Abstract
Chronic infection with human CMV may contribute to poor vaccine efficacy in older adults. We assessed the effects of CMV serostatus on Ab quantity and quality, as well as cellular memory recall responses, after two and three SARS-CoV-2 mRNA vaccine doses, in older adults in assisted living facilities. CMV serostatus did not affect anti-Spike and anti–receptor-binding domain IgG Ab levels, nor neutralization capacity against wild-type or β variants of SARS-CoV-2 several months after vaccination. CMV seropositivity altered T cell expression of senescence-associated markers and increased effector memory re-expressing CD45RA T cell numbers, as has been previously reported; however, this did not impact Spike-specific CD4+ T cell memory recall responses. CMV-seropositive individuals did not have a higher incidence of COVID-19, although prior infection influenced humoral immunity. Therefore, CMV seropositivity may alter T cell composition but does not impede the durability of humoral protection or cellular memory responses after SARS-CoV-2 mRNA vaccination in older adults.
Key Points
CMV-seropositive older adults have more EMRA and terminally differentiated T cells.
CMV seropositivity does not prevent Ab maintenance after SARS-CoV-2 vaccination.
CMV seropositivity does not impede SARS-CoV-2 vaccine T cell memory recall responses.
Introduction
Aging is associated with an increased frequency of viral respiratory infections and postinfection sequelae (1), as well as reduced efficacy and longevity of protective immunity after vaccination (2). Early in the COVID-19 pandemic, age was identified to be the most significant factor contributing to morbidity and mortality (3), and it was unclear how effective vaccines against the novel SARS-CoV-2 virus would be in older adults. In this population, vaccines that target a memory response (e.g., herpes zoster) are often more immunogenic and effective at preventing illness than vaccines that target viral Ags that are antigenically distant from previous circulating strains (e.g., some seasonal influenza vaccines) (4–7). SARS-CoV-2 mRNA vaccines have been shown to be protective in older adults (8–10), as they are effective at generating cellular and Ab-mediated immunity (11–14). However, in older adults, immune responses after vaccination are generally quite heterogeneous, and immunity may wane faster than in younger adults (11, 13).
In older adults, both immunosenescence and inflammation are thought to influence the immunogenicity of vaccines and longevity of protective immune responses after vaccination (15, 16). Many studies have also implicated human CMV as a significant contributor to age-associated immune dysfunction. CMV is a common and persistent β-herpesvirus, found in ∼60–90% of adults worldwide, and seropositivity increases with age (17, 18). CMV infection is typically asymptomatic in immunocompetent individuals, but its accompanying chronic immune activation fundamentally alters immune cell composition and function. There is a consensus that CMV seropositivity has a long-term impact on the maturation and composition of immune cells, including increased numbers and prevalence of CD8+ T cells, with expansion of CMV-specific effector and memory cells at the expense of naive T cells (19, 20). Age-associated immunosenescence likewise contributes to similar changes within the T cell repertoire, reducing naive T cells and increasing memory T cell populations, which have impaired proliferation, differentiation, and effector functions (21, 22). A dysfunctional T cell repertoire may also have significant effects on B cell proliferation, differentiation, and maturation (23). Accordingly, CMV seropositivity has been implicated as an exacerbating factor in age-associated immune remodeling and interindividual immune diversity (24–27), as well as a modifying factor that may compromise infection outcomes and quality and longevity of immune protection after vaccination (28).
Although there is a paucity of data to date, CMV reactivation in older adults has been suggested to contribute to more severe COVID-19 (29–33), as CMV seropositivity has been associated with impaired Ab production and cellular memory recall responses (34–36). However, CMV seropositivity has also been reported to enhance cellular and Ab immune responses to unrelated bacteria or viruses, through diversification of CD8+ TCRs and augmentation of basal inflammation (37–40). CMV seropositivity has, in addition, been associated with a reduced humoral response to inactivated split influenza virus vaccines (41–44) and viral vector–based Ebola vaccines (45). However, a recent meta-analysis reported that there was insufficient evidence that CMV-seropositive individuals have decreased Ab production after influenza vaccination (46). Recent data also show that CMV seropositivity in young adults does not affect Ab or cellular responses after vaccination with the adenovirus-based vector vaccine ChAdOx1 nCoV-19 (47). These conflicting reports may suggest context-, time-, and age-dependent effects of chronic CMV infection on immune function. SARS-CoV-2 mRNA vaccines have been widely deployed in Canada, particularly in older adults. Whether CMV seropositivity impacts SARS-CoV-2 mRNA vaccine efficacy and durability of immunity is not yet known. In this study, we investigated effects of CMV serostatus on vaccine-associated humoral protection and cellular memory recall responses several months after two and three doses of SARS-CoV-2 mRNA vaccines in older adults. We found that CMV serostatus does not impede Ab or cellular responses to SARS-CoV-2 vaccination in older adults.
Materials and Methods
Participant recruitment and blood collection
Participants in the COVID in Long-Term Care Study (https://covidinltc.ca) were recruited from assisted living facilities (17 nursing homes and 8 retirement homes) in Ontario, Canada, between March and December 2021. All protocols were approved by the Hamilton Integrated Research Ethics Board and other site-specific research ethics boards, and informed consent was obtained. Venous blood was drawn in anti-coagulant–free vacutainers for isolation of serum, as per standard protocols (48). Venous blood was drawn in heparin-coated vacutainers for immunophenotyping and T cell activation assays. Blood was collected at least 7 d after two and/or three mRNA vaccine doses. Participants received two doses of Moderna Spikevax (100 µg; mRNA-1273) or Pfizer Comirnaty (30 µg; BNT162b2) as per the recommended schedules, and a third mRNA vaccine dose in Fall 2021 at least 6 mo from the last dose, as per Province of Ontario guidelines (49). For this study, humoral and cellular data were retrospectively assessed in the context of CMV serostatus from a cohort of 186 participants, 65 y of age and older. Blood was drawn after two and three mRNA vaccine doses from 47 cohort participants. Participant cohort demographics are summarized in Table I.
Table I.
Participant demographics
| CMV Seronegative (n = 74) | CMV Seropositive (n = 159) | Statistical Assessment | |
|---|---|---|---|
| Age (y), median ± SD (range) | 85 ± 6.8 (65–93) | 86 ± 7.7 (65–101) | p = 0.1265a |
| Sex (frequency) | 67.6% Female (n = 50) | 69.8% Female (n = 111) | p = 0.7618b |
| Sample size postdose 2 | n = 51 | n = 108 | — |
| Second dose vaccine combination (frequency) | 47.1% Moderna-Moderna (n = 24) 52.9% Pfizer-Pfizer (n = 27) |
35.8% Moderna-Moderna (n = 39) 60.6% Pfizer-Pfizer (n = 66) 2.75% Pfizer-Moderna (n = 3) |
p = 0.2968b |
| Days between dose 1 and dose 2 (mean ± SD) | 32.2 ± 20.8 | 30.4 ± 17.9 | p = 0.6697a |
| Days since second dose to blood collection (mean ± SD) | 179.8 ± 50.1 | 175.4 ± 64.1 | p = 0.9041a |
| Sample size postdose 3c | n = 23 (17 repeated) | n = 51 (30 repeated) | — |
| Third dose vaccine combination (frequency) | 65.2% Moderna-Moderna-Moderna (n = 15) 34.8% Pfizer-Pfizer-Pfizer (n = 8) |
56.9% Moderna-Moderna-Moderna (n = 29) 43.1% Pfizer-Pfizer-Pfizer (n = 22) |
p = 0.6115b |
| Days between dose 2 and dose 3 (mean ± SD) | 203.9 ± 21.0 | 211.7 ± 12.1 | p = 0.2689a |
| Days since third dose to blood collection (mean ± SD) | 82.0 ± 17.3 | 83.7 ± 11.5 | p = 0.7597a |
| Prior COVID-19d | 37.8% (n = 28) | 34.4% (n = 55) | p = 0.6609a |
| Only positive for nasopharyngeal PCR test | 28.6% (n = 8) | 36.4% (n = 20) | p = 0.6244b |
| Only positive for anti-nucleocapsid Abs | 39.3% (n = 11) | 40.0% (n = 22) | p > 0.9999b |
| Positive for PCR test and anti-nucleocapsid Abs | 32.1% (n = 9) | 23.6% (n = 13) | p = 0.7977b |
Student t test with Welch’s correction or Mann–Whitney U test by normality.
Fisher’s exact test. Comparisons for vaccines postdose 2 were calculated for Moderna-Moderna versus Pfizer-Pfizer.
Forty-seven participants had blood drawn postdose 2 and postdose 3.
Participants were considered positive for prior COVID-19 infection when they had a recorded positive nasopharyngeal PCR test at a date prior to blood collection and/or seropositivity for anti-nucleocapsid IgG or IgA Abs at time of blood collection for Ab and cellular assays.
Determination of CMV serostatus
CMV seropositivity was determined by ELISA with a human anti-CMV IgG ELISA kit (CMV) (no. ab108639, Abcam) as per the manufacturer’s instructions. Serum samples from a participant’s first blood draw were diluted 1:40 and assessed in duplicate. Samples with a CMV IgG index above or equal to the positive standard were classified as CMV seropositive, whereas samples with a CMV IgG index less than the positive standard were classified as CMV seronegative.
Whole-blood immunophenotyping
Circulating immune cell populations were quantitated in whole blood using fluorophore-conjugated mAbs by multicolor flow cytometry with a CytoFLEX LX (four lasers, Beckman Coulter), as per standard protocols (48, 50, 51). CountBright absolute counting beads (no. C36950, Invitrogen/Life Technologies) were used to determine absolute cell counts. Data were analyzed with FlowJo v10.8.1 (Tree Star), following a previously published gating strategy to identify T cell populations (50). Five main subsets of human CD8+ and CD4+ T cells (naive [N], central memory [CM], effector memory [EM], EM re-expressing CD45RA [EMRA], and terminally differentiated [TD]) were identified by their expression of CD45RA, CCR7, CD28, and/or CD57. CD8N and CD4N were classified as CD45RA+CCR7+, CD8CM and CD4CM as CD45RA−CCR7+, CD8EM and CD4EM as CD45RA−CCR7−, CD8EMRA and CD4EMRA as CD45RA+CCR7−, and CD8TD and CD4TD were classified as CD45RA+CCR7−CD28−CD57+, as per standard protocols (52).
Assessment of T cell memory responses to SARS-CoV-2 Spike by an activation-induced marker assay
Ag-specific T cell recall responses were evaluated by an activation-induced marker (AIM) assay as per established protocols (50). Each participant sample was stimulated with a Spike glycoprotein SARS-CoV-2 peptide pool (1 μg/ml) containing overlapping peptides of the complete immunodominant sequence domain (no. 130-126-701, Miltenyi Biotec) as well as influenza hemagglutinin (HA) peptides (4 µl of 0.12 μg/µl HA; AgriFlu, Afluria Tetra inactivated influenza vaccine 2020–2021 season, Seqirus, Maidenhead, U.K.). A negative medium control (unstimulated) and positive stimulation control (polyclonal stimulation with CytoStim at 0.5 µl/well; no. 130-092-173, Miltenyi Biotec) were included with each sample. For each of these four conditions, 100 µl of heparinized venous blood was incubated with an equal volume of IMDM with GlutaMAX supplement (no. 31980030, Invitrogen/Life Technologies) for 44 h in 96-well flat bottom plates at 37°C. Samples were stained with fluorophore-conjugated mAbs and assessed with a CytoFLEX LX (4 lasers, Beckman Coulter, Brea, CA) as previously described (50). Data were analyzed with FlowJo, following a previously published gating strategy to identify AIM+ T cells (50). Activated T cells (AIM positive) were identified by their coexpression of CD25 and CD134 (OX40) on CD4+ T cells (53, 54) and coexpression of CD69 and CD137 (4-1BB) on CD8+ T cells (55). Samples with a T cell count of at least 20 events and ≥2-fold above the unstimulated sample (negative control; i.e., stimulation index ≥ 2) were defined as AIM-positive. Expression of CXCR3 (CD183; Brilliant Violet 421, no. 353716, BioLegend), CCR4 (CD194; Brilliant Violet 605, no. 359418, BioLegend), and CCR6 (CD196; Brilliant Violet 785, no. 353422, BioLegend) was used to identify Th1 (CXCR3+CCR6−CCR4−), Th2 (CXCR3−CCR4+CCR6−), and Th17 (CXCR3−CCR4+CCR6+) AIM+CD4+ T cell subsets.
