipitation buffer (NETT–0.5% BSA containing 50 μg of E-64 per ml and 0.4 mM PMSF). Beads were centrifuged (16,000 × g, 1 min); the supernatant was discarded and then washed as outlined above. Pelleted beads were added to an equal volume of 1× SDS sample buffer and boiled for 10 min with vortex mixing at 5 min. Following centrifugation (16,000 × g, 1 min), the supernatant was run on a prepoured SDS–7.5% polyacrylamide gel and transferred onto nitrocellulose (Life Technologies). Blots were blocked in 5% milk protein–Tris-buffered saline containing 0.05% Tween 20 (TTBS) for 2 h at room temperature, incubated with a 1:5,000 dilution of avidin-horseradish peroxidase in 2% milk protein–TTBS (Bio-Rad Laboratories, Hercules, Calif.) for 1 h, washed three times with TTBS for 5 min and once with TBS for 7 min, and developed with enhanced chemiluminescence ECL reagents (Amersham Life Science).
Flow cytometry.
HM1 trophozoites (105) at stationary phase were harvested following 3 days of growth in YI-S medium. Trophozoites were pelleted, washed three times with PBS, and resuspended in 0.5% BSA–PBS containing a 1:100 dilution of MAb 1G7, 3F4, 7F4, or isotype control CLB for 1 h on ice. Cells were washed three times with PBS and incubated with a 1:100 dilution of goat anti-mouse-fluorescein isothiocyanate in 0.5% BSA–PBS (Bio-Rad) for 30 min on ice. Cells were washed three times with PBS and finally resuspended in 2% paraformaldehyde–PBS until analysis by flow cytometry (Coulter Epics Elite ESP) for fluorescence intensity.
CHO binding studies.
CHO cells were trypsinized and resuspended in minimal essential medium, centrifuged (1,000 × g for 3 min), and washed twice in DPBS. The cell pellet was resuspended in either DPBS, DPBS–55 mM glucose, DPBS–55 mM mannose, DPBS–55 mM d-galactose, or DPBS-purified native lectin (1, 10, or 50 ng/ml). All sugars were obtained from Sigma-Aldrich (Oakville, Ontario, Canada). The final suspension of cells was counted on a hemocytometer, and concentrations were adjusted to 106 cells/ml; 5 × 105 cells were aliquoted into 12- by 75-mm polystyrene culture tubes. PDI-treated in vitro-translated protein (100,000 cpm; determined as described above) was added and incubated with the cells at 4°C for 60 min. After incubation, the cells were washed twice in DPBS, DPBS-sugar, or DPBS-native lectin. The washed cells were layered onto a 4:1 mixture of silicon oil (Accumetric, Elizabeth, Ky.) and mineral oil (Sigma-Aldrich) in a 1.5-ml microcentrifuge tube. The cells were pelleted at 9,000 × g for 1 min. The tips of the microcentrifuge tubes containing the cell pellets were cut off and dropped into 10 ml of scintillation fluid (CytoScint) and counted. Absolute counts were at least 30-fold higher than background. All binding experiments were carried out in duplicate or triplicate, and results are presented as means ± standard errors (SE) (n = 3).
GalNAc-BSA binding studies.
The glyconeoconjugate GalNAc19BSA (Sigma-Aldrich) was resuspended in PBS at 1 mg/ml. GalNAc19BSA (50 μg per well) was added to Immulon 2 Removawell strips (Dynatech Laboratories, Inc., Chantilly, Va.) and incubated at room temperature for 2 h. The unbound glyconeoconjugate was then pipetted and discarded. Control wells were coated with 50 μg of BSA (Sigma-Aldrich) in the same way. Equal counts (100,000 cpm) of 35S-labeled in vitro translated and PDI-refolded lectin heavy subunit were resuspended in 0.1% BSA–PBS, added to GalNAc19BSA and BSA (control well) in parallel, and incubated for 2 h at room temperature. The unbound lectin was then discarded, and the wells were washed three times with PBS. The wells were snapped off each of the Removawell strips, added to scintillation vials (5 ml; CytoScint), and counted (counts per minute, with <3% error per reading). GalNAc-specific binding was determined as follows: GalNAc-specific binding (cpm) = lectin bound to GalNAc19BSA (cpm) − lectin bound to BSA (cpm).
