Abstract
Purpose
Ophthalmic surgery involves the manipulation of micron-level sized structures such as the internal limiting membrane where tactile sensation is practically absent. All humans have physiologic tremors that are of low amplitude and not discernible to the naked eye; they do not adversely affect the majority of the population’s daily functioning. However, during microsurgery, such tremors can be problematic. In this review, we focus on the impact of physiological tremors on ophthalmic microsurgery and offer a comparative discussion on the impact of such tremors on other surgical specialties.
Methods
A single investigator used the MEDLINE database (via PubMed) to search for and identify articles for inclusion in this systematic review. Ten key factors were identified as potentially having an impact on tremor amplitude: beta-blockers, muscle fatigue, robotic systems, handheld tools/micromanipulators, armrests/wrist supports, caffeine, diet, sleep deprivation, consuming alcohol, and workouts (exercise). These key terms were then searched using the advanced Boolean search tool and operators (i.e., AND, OR) available on PubMed: (*keyword*) AND (surgeon tremor OR microsurgery tremor OR hand steadiness OR simulator score).
Results
Ten studies attempted to quantify the baseline severity of operator physiologic tremor. Approximately 89% of studies accessing the impact of tremors on performance in regards to surgical metrics reported an improvement in performance compared to 57% of studies concluding that tremor elimination was of benefit when considering procedural outcomes.
Conclusions
Robotic technology, new instruments, exoskeletons, technique modifications, and lifestyle factors have all demonstrated the potential to assist in overcoming tremors in ophthalmology.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00417-022-05718-2.
Keywords: Physiologic tremors, Ophthalmology, Robotics, Instruments, Exoskeletons, Technique modifications, Lifestyle factors
Introduction
Ophthalmic surgery involves the manipulation of micron-level sized structures such as the internal limiting membrane where tactile sensation are practically absent and subsequently the procedure is performed solely on the basis of visual feedback [1]. Negotiating such microsurgeries understandably requires a great deal of precision and maybe limited by a surgeon’s physiological tremor.
A tremor is defined as a rhythmic and involuntary movement of any body part and is the most common movement disorder [2]. Tremors can broadly be divided into two main categories: physiological and pathological. Physiological tremor are bilateral low-amplitude tremors with an average frequency of 7.7 Hz [3, 4]. All humans have a physiologic tremor, which may be unnoticed unless testing is conducted [5]. As physiological tremors are of low amplitude and not discernible to the naked eye, they do not adversely affect the majority of the human population’s daily functioning or cause limitation in their activities of daily living. However, during microsurgery, such tremors can be problematic. An accentuated physiologic tremor is differentiated from a pathological tremor on clinical grounds (i.e., is visible) and in the presence of magnifying factors. Stressors that may magnify physiological tremors include drug-induced (e.g., caffeine or anti-depressants), metabolic (e.g., hyperthyroidism and hypoglycemia), and anxiety [6].
Physiological tremors can be accentuated by postural movements and muscle contraction [7]. Lakie et al. found that large changes in posture had no effect on severity of tremor,however, slow wrist flexion and extension maneuvers significantly worsened the magnitude of tremor recorded [8]. In the case of contraction tremors, one study concluded isometric and tonic contraction both result in worsening of an individual’s physiologic tremor [9]. Another factor affecting the severity of one’s physiologic tremor is fatigue. Chandra et al. reported that increasing fatigue over time resulted in an increase in hand tremor during simulated laparoscopic surgery tasks [10]. Based on these findings, postural movements, muscle contraction (isometric and tonic), and fatigue may be key modifiable factors to reduce the impact of tremors in ophthalmology. In this review, we focus on the impact of physiological tremors on ophthalmic microsurgery and offer a comparative discussion on the impact of such tremors on other surgical specialties that employ microsurgeries. Secondly, this review aims to summarize the many strategies employed to overcome these tremors and make ophthalmic microsurgery more efficient and safer.
Methods
Protocol
The PRISMA (Preferred Reporting Items for Systematic Re-views and Meta-Analyses) criterion was used as the structural basis for this systematic review. The review was aimed at identifying existing literature on the impact of tremor on ophthalmic surgery and to explore the evidence surrounding various modalities for overcoming tremors in ophthalmology.
Search strategy
A single investigator used the MEDLINE database (via PubMed) to search for and identify articles for inclusion in this systematic review. Additionally, the reference lists from identified articles were reviewed and relevant articles were included if they were deemed appropriate. Through an initial literature search, 10 key factors were identified as potentially having an impact on tremor amplitude: beta-blockers, muscle fatigue, robotic systems, handheld tools/micromanipulators, armrests/wrist supports, caffeine, diet, sleep deprivation, consuming alcohol, and workouts (exercise). These key terms were then searched using the advanced Boolean search tool and operators (i.e., AND, OR) available on PubMed: (*keyword*) AND (surgeon tremor OR microsurgery tremor OR hand steadiness OR simulator score). There were no date restrictions applied to the database searches, with all articles published until June 15, 2020 being considered. Only articles published in English were included in the review.
Study selection
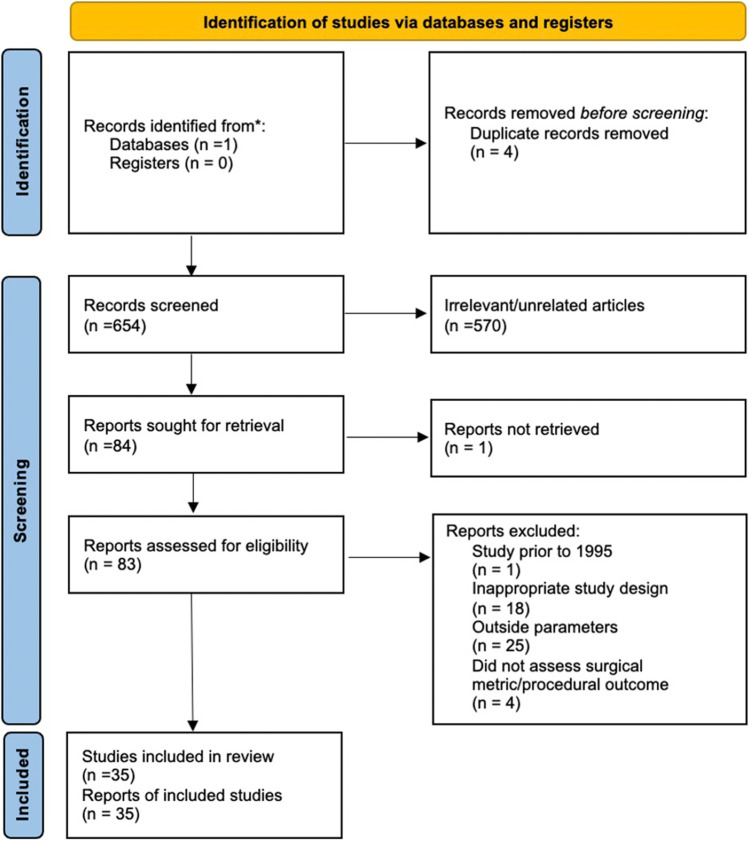
Through the “save” option, each keyword’s search results were downloaded in the .CSV format. A manual assessment of the titles and abstracts from these article lists were then used to filter for relevant articles and remove all duplicate articles identified. All articles deemed relevant were subsequently transferred to a separate Microsoft Excel Spreadsheet. This process generated a database consisting of 84 articles that either evaluated the impact of the selected factors on tremors and/or on surgical performance being selected in the first phase of screening. The final stage of screening involved accessing the full texts of all the 83 articles in order to identify studies measuring the impact of tremors on surgical metrics and/or procedural outcomes which resulted in 35 articles being identified (see Figure 1 for PRISMA flowchart for study identification).
Figure 1.
PRISMA flow diagram for identification of studies [11]
Eligibility criteria
The final stage of screening selected articles based on the following criterion: (1) articles must be laboratory studies, prospective studies, animal studies or retrospective studies; (2) must discuss or suggest one of the ten key factors mentioned above as having an implication on tremor and the outcome being accessed; and (3) outcomes can be in the form of surgical metrics (e.g., accuracy, error rate, time to completion) or procedural outcomes (e.g., successful completion rate or post-procedure complications). The only exclusion criteria were if articles were review based (i.e., comprehensive reviews, systematic reviews, and meta-analyses), case reports or patient/animal series, and if they were published prior to 1995. All studies with medical students, resident surgeons, consultant surgeons, and non-surgeon participants were included.
