Abstract
Candida albicans (C. albicans) is an opportunistic pathogen, which causes superficial infection and can lead to mortal systemic infections, especially in immunocompromised patients. The incidence of C. albicans infections is increasing and there are a limited number of antifungal drugs used in treatment. Therefore, there is an urgent need for new and alternative antifungal drugs. Pomegranate rind extract (PRE) is known for its broad-spectrum antimicrobial activities, including against C. albicans and recently, PRE and Zn (II) have been shown to induce synergistic antimicrobial activity against various microbes. In this study, the inhibitory activities of PRE, Zn (II) and PRE in combination with Zn (II) were evaluated against C. albicans. Antifungal activities of PRE and Zn (II) were evaluated using conventional microdilution methods and the interaction between these compounds was assessed by in vitro checkerboard and time kill assays in planktonic cultures. The anti-biofilm activities of PRE, Zn (II) and PRE in combination with Zn (II) were assessed using confocal laser scanning microscopy, with quantitative analysis of biofilm biomass and mean thickness analysed using COMSTAT2 analysis. In addition, antimicrobial interactions between PRE and Zn (II) were assayed in terms reactive oxygen species (ROS) production by C. albicans. PRE and Zn (II) showed a potent antifungal activity against C. albicans, with MIC values of 4 mg/mL and 1.8 mg/mL, respectively. PRE and Zn (II) in combination exerted a synergistic antifungal effect, as confirmed by the checkerboard and time kill assays. PRE, Zn (II) and PRE and Zn (II) in combination gave rise to significant reductions in biofilm biomass, although only PRE caused a significant reduction in mean biofilm thickness. The PRE and Zn (II) in combination caused the highest levels of ROS production by C. albicans, in both planktonic and biofilm forms. The induction of excess ROS accumulation in C. albicans may help explain the synergistic activity of PRE and Zn (II) in combination against C. albicans in both planktonic and biofilm forms. Moreover, the data support the potential of the PRE and Zn (II) combination as a novel potential anti-Candida therapeutic system.
Subject terms: Drug discovery, Microbiology, Plant sciences
Introduction
The prevalence of fungal infection has steadily increased as a result of the extensive use of hormones, immunosuppressants and broad-spectrum antibiotics1. Candida is a yeast-like fungus that normally lives on skin and inside the body such as in the mouth, throat, gut, and vagina, without causing any problems. However, uncontrolled proliferation leads to candidiasis, which is a broad term that refers to a group of infections affecting cutaneous, mucosal or deep-seated tissues2,3. Oral candidiasis can progress to severe stomatitis, which can lead to life-threatening bloodstream and tissue infections4. Intraocular Candida infection and subsequent ocular candidiasis can have devastating visual consequences5. Candida is also the causative agent of vulvovaginal candidiasis, which has the highest incidence of any single infectious diseases on the world6. Azole, polyenes, and echinocandins are currently the most often used medications to treat candidiasis7. However, the extensive use of these medications may exacerbate multidrug resistance and significantly diminish treatment efficacy8,9. In addition, side effects such as nephrotoxicity, hepatotoxicity, haemolytic anaemia, are limitations in the treatment of Candida infections10,11. As a result, novel antifungal drugs for the treatment of Candida-related infections are urgently needed.
Many natural compounds have applications as antifungal therapies, due to their potencies, abundant supplies and low toxicities12. The pomegranate, fruit of Punica granatum L., has a long folkloric history of treating infections, and antimicrobial activity has been reported in recent times against different microbes, including C. albicans13–15. It was shown that pomegranate peel/rind extract inhibit fungal growth by compromising the cell wall and the cytoplasmic membrane16,17. The antimicrobial activity of pomegranate extracts has been largely attributed to its polyphenolic content, in particular the hydrolysable ellagitannin, punicalagin, which is particularly abundant in the fruit rind or exocarp18,19. Recently, the enhancement of the antimicrobial activity of PRE has been explored by the co-application of Zn (II), with significant synergistic (potentiated) virucidal activity having been found against Herpes simplex virus (HSV)20 and bactericidal activity against Micrococcus luteus21, methicillin-resistant Staphylococcus aureus (MRSA), Escherichia coli, Pseudomonas aeruginosa and S. epidermidis22. The significance of combination therapy in combating resistance is known, including that for C. albicans23. As pomegranate extract was previously shown to inhibit C. albicans biofilm formation14, the aim of this study was to determine if activity against C. albicans could be similarly enhanced using PRE and Zn (II) in combination against planktonic and pre-formed biofilms of C. albicans. In addition, PRE and PRE and Zn (II) in combination were investigated for their abilities to promote reactive oxygen species (ROS) production and oxidative stress in C. albicans.
Results
Characterisation of PRE
Total phenolic content was found as 496 mg of TAE/g of freeze-dried PRE. HPLC chromatogram of PRE showed two major peaks of punicalagin with the equilibrium constant 1.76:1 (K = [B]/[a]), and the amount of punicalagin was determined as 170 mg/g of freeze-dried PRE, using the standard curve obtained with standard punicalagin (Fig. 1).
Figure 1.
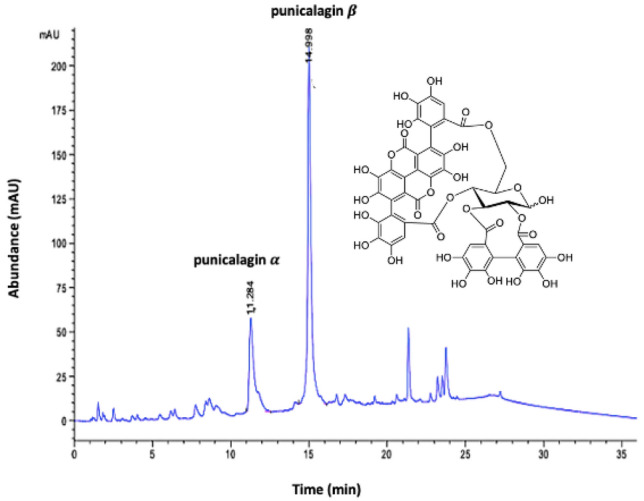
HPLC chromatogram of pomegranate rind extract (PRE) highlighting the major ellagitannin anomers α and β punicalagin; inset: the chemical structure of punicalagin.
