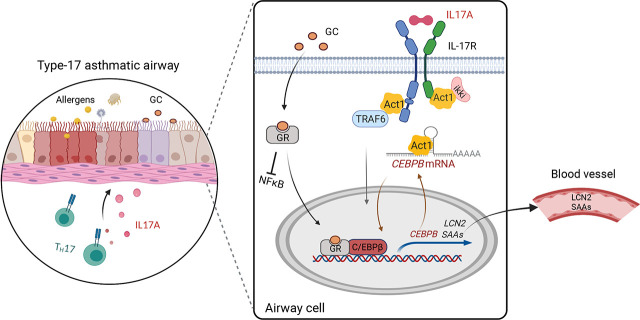
Abstract
IL-17A plays an important role in the pathogenesis of asthma, particularly the neutrophilic corticosteroid (CS)-resistant subtype of asthma. Clinical studies suggest that a subset of asthma patients, i.e., Th17/IL-17A–mediated (type 17) CS-resistant neutrophilic asthma, may improve with Th17/IL-17A pathway blockade. However, little is known about the mechanisms underlying type 17 asthma and CS response. In this article, we show that blood levels of lipocalin-2 (LCN2) and serum amyloid A (SAA) levels are positively correlated with IL-17A levels and are not inhibited by high-dose CS usage in asthma patients. In airway cell culture systems, IL-17A induces these two secreted proteins, and their induction is enhanced by CS. Furthermore, plasma LCN2 and SAA levels are increased in mice on a preclinical type 17 asthma model, correlated to IL-17A levels, and are not reduced by glucocorticoid (GC). In the mechanistic studies, we identify CEBPB as the critical transcription factor responsible for the synergistic induction of LCN2 and SAA by IL-17A and GC. IL-17A and GC collaboratively regulate CEBPB at both transcriptional and posttranscriptional levels. The posttranscriptional regulation of CEBPB is mediated in part by Act1, the adaptor and RNA binding protein in IL-17A signaling, which directly binds CEBPB mRNA and inhibits its degradation. Overall, our findings suggest that blood LCN2 and SAA levels may be associated with a type 17 asthma subtype and provide insight into the molecular mechanism of the IL-17A–Act1/CEBPB axis on these CS-resistant genes.
Visual Abstract
Key Points
Blood LCN2 and SAA levels may be associated with a type 17 asthma subtype.
LCN2 and SAAs are steroid-resistant IL-17A target genes in airway cells.
IL-17A–Act1/CEBPB axis is an important regulatory mechanism of LCN2 and SAAs.
Introduction
Asthma is an inflammatory airway disease, often characterized into Th2-high or Th2-low subtypes. Many studies show that IL-17A, a cytokine released by Th17 cells, plays a pathogenic role in a subtype of asthma characterized by neutrophilic inflammation and corticosteroid (CS) resistance (1–7). IL-17A signaling deficits or blockade decreases inflammation and airway hyperresponsiveness in murine allergen-induced asthma models (8–14). IL-17A is present in asthmatic lung tissues, and levels are positively correlated to neutrophilic airway inflammation (2,3, 5–7, 15, 16). Single-nucleotide polymorphisms of IL17A are linked to asthma susceptibility (17, 18). Clinical studies support the idea of an asthma subtype that responds to Th17/IL-17A pathway blockade (19, 20). These preclinical and clinical findings point to the existence of a Th17/IL-17A–mediated (type 17) CS-resistant neutrophilic asthma that is more difficult to treat than Th2-type asthma, but little is known of mechanisms underlying this type of asthma.
IL-17A signals through a heterogeneous IL-17R complex (composed of IL-17RA and IL-17RC) to induce the production of proinflammatory cytokines and chemokines (21, 22). This is achieved through the activation of transcription factors (e.g., NF-κB, C/EBPs, IκBζ) and posttranscriptional regulation, including stabilization of a specific set of mRNAs (23). Many IL-17A–induced transcripts possess AU-rich elements (AREs) or other stability-determining sequences in the 3′ untranslated region (UTR). In this context, Act1 (encoded by TRAF3IP2), a proximal IL-17R adaptor molecule, directly binds stem-loop RNA structures through its SEF/IL-17R (SEFIR) domain to stabilize target mRNAs on IL-17A stimulation (24). Act1 binds to the SEFIR binding element (SBE), which is located proximal to AREs and interacts with ARE binding proteins (e.g., HuR, Arid5a), to promote the expression of IL-17A target mRNAs (24–26).
C/EBPβ (encoded by CEBPB) is a transcription factor that regulates gene expression in lung pathological conditions (e.g., chronic obstructive pulmonary disease, pulmonary fibrosis, and LPS-induced neutrophilia) (27–32). Previous studies have shown that IL-17A and glucocorticoid (GC) signaling induce posttranscriptional modifications of CEBPB that alter its DNA binding activity for target gene expression (30, 31). Prior studies suggest that CEBPB expression is upregulated in asthmatics (32), and that CEBPB deficiency attenuates airway neutrophilic inflammation in murine models (33, 34).
Lipocalin-2 (LCN2), originally identified as a protein secreted by neutrophils, is produced by multiple cell types (35), and its expression is regulated by IL-17A and GC (36, 37). The LCN2 promoter contains NF-κB and CEBP binding sites, as well as a GC response element. LCN2 plays a pivotal role in the innate immune defense against bacterial infection but is also an important mediator for which both anti-inflammatory and proinflammatory functions have been reported (38–41). In murine topical imiquimod-treated psoriatic skin, i.p. injection of LCN2 increases the production of Th17 cytokines/chemokines and exacerbates Th17-mediated skin inflammation (42). LCN2 is highly induced in inflammatory states and has been used as a biomarker to predict disease onset/progression in a variety of diseases (39, 43–45), including chronic obstructive pulmonary disease characterized by CS-resistant neutrophilic airway inflammation.
The serum amyloid A (SAA) family includes four closely linked genes (SAA1, SAA2, SAA3, and SAA4). SAA1 and SAA2 are acute-phase response proteins and have been used as biomarkers for inflammation (46, 47). Given the amino acid homology and tissue distribution, mouse SAA3 is most similar to the human isoform SAA1 (70% amino acid identity) and is considered a human ortholog. Although SAA proteins are highly induced in many inflammatory states and cancers, their functions are less studied. SAA acts as a soluble pattern recognition receptor that drives pulmonary type 2 immunity. Recent studies suggest that SAAs promote pathogenic Th17 differentiation and IL-17A production from CD4 T cells, leading to exacerbated Th17/IL-17A–mediated diseases (e.g., inflammatory bowel disease and experimental autoimmune encephalomyelitis) (48–50).
In this study, we investigate IL-17A levels in relationship to LCN2 and SAA levels in asthma patients who are on a variety of doses of CS medications. Furthermore, we study the effects of IL-17A and GCs on the production of LCN2 and SAA in human and mouse airway cells in vitro and in mice on a preclinical type 17 CS-resistant neutrophilic asthma model. To elucidate mechanisms, we assess the regulatory role of CEBPB in the expression of LCN2 and SAAs and transcriptional and posttranscriptional regulation of CEBPB, including the role of Act1, the adapter and RNA binding protein in IL-17A signaling.
Materials and Methods
Human subjects
The study population included 27 subjects with asthma and 13 healthy control subjects. Asthma severity was defined as per the Proceedings of the American Thoracic Society Workshop on Refractory Asthma, with major and minor characteristics (51). Exclusion criteria included current smoking history or smoking history within 1 y, former smokers with >5 pack-year total history, pregnancy, and HIV infection. The protocol complies with the Declaration of Helsinki and is approved by the Cleveland Clinic Institutional Review Board. All participants signed an informed consent.
Cell culture
Human primary airway smooth muscle cells (ASMCs; hASMCs) were purchased from ATCC (PCS-301-010). Mouse ASMCs (mASMCs) were isolated as described previously (52). Both hASMCs and mASMCs were cultured in DMEM/Ham's F12 medium supplemented with 10% FCS, 100 U/ml penicillin, and 100 μg/ml streptomycin. Murine and human IL-17A were used at 100 ng/ml, and dexamethasone (DEX; sc-29059A; Santa Cruz) was used at 1 μM.
Mice
Wild-type (WT) C57BL/6 mice were purchased from Jackson Laboratories. LSL–hemagglutinin tag (HA)–Act1 knock-in mice were generated by knocking a LoxP-stop-LoxP-Traf3ip2 cDNA-HA-poly(A) cassette into the endogenous Act1 locus; LSL–HA–Act1f/f knock-in mice were bred with UBC-Cre-ERT2 mice to generate UBC-Cre-ERT2/LSL-HA-Act1f/+ mice that express HA-tagged Act1 (53). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Cleveland Clinic and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication No. 8023, revised 1978).
House dust mite–CFA type 17 asthma model
The method was described in our previously published work (14). Eight-week-old female WT C57BL/6 mice were sensitized s.c. with house dust mites (HDMs; 100 μg/mouse; Dermatophagoides farina; Greer Laboratories) in CFA on day 0 and subsequently challenged (intranasally) with HDMs (100 μg/mouse) on day 14. DEX (0.3 mg/kg per mouse, i.p.) and SBE WT or SBE mutant (SBE-mut) aptamer (5 nmol/mouse, intranasally, mixed with 5 μl of TransIT-TKO reagent [Mirus Bio] and PBS in a total volume of 40 μl) or IL-17A neutralizing mAb (100 μg i.p. per mouse; BioXCell) were administered to mice 1 h before HDM challenge. SBE WT and SBE-mut aptamer were ordered from Integrated DNA Technologies as described previously (24). Bronchoalveolar lavage (BAL) cell counting and tissue collection were performed 24 h after the last HDM challenge.