Data extracted
For this review, every study deemed to fit the criteria for inclusion had the following data extracted: title of publications, authors and affiliations, country of study, year of study, strategy to overcome impact of tremors, surgical metric or procedural outcome accessed, impact on metric or outcome, specialty, and number of operating participants.
The findings of this review are presented in narrative form, as no data was found to be appropriate for pooling for statistical analysis. Furthermore, due to the nature of the studies (limited sample sizes) and the considerable variation in assessed outcomes (several studies considering subjective outcomes), there likely is a degree of publication bias to consider.
Assessment of study evidence
All of the selected articles were then accessed by the reviewer (G. S.) and assigned a level of evidence according to The Oxford 2011 Levels of Evidence (OCEBM) [12]. The assigned grades were than checked by a second reviewer (W. C.) and any discrepancies were discussed in order to arrive at a final grade.
Results
From the final data set 10 studies attempted to quantify the baseline severity of operator physiologic tremor (Table 1). Techniques to gauge the severity of tremor included subjective severity while undertaking surgical procedures, ability to maintain a specific offset distance from a target, and deviation from a stationary target when attempting to maintain position for a set time. As seen in Table 1 there was considerable variability in the methods of assessing baseline tremor and the units of measurement of baseline severity. Therefore, it was not deemed feasible or reliable to calculate a mean baseline tremor (e.g., in Hz).
Table 1.
Studies reporting baseline tremor severity during surgical procedures and/or simulation tasks
| Author | Method/unit of tremor measurement | Score |
|---|---|---|
| Feng et al. [13] | Subjective microvascular tremor scale | N: 2.4, E: 0.9* |
| Nakano et al. [14] | Average pointing error + maximum error (μm) | A: 70, M: >300 |
| Yang et al. [15] | RMS for pointing task (μm) | 112 |
| Song et al. [16] | RMS for drift from a defined offset height (μm) | L: 43.4, S: 36.0 |
| Maclachlan et al. [17] | 3D maximum error (μm) | N: 264, E: 318 |
| Song et al. [18] | Tool tip motion towards target (μm) | 1000 |
| Zhang et al. [19] | Average deviation of tool tip (μm) | 23.7 |
| Yang et al. [20] | RMSE for holding tip still (μm) | 90 |
| Maximum error for holding tip still (μm) | 240 | |
| Okamura et al. [21] | Mean tremor amplitude without FMA (pix) | 699.4 |
| Subjective tremor score (pts) | 3 | |
| Csókay et al. [22] | Instrument tip movements (mm) | 0.6 |
Table 1 aims to highlight the significant differences in method of tremor severity measurement within included studies. RMS root mean square, RMSE root-mean-square error, N novice operator, E expert operator, L longer time, S shorter time, A average, M maximum, μm micrometers, pts points, pix pixels
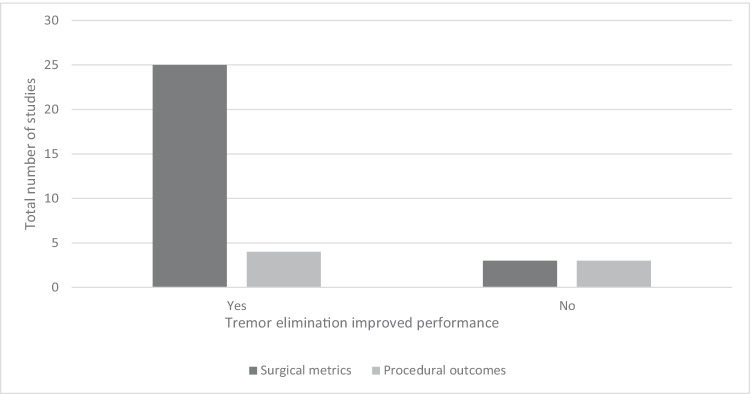
Figure 2 highlights that approximately 89% of studies accessing the impact of tremors on performance in regards to surgical metrics reported an improvement in performance upon reduction and/or elimination of operator physiological tremor. As seen in Table 2, there were 12 ophthalmology specific studies identified, of which 11 found an improvement in performance when eliminating tremors. Two studies employed medications for tremor reduction, one used a robotic surgical system, and nine studies evaluated surgical metric performance when a handheld tremor reduction tool was used.
Figure 2.
Comparison of studies evaluating if tremor elimination improved performance when considering surgical metrics vs. procedural outcomes
Table 2.
Ophthalmology-based studies reporting the impact of physiologic tremors on surgical metrics
| Author | Strategy to reduce tremors | Surgical metric | Improved performance | Speciality | Evidence quality | Year | Country | n |
|---|---|---|---|---|---|---|---|---|
| Roizenblatt et al. [23] | Beta-blocker (tremor reducing), caffeine (tremor increasing) | Simulator score | Yes (beta-blocker) | Ophthalmology | II | 2020 | Brazil | 15 |
| Pointdujoir et al. [24] | Beta-blocker (propranolol) | Simulator score | Yes | Ophthalmology | III | 2011 | America | 18 |
| Nakano et al. [14] | Robotic surgical system | Stability and accuracy | Yes | Ophthalmology | IV | 2009 | Japan | NS |
| Yang et al. [15] | “Micron” handheld robotic micromanipulator | Physiologic tremor filtration and RMS error | Yes | Ophthalmology | IV | 2015 | America | 1 |
| Song et al. [16] | Swept source optical coherence tomography-based “SMART” handheld tool | Maintained tool tip offset distance | Yes | Ophthalmology | IV | 2012 | America | 2 |
| Maclachlan et al. [17] | Actively stabilized handheld tool | Position error on several error metrics | Yes | Ophthalmology | IV | 2013 | America | 6 |
| Gonenc et al. [25] | “Micron” handheld robotic micromanipulator | Magnitude of forces during membrane peeling of a phantom bandage and membrane on raw chicken egg | Yes | Ophthalmology | IV | 2012 | America | 1 |
| Song et al. [18] | Fibre-optic OCT sensor-guided SMART microforceps | Targeted grasping and peeling performance | Yes | Ophthalmology | IV | 2013 | America | NS |
| Zhang et al. [19] | Hand-held instrument with active tremor compensation | Tip force applied while peeling inner shell membrane of chicken egg | Yes | Ophthalmology | IV | 2020 | China | 1 |
| Gonenc et al. [26] | Micron with force sensing | Peeling force and peeling speed on phantom bandages and eggs | Yes | Ophthalmology | IV | 2014 | America | 1 |
| Yang et al. [20] | Micron with force sensing | RMS error for holding-still and circle tracing in eye phantom and rubber target | Yes | Ophthalmology | IV | 2013 | America | 1 |
| Okamura et al. [21] | Freely movable arm rest (FMA) | Arm fatigue, subjective tremor score, and suturing accuracy | No | Ophthalmology | IV | 2020 | Japan | 8 |
RMS root mean square, RMSE root-mean-square error, N novice, E expert, L longer time, S shorter time, A average, M maximum, MTS microvascular tremor scale, TTC time to completion, TLM transoral laser microsurgery, RVBS retinal vein bypass surgery, NS not specified
Comparatively, there were only 16 studies identified from other surgical specialities within medicine; approximately 81% of these studies reported an improvement in surgical metric performance when a tremor eliminating strategy was employed (See Online Resource 1) [10, 17, 27–40]. The literature evaluating the impact of tremor elimination on procedural outcomes was far scarcer, with only seven studies identified (Table 3). Overall, only 4/7 (approximately 57%) of studies concluded that tremor elimination was of benefit when considering procedural outcomes. The two ophthalmology studies identified both employed the use of surgical robotic systems to eliminate tremors and both reported statistically significant findings for tremor elimination improving procedural outcomes.
Table 3.