Determination of C. albicans susceptibility and antifungal interaction of PRE and Zn (II)
Both PRE and Zn (II) inhibited C. albicans growth using the broth dilution assay, with concentrations of 4 mg/mL and 1.8 mg/mL, respectively (Table 1). The antifungal interaction between PRE and Zn (II) was determined as synergistic using the checkerboard assay (FICI = 0.125) (Table 1). This combination also exerted synergistic antifungal activity at 240 min using the time kill assay, which showed more than 2 log reduction compared to PRE or Zn (II) alone (Fig. 2).
Table 1.
MIC and FICI values for PRE and Zn (II) alone and in combination against C. albicans (ATCC 90028), determined by broth dilution and checkerboard method.
| MIC | MIC in combination | FIC | FICI | |
|---|---|---|---|---|
| PRE (mg/mL) | 4 ± 1.8 | 0.25 ± 0.115 | 0.0625 ± 0.03 | – |
| Zn (II) (mg/mL) | 1.8 ± 0.75 | 0.112 ± 0.05 | 0.0625 ± 0.03 | – |
| PRE + Zn (II) | – | – | 0.125 ± 0.05 (synergism) |
Figure 2.
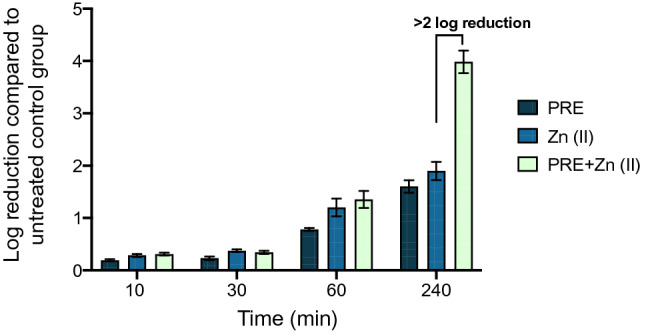
Log reduction of C. albicans (ATCC 90028) after incubation of PRE (4 mg/mL), Zn (II) (1.8 mg/mL), and PRE and Zn (II) (4 mg/mL + 1.8 mg/mL) in combination, over a range of contact times (10 min, 30 min, 60 min and 240 min) at 37 °C.
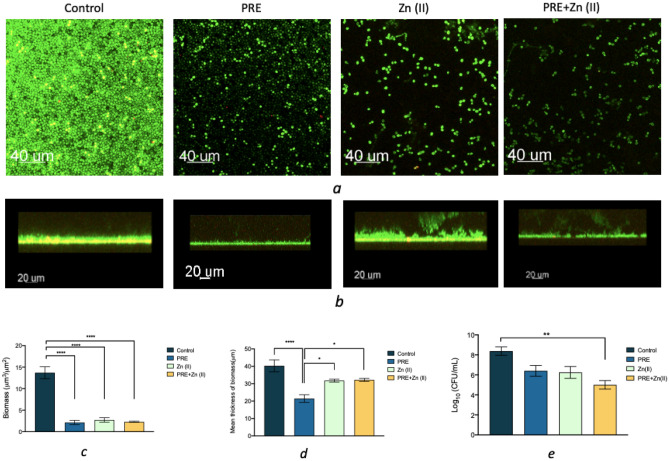
Confocal microscopy and COMSTAT2 analysis of C. albicans biofilms in the presence of PRE, Zn (II) and PRE/Zn (II) combined
The effects of PRE, Zn (II) and PRE and Zn (II) combination on pre-formed C. albicans biofilms were evaluated by confocal microscopy, with quantitative analysis performed using COMSTAT2 analysis. Control cultures (without treatment) formed a dense biofilm (biomass ≃ 15 µm3/µm2 and mean thickness ≃ 40 µm). A substantial decrease in biomass was observed in the presence of PRE, Zn (II) and PRE and Zn (II) combined (all p < 0.0001). However, a significant reduction in biofilm thickness was only observed in the presence of PRE, compared to both the untreated controls (p < 0.0001) and other treatment groups, Zn (II) and PRE/Zn (II) (both p < 0.05) (Fig. 3).
Figure 3.
(a and b) CLSM images of C. albicans biofilm after incubation with PRE (4 mg/mL), Zn (II) (1.8 mg/mL) and PRE and Zn (II) combined (4 mg/mL + 1.8 mg/mL), at 37 °C for 24 h. (c and d) Comparison of different variables of C. albicans biofilm using CLSM/COMSTAT2 analysis. (e) Log10 values of C. albicans biofilm a after incubation with PRE, Zn (II) and PRE and Zn (II) combined, at 37 °C for 24 h. Significance indicated by *, where *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Data represented as mean ± SEM (n = 3).
ROS production by C. albicans in the presence of PRE, Zn (II) and PRE and Zn (II) combined
ROS production was measured in planktonic and biofilm forms of C. albicans using DCFH-DA, which is hydrolyzed to DCF by intracellular esterases producing a high intensity green fluorescence. Fluorescence intensity was quantified via COMSTAT2 and normalized to total fungal biomass. ROS production was observed in all experimental groups, under both planktonic and biofilm conditions, although C. albicans biofilms resulted in higher ROS production than its planktonic equivalents (Fig. 4a,b). PRE and Zn (II) alone applications caused higher ROS generation, compared to untreated controls. However, PRE and Zn (II) in combination caused a substantial increase in ROS production by C. albicans, compared to untreated controls and the PRE and Zn (II) alone treatments, under both planktonic (Fig. 4c) and biofilm conditions (Fig. 4d) normalised to total biomass.
Figure 4.
