Abstract
An in vitro culture system of bovine vaginal epithelial cells (BVECs) was developed to study the cytopathogenic effects of Tritrichomonas foetus and the role of lipophosphoglycan (LPG)-like cell surface glycoconjugates in adhesion of parasites to host cells. Exposure of BVEC monolayers to T. foetus resulted in extensive damage of monolayers. Host cell disruption was measured quantitatively by a trypan blue exclusion assay and by release of 3H from [3H]thymidine-labeled host cells. Results indicated contact-dependent cytotoxicity of host cells by T. foetus. The cytopathogenic effect was a function of T. foetus density. Metronidazole- or periodate-treated T. foetus showed no damage to BVEC monolayers. A related human trichomonad, Trichomonas vaginalis, showed no cytotoxic effects, indicating species-specific host-parasite interactions. A direct binding assay was developed and used to investigate the role of a major cell surface LPG-like molecule in host-parasite adhesion. The results of competition experiments showed that the binding to BVECs was displaceable, was saturable, and yielded a typical binding curve, suggesting that specific receptor-ligand interactions mediate the attachment of T. foetus to BVECs. Progesterone-treated BVECs showed enhanced parasite binding. T. foetus LPG inhibited the binding of T. foetus to BVECs; the LPG from T. vaginalis and a variety of other glycoconjugates did not. These data imply specificity of LPG on host-parasite adhesion. Periodate-treated parasites showed no adherence to host cells, indicating the involvement of carbohydrate containing molecules in the adhesion process.
Bovine trichomoniasis is a sexually transmitted disease caused by a flagellated protozoan, Tritrichomonas foetus. In cows the disease is associated with infertility, vaginitis, endometritis, abortion, and sometimes pyometra (7, 12, 13). Bovine trichomoniasis causes considerable economic loss in the United States as well as in other parts of the world (7, 15, 19, 32, 34). Initially, the parasites are transmitted from the bull to the cow during coitus. Thus, the trichomonads first encounter vaginal epithelial cells and the organisms subsequently migrate to the uterus. The parasite is confined to the epithelial cells of the endometrium and placenta. In cows, the life span of T. foetus is self-limiting, and bulls, which remain infected for life, represent a repository of infection (4, 7).
It has been reported that T. foetus and the related human pathogen Trichomonas vaginalis adhere to host cells and damage them through a contact-dependent cytotoxic mechanism (1–5, 9, 13, 18). There are only a few published reports that illustrate the cytopathogenic effect of T. foetus on mammalian cells (9, 10, 25). However, those studies did not use natural bovine target cells; instead, they used cell lines such as HeLa cells (a human cervical cell line), bovine lymphosarcoma cells, and Madine-Darby canine kidney epithelial (MDCK) cells. Furthermore, although it has been suggested that T. foetus can directly damage bovine placental tissue and probably causes severe cell destruction (8, 22), little information was provided to show the cytotoxicity against the natural specific host targets, bovine vaginal epithelial cells. Parasitism of specific host target cells by T. foetus is a critical step in establishing bovine trichomoniasis.
The ability of trichomonads to adhere to host cells plays an integral role in establishing infections, and such interactions may be mediated by glycoconjugates. T. foetus parasites contain several surface carbohydrate-containing antigens, some of which have been reported to play an important role in the pathogenesis of bovine trichomoniasis (6, 9, 10, 12, 13, 16). Corbeil and coworkers (12, 13, 16) isolated a trichomonad cell surface glycoconjugate (TF1.17) of approximately 50 to 70 kDa and have shown that corresponding monoclonal antibodies (MAbs TF1.17 and TF1.15) inhibit adhesion of parasites to host cells. Burgess et al. have also isolated a parasite surface glycoconjugate, Tf190 (9, 10), which has been demonstrated to inhibit adsorption of the parasite to host cells. Furthermore, we have isolated and partially characterized a novel lipophosphoglycan (LPG) from T. foetus (26, 27) of approximately 50 to 70 kDa. This molecule, designated TF-LPG, is the major glycoconjugate (2 × 106 to 3 × 106 copies/organism) on the cell surface of the parasite and is anchored to the surface by an inositol-phosphoceramide moiety.
Since T. foetus parasitizes bovine vaginal epithelial cells (BVECs), and the parasites possess a major LPG-like glycoconjugate, we have studied the interactions of purified LPG with cultured BVECs. For a full understanding of the mechanism of infection, it is essential to study the cytopathogenic nature and specificity of T. foetus infection by using the specific host target cells, BVECs. To facilitate these studies, it was necessary to develop a cell culture system of BVECs that was devoid of fibroblasts and could be stored frozen and thawed for use. In this report, we demonstrate (i) the culturing of BVECs, (ii) hormonal effects on adhesion of T. foetus to BVECs, (iii) the involvement of LPG-like glycoconjugates in the attachment of T. foetus to BVECs, and (iv) the cytotoxicity of T. foetus toward host cells. These studies provide important information regarding host-parasite interactions and the biological attributes of cell surface glycoconjugates such as LPG in the pathogenesis of bovine trichomoniasis.
MATERIALS AND METHODS
Trichomonads.
T. foetus KV1 (ATCC 30924), D1 (obtained from L. Corbeil, University of California, San Diego), and Montana (obtained from D. Burgess, Montana State University) were grown in Diamond’s medium (14) with 10% heat-inactivated fetal calf serum (HyClone Laboratories, Inc.) at 37°C in screw-capped 100- and 500-ml serum bottles. T. vaginalis CDC-85 (ATCC 50143) was also grown in Diamond’s medium. The initial pHs were 7.2 for T. foetus and 6.2 for T. vaginalis and the inoculum was 106 ml−1. Organisms were counted at 24 h (Coulter counter model ZF; Coulter Electronics), harvested in late log phase (24 h) by centrifugation (5,000 rpm), and washed twice with cold phosphate-buffered saline (PBS; pH 7.2). T. foetus KV1 was used in most of the binding experiments; the other parasite strains were used to evaluate adhesion specificity to BVECs. For binding experiments, T. foetus parasites were radiolabeled with [35S]cysteine-methionine (Tran35S-label metabolic reagent; 2 mCi/100-ml culture) 12 to 14 h prior to harvest.
