Abstract
Diverse communities of groundwater-dwelling organisms (i.e., stygobionts) are important for human wellbeing; however, we lack an understanding of the factors driving their distributions, making it difficult to protect many at-risk species. Therefore, our study objective was to determine the landscape factors related to the occurrence of cavefishes and cave crayfishes in the Ozark Highlands ecoregion, USA. We sampled cavefishes and cave crayfishes at 61 sampling units using both visual and environmental DNA surveys. We then modeled occurrence probability in relation to lithology and human disturbance while accounting for imperfect detection. Our results indicated that occurrence probability of cave crayfishes was negatively associated with human disturbance, whereas there was a weak positive relationship between cavefish occurrence and disturbance. Both cavefishes and cave crayfishes were more likely to occur in limestone rather than dolostone lithology. Our results indicate structuring factors are related to the distribution of these taxa, but with human disturbance as a prevalent modifier of distributions for cave crayfishes. Limiting human alteration near karst features may be warranted to promote the persistence of some stygobionts. Moreover, our results indicate current sampling efforts are inadequate to detect cryptic species; therefore, expanding sampling may be needed to develop effective conservation actions.
Subject terms: Ecology, Conservation biology
Introduction
Groundwater obligate organisms (hereafter stygobionts1) are important to human wellbeing2. Diverse stygobiont communities support healthy groundwater ecosystems that humans rely on for drinking water and food production3–5. Moreover, some stygobionts are model organisms of evolutionary and human health studies. For example, groundwater amphipods in the genus Niphargus have been used to understand evolutionary ecology because of the high variability in their biological and life-history traits and the diversity of habitats in which they are found6. Additionally, some cavefishes are model organisms for examining insulin resistance, which has potential implications for diabetes research7. Unfortunately, many groundwater species are at risk of extinction.
Stygobiont populations have inherent risks of extinction that are exacerbated by human threats. Many stygobiont species have narrow ranges8,9, are long-lived (e.g.10,11), reach sexual maturity at a later age (e.g.12,13), and lay fewer and larger eggs (e.g.14–16)—traits which are often associated with increased extinction risk17,18. Additionally, stygobiont persistence is threatened by land-use changes (e.g., agriculture and urbanization), direct human contact (e.g., trampling), habitat loss (e.g., groundwater overexploitation), and climate change, among other threats2,19,20. In fact, about 70% of subterranean fauna are listed as threatened, vulnerable, or extinct21. Our ability to address these threats and conserve and manage stygobiotic diversity is hindered by limited knowledge of the ecological drivers of stygobiont distributions (i.e., the Wallacean shortfall2,19,22).
Occurrence is a fundamental ecological state variable that provides basic information necessary for conservation decisions23. Occurrence may be a useful surrogate for abundance when it is difficult or impossible to estimate population sizes (e.g., a high occurrence probability may reflect high abundance23), which is common for many subterranean species2. In particular, knowing a species’ distribution is useful for directing sampling efforts24, predicting how species respond to climate change25, calculating invasion or extinction risk26, and prioritizing locations for conservation efforts27. Ultimately, understanding changes in the abundances and distributions of stygobionts will be critical for mitigating biodiversity loss in groundwater habitats28. For example, Domínguez-Domínguez et al.29 mapped the distribution of Goodeine fishes in Mexico to determine which springs should be protected to promote their persistence.
At least 469 stygobiont species occur in the United States and Canada21, but studies examining the factors shaping the distributions of stygobionts have been limited largely to Europe (see30 for an overview of these studies). These studies have demonstrated that subterranean species distributions are related to glaciation31,32, geology33, climate34, land use35,36, above-ground vegetation37, and elevation38. These factors may influence stygobiont occurrence specifically or regulate fine-scale features that further define species distributions39,40. For example, geology can influence groundwater chemistry, hydrology, and local habitat availability31,33.
The Ozark Highlands ecoregion has high stygobiotic diversity21, which faces potential threats from human land uses (e.g., the land is 31% and 7% agricultural and urban, respectively41). Therefore, our study goal was to identify the relationship between occurrence of cavefishes and cave crayfishes and landscape variables (i.e., land use, elevation, vegetation index, and lithology). We did not include climate and glaciation in our assessment as those factors would be more relevant at coarser spatial scales. Our research can help managers of karst resources prioritize sites for conservation and management efforts and guide efforts to locate new populations in areas with high occurrence probability. Further, our results add to the limited body of knowledge concerning stygobiont distributions in North America.