Measurements of anti–SARS-CoV-2 Abs and neutralizing capacity
Serum anti–SARS-CoV-2 Spike protein and receptor-binding domain (RBD) IgG, IgA, and IgM Abs were measured by a validated ELISA as previously described (50, 56), with assay cutoff 3 SD above the mean of a pre-COVID-19 population from the same geographic region. Ab neutralization capacity was assessed by cell culture assays with Vero E6 (ATCC CRL-1586) cells and live SARS-CoV-2, with data reported as geometric microneutralization titer at 50% (MNT50), which ranged from below detection (MNT50 = 5; 1:10 dilution) to MNT50 = 1280 (56). Ab neutralization was measured against the ancestral strain of SARS-CoV-2 and the β variant of concern (B.1.351). The β variant was obtained through BEI Resources (National Institute of Allergy and Infectious Diseases, National Institutes of Health: SARS-related coronavirus 2, isolate hCoV-19/South Africa/KRISP-K005325/2020, NR-54009, contributed by Alex Sigal and Tulio de Oliveira).
Determination of prior SARS-CoV-2 infection
Due to demand for and limits on availability of testing, participants were not consistently tested for COVID-19 by a nasopharyngeal swab PCR assay, even when they were symptomatic. Serological testing for anti-nucleocapsid SARS-CoV-2 IgG and IgA Abs was performed on all collected samples by the ELISA described above (50, 56), using assay wells coated with 2 µg/ml nucleocapsid Ag (Jackson ImmunoResearch Laboratories). Participants were identified to have had COVID-19 when they were seropositive for IgG or IgA anti-nucleocapsid Abs and had a documented positive nasopharyngeal PCR test prior to any blood collection, as summarized in Table I.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism version 9 (GraphPad Software, San Diego, CA). Two-group comparisons of dose and CMV seropositivity or prior COVID-19 and CMV seropositivity were assessed by two-way ANOVA. Differences between CMV-seropositive and CMV-seronegative group Ab levels, Ab neutralization capacity, T cell immunophenotype, and T cell memory recall responses were assessed by a Student t test with Welch’s correction or a Mann–Whitney U test, according to data normality. The p values are reported as two-tailed, and p values <0.05 were considered significant.
Results
Participant demographics
Serum anti-CMV IgG Abs were measured by ELISA, and 69.4% (n = 129/186) of participants were found to be CMV seropositive. Age and sex distribution were similar between seropositive (median 85 ± 6.8 y, 67.6% female) and seronegative (median 86 ± 7.7 y, 69.8% female) participants (Table I). Blood samples were collected at a median of 179.8 d (CMV seronegative) and 175.4 d (CMV seronegative) after two doses of Moderna Spikevax (100 µg; mRNA-1273) or Pfizer Comirnaty (30 µg; BNT162b2) administered as per the manufacturer-recommended schedules. In Ontario, Canada, third dose vaccinations were recommended for older adults in congregate living beginning in August 2021 when they were >6 mo after their second vaccinations (57). Participants received third doses in August–September 2021, and blood samples were collected at a median of 82.0 d (CMV seronegative) or 83.7 d (CMV seropositive) after third doses. Participants were classified as having had a previous SARS-CoV-2 infection when they had a documented positive PCR test and/or were seropositive for IgG or IgA nucleocapsid Abs. A positive nasopharyngeal PCR test and/or serum anti-nucleocapsid IgG or IgA Abs were reported in 37.8% of CMV-seronegative participants and 34.4% of CMV-seropositive participants.
CMV seropositivity does not impede anti–SARS-CoV-2 Ab production or neutralization in older adults
To assess the effects of CMV serostatus on Ab responses after SARS-CoV-2 vaccination, serum anti–SARS-CoV-2 Spike and RBD IgG, IgA, and IgM Ab levels were measured by ELISA (Fig. 1, Table II). The number of responders (i.e., individuals with Abs above the threshold limit of detection) significantly increased between post-second and post-third dose measurements of anti-Spike IgG, IgA, and IgM Abs, as well as anti-RBD IgG Abs, but not anti-RBD IgA or IgM Abs (Table II). For example, 5–7 mo after second dose vaccinations, anti-Spike IgG Abs were detected in 88.7% of participants, and anti-RBD IgG Abs were detected in 63.5% of participants. Approximately 3 mo after the third vaccine dose, 97.3 and 94.6% of participants had detectable anti-Spike IgG and anti-RBD IgG, respectively. CMV seropositivity did not impact the frequency of responders for anti-Spike and anti-RBD IgG, IgA, and IgM Abs. Accordingly, two-group analyses showed a main effect of vaccine dose, but not CMV serostatus, on serum anti-Spike IgG (Fig. 1A) and anti-RBD IgG (Fig. 1D) Abs, anti-Spike IgA (Fig. 1B) and anti-RBD IgA (Fig. 1E) Abs, as well as anti-Spike IgM (Fig. 1C) Abs. There were no significant main effects of vaccine dose or CMV serostatus on anti-RBD IgM (Fig. 1F) Abs. Therefore, CMV seropositivity does not impede maintenance of antiviral Abs several months after two or three doses of SARS-CoV-2 mRNA vaccines.
FIGURE 1.
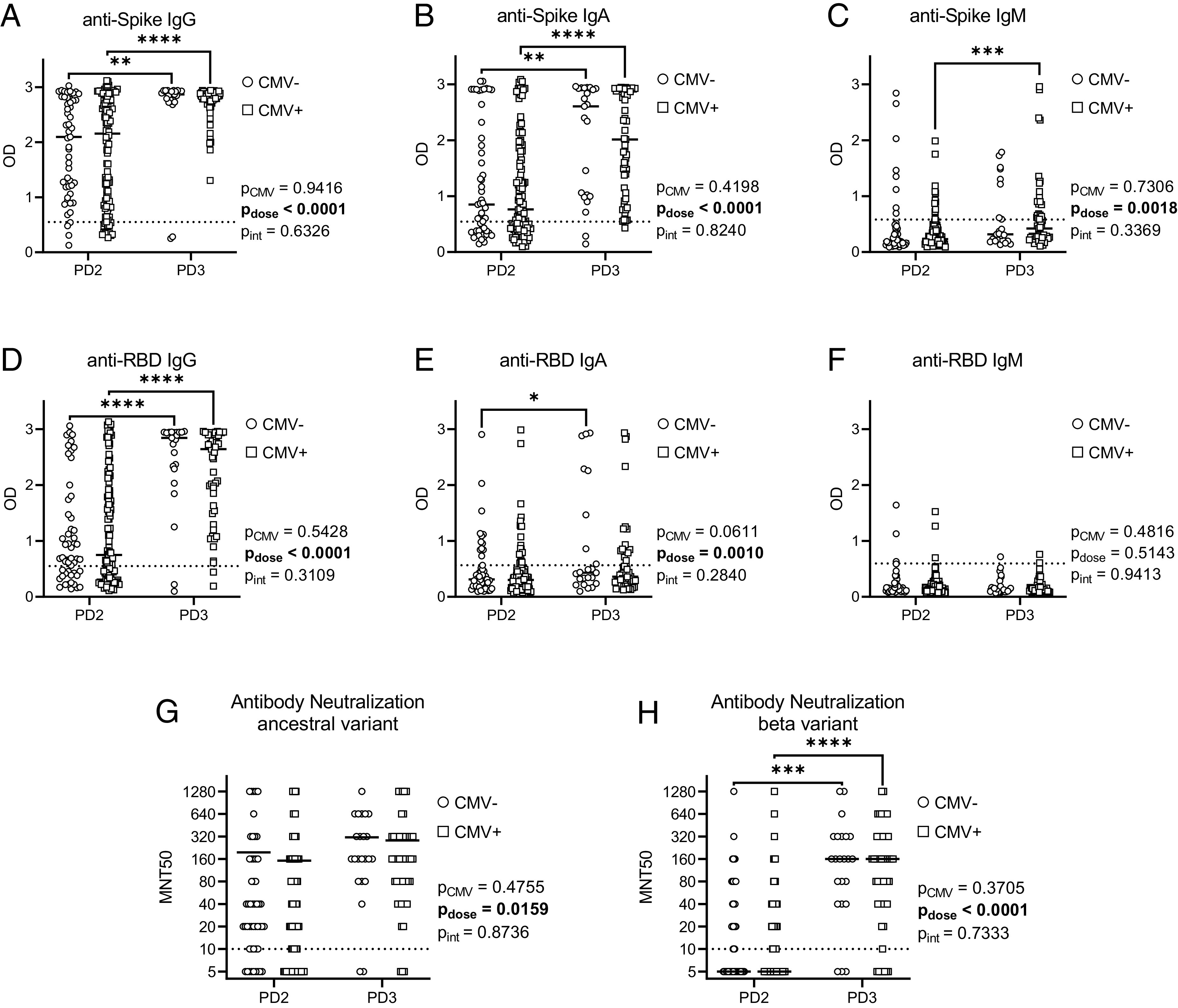
Abs and neutralization capacity by CMV serostatus after two and three COVID-19 vaccines in older adults. Serum SARS-CoV-2 anti-Spike and anti-RBD Abs were detected by ELISA, and Ab neutralization capacity was assessed by MNT50 with live SARS-CoV-2 virus, postdose 2 (PD2), and postdose 3 (PD3) vaccination in CMV-seronegative (−ve) and CMV-seropositive (+ve) individuals. (A–C) Anti-Spike Abs: IgG (A), IgA (B), and IgM (C). (D–F) Anti-RBD Abs: IgG (D), IgA (E), and IgM (F). (G and H) Ab neutralization capacity was assessed against ancestral (G) and β variant (H) SARS-CoV-2. CMV-PD2, n = 51; CMV-PD3, n = 23; CMV+PD2, n = 108; CMV+PD3, n = 51. Dotted lines indicate the threshold of detection. Each data point indicates an individual participant, with the center line at the median. Associations between CMV serostatus and vaccine dose were assessed by two-way ANOVA, with a Tukey’s test post hoc analysis of significant main effects. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Table II.
Responders to vaccination by CMV serostatus: Abs
| Ab | Vaccine Doses | Total Responder Frequency | Responder Frequency by CMV Seropositivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Frequency (%) |
p (Fisher’s Exact Test) |
Seronegative | Seropositive |
p (Fisher’s Exact Test) |
||||
| n | Frequency (%) | n | Frequency (%) | ||||||
| anti-Spike IgG | 2 | 141/159 | 88.7 | 0.0415 | 47/51 | 92.2 | 94/108 | 87.0 | 0.4287 |
| 3 | 72/74 | 97.3 | 21/23 | 91.3 | 51/51 | 100 | 0.0937 | ||
| anti-Spike IgA | 2 | 98/159 | 61.6 | <0.0001 | 33/51 | 64.7 | 65/108 | 60.2 | 0.6053 |
| 3 | 71/74 | 95.9 | 21/23 | 91.3 | 50/51 | 98.0 | 0.2264 | ||
| anti-Spike IgM | 2 | 20/159 | 12.6 | <0.0001 | 8/51 | 15.7 | 12/108 | 11.1 | 0.4476 |
| 3 | 28/74 | 37.8 | 8/23 | 34.8 | 20/51 | 39.2 | 0.7992 | ||
| anti-RBD IgG | 2 | 101/159 | 63.5 | <0.0001 | 34/51 | 66.7 | 67/108 | 62.0 | 0.6011 |
| 3 | 70/74 | 94.6 | 21/23 | 91.3 | 49/51 | 96.1 | 0.5837 | ||
| anti-RBD IgA | 2 | 35/159 | 22.0 | 0.0760 | 12/51 | 23.5 | 23/108 | 21.3 | 0.8380 |
| 3 | 25/74 | 33.8 | 8/23 | 13.0 | 17/51 | 33.3 | >0.9999 | ||
| anti-RBD IgM | 2 | 6/159 | 3.77 | >0.9999 | 3/51 | 5.88 | 3/108 | 2.78 | 0.3863 |
| 3 | 3/74 | 4.05 | 1/23 | 4.35 | 2/51 | 3.92 | >0.9999 | ||
To examine potential effects of CMV serostatus on Ab function, serum Ab neutralization capacity was assessed by MNT50 assays against live ancestral (wild-type) and β variant SARS-CoV-2 (Fig. 1G, 1H). Vaccines were designed against the wild-type virus, whereas the β variant contains mutations that confer increased transmissibility and immune evasion (58). Neutralization of ancestral and β variant SARS-CoV-2 ranged from below the detection limit to MNT50 = 1280, although mean neutralization was consistently higher against the ancestral virus compared with the β variant after two and three vaccine doses. Neutralization capacity was similar between CMV-seropositive and CMV-seronegative individuals against both ancestral and β variant SARS-CoV-2, although there was a main effect of dose on neutralization capacity. In particular, significant increases in serum Ab neutralization were observed against the β variant between second and third dose vaccinations. Anti-Spike and anti-RBD IgG levels moderately correlated with Ab neutralization capacity, irrespective of CMV serostatus (Table III). As well, modest correlations were generally observed between ancestral SARS-CoV-2 neutralization capacity and anti-Spike and anti-RBD IgA Abs in both CMV-seropositive and CMV-seronegative participants. Therefore, CMV seropositivity does not compromise maintenance of vaccine-elicited Ab neutralization of SARS-CoV-2 several months after two or three vaccine doses.