For inhibition experiments, equal amounts of 35S-lectin were preincubated with asialofetuin (25 or 50 μM) or the irrelevant glycoprotein holotransferrin (50 μM) and incubated for 1 h at room temperature with end-over-end rotation. Lectin and inhibitor were then added to glyconeoconjugate- or BSA-coated wells and analyzed as described above. Radiolabeled protein added in each binding experiment was standardized by trichloroacetic acid (TCA) precipitation. SDS-PAGE of the same proteins was carried out to further ensure specific translation. All GalNAc-BSA binding experiments were performed at least three times in duplicate or triplicate.
Densitometric analysis.
All films were photographed (Gel Documentation System; U.V.P. Inc., San Gabriel, Calif.) and analyzed with software by Scion Image (Scion Corp., Frederick, Md.).
RESULTS
Immunoprecipitation of FL and CR Hgl2.
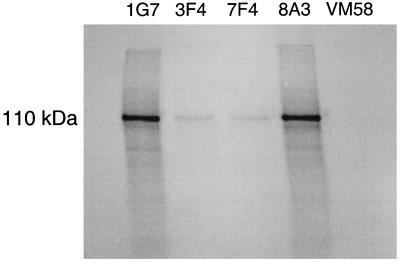
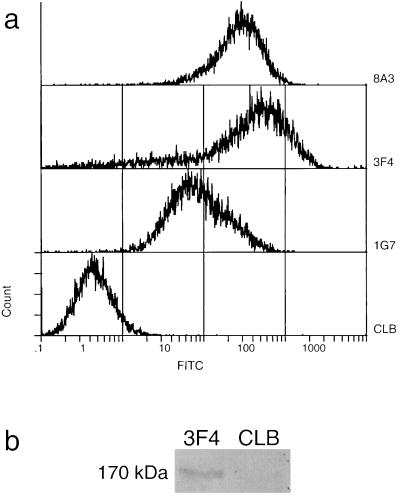
Both FL and CR Hgl2 were translated in vitro in a rabbit reticulocyte lysate system. FL Hgl2 migrated at ∼170 kDa, CR1 Hgl2 migrated at ∼110 kDa, and CR2 Hgl2 migrated at ∼70 kDa by SDS-PAGE under denaturing and reducing conditions. To ensure that lectin translation products were immunoreactive, 35S-labeled FL, CR1, and CR2 Hgl2 were immunoprecipitated with a panel of antilectin MAbs. Equal amounts of radiolabeled protein were added to protein G-Sepharose-coupled MAb, coincubated, and washed, and bound protein was run on a 7.5% polyacrylamide gel under both reducing and denaturing conditions. FL, CR1, and CR2 Hgl2 were immunoprecipitated with 1G7 and 8A3 and, to a lesser extent, with 3F4 and 7F4 (Fig. 1 shows data for CR1 Hgl2). In agreement with previous findings (12), the strong immunoreactivity of 1G7 and 8A3 with the translated proteins suggests that they recognize linear epitopes, while MAb 3F4 and 7F4 are likely conformationally dependent. In support of this conclusion, unfixed HM1 trophozoites expressing the Gal/GalNAc lectin were shown to be immunoreactive with 3F4 but not isotype control MAb CLB (Fig. 2A). Moreover, the heavy subunit of the lectin was specifically immunoprecipitated by 3F4 under native conditions, suggesting that 3F4 recognizes the heavy subunit when conformationally intact on the trophozoite (Fig. 2B). The observed 170-kDa band was confirmed to be the lectin heavy subunit by Western immunoblotting (data not shown).
FIG. 1.
Immunoprecipitation of in vitro-synthesized 35S-labeled CR1 Hgl2. CR1 Hgl2 was immunoprecipitated with antilectin MAbs 1G7 (IgG2b), 3F4 (IgG1), 7F4 (IgG2b), and 8A3 (IgG1) and irrelevant isotype VM58 (IgG1).