Methods
Study area
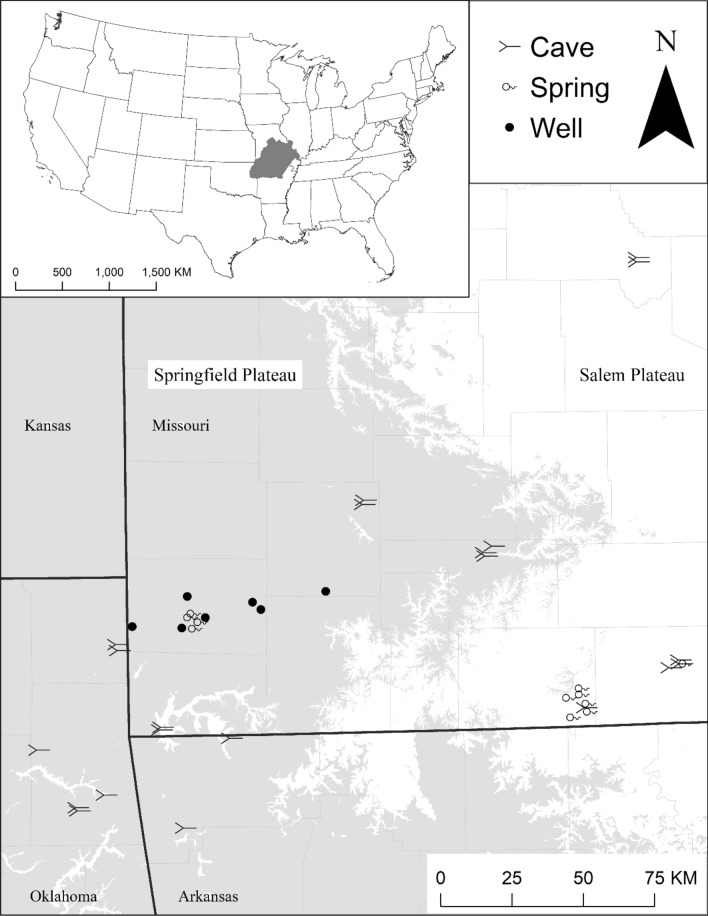
We sampled caves, springs, and wells of the Ozark Highlands ecoregion of Missouri, Arkansas, and Oklahoma in the United States (Fig. 1). The Ozark Highlands ecoregion is relatively wet (97–122 cm of precipitation annually) with moderate temperatures (13–16 °C average annual temperature42). Many lowland areas have been converted from native, warm-season grasses and oak, hickory, and pine forest to agriculture, whereas many upland areas remain forested43. The primary lithologies are limestone and dolomite that through dissolution over time have resulted in cave and spring features emblematic of karst topography44.
Figure 1.
Environmental DNA and visual surveys were conducted for cavefishes and cave crayfishes at 61 sampling units within 21 caves, 12 springs, and seven wells across the Ozarks Highlands ecoregion, USA (dark gray of inset). The lighter gray and white shading on the map represent the Springfield Plateau (i.e., limestone) and Salem Plateau (i.e., dolostone) physiographic regions, respectively. This map was created using ArcGIS software (version 10.4, ESRI, https://www.esri.com/) by Esri. ArcGIS and ArcMap are the intellectual property of Esri and are used herein under license.
Study species
The Ozark Highlands ecoregion is a hotspot of stygobiotic diversity, including snails, copepods, ostracods, amphipods, isopods, amphibians, fishes, and crayfishes21. Our study focused on two species of fishes: the Ozark Cavefish Troglichthys rosae45,46 and Salem Plateau Cavefish Typhlichthys eigenmanni47 and five species of cave crayfishes: Benton County Cave Crayfish Cambarus aculabrum48,49, Bristly Cave Crayfish C. setosus50,51, Delaware County Cave Crayfish C. subterraneus52,53, Oklahoma Cave Crayfish C. tartarus54,55, and Caney Mountain Cave Crayfish Orconectes stygocaneyi56. Although little is known about many of these species, descriptions are quite similar due to convergent evolution (e.g., cryptic behavior, habitat generalists, albinistic, and reduced eyes). Therefore, for our statistical analyses, we treated the species of cavefishes as one taxon, cavefishes, and the species of cave crayfishes as one taxon, cave crayfishes.
Study design
We conducted both environmental DNA (eDNA) and visual surveys for cavefishes and cave crayfishes at 61 discrete habitat patches (hereafter sampling units) within 21 caves, 12 springs, and seven wells (hereafter sites; Fig. 1). Wells were holes dug at old homesites to access groundwater, caves were underground access to groundwater, and springs were areas where the groundwater met the surface. In most instances, a cave, spring, or well was considered a single site; however, two sites were located in the same cave because they represented two rivers with different hydrologic regimes57. We selected one to five sampling units at each site as described by Mouser et al.58. Each sampling unit was selected based on hydrologic barriers such as waterfalls or shallow riffles and was separated by at least one habitat patch that was not sampled. For example, wells, springs, and caves with a single pool of water were all considered a single sampling unit, but larger caves with complex habitat had multiple sampling units. Sampling units were surveyed on one to five occasions from February–May 2017 before spring flooding potentially caused changes in species occurrence.