Studies evaluating procedural outcomes
| Author | Strategy | utcome | Improved performance | Specialty | Evidence | Year | Country | n |
|---|---|---|---|---|---|---|---|---|
| Bernie et al. [41] | Da Vinci surgical system | TTC, blood loss, hospital stay, complications | Yes (hospital stay + number of complications) | Urology | IV | 2005 | America | 1 |
| Chen et al. [42] | Robotic surgical system | RVBS success rate in ex vivo porcine eyes | Yes | Ophthalmology | IV | 2017 | China | 1 |
| Knight et al. [43] | Zeus surgical system | Patency of rat femoral artery anastomoses | No | Pediatric surgery | IV | 2005 | America | NS |
| You et al. [44] | Da Vinci surgical system | TTC, hospital stay, complications, and mortality | No | General surgery | III | 2013 | Korea | 1 |
| Fleming et al. [45] | Co-op robotic surgical assistant | Time to cannulation, successful cannulation, and successfully maintained cannulation | Yes | Ophthalmology | IV | 2008 | America | 19 |
| Csókay et al. [22] | I–III finger support, which holds the instruments on Bethlehem bridge | Complication rate from 23 cases | Yes | Neurosurgery | III | 2009 | Hungary | NS |
| Basaran et al. [40] | Fatigue and sleep deprivation | Anastomosis patency rates | No (less fatigue) | Plastic surgery | III | 2015 | Turkey | 1 |
RMS root mean square, RMSE root-mean-square error, N novice, E expert, L longer time, S shorter time, A average, M maximum, MTS microvascular tremor scale, TTC time to completion, TLM transoral laser microsurgery, RVBS retinal vein bypass surgery, NS not specified
Discussion
Overall, our findings tend to support the notion that physiologic tremor reduction has a positive impact on surgical performance when considering surgical metrics or procedural outcomes across many specialties, including ophthalmology in particular.
Two studies from Table 1 (evaluating operator baseline tremor) differentiated between the baseline tremors of novices and expert participants. Feng et al. noted that the mean tremor score of their microvascular naïve precipitants was 2.40 during freehand completion of tasks (statically significant). However, their single microvascular expert operator had a score of 0.86; this was not statically significant given a sample size of 1 [13]. Interestingly, Machlachlan et al.’s study noted that the 3D maximum error was higher in surgeon participants compared to their less experienced counterparts at baseline/without tremor eliminating intervention (264 μm versus 318 μm, respectively). External to the study, one study comparing the performance of residents to their more experienced consultant anterior segment colleagues noted that the specialist cohort had superior anti-tremor simulator scores [46]. Overall, data comparing the baseline physiologic tremors of expert ophthalmic surgeons to novices is very limited. The more robust data tends to suggest that novice surgeons have a greater baseline physiologic tremor than their more experienced counterparts. This may be attributed to greater time spent training (therefore more repetition), resulting in less stress and greater familiarity with specific maneuvers intraprocedurally.
Medications
A potential solution to overcoming tremors in ophthalmology may be through the use of medications. Propranolol is commonly used for managing hypertension, cardiac arrhythmias, myocardial infarction, migraine, portal hypertension, anxiety, essential tremors, and hyperthyroidism [47]. Elman et al. discovered a dose prior to operating reduced the tremors of ophthalmology residents [48]. Similarly, another study demonstrated that propranolol resulted in a 22% decrease in the magnitude of the tremors of ophthalmologists [49]. Lubahn et al. concluded that another beta antagonist (timolol) was not effective in alleviating hand tremors [50]. Arnold et al. also reported that timolol was only effective in reducing caffeine-induced tremors of ophthalmology trainees [51]. An additional consideration to the use of beta-blockers for tremor control is the potential for side effects. There was a total of seven beta-blocker studies identified in this review and only three had discussion on adverse effects. Arnold et al. reported two (out of 16 participants) withdrew due to unspecified side effects, whereas two other studies stated no participants experienced any adverse effects [48, 49, 51]. These findings, in addition to 2/3 studies reporting that a beta-blocker was effective in improving surgical performance through tremor reduction, allude to select beta-blockers being effective in reducing tremors and subsequently improving intraoperative performance. Presently data is extremely limited and larger, more robust studies are needed to confirm if this reduction in tremors offers improved surgical performance without side effects.
Fatigue
Ophthalmology surgeries can often be completed in less than 30 min [52]. During this time a high degree of concentration is required and this over the course of a day can result in increasing fatigue. The onset of physical fatigue over the course of a day can result in the increase in amplitude of a surgeon’s physiological tremor. One study demonstrated that muscle-cooling was effective in reducing the amplitude of fatigue-induced tremors [27]. The trial demonstrated a statistically significant reduction in tremor amplitude of experienced surgeons when wearing a muscle cooling suit (5 °C). The trial failed to show statistical significance for trainees. If larger studies are conducted and the results continue to be promising, muscle-cooling suits may have a role to play in the reduction of ophthalmologists’ tremors. One key limitation to consider is the design of the cooling suit itself, the physical specifications of the suit may impair intrinsic hand dexterity and thereby limit its use in microsurgery.
Sleep plays a vital role in maintaining optimal functioning of the human body. It is recommended that individuals between the ages 18 and 60 obtain at least 7 h of sleep each night [53]. Presently, no literature exists that quantifies the impact of sleep on an ophthalmologist’s physiologic tremor. A study of ophthalmology residents did however conclude that acute sleep deprivation did not have an impact on overall performance of a simulated anterior segment surgical task [54]. Basaran et al.’s findings suggest that there was a decrease in anastomosis success when comparing the procedures done in the morning versus those done at the end of the day (fatigued) or the following morning without sleeping, however, their results did not demonstrate statistical significance [40]. They did however report a statistically significant reduction in error score, autopsy score, and global rating score when comparing the evening and morning groups. These findings suggest that sleep may play a role in decreasing surgical performance. In contrast, several studies not included in the final data have concluded that sleep deprivation did not demonstrate a detectable decrease in surgical performance [55–57]. Based on the data available it is difficult to ascertain if sleep deprivation has a significant impact on the physiological tremors and surgical performance of ophthalmologists.
Robotic surgical systems
Recent times have seen increasing research and attempts to incorporate robotics into ophthalmic surgery in order to improve patient outcomes [58]. This study identified a total of 14 studies (10 evaluating surgical metrics and 4 evaluating outcomes) assessing the impact of tremor on surgical performance through the use of robotic surgical systems. Approximately 65% of the studies concluded that there was a benefit to surgical performance. Three of the studies were noted to state that there was no benefit to performance through the use of tremor filtering robotic systems. Interestingly, a fourth study by Prasad et al. suggested that the motion scaling, rather than tremor cancellation offered by robotic surgical systems, improves surgical accuracy.
Only two of the robotic surgery studies identified were ophthalmology based, both of which evaluated or used their self-designed systems. External to the review, case reports have been published of the da Vinci system being successfully used to perform used for pterygium surgery, penetrating keratoplasty, and amniotic membrane transplant surgery in humans [59–61].
From the review of literature, seven studies evaluating the impact of tremor elimination on surgical outcomes were deemed appropriate for inclusion (see Table 3). Notably, five of these studies employed the use of robotic surgical systems.
In 2005, Knight et al. compared the use of the tremor eliminating Zeus Surgical System to traditional freehand femoral artery anastomosis in rats [43]. They reported that the robotic system offered no measurable benefit when considering surgical outcomes, including success of femoral artery anastomosis patency or leakage rates after 3 min. In a more ophthalmology specific context, Fleming et al. evaluated outcomes of a robotic system being employed to cannulate chorioallantoic membranes of chicken embryos (imitating retinal microsurgery) [45]. They concluded with learning time that the robotic system improved both maintenance of cannulation and time for successful cannulation. Similarly, Chen et al. compared the efficacy of a custom-made retinal microsurgery robotic system to the conventional free-hand approach, assessed via the successful completion of retinal vein bypass surgery (RVBS), using porcine eyes [42]. They found that the tremor eliminating system significantly improved RVBS success rates compared to the free-hand approach (35% vs. 5%, respectively). While this data is very limited, tremor elimination using robotic systems do appear to be beneficial in enhancing surgical outcomes.