TF-LPG.
TF-LPG was isolated as previously described (25). Briefly, the delipidated residue of organisms was extracted with solvent E (H2O, ethanol, diethyl ether, pyridine, NH4OH, 15:15:5:1:0.017). The extract was treated with pronase and purified by Sepharose CL-4B and octyl-Sepharose column chromatography, followed by methanol precipitation. The purity of TF-LPG was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Culture of BVECs.
Small fragments of vaginal mucosa dissected from bovine fetal reproductive tracts were obtained from fetuses of crown rump length 75 to 90 cm (corresponding roughly to the last month of gestation). The fragments (ca. 0.5 mm) were washed and placed in chick embryo extract (GIBCO) before being transferred to 100-μl drops of chick plasma in plastic petri dishes. After 30 min at room temperature to allow clot formation, culture medium (Williams complete medium [GIBCO] [21, 31] fortified with charcoal-stripped fetal bovine serum, epidermal growth factor, insulin, transferrin, selenium, and antibiotic-antimycotic) was added. Dishes were incubated at 38°C in an atmosphere of 5% CO2 in air. The explants were removed after a corona of 5 to 8 mm had formed, usually within 1 to 2 weeks. Fibroblasts and epithelial cells were separated by differential trypsinization. Cultured cells were washed with Ca- and Mg-free buffer and then exposed to 0.05% trypsin and 0.53 mM EDTA in Ca- and Mg-free buffer. The cells were kept under microscopic observation while the fibroblasts rounded up and became detached. The flasks were tapped to loosen the detached fibroblasts, which were removed by aspiration. Trypsin was inactivated by addition of serum-containing medium. This procedure was repeated if necessary. The purity of epithelial cells was determined by using an Histostain-SP immunostaining kit (Zymed Laboratories) and anticytokeratin MAb (Boehringer Mannheim). Contamination of fibroblasts was identified by staining with a MAb against vimentin (Dako). Vaginal epithelial cells were cultured to confluence and subcultured. For experimental studies, cells were subcultured in 24-well cluster plates, and experiments were performed when confluence was reached. At this time, each well contained approximately (4 ± 0.45 [standard deviation]) × 105 cells. These cells can be frozen and rethawed for further experimentation as needed.
Binding assays.
Binding of T. foetus to BVECs was performed as follows. BVECs were subcultured in 24-well plates and allowed to become confluent in Williams medium (∼[4 ± 0.45] × 105 cells). Radiolabeled trichomonads were washed twice in PBS (pH 7.2) and once in PBS with 1% fetal bovine serum (PBS-F) and then suspended to the desired densities in a mixture of 2 parts of Williams medium (21, 31) (pH 7.4) and 1 part of PBS-F (W-PBSF). Parasites and BVECs were always equilibrated for 15 to 20 min in W-PBSF at 37°C prior to coincubation. We investigated several other media (Diamond’s, Dulbecco modified Eagle, RPMI, etc.) and several different pHs (from 6.0 to 8.0) to optimize adhesion of T. foetus to BVECs. It has been reported that in vivo the pH of bovine vaginal epithelium is approximately 7.6 (13). We found that W-PBSF is best for our experimental purposes (in terms of minimizing pH changes and viability of parasites during incubation period). For binding studies, ∼4 × 106 35S-labeled parasites were added to confluent BVECs and incubated for 30 min (37°C, 5% CO2 in air). The total volume was adjusted to 1.2 ml. At the end of the incubation period, the wells were washed gently five times with warm PBS-F (37°C). The BVECs were solubilized with 1 N NaOH, and the amount of adherent radioactivity was determined by liquid scintillation counting. For competition experiments with LPG, different amounts of LPG (2 to 120 μg) were added to BVECs and equilibrated for 15 min (37°C, 5% CO2 air) before the addition of parasites. All experimental points were performed in quadruplicate, and at least one sample was always visualized by phase-contrast microscopy to examine attachment, parasite viability, and motility. The mean of the data was presented for each experiment. Each experiment was repeated three times. In control experiments, other carbohydrate-containing components such as LPGs from other parasites, glycoproteins, glycolipids, lipopolysaccharide from bacteria, and saccharides (25 to 50 μg/wells) were added to each well. To examine the saturation of BVEC membrane sites, the following experiments were performed. Fixed amounts (4 × 106) of 35S-labeled parasites were added to BVECs along with various densities of unlabeled parasites (the ratio of labeled to unlabeled organisms ranged from 1:0.1 to 1:10 in one experiment and 1:1 to 1:100 in other experiments).
Hormone-treated BVECs.
BVECs were treated with progesterone (50 ng/ml), estradiol (200 pg/ml), and progesterone plus estradiol for 5 days before confluence was reached. Radiolabeled parasites were added to the wells containing hormone-treated and normal BVECs. After incubation for 30 min, the wells were washed with PBS-F and counted for radioactivity to determine percent adhesion as described above.
Chemical treatment of T. foetus for binding and cytotoxicity experiments.
In some cases, the PBS-washed parasites were treated with metronidazole (200 μg/ml for 5 min) or periodate (10 mM in 50 mM sodium acetate buffer [pH 4.5] for 10 min) at room temperature. Under these conditions, parasites became nonmotile but not lysed and appeared to retain their cellular integrity as visualized by phase-contrast microscopy. Chemically treated parasites were further washed twice with PBS-F and resuspended in a W-PBSF before being added to the wells containing BVECs.
Cytotoxicity of BVECs mediated by T. foetus.
Two assay methods were used to assess the cytotoxicity of parasites: (i) the colorimetric trypan blue exclusion assay and (ii) assay of the release of radioactivity from [3H]thymidine-labeled host cells, as reported earlier for T. vaginalis (1, 3).
(i) Colorimetric assay.