Species surveys
We collected two water samples (≈ 1-L each) for eDNA analysis following Mouser et al.58. Briefly, water was collected from the water column, filtered across 0.45-μm cellulose-nitrate filters, and the filters were stored in Longmire’s buffer59. We sterilized sampling equipment by immersion in 50% bleach and rinsing in deionized water. Gear was sterilized between sites and, when possible, between sampling units. We filtered distilled water between sites on four occasions to provide negative field controls. After eDNA collection, two observers walked or crawled the entire sampling unit, while carefully searching the whole wetted area of springs and caves for cave crayfishes or cavefishes following Graening et al.46,49. We also visually surveyed hand-dug wells in their entirety for one to six minutes using a spotlight both before and after water samples were collected.
Detection and occurrence covariates
We selected variables hypothesized to influence stygobiont occurrence and detection probability. We calculated a human disturbance index and recorded dominant lithology associated with each site (i.e., sampling units nested within sites received the same values) to estimate occurrence probability of cavefishes and cave crayfishes. We used land-use data to calculate site-specific human disturbance indices following Mouser et al.60. Land-use data were acquired from the 2011 National Land Cover Database41. We used ArcMap (version 10.4, ESRI, Redlands, CA) to create 500-m buffers around each site to assess local disturbance. The proportion of each land-use type within the buffers was calculated and multiplied by the following coefficients: open-space development (1.83), low-intensity development (7.31), medium-intensity development (7.31), high-intensity development (8.67), pasture or hay (2.99), cultivated crops (4.54), and undisturbed (1.00, all other categories). The resulting values were summed across all land-use categories to obtain a final disturbance index for each site. We also calculated the difference between the highest and lowest elevation61 and the average normalized difference vegetation index (NDVI) for 201662 within the 500-m buffers. Finally, we assigned each site to a lithology category based on the predominant rock type (i.e., limestone or dolostone) within the buffers around each site63. To account for variable detection probability, we visually estimated the following covariates for each sampling unit (see58 for complete details): water volume (1.0 m3), water-column velocity (flowing or not flowing), and substrate (coarse or fine).
eDNA analysis
We performed eDNA analysis according to Mouser et al.58. We designed quantitative Polymerase Chain Reaction (qPCR) Taqman® assays to amplify the DNA of each of the study species. Not all assays developed were species-specific; thus, we sequenced a subset of the positive field samples to confirm species identity. We extracted eDNA from the filters using a Qiagen DNeasy® Blood and Tissue Kit by following the “purification of total DNA from crude lysates” protocol with the modifications found in Mouser et al.58. Major modifications included doubling the reagents in steps one to four and decreasing the final elution buffer to 125 μl. We initially extracted a single filter from each sampling unit and our extraction protocol resulted in two subsamples per sampling unit. We amplified eDNA using qPCR. Each subsample was run in triplicate, which resulted in an initial six pseudoreplicates for each sampling unit. We processed the filters until any pseudoreplicates were positive or all were negative for a sampling unit. If any pseudoreplicates were positive, we considered the site positive for the species. We also ran three negative plate controls and a single positive plate control during each qPCR run. The qPCR run was discarded if any of the negative controls amplified.
Statistical analysis
We used occupancy modeling64,65 to estimate occurrence probability of cavefishes and cave crayfishes while accounting for detection probability. Occurrence for taxa i at sampling unit j was treated as partially observed, with zij = 1 if the species was truly present and zij = 0 if the species was truly absent. The detection of taxa i at sampling unit j for survey k was conditional on both the true occurrence state and detection probability p. Both processes were modeled using a Bernoulli distribution and can be written as:
where Ψ is occurrence probability.
We modeled variation in Ψ and p using linear models64. We examined taxa-dependent occurrence probability by allowing each occurrence covariate (i.e., human disturbance and lithology) to vary by taxa. Elevation range and NDVI were not included in the model due to strong correlations with disturbance (Pearson’s pairwise correlation coefficient > 0.65). Lithology and human disturbance were not correlated (point-biserial correlation = − 0.12). Taxa-dependent and sampling-dependent detection probability was modeled by allowing the environmental covariates water volume, water-column velocity, and substrate to vary by taxa and survey method. We also accounted for detection probability by allowing water velocity to vary by survey method. For both models, we used a means parameterization66,67 for taxa. This parameterization yields the same model estimates as the dummy variable approach, but provides independent coefficients for each taxon (i.e., the coefficients for the alternate taxa do not represent the difference with reference taxa, but rather the actual estimate). Lithology, survey method, water-column velocity, and substrate were treated as dummy variables with dolostone, eDNA, not flowing, and coarse substrate as reference levels. None of the detection variables were highly correlated58. Both water volume and human disturbance were natural-log transformed due to right-skewed distributions. The detection model can be written as:
where α1 is the taxa intercept, α2 is the velocity main effect coefficient, α3 is the survey method coefficient, α4 is the survey method and velocity interaction coefficient, α5 is the substrate coefficient, α6 is the volume coefficient, α7 is the survey method and substrate interaction coefficient, α8 is the survey method and volume interaction coefficient, X1 is velocity, X2 is method, X3 is substrate, and X4 is volume.