FIG. 2.
Immunoreactivity of 3F4 with the native lectin heavy subunit. (a) Indirect immunofluorescence of HM1:IMSS trophozoites by flow cytometric analysis. Primary antilectin MAbs 1G7, 3F4, and 8A3 (CLB was the isotype control used to measure background) were monitored by secondary goat anti-mouse-fluorescein isothiocyanate. Data depict fluorescence intensity on a logarithmic scale for 104 trophozites from a representative experiment. (b) Immunoprecipitation of biotinylated native lectin heavy subunit (170 kDa) from solubilized amebic membranes, using antilectin MAb 3F4 (IgG1) and irrelevant isotype control CLB (IgG1). Immunoprecipitates were subject to SDS-PAGE on a 7.5% gel, transferred to nitrocellulose, and probed with avidin-horseradish peroxidase.
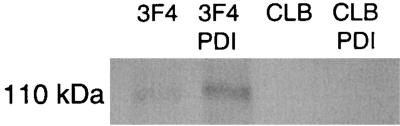
To improve detection of in vitro-translated Hgl2 by putative conformationally dependent MAb 3F4, translation products were treated with the folding catalyst PDI in the presence of glutathione redox buffer. The thioredoxin PDI has been shown to promote native pairing of cysteines required to refold several proteins (7, 27). Consistent with proper refolding, PDI-treated FL and CR Hgl2 demonstrated reproducibly improved immunoreactivity with 3F4 (P < 0.05 by one-way analysis of variance [n = 3], based on densitometric analysis) compared to no PDI treatment (Fig. 3 shows data for CR1 Hgl2). The same experiment performed with isotype control MAb CLB showed no difference in immunoreactivity (Fig. 3). Furthermore, MAbs 1G7 and 8A3, reported to recognize linear epitopes (12), showed no difference in immunoreactivity with and without PDI treatment (data not shown).
FIG. 3.
Immunoprecipitation of PDI-treated 35S-CR1 Hgl2. CR1 Hgl2 (110 kDa) was immunoprecipitated with and without PDI treatment by the antilectin MAb 3F4 (IgG1) and the irrelevant isotype CLB (IgG1).
Binding of FL and CR Hgl2 to CHO cells.
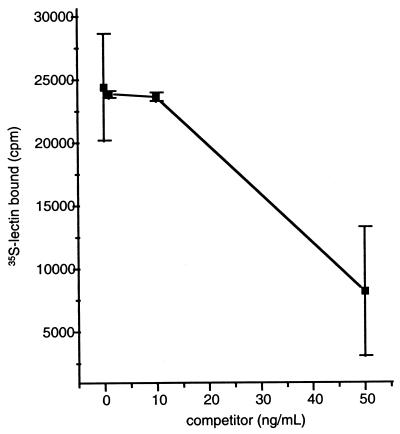
CHO binding studies were performed with PDI-refolded 35S-Hgl2. TCA precipitation was used to quantitate radiolabel incorporation and standardize each protein synthesis to ensure that differences in binding were not due to discrepancies in translation efficiency. Consistent with the established carbohydrate specificity of the amebic lectin (23), FL and CR1 Hgl2 bound to CHO cells with binding specifically inhibited (60 ± 4.5%; P < 0.005; t test) by 55 mM galactose but not by 55 mM glucose or 55 mM mannose. In contrast, radiolabeled protein outside the cysteine-rich domain (residues 1 to 353) did not exhibit galactose-inhibitable binding. As further evidence for specificity, native purified hololectin (260 kDa) significantly competed for binding of FL Hgl2 with a 50% inhibitory concentration (IC50) of 39.6 ng/ml (Fig. 4).
FIG. 4.
Competitive inhibition of 35S-CR1 Hgl2 binding to CHO cells by native hololectin (IC50 = 39.6 ng/ml). Data are presented as means ± SE for duplicate determinations.
Binding of FL and CR Hgl2 to GalNAc-BSA.