Literature on the efficacy of tremor eliminating robotic systems on procedural outcomes in human subjects is also similarly scarce. Bernie et al. found that the patients who underwent robotic pyeloplasty had lower post-operative complications and length of hospital stay compared to the traditional approach [41]. In contrast to this, You et al. found that the Da Vinci Surgical System was relatively equivocal to laparoscopic adrenalectomy when considering surgical outcomes such as completion time, complications, mortality, and length of hospital stay [44].
The Intraocular Robotic Interventional Surgical System (IRISS) is another robotic surgical system currently under development; unlike the da Vinci it is specifically designed for use in ophthalmology. Wilson et al. created the IRISS to be employed as both a master-slave manipulator and a fully automated robot. The IRISS was demonstrated to be able to successfully perform a range of ocular procedures including vitrectomies, retinal-vein cannulation, and lens aspiration on pig porcine eyes [62]. Chen et al. also used the IRISS to perform semi-automated OCT-guided cataract surgeries,their system successfully achieved complete extraction in over 80% of pig eyes and in the remaining eyes almost complete extraction was achieved, with no reported capsule ruptures [63]. The IRISS has thus far shown great promise in successfully performing several ophthalmic procedures on pig porcine eyes,with further development its widespread incorporation may play a vital role in future ophthalmic practice and elimination of challenges associated with operator physiologic tremor.
Another robotic system showing a great deal of promise is the Steady Hand Eye Robot 2 (SHER2), and similarly to the IRISS, it is specifically designed for ophthalmic microsurgery. The robot actuates and mimics the operator’s movements with the robotic arm and filters out any detected tremors. Gonenc et al. demonstrated that the SHER tool reduced oscillations of 2–15 Hz in magnitude by over 50% while peeling the inner shell membrane (ISM) of chicken embryos [64]. Data on the SHER2 is currently limited, however with further research and development it may be the solution to addressing the challenges associated with a surgeon’s physiological tremor.
Robotic devices
This review of literature identified a total of 10 articles discussing the use of tremor reducing micromanipulators and their impact on surgical performance. Six of the studies assessed a micromanipulation handheld tool known as the “Micron” with/without additional modifications to assist retinal surgeons [15, 17, 20, 25, 26, 36]. The development of the Micron commenced in 1996 and Riviere et al. published the earliest literature on a Micron prototype impacting operator tremor 7 years later [65, 66]. They described the Micron’s handle being separated from the tip by piezoelectric actuators, in doing so allowing for the tip to move independently to the handle. The earliest version of the Micron identified in this study was published by Choi et al. in 2007 [36]. In this version an optical sensing system and orthogonal accelerometers were used to provide feedback and allow the tip to adjust for deviation from target point due to tremor. Several subsequent studies have been published incorporating additional technology such as a force-sensing needle tip in order to further improve the Micron instrument [20, 26]. Notably, Yang et al. reported that the Micron resulted in an approximately 90% reduction in physiologic tremor during a pointing task and error less than 25 μm during circle tracing. They concluded that this reduction in error was well within parameters for safe laser photocoagulation in Diabetic Retinopathy patients [15]. The results of the remaining five studies assessing the use of the Micron also demonstrated an improvement in surgical metric performance as a result of the tremor reduction offered by the use of the micromanipulator. While present literature is promising, it is important to note that all of the studies are based on phantom models or animal studies and more robust safety studies will be required prior to its realistic implementation into ophthalmic practice.
Similarly, Song et al. developed a “SMART” (Smart Micromanipulation Aided Robotic-surgery Tool) tool to assist vitreoretinal surgeons [16]. Their results also demonstrated that surgeon hand tremor was reduced with the use of the SMART tool. From a surgical metric point of view, its use increased tool stability, thereby helping maintain a constant stand-off height from the chick embryo’s membrane. In the following year, Song et al. also developed SMART OCT-guided microforceps for microsurgery, which was also reported to improve surgical performance by providing superior grasping and peeling compared to freehand use [18]. The incorporation of robot master-slave systems may make such devices obsolete in leading centers, however, these tools are a considerably more economical alternative in less wealthy geographic regions.
In vitreoretinal surgery, closure of the forceps during membrane peeling in order to manipulate retinal structures requires delicate precision and controlled movements [36]. The presence of a physiologic tremor can interfere with efficient completion of this process. Romano et al. reported the use of foot controllers for closure of pneumatic forceps rather than conventional manual hand-controlled closure [67]. The use of a foot controller eliminates the impact of physiologic tremors on the ability of a surgeon to grasp and manipulate a patient’s retinal membrane. The initial results are promising, with no retinal breaks found intraoperatively or post-operatively.
Chan et al. developed a foot-controlled endoscope holder to assist with sinus surgery [68]. The device involves the use of a foot control which consists of an inertial measurement unit (IMU) and Bluetooth capabilities to connect to the robotic arm. The robot arm consists of four points of joint movement. Inversion and eversion of the foot allow the surgeon to navigate through each joint and select the specific joint they would like to move. Raising their heel off the ground and moving it left or right allows the specific joint selected to be manipulated. This extra arm coupled with the surgeon’s two hands produce a three-hand technique, theoretically offering a great deal of precision and possible tremor reduction. This design is currently in its developmental stage and data is not yet available to determine how significant of a tremor reduction this additional hand may offer.
Exoskeletons
The review of literature conducted did not identify any current exoskeletons being employed to overcome the impact of tremor on microsurgery; however, their potential in reducing the severity of pathological tremors presents a possibility of their future use to bolster ophthalmic surgeon performance. An example of the exoskeleton is the wearable orthosis for tremor assessment and suppression (WOTAS), which showed a 40% tremor reduction for all 10 participants with pathologic tremors [69]. In its active mode (greatest reduction in tremor), the WOTAS generates forces that directly oppose the direction of the tremor, thereby cancelling out motion. Gallego et al. demonstrated a neuroprosthesis that was effective in reducing the amplitude of tremor by an average of 52% [70]. The neuroprosthesis delivers an injecting current via transcutaneous patches to trigger an antagonistic contraction of muscles, subsequently cancelling the motion of the tremor. Though presently in its infancy, these exoskeletons may play a key role in overcoming tremors in ophthalmology. They may be particularly beneficial in areas where it is not possible to use a da Vinci system (e.g., financial constraints in developing countries). Much like the cooling suits, the design of the exoskeletons may limit the dexterity of ophthalmologists and subsequently prevent its incorporation into ophthalmic microsurgery.
Ergonomic modifications
The alteration of instrument grip, finger configuration, and the use of orthotic supports have been demonstrated to reduce the impact of tremor during microsurgery. Csókay et al. describe a microsurgical technique which involves the use of 1st–3rd fingertip method with the addition of a Bethlehem bridge (support orthosis) during microneurosurgery [22]. Their comparison of 23 human cases with and without the technique demonstrated a reduction in complications (i.e., improvement in surgical outcomes). Another study reported that robotic arm holder known as the “EXPERT” decreased surgeon fatigue and decreased difficulty in performing microneurosurgical procedures [39]. In the 3 years prior to Goto et al.’s publication, the EXPERT was used in 13 procedures on live patients and demonstrated no associated complications or mortality. In contrast to this, the previously discussed article by Okamura et al. found that their FMA (freely moveable armrest) did not improve performance through reduction of hand tremor [21].
Anecdotally, resting the hand on the brow with a superior approach is also believed helpful for tremor reduction during ophthalmic surgery. Prior to the 1990s the superior approach was the approach of choice for cataract surgeries. In the early 1990s Dr I. Fine described a clear corneal incision with a temporal approach [71]. This approach likely concedes a significant advantage of tremor reduction due to wrist support but offers greater technical benefit including improved ability for the patient to fixate on the microscope light due to an unobstructed visual axis, improved exposure to the surgical limbus, and being more astigmatically neutral [72]. Data on the use of wrist supports and technique alterations is very limited, alterations in ergonomics to minimize fatigue may be key in reducing the impact of tremors during ophthalmic surgery.