Differential cell counting and histology staining
BAL cell counts were determined using a cytospin slide preparation after a Diff-Quik Giemsa stain. For histological analysis, mouse lung tissue was fixed in 10% buffered formalin and then subjected to H&E staining.
RNA electrophoretic mobility shift assay
The methods for probe preparation, RNA electrophoretic mobility shift assay (REMSA), and aptamer competition were developed and described previously (24). In brief, fragments containing the 3′-UTR sequences of mouse Cebpb mRNA (Cebpb240: nt 143–282; Cebpb180: nt 203–382; Cebpb90: nt 293–382) were generated by PCR and cloned into the pGEM-3ZF(+) vector (Promega) through the EcoRI and BamHI sites. Radiolabeled probes were in vitro transcribed using T7 RNA polymerase (Promega) and 1 mM GTP, 1 mM ATP, 1 mM CTP, 0.005 mM UTP, and 25 μCi of [32P]-labeled UTP. Labeled probes (10 fmol) were incubated with increasing amounts of purified protein. The reaction conditions were as previously described (24). For competition reactions, REMSA was performed with increasing concentrations of SBE (Cxcl1-SBE and SBE mutant) aptamers (24). Complexes were resolved on either 4 or 6% nondenaturing polyacrylamide gels. The gels were dried, and the appearance of complexes was visualized by exposure to BioMax MR film. The expression and purification of Act1-SEFIR and Act1-SEFIR mutant (K407/524/526/527A) were performed as previously described (24).
ELISA
Human IL-17A (DY317; R&D Systems), LCN2 (Hu9225; Biotang), SAA1 (ab100635; Abcam), CXCL1 (DY275; R&D Systems), and C-reactive protein (CRP; KHA0031; Invitrogen) levels were measured by using commercial ELISA kits according to the manufacturer’s instructions. Mouse IL-17A, LCN2, SAA3, and CXCL1 levels were measured by using commercial ELISA kits (ab199081 [Abcam], EMLCN2 [Invitrogen], EZMSAA3-12K [EMD Millipore], and DY453 [R&D Systems]) according to the manufacturer’s instructions.
Western blotting
Cells were washed with ice-cold PBS three times and lysed in lysis buffer (1% Triton X-100, 50 mM Tris–HCl [pH 7.4], 150 mM NaCl, 12.5 mM β-glycerophosphate, 1.5 mM MgCl2, 10 mM NaF, 2 mM DTT, 2 mM sodium orthovanadate, 2 mM EGTA, and Protease Inhibitor Cocktail [Roche]). Cell extracts were centrifuged at 12,000 rpm for 10 min at 4°C. Protein concentration was normalized with Bio-Rad Protein Assay Kit. The following Abs were used for Western blots: anti-C/EBPβ (sc-7962; Santa Cruz Biotechnology), anti–β-actin (sc-8432; Santa Cruz Biotechnology), and goat anti-mouse IgG-HRP (7076, polyclonal; Cell Signaling Technology) were used as secondary Abs.
mRNA decay assay
A549 or mASMCs were treated with 5 μg/ml actinomycin D (ActD) in the presence or absence of IL-17A. Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer's instructions, then subjected to real-time PCR (RT-PCR). The values were normalized to those of GAPDH mRNA.
Quantitative RT-PCR
Total RNA was isolated with TRIzol reagent (Invitrogen). The cDNA was synthesized with oligo dT (Applied Biosystems) and M-MLV reverse transcriptase (Invitrogen). RT-PCR was performed with a SYBR Green PCR Master Mix kit (Applied Biosystems). The RT-PCR primers are listed in Supplemental Table I.
RNA immunoprecipitation
The ability of Act1 to bind to RNA in vivo was assessed as described previously (24). In brief, 10 × 106 mASMCs isolated from UBC-Cre-ERT2/LSL-HA-Act1f/+ mice were left untreated or treated with IL-17A (50 ng/ml) for 3 h. Cells were fixed in 0.1% formaldehyde for 15 min at room temperature, whereupon the cross-linking reaction was stopped with glycine (pH 7; 0.25 M). The cells were harvested in 2 ml RIPA buffer and sonicated. After centrifugation, the supernatant was immunoprecipitated overnight at 4°C with Dynabeads (Invitrogen) preincubated with 20 μg anti-M2 (D6W5B; Cell Signaling Technology) or anti-IgG Ab. The beads were washed five times with 1 ml RIPA buffer and resuspended in 150 μl RNA immunoprecipitation (RIP) elution buffer. Cross-linking was reversed by incubation at 70°C for 45 min. RNA was purified from immunoprecipitants with TRIzol (Invitrogen). The cDNAs were synthesized, and 10% of the reverse transcriptase product was subjected to quantitative RT-PCR (qRT-PCR). Primers used for qRT-PCR are forward: 5′-TCGAACCCGCGGACTGCAAG-3′, reverse: 5′-CGACGACGACGTGGACAGGC-3′.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) Assay Kit (Cat# 17-295; Millipore) was used to examine the binding of the GC receptor (GR; encoded by NR3C1), C/EBPB, and p65 (RELA) to mouse Lcn2, Saa3, and Cebpb promoters on DEX and IL-17A treatment. In brief, mASMCs were grown to 100% confluence and treated with DEX (1 µM) and/or IL-17A (100 ng/ml) for 4 h. Cells were fixed with formaldehyde at a final concentration of 1% for 10 min at 37°C. Cells were then harvested in SDS lysis buffer and sonicated to shear DNA to length 100–200 bp. The sonicated cell supernatant was then diluted in ChIP dilution buffer and immunoprecipitated with anti-GR Ab (Clone D6H2L; Cell Signaling Technology), anti-C/EBPB (sc-7962; Santa Cruz), anti-p65 (sc-8008; Santa Cruz), or IgG. DNA was eluted and purified, followed by PCR amplification with primers spanning the putative RELA, NR3C1, or CEBPB binding sites of Lcn2, Saa3, and Cebpb promoters. The RT-PCR primers used are listed in Supplemental Table I.
Statistics
Unless otherwise specified, statistical analysis was performed using Student t test between two groups; one-way ANOVA (parametric) followed by Tukey’s multiple-comparisons test or Kruskal–Wallis test (nonparametric) followed by Dunn’s multiple-comparisons test. All bar graphs show mean and SEM, as indicated in each figure legend. Pearson correlation test was performed for correlation analysis. χ2/Fisher’s exact test was used to compare qualitative variables between groups. All tests and calculations were performed using GraphPad Prism (version 9).
Results
Plasma LCN2 and SAA1 levels correlate with IL-17A levels and resist high-dose steroids in patients with asthma
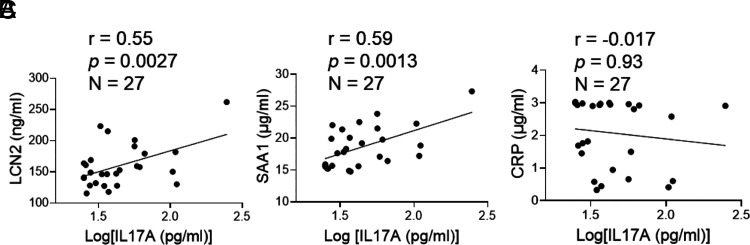
LCN2 and SAA1 are IL-17A target genes and are considered circulating biomarkers for a variety of inflammatory disorders (43, 44, 46, 47). In this study, we measured IL-17A, LCN2, and SAA1 in plasma samples of patients with asthma (n = 27) and healthy control subjects (n = 13) (Table I). Patients with asthma are further categorized as no/low-dose and high-dose groups based on steroid usage. There are no significant differences in demographics (age, gender, race, and ethnicity) between control and asthma groups or between no/low-dose and high-dose groups. In the asthma group, plasma IL-17A (p = 0.019) and SAA1 (p = 0003) concentrations are considerably greater than in the control group. Although high-dose steroids inhibit Th2 indicators (e.g., blood eosinophils [p = 0.016], IgE [p = 0.02], and percentage of fractional exhaled nitric oxide (FENO) [p = 0.09]), plasma IL-17A, LCN2, and SAA1 are unaffected. Blood LCN2 and SAA1 levels are positively correlated with IL-17A levels in all patients with asthma (Fig. 1A–C); however, the amount of plasma CRP, a nonspecific biomarker for inflammation, is not. It should be noted that the high-dose steroid group has higher sputum neutrophils and IgE than the low-dose group (45 ± 7 versus16.7 ± 8, p < 0.038; 2.5 ± 0.1 versus 2.0 ± 0.1, p < 0.02, respectively), indicating the adverse effect of high-dose steroid on asthma. The earlier evidence implicates that LCN2 and SAA1 are worth further investigation to see whether they could serve as specific molecular markers for Th17/IL-17–mediated (type 17) asthma in humans.
Table I.