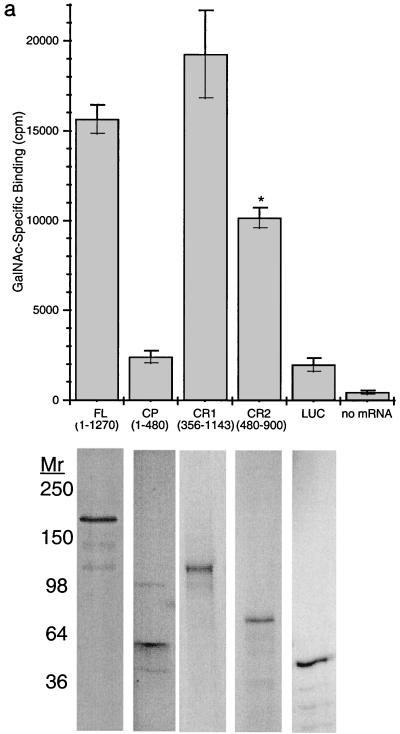
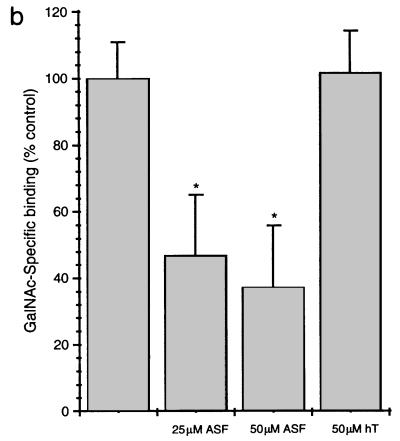
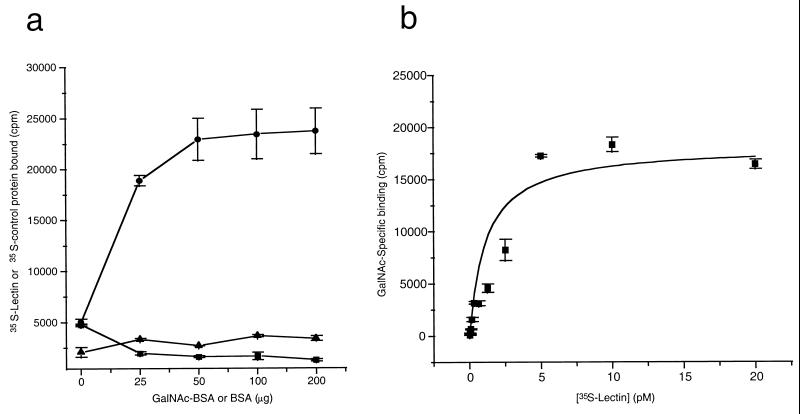
To confirm and extend these findings, 35S-labeled FL, CR1, CR2, and CP (1–480) Hgl2 were PDI refolded and added to microtiter plate wells coated with the glyconeoconjugate GalNAc19BSA or with BSA alone. As with CHO cell experiments, TCA precipitation was used to quantitate radiolabel incorporation for the various constructs and standardize each radiolabeled protein for binding studies. GalNAc-specific binding was observed for FL, CR1, and CR2 Hgl2, but no significant binding was observed for CP Hgl2, the irrelevant protein luciferase, or control translation reactions performed in the absence of exogenous mRNA (Fig. 5a). Results similar to those in Fig. 5a were obtained for experiments performed with either l-[35S]cysteine or [35S]methionine, suggesting that TCA precipitation was an effective method of standardizing radiolabel incorporation for the different proteins (data not shown). CR2 Hgl2 exhibited lower maximal binding (Bmax) (P < 0.05, n = 3) than FL Hgl2 and CR1 Hgl2, which bound equally well to GalNAc-BSA. Binding of CR1 Hgl2 to GalNAc19BSA was significantly inhibited by 25 and 50 μM asialofetuin (53.1 and 64.8% inhibition, respectively) but not by equimolar amounts of an irrelevant glycoprotein (holotransferrin at 50 μM) (Fig. 5B). Binding of 35S-labeled CR Hgl2 was dependent on the concentration of GalNAc19BSA coated in each well and was saturable (Fig. 6a). Background binding to BSA was not dependent on the concentration of BSA coated. Furthermore, the irrelevant control protein luciferase failed to bind GalNAc19BSA in concentration-dependent manner (Fig. 6a). To estimate the affinity of binding, 35S-CR Hgl2 (90.54 mCi/nmol) was added in a dose-dependent manner to GalNAc-BSA and BSA (background control). Specific binding was determined and the least squares fit of the data to a rectangular hyperbola was used to calculate the KD (0.85 ± 0.37 pM) and Bmax (18,125 ± 2,102 cpm) (Fig. 6b).