CLSM images of C. albicans in planktonic (a) and biofilm form (b) incubated with PRE (4 mg/mL), Zn (II) (1.8 mg/mL) and PRE and Zn (II) combined (4 mg/mL + 1.8 mg/mL) for 24 h. Blue channel shows calcofluor in C. albicans cells walls and green channel shows ROS production in C. albicans cells. Graphs shows ROS levels for C. albicans in planktonic form (c) and biofilm form (d) normalised to total biomass. Significance indicated by *, where **p < 0.01, ***p < 0.001, and ****p < 0.0001. Data represented as mean ± SEM (n = 3).
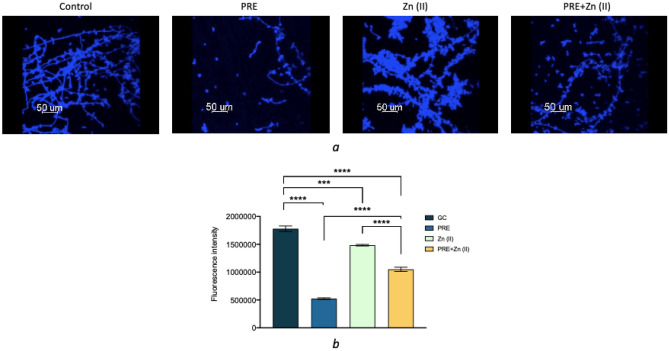
Hyphal growth of C. albicans biofilms in the presence of PRE, Zn (II) and PRE and Zn (II) combined
PRE showed the highest inhibitory activity against C. albicans hyphal growth (Fig. 5a). While PRE and Zn (II) in combination caused an inhibition in hyphal growth, this inhibitory effect was substantially lower than PRE alone. The fluorescence intensities were significantly reduced in the presence of PRE, Zn (II) and PRE and Zn (II) combined, compared to untreated controls (p < 0.001 for Zn (II) vs untreated controls and p < 0.0001 for PRE and PRE + Zn (II) vs untreated controls). However, PRE exhibited the lowest fluorescence intensities, with significant differences between biofilms treated with PRE, versus the untreated control and other treatments (p < 0.0001) (Fig. 5b).
Figure 5.
(a) CLSM imaging of PRE (4 mg/mL), Zn (II) (1.8 mg/mL) and PRE and Zn (II) combined (4 mg/mL + 1.8 mg/mL) treated and untreated C. albicans biofilms, stained with Calcofluor-White to identify hyphal formation (for C. albicans cell walls in blue, scale bar = 50 µm). (b) The fluorescence intensities of Confocal Microscopy images. Significance indicated by *, where ***p < 0.001, and ****p < 0.0001. Data represented as mean ± SEM (n = 3).
Discussion
The frequency of mucosal and systemic Candida species infections has increased in recent years, due to various factors including the increasing number of immunocompromised patients, illnesses, and widespread use of broad-spectrum antibiotics24. The increasing rate of drug resistance and the low number of antifungal drugs in clinical development highlights the difficulty in treating C. albicans infections25. As a result, the need for new antifungal compounds has become a major priority worldwide26.
Pomegranate has a long history in the treatment of microbial infections and recently, pomegranate extracts have been reported to possess antimicrobial, anti-inflammatory and wound healing activities27–31. Pomegranate extract exerted a broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria, viruses, protozoa, in addition to fungal strains, including C. albicans20,31,32. Beneficial health activities of pomegranate extracts have been largely attributed to high amounts of hydrolysable tannins, especially punicalagins, punicalin, and ellagic acid33–36. These hydrolysable tannins have been shown to precipitate proteins present in the cell membrane which causes disruption of the cytoplasmic membrane, inhibition of nucleic acids, limitation of energy metabolism, cell lysis and death33,37. In this study, C. albicans was found to be susceptible to PRE and Zn (II) with MIC values of 4 mg/mL and 1.8 mg/mL, respectively. C. albicans can grow in three different morphological forms: budding yeast, pseudohyphal and hyphal. This morphological adaptivity gives C. albicans an edge in the formation of biofilms on medical device surfaces, resulting in biofilm-associated illnesses38. We also evaluated the antibiofilm activity of PRE, Zn (II) and PRE and Zn (II) in combination, via Live/Dead staining analysis by Confocal Microscopy. Microscopy images and quantitative COMSTAT2 analysis demonstrated the disruptive effects of PRE, Zn (II), and PRE and Zn (II) in combination on C. albicans biofilms. C. albicans generated high-density biofilms in the absence of test compounds and their combination. Only PRE induced significant decreases in the mean thickness of C. albicans biofilms, despite all treatments resulting in large reductions in biofilm biomass. In this study, we further investigated the influence of PRE, Zn (II) and PRE and Zn (II) in combination on the morphological forms of the C. albicans biofilm using calcofluor white staining. PRE was the most efficient compound at inhibiting hyphae formation, demonstrating similar responses to its effect on C. albicans biofilm mean thickness, as C. albicans hyphal formation would contribute to biofilm thickness overall. However, only PRE and Zn (II) in combination caused a substantial reduction in the CFU of C. albicans cells in biofilm form. This could be result of synergistic fungicidal action of PRE and Zn (II) on C. albicans cells, as this combination showed a synergistic antifungal activity in both checkerboard and time-kill assays. Previously, PRE and Zn (II) in combination showed a synergistic antimicrobial activity in vitro against a range of bacteria and HSV20–22. PRE and copper combination exhibited an enhanced antimicrobial activity against E. coli, P. aeruginosa and P. mirabilis39. The synergistic antifungal activity found in the current study has great importance as combinational antimicrobial therapy of C. albicans infections results in lower toxicity and decreased rate of drug resistance40. Similarly, vanillin complex (a phenolic molecule) coupled with various metal ions has previously potentiated antibacterial efficacy against S. aureus, E. coli, K. pneumoniae, P. aeruginosa, and C. albicans41.