Demographic data of asthmatic and control participants
| Asthma Based on Steroid Dose | ||||||
|---|---|---|---|---|---|---|
| Control (n = 13) | Asthma (n = 27) | p Value | No/Low Dose (n = 8) | High Dose (n = 19) | p Value | |
| Demographics | ||||||
| Age, y | 38.3 (3.3) | 45.3 (2.8) | 0.11 | 38.0 (5.9) | 48 (2.8) | 0.08 |
| Gender, female/male (n) | 9/4 | 21/6 | 0.55 | 7/1 | 14/5 | 0.43 |
| Race, African/others/White | 2/0/11 | 10/2/15 | 0.23 | 3/0/5 | 7/2/10 | 0.81 |
| Ethnicity, Hispanic/no Hispanic | 0/13 | 2/25 | 0.31 | 0/8 | 2/17 | 0.19 |
| Severity | 63 (17) | 0 (0) | 89 (17) | 0.0001 | ||
| Lung functions | ||||||
| FEV1, % | 73 (4) | 87 (7) | 69 (4) | 0.022 | ||
| FEV1/FVC, % | 82 (3) | 88 (6) | 80 (3) | 0.263 | ||
| Asthma medication | ||||||
| High-dose inhaled steroids, % (n) | 60 (16) | 0 (0) | 84 (16) | 0.0001 | ||
| Systemic steroids, % (n) | 15 (4) | 0 (0) | 21 (4) | 0.159 | ||
| Total high-dose steroids, % (n) | 70 (19) | 0 (0) | 84 (16) | 0.0001 | ||
| Th2-related markers | ||||||
| Blood eosinophils, % | 2.8 (0.3) | 4.0 (0.5) | 2.4 (0.3) | 0.016 | ||
| FENO, ppb | 29 (3.8) | 41 (12) | 26 (3.2) | 0.09 | ||
| IgE, log kU/l | 2.3 (0.1) | 2.0 (0.1) | 2.5 (0.1) | 0.02 | ||
| Th17-related markers | ||||||
| IL-17A, pg/ml | 30.4 (3.1) | 53.5 (8.9) | 0.019 | 45.0 (10.2) | 57.2 (12.0) | 0.45 |
| LCN2, ng/ml | 152 (7) | 160 (7) | 0.418 | 147 (8) | 166 (10) | 0.14 |
| SAA1, ng/ml | 15.6 (0.4) | 18.6 (0.7) | 0.0003 | 17.4 (0.8) | 19.1 (0.8) | 0.14 |
| Blood neutrophils, % | 59 (2) | 58 (3) | 59 (2) | 0.82 | ||
| Sputum neutrophils, % | 41 (7) | 16.7 (8) | 45 (7) | 0.038 | ||
| CRP, µg/ml | 1.18 (0.2) | 2.07 (0.2) | 0.009 | 2.1 (0.4) | 2.1 (0.3) | 0.89 |
Data are represented as mean (SEM). FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
FIGURE 1.
Plasma LCN2 and SAA1 levels correlate with IL-17A in patients with asthma. The correlations between plasma LCN2, SAA1, CRP, and IL-17A (A–C) were performed using the Pearson correlation test.
LCN2 and SAAs are CS-resistant IL-17A target genes in vitro and in vivo
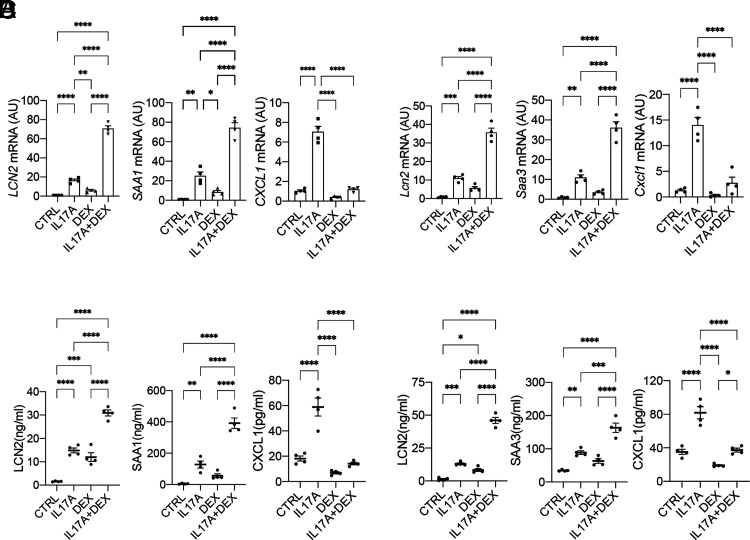
In a previous study, we identified a group of IL-17A target genes that are resistant to DEX (a synthetic GC drug) using RNA sequencing in hASMCs treated with sham placebo control, IL-17A, DEX, or in combination (GSE135730; https://www.ncbi.nlm.nih.gov/geo/) (14). In this study, in independent experiments, we validated LCN2 and SAAs (human SAA1 and mouse ortholog SAA3) as IL-17A target genes that are synergistically induced by DEX using RT-PCR and ELISA in hASMCs and mASMCs (Fig. 2A–D). This is in contrast with the suppression of CXCL1, a well-known IL-17A–induced chemokine. We observed a similar induction pattern in A549 cells (a human lung epithelial cell line) (Supplemental Fig. 1A, 1B), suggesting that LCN2 and SAAs can be induced by IL-17A/DEX in multiple cell types found in the airway.
FIGURE 2.
LCN2 and SAA are steroid-resistant IL-17A target genes. (A and B) hASMCs were untreated (CTRL) or treated for 24 h with human IL-17A (100 ng/ml), DEX (1 μM), or both. (C and D) mASMCs were untreated (CTRL) or treated with IL-17A (100 ng/ml), DEX (1 μM), or IL-17A+DEX for 24 h. The mRNA and protein levels were then analyzed by RT-PCR (A and C) and ELISA (B and D), respectively (n = 4 independent plates of cells). Data represent mean ± SEM. One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
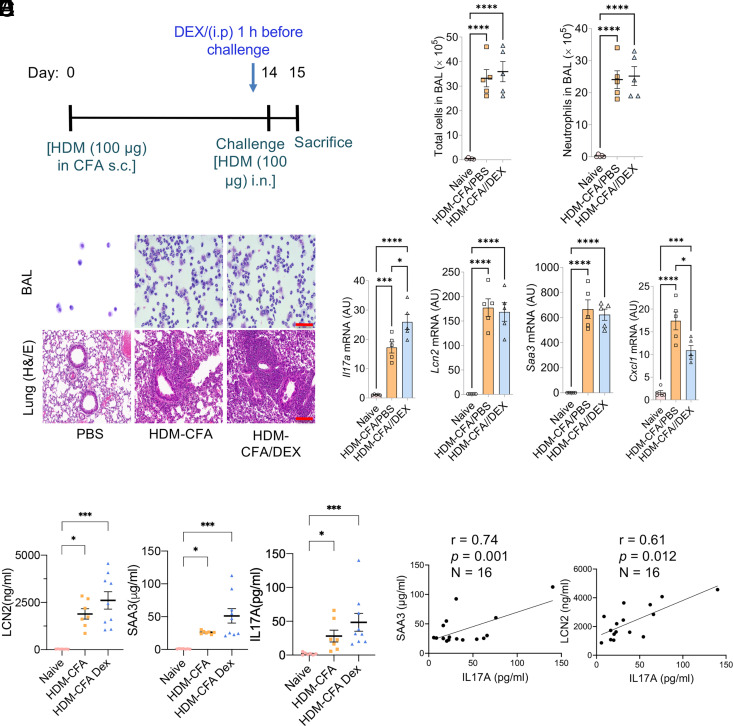
We then used a type 17 murine asthma model (induced by HDM-CFA) that recapitulates human neutrophilic asthma to assess IL-17A downstream targets and CS effects (Fig. 3A–D). Mice on this model exhibit prevalent neutrophilic inflammation (>80% neutrophils in BAL), high levels of IL-17A, and neutrophil-promoting genes (Csf3, Cxcl1, and Cxcl2), and the pathological features of the lung are resistant to DEX treatment (14). We also found that expressions of Lcn2 and Saa3 are highly upregulated and resistant to DEX treatment at mRNA and protein levels in lung tissue and plasma (Fig. 3D, 3E). In contrast, Cxcl1, a steroid-sensitive IL-17A target, is upregulated in the type 17 model but is suppressed by DEX treatment. The plasma LCN2 and SAA3 levels are highly correlated to IL-17A levels, similar to our findings in patients with asthma (Fig. 3F).
FIGURE 3.
The expressions of Lcn2 and Saa3 were resistant to steroid treatment in the preclinical type 17 severe asthma model. (A–C) Eight-week-old female WT C57BL/6 female mice were subjected to the HDM-CFA acute asthma model. PBS or DEX was administered to the mice (as described in Materials and Methods). Twenty-four hours after the challenge, total cell and neutrophil counts in the BAL were quantified (B), representative BAL cells were prepared by cytospin, and lung tissues were subjected to histochemical staining as indicated (C). All scale bars (red), 100 μm. (D) mRNA expression of lung tissues was quantified by RT-PCR. (E) Plasma LCN2, SAA3, and IL-17A levels were measured using ELISA. One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test or Kruskal–Wallis test (nonparametric) followed by Dunn’s multiple-comparisons test. (F) The correlation between plasma LCN2 or SAA3 and IL-17A was performed using the Pearson correlation test. For (B), (D) and (E), *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. AU, fold induction relative to unchallenged control mice.