Table III.
Associations of Ab and neutralization responses after vaccination by CMV serostatus
| Ab | MNT50 Wild-Type | MNT50 β Variant | |||
|---|---|---|---|---|---|
| CMV−ve | CMV+ve | CMV−ve | CMV+ve | ||
| Postdose 2 | IgG Spike |
**** r = 0.8367 |
**** r = 0.8316 |
**** r = 0.6963 |
**** r = 0.7541 |
| IgG RBD |
**** r = 0.7394 |
**** r = 0.8291 |
**** r = 0.6848 |
**** r = 0.6981 |
|
| IgA Spike |
**** r = 0.6222 |
**** r = 0.6152 |
**** r = 0.6402 |
**** r = 0.5254 |
|
| IgA RBD |
** r = 0.4311 |
**** r = 0.4572 |
** r = 0.4813 |
*** r = 0.3639 |
|
| Postdose 3 | IgG Spike |
*** r = 0.6923 |
*** r = 0.5004 |
*** r = 0.6545 |
*** r = 0.5038 |
| IgG RBD |
**** r = 0.8207 |
**** r = 0.5770 |
**** r = 0.7475 |
**** r = 0.6926 |
|
| IgA Spike |
** r = 0.6324 |
* r = 0.3228 |
* r = 0.4550 |
NS | |
| IgA RBD |
**** r = 0.7750 |
* r = 0.3081 |
*** r = 0.7051 |
* r = 0.3575 |
|
Spearman’s correlation coefficient is reported. CMV−ve, CMV negative; CMV+ve, CMV positive.
p < 0.05,
p < 0.01,
p < 0.001,
p < 0.0001.
As serum was collected from some participants after both second and third vaccine doses, these paired data were also assessed independently (Supplemental Fig. 1). Consistent with our pooled participant data, there were main effects of vaccine dose but not CMV serostatus on anti-Spike and anti-RBD IgG and IgA serum Ab levels. We did observe an interaction between CMV serostatus and vaccine dose on anti-RBD IgM measurements by intraindividual analysis, but most participants had Ab levels below the threshold. Intraindividual analyses also showed that the number of vaccine doses, but not CMV serostatus, had a significant effect on Ab neutralization capacity against wild-type and β variant SARS-CoV-2. Therefore, CMV seropositivity does not significantly impact intraindividual changes in Ab levels or neutralization capacity between two and three doses of SARS-CoV-2 mRNA vaccines.
We next considered postdose 2 and postdose 3 vaccination Ab measurements in the context of prior SARS-CoV-2 infection (Fig. 2). As summarized in Table I, the incidence of COVID-19 (prior to blood collections) was similar between CMV-seronegative and CMV-seropositive participants. We observed a main effect of prior SARS-CoV-2 infection on anti-Spike and anti-RBD IgG, IgA, and IgM Abs after two doses of mRNA vaccines, as well as anti-Spike and anti-RBD IgG and IgA, but not IgM, serum Abs after three vaccine doses. We observed an interaction between prior COVID-19 and CMV serostatus for anti-Spike and anti-RBD IgA Abs postdose 2 and postdose 3, and IgM Abs postdose 2. There was a main effect of CMV serostatus on anti-Spike IgM Abs, but most individuals had levels below the detection threshold. We in addition observed main effects of prior SARS-CoV-2 infection, but not CMV serostatus, on Ab neutralization of ancestral SARS-CoV-2 after two and three vaccine doses, and the β variant after two, but not three, vaccine doses. Collectively, these data indicate that CMV serostatus does not appear to have a major impact on the longevity of circulating anti-Spike or anti-RBD IgG Abs, or total serum Ab neutralization capacity, after SARS-CoV-2 infection or vaccination.
FIGURE 2.
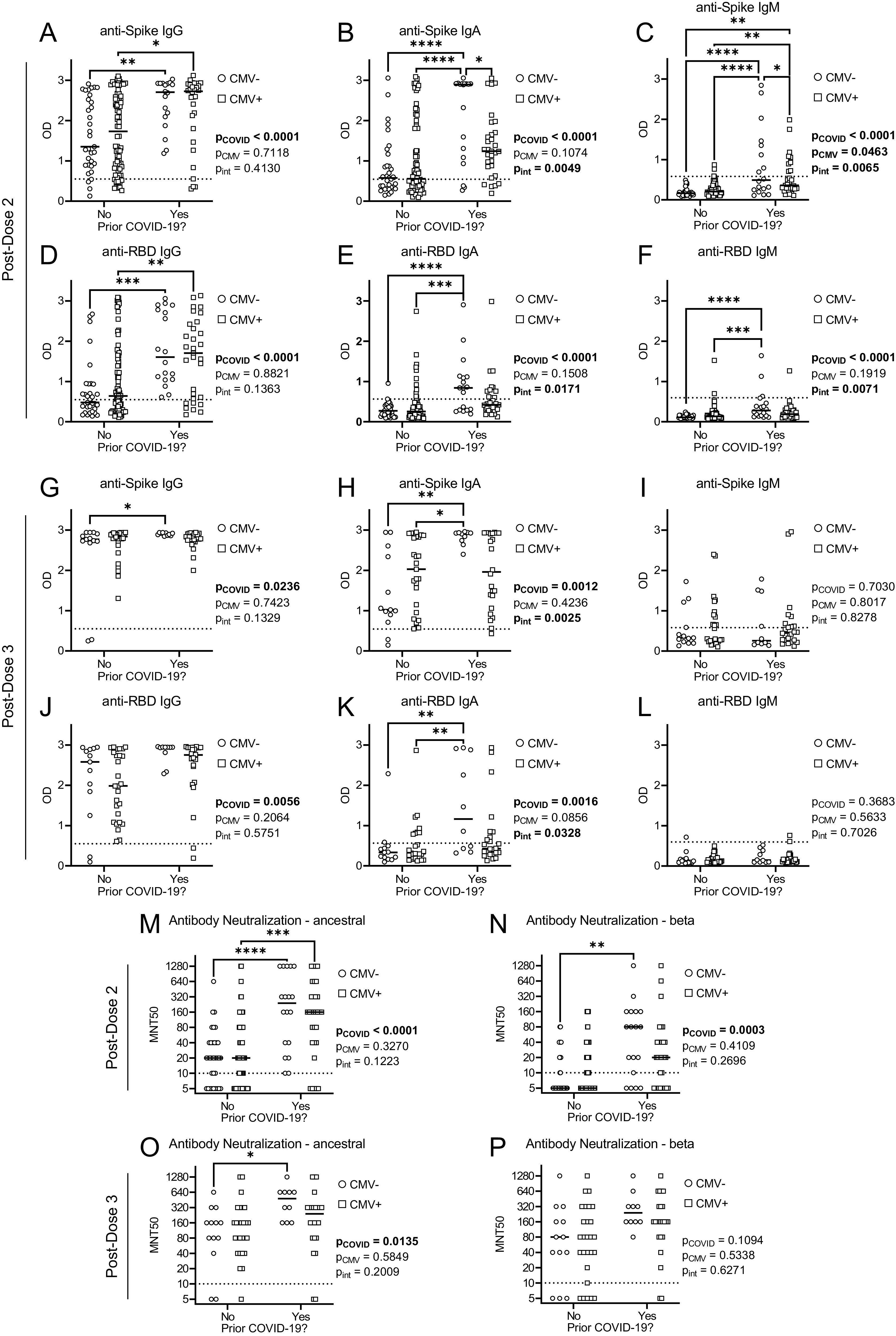
Abs and neutralization capacity by CMV serostatus and prior SARS-CoV-2 infection after two and three COVID-19 vaccines in older adults. SARS-CoV-2 anti-Spike and anti-RBD Abs were measured in serum of CMV-seronegative (CMV−) and CMV-seropositive (CMV+) individuals by ELISA, and serum Ab neutralization capacity was assessed by MNT50 with live SARS-CoV-2 virus. Data are stratified by prior SARS-CoV-2 infection history. (A–F) Postdose 2: anti-Spike IgG (A), IgA (B), and IgM (C) Abs, and anti-RBD IgG (D), IgA (E), and IgM (F) Abs. (G–L) Postdose 3: anti-Spike IgG (G), IgA (H) and IgM (I) Abs, and anti-RBD IgG (J), IgA (K), and IgM (L) Abs. (M and N) Postdose 2 SARS-CoV-2 neutralization: ancestral (M) and β variant (N). (O and P) Postdose 3 SARS-CoV-2 neutralization: ancestral (O) and β variant (P). PD2: CMV−No, n = 33; CMV−Yes, n = 18; CMV+No, n = 77; CMV+Yes, n = 31. PD3: CMV−No, n = 13; CMV−Yes, n = 10; CMV+No, n = 27; CMV+Yes, n = 24. Dotted lines indicate the threshold of detection. Each data point indicates an individual participant, with the center line at the median. Associations between CMV serostatus and prior COVID-19 were assessed by two-way ANOVA, with a Tukey’s test post hoc analysis of significant main effects and interactions. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
CMV serostatus influences peripheral CD4+ and CD8+ T cell immunophenotype in older adults
To examine the impact of CMV seropositivity on the T cell repertoire, whole-blood CD4+ and CD8+ T cell composition was quantitated and the surface expression of CD28 and CD57 was measured by flow cytometry (Fig. 3, Supplemental Fig. 2). Chronic T cell activation and an altered T cell repertoire are characteristics of CMV seropositivity (20, 38, 59). Accordingly, CMV-seropositive individuals had significant changes to their peripheral blood T cell composition. We found no changes in numbers of circulating total leukocytes, total CD4+ T cells, or CD4N, CD4EM, CD8N, or CD8EM T cell populations by CMV serostatus. However, CMV seropositivity increased numbers of total CD8+ T cells, as well as CD4EMRA, CD4TD, CD8EMRA, and CD8TD T cells, and decreased numbers of CD4CM and CD8CM T cells.
FIGURE 3.