The mechanism for the synergistic antimicrobial activity of pomegranate extracts and metal salts is not fully understood, but suggested mechanisms involve the phenolic chemicals forming a complex with metal ions, which has increased antimicrobial action42. Also, interaction on the microbe surface could disrupt efflux pump activity, or tannin/Zn endocytosis facilitated where a low pH is employed such that surface charge and electrostatic repulsion is reduced21. However, the time kill data alludes to a diffusional aspect as the synergy is not observed in this work until 240 min contact time.
Phenolic compounds, such as those found in pomegranate, are generally known for antioxidative activities43–45. However, phenolic compounds can also exhibit pro-oxidative behaviour, depending on such factors as concentration and the intra-cellular environment, including the presence of metal ions at the site of free radical generation. It is claimed that whether phenolic compounds act as antioxidants or pro-oxidants is influenced by the presence of transition metal ions, e.g. iron, copper and zinc46. For instance, pro-oxidative activity has been reported for curcumin with copper. In the presence of copper, curcumin showed an apoptosis on cancer cells by increasing secretion of ROS molecules47–49. Thus, a possible mechanism behind the synergistic antifungal activity of PRE and Zn (II) combined was evaluated, in terms of the oxidative stress responsiveness of C. albicans. In the current study, it was shown that both PRE and Zn (II) did not induce the ROS production of C. albicans cells, when they were applied alone and they did produce a similar level of ROS in both planktonic and biofilm level, compared to untreated controls. However, the combination of PRE and Zn (II) was shown to substantially elevate ROS production, compared to untreated controls and when PRE and Zn (II) were applied alone—these high levels would be expected to be harmful to the cells, causing death.
It has been reported that compounds that induce ROS production could act as promising antifungal agents50. ROS have apoptotic effects on different cell types, including C. albicans, and studies have reported ROS-induced C. albicans apoptosis in the presence of acetic acid, resveratrol, farnesol, and antimicrobial peptides51–54. In addition to their target specific actions, the fungicidal activity of routinely used antifungal drugs, such as azoles, has been linked to their increased ROS effects50. Furthermore miconazole-tolerant Candida cells have a high amount of ROS inactivating activity55. C. albicans have enzymatic and non-enzymatic antioxidant defence mechanisms, such as superoxide dismutase (SOD), that plays a key role in C. albicans virulence56,57. This elevated ROS level could be a result of the inhibition of C. albicans endogenous antioxidant system or the pro-oxidant activity of PRE and Zn (II) in combination. ROS can affect a wide range of biological molecules, including nucleic acids, proteins, and lipids58. The pro-oxidative characteristics of PRE and Zn (II) in combination may inhibit mitochondrial respiration enzymes, including NADH oxidase and succino-oxidase59. However, pinpointing the particular processes responsible for the antifungal efficacy of this combination against C. albicans is difficult, and more research is needed to have a better understanding of the role of ROS in the synergistic antifungal activity of PRE and Zn (II).
There are a limited number of effective antifungal medications commonly used, with new treatments against fungal infections urgently needed. Natural products, in particular polyphenols, are becoming more appealing as an alternative agent in the treatment of infectious disorders, because of their broad-spectrum antibacterial and antifungal effects, low toxicities and low costs60,61. In addition, combination drug therapy is known to inhibit resistance mechanisms.
To conclude, this work successfully demonstrated that the polyphenol-rich composition of PRE and the addition of Zn (II), which is already known for its synergistic antibacterial, antiviral and anti-inflammatory properties, could also make this combination a promising novel treatment for C. albicans infections15,20–22,29. Taken together with previous results, the results reported herein against C. albicans supports the development of a novel broadspectrum anti-infective system.
Materials and methods
Materials
Pomegranates (of Spanish origin) were obtained from a local supermarket, therefore collection of plant material, complied with relevant institutional, national, and international guidelines and legislation. Zinc sulphate heptahydrate (ZnSO4·7H2O) and potassium hydrogen phthalate were obtained from ThermoFisher Scientific (Loughborough, UK). Mueller–Hinton broth (MH broth), Mueller–Hinton agar (MH agar), brain–heart infusion agar (BH agar), brain–heart infusion broth (BH broth) were obtained from Oxoid Ltd. (Basingstoke, UK) and Saboraud dextrose 4% agar (SDA) was obtained from VWR chemicals (Leuven, Belgium). Live/Dead Baclight™ Bacterial Viability Kit was obtained from Invitrogen Molecular Probes (Paisley, UK). Calcifluor white stain and DCFH-DA probe were obtained from Sigma-Aldrich (Poole, UK).
Preparation of pomegranate rind extract (PRE)
Fresh fruits of pomegranate were washed (Punica granatum L., Spanish origin) and pomegranate rind separated from its arils. The rind was cut to approximately 2 cm2 pieces with a scalpel, before blending in deionised water (25% w/v). The homogenous rind solution was boiled for 10 min, centrifuged 4 times (5980×g at 4 °C for 30 min, Heraeus Multifuge 3S/3S-R centrifuge), and filtered through a Whatman 0.45 µm nylon membrane filter. The supernatant was collected, freeze-dried, and then stored at − 20 °C until stock solutions were prepared in pH 4.5 phthalate buffer62. The total phenolic content of PRE was found using Folin-Ciocalteu assay with the method described before and the result was shown as tannic acid equivalents (TAE) per gram of freeze-dried PRE63. The punicalagin amount in PRE was determined by high pressure liquid chromatography according to the method of Seeram et al.64.
Media used and growth condition of Candida albicans
Candida albicans was grown on SDA for 24 h at 37 °C. Few colonies from the agar plate were propogated in MH broth and the turbidity of C. albicans cells were adjusted with MHB to optical density (600 nm) value of 0.1. Then cell suspension finally diluted with MH broth obtain approximately 1 × 106 cells/mL of C. albicans to use in the experiments.