IL-17A/DEX synergistically induce the expression of Lcn2 and Saa3 through upregulating CEBPB
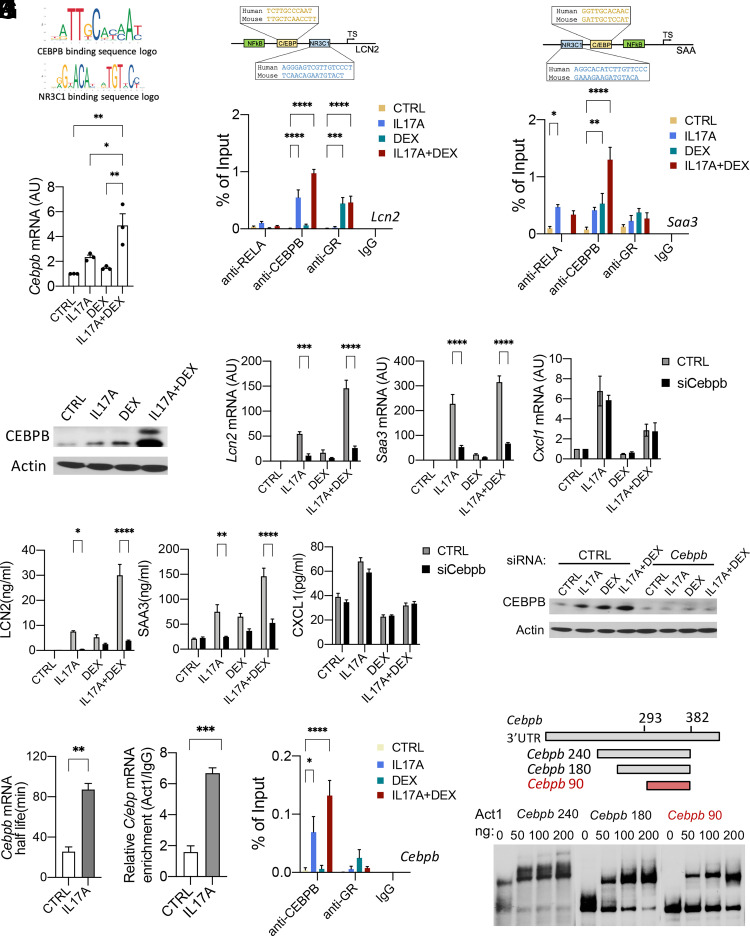
To investigate the mechanisms of the synergistic induction of Lcn2 and Saa3 by IL-17A and DEX, we used primary mASMCs, which are highly responsive to IL-17A and DEX. IL-17A induces gene expression through the activation of transcription factors (e.g., NF-κB, C/EBPs, IκBζ) and posttranscriptional stabilization of mRNA. Although IL-17A signaling is known to regulate mRNA stability of a set of inflammatory genes (e.g., Cxcl1), it has little impact on the transcripts of Lcn2 and Saa3 because both transcripts are very stable (25, 54) (Supplemental Fig. 1C, 1D). Thus, we focused on studying the transcriptional control of Lcn2 and Saa3. Using JASPAR (an open-access database of curated, nonredundant transcription factor binding profiles; http://jaspar.genereg.net) (55), we identified putative binding sites for multiple transcription factors (including NF-κB [RELA], GR [NR3C1], and CEBPB) in the proximal promoter regions of mouse Lcn2 and Saa3 (Fig. 4A–C). These sites are highly conserved between human and mouse orthologs. The direct interaction between the promoter regions of Lcn2 and Saa3 and transcription factors (RELA, NR3C1, and CEBPB) was confirmed by ChIP-qPCR analysis in mASMCs (Fig. 4B, 4C). IL-17A induced the binding of both RELA and CEBPB to Lcn2 and Saa3 promoters. While DEX suppressed RELA binding, it enhanced the binding of CEBPB and NR3C1 to the promoter regions of Lcn2 and Saa3. Interestingly, we observed synergistic induction of CEBPB binding to Lcn2 and Saa3 promoters when mASMCs were costimulated with DEX and IL-17A, suggesting this is a key mechanism responsible for the synergistic induction of Lcn2 and Saa3 by IL-17A and DEX.
FIGURE 4.
IL-17A/DEX synergistically induces the expression of Lcn2 and Saa3 through upregulating CEBPB. (A–C) Schematic diagrams showing the putative binding sites for multiple transcription factors (including NF-κB, NR3C1, and C/EBP) in the mouse and human LCN2 and SAA promoters. (B and C) ChIP-qPCR of RELA, CEBPB, and NR3C1 occupancy at the promoter of mouse Lcn2 and Saa3. Nucleic extracts from untreated (CTRL) and treated mASMCs were immunoprecipitated with IgG, anti-RELA, anti-CEBPB, or anti-GR, followed by DNA purification and RT-PCR quantitation with primers spanning the putative RELA, CEBPB, or NR3C1 binding sites of the Saa3 and Lcn2 promoter. (D and E) mASMCs were untreated (CTRL) or treated with IL-17A (100 ng/ml), DEX (1 μM), or IL-17A+DEX for 24 h. mRNA expression of Cebpb was measured by RT-PCR analysis (D). Protein levels were examined by Western blotting (E). (F and G) mASMCs were transfected with pooled small interfering RNAs (siRNAs) targeting CEBPB or scrambled control and treated as indicated for 24 h. The mRNA and protein levels were then analyzed by RT-PCR (F) and ELISA (G), respectively. (H) Western blot analysis of CEBPB in mASMCs transfected with pooled siRNAs targeting Cebpb or scrambled control and treated as indicated for 24 h. (I) mASMCs were pretreated with DEX for 4 h and then treated with ActD (5 μM) either alone (CTRL) or in the presence of IL-17A (100 ng/ml) for 25, 50, 70, and 100 min, then subjected to RT-PCR analysis (n = 3 independent plates of cells). The indicated mRNA levels were normalized to those of Gapdh and are presented as half-life. Data represent mean ± SEM (n = 3 biological replicates). (J) Primary mASMCs isolated from UBC-Cre-ERT2/LSL-HA-Act1f/+ mice were treated with IL-17A or sham control (CTRL) for 3 h and subjected to RIP with IgG or anti-HA. Cebpb mRNA was analyzed by qRT-PCR. Relative values normalized against IgG control are shown. (K) ChIP analysis of IL-17A/DEX–induced binding of CEBPB and NR3C1 to Cebpb promoter as described in (B) and (C). (L) REMSA for the binding of purified recombinant Act1 SEFIR to the serial-deletion mutants of Cebpb240 (142–382 nt). For (D), data represent mean ± SEM. One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. For (B), (C), and (K), data represent mean ± SEM. Two-way ANOVA was performed to compare each treated group with the control group, followed by Dunnett's multiple-comparisons test. For (F) and (G), statistical analysis was performed using two-tailed Student t test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. For (I) and (J), data represent mean ± SEM. Statistical analysis was performed using a two-tailed Student’s t test. *p < 0.05, **p < 0.01, ***p < 0.001. All data are representative of three independent experiments. AU, fold induction relative to unchallenged control mice; TS, transcription start site.
We next investigated the role of Cebpb in the synergistic induction of Lcn2 and Saa3 by IL-17A and DEX. We found that CEBPB itself was a CS-resistant IL-17A target gene from our previous RNA sequencing study using human ASMCs (14). We confirmed that IL-17A/DEX could synergistically induce Cebpb at both RNA and protein levels in mASMCs (Fig. 4D, 4E). In addition, Cebpb knockdown abolished synergistic induction of Lcn2 and Saa3 by IL-17A/DEX (Fig. 4F–H), implicating that Cebpb is the critical transcription factor that is responsible for the synergy between IL-17A and DEX on the expression of Lcn2 and Saa3. In contrast, knockdown of Cebpb mRNA did not abolish the induction of Cxcl1 by IL-17A or the suppression of Cxcl1 by DEX, implying Cebpb is not the critical transcription factor for Cxcl1 in response to IL-17A and DEX. We also performed CEBPB knockdown in hASMCs and human airway epithelial cell line A549 to examine the importance of CEBPB in the synergistic induction of LCN2 and SAA1 by IL-17A and DEX (Supplemental Fig. 2A–C). Likewise, we found that CEBPB mRNA knockdown abolished the induction of LCN2 and SAA1 by IL-17A/DEX, while having no significant effect on the production of CXCL1 in human cells.
The promoter of CEBPB contains binding sites for C/EBP and NR3C1 (Fig. 4F), suggesting that CEBPB may cooperate with NR3C1 on its own promoter to amplify itself. Using ChIP-qPCR analysis in mASMCs, we found that IL-17A and DEX induced CEBPB binding to its own promoter in a synergistic manner (Fig. 4F). These results indicate that IL-17A and DEX cooperatively upregulate CEBPB expression at the transcriptional level through induction of CEBPB binding to its own promoter.