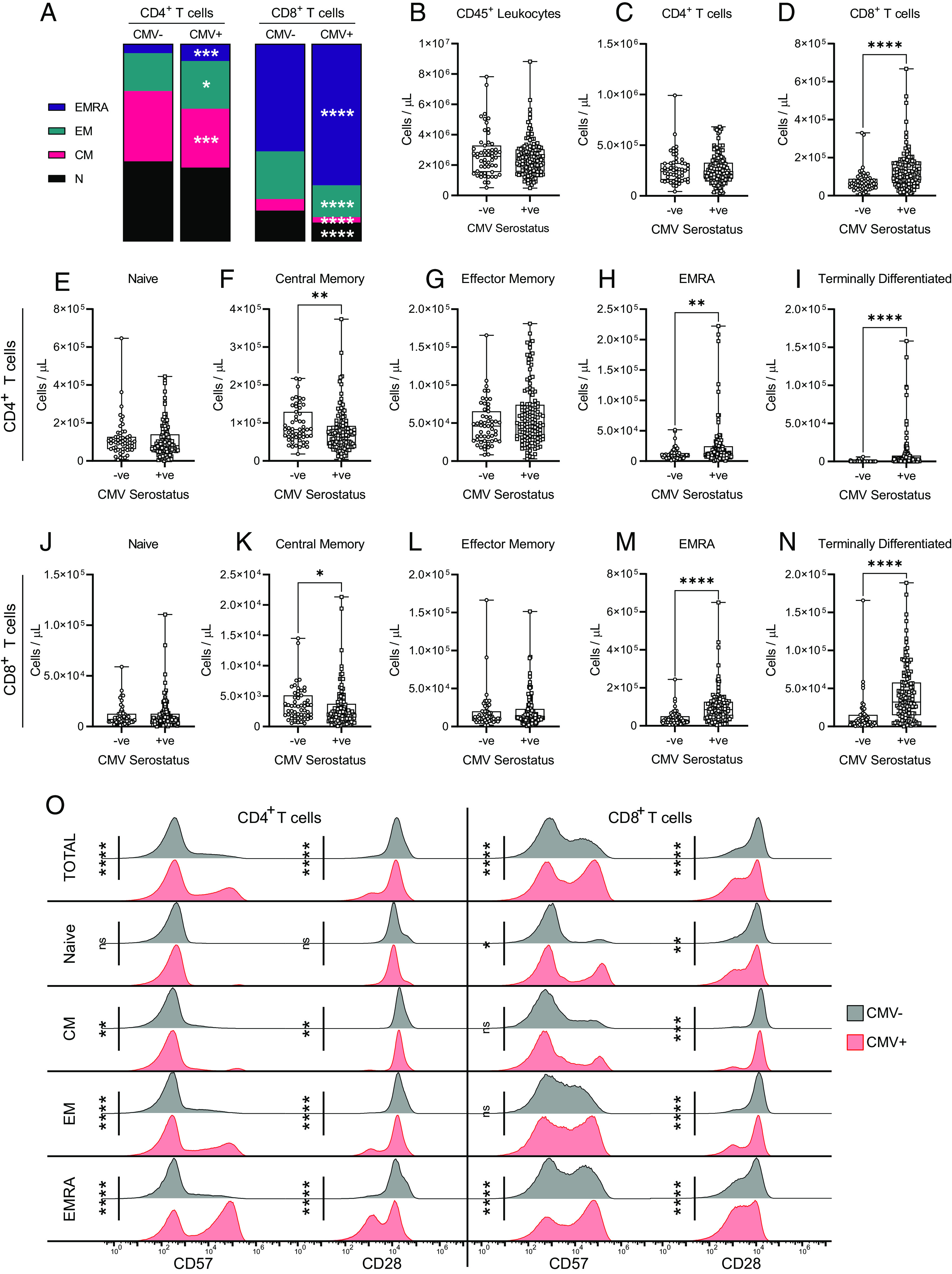
Effect of CMV serostatus on the circulating T cell repertoire in older adults. T cell populations in whole blood were assessed by flow cytometry in CMV-seronegative (−ve) and CMV-seropositive (+ve) individuals. (A) Relative prevalence of CD4+ and CD8+ T cell subsets by CMV serostatus (also see Supplemental Fig. 2). (B–D) Absolute cell counts of (B) total leukocytes, (C) total CD4+ T cells, and (D) CD8+ T cells. (E–I) Absolute cell counts of CD4+ T cells: (E) naive, (F) central memory, (G) effector memory, (H) EMRA, and (I) terminally differentiated. (J–N) Absolute cell counts of CD8+ T cells: (J) naive, (K) central memory, (L) effector memory, (M) EMRA, and (N) terminally differentiated. (O) Surface geometric mean expression of CD57 and CD28 on CD4+ and CD8+ T cells by CMV serostatus. Blood was assessed either postdose 2 or postdose 3 for each participant. CMV−ve, n = 56; CMV+ve, n = 128. Each data point in (B)–(N) indicates an individual participant, and data are presented as box-and-whisker plots, minimum to maximum, with the center line at the median. The surface marker expression in (O) was visualized by concatenating uncompensated events in FlowJo for each participant and indicated T cell population grouped according to CMV serostatus, and then geometric mean fluorescence intensity expression data of each CMV group were overlaid onto the same histogram plot. Associations between T cell subsets and CMV serostatus were assessed by a Student t test with Welch’s correction or a Mann–Whitney U test, according to normality. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
CD28 is a costimulatory molecule that contributes to TCR Ag–mediated activation of T cells, whereas CD57 is a marker of TD T cells as well as an indicator of immune senescence (60). Repeated T cell activation is associated with upregulation of CD57 and a reduction in CD28 expression (61–63). Consistent with these prior data, comparisons of CD28 and CD57 expression on T cell populations by CMV serostatus in our cohort of older adults (Fig. 3O) revealed increased CD57 expression and reduced CD28 expression on total CD4+ and CD8+ T cell populations, as well as more specifically CD4CM, CD4EM, CD4EMRA, CD8N, and CD8EMRA T cells, in CMV-seropositive individuals. Expression of CD28 was also decreased on CD8CM and CD8EM cells of CMV-seropositive individuals, although their expression of CD57 was not influenced by CMV serostatus. CMV serostatus did not alter CD57 or CD28 expression on CD4N T cells.
As even mild COVD-19 can have lasting effects on immune cell composition (50), we also considered combined effects of prior SARS-CoV-2 infection and CMV serostatus on T cell composition (Fig. 4, Supplemental Fig. 2). Prior SARS-CoV-2 infection was associated with lower total leukocyte counts, and an interaction was observed between CMV serostatus and prior COVID-19 that influenced the numbers of CD8TD cells. Otherwise, there were no significant main effects of prior SARS-CoV-2 infection on absolute cell numbers nor the prevalence of the assessed CD4+ or CD8+ T cell populations.
FIGURE 4.
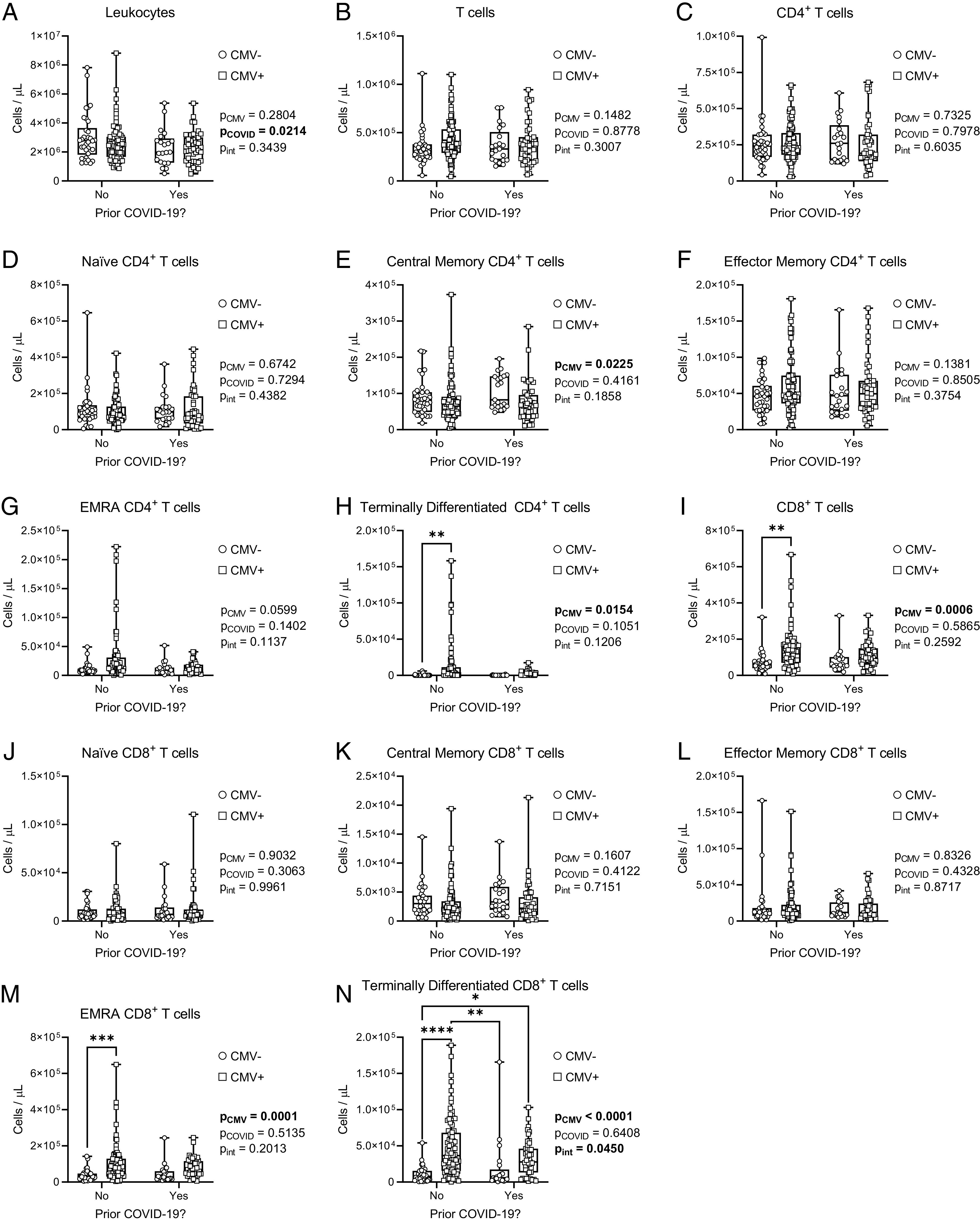
Effects of CMV serostatus and prior COVID-19 on the circulating T cell repertoire in older adults. T cell populations in whole blood were assessed by flow cytometry in CMV-seronegative (CMV−) and CMV-seropositive (CMV+) individuals. Data are stratified by prior SARS-CoV-2 infection history. (A and B) Absolute cell counts of (A) total leukocytes and (B) total T cells. (C–H) Absolute cell counts of CD4+ T cells: (C) total, (D) naive, (E) central memory, (F) effector memory, (G) EMRA, and (H) terminally differentiated. (I–N) Absolute cell counts of CD8+ T cells: (I) total, (J) naive, (K) central memory, (L) effector memory, (M) EMRA, and (N) terminally differentiated. Blood was assessed either postdose 2 or postdose 3 for each participant. CMV−No, n = 35; CMV−Yes, n = 21; CMV+No, n = 84; CMV+Yes, n = 44. Each data point indicates an individual participant. Data are presented as box-and-whisker plots, minimum to maximum, with the center line at the median. Associations between CMV serostatus and prior COVID-19 were assessed by two-way ANOVA, with a Tukey’s test post hoc analysis of significant main effects and interactions. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
In summary, there are significant changes to the relative composition and phenotype of peripheral blood CD8+ T cell and CD4+ T cell subsets between CMV-seronegative and CMV-seropositive older adults, irrespective of prior COVID-19. The observed expansion of EMRA and TD T cells, as well as reduced surface expression of the costimulatory molecule CD28 on CD8N T cells in particular, may influence vaccine-specific T cell responses.
CMV serostatus influences CD4+ and CD8+ T cell SARS-CoV-2 Ag-induced recall responses in older adults
An AIM assay was used to examine T cell memory responses by stimulation with the SARS-CoV-2 Spike Ag after second and third dose SARS-CoV-2 mRNA vaccinations (Fig. 5, Table IV). SARS-CoV-2 vaccines are unusual in that healthy adults generate strong CD4+ T cell memory recall responses, but weaker CD8+ T cell memory responses (64). We also made similar observations in older adults. Most study participants had CD4+ T cell responses to SARS-CoV-2 Spike (postdose 2, 93.1%; postdose 3, 95.9%), but only 19.5% of participants had Spike-elicited CD8+ memory T cell responses after two vaccine doses, although this increased to 29.7% of participants after three vaccine doses (Table IV). CMV serostatus did not influence the number of individuals with SARS-CoV-2 Spike-AIM+CD4+ T cells or Spike-AIM+CD8+ T cells after second or third dose vaccinations. Grouped analyses revealed a significant main effect of CMV serostatus on the frequency of Spike-AIM+CD8+ T cells (Fig. 5A). However, despite greater variance of data in CMV-seropositive individuals, post hoc analyses by CMV serostatus were not significant postdose 2 or postdose 3. Intraindividual paired analyses also showed no main effects of vaccine dose or CMV serostatus on activation of Spike-AIM+CD8+ T cells (Supplemental Fig. 3A). Prior COVID-19 did not influence the prevalence of Spike-AIM+CD8+ T cells after two or three vaccine doses, although CMV seropositivity contributed to increased Spike-AIM+CD8+ T cell activation postdose 2 (Fig. 6). Grouped analyses on a population and intraindividual basis showed a main effect of vaccine dose (Fig. 5B, Supplemental Fig. 3B), but the prevalence of Spike-AIM+CD4+ T cells was likewise not different by CMV serostatus (Fig. 5B) or by prior COVID-19 (Fig. 6B, 6G). Therefore, CMV serostatus contributes to increased CD8+ T cell, but not CD4+ T cell, memory recall responses to the SARS-CoV-2 Spike protein several months after second and third dose vaccinations.
FIGURE 5.