Anti-Candida susceptibility of PRE and Zn (II)
Broth dilution assays were performed to find the minimum inhibitory concentration (MIC) of PRE and Zn (II). C. albicans (1 × 106 cells/mL) in MHB were incubated with equal volume of serially two-fold diluted tested agents aerobically at 37 °C for 24 h. The concentrations that caused no turbidity were subsequently determined as MIC values65,66.
Chequerboard assay
Antimicrobial activity interaction between PRE and Zn (II) was determined using the chequerboard assay67. Eight doubling dilutions of PRE and Zn (II) were prepared in MH broth and combined (final volume 100 µL) and diluted tested agents were inoculated with C. albicans at a density of 1 × 106 cells/mL in each well. Experimental plate was incubated for 24 h at 37 °C in ambient air. The fractional inhibitory concentration index (FICI) for PRE and Zn (II) were calculated using the formula:
where FICPRE is the MIC of PRE in combination/MIC of PRE alone, and FICZn(II) is the MIC of Zn (II) in combination/MIC of Zn (II) alone. The FICI was interpreted as synergy where FICI ≤ 0.5; no interaction where FICI > 0.5 ≤ 4; or antagonism where FICI > 468.
Time-kill assay
PRE, Zn (II) and PRE in combination with Zn (II) were investigated for their fungicidal and time-killing activities by measuring viable cell counts. C. albicans (1 × 106 cells/mL) was incubated with PRE (2 mg/mL and 4 mg/mL), Zn (II) (0.9 mg/mL and 1.8 mg/mL) and PRE in combination with Zn (II) (2 mg/mL PRE with 0.9 mg/mL Zn (II) and 4 mg/mL PRE with 1.8 mg/mL Zn (II)) at 37 °C. The number of viable cells were obtained by colony counting at specified timepoints (10, 30, 60 and 240 min)69. The results were presented as the mean values number of colony forming units (CFU) of quadruplicate measurements from three independent experiments.
Effects on C. albicans biofilms via confocal microscopy
The effects of PRE, Zn (II), and PRE and Zn (II) combined on pre-existing 24 h C. albicans biofilms was investigated using a Live/Dead BacLight™ Bacterial Viability test (Greiner Bio One Ltd., Stonehouse, UK). This assay was performed based on the method previously described by Powell et al.70. Briefly, C. albicans biofilms were generated in a glass-bottomed, 96-well plates by inoculating 100 µL of C. albicans at a density of 1 × 108 cells/mL per well. After aerobic incubation at 37 °C for 24 h, the supernatant was replaced with PRE (MIC), Zn (II) (MIC) and PRE in combination with Zn (II) (MIC + MIC) for another 24 h incubation. According to the manufacturer's instructions, each plate was subsequently stained with the Live/Dead BacLight™ Bacterial Viability Kit and observed using the Leica TCS SP5 Confocal Microscope (Leica Microsystems Ltd., Milton Keynes, UK). Images were captured with an 63× oil objective and a 1 µm z-step size. COMSTAT2 plugin with ImageJ analysis software, Version 2.1.0 (US National Institutes of Health, Bethesda, Maryland, USA)71 was used to analyse Z-stack pictures produced using Bitplane’s Imaris Programme (Concord, MA, USA). Results were expressed as mean ± SEM (n = 12).
Reactive oxygen species (ROS) production by C. albicans during and following biofilm formation
The intracellular ROS production of C. albicans was determined by CLSM, using dichloro-dihydrofluorescein diacetate (DCFH-DA) in a glass bottomed, 96-well plates during and following biofilm formation72. C. albicans biofilms were developed as described above and ROS production during biofilm formation was assessed by incubating C. albicans cells with PRE, Zn (II) and PRE in combination with Zn (II) aerobically at 37 °C for 24 h. Then, each supernatant was discarded and the plates were first stained with 5 µL of Calcofluor-White (0.05% v/v, Sigma-Aldrich) for 1 min, and excited at 355 nm. Then, 15 µL of 10 µM of DCFH-DA was added and incubated for 15 min in the dark at room temperature, excited at 488 nm, and stained plates were visualised with a Leica TCS SP5 Confocal Microscope (Leica Microsystems Ltd, Milton Keynes, UK). Images were obtained with 60 × 1.8 oil objective with a z-step size of 1 μm. The randomly selected fields of view were analysed using COMSTAT2 software with the NIH-ImageJ analysis software for biomass (μm3/μm2) of two channels, blue for C. albicans cell wall, and green for ROS. The ROS level was normalised to total biomass of Calcofluor-White C. albicans and presented in a graph as mean ± SEM (n = 12).
Effects on C. albicans hyphal growth
Candida albicans biofilms (500 µL of C. albicans cells incubated aerobically at 37 °C for 24 h) were formed in 24-well glass bottomed plates. After incubation, biofilms were treated with 0.5 mL of PRE, Zn (II) and PRE in combination with Zn (II) or untreated for growth control for 24 h. Biofilms were washed with phosphate buffered saline (pH 7.2) and stained with 0.05% (v/v) Calcofluor White for 1 min in the dark and visualised with CLSM. Images were analysed using ImageJ analysis software and the results presented as mean ± SEM of fluorescence intensity73.
Statistical analysis
All experiments were performed in triplicate, using independent microbial cultures for all antimicrobial assays. The results were analysed and graphically presented using GraphPad Prism 8.0 software. The one-way ANOVA test with post-test Tukey correction was used and p < 0.05 was considered statistically significant.
Acknowledgements
We would like to thank the Turkish Government for supporting this work, and also Ms Lydia C. Powell for assistance with the CLSM imaging.