Act1 stabilizes Cebpb mRNA through direct binding to the 3′-UTR of Cebpb transcript
In addition to transcriptional regulation, we investigated the stabilization mechanisms of synergistic induction of Cebpb by IL-17A/DEX. IL-17A stabilizes mRNA posttranscriptionally (24, 26, 56–58). We performed a Cebpb mRNA decay assay using ActD (a transcription inhibitor) followed by DEX treatment in mASMCs (Fig. 4G). Cebpb mRNA decayed at a slower rate in the presence of IL-17A as compared with that in the absence of IL-17A, indicating that IL-17A signaling enhanced the stability of DEX-induced Cebpb transcript. We previously showed that Act1, the adapter molecule of IL-17R, is an RNA binding protein that can directly bind to 3′-UTR of the select transcripts to promote mRNA stabilization (24). To determine whether Cebpb mRNA is a new binding target of Act1, we stimulated primary mASMCs isolated from UBC-Cre-ERT2/LSL-HA-Act1f/+ mice (expressing HA-tagged Act1) with or without IL-17A, followed by RIP with anti-HA Ab. As shown in (Fig. 4H, Cebpb mRNA was selectively retained by anti-HA Ab, but not isotype control IgG; in addition, the association of Cebpb transcript with Act1 was increased after IL-17A stimulation, indicating Cebpb mRNA is an Act1 binding target. The interaction between Act1 protein and the 3′-UTR of Cebpb mRNA was also confirmed by REMSA using purified Act1–WT protein (Fig. 4I). We mapped the Act1 binding region of Cebpb mRNA 3′-UTR to nt 293–382, which is evolutionarily conserved and forms a secondary stem-loop structure (Supplemental Fig. 3A). As a specificity control, Act1 RNA binding mutant (that lost RNA binding activity) (24) failed to bind Cebpb mRNA 3′-UTR (Supplemental Fig. 3B). The binding region is adjacent to the reported AREs, which are one of the major cis-regulatory elements that mediate its mRNA destabilization (Supplemental Fig. 3C).
Blocking Act1 binding to Cebpb transcript reduces blood LCN2 and SAA3 in mice in the type 17 severe asthma model
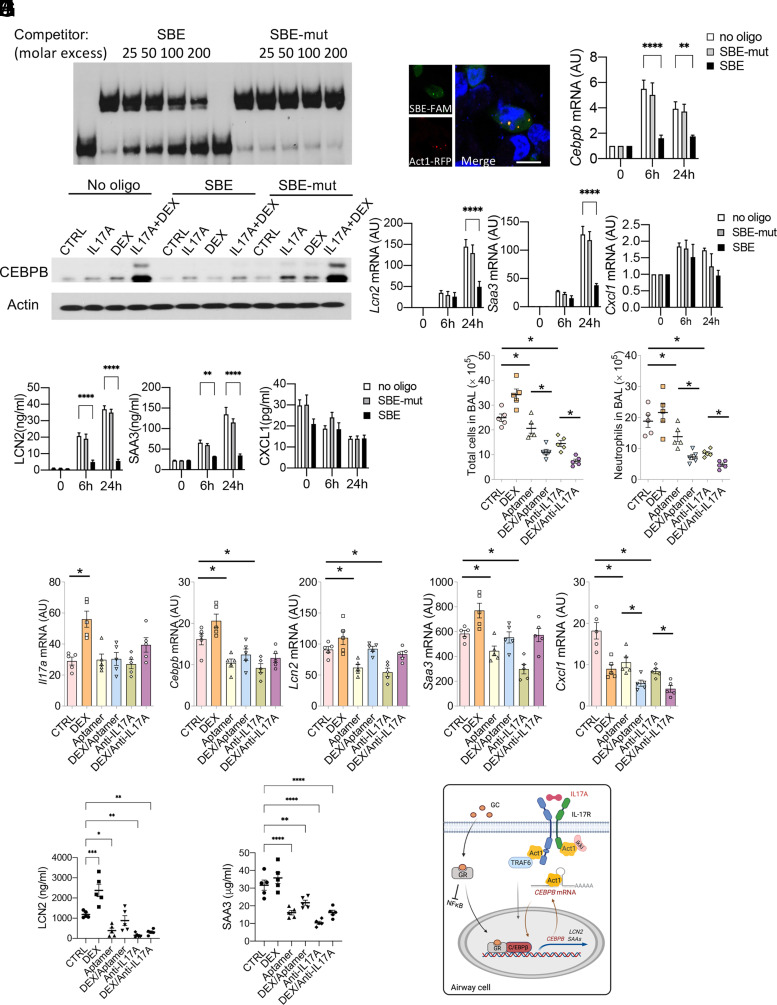
We developed an SBE RNA aptamer derived from Act1’s SBE on the 3′-UTR of Cxcl1, which can disrupt the interaction between Act1 and its inflammatory target transcripts (24). The SBE on the 3′-UTR of Cebpb shares a similar secondary stem-loop structure to that on the 3′-UTR of Cxcl1. We found that this SBE aptamer (but not SBE-mut, which lost binding to Act1) can outcompete Act1 SEFIR's binding to the Cebpb 3′-UTR in REMSA (Fig. 5A). In addition, the transfected fluorescent SBE aptamers colocalized with Act1 in the cytoplasm of mASMCs (Fig. 5B). Moreover, SBE RNA aptamer (but not SBE-mut) inhibits IL-17A/DEX–induced Cebpb expression at both RNA and protein levels (Fig. 5C, 5D), resulting in reduced expression of Lcn2 and Saa3 in mASMCs, while having no effect on the production of Cxcl1 in mASMCs (Fig. 5E, 5F).
FIGURE 5.
Blocking Act1 binding to Cebpb transcript reduces expression of Lcn2 and Saa3 in mice on type 17 severe asthma model. (A) SBE RNA aptamers (Cxcl1-SBE and SBE mutant) were used to compete with Cebpb probe (203–382 nt) for binding to Act1 SEFIR. (B) Confocal imaging of RFP-Act1 and fluorescein amidite–labeled SBE aptamer (SBE-FAM) in transfected mouse ASMCs. Blue, DAPI nuclear staining. Scale bars, 20 μm. (C–F) mASMCs transfected with 100 pmol SBE RNA aptamers or no oligonucleotide control were subjected to stimulation with DEX+IL-17A for 0, 6, or 24 h. mRNA expression of Cebpb (C) was then analyzed by RT-PCR. Protein levels were measured by Western blot (D). The mRNA and protein levels of Lcn2 and Saa3 were analyzed by RT-PCR (E) and ELISA (F). (G–I) Eight-week-old WT C57BL/6 female mice were sensitized and challenged with HDM, then subjected to treatment as indicated (n = 5 mice/group). Total and neutrophil cell numbers in the BAL of the indicated mice (n = 5 mice/group) were quantified (G). mRNA expression of lung tissues was quantified by RT-PCR (H). The levels of IL-17A, SAA3, and LCN2 in the serum were measured using ELISA (I). For (C), (E), and (F), data represent mean ± SEM. Two-way ANOVA was performed to compare each treated group with no oligo group, followed by Dunnett's multiple-comparisons test. For (G)–(I), data represent mean ± SEM. One-way ANOVA was performed, followed by Tukey’s multiple-comparisons test. All data are representative of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. (J) Model of IL-17A and glucocorticoid signaling for the induction of steroid-resistant IL-17A target genes (CEBPB, LCN2, and SAA). On stimulation with glucocorticoid, IL-17A–induced NF-κB was inhibited, and instead GR cooperates with CEBPB to amplify the expression of SR genes. In addition, IL-17A induces Act1 binding and stabilization of CEBPB mRNA to enhance gene expression.
Previously, we showed intranasal administration of SBE RNA aptamer enabled efficient tissue penetration (24). We hence examined the efficacy of this RNA aptamer for inhibiting IL-17A/DEX–induced CEBPB and its targets (LCN2 and SAA3) in physiological conditions. We treated mice with SBE aptamer, DEX, anti–IL-17A neutralizing Ab, or in combination in the HDM-CFA type 17 severe asthma model. Administration of SBE aptamer to mice suppressed the expression of Cebpb, Lcn2, and Saa3 in the lung tissues and inhibited blood LCN2 and SAA3 levels regardless of DEX treatment. Furthermore, SBE aptamer decreased neutrophilic inflammation in the airway (as measured by BAL cell counts) and enhanced DEX sensitivity to the same extent that anti–IL-17A neutralizing Ab did (Fig. 5G–I). Taken together, these findings suggest that the IL-17A–Act1–CEBPB axis is responsible for CS-resistant IL-17A targets that promote the type 17 severe asthma, opening the possibility of targeting Act1 binding to CEBPB mRNA in future therapeutic approaches (Fig. 5J).
Discussion
There are only limited human studies that demonstrate the beneficial effects of Th17/IL-17 blockage on asthma, even though genetic and preclinical mouse studies have provided strong evidence that IL-17A signaling is a targetable mechanism for the treatment of asthma (59). This is partly due to the lack of molecular biomarkers for patient classification and identification of better responders to IL-17A target treatments. In a randomized, double-blind, placebo-controlled phase II study, the administration of brodalumab (a human mAb that binds to the IL-17RA) did not show any treatment effects in the overall study population (composed of inadequately controlled moderate-to-severe asthmatics); however, a positive response was seen in patients with high bronchodilator reversibility in one dose group (19). In a case study, a patient with persistent asthma and psoriasis showed improved asthma-associated inflammation and was no longer dependent on asthma maintenance medications after treatment with ustekinumab, a human mAb that inhibits Th1 and Th17 by binding the p40 subunit of IL-12 and IL-23 (20). In this study, we found that IL-17A induces the production of two secreted proteins, LCN2 and SAA1, in airway cells, and that this induction is greatly enhanced when CSs are present. Blood IL-17A levels and LCN2 or SAA levels have a strong positive correlation in preclinical and human research. Furthermore, CS does not reduce plasma LCN2 or SAA levels. Th2 indicators, including blood eosinophils and FENO, in contrast, are reduced by CS. As a result, LCN2 and SAAs, which are CS-resistant IL-17A targets, could be useful molecular indicators for endotyping type 17 asthma for therapies targeting IL-17A.