Similarly, the occurrence model can be written as:
where β1 is the disturbance index coefficient, β2 is the lithology coefficient, X1 is the disturbance index, and X2 is lithology. All covariates were standardized to a mean of zero and standard deviation of one.
We fit the detection and occurrence models using the program JAGS68 called from the statistical software R69 using the package jagsUI70. We used broad uniform priors for model parameters67. Posterior distributions for coefficients were estimated using Markov chain Monte Carlo methods using two chains of 55,000 iterations each after a 5000-iteration burn-in phase and no thinning. We calculated 95% highest density intervals (HDIs; i.e., the probability the true parameter is within the interval) for each coefficient and evaluated plots of the posterior distributions to examine support for relationships with cavefish and cave crayfish occurrence probability. We assessed convergence using the Brooks–Gelman–Rubin statistic (71). values < 1.1 indicate adequate mixing of chains70,72. Model fit was assessed using a Bayesian p-value. A Bayesian p-value between 0.10–0.90 suggests adequate fit67,73,74.
Results
Species surveys
Cavefishes were observed in more sampling units than cave crayfishes. We detected Ozark Cavefish (i.e., either positive for the species DNA or a visual confirmation) at 31 of 55 sampling units (24 sites) and Salem Plateau Cavefish at four of six sampling units (two sites) where they are hypothesized or known to occur. The small number of sampling units where Salem Plateau Cavefish was observed was an artifact of only sampling a single cave from its much larger distribution. We detected Ozark Cavefish at 6 sites where they have not been previously detected using eDNA surveys. The Bristly Cave Crayfish had the largest distribution of the cave crayfishes and was observed at 12 sampling units (nine sites) where they are hypothesized or known to occur. We detected Benton County Cave Crayfish at four of six sampling units (two sites), Delaware County Cave Crayfish at one of two sampling units (one site), Oklahoma Cave Crayfish at all 6 sampling units (three sites), and Caney Mountain Cave Crayfish at two of four sampling units (two sites) where they are hypothesized or known to occur. We detected Caney Mountain Cave Crayfish at a site where it had not been previously detected using eDNA surveys. All of the negative controls collected in the field were negative, indicating our decontamination protocol was adequate and the absence of false positives.
Detection and occurrence covariates
Lithology and human disturbance were included in our analysis as occurrence covariates while using water volume, water-column velocity, and substrate as covariates to account for imperfect sampling detection. Our sampling units were located within limestone (n = 43) and dolostone lithologies (n = 18). Human disturbance index values ranged from 1.00 to 7.79 (mean ± SD = 2.02 ± 0.99), where 1.00 would represent undisturbed and 8.67 would represent the most highly disturbed via the index. Water volume ranged from 0.6 to 800.0 m3 (mean ± SD = 64.0 ± 130.2). One hundred twenty-eight surveys were classified as having flowing water and 105 with water not flowing. Thirty-four sampling units had coarse substrate and 27 sampling units had fine substrate.
Statistical analysis
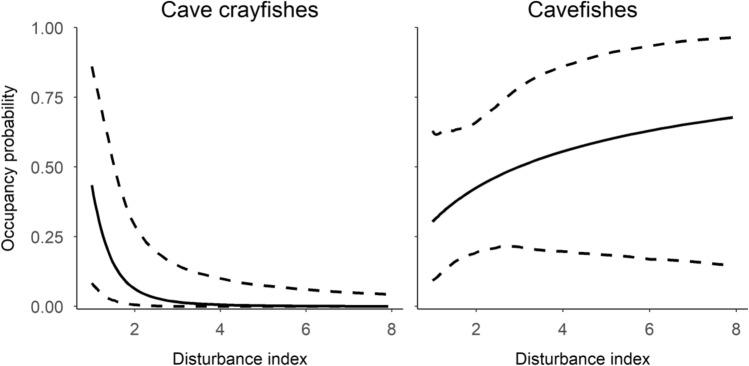
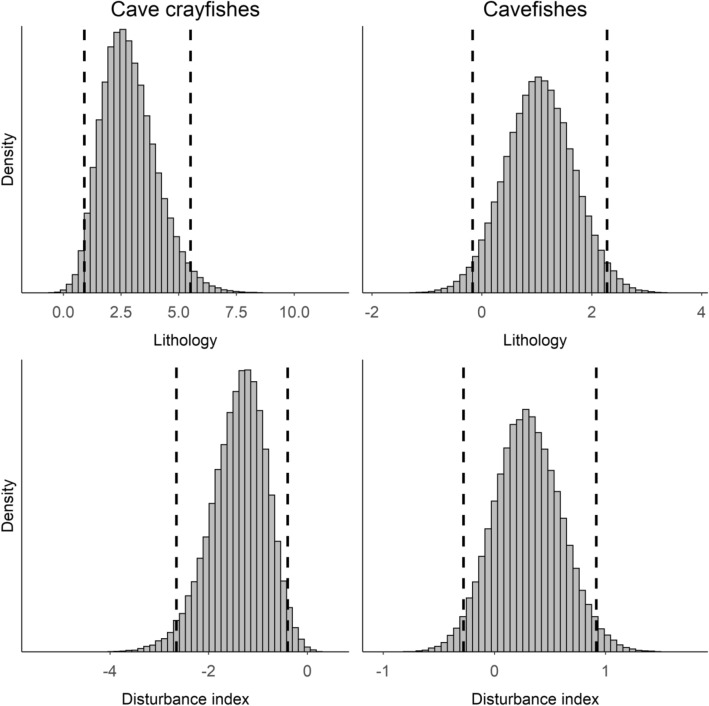