For each experimental condition, BVECs in 24-well plates were equilibrated in W-PBSF for 15 min at 37°C (under 5% CO2) before the addition of parasites. Approximately 8 × 105 parasites were added to monolayers (4.0 × 105 cells) and incubated for 2 to 24 h. For control experiments, parasites were not added to BVECs in the wells. At the end of incubation periods, the wells were gently washed two times with warm PBS, and remaining cells were fixed to the wells with 2% (wt/vol) formaldehyde in PBS for 10 min. The wells were washed with PBS and stained with 0.13% crystal violet as reported earlier (1). The stained product was subsequently washed twice with distilled water and air dried. The stained cells were finally solubilized in 1% (wt/vol) SDS in 50% (vol/vol) ethanol, and the intensity of staining was read at a wavelength of 570 nm. Each experiment was performed in quadruplicate, and the mean of the data was presented. The nature and extent of host cell damage caused by parasites were also assessed by using a phase-contrast inverted microscope. Cytotoxicity was calculated as 1 − (E/C); i.e., all measurements of experimental (E) samples (A570) were indexed to those of control (C) samples (E/C), which showed no loss of cells from the well, and subtracted from 1.0. In control experiments, we measured the cytotoxicity to BVECs of a related human trichomonad, T. vaginalis (8 × 105 parasites/4.0 × 105 BVECs/well). We also examined the cytotoxic effect of T. foetus parasites which had been pretreated by exposure to periodate (10 mM) or metronidazole (200 μg/ml). The incubation times for the above experiments ranged from 3 to 24 h. In all of these experiments, the total amounts of T. vaginalis and metronidozole- or periodate-treated T. foetus were the same (8 × 105 parasites/well) as it was for untreated T. foetus.
(ii) Release of 3H by host cells.
It has been reported that target cells labeled with radioactive DNA precursors released labeled DNA in the presence of the relevant pathogenic organisms, indicating that the microbes damaged the membrane of the host cells (3, 20). We used [3H]thymidine to label the BVEC monolayers in order to assess the damage of host cells by T. foetus. Confluent monolayers of BVECs were labeled with [3H]thymidine (8 μCi/well; specific activity, 40 to 60 Ci/mmol; ICN) overnight. After gentle removal of media, wells were washed with W-PBSF prior to the addition of different amounts of parasites for the desired length of time. Control experiments contained no parasites. At the end of the experimental periods, the incubation medium was collected and the release of 3H was determined by liquid scintillation counting.
Contact-dependent cytopathic effect.
To test the hypothesis that the cytopathic effect of T. foetus on BVECs is contact dependent, four experimental groups were studied. In the first group, T. foetus (4 × 106 parasites/well) was added to monolayers of BVECs. The second group consisted of parasites separated from the cell monolayer by a permeable collagen membrane (CoStar). The third and fourth groups were control groups with parasites only and BVECs only, respectively. There were 12 replicates for each group. The parasites and BVECs were always equilibrated for 15 min in incubation buffer (W-PBSF) before the beginning of experiments. Plates were incubated for the desired lengths of times. An inverted phase-contrast Nikon microscope was used to evaluate contact-dependent cytopathogenicity. Data were recorded every 6 h for 72 to 100 h.
RESULTS
Earlier studies on the recognition and binding of T. foetus parasites to mammalian cells used HeLa cells (9, 10), MDCK cells (25), and primary epithelial cells from scrapings of vaginal surfaces (13). Adhesion is regarded as an essential feature in the pathogenesis of microorganisms, and it has been shown that both bovine and human trichomonads attach to HeLa cells. Although significant data were obtained with each of the above culture systems, they did not study adhesion to natural host cells. They used either unnatural model systems or BVECs that were contaminated with other cells, primarily fibroblasts. Therefore, to define host specificity more fully, we have established an in vitro culture system of BVECs (Fig. 1A) to study T. foetus cytoadhesion. The results of keratin and vimentin staining of BVECs showed that usually more than 93% of the cells were epithelial cells. This dramatic enrichment was possible because fibroblasts were more readily removed by short-term trypsin exposure, while vaginal epithelial cells adhered avidly to the plastic. This is the first study of its kind where relatively pure BVECs have been subcultured for experimental purposes. An additional advantage of this system is that cells can be frozen and thawed for use as needed.
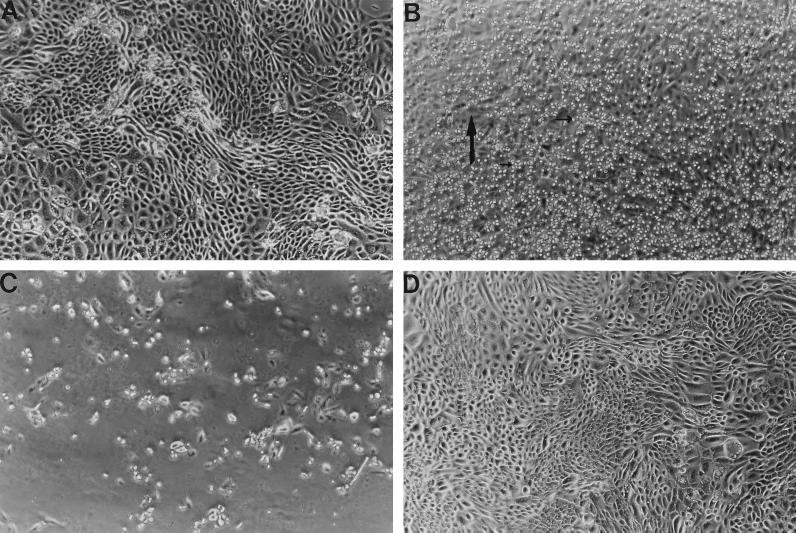
FIG. 1.
(A) BVECs not exposed to T. foetus. (B) BVECs coincubated with T. foetus for 30 min and then washed three times. Note abundant adhesion of parasites to the monolayer. Large and small arrows indicate BVECs and parasites, respectively. (C) BVECs coincubated with T. foetus for 48 h. Monolayers are completely disrupted, and only dead parasites are observed. (D) BVECs exposed to periodate-treated T. foetus for 48 h. No adherence of parasites and no destruction of monolayers are apparent. (E) BVECs exposed to metronidazole-treated T. foetus for 48 h. Only a few parasites adhere to BVECs, and there is no destruction of monolayers.