Mechanistically, IL-17A and GC collaboratively regulate transcription and mRNA stability of CEBPB, the crucial transcription factor that is responsible for the synergistic induction of LCN2 and SAA by IL-17A and GC. Act1, the adaptor and RNA binding protein in IL-17A signaling, directly binds CEBPB mRNA and inhibits its degradation. Both IL-17A and GC induce CEBPB protein modifications, alter its DNA-binding ability, and thus regulate expression of their target genes (30, 31). LCN2 and SAA transcripts are very stable, and CEBPB-dependent transcriptional regulation is pivotal to their cumulative expression. In contrast, CEBPB mRNA is not stable and requires IL-17A–mediated posttranscriptional stabilization that involves direct binding of CEBPB mRNA by Act1. Overall, these findings identify the IL-17A/Act1-CEBPB axis as a critical mechanism in the synergistic induction of proinflammatory targets LCN2 and SAAs.
LCN2 and SAAs have been reported to play important roles in Th17/IL-17A–mediated inflammation (42, 48, 49). LCN2 administered i.p. increases Th17 cytokines/chemokines (e.g., IL-17A, IL-22, CCL20, CXCL1) expression and exacerbates Th17-mediated skin inflammation (42). The SAAs have been found to promote a distinct pathogenic Th17 cell polarization (48) and boost the expression of IL-17A in both CD4 T cells and γδ T cells in a chronic mouse model of SAA exposure (49), presumably giving positive feedback on the Th17/IL-17A axis. Through feed-forward processes, the synergistic production of LCN2 and SAAs by IL-17A and DEX may further enhance type 17 airway inflammation.
The expression and biological functions of LCN2 and SAAs in asthma have been described. LCN2 level is lower in nasal secretions of patients with asthma than that in healthy controls (60). In contrast, LCN2 expression of PBMCs is higher in neutrophil-predominant asthma as compared with that in other asthma phenotypes (61). LCN2 has been found to serve a protective role in a type 2 allergic asthma model because of its proapoptotic action on lung inflammatory cells (62). SAA1 acts as a soluble pattern recognition receptor for conserved fatty acid–binding protein present in common mite allergens that activate type 2 immunity at mucosal surfaces, prompting the epithelial release of the type 2–promoting cytokine IL-33 (63). However, the specific role of LCN2 and SAAs in type 17 asthma needs to be further explored by using genetically defective animals and Th17/IL-17A–mediated neutrophilic asthma patients. Interestingly, a recent study implies that the SAA1 level is a possible biomarker for neutrophilic airway inflammation in adult patients with asthma, which is congruent with our findings (64).
The Cxcl1 mRNA-derived SBE RNA aptamer used in this study was previously shown to suppress the expression of several inflammatory genes (including Cxcl1) and reduce neutrophil infiltration in the type 17 murine asthma model (24). Although DEX treatment can effectively inhibit neutrophil-mobilizing chemokine Cxcl1 expression, it fails to inhibit neutrophilic airway inflammation, suggesting that there must be other CS-resistance mechanisms operating in type 17 asthma. In this study, our findings point to the IL-17A/DEX–induced transcription factor CEBPB as a mechanism for the observed CS resistance. In support of this, destabilization of Cebpb mRNA by SBE aptamer inhibition is sufficient to reduce the expression of its downstream targets (LCN2 and SAAs) in both in vitro cell culture and in vivo mouse studies. Importantly, administration of SBE aptamer resulted in reduced neutrophilic inflammation in the airway and rendered DEX sensitivity in mice on the type 17 asthma model, which parallels findings of anti–IL-17A neutralizing Ab in clinical trials (65). This opens the possibility for targeting Act1 binding to the CEBPB transcript as a potential new therapy for type 17 asthma.
Despite lack of clinical studies on IL-17A signaling inhibition in type 17 CS-resistant neutrophilic asthma, Abs targeting IL-17A or IL-17R (secukinumab, ixekizumab, brodalumab) have been proven to be very effective for the treatment of IL-17A–dependent inflammatory diseases, such as psoriasis, psoriatic arthritis, and ankylosing spondylitis (66–70). However, increasing evidence reveals that systemic inhibition with IL-17A/IL-17R Abs may impair host defense and lead to adverse effects. RNA aptamers offer several advantages over Abs (e.g., small size for better tissue penetration, low production cost, easy chemical modification, and lack of immunogenicity) and are emerging as a promising new class of drugs (70). The SBE RNA aptamer used in this study may offer advantages over IL-17A or IL-17RA neutralizing Abs (which target global IL-17A signaling) because the aptamer selectively inhibits the IL-17A–mediated mRNA stability pathway and attenuates the expression of only a subset of IL-17A target genes (e.g., LCN2, SAAs, CEBPB), thus minimizing side effects associated with anti–IL-17A/IL-17R biologics. Although the direct functions of IL-17A, SAAs, LCN2, and CEBPB in type 17 CS-resistant neutrophilic asthma remain to be further explored, our studies provide potential background evidence for alternate therapeutics not currently in use for type 17 inflammatory diseases, including CS-resistant neutrophilic asthma.
In the human study, there is no difference in plasma levels of IL-17A, LCN2, and SAA1 between asthmatics on low and high doses, indicating these proteins are resistant to steroid treatment. However, we did not observe that LCN2 level is enhanced in asthma patients as we saw in type 17 asthmatic mice. This may be because of the limited sample size and heterogeneity of human patients. The failure to observe the elevated LCN2 and SAAs on steroid treatment in patients and mice demonstrates the more complex regulatory mechanisms in in vivo settings. Nonetheless, our results warrant further investigation to see whether LCN2 and SAAs can be used as molecular markers for defining type 17 asthma, given their steroid-resistance character and association with IL-17A levels. Future clinical studies with a larger cohort of patients will elucidate the relationship between these CS genes and type 17 CS-resistant neutrophilic asthma.
Supplementary Material
Acknowledgments
For the human study, we acknowledge the Lerner Research Institute Human Biorepository Core; in particular, we appreciate Marybeth Boyle’s assistance with the human samples. For animal studies, we thank the staff of the Cleveland Clinic animal facility. We also thank Allison Janocha and Vanessa Salazar for reviewing the manuscript.
This work was supported by the National Heart, Lung, and Blood Institute, National Institutes of Health Grants HL103453 and HL144497.
The online version of this article contains supplemental material.
- ActD
- actinomycin D
- ARE
- AU-rich element
- ASMC
- airway smooth muscle cell
- BAL
- bronchoalveolar lavage
- ChIP
- chromatin immunoprecipitation
- CRP
- C-reactive protein
- CS
- corticosteroid
- DEX
- dexamethasone
- FENO
- fractional exhaled nitric oxide
- GC
- glucocorticoid
- GR
- glucocorticoid receptor
- HA
- hemagglutinin tag
- hASMC
- human primary airway smooth muscle cell
- HDM
- house dust mite
- LCN2
- lipocalin-2
- mASMC
- mouse airway smooth muscle cell
- qPCR
- quantitative PCR
- qRT-PCR
- quantitative real-time PCR
- REMSA
- RNA electrophoretic mobility shift assay
- RIP
- RNA immunoprecipitation
- RT-PCR
- real-time PCR
- SAA
- serum amyloid A
- SBE
- SEFIR binding element
- SBE-mut
- mutant SBE
- SEFIR
- SEF/IL-17R
- UTR
- untranslated region
- WT
- wild type
Disclosures
The authors have no financial conflicts of interest.