Author contributions
Conceptualization: C.M.H. Methodology: C.M.H., R.M., A.J.S. Validation: C.M.H., R.M., R.L.M., A.J.S. Formal analysis: V.C., C.M.H., R.M., R.L.M., A.J.S. Investigation: V.C., R.L.M. Resources: C.M.H., R.M., A.J.S. Data curation: V.C., R.L.M. Writing—original draft preparation: V.C. Writing—review and editing: C.M.H., R.M., R.L.M., A.J.S. Supervision: C.M.H., R.M., R.L.M., A.J.S. Project administration, funding acquisition: C.M.H.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lombardi A, Ouanounou A. Fungal infections in dentistry: Clinical presentations, diagnosis, and treatment alternatives. Oral. Surg. Oral Med. Oral. Pathol. Oral. Radiol. 2020;130:533–546. doi: 10.1016/j.oooo.2020.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Poulain D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015;41:208–217. doi: 10.3109/1040841X.2013.813904. [DOI] [PubMed] [Google Scholar]
- 3.McCarty TP, Pappas PG. Invasive candidiasis. Infect. Dis. Clin. 2016;30:103–124. doi: 10.1016/j.idc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Lewis MA, Williams DW. Diagnosis and management of oral candidosis. Br. Dent. J. 2017;223:675–681. doi: 10.1038/sj.bdj.2017.886. [DOI] [PubMed] [Google Scholar]
- 5.Gopinathan U, Sharma S, Garg P, Rao GN. Review of epidemiological features, microbiological diagnosis and treatment outcome of microbial keratitis: Experience of over a decade. Indian J. Ophthalmol. 2009;57:273. doi: 10.4103/0301-4738.53051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foxman B, Muraglia R, Dietz JP, Sobel JD, Wagner J. Prevalence of recurrent vulvovaginal candidiasis in 5 European countries and the United States: Results from an internet panel survey. J. Low. Genit. Tract Dis. 2013;17:340–345. doi: 10.1097/LGT.0b013e318273e8cf. [DOI] [PubMed] [Google Scholar]
- 7.Quindós G, Gil-Alonso S, Marcos-Arias C, Sevillano E, Mateo E, Jauregizar N, Eraso E. Therapeutic tools for oral candidiasis: Current and new antifungal drugs. Med. Oral. Patol. Oral. Cir. Bucal. 2019;24:e172–e180. doi: 10.4317/medoral.22978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodrigues CF, Rodrigues ME, Henriques MC. Promising alternative therapeutics for oral candidiasis. Curr. Med. Chem. 2019;26:2515–2528. doi: 10.2174/0929867325666180601102333. [DOI] [PubMed] [Google Scholar]
- 9.Millsop JW, Fazel N. Oral candidiasis. Clin. Dermatol. 2016;34:487–494. doi: 10.1016/j.clindermatol.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 10.Odds FC, Brown AJ, Gow NA. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003;11:272–279. doi: 10.1016/s0966-842x(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 11.Deray G. Amphotericin B nephrotoxicity. J. Antimicrob. Chemother. 2002;49(suppl_1):37–41. doi: 10.1093/jac/49.suppl_1.37. [DOI] [PubMed] [Google Scholar]
- 12.Ansari MA, Fatima Z, Hameed S. Sesamol: A natural phenolic compound with promising anticandidal potential. J. Pathogens. 2014;2014:895193. doi: 10.1155/2014/895193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duman AD, Ozgen M, Dayisoylu KS, Erbil N, Durgac C. Antimicrobial activity of six pomegranate (Punica granatum L.) varieties and their relation to some of their pomological and phytonutrient characteristics. Molecules. 2009;14:1808–1817. doi: 10.3390/molecules14051808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakkiyaraj D, Nandhini JR, Malathy B, Pandian SK. The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling. 2013;29:929–937. doi: 10.1080/08927014.2013.820825. [DOI] [PubMed] [Google Scholar]
- 15.Celiksoy, V., Heard, C.M. Antimicrobial potential of pomegranate extracts. in Pomegranate Feb 11. IntechOpen (2021).
- 16.Benslimane S, Rebai O, Djibaoui R, Arabi A. Pomegranate Peel Extract Activities as Antioxidant and Antibiofilm against Bacteria Isolated from Caries and Supragingival Plaque. Jordan J. Biol. Sci. 2020;2020:13. [Google Scholar]
- 17.Lu J, Ding K, Yuan Q. Determination of punicalagin isomers in pomegranate husk. Chromatographia. 2008;68:303–306. [Google Scholar]
- 18.Anibal PC, Peixoto IT, Foglio MA, Höfling JF. Antifungal activity of the ethanolic extracts of Punica granatum L. and evaluation of the morphological and structural modifications of its compounds upon the cells of Candida spp. Braz. J. Microbiol. 2013;44:839–848. doi: 10.1590/S1517-83822013005000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar KP, Samlin SS, Siva B, Sudharshan R, Vignesswary A, Divya K. Punica granatum as a salutiferous superfruit in the treatment of oral candidiasis—An in-vitro study. J. Oral Maxillofac. Pathol. JOMFP. 2020;24:188. doi: 10.4103/jomfp.JOMFP_268_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houston DM, Bugert JJ, Denyer SP, Heard CM. Correction: Potentiated virucidal activity of pomegranate rind extract (PRE) and punicalagin against Herpes simplex virus (HSV) when co-administered with zinc (II) ions, and antiviral activity of PRE against HSV and aciclovir-resistant HSV. PLoS ONE. 2017;12(11):e0188609. doi: 10.1371/journal.pone.0179291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Celiksoy V, Moses RL, Sloan AJ, Moseley R, Heard CM. Synergistic in vitro antimicrobial activity of pomegranate rind extract and zinc (II) against Micrococcus luteus under planktonic and biofilm conditions. Pharmaceutics. 2021;13(6):851. doi: 10.3390/pharmaceutics13060851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alrashidi A, Jafar M, Higgins N, Mulligan C, Varricchio C, Moseley R, Celiksoy V, Houston DM, Heard CM. A time-kill assay study on the synergistic bactericidal activity of pomegranate rind extract and Zn (II) against Methicillin-resistant Staphylococcus aureus (MRSA), Staphylococcus epidermidis, Escherichia coli, and Pseudomonas aeruginosa. Biomolecules. 