Our final model indicated that lithology and human disturbance influenced the occurrence of both cavefishes and cave crayfishes (Table 1, Figs. 2, 3) after accounting for the influence of water volume, substrate, and water velocity on detection probability (Table 2). Both taxa were more likely to occur in limestone compared to dolostone lithology based on the posterior distributions having the greatest density at values than > zero (Fig. 3). Cave crayfish occurrence probability decreased sharply with small increases in human disturbance (Fig. 2). The posterior distribution and 95% HDI for the disturbance slope also supported a strong negative relationship with cave crayfish occurrence (Fig. 3). There was slight evidence of a positive relationship between disturbance and cavefish occurrence based on the posterior distribution (Figs. 2, 3). Detection of cave crayfishes decreased when sampling in locations with higher water volume (Table 2). Cavefish detection decreased when using visual surveys in sampling units classified by fine rather than coarse substrates. Lastly, detection probability of both taxa decreased when using visual surveys in flowing water. Values of indicated adequate mixing of chains. The Bayesian p-value was 0.47, which indicated adequate model fit.
Table 1.
Estimated occurrence probability of cavefishes and cave crayfishes while accounting for imperfect detection derived from an occupancy model.
| Parameter | Mean ± SD | 95% HDI |
|---|---|---|
| Cave crayfish intercept | − 2.45 ± 1.09 | − 4.59, − 0.61 |
| Cavefish intercept | − 0.35 ± 0.55 | − 1.47, 0.62 |
| Cave crayfish disturbance index | − 1.37 ± 0.57 | − 2.59, − 0.36 |
| Cavefish disturbance index | 0.30 ± 0.31 | − 0.33, 0.87 |
| Cave crayfish lithology | 2.83 ± 1.20 | 0.88, 5.30 |
| Cavefish lithology | 1.05 ± 0.64 | − 0.25, 2.32 |
HDI highest density interval.
Figure 2.
The modeled relationship between human disturbance and occurrence probability of cavefishes and cave crayfishes. Lower numbers along the x-axis indicate less human disturbance, whereas higher numbers indicate higher human disturbance. Solid lines depict the modeled relationship and dotted lines reflect 95% confidence limits (i.e., the uncertainty around the estimated occurrence probability). The categorical variable lithology was set to dolostone.
Figure 3.
Posterior distributions for the coefficients from our model used to predict occurrence probability of cavefishes and cave crayfishes. The values on the x-axis represent parameter estimates for each covariate. The dotted lines are 95% highest density intervals. The categorical variable lithology was set to dolostone.
Table 2.
Estimated detection probability of cavefishes and cave crayfishes in relation to sampling method and environmental covariates derived from an occupancy model.
| Parameter | Mean ± SD | 95% HDI |
|---|---|---|
| Cave crayfish | − 0.17 ± 0.31 | − 0.88, 0.42 |
| Cavefish | − 0.08 ± 0.25 | − 0.61, 0.47 |
| Velocity | 0.74 ± 0.35 | 0.08, 1.47 |
| Cave crayfish method | 0.73 ± 0.57 | − 0.32, 1.84 |
| Cavefish method | − 0.67 ± 0.48 | − 1.60, 0.28 |
| Method × velocity | − 1.54 ± 0.54 | − 2.58, − 0.53 |
| Cave crayfish substrate | − 1.11 ± 0.78 | − 2.68, 0.33 |
| Cavefish substrate | 0.93 ± 0.41 | 0.12, 1.71 |
| Cave crayfish volume | − 0.04 ± 0.23 | − 0.46, 0.45 |
| Cavefish volume | − 0.11 ± 0.16 | − 0.44, 0.17 |
| Cave crayfish substrate × method | 0.54 ± 1.04 | − 1.40, 2.57 |
| Cavefish substrate × method | − 1.86 ± 0.71 | − 3.23, − 0.44 |
| Cave crayfish volume × method | − 1.15 ± 0.39 | − 1.85, − 0.36 |
| Cavefish volume × method | − 0.41 ± 0.27 | − 0.94, 0.10 |
HDI highest density interval.