References
- 1. Al-Ramli W., Préfontaine D., Chouiali F., Martin J. G., Olivenstein R., Lemière C., Hamid Q.. 2009. T(H)17-associated cytokines (IL-17A and IL-17F) in severe asthma. J. Allergy Clin. Immunol. 123: 1185–1187. [DOI] [PubMed] [Google Scholar]
- 2. Bullens D. M., Truyen E., Coteur L., Dilissen E., Hellings P. W., Dupont L. J., Ceuppens J. L.. 2006. IL-17 mRNA in sputum of asthmatic patients: linking T cell driven inflammation and granulocytic influx? Respir. Res. 7: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ricciardolo F. L. M., Sorbello V., Folino A., Gallo F., Massaglia G. M., Favatà G., Conticello S., Vallese D., Gani F., Malerba M., et al. 2017. Identification of IL-17F/frequent exacerbator endotype in asthma. J. Allergy Clin. Immunol. 140: 395–406. [DOI] [PubMed] [Google Scholar]
- 4. Sorbello V., Ciprandi G., Di Stefano A., Massaglia G. M., Favatà G., Conticello S., Malerba M., Folkerts G., Profita M., Rolla G., Ricciardolo F. L.. 2015. Nasal IL-17F is related to bronchial IL-17F/neutrophilia and exacerbations in stable atopic severe asthma. Allergy 70: 236–240. [DOI] [PubMed] [Google Scholar]
- 5. Agache I., Ciobanu C., Agache C., Anghel M.. 2010. Increased serum IL-17 is an independent risk factor for severe asthma. Respir. Med. 104: 1131–1137. [DOI] [PubMed] [Google Scholar]
- 6. Zheng R., Wang F., Huang Y., Xiang Q., Dai H., Zhang W.. 2021. Elevated Th17 cell frequencies and Th17/Treg ratio are associated with airway hyperresponsiveness in asthmatic children. J. Asthma 58: 707–716. [DOI] [PubMed] [Google Scholar]
- 7. Bullone M., Carriero V., Bertolini F., Folino A., Mannelli A., Di Stefano A., Gnemmi I., Torchio R., Ricciardolo F. L. M.. 2019. Elevated serum IgE, oral corticosteroid dependence and IL-17/22 expression in highly neutrophilic asthma. Eur. Respir. J. 54: 1900068. [DOI] [PubMed] [Google Scholar]
- 8. Chesné J., Braza F., Chadeuf G., Mahay G., Cheminant M. A., Loy J., Brouard S., Sauzeau V., Loirand G., Magnan A.. 2015. Prime role of IL-17A in neutrophilia and airway smooth muscle contraction in a house dust mite–induced allergic asthma model. J. Allergy Clin. Immunol. 135: 1643–1645.e5. [DOI] [PubMed] [Google Scholar]
- 9. Kudo M., Melton A. C., Chen C., Engler M. B., Huang K. E., Ren X., Wang Y., Bernstein X., Li J. T., Atabai K., et al. 2012. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat. Med. 18: 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wilson R. H., Whitehead G. S., Nakano H., Free M. E., Kolls J. K., Cook D. N.. 2009. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am. J. Respir. Crit. Care Med. 180: 720–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liu C., Zhu L., Fukuda K., Ouyang S., Chen X., Wang C., Zhang C. J., Martin B., Gu C., Qin L., et al. 2017. The flavonoid cyanidin blocks binding of the cytokine interleukin-17A to the IL-17RA subunit to alleviate inflammation in vivo. Sci. Signal. 10: eaaf8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Swaidani S., Bulek K., Kang Z., Gulen M. F., Liu C., Yin W., Abbadi A., Aronica M., Li X.. 2011. T cell-derived Act1 is necessary for IL-25-mediated Th2 responses and allergic airway inflammation. J. Immunol. 187: 3155–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Swaidani S., Bulek K., Kang Z., Liu C., Lu Y., Yin W., Aronica M., Li X.. 2009. The critical role of epithelial-derived Act1 in IL-17- and IL-25-mediated pulmonary inflammation. J. Immunol. 182: 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ouyang S., Liu C., Xiao J., Chen X., Lui A. C., Li X.. 2020. Targeting IL-17A/glucocorticoid synergy to CSF3 expression in neutrophilic airway diseases. JCI Insight 5: e132836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chakir J., Shannon J., Molet S., Fukakusa M., Elias J., Laviolette M., Boulet L.-P., Hamid Q.. 2003. Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-β, IL-11, IL-17, and type I and type III collagen expression. J. Allergy Clin. Immunol. 111: 1293–1298. [DOI] [PubMed] [Google Scholar]
- 16. Molet S., Hamid Q., Davoine F., Nutku E., Taha R., Pagé N., Olivenstein R., Elias J., Chakir J.. 2001. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 108: 430–438. [DOI] [PubMed] [Google Scholar]
- 17. Du J., Han J. C., Zhang Y. J., Qi G. B., Li H. B., Zhang Y. J., Cai S.. 2016. Single-nucleotide polymorphisms of IL-17 gene are associated with asthma susceptibility in an Asian population. Med. Sci. Monit. 22: 780–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silva M. J., de Santana M. B. R., Tosta B. R., Espinheira R. P., Alcantara-Neves N. M., Barreto M. L., Figueiredo C. A., Costa R. D. S.. 2019. Variants in the IL17 pathway genes are associated with atopic asthma and atopy makers in a South American population. Allergy Asthma Clin. Immunol. 15: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Busse W. W., Holgate S., Kerwin E., Chon Y., Feng J., Lin J., Lin S. L.. 2013. Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am. J. Respir. Crit. Care Med. 188: 1294–1302. [DOI] [PubMed] [Google Scholar]
- 20. Amarnani A., Rosenthal K. S., Mercado J. M., Brodell R. T.. 2014. Concurrent treatment of chronic psoriasis and asthma with ustekinumab. J. Dermatolog. Treat. 25: 63–66. [DOI] [PubMed] [Google Scholar]
- 21. Toy D., Kugler D., Wolfson M., Vanden Bos T., Gurgel J., Derry J., Tocker J., Peschon J.. 2006. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 177: 36–39. [DOI] [PubMed] [Google Scholar]
- 22. Shen F., Gaffen S. L.. 2008. Structure-function relationships in the IL-17 receptor: implications for signal transduction and therapy. Cytokine 41: 92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gu C., Wu L., Li X.. 2013. IL-17 family: cytokines, receptors and signaling. Cytokine 64: 477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Herjan T., Hong L., Bubenik J., Bulek K., Qian W., Liu C., Li X., Chen X., Yang H., Ouyang S., et al. 2018. IL-17-receptor-associated adaptor Act1 directly stabilizes mRNAs to mediate IL-17 inflammatory signaling. Nat. Immunol. 19: 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Amatya N., Childs E. E., Cruz J. A., Aggor F. E. Y., Garg A. V., Berman A. J., Gudjonsson J. E., Atasoy U., Gaffen S. L.. 2018. IL-17 integrates multiple self-reinforcing, feed-forward mechanisms through the RNA binding protein Arid5a. Sci. Signal. 11: eaat4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herjan T., Yao P., Qian W., Li X., Liu C., Bulek K., Sun D., Yang W. P., Zhu J., He A., et al. 2013. HuR is required for IL-17-induced Act1-mediated CXCL1 and CXCL5 mRNA stabilization. J. Immunol. 191: 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mori M., Bjermer L., Erjefält J. S., Stampfli M. R., Roos A. B.. 2015. Small airway epithelial-C/EBPβ is increased in patients with advanced COPD. Respir. Res. 16: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu S. S., Lv X. X., Liu C., Qi J., Li Y. X., Wei X. P., Li K., Hua F., Cui B., Zhang X. W., et al. 2019. Targeting degradation of the transcription factor C/EBPβ reduces lung fibrosis by restoring activity of the ubiquitin-editing enzyme A20 in macrophages. Immunity 51: 522–534.e7. [DOI] [PubMed] [Google Scholar]
- 29. Roos A. B., Barton J. L., Miller-Larsson A., Dahlberg B., Berg T., Didon L., Nord M.. 2012. Lung epithelial-C/EBPβ contributes to LPS-induced inflammation and its suppression by formoterol. Biochem. Biophys. Res. Commun. 423: 134–139. [DOI] [PubMed] [Google Scholar]
- 30. Berg T., Didon L., Barton J., Andersson O., Nord M.. 2005. Glucocorticoids increase C/EBPbeta activity in the lung epithelium via phosphorylation. Biochem. Biophys. Res. Commun. 334: 638–645. [DOI] [PubMed] [Google Scholar]
- 31. Sønder S. U., Paun A., Ha H. L., Johnson P. F., Siebenlist U.. 2012. CIKS/Act1-mediated signaling by IL-17 cytokines in context: implications for how a CIKS gene variant may predispose to psoriasis. J. Immunol. 188: 5906–5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borger P., Matsumoto H., Boustany S., Gencay M. M., Burgess J. K., King G. G., Black J. L., Tamm M., Roth M.. 2007. Disease-specific expression and regulation of CCAAT/enhancer-binding proteins in asthma and chronic obstructive pulmonary disease. [Published erratum appears in 2021 J. Allergy Clin. Immunol. 148: 1088.] J. Allergy Clin. Immunol. 119: 98–105. [DOI] [PubMed] [Google Scholar]
- 33. Roos A. B., Nord M.. 2012. The emerging role of C/EBPs in glucocorticoid signaling: lessons from the lung. J. Endocrinol. 212: 291–305. [DOI] [PubMed] [Google Scholar]
- 34. Didon L., Barton J. L., Roos A. B., Gaschler G. J., Bauer C. M., Berg T., Stämpfli M. R., Nord M.. 2011. Lung epithelial CCAAT/enhancer-binding protein-β is necessary for the integrity of inflammatory responses to cigarette smoke. Am. J. Respir. Crit. Care Med. 184: 233–242. [DOI] [PubMed] [Google Scholar]
- 35. Devireddy L. R., Gazin C., Zhu X., Green M. R.. 2005. A cell-surface receptor for lipocalin 24p3 selectively mediates apoptosis and iron uptake. Cell 123: 1293–1305. [DOI] [PubMed] [Google Scholar]
- 36. Ferreira M. C., Whibley N., Mamo A. J., Siebenlist U., Chan Y. R., Gaffen S. L.. 2014. Interleukin-17-induced protein lipocalin 2 is dispensable for immunity to oral candidiasis. Infect. Immun. 82: 1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Owen H. C., Roberts S. J., Ahmed S. F., Farquharson C.. 2008. Dexamethasone-induced expression of the glucocorticoid response gene lipocalin 2 in chondrocytes. Am. J. Physiol. Endocrinol. Metab. 294: E1023–E1034. [DOI] [PubMed] [Google Scholar]