A number of BVEC culture conditions were optimized. For example, pH is a critical element for both host cell growth and parasite viability. The pH of bovine vaginal epithelium in vivo has been estimated to be approximately 7.6 (13). The initial pH for T. foetus growth medium is 7.2, and the BVECs are grown in Williams medium (pH 7.4). It has been shown that motility of T. foetus is maintained best in PBS-F (pH 7.2) and that a suitable pH for the adherence of T. foetus to vaginal epithelial cells is between pH 6.0 and 7.5 (13). Therefore, we used a mixture of Williams medium and PBS-F (2:1). These conditions support the viability of both host cells and parasites and minimize pH changes during the incubation period. Other media such as RPMI, Dulbecco modified Eagle, and Diamond’s failed to provide satisfactory results in our studies.
Specificity of T. foetus adherence to BVECs.
Phase-contrast microscopy showed that early in the adhesion process, T. foetus used its posterior flagellum to adhere to the host cell. Subsequently, the cell body appears apposed to the host cell. Figure 1B shows T. foetus parasites adhered to BVECs. Once a viable culture system was established, it was used to study the specificity of parasite-host interactions. BVECs were incubated with T. foetus or T. vaginalis parasites in separate culture wells for times ranging from 30 min to 4 days and visualized by phase-contrast microscopy. T. foetus parasites adhere to BVECs with much stronger avidity than do T. vaginalis parasites, indicating species specific host-parasite interactions. These observations also imply that surface molecules from T. foetus mediate the binding of T. foetus to BVECs, but cell surface molecules from T. vaginalis do not support strong binding. This point is further supported by binding studies described below.
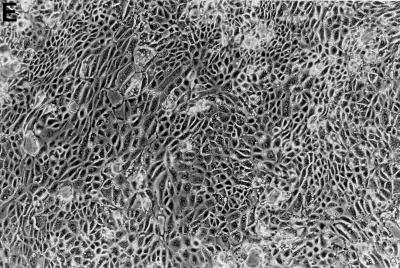
Bovine vaginal fibroblasts (BVF) (Fig. 2A) were cultured separately to examine host cell specificity. T. foetus parasites were incubated with confluent BVF monolayers and allowed to adhere for 3 h to 5 days. In one set of experiments, cells were washed three times with PBS after 3 h and examined under the microscope. Conspicuously, fewer parasites appeared to adhere to BVF (Fig. 2B) than to BVECs (Fig. 1B). In a second set of experiments, parasites were allowed to adhere for 96 h, washed, and examined under the microscope. There was no damage to BVF monolayers even after 96 h (Fig. 2D), and monolayers appeared similar to control BVF (Fig. 2C). In a group of experiments in which the cultures were unwashed, coincubation of parasites with BVF for 5 days showed no disruption to fibroblast monolayers (data not shown). Parasites remained alive and motile, and significant multiplication occurred. These results clearly indicate host cell specificity and suggest that BVF probably provide nutrients for parasite survival.
FIG. 2.
(A) BVF after washing (control group). (B) BVF exposed to T. foetus for 3 h and then washed three times. Note relatively sparse adhesion of parasites to cell. (C) Control BVF 96 h after washing. The cell monolayer is intact and in good condition. (D) BVF coincubated with T. foetus for 96 h and then washed. Parasites remain alive without inflicting conspicuous damage on the monolayers.
Cytopathogenic effects of T. foetus on BVECs.
Having established a functional culture system for BVECs allowed us to investigate cytotoxicity directly. Initial observations were made by phase-contrast microscopy. In one experimental group, T. foetus coincubated with BVECs caused severe disruption of the BVEC monolayer around 48 h, followed soon after by death of the parasites (Fig. 1C). The median survival time for the parasites was 48 h. After death of all of the parasites, the epithelial monolayer became reestablished in some wells by outgrowth of surviving epithelial cells (at approximately 120 h), which had not been evident 24 to 48 h previously. When T. foetus parasites were cultivated under identical conditions but in the absence of BVECs (group 3), the median survival time was also 48 h. Subjectively, however, the cells appeared more sluggish in mobility than parasites cocultured with BVEC. When the T. foetus parasites were physically removed from the BVEC monolayer by a permeable filter (group 2), the epithelial cells suffered no visible damage. Interestingly, parasite survival time was significantly increased (P = 0.001, Kruskal-Wallis analysis of variance followed by Student-Neuman-Kuels post hoc test) to a median of 96 h. In a control group, BVECs in the absence of parasites showed no cytopathic effects.
These observations imply that the cytopathic effect of T. foetus on BVECs is contact dependent and also that some factor related to coculture extends the motile life span of the parasites. The latter effect may be as simple as a lowering of the oxygen tension by the epithelial cells, or it may be the result of a more complex interaction.
As shown in Fig. 1B, many more untreated parasites than either metronidazole- or periodate-treated parasites adhere to BVECs. In a different set of coincubation experiments (30 min to 48 h) with chemically exposed T. foetus, a dramatic reduction of parasite adherence to host cells was observed (Fig. 1D and E). There was no evident damage of BVEC monolayers by T. foetus treated with periodate (Fig. 1D) or metronidazole (Fig. 1E). The results show that some metronidazole-treated parasites adhered to host cells but did not destroy them, consistent with our observation (described below) that metronidazole-killed parasites displace some binding. The fact that metronidazole-treated parasites adhere to host cells suggest that adhesion of T. foetus is necessary but not sufficient to cause damage to BVECs. The effect of periodate suggests that parasite surface glycoconjugates are involved in host-parasite interactions.
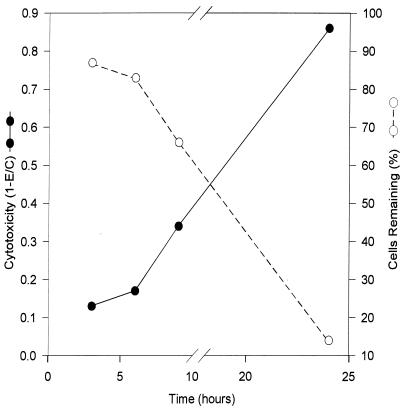
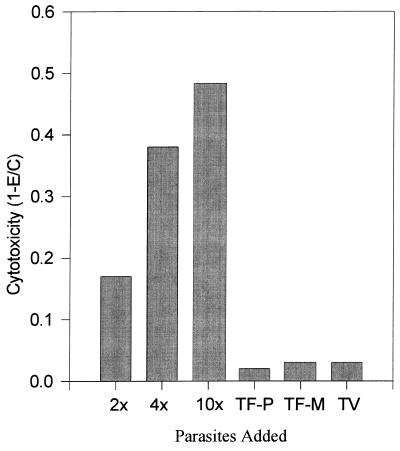
The cytopathic effect of T. foetus on BVEC monolayers was further studied in two quantitative assays. The first is a trypan blue exclusion assay described by Alderete and Pearlman (3). Figure 3 depicts the kinetics of damage to BVECs by T. foetus. Cytotoxic effects were seen as early as 3 h after parasite exposure, and nearly complete destruction occurred by 24 h. We also examined cytotoxicity at higher parasite densities. As shown in Fig. 4, increasing the parasite-to-host cell ratios (2:1, 4:1, and 10:1) intensified the cytotoxic effect measured at 6 h. Clearly, the cytopathogenic effect is a function of T. foetus density. The 6-h point was chosen to show the maximal effect on cytotoxicity (from 15 to 50%) and to preclude significant multiplication of parasites during the experiment (9 to 24 h).