Lifestyle factors
The consumption of coffee is extremely common among doctors and even more prevalent in those working in surgical specialties [73]. While caffeine consumption temporarily improves alertness and cognitive functioning, it also adversely affects the severity of an individual’s physiologic tremor. Urso-Baiarda et al. double-blind crossover study observing end-to-end vessel anastomosis reported that caffeine had a deleterious effect on performance [38]. Another trial of vitreoretinal surgeons noted deleterious effects on Eyesi Surgical Simulator performance following lose-dose caffeine consumption [23]. Evidence on whether caffeine consumption impacts on surgical performance is currently limited, it is still recommended that caffeine intake immediately prior to surgery should be avoided.
Literature on the impact of diet on physiological tremors is not available to the best of our knowledge, however following a Mediterranean diet has been reported to be beneficial in the management of patients suffering from essential tremors [74]. An American study reported that both upper body only (i.e., using free weight/machine to train chest, shoulders, and arms) and aerobic exercise (i.e., running, cycling, stair climbing) for 30–60 min significantly increased microsurgical hand tremor immediately after exercise [75]. They found that the participants returned to their baseline tremor 2 h post-upper body only exercise and 4 h post-aerobic exercise. Physical activity has been demonstrated to increase circulating levels of catecholamines by 1.5 to >20 times (dependent on duration and intensity) within the body, leading to an increase in muscle activity and subsequent worsening of physiologic tremor [76]. Based on this finding avoiding exercise 2 to 4 h prior to performing ophthalmic microsurgery may be beneficial in minimizing the severity of a proceduralist’s tremor, however, it is worth noting that no studies have directly evaluated the impact of preceding exercise on surgical performance in terms of metrics or procedural outcomes. In the case of pathological tremors resistance training has demonstrated benefit in reducing the tremor amplitude of individual’s suffering from essential tremors [77–79]. Similarly, active and passive lower body cycling have demonstrated an improvement in Parkinson’s disease patients [80].
Recent times have seen increasing focus on yoga, mindfulness, and deep breathing exercises as potential tremor reduction strategies in microsurgery. These activities have demonstrated efficacy in reducing stress and improving general well-being [81, 82]. A study of 50 surgical residents found an objective reducing in hand tremor in participants when assessing their tremors prior to and after pranayama yoga [83]. Pranyama yoga involves four main principles: a stepwise reduction in breathing rate, aiming for 1:2 ratio of length of inspiration vs. expiration, holding ones breathing for twice as long as expiration, and focusing on the noise produced by air moving through the glottis. This paper by La Padula et al. is the first of its kind demonstrating tremor reduction following yoga/deep breathing exercises.
Alcohol consumption has been reported to be beneficial in the transient reduction of tremors in patients suffering from essential tremors (ET) [84]. Similarly, Lakie et al. found that alcohol consumption caused a significant transient reduction in physiological tremor at the wrist [85]. While alcohol consumption does demonstrate improvement in physiological tremor, its consumption immediately prior to and the day before surgery has been found to be detrimental to surgical performance [86]. Furthermore, Gallagher et al. reported that excessive alcohol consumption the night prior to operating resulted in a decreased surgical performance until at least 4 pm the following day [87]. These findings suggest that though alcohol may be beneficial in reducing the magnitude of an operator’s physiologic tremor, it is detrimental to overall surgical performance and as such should be avoided the day prior and immediately before surgery.
While literature is limited, there is data suggesting that smoking cigarettes may acutely worsen an individual’s tremor dating back to the 1970s [88]. Following this (in 1983), Shiffman et al. reported that smoking two cigarettes doubled participant’s tremor scores [89]. Another American study found that the smoking cohort had a greater tremor score when assessing both static and kinetic tremors in comparison with their non-smoking counterparts. The findings from these studies tend to suggest that smoking may be detrimental to surgeon hand tremor and thus performance, however, no study directly evaluating this was identified from the review of literature.
Broadly, recreational drugs such as cocaine, methamphetamines, ecstasy, and cannabis are strictly prohibited for use by surgeons. A review of literature did not identify any studies evaluating the use of these illicit substances and the impact upon surgical performance. In the case of methamphetamines and ecstasy, one study found that stimulant users self-reported negative impacts on fine hand control, in addition to worsened tremors [90]. Interestingly cannabidiol has been demonstrated to reduce anxiety and tremor amplitude [91]. The use of cannabis has also been trialed as a tremor-reducing strategy in pathologic tremors (e.g., Parkinson’s disease, multiple sclerosis), thus far demonstrating no benefit [92–94]. Flavel et al. explored the impact of these substances on resting and active tremors of participants using an accelerometer [95]. They found that during movement, tremor amplitude was significantly increased in ecstasy users compared to non-drug users. Interestingly, cannabis or amphetamine-like drugs were not noted to worsen tremors. In contrast, Bauer found that participants with cocaine dependence had greater hand tremors than their non-dependent counterparts [96]. While research on the impact of recreational substances on physiologic tremor amplitude is inconclusive, it is unlikely that significant literature exploring this topic further would be easily attainable due to ethical considerations.
In addition to this, an attempted review of literature to evaluate the impact of underlying health conditions (e.g., stroke or muscle atrophy) on surgeon tremor did not yield any significant literature. Furthermore, there does not appear to be any data evaluating the impact of therapies owing to specific pathologies and operator tremor.
Limitations
One of the limitations of this review is the limited quality of published literature included. The majority of studies identified were Level IV and V evidence; thereby the strength of conclusions made have limited validity. The reasons for most studies being assigned lower levels of evidence include limited number of participants, studies with solely non-surgeon participants, subjects were phantom models or animals, and poor study design.
Another limitation of this review is a majority of studies originated from America. While the training of ophthalmologists and surgeons in America is likely comparable to countries such as the United Kingdom and Australia, the training of specialists in regions such as Asia and South America may be different. It is currently unclear the impact geographic training locations have on surgical performance and if the narrow geographic profile of countries included in this study may skew the findings noted.
A considerable proportion of the literature included is based on specialties other than ophthalmology. Although specialties such as neurosurgery may have some similarities to surgical approaches and techniques used in ophthalmology, several of the studies included in the review were based on General Surgery and the transferability of these findings is limited. While findings suggest that tremor reduction improves surgical performance in ophthalmic surgery, no robust evidence is available with regards to directly examining the effect of intraoperative tremor on surgical outcomes in live patients at this point in time.
Another limitation to this study was the significant variability in the methodology of tremor measurement. Strategies used to evaluate operator tremors included subjective reporting, positional sensors, software within surgical simulators, and motion tracking software. At present, there is no clear consensus as to which method is optimal for use in future studies. Furthermore, there is no data clearly identifying which specific parameters are the most robust indicators of measuring tremor severity (e.g., deviation from target point or maximum error while undertaking a specific surgical maneuver). Given the lack of consensus, exploring which methodology of tremor measurement and detection is most accurate warrants further exploration. This would allow for the design of more robust and consistent projects in the future.
Conclusion
Ophthalmologists are required to operate at the peak of their physiological limits and have impeccable control of their physiologic tremor due to the precision and dexterity required to undertake eye surgery. Operating in these conditions is extremely challenging and leaves little margin for error. In recent times there has been increasing research to suppress a microsurgeon’s tremor. Robotic technology, new instruments, exoskeletons, technique modifications, and lifestyle factors have all demonstrated the potential to assist in overcoming tremors in ophthalmology. Presently, the main barrier to the incorporation of such technologies into widespread commercial use is the lack of published data on success and safety in human subjects. However, with continued development, the incorporation of these strategies into clinical practice is promising.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
W. C. conceptualized the review topic. G. S. conducted the search for literature, extracted relevant points of discussion, analyzed currently reported statistics, updated reference lists, and contributed to writing of the report. W. C., W. W., M. S., R. C., and D. S. were involved in analyzing the current literature, drafting, and contributed to writing of the review. All co-authors approved of the review being submitted for publication.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Declarations
Ethics approval
This article does not contain any studies with human participants and/or animals performed by any of the authors. Given this is a review article and there were no animal or human participants, ethics approval was not required/sought.