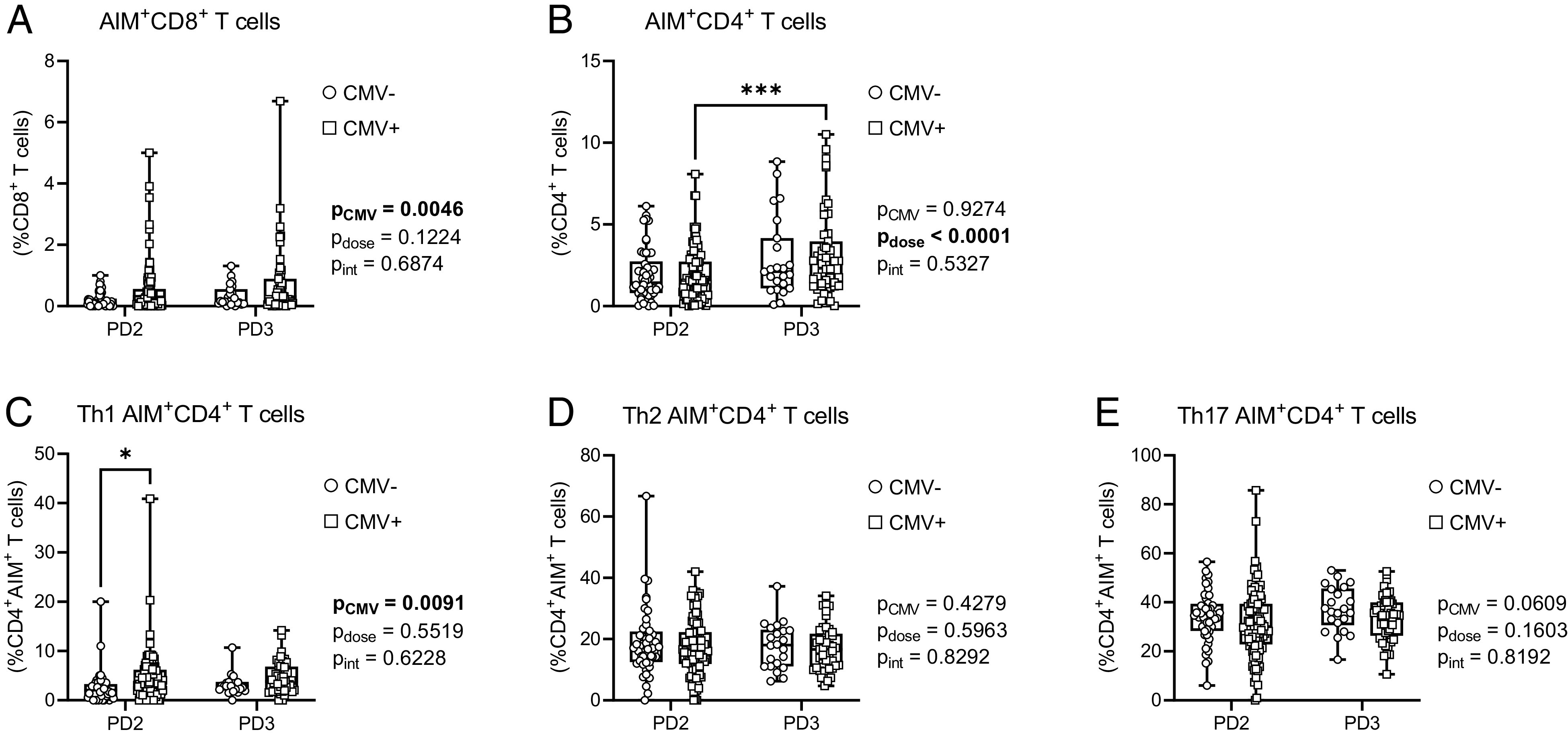
CD4+ and CD8+ T cell AIM responses to SARS-CoV-2 Spike in older adults. T cell memory responses to SARS-CoV-2 Spike were assessed by AIM assay in CMV-seronegative (CMV−) and CMV-seropositive (CMV+) individuals postdose 2 (PD2) and postdose 3 (PD3) SARS-CoV-2 vaccination. (A) AIM+CD8+ T cells (expressing CD69 and CD137) were measured as a proportion of total CD8+ T cells. (B) AIM+CD4+ T cells (expressing CD25 and OX40) were measured as a proportion of total CD4+ T cells. (C–E) AIM+CD4+ T cell Th1 (C), Th2 (D), and Th17 (E) subsets. Each data point indicates an individual participant. For (A) and (B), data from all individuals are graphed irrespective of whether they meet cutoff requirements for a “positive” result. For (C)–(E), only data from individuals with positive Spike-AIM+CD4+ T cell memory recall responses were graphed (Table IV). CMV−PD2, n = 51; CMV−PD3, n = 23; CMV+PD2, n = 108; CMV+PD3, n = 51. Data are presented as box-and-whisker plots, minimum to maximum, with the center line at the median. Associations between CMV serostatus and vaccine dose were assessed by two-way ANOVA, with a Tukey’s test post hoc analysis of significant main effects. *p < 0.05, ***p < 0.001.
Table IV.
Responders to vaccination by CMV serostatus: SARS-CoV-2 Spike T cell memory recall responses
| T Cell Population | Vaccine Doses | Total Responder Frequency | Responder Frequency by CMV Seropositivity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Frequency (%) | p (Fisher’s Exact Test) | Seronegative | Seropositive | p (Fisher’s Exact Test) | ||||
| n | Frequency (%) | n | Frequency (%) | ||||||
| Spike-AIM+CD8+ | 2 | 31/159 | 19.5 | 0.0943 | 6/50 | 12.0 | 24/108 | 22.2 | 0.1898 |
| 3 | 22/74 | 29.7 | 3/23 | 13.0 | 19/51 | 37.3 | 0.0532 | ||
| Spike-AIM+CD4+ | 2 | 148/159 | 93.1 | 0.5570 | 45/50 | 90.0 | 102/108 | 94.4 | 0.3262 |
| 3 | 71/74 | 95.9 | 22/23 | 95.7 | 49/51 | 96.1 | >0.9999 | ||
FIGURE 6.
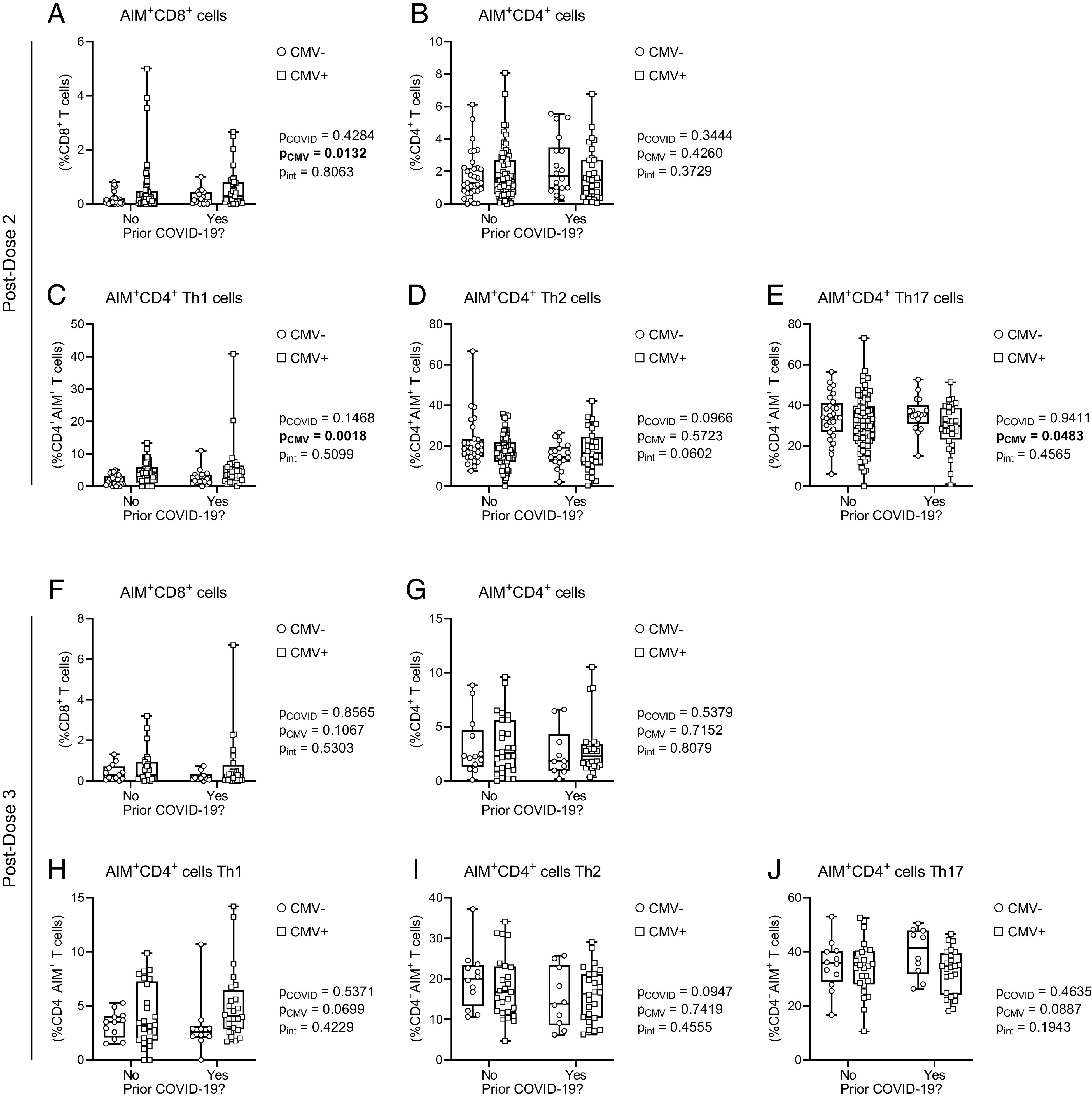
CD4+ and CD8+ T cell AIM responses to SARS-CoV-2 Spike after COVID-19 in older adults by CMV serostatus. T cell memory responses to SARS-CoV-2 Spike were assessed by AIM assay in CMV-seronegative (CMV−) and CMV-seropositive (CMV+) individuals. Data are stratified by prior SARS-CoV-2 infection history. (A–E) Postdose 2: (A) AIM+CD8+ T cells (expressing CD69 and CD137) were measured as a proportion of total CD8+ T cells; (B) AIM+CD4+ T cells (expressing CD25 and OX40) were measured as a proportion of total CD4+ T cells; AIM+CD4+ T cell Th1 (C), Th2 (D), and Th17 (E) subsets. (F–I) Postdose 3: (F) AIM+CD8+ T cells as a proportion of total CD8+ T cells; (G) AIM+CD4+ T cells as a proportion of total CD4+ T cells; AIM+CD4+ T cell Th1 (H), Th2 (I), and Th17 (J) subsets. For (C)–(E) and (H)–(J), only data from individuals with positive Spike-AIM+CD4+ T cell memory recall responses were graphed (Table IV). PD2: CMV−No, n = 33; CMV−Yes, n = 18; CMV+No, n = 77; CMV+Yes, n = 31. PD3: CMV−No, n = 13; CMV−Yes, n = 10; CMV+No, n = 27; CMV+Yes, n = 24. Each data point indicates an individual participant. Data are presented as box-and-whisker plots, minimum to maximum, with the center line at the median. Associations between CMV serostatus and prior COVID-19 were assessed by two-way ANOVA, with a Tukey’s test post hoc analysis of significant main effects.
CD4+ memory T cells are comprised of a number of different functional subsets, including Th1, Th2, and Th17 cells (65), which were further characterized. There was a significant effect of CMV serostatus on the frequency of Th1 Spike-AIM+CD4+ T cells, with post hoc analyses showing an increase in CMV-seropositive individuals after two but not three vaccine doses (Fig. 5C, Supplemental Fig. 3C). Th2 and Th17 Spike-AIM+CD4+ T cell frequencies were not influenced by CMV serostatus (Fig. 5D, 5E), although paired analyses by dose and CMV serostatus suggested a significant increase in Th17 responses in CMV-seropositive individuals after third vaccine doses (Supplemental Fig. 3E). Interestingly, when we considered these data in the context of prior SARS-CoV-2 infection, CMV serostatus had a main effect on the prevalence of Spike-AIM+CD4+ Th1 and Th17 T cells, which increased and decreased, respectively, with CMV seropositivity, although only postdose 2 (Fig. 6A, 6E), suggesting dose-dependent modulation of vaccine-associated T cell memory responses by CMV serostatus.