Discussion
We found that occurrence of some Ozark stygobionts had a strong negative relationship with human disturbance, which could be explained by associated physicochemical changes in the groundwater habitat. Higher human disturbance values reflected increased proportions of urban and agricultural land use, which can result in decreased water quality and altered hydrology in surface streams75,76. Impaired surface water will likely lead to poor water quality in aquifers because surface streams are the primary recharge sources in the Ozark Highlands77. Cavefishes showed a slight positive relationship with disturbance, which is surprising because they are thought to have low tolerance to water quality degradation46. In contrast to cavefishes, we found that cave crayfishes had a strong negative relationship with human disturbance. Cave crayfishes may be more influenced than cavefishes by human-induced landscape changes because of pesticide applications in urban and agricultural settings that specifically target arthropods78,79. Follow-up testing of the chemical constituents present in runoff may be warranted to better understand mechanisms on species persistence. The relationships observed between stygobiont occurrence and human disturbance, and geology could also be explained by food availability because higher human disturbance would result in less above-ground vegetation and less food availability underground30. Other studies have shown that above-ground vegetation (e.g., forest cover) is related to subterranean species occurrence34,37.
The lithology associated with our sites also influenced the occurrence of cavefishes and cave crayfishes. In other studies of subterranean species’ distributions, lithology was hypothesized to represent habitat availability, water chemistry, and physical barriers31,33. Both lithology types found in our study area represent karst habitat that are chemically and physically capable of supporting stygobionts, so physical barriers between the lithology types could be the actual driver of the relationship. The areas we classified as dolostone lithology correspond roughly with the Salem Plateau, whereas the areas we classified as limestone lithology correspond with the Springfield Plateau (Fig. 1). The hydrogeological differences between the Salem and Springfield plateaus could serve as a physical barrier that limits species to a particular region80. However, we detected Ozark Cavefish via eDNA in the Salem Plateau, and they are currently thought to occur only in the Springfield Plateau46,80. The cave crayfishes appear to be more isolated by lithology as individual species more closely align with the Salem or Springfield plateaus.
Our results can help guide future conservation efforts for stygobionts. Many populations of stygobionts occur near rapidly expanding cities or near fields used for agriculture, and in some instances, most, if not all, of their known range is threatened by human land use (e.g.81–83). We found that cave crayfishes have a strong negative relationship with human disturbance; therefore, limiting agricultural and urban development in karst locations is worth considering if the goal is to protect at-risk populations. Human disturbance can be reduced through the implementation of freshwater protected areas that completely exclude development and are designed specifically to protect key ecosystem processes84. When completely excluding development from karst areas is not feasible, agricultural and urban best management practices can be implemented to reduce pollution of groundwater while allowing human activities to continue85. We also found that some species might occur outside of their known range. For example, Ozark Cavefish are currently thought to be restricted to the Springfield Plateau46,80. However, we detected their DNA in the Salem Plateau. Current sampling efforts are often not adequate to detect cryptic stygobiont species58; therefore, expanding sampling efforts might be needed if the goal is to make effective conservation decisions2.
We have identified that human changes and lithology play important roles in structuring the distribution of both cavefishes and cave crayfishes. However, cavefishes generally receive more conservation focus than cave crayfishes as indicated by federal listing (i.e., half of cavefishes are federally listed, whereas < 10% of cave crayfishes are listed). Our results indicate that cave crayfishes may be more sensitive to land-use changes than is reflected by their listing status. Cavefishes may be equally sensitive to land-use changes, but our results may have indicated a positive relationship with disturbance because disturbance is positively correlated with other factors that influence cavefish occurrence (e.g., baseflow). Our results indicate that protecting both cavefishes and cave crayfishes begins with improved sampling efforts to understand where these species occur and then protecting populations from human-induced landscape changes.
Acknowledgements
This research is a contribution of the Oklahoma Cooperative Fish and Wildlife Research Unit (U.S. Geological Survey, Oklahoma Department of Wildlife Conservation, Oklahoma State University, and Wildlife Management Institute cooperating). Special thanks to David Ashley, Doug Novinger, Michael Slay, Richard Stark, and Jacob Westhoff for contributing genetic samples and helping secure karst access. We also thank the resource managers and private landowners that allowed us to access their lands. Field and laboratory assistance were provided by Victoria Dropps, Megan Judkins, Andy Miller, Sam Schneider, Denise Thomson, Meaghan Wedgeworth, Jodie Wiggins, and Chris Wood. An animal care and use protocol was not required for this research because the fish were not handled by the investigators. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author contributions
S.K.B. and R.A.V.D.B. conceptualized the project; all authors contributed to the experimental design; J.B.M. and S.K.B. collected the field data; J.B.M. and M.L.N. performed the molecular work; J.B.M. and R.M. analyzed these data; and all authors contributed to the manuscript writing.