Informed consent
There were no human participants and/or animals involved in the study; therefore, no informed consent process was applicable in this review article.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cutler N, Balicki M, Finkelstein M, et al. Auditory force feedback substitution improves surgical precision during simulated ophthalmic surgery. Invest Ophthalmol Vis Sci. 2013;54(2):1316–1324. doi: 10.1167/iovs.12-11136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis ED. Tremor. Continuum (Minneap Minn) 2019;25(4):959–975. doi: 10.1212/con.0000000000000748. [DOI] [PubMed] [Google Scholar]
- 3.Bötzel K, Tronnier V, Gasser T. The differential diagnosis and treatment of tremor. Deutsches Arzteblatt Int. 2014;111(13):225–236. doi: 10.3238/arztebl.2014.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raethjen J, Pawlas F, Lindemann M, et al. Determinants of physiologic tremor in a large normal population. Clin Neurophysiol. 2000;111(10):1825–1837. doi: 10.1016/s1388-2457(00)00384-9. [DOI] [PubMed] [Google Scholar]
- 5.Elias WJ, Shah BB. Tremor. Jama. 2014;311(9):948–954. doi: 10.1001/jama.2014.1397. [DOI] [PubMed] [Google Scholar]
- 6.Crawford P, Zimmerman EE. Differentiation and diagnosis of tremor. Am Fam Physician. 2011;83(6):697–702. [PubMed] [Google Scholar]
- 7.Jankovic J, Fahn S. Physiologic and pathologic tremors. Diagnosis, mechanism, and management. Ann Intern Med. 1980;93(3):460–465. doi: 10.7326/0003-4819-93-3-460. [DOI] [PubMed] [Google Scholar]
- 8.Lakie M, Vernooij CA, Osborne TM, et al. The resonant component of human physiological hand tremor is altered by slow voluntary movements. J Physiol. 2012;590(10):2471–2483. doi: 10.1113/jphysiol.2011.226449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jalaleddini K, Nagamori A, Laine CM, et al. Physiological tremor increases when skeletal muscle is shortened: implications for fusimotor control. J Physiol. 2017;595(24):7331–7346. doi: 10.1113/JP274899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandra S, Hayashibe M, Thondiyath A. Dominant component in muscle fatigue induced hand tremor during laparoscopic surgical manipulation. Conf Proc IEEE Eng Med Biol Soc. 2014;2014:6539–6542. doi: 10.1109/embc.2014.6945126. [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Howick J, Chalmers I, Glasziou P, et al (2011) Oxford centre for evidence-based medicine 2011 levels of evidence. Centre for Evidence-Based Medicine. Retrieved July from https://www.cebm.net/wp-content/uploads/2014/06/CEBM-Levels-of-Evidence-2.1.pdf
- 13.Feng AL, Razavi CR, Lakshminarayanan P, et al. The robotic ENT microsurgery system: a novel robotic platform for microvascular surgery. Laryngoscope. 2017;127(11):2495–2500. doi: 10.1002/lary.26667. [DOI] [PubMed] [Google Scholar]
- 14.Nakano T, Sugita N, Ueta T, et al. A parallel robot to assist vitreoretinal surgery. Int J Comput Assist Radiol Surg. 2009;4(6):517–526. doi: 10.1007/s11548-009-0374-2. [DOI] [PubMed] [Google Scholar]
- 15.Yang S, MacLachlan RA, Riviere CN. Manipulator design and operation for a six-degree-of-freedom handheld tremor-canceling microsurgical instrument. IEEE ASME Trans Mechatron. 2015;20(2):761–772. doi: 10.1109/tmech.2014.2320858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song C, Gehlbach PL, Kang JU. Swept source optical coherence tomography based smart handheld vitreoretinal microsurgical tool for tremor suppression. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1405–1408. doi: 10.1109/embc.2012.6346202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maclachlan RA, Becker BC, Tabarés JC, et al. Micron: an actively stabilized handheld tool for microsurgery. IEEE Trans Robot. 2012;28(1):195–212. doi: 10.1109/tro.2011.2169634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song C, Park DY, Gehlbach PL, et al. Fiber-optic OCT sensor guided “SMART” micro-forceps for microsurgery. Biomed Opt Express. 2013;4(7):1045–1050. doi: 10.1364/boe.4.001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang T, Gong L, Wang S, et al. Hand-held instrument with integrated parallel mechanism for active tremor compensation during microsurgery. Ann Biomed Eng. 2020;48(1):413–425. doi: 10.1007/s10439-019-02358-2. [DOI] [PubMed] [Google Scholar]
- 20.Yang S, Wells TS, Maclachlan RA, et al. Performance of a 6-degree-of-freedom active microsurgical manipulator in handheld tasks. Conf Proc IEEE Eng Med Biol Soc. 2013;2013:5670–5673. doi: 10.1109/embc.2013.6610837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okamura C, Kojima T, Tokiwa S, et al. Microscopic ophthalmic surgery using freely movable arm support robot: basic experiment and clinical experience. Ophthalmic Res. 2020 doi: 10.1159/000506645. [DOI] [PubMed] [Google Scholar]
- 22.Csókay A, Valálik I, Jobbágy A. Early experiences with a novel (robot hand) technique in the course of microneurosurgery. Surg Neurol. 2009;71(4):469–472. doi: 10.1016/j.surneu.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Roizenblatt M, Marin DGB, V, Grupenmacher, AT, , et al. Association of weight-adjusted caffeine and β-blocker use with ophthalmology fellow performance during simulated vitreoretinal microsurgery. JAMA Ophthalmol. 2020 doi: 10.1001/jamaophthalmol.2020.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pointdujour R, Ahmad H, Liu M, et al. Beta-blockade affects simulator scores. Ophthalmology. 2011;118(9):1893–1893.e1893. doi: 10.1016/j.ophtha.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Gonenc B, Balicki MA, Handa J, et al. Evaluation of a micro-force sensing handheld robot for vitreoretinal surgery. Rep U S. 2012;2012:4125–4130. doi: 10.1109/iros.2012.6385715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gonenc B, Gehlbach P, Handa J, et al. Motorized force-sensing micro-forceps with tremor cancelling and controlled micro-vibrations for easier membrane peeling. Proc IEEE RAS EMBS Int Conf Biomed Robot Biomechatron. 2014;2014:244–251. doi: 10.1109/BIOROB.2014.6913784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen L, Dancisak M, Korndorffer J. Muscle-cooling intervention to reduce fatigue and fatigue-induced tremor in novice and experienced surgeons: a preliminary investigation. Surg J (New York, NY) 2016;2(4):e126–e130. doi: 10.1055/s-0036-1594246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deshpande N, Peretti G, Mora F, et al. Design and study of a next-generation computer-assisted system for transoral laser microsurgery. OTO Open. 2018;2(2):2473974x18773327. doi: 10.1177/2473974x18773327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willems JI, Shin AM, Shin DM, et al. A comparison of robotically assisted microsurgery versus manual microsurgery in challenging situations. Plast Reconstr Surg. 2016;137(4):1317–1324. doi: 10.1097/prs.0000000000002030. [DOI] [PubMed] [Google Scholar]
- 30.Mitsuishi M, Morita A, Sugita N, et al. Master-slave robotic platform and its feasibility study for micro-neurosurgery. Int J Med Robot. 2013;9(2):180–189. doi: 10.1002/rcs.1434. [DOI] [PubMed] [Google Scholar]
- 31.Prasad SM, Prasad SM, Maniar HS, et al. Surgical robotics: impact of motion scaling on task performance. J Am Coll Surg. 2004;199(6):863–868. doi: 10.1016/j.jamcollsurg.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Ruiz A, Gagner M, Miller JH, et al. Manual vs robotically assisted laparoscopic surgery in the performance of basic manipulation and suturing tasks. Arch Surg. 1998;133(9):957–961. doi: 10.1001/archsurg.133.9.957. [DOI] [PubMed] [Google Scholar]