FIG. 3.
Time course of T. foetus cytotoxicity. Cytotoxicity in BVEC monolayers, determined by trypan blue exclusion assay. Each well contained 8 × 105 parasites and 4 × 105 BVECs. Cytotoxicity was calculated as described in Materials and Methods.
FIG. 4.
Concentration dependence of cytotoxicity of BVEC monolayers in the presence of increasing ratios of T. foetus to host cells (2-, 4-, and 10-fold). Controls include T. foetus treated with periodate (TF-P) and metronidazole (TF-M) and the related human trichomonad, T. vaginalis (TV). In controls, the ratios of parasites to BVECs was 2:1. The incubation time was for 6 h. Similar results were obtained when TF-P, TF-M, and TV were incubated with BVECs for 24 h. However, 80 to 85% of the BVEC monolayers were destroyed at 24 h in presence of T. foetus. For the formula used to determine cytotoxicity, see Materials and Methods.
The trypan blue assay was also used to study the effects of periodate and metronidazole treatment of T. foetus on cytotoxicity (Fig. 4). These treatments nearly eliminated cytotoxicity over the course of 6 to 24 h. However, microscopic examination showed that some metronidazole-treated parasites adhere to host cells, although no damage to host cells was observed (Fig. 1E). Periodate-treated T. foetus showed no adherence to host cells (Fig. 1D) during microscopic examination, suggesting involvement of carbohydrate-containing molecules in the adhesion processes. In a control experiment, incubation of the pathogenic human trichomonad T. vaginalis with BVECs showed no cytotoxic effects, indicating species-specific host-parasite interactions.
We also examined the release of 3H from [3H]thymidine-labeled host cells incubated with T. foetus (data not shown). T. foetus in contact with radiolabeled BVECs showed detectable levels of 3H release (1.5, 2.5, 3.4, and 5.6 times control levels at 3, 6, 9, and 24 h, respectively) over a 24-h period. In control experiments, radiolabeled BVEC monolayers in the absence of T. foetus showed no release of radioactive material. An increased ratio of parasites to BVECs (2:1, 10:1, and 20:1) showed greater release of 3H (1.5, 3, and 3.5 times control levels, respectively). These observations are entirely consistent with results of the microscopic and trypan blue assays.
Involvement of LPG in the attachment of T. foetus to BVECs.
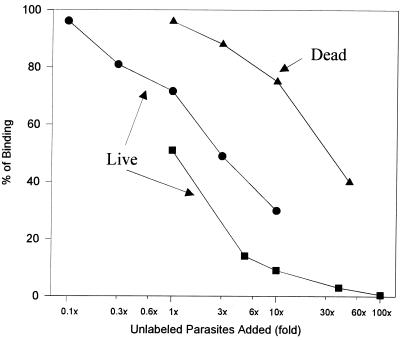
Having obtained microscopic evidence that the binding of parasites is species specific, we developed a direct binding assay to examine the role of individual molecules in host-parasite adhesion. Significant evidence implicates LPG in the attachment of T. foetus to host cells. Therefore, binding studies were performed to directly test the hypothesis that LPG mediates attachment to BVECs. Initially, we studied the binding characteristics of radiolabeled T. foetus to BVECs. The results of competition experiments show that the binding to BVECs is displaceable, is saturable, and yields a typical binding curve (Fig. 5). A 100-fold excess of unlabeled parasites completely displaces binding of radiolabeled organisms. These results suggest that specific receptor-ligand interactions mediate the attachment of T. foetus to BVECs.
FIG. 5.
Inhibition of radiolabeled T. foetus adhesion to BVECs by unlabeled T. foetus. Live unlabeled parasites and parasites killed with metronidazole (200 μg/ml, 20 min) were incubated with BVECs and radiolabeled T. foetus as described in Materials and Methods. Dead parasites were washed three times with PBS-F before coincubation with labeled parasites. Various densities of unlabeled parasites were coincubated with labeled parasites (4 × 106) in a total volume of 1.2 ml for 30 min at 37°C (5% CO2). Binding was determined as described in the text.
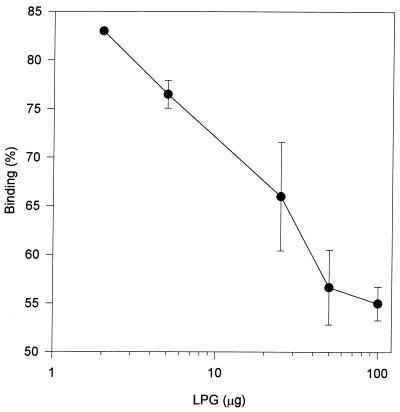
The studies were extended to include the effects of purified LPG on parasite adhesion (Fig. 6). Inhibition is observed even with amounts as small as 2 μg of purified LPG. As summarized in Table 1, the binding of parasites to BVECs is inhibited 40 to 50% by TF-LPG. In contrast, LPGs from T. vaginalis, Leishamania donovani, and Trypanosoma cruzi do not inhibit adhesion. Furthermore, mild acid treatment (0.04 M trifluoroacetic acid, 100°C, 12 min) of TF-LPG destroyed its inhibitory activity. A variety of other glycoproteins, glycolipids, and saccharides (25 to 50 μg/well) were also ineffective at displacing parasites in the assay. These data demonstrate the specificity of LPG on host-parasite adhesion and support the microscropic observations shown in Fig. 1B.