To determine whether the observed contributions of CMV serostatus to increased AIM+CD8+ T cell and AIM+CD4+ Th1 T cell memory responses are consistent across different stimuli, we also examined T cell AIM memory responses after TCR-independent polyclonal stimulation with CytoStim and stimulation with influenza HA Ags (Fig. 7). As we observed for SARS-CoV-2 Spike-activated memory T cells, both CytoStim and HA stimulation resulted in an increased prevalence of AIM+CD8+ T cells in CMV-seropositive individuals (Fig. 7A, 7F), although AIM+CD4+ T cell frequency was not affected by CMV serostatus (Fig. (7B, 7G). Prior COVID-19 did not influence the frequencies of AIM+ T cells (Supplemental Fig. 4). These data show that the effects of CMV seropositivity on AIM+CD8+ and AIM+CD4+ T cell frequencies are consistent across different stimuli. CytoStim-stimulated CD4+ T cells, similar to Spike-stimulated CD4+ T cells, also showed distinct Th1-skewed AIM+CD4+ T cell responses, although this was not observed after HA stimulation. These data collectively suggest that CMV serostatus alters the T cell response to polyclonal activation (i.e., with CytoStim) and has differential effects on the recall response of memory T cells to specific Ags (i.e., influenza HA or SARS-CoV-2 Spike). However, CMV serostatus does not alter the ability of older adults to generate lasting CD4+ or CD8+ T cell memory, nor the incidence of T cell memory recall activation in response to SARS-CoV-2 Spike protein after mRNA vaccination.
FIGURE 7.
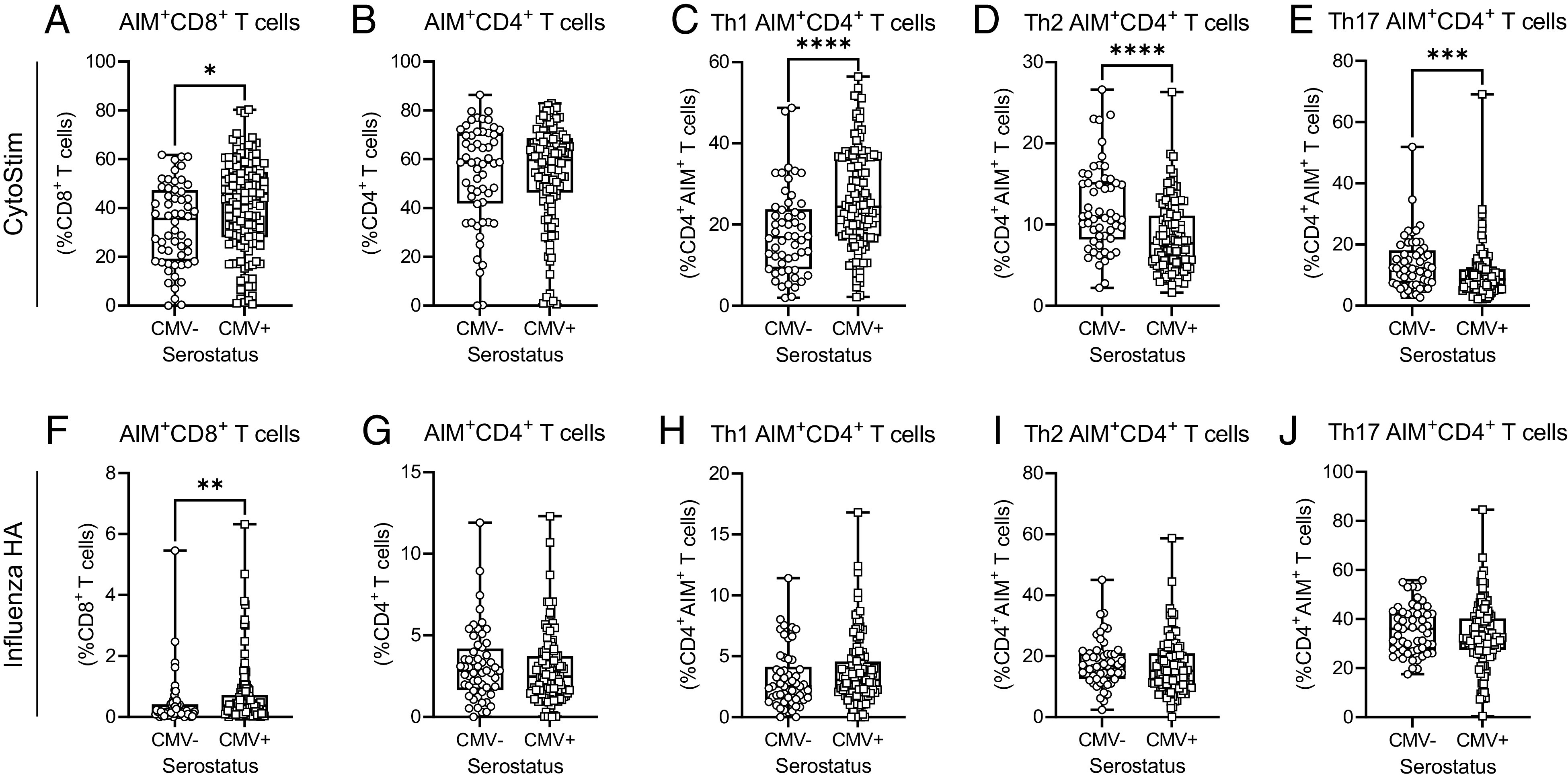
CD4+ and CD8+ T cell AIM responses to CytoStim and influenza HA in older adults. AIM assays were assessed by flow cytometry analysis after whole-blood stimulation. (A–E) CytoStim-induced responses: (A) AIM+CD8+ T cells (expressing CD69 and CD137) as a proportion of total CD8+ T cells; (B) AIM+CD4+ T cells (expressing CD25 and OX40) as a proportion of total CD4+ T cells; AIM+CD4+ T cell Th1 (C), Th2 (D), and Th17 (E) subsets. (F–J) Influenza HA-induced responses: (F) AIM+CD8+ T cells as a proportion of total CD8+ T cells; (G) AIM+CD4+ T cells as a proportion of total CD4+ T cells; AIM+CD4+ T cell Th1 (H), Th2 (I), and Th17 (J) subsets. Each data point indicates an individual participant. Data were pooled from all blood collections, and for participants with two different collection time points, data are only included from postdose 2 assessments. CMV−, n = 57; CMV+, n = 130. For (C)–(E), only data from individuals with >5% CytoStim AIM+CD4+ T cells were graphed. For (H)–(J), only data from individuals with >0% influenza HA AIM+CD4+ T cells were graphed. Data are presented as box-and-whisker plots, minimum to maximum, with the center line at the median. Associations between T cell responses and CMV serostatus were assessed by a Student t test with Welch’s correction or a Mann–Whitney U test, according to normality. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Discussion
Our data suggest that despite being a significant modifier of peripheral blood T cell composition and phenotype, CMV seropositivity in older adults does not have a negative impact on the longevity of vaccine-elicited Ab quantity and quality, or T cell memory recall responses, several months after second or third doses of SARS-CoV-2 mRNA vaccines. Yet, we found that there were subtle changes in Ab and cellular responses in CMV-seropositive individuals between vaccine doses and in individuals with prior COVID-19. Our study cohort included participants from multiple assisted living facilities, and it did not exclude individuals with particular health conditions (e.g., cancer, diabetes, cardiovascular disease, autoimmune disorders) or prescribed medications (e.g., immune-modulating drugs). Thus, any observed effects of CMV needed to be sufficiently robust to overcome potential effects of those other factors.
Our observations of changes in the peripheral T cell repertoire in CMV-seropositive individuals, and CD4+ and CD8+ T cell expression of CD28 and CD57, are consistent with prior publications that reported expansion of exhausted CD4EMRA and CD8EMRA T cells in CMV-seropositive healthy community-dwelling adults (41, 43). There are conflicting reports as to whether CMV seropositivity is associated with a reduction in naive CD4+ and CD8+ T cells. In this investigation, we observed similar numbers of CD4N and CD8N T cells in seropositive and seronegative individuals, and in particular similar expression of CD57 and CD28 on CD4N T cells. Our data therefore suggest that CMV seropositivity does not influence the availability or capacity of circulating naive T cells to respond and generate memory responses to Ags from novel viruses such as SARS-CoV-2 in older adults.
CMV seropositivity in older adults has been associated with lower frequencies of memory T cells in response to seasonal influenza, although acute infection T cell responses were unchanged (66), and there are conflicting data as to whether CMV seropositivity enhances or impairs influenza virus-specific T cell responses (33, 41, 67, 68). We found that T cell memory recall responses to influenza HA, and moreover SARS-CoV-2 Spike, were similar in CMV-seropositive and CMV-seronegative individuals. Our findings are concordant with observations from a previous study that found CMV serostatus did not alter the ability of older adults to generate memory responses to the (at the time) newly emergent West Nile virus (48). Furthermore, it has been reported that T cell memory responses to the SARS-CoV-2 Spike protein are boosted in convalescent younger adults after vaccination (69, 70). We identified an increase in Spike-specific CD4+ T cell memory responses between two and three mRNA vaccine doses in our older adult cohort, but we did not observe increased CD8+ or CD4+ T cell memory responses in convalescent older adults after vaccination. This may in part be because our analyses of effects of prior COVID-19 were not restricted to a particular time frame after SARS-CoV-2 infection. Importantly, we acknowledge that there may be early effects of CMV seropositivity on the initial generation of Abs or cellular memory that are not apparent at the assessed time points in this study, which were several months since vaccination. Irrespective, these findings suggest that although combined effects of infection and subsequent vaccination may differ by age, older adults can elicit memory T cell responses to infection and vaccination against newly emergent viruses such as SARS-CoV-2.
We observed a distinct Th1 bias after polyclonal T cell activation and in response to the immunodominant regions of the Spike Ag after two doses, but not three doses, of SARS-CoV-2 mRNA vaccines. Th1 skewing of the cellular immune response after influenza virus vaccination has been previously noted in CMV-seropositive infants and young adults and mice (68, 71), as well as in older adults (72). Strong Th1 CD4+ T cell responses have been associated with lower disease severity in unvaccinated COVID-19 patients (73), and SARS-CoV-2 Spike-elicited CD4+ T cell memory responses in unvaccinated convalescent individuals have also been identified to be primarily Th1 differentiated (69, 70). However, we did not identify a main effect of prior COVID-19, or an interaction of CMV serostatus and prior COVID-19, on the prevalence of CD4+ T cell Th1 responses in vaccinated older adults. These data indicate that in older adults CMV seropositivity is associated with Th1-biased CD4+ T cell responses, which are not further modified by prior SARS-CoV-2 infection. Our observations also suggest that Spike-specific T cell memory recall responses change between two and three vaccine doses in older adults, congruent with observations of changes in memory T cell phenotype between vaccine doses in younger adults (69).
Our data also show that CMV seropositivity does not prevent production of anti-Spike or anti-RBD IgG, IgA, or IgM Abs after SARS-CoV-2 mRNA vaccination, although we did observe interaction effects between CMV seropositivity and prior COVID-19 both postdose 2 and postdose 3 for anti-Spike and anti-RBD IgA Abs, and postdose 2 for IgM Abs. These interactions may be reflective of our limited sample size or differences in time since infection between CMV-seropositive and CMV-seronegative individuals. CMV-seropositive individuals have been reported to have increased B cell proliferation and mutations within the IgH sequences of IgM and IgG, but not IgA, isotypes (74). CMV serostatus could also have a larger effect on maturation of the Ab response via isotype switching, and thus isotype composition after viral infection, which may contribute to our observations, but to our knowledge this has not been extensively explored. Furthermore, our observations are from individuals vaccinated with mRNA vaccines, which were predominately used in older adults in assisted living facilities in Ontario, Canada. The effects of CMV-associated immune dysfunction on the quality and durability of humoral and cellular immune responses after vaccination may be ameliorated with mRNA vaccines, but other vaccine platforms (e.g., inactivated or attenuated whole virus, viral vector, or protein subunit) may have differential outcomes, which should be investigated in future studies.
Early in the pandemic, CMV seropositivity was associated with increased risk of hospitalization in COVID-19 patients (75), and CMV reactivation was later reported to have a higher incidence in patients in intensive care (76). More recently it was reported that unvaccinated individuals with latent CMV, irrespective of anti-CMV Ab levels, age, and sex, are at higher risk of SARS-CoV-2 infection and hospitalization (77). In particular, the exhausted T cells present in CMV-seropositive individuals have been predicted to contribute to more severe COVID-19 pathophysiology (78). Our data do not preclude the possibility that CMV-associated remodeling of innate and adaptive immunity in older adults may contribute to the pathogenesis and severity of SARS-CoV-2 infection. CMV serostatus may in addition impact humoral or cellular protection after vaccination against breakthrough infections with current or emerging variants of concern.