- 33.Moorthy K, Munz Y, Dosis A, et al. Dexterity enhancement with robotic surgery. Surg Endosc. 2004;18(5):790–795. doi: 10.1007/s00464-003-8922-2. [DOI] [PubMed] [Google Scholar]
- 34.Krapohl BD, Reichert B, Machens HG, et al. Computer-guided microsurgery: surgical evaluation of a telerobotic arm. Microsurgery. 2001;21(1):22–29. doi: 10.1002/1098-2752(2001)21:1<22::aid-micr1004>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 35.Moore LJ, Wilson MR, Waine E, et al. Robotically assisted laparoscopy benefits surgical performance under stress. J Robot Surg. 2015;9(4):277–284. doi: 10.1007/s11701-015-0527-y. [DOI] [PubMed] [Google Scholar]
- 36.Choi DY, Sandoval R, MacLachlan RA, et al. Test of tracing performance with an active handheld micromanipulator. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:3638–3641. doi: 10.1109/iembs.2007.4353119. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan M, Deshpande N, Pacchierotti C, et al. A robotic microsurgical forceps for transoral laser microsurgery. Int J Comput Assist Radiol Surg. 2019;14(2):321–333. doi: 10.1007/s11548-018-1887-3. [DOI] [PubMed] [Google Scholar]
- 38.Urso-Baiarda F, Shurey S, Grobbelaar AO. Effect of caffeine on microsurgical technical performance. Microsurgery. 2007;27(2):84–87. doi: 10.1002/micr.20311. [DOI] [PubMed] [Google Scholar]
- 39.Goto T, Hongo K, Yako T, et al. The concept and feasibility of EXPERT: intelligent armrest using robotics technology. Neurosurgery. 2013;72(Suppl 1):39–42. doi: 10.1227/NEU.0b013e318271ee66. [DOI] [PubMed] [Google Scholar]
- 40.Basaran K, Mercan ES, Aygit AC. Effects of fatigue and sleep deprivation on microvascular anastomoses. J Craniofac Surg. 2015;26(4):1342–1347. doi: 10.1097/scs.0000000000001719. [DOI] [PubMed] [Google Scholar]
- 41.Bernie JE, Venkatesh R, Brown J, et al. Comparison of laparoscopic pyeloplasty with and without robotic assistance. Jsls. 2005;9(3):258–261. [PMC free article] [PubMed] [Google Scholar]
- 42.Chen YQ, Tao JW, Li L, et al. Feasibility study on robot-assisted retinal vascular bypass surgery in an ex vivo porcine model. Acta Ophthalmol. 2017;95(6):e462–e467. doi: 10.1111/aos.13457. [DOI] [PubMed] [Google Scholar]
- 43.Knight CG, Lorincz A, Cao A, et al. Computer-assisted, robot-enhanced open microsurgery in an animal model. J Laparoendosc Adv Surg Tech A. 2005;15(2):182–185. doi: 10.1089/lap.2005.15.182. [DOI] [PubMed] [Google Scholar]
- 44.You JY, Lee HY, Son GS, et al. Comparison of robotic adrenalectomy with traditional laparoscopic adrenalectomy with a lateral transperitoneal approach: a single-surgeon experience. Int J Med Robot. 2013;9(3):345–350. doi: 10.1002/rcs.1497. [DOI] [PubMed] [Google Scholar]
- 45.Fleming I, Balicki M, Koo J, et al. Cooperative robot assistant for retinal microsurgery. Med Image Comput Comput Assist Interv. 2008;11(Pt 2):543–550. doi: 10.1007/978-3-540-85990-1_65. [DOI] [PubMed] [Google Scholar]
- 46.Mahr MA, Hodge DO. Construct validity of anterior segment anti-tremor and forceps surgical simulator training modules: attending versus resident surgeon performance. J Cataract Refract Surg. 2008;34(6):980–985. doi: 10.1016/j.jcrs.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 47.Srinivasan AV. Propranolol: a 50-year historical perspective. Ann Indian Acad Neurol. 2019;22(1):21–26. doi: 10.4103/aian.AIAN_201_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elman MJ, Sugar J, Fiscella R, et al. The effect of propranolol versus placebo on resident surgical performance. Transactions of the American Ophthalmological Society. 1998;96:283–294. [PMC free article] [PubMed] [Google Scholar]
- 49.Humayun MU, Rader RS, Pieramici DJ, et al. Quantitative measurement of the effects of caffeine and propranolol on surgeon hand tremor. Arch Ophthalmol. 1997;115(3):371–374. doi: 10.1001/archopht.1997.01100150373010. [DOI] [PubMed] [Google Scholar]
- 50.Lubahn JD, Dickson BG, Cooney TE. Effect of timolol vs. a postural orthotic on hand tremor during microsurgery. Microsurgery. 2002;22(6):273–276. doi: 10.1002/micr.10049. [DOI] [PubMed] [Google Scholar]
- 51.Arnold RW, Springer DT, Engel WK, et al. The effect of wrist rest, caffeine, and oral timolol on the hand steadiness of ophthalmologists. Ann Ophthalmol. 1993;25(7):250–253. [PubMed] [Google Scholar]
- 52.Rothschild P-R, Grabar S, Le Dû B, et al. Patients’ subjective assessment of the duration of cataract surgery: a case series. BMJ Open. 2013;3(5):e002497. doi: 10.1136/bmjopen-2012-002497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watson NF, Badr MS, Belenky G, et al. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):843–844. doi: 10.5665/sleep.4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erie EA, Mahr MA, Hodge DO, et al. Effect of sleep deprivation on the performance of simulated anterior segment surgical skill. Can J Ophthalmol. 2011;46(1):61–65. doi: 10.3129/i10-112. [DOI] [PubMed] [Google Scholar]
- 55.Olasky J, Chellali A, Sankaranarayanan G, et al. Effects of sleep hours and fatigue on performance in laparoscopic surgery simulators. Surg Endosc. 2014;28(9):2564–2568. doi: 10.1007/s00464-014-3503-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Veddeng A, Husby T, Engelsen IB, et al. Impact of night shifts on laparoscopic skills and cognitive function among gynecologists. Acta Obstet Gynecol Scand. 2014;93(12):1255–1261. doi: 10.1111/aogs.12496. [DOI] [PubMed] [Google Scholar]
- 57.Jakubowicz DM, Price EM, Glassman HJ, et al. Effects of a twenty-four hour call period on resident performance during simulated endoscopic sinus surgery in an accreditation council for graduate medical education-compliant training program. Laryngoscope. 2005;115(1):143–146. doi: 10.1097/01.mlg.0000150689.77764.ad. [DOI] [PubMed] [Google Scholar]
- 58.Channa R, Iordachita I, Handa JT. Robotic vitreoretinal surgery. Retina (Philadelphia, Pa) 2017;37(7):1220–1228. doi: 10.1097/IAE.0000000000001398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chammas J, Sauer A, Pizzuto J, et al. Da Vinci Xi robot-assisted penetrating keratoplasty. Transl Vis Sci Technol. 2017;6(3):21–21. doi: 10.1167/tvst.6.3.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bourcier T, Chammas J, Becmeur PH, et al. Robotically assisted pterygium surgery: first human case. Cornea. 2015;34(10):1329–1330. doi: 10.1097/ico.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 61.Bourcier T, Becmeur PH, Mutter D. Robotically assisted amniotic membrane transplant surgery. JAMA Ophthalmol. 2015;133(2):213–214. doi: 10.1001/jamaophthalmol.2014.4453. [DOI] [PubMed] [Google Scholar]
- 62.Wilson JT, Gerber MJ, Prince SW, et al (2018) Intraocular robotic interventional surgical system (IRISS): mechanical design, evaluation, and master-slave manipulation. Int J Med Robot 14(1). 10.1002/rcs.1842 [DOI] [PubMed]
- 63.Chen CW, Lee YH, Gerber MJ, et al. Intraocular robotic interventional surgical system (IRISS): semi-automated OCT-guided cataract removal. Int J Med Robot. 2018;14(6):e1949. doi: 10.1002/rcs.1949. [DOI] [PubMed] [Google Scholar]
- 64.Gonenc B, Handa J, Gehlbach P, et al (2013) A comparative study for robot assisted vitreoretinal surgery: micron vs. the steady-hand robot IEEE International Conference on Robotics and Automation : ICRA : [proceedings]. IEEE International Conference on Robotics and Automation. pp 4832–4837. 10.1109/ICRA.2013.6631266 [DOI] [PMC free article] [PubMed]