FIG. 6.
Inhibition of T. foetus adhesion to BVECs with TF-LPG. Approximately 4 × 106 35S-labeled T. foetus parasites were added to confluent BVECs containing variable concentrations of TF-LPG in a total volume of 1 ml and incubated for 30 min at 37°C (5% CO2). Binding was determined as described in the text.
TABLE 1.
Inhibition of T. foetus binding to BVECs with TF-LPG and several other glyosylated compoundsa
| Compound (25–50 μg/well) |
% Inhibition |
|---|---|
| TF-LPG | 40–50 |
| TF-LPG, mild acid treated | 1–2 |
| T. vaginalis LPG | 2 |
| Lipopolysaccharide (bacteria) | 1–2 |
| L. donovani LPG | 0 |
| T. cruzi 35/50 LPG | 0 |
| Cerebrosides | 0 |
| Ovalbumin | 1 |
| Disaccharide-trisacchide mix | 0 |
BVECs were incubated (10 min, 37°C) with individual carbohydrate-containing components prior to the addition of 35S-labeled T. foetus parasites (4 × 106) to each well. Binding was determined as described in the text.
Because live parasites were used in these studies, it is possible that some of the binding displacement was due to their metabolic activity. To demonstrate that the binding displacement is not due to changes in pH or to secretion of metabolites from parasites, we performed an identical competition experiment with unlabeled T. foetus killed by treatment with metronidazole (the ratios of 35S-labeled to unlabeled dead parasites ranged from 1:1 to 1:50). The results show that the unlabeled dead parasites (50-fold) displaced binding up to 60% (Fig. 5). This result further reinforces our hypothesis that surface molecules such as LPG are involved in adhesion of T. foetus to BVECs.
Hormonal effects on adhesion to T. foetus to BVECs.
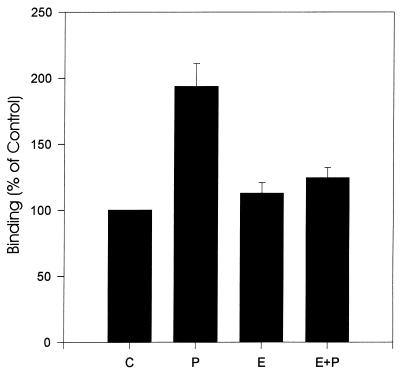
Since bovine infection with T. foetus is very likely influenced by the estrus cycle, we examined the effects of steroid hormones on the adhesion of parasites to BVECs. As shown in Fig. 7, a greater number of parasites bound to progesterone-treated BVECs (P < 0.05 by Student’s t test). The effect of treatment of BVECs with estrogen alone and estrogen plus progesterone was not statistically significant.
FIG. 7.
Adhesion of T. foetus to BVECs treated with hormone. C, control (untreated cells); P, progesterone-treated BVECs; E, estradiol-treated cells; E+P, estradiol-plus-progesterone-treated cells.
DISCUSSION
Knowledge of the nature of host cell cytotoxicity by T. foetus is critical to a complete understanding of pathogenesis. Cytotoxicity is a major consequence of T. foetus infection. We have used microscopy, spectrophotometry, and 3H release assays to measure the cytotoxicity of parasites to BVECs. The incubation of live T. foetus with BVEC monolayers showed disruption of host cells within 3 h and resulted in a total loss of cells after extended exposure to parasites. These results, along with the initial contact dependence experiment (using parasites separated from BVECs by a permeable collagen membrane insert) imply contact-dependent mechanisms. In the absence of direct contact, there is no damage to host cell monolayers. It is interesting that the parasites survived longer when a barrier was placed between the host cells and parasites. It is possible that the epithelial cells provide some nutrients or a partially anaerobic environment that enhances the survival of T. foetus parasites.
Several cell lines such as HeLa and MDCK cells have been used for cell-trichomonad interaction studies. Those cell lines are parasitized by both T. foetus and T. vaginalis (3, 9, 10, 25). Using our BVEC culture system, we have clearly demonstrated host-parasite specificity since the related human trichomonad T. vaginalis showed no damage to BVECs. Likewise, coincubation of T. foetus parasites with human vaginal epithelial cells resulted in no cytotoxic effect (30). The fact that T. foetus parasites do not destroy BVF clearly indicates host-cell specificity. Silva-Filho and deSouza (25) suggested that trichomonads exert their pathogenic effects on epithelial MDCK cells in culture either by direct contact or by the release of certain components. It is possible that certain proteases and glycosidases found in trichomonad extracts play a role in modulating the interactions of trichomonads with epithelial cells. Thus, it has been reported that the addition of protease inhibitors to the incubation medium decreased epithelial cell disruption by T. foetus (9, 10, 25). Burgess et al. (9) have indicated that some factors, devoid of protease activity, released by T. foetus parasites appeared to be involved in the cytotoxicity of HeLa cells. However, these factors were not characterized or defined. A number of microorganisms have been reported to produce extracellular components which are cytotoxic (11, 17, 20, 33). Our results suggest, however, that cell destruction by T. foetus is a contact-dependent mechanism. It is not known whether T. foetus parasites produce cytotoxic material upon contact with host cells.
The results described in this report demonstrate that we have developed a BVEC culture system which allows quantification of host-parasite binding and can be used to study the mechanism of T. foetus binding to host cells. It is likely that parasite adhesion is a complex process, and the new assay will facilitate studies aimed at determining which molecules are directly involved. Although the data presented here provide strong evidence for the involvement of TF-LPG in adhesion, other studies have suggested that additional surface molecules may be involved in parasite adhesion. Thus, Arroyo et al. (5) and Alderete et al. (2) suggested that four surface proteins of T. vaginalis are involved in adhesion of parasites to HeLa cells, and antibodies directed against these proteins reduced adhesion by approximately 50%. Corbeil and coworkers (6, 12, 13, 16) demonstrated that the surface antigen TF1.17 of T. foetus is involved in adhesion of parasites to epithelial cells obtained from scrapings of bovine vagina. The cytotoxicity of T. foetus to VECs was not determined. The MAbs (TF1.17 and TF1.15) directed against antigen TF1.17 have been shown to immobilize, agglutinate, and prevent adherence of T. foetus parasites to vaginal epithelial cells (13). In an assay based on phase-contrast microscopy, the two MAbs inhibit the adhesion of parasites up to 73% (16). Antigen TF1.17 has been reported to possess the characteristics of a glycoprotein (based on thymol staining and broad diffuse nature of this band) with an approximate molecular mass of 50 to 70 kDa (17, 18). Similarly, Singh (26) reported that TF-LPG has mass of 50 to 70 kDa as determined by SDS-PAGE. In fact, recent evidence indicates that MAbs TF1.17 and TF1.15 react strongly with TF-LPG (28, 29). Furthermore, cattle infected with T. foetus show a strong antibody response to TF-LPG (29). It appears, therefore, that there is an as yet undefined relationship between LPG and TF1.17.