2021;11(12):1889. doi: 10.3390/biom11121889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simonson AW, Mongia AS, Aronson MR, Alumasa JN, Chan DC, Lawanprasert A, Howe MD, Bolotsky A, Mal TK, George C, Ebrahimi A. Pathogen-specific antimicrobials engineered de novo through membrane-protein biomimicry. Nat. Biomed. Eng. 2021;5(5):467–480. doi: 10.1038/s41551-020-00665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan L, Yang C, Tang J. Disruption of the intestinal mucosal barrier in Candida albicans infections. Microbiol. Res. 2013;168:389–395. doi: 10.1016/j.micres.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Özçelik B, Çıtak S, Cesur S, Abbasoglu U. In vitro susceptibility of Candida species isolated from cancer patients to some antifungal agents. Drug Metabol. Drug Interact. 2004;20:101–108. doi: 10.1515/dmdi.2004.20.1-2.1. [DOI] [PubMed] [Google Scholar]
- 26.Silva FC, Chalfoun SM, Siqueira VM, Botelho DM, Lima N, Batista LR. Evaluation of antifungal activity of essential oils against potentially mycotoxigenic Aspergillus flavus and Aspergillus parasiticus. Rev. Bras. 2012;22:1002–1010. [Google Scholar]
- 27.Celiksoy V, Moses RL, Sloan AJ, Moseley R, Heard CM. Evaluation of the in vitro oral wound healing effects of pomegranate (Punica granatum) rind extract and punicalagin, in combination with Zn (II) Biomolecules. 2020;10(9):1234. doi: 10.3390/biom10091234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail T, Sestili P, Akhtar S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012;143:397–405. doi: 10.1016/j.jep.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Houston DM, Bugert J, Denyer SP, Heard CM. Anti-inflammatory activity of Punica granatum L. (Pomegranate) rind extracts applied topically to ex vivo skin. Eur. J. Pharm. Biopharm. 2017;112:30–37. doi: 10.1016/j.ejpb.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 30.Xiang Q, Li M, Wen J, Ren F, Yang Z, Jiang X, Chen Y. The bioactivity and applications of pomegranate peel extract: A review. J. Food Biochem. 2022;6:e14105. doi: 10.1111/jfbc.14105. [DOI] [PubMed] [Google Scholar]
- 31.Belgacem I, Li Destri Nicosia MG, Pangallo S, Abdelfattah A, Benuzzi M, Agosteo GE, Schena L. Pomegranate peel extracts as safe natural treatments to control plant diseases and increase the shelf-life and safety of fresh fruits and vegetables. Plants. 2021;10:453. doi: 10.3390/plants10030453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassiri-Jahromi, S., Katiraee, F., Hajimahmoodi, M., Mostafavi, E., Talebi, M., Pourshafie, M.R. In vitro antifungal activity of various Persian cultivars of Punica granatum L. extracts against Candida species. Jundishapur J. Nat. Pharm. Products. 10(3) (2015).
- 33.Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582. doi: 10.1128/cmr.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel C, Dadhaniya P, Hingorani L, Soni MG. Safety assessment of pomegranate fruit extract: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2008;46(8):2728–2735. doi: 10.1016/j.fct.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 35.Bialonska D, Kasimsetty SG, Schrader KK, Ferreira D. The effect of pomegranate (Punica granatum L.) byproducts and ellagitannins on the growth of human gut bacteria. J. Agric. Food Chem. 2009;57:8344–8349. doi: 10.1021/jf901931b. [DOI] [PubMed] [Google Scholar]
- 36.Glazer I, Masaphy S, Marciano P, Bar-Ilan I, Holland D, Kerem Z, Amir R. Partial identification of antifungal compounds from Punica granatum peel extracts. J. Agric. Food Chem. 2012;60:4841–4848. doi: 10.1021/jf300330y. [DOI] [PubMed] [Google Scholar]
- 37.Cushnie TT, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int. J. Antimicrob. Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 38.Sudbery PE. Growth of Candida albicans hyphae. Nat. Rev. Microbiol. 2011;9(10):737–748. doi: 10.1038/nrmicro2636. [DOI] [PubMed] [Google Scholar]
- 39.McCarrell EM, Gould SW, Fielder MD, Kelly AF, El Sankary W, Naughton DP. Antimicrobial activities of pomegranate rind extracts: Enhancement by addition of metal salts and vitamin C. BMC Complement. Altern. Med. 2008;8:1–7. doi: 10.1186/1472-6882-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis RE, Kontoyiannis DP. Rationale for combination antifungal therapy. Pharmacother. J. Hum. Pharmacol. Drug Therapy. 2001;21(8P2):149S–164S. doi: 10.1592/phco.21.12.149s.34505. [DOI] [PubMed] [Google Scholar]
- 41.Nair MS, Joseyphus RS. Synthesis and characterization of Co (II), Ni (II), Cu (II) and Zn (II) complexes of tridentate Schiff base derived from vanillin and DL-α-aminobutyric acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008;70:749–753. doi: 10.1016/j.saa.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Bravo A, Anacona JR. Metal complexes of the flavonoid quercetin: Antibacterial properties. Transition Met. Chem. 2001;26:20–23. [Google Scholar]
- 43.Ali HM, Abo-Shady A, Sharaf Eldeen HA, Soror HA, Shousha WG, Abdel-Barry OA, Saleh AM. Structural features, kinetics and SAR study of radical scavenging and antioxidant activities of phenolic and anilinic compounds. Chem. Central J. 2013;7:1–9. doi: 10.1186/1752-153X-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res./Fund. Mol. Mech. Mutagenesis. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 45.Rice-Evan C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 46.Yoshino M, Haneda M, Naruse M, Htay HH, Tsubouchi R, Qiao SL, Li WH, Murakami K, Yokochi T. Prooxidant activity of curcumin: Copper-dependent formation of 8-hydroxy-2′-deoxyguanosine in DNA and induction of apoptotic cell death. Toxicol. In Vitro. 2004;18:783–789. doi: 10.1016/j.tiv.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 47.Leung MHM, Harada T, Kee TW. Delivery of curcumin and medicinal effects of the copper (II)-curcumin complexes. Curr. Pharm. Des. 2013;19:2070–2083. doi: 10.2174/138161213805289237. [DOI] [PubMed] [Google Scholar]