- 65.Riviere CN, Thakor NV. Modeling and canceling tremor in human-machine interfaces. IEEE Eng Med Biol Mag. 1996;15(3):29–36. doi: 10.1109/51.499755. [DOI] [Google Scholar]
- 66.Riviere CN, Wei Tech A, Khosla PK. Toward active tremor canceling in handheld microsurgical instruments. IEEE Trans Robot Autom. 2003;19(5):793–800. doi: 10.1109/TRA.2003.817506. [DOI] [Google Scholar]
- 67.Romano MR, Vallejo-Garcia JL, Randazzo A, et al. Pneumatic tools for vitreoretinal surgery. Clin Ophthalmol (Auckland, NZ) 2012;6:385–387. doi: 10.2147/OPTH.S28496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chan JY, Leung I, Navarro-Alarcon D, et al. Foot-controlled robotic-enabled endoscope holder for endoscopic sinus surgery: a cadaveric feasibility study. Laryngoscope. 2016;126(3):566–569. doi: 10.1002/lary.25634. [DOI] [PubMed] [Google Scholar]
- 69.Rocon E, Gallego JÁ, Belda-Lois JM, et al. Biomechanical loading as an alternative treatment for tremor: a review of two approaches. Tremor and Other Hyperkinetic Movements (New York, NY) 2012;2:02-77-495–491. doi: 10.7916/D82Z147G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gallego JÁ, Rocon E, Belda-Lois JM, et al. A neuroprosthesis for tremor management through the control of muscle co-contraction. J Neuroeng Rehabil. 2013;10:36–36. doi: 10.1186/1743-0003-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fine IH. Clear corneal incisions. Int Ophthalmol Clin. 1994;34(2):59–72. doi: 10.1097/00004397-199403420-00005. [DOI] [PubMed] [Google Scholar]
- 72.Singh K, Misbah A, Saluja P, et al. Review of manual small-incision cataract surgery. Indian J Ophthalmol. 2017;65(12):1281–1288. doi: 10.4103/ijo.IJO_863_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giesinger K, Hamilton DF, Erschbamer M, et al. Black medicine: an observational study of doctors’ coffee purchasing patterns at work. BMJ (Clin Res ed.) 2015;351:h6446–h6446. doi: 10.1136/bmj.h6446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scarmeas N, Louis ED. Mediterranean diet and essential tremor A case-control study. Neuroepidemiology. 2007;29(3–4):170–177. doi: 10.1159/000111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu PA, Cooley BC. Effect of exercise on microsurgical hand tremor. Microsurgery. 2003;23(4):323–327. doi: 10.1002/micr.10156. [DOI] [PubMed] [Google Scholar]
- 76.Zouhal H, Jacob C, Delamarche P, et al. Catecholamines and the effects of exercise, training and gender. Sports Med. 2008;38(5):401–423. doi: 10.2165/00007256-200838050-00004. [DOI] [PubMed] [Google Scholar]
- 77.Bilodeau M, Keen DA, Sweeney PJ, et al. Strength training can improve steadiness in persons with essential tremor. Muscle Nerve. 2000;23(5):771–778. doi: 10.1002/(sici)1097-4598(200005)23:5<771::aid-mus15>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 78.Kavanagh JJ, Wedderburn-Bisshop J, Keogh JW. Resistance training reduces force tremor and improves manual dexterity in older individuals with essential tremor. J Mot Behav. 2016;48(1):20–30. doi: 10.1080/00222895.2015.1028583. [DOI] [PubMed] [Google Scholar]
- 79.Sequeira G, Keogh JW, Kavanagh JJ. Resistance training can improve fine manual dexterity in essential tremor patients: a preliminary study. Arch Phys Med Rehabil. 2012;93(8):1466–1468. doi: 10.1016/j.apmr.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 80.Ridgel AL, Muller MD, Kim CH, et al. Acute effects of passive leg cycling on upper extremity tremor and bradykinesia in Parkinson’s disease. Phys Sportsmed. 2011;39(3):83–93. doi: 10.3810/psm.2011.09.1924. [DOI] [PubMed] [Google Scholar]
- 81.Peterson CT, Bauer SM, Chopra D, et al. Effects of Shambhavi Mahamudra Kriya, a multicomponent breath-based yogic practice (Pranayama), on perceived stress and general well-being. J Evid Based Complement Altern Med. 2017;22(4):788–797. doi: 10.1177/2156587217730934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown RP, Gerbarg PL. Sudarshan Kriya yogic breathing in the treatment of stress, anxiety, and depression: part I — neurophysiologic model. J Altern Complement Med. 2005;11(1):189–201. doi: 10.1089/acm.2005.11.189. [DOI] [PubMed] [Google Scholar]
- 83.La Padula S, Hersant B, Roccaro G, et al. Yoga breathing exercises (pranayama) decrease hand microtremor in young microsurgeons: toward a new paradigm in surgery. Plast Reconstr Surg. 2020;146(5):701e–703e. doi: 10.1097/prs.0000000000007307. [DOI] [PubMed] [Google Scholar]
- 84.Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. 2010;25(14):2274–2284. doi: 10.1002/mds.23240. [DOI] [PubMed] [Google Scholar]
- 85.Lakie M, Frymann K, Villagra F, et al. The effect of alcohol on physiological tremor. Exp Physiol. 1994;79(2):273–276. doi: 10.1113/expphysiol.1994.sp003763. [DOI] [PubMed] [Google Scholar]
- 86.Kirby G, Kapoor K, Das-Purkayastha P, et al. The effect of alcohol on surgical skills. Ann R Coll Surg Engl. 2012;94(2):90–93. doi: 10.1308/003588412X13171221501627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gallagher AG, Boyle E, Toner P, et al. Persistent next-day effects of excessive alcohol consumption on laparoscopic surgical performance. Arch Surg. 2011;146(4):419–426. doi: 10.1001/archsurg.2011.67. [DOI] [PubMed] [Google Scholar]
- 88.Lippold OC, Williams EJ, Wilson CG. Finger tremor and cigarette smoking. Br J Clin Pharmacol. 1980;10(1):83–86. doi: 10.1111/j.1365-2125.1980.tb00505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shiffman SM, Gritz ER, Maltese J, et al. Effects of cigarette smoking and oral nicotine on hand tremor. Clin Pharmacol Ther. 1983;33(6):800–805. doi: 10.1038/clpt.1983.109. [DOI] [PubMed] [Google Scholar]
- 90.Todd G, Burns L, Pearson-Dennett V, et al. Prevalence of self-reported movement dysfunction among young adults with a history of ecstasy and methamphetamine use. Drug Alcohol Depend. 2019;205:107595. doi: 10.1016/j.drugalcdep.2019.107595. [DOI] [PubMed] [Google Scholar]
- 91.de Faria SM, de Morais Fabrício D, Tumas V, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson’s disease. J Psychopharmacol. 2020;34(2):189–196. doi: 10.1177/0269881119895536. [DOI] [PubMed] [Google Scholar]
- 92.Urbi B, Corbett J, Hughes I, et al. Effects of cannabis in Parkinson’s disease: a systematic review and meta-analysis. J Parkinsons Dis. 2022;12(2):495–508. doi: 10.3233/jpd-212923. [DOI] [PubMed] [Google Scholar]
- 93.Fox P, Bain PG, Glickman S, et al. The effect of cannabis on tremor in patients with multiple sclerosis. Neurology. 2004;62(7):1105–1109. doi: 10.1212/01.wnl.0000118203.67138.3e. [DOI] [PubMed] [Google Scholar]
- 94.Nielsen S, Germanos R, Weier M, et al. The use of cannabis and cannabinoids in treating symptoms of multiple sclerosis: a systematic review of Reviews. Curr Neurol Neurosci Rep. 2018;18(2):8. doi: 10.1007/s11910-018-0814-x. [DOI] [PubMed] [Google Scholar]
- 95.Flavel SC, Koch JD, White JM, et al. Illicit stimulant use in humans is associated with a long-term increase in tremor. PLoS ONE. 2012;7(12):e52025–e52025. doi: 10.1371/journal.pone.0052025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bauer LO. Resting hand tremor in abstinent cocaine-dependent, alcohol-dependent, and polydrug-dependent patients. Alcohol Clin Exp Res. 1996;20(7):1196–1201. doi: 10.1111/j.1530-0277.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.