- 38. Wu G., Li H., Zhou M., Fang Q., Bao Y., Xu A., Jia W.. 2014. Mechanism and clinical evidence of lipocalin-2 and adipocyte fatty acid-binding protein linking obesity and atherosclerosis. Diabetes Metab. Res. Rev. 30: 447–456. [DOI] [PubMed] [Google Scholar]
- 39. Abella V., Scotece M., Conde J., Gómez R., Lois A., Pino J., Gómez-Reino J. J., Lago F., Mobasheri A., Gualillo O.. 2015. The potential of lipocalin-2/NGAL as biomarker for inflammatory and metabolic diseases. Biomarkers 20: 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vijay-Kumar M., Aitken J. D., Carvalho F. A., Cullender T. C., Mwangi S., Srinivasan S., Sitaraman S. V., Knight R., Ley R. E., Gewirtz A. T.. 2010. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borkham-Kamphorst E., van de Leur E., Zimmermann H. W., Karlmark K. R., Tihaa L., Haas U., Tacke F., Berger T., Mak T. W., Weiskirchen R.. 2013. Protective effects of lipocalin-2 (LCN2) in acute liver injury suggest a novel function in liver homeostasis. Biochim. Biophys. Acta 1832: 660–673. [DOI] [PubMed] [Google Scholar]
- 42. Hau C. S., Kanda N., Tada Y., Shibata S., Uozaki H., Fukusato T., Sato S., Watanabe S.. 2016. Lipocalin-2 exacerbates psoriasiform skin inflammation by augmenting T-helper 17 response. J. Dermatol. 43: 785–794. [DOI] [PubMed] [Google Scholar]
- 43. Matet A., Jaworski T., Bousquet E., Canonica J., Gobeaux C., Daruich A., Zhao M., Zola M., Meester-Smoor M., Mohabati D., et al. 2020. Lipocalin 2 as a potential systemic biomarker for central serous chorioretinopathy. Sci. Rep. 10: 20175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eagan T. M., Damås J. K., Ueland T., Voll-Aanerud M., Mollnes T. E., Hardie J. A., Bakke P. S., Aukrust P.. 2010. Neutrophil gelatinase-associated lipocalin: a biomarker in COPD. Chest 138: 888–895. [DOI] [PubMed] [Google Scholar]
- 45. Wang X. R., Li Y. P., Gao S., Xia W., Gao K., Kong Q. H., Qi H., Wu L., Zhang J., Qu J. M., Bai C. X.. 2014. Increased serum levels of lipocalin-1 and -2 in patients with stable chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 9: 543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sack G. H. Jr. 2018. Serum amyloid A–a review. Mol. Med. 24: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li H., Xiang X., Ren H., Xu L., Zhao L., Chen X., Long H., Wang Q., Wu Q.. 2020. Serum amyloid A is a biomarker of severe coronavirus disease and poor prognosis. J. Infect. 80: 646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lee J. Y., Hall J. A., Kroehling L., Wu L., Najar T., Nguyen H. H., Lin W. Y., Yeung S. T., Silva H. M., Li D., et al. 2020. Serum amyloid A proteins induce pathogenic Th17 cells and promote inflammatory disease. [Published erratum appears in 2020 Cell 180: 79–91.e16.] Cell 183: 2036–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Anthony D., Seow H. J., Uddin M., Thompson M., Dousha L., Vlahos R., Irving L. B., Levy B. D., Anderson G. P., Bozinovski S.. 2013. Serum amyloid A promotes lung neutrophilia by increasing IL-17A levels in the mucosa and γδ T cells. Am. J. Respir. Crit. Care Med. 188: 179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang M. S., Bowcutt R., Leung J. M., Wolff M. J., Gundra U. M., Hudesman D., Malter L. B., Poles M. A., Chen L. A., Pei Z., et al. 2017. Integrated analysis of biopsies from inflammatory bowel disease patients identifies SAA1 as a link between mucosal microbes with TH17 and TH22 cells. Inflamm. Bowel Dis. 23: 1544–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. American Thoracic Society . 2000. Proceedings of the ATS workshop on refractory asthma: current understanding, recommendations, and unanswered questions. Am. J. Respir. Crit. Care Med. 162: 2341–2351. [DOI] [PubMed] [Google Scholar]
- 52. Bulek K., Chen X., Parron V., Sundaram A., Herjan T., Ouyang S., Liu C., Majors A., Zepp J., Gao J., et al. 2019. IL-17A recruits Rab35 to IL-17R to mediate PKCα-dependent stress fiber formation and airway smooth muscle contractility. J. Immunol. 202: 1540–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ruzankina Y., Pinzon-Guzman C., Asare A., Ong T., Pontano L., Cotsarelis G., Zediak V. P., Velez M., Bhandoola A., Brown E. J.. 2007. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1: 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hao S., Baltimore D.. 2009. The stability of mRNA influences the temporal order of the induction of genes encoding inflammatory molecules. Nat. Immunol. 10: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Khan A., Fornes O., Stigliani A., Gheorghe M., Castro-Mondragon J. A., van der Lee R., Bessy A., Chèneby J., Kulkarni S. R., Tan G., et al. 2018. JASPAR 2018: update of the open-access database of transcription factor binding profiles and its web framework. Nucleic Acids Res. 46: D1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hartupee J., Liu C., Novotny M., Li X., Hamilton T.. 2007. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 179: 4135–4141. [DOI] [PubMed] [Google Scholar]
- 57. Bulek K., Liu C., Swaidani S., Wang L., Page R. C., Gulen M. F., Herjan T., Abbadi A., Qian W., Sun D., et al. 2011. The inducible kinase IKKi is required for IL-17-dependent signaling associated with neutrophilia and pulmonary inflammation. Nat. Immunol. 12: 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sun D., Novotny M., Bulek K., Liu C., Li X., Hamilton T.. 2011. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF). Nat. Immunol. 12: 853–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ramakrishnan R. K., Al Heialy S., Hamid Q.. 2019. Role of IL-17 in asthma pathogenesis and its implications for the clinic. Expert Rev. Respir. Med. 13: 1057–1068. [DOI] [PubMed] [Google Scholar]
- 60. Thijs W., Janssen K., van Schadewijk A. M., Papapoulos S. E., le Cessie S., Middeldorp S., Melissant C. F., Rabe K. F., Hiemstra P. S.. 2015. Nasal levels of antimicrobial peptides in allergic asthma patients and healthy controls: differences and effect of a short 1,25(OH)2 vitamin D3 treatment. PLoS One 10: e0140986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Su M. W., Lin W. C., Tsai C. H., Chiang B. L., Yang Y. H., Lin Y. T., Wang L. C., Lee J. H., Chou C. C., Wu Y. F., et al. 2018. Childhood asthma clusters reveal neutrophil-predominant phenotype with distinct gene expression. Allergy 73: 2024–2032. [DOI] [PubMed] [Google Scholar]
- 62. Dittrich A. M., Krokowski M., Meyer H. A., Quarcoo D., Avagyan A., Ahrens B., Kube S. M., Witzenrath M., Loddenkemper C., Cowland J. B., Hamelmann E.. 2010. Lipocalin2 protects against airway inflammation and hyperresponsiveness in a murine model of allergic airway disease. Clin. Exp. Allergy 40: 1689–1700. [DOI] [PubMed] [Google Scholar]
- 63. Bich T. C. T., Quoc Q. L., Choi Y., Yang E. M., Trinh H. K. T., Shin Y. S., Park H. S.. 2022. Serum amyloid A1: a biomarker for neutrophilic airway inflammation in adult asthmatic patients. Allergy Asthma Immunol. Res. 14: 40–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Smole U., Gour N., Phelan J., Hofer G., Köhler C., Kratzer B., Tauber P. A., Xiao X., Yao N., Dvorak J., et al. 2020. Serum amyloid A is a soluble pattern recognition receptor that drives type 2 immunity. Nat. Immunol. 21: 756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Khokhlovich E., Grant S., Kazani S., Strieter R., Thornton-Wells T., Laramie J., Morgan T., Kennedy S.. 2017. Late Breaking Abstract: the biological pathways underlying response to anti-IL-17A (AIN457; secukinumab) therapy differ across severe asthmatic patients. Eur. Respir. J. 50: OA2897 [Google Scholar]
- 66. Papp K. A., Leonardi C., Menter A., Ortonne J. P., Krueger J. G., Kricorian G., Aras G., Li J., Russell C. B., Thompson E. H., Baumgartner S.. 2012. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N. Engl. J. Med. 366: 1181–1189. [DOI] [PubMed] [Google Scholar]
- 67. Baeten D., Baraliakos X., Braun J., Sieper J., Emery P., van der Heijde D., McInnes I., van Laar J. M., Landewé R., Wordsworth P., et al. 2013. Anti-interleukin-17A monoclonal antibody secukinumab in treatment of ankylosing spondylitis: a randomised, double-blind, placebo-controlled trial. Lancet 382: 1705–1713. [DOI] [PubMed] [Google Scholar]
- 68. Rich P., Sigurgeirsson B., Thaci D., Ortonne J. P., Paul C., Schopf R. E., Morita A., Roseau K., Harfst E., Guettner A., et al. 2013. Secukinumab induction and maintenance therapy in moderate-to-severe plaque psoriasis: a randomized, double-blind, placebo-controlled, phase II regimen-finding study. Br. J. Dermatol. 168: 402–411. [DOI] [PubMed] [Google Scholar]
- 69. Leonardi C., Matheson R., Zachariae C., Cameron G., Li L., Edson-Heredia E., Braun D., Banerjee S.. 2012. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N. Engl. J. Med. 366: 1190–1199. [DOI] [PubMed] [Google Scholar]
- 70. Mease P. J., McInnes I. B., Kirkham B., Kavanaugh A., Rahman P., van der Heijde D., Landewé R., Nash P., Pricop L., Yuan J., et al. FUTURE 1 Study Group . 2015. Secukinumab inhibition of interleukin-17A in patients with psoriatic arthritis. N. Engl. J. Med. 373: 1329–1339. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.