- 48.Gould SW, Fielder MD, Kelly AF, Sankary WE, Naughton DP. Antimicrobial pomegranate rind extracts: enhancement by Cu (II) and vitamin C combinations against clinical isolates of Pseudomonas aeruginosa. Br. J. Biomed. Sci. 2009;66:129–132. doi: 10.1080/09674845.2009.11730258. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Tsao R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016;8:33–42. [Google Scholar]
- 50.Delattin N, Cammue BP, Thevissen K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med. Chem. 2014;6:77–90. doi: 10.4155/fmc.13.189. [DOI] [PubMed] [Google Scholar]
- 51.Phillips AJ, Sudbery I, Ramsdale M. Apoptosis induced by environmental stresses and amphotericin B in Candida albicans. Proc. Natl. Acad. Sci. 2003;100:14327–14332. doi: 10.1073/pnas.2332326100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cho J, Lee DG. The antimicrobial peptide arenicin-1 promotes generation of reactive oxygen species and induction of apoptosis. Biochimica et Biophysica Acta BBA-General Subjects. 2011;1810:1246–1251. doi: 10.1016/j.bbagen.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 53.Zhu J, Krom BP, Sanglard D, Intapa C, Dawson CC, Peters BM, Shirtliff ME, Jabra-Rizk MA. Farnesol-induced apoptosis in Candida albicans is mediated by Cdr1-p extrusion and depletion of intracellular glutathione. PLoS ONE. 2011;6:e28830. doi: 10.1371/journal.pone.0028830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee J, Lee DG. Novel antifungal mechanism of resveratrol: Apoptosis inducer in Candida albicans. Curr. Microbiol. 2015;70:383–389. doi: 10.1007/s00284-014-0734-1. [DOI] [PubMed] [Google Scholar]
- 55.Mah TF. Biofilm-specific antibiotic resistance. Future Microbiol. 2012;7:1061–1072. doi: 10.2217/fmb.12.76. [DOI] [PubMed] [Google Scholar]
- 56.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12:913–922. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 57.Bink A, Vandenbosch D, Coenye T, Nelis H, Cammue BP, Thevissen K. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob. Agents Chemother. 2011;55:4033–4037. doi: 10.1128/AAC.00280-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hwang B, Hwang JS, Lee J, Kim JK, Kim SR, Kim Y, Lee DG. Induction of yeast apoptosis by an antimicrobial peptide, Papiliocin. Biochem. Biophys. Res. Commun. 2011;408:89–93. doi: 10.1016/j.bbrc.2011.03.125. [DOI] [PubMed] [Google Scholar]
- 59.Bohmont C, Aaronson LM, Mann K, Pardini RS. Inhibition of mitochondrial NADH oxidase, succinoxidase, and ATPase by naturally occurring flavonoids. J. Nat. Prod. 1987;50:427–433. doi: 10.1021/np50051a014. [DOI] [PubMed] [Google Scholar]
- 60.Viuda-Martos M, Fernández-López J, Pérez-Álvarez JA. Pomegranate and its many functional components as related to human health: A review. Comprehensive Rev. Food Sci. Food Safety. 2010;9:635–654. doi: 10.1111/j.1541-4337.2010.00131.x. [DOI] [PubMed] [Google Scholar]
- 61.Vučić V, Grabež M, Trchounian A, Arsić A. Composition and potential health benefits of pomegranate: A review. Curr. Pharm. Des. 2019;25:1817–1827. doi: 10.2174/1381612825666190708183941. [DOI] [PubMed] [Google Scholar]
- 62.Houston, D. Towards a Nanomedicine-Based Broad-Spectrum Topical Virucidal Therapeutic System (Doctoral dissertation, Cardiff University, 2011).
- 63.Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007;2:875–877. doi: 10.1038/nprot.2007.102. [DOI] [PubMed] [Google Scholar]
- 64.Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Sep. Purif. Technol. 2005;41:49–55. [Google Scholar]
- 65.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998;64:711–713. doi: 10.1055/s-2006-957563. [DOI] [PubMed] [Google Scholar]
- 66.Wayne, P.A. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI document M27-A2 (2002).
- 67.Endo EH, Ueda-Nakamura T, Nakamura CV, Filho BP. Activity of spray-dried microparticles containing pomegranate peel extract against Candida albicans. Molecules. 2012;17:10094–10107. doi: 10.3390/molecules170910094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Odds FC. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003;52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 69.Miles AA, Misra SS, Irwin JO. The estimation of the bactericidal power of the blood. Epidemiol. Infect. 1938;38:732–749. doi: 10.1017/s002217240001158x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Powell LC, Pritchard MF, Ferguson EL, Powell KA, Patel SU, RyeM PD, Sakellakou SM, Buurma NJ, Brilliant CD, Copping JM, Menzies GE. Targeted disruption of the extracellular polymeric network of Pseudomonas aeruginosa biofilms by alginate oligosaccharides. NPJ Biofilms Microbiomes. 2018;4:1. doi: 10.1038/s41522-018-0056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heydorn A, Nielsen AT, Hentzer M, Sternberg C, Givskov M, Ersbøll BK, Molin S. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 72.LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2ʹ, 7ʹ-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992;5:227–231. doi: 10.1021/tx00026a012. [DOI] [PubMed] [Google Scholar]
- 73.Watanabe H, Azuma M, Igarashi K, Ooshima H. Analysis of chitin at the hyphal tip of Candida albicans using calcofluor white. Biosci. Biotechnol. Biochem. 2005;69:1798–1801. doi: 10.1271/bbb.69.1798. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.