The fact that LPG inhibits binding only up to 40 to 50% and unlabeled parasites (100-fold) displace binding up to 100% suggests that other molecules present on T. foetus surface may also be involved in adhesion. One of the adhesion antigens is TF1.17, described earlier (16); the other is Tf190, identified by Burgess et al. (9, 10). Thus, we examined the effect of MAb 32.3B3.5 (anti-Tf190; obtained from D. Burgess) on the adhesion of T. foetus to BVECs. The MAb inhibits T. foetus binding to BVECs up to 50% (28a). If BVECs are first treated with LPG (40 μg/well) followed by MAb-treated parasites, binding is inhibited up to 60 to 75% (28a). Burgess et al. (9, 10) reported that MAb 32.3B 3.5 lowered the adhesion of parasites to HeLa cells up to 45% in a [3H]uracil-labeled parasite assay. They also observed a reduction in parasite-mediated cytotoxicity. Interestingly, the Tf190 antigen appears to contain monosaccharides and fatty acid with a composition similar to that of TF-LPG (23). These results, along with the observations that the periodate-treated parasites do not adhere or destroy BVECs, strongly suggest the involvement of surface glycoconjugates in the parasitism of T. foetus organisms.
The increased adhesion of parasite to progesterone-treated BVECs is a particularly interesting observation and may have direct relevance in vivo since parasitism peaks during the estrus cycle. The biological significance of this observation may be that it ensures the survival of large numbers of parasites in the luteal (progesterone) phase of the cycle. Therefore, there will be more free parasites in the vaginal discharge during estrus, with a greater chance of infecting the male at the time the cow is receptive to mating. Silva-Filho and Bonilha (24) have reported that α-estradiol enhances the adhesion of the human trichomonad T. vaginalis to MDCK cells.
A thorough examination of the mechanism of host-parasite adhesion requires defined molecules and natural host cells. Two significant advances in the culture system reported here are the purity of the BVECs and the ability to freeze and thaw the cells. This allows the study of host-parasite interactions to take place in a convenient, easily manipulated system. The availability of pure LPG permits studies of its molecular interactions with host cells. Understanding the biochemical nature of TF-LPG will have a direct impact on understanding the domains involved in cell adhesion and may help elucidate the relationships among the TF-LPG, Tf190, and TF1.17 molecules.
The BVEC culture system and binding assay described here will allow further studies of parasite adhesion and infection. Having an assay for investigating the role of specific adhesion molecules will ultimately yield insights into the mechanism of the infection processes. Such an understanding will provide targets for therapeutic treatment and prevention of trichomonal infections.
ACKNOWLEDGMENTS
We thank G. Hayes for useful advice, discussions, and preparation of graphic illustrations. We also thank Suzanne Klaessig for technical assistance and Corinne Duck for the preparation of the manuscript.
This work was supported by the USDA grants 95-2170 and 97-2615 (to B.N.S.).
REFERENCES
- 1.Alderete J F, Garza G E. Specific nature of Trichomonas vaginalis parasitism of host cell surfaces. Infect Immun. 1985;50:701–708. doi: 10.1128/iai.50.3.701-708.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete J F, Lehker M W, Arroyo R. The mechanisms and molecules involved in cytoadherence and pathogenesis of Trichomonas vaginalis. Parastitol Today. 1995;11:70–74. [Google Scholar]
- 3.Alderete J F, Pearlman E. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br J Vener Dis. 1984;60:99–105. doi: 10.1136/sti.60.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews J. Self-limitation and resistance in Trichomonas foetus in cattle. Am J Hyg. 1938;27:149–154. [Google Scholar]
- 5.Arroyo R, Gonzalez-Robles A, Martinez-Palomo A, Alderete J F. Signaling of Trichomonas vaginalis for amoeboid transformation and adhesion synthesis follows cytoadherence. Mol Microbiol. 1993;7:299–309. doi: 10.1111/j.1365-2958.1993.tb01121.x. [DOI] [PubMed] [Google Scholar]
- 6.BonDurant R H, Corbeil R R, Corbeil L B. Immunization of virgin cows with surface antigen TF 1.17 of Tritrichomonas foetus. Infect Immun. 1993;61:1385–1394. doi: 10.1128/iai.61.4.1385-1394.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.BonDurant R H, Honigberg B M. Trichomonads of veterinary importance. In: Krieger J N, editor. Parasitic protozoa. Vol. 9. New York, N.Y: Academic Press; 1994. pp. 111–188. [Google Scholar]
- 8.Burgess D E, Knoblock K F. Identification of Tritrichomonas foetus in secretions of bovine placental tissue with monoclonal antibodies. J Parasitol. 1989;75:977–980. [PubMed] [Google Scholar]