FIG. 5.
Binding of 35S-labeled FL, CP (1–480), CR1, and CR2 Hgl2 to GalNAc19BSA. (a) GalNAc-specific binding (determined as described in the text) of FL, CP, CR1, and CR2 Hgl2, luc (LUC), or samples from control translation reaction without lectin mRNA added. Equivalent amounts of 35S-labeled protein used in each corresponding binding experiment were analyzed by SDS-PAGE and fluorography to ensure specific in vitro synthesis (shown below each corresponding bar). CR2 Hgl2 binds to lower maximal activity than FL or CR1 Hgl2 (∗, P < 0.05). (b) Inhibition of GalNAc-specific binding of cysteine-rich Hgl2 to GalNAc19BSA with asialofetuin (ASF) and holotransferrin (hT) at 25 or 50 μM. Data are means ± SE from three separate experiments performed in duplicate. Statistical significance was determined by one-way analysis of variance (∗, P < 0.005).
FIG. 6.
Binding of 35S-CR1 Hgl2 to GalNAc19BSA. (a) Binding of 35S-labeled protein (100,000 cpm) as a function of GalNAc19BSA concentration for CR1 Hgl2 (circles) or luciferase (triangles). Nonspecific binding of CR Hgl2 to BSA is also shown (squares). (b) Specific binding (determined as described in the text) of 35S-CR Hgl2 (90.54 mCi/nmol) to 50 μg of GalNAc19BSA as a function of the amount of radiolabeled lectin added to each well. The line represents the least squares fit of the data to a rectangular hyperbola with the KD (0.85 ± 0.37 pM) and Bmax (18,125 ± 2,103 cpm). Data presented are means ± SE for duplicate determinations.
DISCUSSION
An understanding of the molecular basis for amebic adherence may provide insight into the pathogenesis of invasive disease and a possible basis for the design of interventions that would disrupt amebic attachment and cytolysis. For instance, delineating the carbohydrate-binding capacity of the E. histolytica Gal/GalNAc lectin may facilitate the rational design of recombinant protein or DNA vaccines (33). To date, there has been no direct evidence supporting the assumption that the heavy subunit of the Gal/GalNAc lectin contains the carbohydrate-binding domain(s). In this study, we have localized the carbohydrate-binding domains of the E. histolytica Gal/GalNAc lectin to CR1 of the heavy subunit. This is the first direct evidence that the cysteine-rich region of the 170-kDa subunit is sufficient for high-affinity carbohydrate binding.
The in vitro-synthesized Gal/GalNAc lectin heavy subunit was specifically recognized by anti-lectin MAbs that bind linear epitopes (1G7 and 8A3), in agreement with previous observations that 1G7 and 8A3 recognize bacterially produced recombinant fragments of the 170-kDa subunit when reduced and denatured (12). PDI treatment of the translated heavy subunit improves its immunoreactivity with the putative conformationally-dependent anti-lectin MAb, 3F4, suggesting that it has been folded into a more native conformation. The folding catalyst PDI has been previously used to achieve functional native proteins following in vitro synthesis (7, 27). Binding studies using 35S-labeled FL and CR Hgl2 indicated that millimolar concentrations of the monosaccharide galactose was able to specifically inhibit binding to CHO cells. Moreover, purified native hololectin competed binding of 35S-lectin to CHO cells in a dose-dependent fashion. The fragment containing residues 1 to 353 failed to bind CHO cells in a galactose-dependent manner. These results suggested that PDI-refolded FL and CR Hgl2 are functional.