Data availability
The dataset generated and analyzed during the current study is available in the SHAREOK repository [10.22488/okstate.22.000004].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sket B. Can we agree on an ecological classification of subterranean animals? J. Nat. Hist. 2008;42:1549–1563. doi: 10.1080/00222930801995762. [DOI] [Google Scholar]
- 2.Mammola S, et al. Scientists’ warning on the conservation of subterranean ecosystems. Bioscience. 2019;69:641–650. doi: 10.1093/biosci/biz064. [DOI] [Google Scholar]
- 3.Boulton AJ, Fenwick GD, Hancock PJ, Harvey MS. Biodiversity, functional roles and ecosystem services of groundwater invertebrates. Invertebr. Syst. 2008;22:103–116. doi: 10.1071/IS07024. [DOI] [Google Scholar]
- 4.Danielopol DL, Griebler C. Changing paradigms in groundwater ecology—From the ‘living fossils’ tradition to the ‘new groundwater ecology’. Int. Rev. Hydrobiol. 2008;93:565–577. doi: 10.1002/iroh.200711045. [DOI] [Google Scholar]
- 5.Griebler C, Malard F, Lefébure T. Current developments in groundwater ecology—From biodiversity to ecosystem function and services. Curr. Opin. Biotechnol. 2014;27:159–167. doi: 10.1016/j.copbio.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 6.Fišer C. Niphargus—A model system for evolution and ecology. In: Culver DC, White WB, Pipan T, editors. Encyclopedia of Caves. Academic Press; 2019. pp. 746–755. [Google Scholar]
- 7.Riddle MR, et al. Insulin resistance in cavefish as an adaptation to a nutrient-limited environment. Nature. 2018;555:647–651. doi: 10.1038/nature26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibert J, Culver DC, Dole-Olivier M, Malard F, Christman MC, Deharveng L. Assessing and conserving groundwater biodiversity: Synthesis and perspectives. Freshw. Biol. 2009;54:930–941. doi: 10.1111/j.1365-2427.2009.02201.x. [DOI] [Google Scholar]
- 9.Trontelj P, et al. A molecular test for cryptic diversity in ground water: How large are the ranges of macro stygobionts? Freshw. Biol. 2009;54:727–744. doi: 10.1111/j.1365-2427.2007.01877.x. [DOI] [Google Scholar]
- 10.Cooper JE. Ecological and Behavioral Studies in Shelta Cave, Alabama, with Emphasis on Decapod Crustaceans. University of Kentucky; 1975. [Google Scholar]
- 11.Voituron Y, de Fraipont M, Issartel J, Guillaume O, Clobert J. Extreme lifespan of the human fish (Proteus anguinus): A challenge for ageing mechanisms. Biol. Lett. 2011;7:105–107. doi: 10.1098/rsbl.2010.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Poulson TL. Cave adaptation in amblyopsid fishes. Am. Midl. Nat. 1963;70:257–290. doi: 10.2307/2423056. [DOI] [Google Scholar]
- 13.Venarsky MP, Huryn AD, Benstead JP. Re-examining extreme longevity of the cave crayfish Orconectes australis using new mark–recapture data: A lesson on the limitations of iterative size-at-age models. Freshw. Biol. 2012;57:1471–1481. doi: 10.1111/j.1365-2427.2012.02812.x. [DOI] [Google Scholar]
- 14.Culver DC, Kane TC, Fong DW. Adaptation and Natural Selection in Caves: The Evolution of Gammarus minus. Harvard University Press; 1995. [Google Scholar]
- 15.Niemiller ML, Poulson TL. Subterranean fishes of North America: Amblyopsidae. In: Trajano E, Bichuette ME, Kapoor BG, editors. Biology of Subterranean Fishes. CRC Press; 2010. pp. 169–280. [Google Scholar]
- 16.Fišer C, Zagmajster M, Zakšek V. Coevolution of life history traits and morphology in female subterranean amphipods. Oikos. 2013;122:770–778. doi: 10.1111/j.1600-0706.2012.20644.x. [DOI] [Google Scholar]
- 17.Purvis A, Gittleman JL, Cowlishaw G, Mace GM. Predicting extinction risk in declining species. Proc. Biol. Sci. 2000;267:1947–1952. doi: 10.1098/rspb.2000.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson RG, et al. Life history and spatial traits predict extinction risk due to climate change. Nat. Clim. Change. 2014;4:217–221. doi: 10.1038/nclimate2113. [DOI] [Google Scholar]
- 19.Niemiller ML, Bichuette E, Taylor SJ. Conservation of cave fauna in Europe and the Americas. In: Moldovan OT, Kovac L, Halse S, editors. Ecological Studies: Cave Ecology. Springer; 2018. pp. 451–478. [Google Scholar]
- 20.Niemiller ML, Taylor SJ. Protecting cave life. In: Culver DC, White WB, Pipan T, editors. Encyclopedia of Caves. Academic Press; 2019. pp. 822–829. [Google Scholar]