- 9.Burgess D E, Knoblock K F, Daugherty T, Robertson N P. Cytotoxic and hemolytic effects of Tritrichomonas foetus on mammalian cells. Infect Immun. 1990;58:3627–3632. doi: 10.1128/iai.58.11.3627-3632.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burgess D E, McDonald C M. Analysis of adhesion and cytotoxicity of Tritrichomonas foetus towards mammalian cells with monoclonal antibodies. Infect Immun. 1992;60:4253–4259. doi: 10.1128/iai.60.10.4253-4259.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinkenbeard K D, Mosier D A, Confer W W. Transmembrane pore size and role of cell swelling in cytotoxicity caused by Pasteurella hemolytica leukotoxin. Infect Immun. 1989;57:420–425. doi: 10.1128/iai.57.2.420-425.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbeil L B. Vaccination strategies against Tritrichomonas foetus. Parasitol Today. 1994;10:103–106. doi: 10.1016/0169-4758(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 13.Corbeil L B, Hodgson J L, Jones D W, Corbeil R R, Widders P R, Stephens L R. Adherence of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect Immun. 1989;57:2158–2165. doi: 10.1128/iai.57.7.2158-2165.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond L. Techniques of axenic cultivation of Entamoeba histolytica Schaudubb 1903 and E. histolytica-like amoebae. J Parasitol. 1968;54:1047–1056. [PubMed] [Google Scholar]
- 15.Fitzgerald P R. Bovine trichomoniasis. Vet Clin North America Food Anim Pract. 1986;2:277–282. doi: 10.1016/s0749-0720(15)31237-8. [DOI] [PubMed] [Google Scholar]
- 16.Hodgson J L, Jones D W, Widders P R, Corbeil L B. Characterization of Tritrichomonas foetus antigens by use of monoclonal antibodies. Infect Immun. 1990;58:3078–3083. doi: 10.1128/iai.58.9.3078-3083.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keen M G, Hoffman P S. Characterization of a Legionella pneumonophilia extracellular protease exhibiting hemolytic and cytotoxic activities. Infect Immun. 1989;57:732–738. doi: 10.1128/iai.57.3.732-738.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krieger J N, Ravdin J I, Rein M F. Contact-dependent cytopathogenic mechanism of Trichomonas vaginalis. Infect Immun. 1985;50:778–786. doi: 10.1128/iai.50.3.778-786.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvasnicka W G, Taylor R E L, Huang J C, Hank D, Tronstad R J, Bogworth A, Hall M R. Investigations of incidence of bovine trichomoniasis in Nevada and the efficacy of immunizing cattle with vaccines containing Tritrichomonas foetus. Theriogenology. 1989;31:963–971. doi: 10.1016/0093-691x(89)90479-2. [DOI] [PubMed] [Google Scholar]
- 20.Ravdin J E, Croft B Y, Guerrant R L. Cytopathic mechanisms of Entamoeba histolytica. J Exp Med. 1980;152:377–390. doi: 10.1084/jem.152.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiser R F. The effect of selected surfactants on baboon vaginal epithelial organ and tissue culture in vitro. Ph.D. thesis. Ithaca, N.Y: Cornell University; 1993. [Google Scholar]
- 22.Rhyan J C, Stackhouse L L, Quinn W J. Fetal and placental lesions in bovine abortion due to Tritrichomonas foetus. Vet Pathol. 1988;25:350–355. doi: 10.1177/030098588802500503. [DOI] [PubMed] [Google Scholar]
- 23.Shaia C I, Voyich J, Gillis S J, Singh B N, Burgess D E. Purification and expression of the Tf190 adhesion in Tritrichomonas foetus. Infect Immun. 1998;66:1100–1105. doi: 10.1128/iai.66.3.1100-1105.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva-Filho F C, Bonilha V L. Effect of estrogens on the adhesion of Trichomonas vaginalis to epithelial cells in vitro. Braz J Med Biol Res. 1992;25:9–18. [PubMed] [Google Scholar]
- 25.Silva-Filho F C, deSouza W. The interaction of Trichomonas vaginalis and Tritrichomonas foetus with epithelial cells in vitro. Cell Struct Funct. 1988;13:301–310. doi: 10.1247/csf.13.301. [DOI] [PubMed] [Google Scholar]
- 26.Singh B N. Lipophosphoglycan-like glycoconjugate of Tritrichomonas foetus and Trichomonas vaginalis. Mol Biochem Parasitol. 1993;57:281–294. doi: 10.1016/0166-6851(93)90204-b. [DOI] [PubMed] [Google Scholar]
- 27.Singh B N, Beach D H, Lindmark D G, Costello C E. Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Tritrichomonas foetus. Arch Biochem Biophys. 1994;309:273–280. doi: 10.1006/abbi.1994.1113. [DOI] [PubMed] [Google Scholar]
- 28.Singh B N, Beach D H, Shin S T, Gilbert R O. Abstracts of the 25th Meeting of the Society for Glycobiology. Vol. 7. Washington, D.C: Society for Glycobiology; 1997. Pathobiochemistry of lipophosphoglycan in trichomonad parasites, abstr. 117; p. 1044. [Google Scholar]
- 28a.Singh, B. N., and D. E. Burgess. Unpublished data.
- 29.Singh B N, Corbeil L B. Proceedings of the 79th Conference of Research Workers in Animal Diseases. Ames, Iowa: Iowa State University Press; 1998. Glycosylated antigens of Tritrichomonas foetus, abstr. 114. [Google Scholar]
- 30.Singh B N, Elia G, Gilbert R O. Abstracts of the 46th Annual Meeting of the Society for Gynecological Investigation, vol. 6, no. 7, supplement. Washington, D.C: Society for Gynecological Investigation; 1999. Cytotoxic effects of trichomonas vaginalis on human vaginal epithelial cells, abstr. 447; pp. 160A–161A. [Google Scholar]
- 31.Sobel J D, Tehao R, Bozzola J, Levison M E, Kaye D. Human vaginal epithelial multilayer tissue culture. In Vitro. 1979;15:993–1000. doi: 10.1007/BF02619158. [DOI] [PubMed] [Google Scholar]
- 32.Speer C A, White M W. Bovine trichomoniasis: better diagnostics and control could save beef industry $650 million annually. Large Anim Vet. 1991;46:18–20. [Google Scholar]
- 33.Young J D E, Cohn Z A. Molecular mechanisms of cytotoxicity modified by Entamoeba histolytica: characterizations of a pore-forming protein (PFP) J Cell Biochem. 1985;29:299–308. doi: 10.1002/jcb.240290404. [DOI] [PubMed] [Google Scholar]
- 34.Yule A, Skirrow S Z, BonDurant R H. Bovine trichomoniasis. Parasitol Today. 1989;5:373–377. doi: 10.1016/0169-4758(89)90298-6. [DOI] [PubMed] [Google Scholar]