In conclusion, our data show that CMV serostatus alters the T cell repertoire but does not blunt durability of vaccine-elicited cellular memory responses or humoral responses after two and three doses of SARS-CoV-2 mRNA vaccines in older adults in assisted living facilities. Further research is necessary to disentangle the more subtle effects of CMV serostatus on immunity after vaccination, as well as to assess its role in risk of breakthrough SARS-CoV-2 infections.
Supplementary Material
Acknowledgments
Data in this study were collected by the COVID-in-LTC Study Group. We acknowledge administrative and technical assistance from Tara Kajaks, Ahmad Rahim, Megan Hagerman, Braeden Cowbrough, Komal Aryal, Leslie Tan, Sussan Kianpour, Jodie Turner, and Sheneice Joseph of the COVID-in-LTC Study Group at McMaster University. We thank our participants and their families, as well as staff, for support of this study.
This work was supported by a grant from the Canadian COVID-19 Immunity Task Force and Public Health Agency of Canada awarded to A.P.C. and D.M.E.B. A.P.C. is the Schlegel Chair in Clinical Epidemiology and Aging. D.M.E.B. is the Canada Research Chair in Aging and Immunity. Funding support for this work was provided by grants from the Canadian Institutes of Health Research, the Ontario Research Foundation COVID-19 Rapid Research Fund, and by the Canadian COVID-19 Immunity Task Force awarded to I.N. M.S.M. is supported, in part, by an Ontario Early Researcher Award and a Canada Research Chair in Viral Pandemics.
The online version of this article contains supplemental material.
- AIM
- activation-induced marker
- CM
- central memory
- EM
- effector memory
- EMRA
- EM re-expressing CD45RA
- HA
- hemagglutinin
- MNT50
- microneutralization titer at 50%
- N
- naive
- RBD
- receptor-binding domain
- TD
- terminally differentiated
Other Members of the COVID-in-LTC Study Group
Eric D. Brown,1,2 Gerry Wright,1,2 David C. Bulir,3 Mark Loeb,2,3.4,5 Marek Smieja,3,4,6 Aaron Jones,5,7 Parminder Raina,5,8 Chris Verschoor,5,9,10 Janet E. McElhaney (posthumous),9,10 Kevin Brown,11,12 George A. Heckman,13,14 John P. Hirdes,13,14 Michael P. Hillmer,15,16 Ahmad Von Schlegell,17,18 Nathan M. Stall,19,20 Kevin Stinson,21 and Arthur Sweetman22.
1Department of Biochemistry & Biomedical Sciences, McMaster University, Hamilton, Ontario, Canada. 2Michael G. DeGroote Institute for Infectious Disease Research, McMaster University, Hamilton, Ontario, Canada. 3Department of Pathology and Molecular Medicine, McMaster University, Hamilton, Ontario, Canada. 4Department of Medicine, Michael G. DeGroote School of Medicine, McMaster University, Hamilton, Ontario, Canada. 5Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, Canada. 6St. Joseph’s Healthcare Hamilton, Hamilton, Ontario, Canada. 7Institute for Clinical Evaluative Sciences (ICES), Toronto, Ontario, Canada. 8McMaster Institute for Research on Aging, McMaster University, Hamilton, Ontario, Canada. 9Health Science North Research Institute, Sudbury, Ontario, Canada. 10Northern Ontario School of Medicine, Sudbury, Ontario, Canada. 11Dalla Lana School of Public Health, University of Toronto, Ontario, Canada. 12Public Health Ontario, Ontario, Canada. 13School of Public Health and Health Systems, University of Waterloo, Waterloo, Ontario, Canada. 14Schlegel Research Institute for Aging, Waterloo, Ontario, Canada. 15Ontario Ministry of Health, Toronto, Canada. 16Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada. 17Schlegel Villages, Ontario, Canada. 18Trillium Health Partners, Ontario, Canada. 19Department of Medicine, Division of General Internal Medicine and Geriatrics, University of Toronto, Toronto, Ontario, Canada. 20Sinai Health, Toronto, Ontario, Canada. 21St. Mary’s General Hospital, Kitchener, Ontario, Canada. 22Department of Economics, McMaster University, Hamilton, Ontario, Canada.
Disclosures
The authors have no financial conflicts of interest.
References
- 1. Kline K. A., Bowdish D. M.. 2016. Infection in an aging population. Curr. Opin. Microbiol. 29: 63–67. [DOI] [PubMed] [Google Scholar]
- 2. Lord J. M. 2013. The effect of ageing of the immune system on vaccination responses. Hum. Vaccin. Immunother. 9: 1364–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banerjee A., Pasea L., Harris S., Gonzalez-Izquierdo A., Torralbo A., Shallcross L., Noursadeghi M., Pillay D., Sebire N., Holmes C., et al. 2020. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet 395: 1715–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cunningham A. L., Lal H., Kovac M., Chlibek R., Hwang S.-J., Díez-Domingo J., Godeaux O., Levin M. J., McElhaney J. E., Puig-Barberà J., et al. ZOE-70 Study Group . 2016. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N. Engl. J. Med. 375: 1019–1032. [DOI] [PubMed] [Google Scholar]
- 5. Lal H., Cunningham A. L., Godeaux O., Chlibek R., Diez-Domingo J., Hwang S. J., Levin M. J., McElhaney J. E., Poder A., Puig-Barberà J., et al. ZOE-50 Study Group . 2015. Efficacy of an adjuvanted herpes zoster subunit vaccine in older adults. N. Engl. J. Med. 372: 2087–2096. [DOI] [PubMed] [Google Scholar]
- 6. Okoli G. N., Racovitan F., Abdulwahid T., Righolt C. H., Mahmud S. M.. 2021. Variable seasonal influenza vaccine effectiveness across geographical regions, age groups and levels of vaccine antigenic similarity with circulating virus strains: a systematic review and meta-analysis of the evidence from test-negative design studies after the 2009/10 influenza pandemic. Vaccine 39: 1225–1240. [DOI] [PubMed] [Google Scholar]
- 7. Rondy M., El Omeiri N., Thompson M. G., Levêque A., Moren A., Sullivan S. G.. 2017. Effectiveness of influenza vaccines in preventing severe influenza illness among adults: a systematic review and meta-analysis of test-negative design case-control studies. J. Infect. 75: 381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown K., Stall N. M., Vanniyasingam T., Buchan S. A., Daneman N., Hillmer M. P., Hopkins J., Johnstone J., Maltsev A., McGeer A., et al. Early impact of Ontario’s COVID-19 vaccine rollout on long-term care home residents and health care workers. Available at: 10.47326/ocsat.2021.02.13.1.0. Accessed: March 19, 2022. [DOI]
- 9. Salcher-Konrad M., Smith S., Comas-Herrera A.. 2021. Emerging evidence on effectiveness of COVID-19 vaccines among residents of long-term care facilities. J. Am. Med. Dir. Assoc. 22: 1602–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung H., He S., Nasreen S., Sundaram M. E., Buchan S. A., Wilson S. E., Chen B., Calzavara A., Fell D. B., Austin P. C., et al. Canadian Immunization Research Network (CIRN) Provincial Collaborative Network (PCN) Investigators . 2021. Effectiveness of BNT162b2 and mRNA-1273 covid-19 vaccines against symptomatic SARS-CoV-2 infection and severe covid-19 outcomes in Ontario, Canada: test negative design study. BMJ 374: n1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Breznik J. A., Zhang A., Huynh A., Miller M. S., Nazy I., Bowdish D. M. E., Costa A. P.. 2021. Antibody responses 3–5 months post-vaccination with mRNA-1273 or BNT163b2 in nursing home residents. J. Am. Med. Dir. Assoc. 22: 2512–2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang A., Breznik J. A., Clare R., Nazy I., Miller M. S., Bowdish D. M. E., Costa A. P.. 2022. Antibody responses to third-dose mRNA vaccines in nursing home and assisted living residents. J. Am. Med. Dir. Assoc. 23: 444–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brockman M. A., Mwimanzi F., Lapointe H. R., Sang Y., Agafitei O., Cheung P. K., Ennis S., Ng K., Basra S., Lim L. Y., et al. 2022. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. J. Infect. Dis. 225: 1129–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weng N. P., Pawelec G.. 2021. Validation of the effectiveness of SARS-CoV-2 vaccines in older adults in “real-world” settings. Immun. Ageing 18: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fulop T., Larbi A., Dupuis G., Le Page A., Frost E. H., Cohen A. A., Witkowski J. M., Franceschi C.. 2018. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol. 8: 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fulop T., Pawelec G., Castle S., Loeb M.. 2009. Immunosenescence and vaccination in nursing home residents. Clin. Infect. Dis. 48: 443–448. [DOI] [PubMed] [Google Scholar]
- 17. Zuhair M., Smit G. S. A., Wallis G., Jabbar F., Smith C., Devleesschauwer B., Griffiths P.. 2019. Estimation of the worldwide seroprevalence of cytomegalovirus: a systematic review and meta-analysis. Rev. Med. Virol. 29: e2034. [DOI] [PubMed] [Google Scholar]
- 18. Pawelec G., McElhaney J. E., Aiello A. E., Derhovanessian E.. 2012. The impact of CMV infection on survival in older humans. Curr. Opin. Immunol. 24: 507–511. [DOI] [PubMed] [Google Scholar]
- 19. Sylwester A. W., Mitchell B. L., Edgar J. B., Taormina C., Pelte C., Ruchti F., Sleath P. R., Grabstein K. H., Hosken N. A., Kern F., et al. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202: 673–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chidrawar S., Khan N., Wei W., McLarnon A., Smith N., Nayak L., Moss P.. 2009. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol. 155: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sansoni P., Vescovini R., Fagnoni F., Biasini C., Zanni F., Zanlari L., Telera A., Lucchini G., Passeri G., Monti D., et al. 2008. The immune system in extreme longevity. Exp. Gerontol. 43: 61–65. [DOI] [PubMed] [Google Scholar]
- 22. Goronzy J. J., Weyand C. M.. 2017. Successful and maladaptive t cell aging. Immunity 46: 364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dörner T., Radbruch A.. 2007. Antibodies and B cell memory in viral immunity. Immunity 27: 384–392. [DOI] [PubMed] [Google Scholar]
- 24. Yan Z., Maecker H. T., Brodin P., Nygaard U. C., Lyu S. C., Davis M. M., Nadeau K. C., Andorf S.. 2021. Aging and CMV discordance are associated with increased immune diversity between monozygotic twins. Immun. Ageing 18: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brodin P., Jojic V., Gao T., Bhattacharya S., Angel C. J., Furman D., Shen-Orr S., Dekker C. L., Swan G. E., Butte A. J., et al. 2015. Variation in the human immune system is largely driven by non-heritable influences. Cell 160: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mekker A., Tchang V. S., Haeberli L., Oxenius A., Trkola A., Karrer U.. 2012. Immune senescence: relative contributions of age and cytomegalovirus infection. PLoS Pathog. 8: e1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Olsson J., Wikby A., Johansson B., Löfgren S., Nilsson B. O., Ferguson F. G.. 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev. 121: 187–201. [DOI] [PubMed] [Google Scholar]
- 28. Crooke S. N., Ovsyannikova I. G., Poland G. A., Kennedy R. B.. 2019. Immunosenescence and human vaccine immune responses. Immun. Ageing 16: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kadambari S., Klenerman P., Pollard A. J.. 2020. Why the elderly appear to be more severely affected by COVID-19: the potential role of immunosenescence and CMV. Rev. Med. Virol. 30: e2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Moss P. 2020. “The ancient and the new”: is there an interaction between cytomegalovirus and SARS-CoV-2 infection? Immun. Ageing 17: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Söderberg-Nauclér C. 2021. Does reactivation of cytomegalovirus contribute to severe COVID-19 disease? Immun. Ageing 18: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y., Klein S. L., Garibaldi B. T., Li H., Wu C., Osevala N. M., Li T., Margolick J. B., Pawelec G., Leng S. X.. 2021. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res. Rev. 65: 101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Merani S., Pawelec G., Kuchel G. A., McElhaney J. E.. 2017. Impact of aging and cytomegalovirus on immunological response to influenza vaccination and infection. Front. Immunol. 8: 784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cicin-Sain L., Brien J. D., Uhrlaub J. L., Drabig A., Marandu T. F., Nikolich-Zugich J.. 2012. Cytomegalovirus infection impairs immune responses and accentuates T-cell pool changes observed in mice with aging. PLoS Pathog. 8: e1002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smithey M. J., Li G., Venturi V., Davenport M. P., Nikolich-Žugich J.. 2012. Lifelong persistent viral infection alters the naive T cell pool, impairing CD8 T cell immunity in late life. J. Immunol. 189: 5356–5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Khan N., Hislop A., Gudgeon N., Cobbold M., Khanna R., Nayak L., Rickinson A. B., Moss P. A.. 2004. Herpesvirus-specific CD8 T cell immunity in old age: cytomegalovirus impairs the response to a coresident EBV infection. J. Immunol. 173: 7481–7489. [DOI] [PubMed] [Google Scholar]