To confirm and extend these observations, a more potent multivalent ligand was evaluated. Previous studies using glyconeoconjugates have shown that they bind E. histolytica membranes with high affinity (KD = 10 ± 3 nM), and this is likely due to the clustered arrangement of the multiple GalNAc residues present on the glyconeoconjugate (1). GalNAc39BSA was able to inhibit binding of E. histolytica membranes to the same radioligand at 200,000-fold-lower concentrations than GalNAc alone (1). Here we show that in vitro-synthesized FL, CR1, and CR2 Hgl2 bind to GalNAc19BSA in a GalNAc-specific manner. Equivalent amounts of radiolabeled firefly luciferase or samples from control translation reactions (no added lectin mRNA) synthesized in identical fashion did not bind GalNAc19BSA. Binding of FL and CR1 Hgl2 to the glyconeoconjugate occurred with the same maximal activity, suggesting that CR2 Hgl contains all carbohydrate-binding activity. However, the smaller CR2 fragment bound with lower maximal activity, suggesting that residues between 356 and 480 and between 900 to 1143 contribute to high-affinity carbohydrate binding. Binding of CR1 was concentration dependent, appeared to be saturable, and was specifically inhibited by asialofetuin. The terminal GalNAc-containing glycoprotein asialofetuin (IC50 of ∼30 μM) binds to E. histolytica membrane-bound lectin but with lower affinity than glyconeoconjugates like GalNAc39BSA (IC50 = 0.005 μM) (1). Additionally, commercial preparations of asialofetuin are often incompletely desialylated and may therefore contribute to incomplete inhibition by this compound. The binding affinity seen for CR1 Hgl2 (KD = 0.85 ± 0.37 pM) was similar to that observed for the native lectin resident on amebic membranes when bound to mucin (KD = 8.2 × 10−11 M), a naturally occurring glyconeoconjugate found in the human colon (4). These data are consistent with distant residues contained within CR1 Hgl2 contributing to high-affinity sugar binding conferred by the Gal/GalNAc lectin and may explain prior conflicting evidence about the location of a carbohydrate-binding domain (10, 11). The experimental strategy presented here provides a useful alternative approach to further delineating the carbohydrate-binding capacity of the 170-kDa subunit.
ACKNOWLEDGMENTS
This work was supported by Medical Research Council of Canada grant MT-12665 (K.C.K.) and National Institutes of Health grant AI 18841 (J.I.R.). K.C.K. is a recipient of a career award from the Ontario Ministry of Health. D.R.P. is funded by an M.D./Ph.D. studentship from the Medical Research Council of Canada.
We thank William Petri (University of Virginia) for the kind gift of MAbs 1G7 and 8A3, Cheryl Smith for assistance with flow cytometry, and Kris Chadee (McGill University) and Ian Crandall (University of Toronto) for helpful discussion and advice.