- 37. Barton E. S., White D. W., Cathelyn J. S., Brett-McClellan K. A., Engle M., Diamond M. S., Miller V. L., Virgin H. W. IV. 2007. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature 447: 326–329. [DOI] [PubMed] [Google Scholar]
- 38. Terrazzini N., Bajwa M., Vita S., Thomas D., Smith H., Vescovini R., Sansoni P., Kern F.. 2014. Cytomegalovirus infection modulates the phenotype and functional profile of the T-cell immune response to mycobacterial antigens in older life. Exp. Gerontol. 54: 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pera A., Campos C., Corona A., Sanchez-Correa B., Tarazona R., Larbi A., Solana R.. 2014. CMV latent infection improves CD8+ T response to SEB due to expansion of polyfunctional CD57+ cells in young individuals. [Published erratum appears in 2014 PLoS One 9: e96971.] PLoS One 9: e88538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Smithey M. J., Venturi V., Davenport M. P., Buntzman A. S., Vincent B. G., Frelinger J. A., Nikolich-Žugich J.. 2018. Lifelong CMV infection improves immune defense in old mice by broadening the mobilized TCR repertoire against third-party infection. Proc. Natl. Acad. Sci. USA 115: E6817–E6825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Derhovanessian E., Theeten H., Hähnel K., Van Damme P., Cools N., Pawelec G.. 2013. Cytomegalovirus-associated accumulation of late-differentiated CD4 T-cells correlates with poor humoral response to influenza vaccination. Vaccine 31: 685–690. [DOI] [PubMed] [Google Scholar]
- 42. Saurwein-Teissl M., Lung T. L., Marx F., Gschösser C., Asch E., Blasko I., Parson W., Böck G., Schönitzer D., Trannoy E., Grubeck-Loebenstein B.. 2002. Lack of antibody production following immunization in old age: association with CD8+CD28− T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J. Immunol. 168: 5893–5899. [DOI] [PubMed] [Google Scholar]
- 43. Frasca D., Diaz A., Romero M., Landin A. M., Blomberg B. B.. 2015. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine 33: 1433–1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Trzonkowski P., Myśliwska J., Szmit E., Wieckiewicz J., Lukaszuk K., Brydak L. B., Machała M., Myśliwski A.. 2003. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination—an impact of immunosenescence. Vaccine 21: 3826–3836. [DOI] [PubMed] [Google Scholar]
- 45. Bowyer G., Sharpe H., Venkatraman N., Ndiaye P. B., Wade D., Brenner N., Mentzer A., Mair C., Waterboer T., Lambe T., et al. 2020. Reduced Ebola vaccine responses in CMV+ young adults is associated with expansion of CD57+KLRG1+ T cells. J. Exp. Med. 217: e20200004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van den Berg S. P. H., Warmink K., Borghans J. A. M., Knol M. J., van Baarle D.. 2019. Effect of latent cytomegalovirus infection on the antibody response to influenza vaccination: a systematic review and meta-analysis. Med. Microbiol. Immunol. (Berl.) 208: 305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sharpe H. R., Provine N. M., Bowyer G. S., Moreira Folegatti P., Belij-Rammerstorfer S., Flaxman A., Makinson R., Hill A. V. S., Ewer K. J., Pollard A. J., et al. 2022. CMV-associated T cell and NK cell terminal differentiation does not affect immunogenicity of ChAdOx1 vaccination. JCI Insight 7: e154187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Verschoor C. P., Kohli V., Balion C.. 2018. A comprehensive assessment of immunophenotyping performed in cryopreserved peripheral whole blood. Cytometry B Clin. Cytom. 94: 662–670. [DOI] [PubMed] [Google Scholar]
- 49. Ontario Ministry of Health . 2021. COVID-19 vaccine third dose recommendations. Queen’s Printer for Ontario, Toronto. Available at: https://www.health.gov.on.ca/en/pro/programs/publichealth/coronavirus/docs/vaccine/COVID-19_vaccine_third_dose_recommendations.pdf. Accessed: December 15, 2021.
- 50. Kennedy A. E., Cook L., Breznik J. A., Cowbrough B., Wallace J. G., Huynh A., Smith J. W., Son K., Stacey H., Ang J., et al. 2021. Lasting changes to circulating leukocytes in people with mild SARS-CoV-2 infections. Viruses 13: 2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Loukov D., Karampatos S., Maly M. R., Bowdish D. M. E.. 2018. Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, BMI and pain. Osteoarthritis Cartilage 26: 255–263. [DOI] [PubMed] [Google Scholar]
- 52. Larbi A., Fulop T.. 2014. From “truly naïve” to “exhausted senescent” T cells: when markers predict functionality. Cytometry A 85: 25–35. [DOI] [PubMed] [Google Scholar]
- 53. Zaunders J. J., Munier M. L., Seddiki N., Pett S., Ip S., Bailey M., Xu Y., Brown K., Dyer W. B., Kim M., et al. 2009. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40). J. Immunol. 183: 2827–2836. [DOI] [PubMed] [Google Scholar]
- 54. Seddiki N., Cook L., Hsu D. C., Phetsouphanh C., Brown K., Xu Y., Kerr S. J., Cooper D. A., Munier C. M., Pett S., et al. 2014. Human antigen-specific CD4+CD25+CD134+CD39+ T cells are enriched for regulatory T cells and comprise a substantial proportion of recall responses. Eur. J. Immunol. 44: 1644–1661. [DOI] [PubMed] [Google Scholar]
- 55. Wolfl M., Kuball J., Ho W. Y., Nguyen H., Manley T. J., Bleakley M., Greenberg P. D.. 2007. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 110: 201–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huynh A., Arnold D. M., Smith J. W., Moore J. C., Zhang A., Chagla Z., Harvey B. J., Stacey H. D., Ang J. C., Clare R., et al. 2021. Characteristics of Anti-SARS-CoV-2 Antibodies in Recovered COVID-19 Subjects. Viruses 13: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. National Advistory Committee on Immunization . 2021. An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI): guidance on booster COVID-19 vaccine doses in Canada—update December 3, 2021. Public Health Agency of Canada. Available at: https://www.canada.ca/content/dam/phac-aspc/documents/services/immunization/national-advisory-committee-on-immunization-naci/guidance-booster-covid-19-vaccine-doses/guidance-booster-covid-19-vaccine-doses.pdf Accessed: December 15, 2021.
- 58. Hirabara S. M., Serdan T. D. A., Gorjao R., Masi L. N., Pithon-Curi T. C., Covas D. T., Curi R., Durigon E. L.. 2022. SARS-COV-2 variants: differences and potential of immune evasion. Front. Cell. Infect. Microbiol. 11: 781429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Klenerman P., Oxenius A.. 2016. T cell responses to cytomegalovirus. Nat. Rev. Immunol. 16: 367–377. [DOI] [PubMed] [Google Scholar]
- 60. Strioga M., Pasukoniene V., Characiejus D.. 2011. CD8+ CD28− and CD8+ CD57+ T cells and their role in health and disease. Immunology 134: 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fletcher J. M., Vukmanovic-Stejic M., Dunne P. J., Birch K. E., Cook J. E., Jackson S. E., Salmon M., Rustin M. H., Akbar A. N.. 2005. Cytomegalovirus-specific CD4+ T cells in healthy carriers are continuously driven to replicative exhaustion. J. Immunol. 175: 8218–8225. [DOI] [PubMed] [Google Scholar]
- 62. Henson S. M., Riddell N. E., Akbar A. N.. 2012. Properties of end-stage human T cells defined by CD45RA re-expression. Curr. Opin. Immunol. 24: 476–481. [DOI] [PubMed] [Google Scholar]
- 63. Brenchley J. M., Karandikar N. J., Betts M. R., Ambrozak D. R., Hill B. J., Crotty L. E., Casazza J. P., Kuruppu J., Migueles S. A., Connors M., et al. 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101: 2711–2720. [DOI] [PubMed] [Google Scholar]
- 64. Neidleman J., Luo X., McGregor M., Xie G., Murray V., Greene W. C., Lee S. A., Roan N. R.. 2021. mRNA vaccine-induced T cells respond identically to SARS-CoV-2 variants of concern but differ in longevity and homing properties depending on prior infection status. eLife 10: e72619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Crotty S. 2015. A brief history of T cell help to B cells. Nat. Rev. Immunol. 15: 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. van den Berg S. P. H., Lanfermeijer J., Jacobi R. H. J., Hendriks M., Vos M., van Schuijlenburg R., Nanlohy N. M., Borghans J. A. M., van Beek J., van Baarle D., de Wit J.. 2021. Latent CMV infection is associated with lower influenza virus-specific memory T-cell frequencies, but not with an impaired T-cell response to acute influenza virus infection. Front. Immunol. 12: 663664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Theeten H., Mathei C., Peeters K., Ogunjimi B., Goossens H., Ieven M., Van Damme P., Cools N.. 2016. Cellular interferon gamma and granzyme B responses to cytomegalovirus-pp65 and influenza N1 are positively associated in elderly. Viral Immunol. 29: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Furman D., Jojic V., Sharma S., Shen-Orr S. S., Angel C. J., Onengut-Gumuscu S., Kidd B. A., Maecker H. T., Concannon P., Dekker C. L., et al. 2015. Cytomegalovirus infection enhances the immune response to influenza. Sci. Transl. Med. 7: 281ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Neidleman J., Luo X., Frouard J., Xie G., Gill G., Stein E. S., McGregor M., Ma T., George A. F., Kosters A., et al. 2020. SARS-CoV-2-specific T cells exhibit phenotypic features of helper function, lack of terminal differentiation, and high proliferation potential. Cell Rep. Med. 1: 100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Grifoni A., Weiskopf D., Ramirez S. I., Mateus J., Dan J. M., Moderbacher C. R., Rawlings S. A., Sutherland A., Premkumar L., Jadi R. S., et al. 2020. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181: 1489–1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Miles D. J., van der Sande M., Jeffries D., Kaye S., Ismaili J., Ojuola O., Sanneh M., Touray E. S., Waight P., Rowland-Jones S., et al. 2007. Cytomegalovirus infection in Gambian infants leads to profound CD8 T-cell differentiation. J. Virol. 81: 5766–5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Felismino E. S., Santos J. M. B., Rossi M., Santos C. A. F., Durigon E. L., Oliveira D. B. L., Thomazelli L. M., Monteiro F. R., Sperandio A., Apostólico J. S., et al. 2021. Better response to influenza virus vaccination in physically trained older adults is associated with reductions of cytomegalovirus-specific immunoglobulins as well as improvements in the inflammatory and CD8+ T-cell profiles. Front. Immunol. 12: 713763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rydyznski Moderbacher C., Ramirez S. I., Dan J. M., Grifoni A., Hastie K. M., Weiskopf D., Belanger S., Abbott R. K., Kim C., Choi J., et al. 2020. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183: 996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang C., Liu Y., Xu L. T., Jackson K. J. L., Roskin K. M., Pham T. D., Laserson J., Marshall E. L., Seo K., Lee J.-Y., et al. 2014. Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. J. Immunol. 192: 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shrock E., Fujimura E., Kula T., Timms R. T., Lee I. H., Leng Y., Robinson M. L., Sie B. M., Li M. Z., Chen Y., et al. MGH COVID-19 Collection & Processing Team . 2020. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science 370: eabd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Simonnet A., Engelmann I., Moreau A. S., Garcia B., Six S., El Kalioubie A., Robriquet L., Hober D., Jourdain M.. 2021. High incidence of Epstein-Barr virus, cytomegalovirus, and human-herpes virus-6 reactivations in critically ill patients with COVID-19. Infect. Dis. Now 51: 296–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Alanio C., Verma A., Mathew D., Gouma S., Liang G., Dunn T., Oldridge D. A., Weaver J. E., Kuri-Cervantes L., Pampena M. B., et al. 2022. Cytomegalovirus latent infection is associated with an increased risk of COVID-19-related hospitalization. J. Infect. Dis. 226: 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Del Valle D. M., Kim-Schulze S., Huang H.-H., Beckmann N. D., Nirenberg S., Wang B., Lavin Y., Swartz T. H., Madduri D., Stock A., et al. 2020. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 26: 1636–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