REFERENCES
- 1.Adler P, Wood S J, Lee Y C, Lee R T, Petri W A, Jr, Schnaar R L. High affinity binding of the Entamoeba histolytica lectin to polyvalent N-acetylgalactosaminides. J Biol Chem. 1995;270:5164–5171. doi: 10.1074/jbc.270.10.5164. [DOI] [PubMed] [Google Scholar]
- 2.Aley S B, Scott W A, Cohn Z A. Plasma membrane of Entamoeba histolytica. J Exp Med. 1980;152:391–404. doi: 10.1084/jem.152.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell D, Chadee K. Survival strategies of Entamoeba histolytica: modulation of cell-mediated immune responses. Parasitol Today. 1997;13:184–189. doi: 10.1016/s0169-4758(97)01022-3. [DOI] [PubMed] [Google Scholar]
- 4.Chadee K, Johnson M L, Orozco E, Petri W A, Jr, Ravdin J I. Binding and internalization of rat colonic mucins by the galactose/N-acetyl-d-galactosamine adherence lectin of Entamoeba histolytica. J Infect Dis. 1988;158:398–406. doi: 10.1093/infdis/158.2.398. [DOI] [PubMed] [Google Scholar]
- 5.Chadee K, Petri W A, Jr, Innes D J, Ravdin J I. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J Clin Investig. 1987;80:1245–1254. doi: 10.1172/JCI113199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond L S, Harlow D R, Cunnick C C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 1978;72:431–432. doi: 10.1016/0035-9203(78)90144-x. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert H F. Protein disulfide isomerase and assisted protein folding. J Biol Chem. 1997;272:29399–29402. doi: 10.1074/jbc.272.47.29399. [DOI] [PubMed] [Google Scholar]
- 8.Guerrant R L, Brush J, Ravdin J I, Sullivan J A, Mandell G L. Interaction between Entamoeba histolytica and human polymorphonuclear neutrophils. J Infect Dis. 1981;143:83–93. doi: 10.1093/infdis/143.1.83. [DOI] [PubMed] [Google Scholar]
- 9.Leippe M, Andra J, Muller-Eberhard H J. Cytolytic and antibacterial activity of synthetic peptides derived from amoebapore, the pore-forming peptide of Entamoeba histolytica. Proc Natl Acad Sci USA. 1994;91:2602–2606. doi: 10.1073/pnas.91.7.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotter H, Zhang T, Seydel K B, Stanley S L, Jr, Tannich E. Identification of an epitope on the Entamoeba histolytica 170-kD lectin conferring antibody-mediated protection against invasive amebiasis. J Exp Med. 1997;185:1793–1801. doi: 10.1084/jem.185.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mann B J, Chung C Y, Dodson J M, Ashley L S, Braga L L, Snodgrass T L. Neutralizing monoclonal antibody epitopes of the Entamoeba histolytica galactose adhesin map to the cysteine-rich extracellular domain of the 170-kilodalton subunit. Infect Immun. 1993;61:1772–1778. doi: 10.1128/iai.61.5.1772-1778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann B J, Lockhart L A. Molecular analysis of the Gal/GalNAc adhesin of Entamoeba histolytica. J Eukaryot Microbiol. 1998;45:13S–16S. doi: 10.1111/j.1550-7408.1998.tb04518.x. [DOI] [PubMed] [Google Scholar]
- 13.Mann B J, Torian B E, Vedvick T S, Petri W A., Jr Sequence of a cysteine-rich galactose-specific lectin of Entamoeba histolytica. Proc Natl Acad Sci USA. 1991;88:3248–3252. doi: 10.1073/pnas.88.8.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCoy J J, Mann B J, Vedvick T S, Pak Y, Heimark D B, Petri W A., Jr Structural analysis of the light subunit of the Entamoeba histolytica galactose-specific adherence lectin. J Biol Chem. 1993;268:24223–24231. [PubMed] [Google Scholar]
- 15.McCoy J J, Mann B J, Vedvick T S, Petri W A., Jr Sequence analysis of genes encoding the light subunit of the Entamoeba histolytica galactose-specific adhesin. Mol Biochem Parasitol. 1993;61:325–328. doi: 10.1016/0166-6851(93)90079-d. [DOI] [PubMed] [Google Scholar]
- 16.Petri W A, Jr, Joyce M P, Broman J, Smith R D, Murphy C F, Ravdin J I. Recognition of the galactose- or N-acetylgalactosamine-binding lectin of Entamoeba histolytica by human immune sera. Infect Immun. 1987;55:2327–2331. doi: 10.1128/iai.55.10.2327-2331.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petri W A, Jr, Schnaar R L. Purification and characterization of galactose- and N-acetylgalactosamine-specific adhesin lectin of Entamoeba histolytica. Methods Enzymol. 1995;253:98–104. doi: 10.1016/s0076-6879(95)53011-8. [DOI] [PubMed] [Google Scholar]
- 18.Petri W A, Jr, Smith R D, Schlesinger P H, Murphy C F, Ravdin J I. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Investig. 1987;80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petri W A, Jr, Snodgrass T L, Jackson T F, Gathiram V, Simjee A E, Chadee K, Chapman M D. Monoclonal antibodies directed against the galactose-binding lectin of Entamoeba histolytica enhance adherence. J Immunol. 1990;144:4803–4809. [PubMed] [Google Scholar]
- 20.Pillai D R, Britten D, Ackers J P, Ravdin J I, Kain K C. A gene homologous to hgl2 of Entamoeba histolytica is present and expressed in Entamoeba dispar. Mol Biochem Parasitol. 1997;87:101–105. doi: 10.1016/s0166-6851(97)00047-9. [DOI] [PubMed] [Google Scholar]
- 20a.Promega Corp. Protocols & applications guide. 2nd ed. Madison, Wis: Promega Corp.; 1995. [Google Scholar]
- 21.Purdy J E, Mann B J, Shugart E C, Petri W A., Jr Analysis of the gene family encoding the Entamoeba histolytica galactose-specific adhesin 170-kDa subunit. Mol Biochem Parasitol. 1993;62:53–59. doi: 10.1016/0166-6851(93)90177-y. [DOI] [PubMed] [Google Scholar]
- 22.Ravdin J I, Croft B Y, Guerrant R L. Cytopathogenic mechanisms of Entamoeba histolytica. J Exp Med. 1980;152:377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ravdin J I, Guerrant R L. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J Clin Investig. 1981;68:1305–1313. doi: 10.1172/JCI110377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ravdin J I, Kelsall B L. Role of mucosal secretory immunity in the development of an amebiasis vaccine. Am J Trop Med Hyg. 1994;50:36–41. [PubMed] [Google Scholar]
- 25.Ravdin J I, Petri W A, Murphy C F, Smith R D. Production of mouse monoclonal antibodies which inhibit in vitro adherence of Entamoeba histolytica trophozoites. Infect Immun. 1986;53:1–5. doi: 10.1128/iai.53.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed S L, Curd J G, Gigli I, Gillin F D, Braude A I. Activation of complement by pathogenic and nonpathogenic Entamoeba histolytica. J Immunol. 1986;136:2265–2270. [PubMed] [Google Scholar]
- 27.Ruddon R W, Bedows E. Assisted protein folding. J Biol Chem. 1997;272:3125–3128. doi: 10.1074/jbc.272.6.3125. [DOI] [PubMed] [Google Scholar]
- 28.Saffer L D, Petri W J. Role of the galactose lectin of Entamoeba histolytica in adherence-dependent killing of mammalian cells. Infect Immun. 1991;59:4681–4683. doi: 10.1128/iai.59.12.4681-4683.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tannich E, Ebert F, Horstmann R D. Molecular cloning of cDNA and genomic sequences coding for the 35-kilodalton subunit of the galactose-inhibitable lectin of pathogenic Entamoeba histolytica. Mol Biochem Parasitol. 1992;55:225–227. doi: 10.1016/0166-6851(92)90144-9. [DOI] [PubMed] [Google Scholar]
- 30.Tannich E, Ebert F, Horstmann R D. Primary structure of the 170-kDa surface lectin of pathogenic Entamoeba histolytica. Proc Natl Acad Sci USA. 1991;88:1849–1853. doi: 10.1073/pnas.88.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tannich E, Scholze H, Nickel R, Horstmann R D. Homologous cysteine proteinases of pathogenic and nonpathogenic Entamoeba histolytica. Differences in structure and expression. J Biol Chem. 1991;266:4798–4803. [PubMed] [Google Scholar]
- 32.Walsh J A. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev Infect Dis. 1986;8:228–238. doi: 10.1093/clinids/8.2.228. [DOI] [PubMed] [Google Scholar]
- 33.Zhang T, Stanley S L., Jr Protection of gerbils from amebic liver abscess by immunization with a recombinant protein derived from the 170-kilodalton surface adhesin of Entamoeba histolytica. Infect Immun. 1994;62:2605–2608. doi: 10.1128/iai.62.6.2605-2608.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]