- 21.Niemiller ML, Taylor SJ, Slay ME, Hobbs HH., III . Biodiversity in the United States and Canada. In: Culver DC, White WB, Pipan T, editors. Encyclopedia of Caves. Academic Press; 2019. pp. 163–176. [Google Scholar]
- 22.Hortal J, et al. Seven shortfalls that beset large-scale knowledge of biodiversity. Annu. Rev. Ecol. Evol. Syst. 2015;46:523–529. doi: 10.1146/annurev-ecolsys-112414-054400. [DOI] [Google Scholar]
- 23.MacKenzie DI, et al. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence. Academic Press; 2018. [Google Scholar]
- 24.Roberto P, Pietro B. Species rediscovery or lucky endemic? Looking for the supposed missing species Leistus punctatissimus through a biogeographer’s eye (Coleoptera, Carabidae) ZooKeys. 2018;740:97–108. doi: 10.3897/zookeys.740.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu C, Mandrak NE, Minns CK. Potential impacts of climate change on the distributions of several common and rare freshwater fishes in Canada. Divers. Distrib. 2005;11:299–310. doi: 10.1111/j.1366-9516.2005.00153.x. [DOI] [Google Scholar]
- 26.Larson ER, Olden JD. Latent extinction and invasion risk of crayfishes in the southeastern United States. Conserv. Biol. 2010;24:1099–1110. doi: 10.1111/j.1523-1739.2010.01462.x. [DOI] [PubMed] [Google Scholar]
- 27.Filipe AF, et al. Selection of priority areas for fish conservation in Guadiana River Basin, Iberian Peninsula. Conserv. Biol. 2004;18:189–200. doi: 10.1111/j.1523-1739.2004.00620.x. [DOI] [Google Scholar]
- 28.Mammola S, et al. Fundamental research questions in subterranean biology. Biol. Rev. 2020;95:1855–1872. doi: 10.1111/brv.12642. [DOI] [PubMed] [Google Scholar]
- 29.Domínguez-Domínguez O, Martínez-Meyer E, Zombrano L, de León GP. Using ecological-niche modeling as a conservation tool for freshwater species: Live-bearing fishes in central Mexico. Conserv. Biol. 2006;20:1730–1739. doi: 10.1111/j.1523-1739.2006.00588.x. [DOI] [PubMed] [Google Scholar]
- 30.Mammola S, Leroy B. Applying species distribution models to caves and other subterranean habitats. Ecography. 2018;41:1194–1208. doi: 10.1111/ecog.03464. [DOI] [Google Scholar]
- 31.Castellarini F, Malard F, Dole-Olivier M, Gibert J. Modelling the distribution of stygobionts in the Jura Mountains (eastern France). Implications for the protection of ground waters. Divers. Distrib. 2007;13:213–224. doi: 10.1111/j.1472-4642.2006.00317.x. [DOI] [Google Scholar]
- 32.Foulquier A, Malard F, Lefébure T, Douady CJ, Gibert J. The imprint of Quaternary glaciers on the present-day distribution of the obligate groundwater amphipod Niphargus virei (Niphargidae) J. Biogeogr. 2008;35:552–564. doi: 10.1111/j.1365-2699.2007.01795.x. [DOI] [Google Scholar]
- 33.Johns T, et al. Regional-scale drivers of groundwater faunal distributions. Freshw. Sci. 2015;34:316–328. doi: 10.1086/678460. [DOI] [Google Scholar]
- 34.Camp CD, Wooten JA, Jensen JB, Bartek DF. Role of temperature in determining relative abundance in cave twilight zones by two species of lungless salamander (family Plethodontidae) Can. J. Zool. 2014;92:119–127. doi: 10.1139/cjz-2013-0178. [DOI] [Google Scholar]
- 35.Korbel KL, Hancock PJ, Serov P, Lim RP, Hose GC. Groundwater ecosystems vary with land use across a mixed agricultural landscape. J. Environ. Qual. 2013;42:380–390. doi: 10.2134/jeq2012.0018. [DOI] [PubMed] [Google Scholar]
- 36.Español C, et al. Does land use impact on groundwater invertebrate diversity and functionality in floodplains? Ecol. Eng. 2017;103:394–403. doi: 10.1016/j.ecoleng.2016.11.061. [DOI] [Google Scholar]
- 37.Christman MC, et al. Predicting the occurrence of cave-inhabiting fauna based on features of the earth surface environment. PLoS One. 2016;11:e0160408. doi: 10.1371/journal.pone.0160408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zagmajster M, et al. Geographic variation in range size and beta diversity of groundwater crustaceans: Insights from habitats with low thermal seasonality. Glob. Ecol. Biogeogr. 2014;23:1135–1145. doi: 10.1111/geb.12200. [DOI] [Google Scholar]
- 39.Poff NL. Landscape filters and species traits: Towards mechanistic understanding and prediction in stream ecology. J. North Am. Benthol. Soc. 1997;16:391–409. doi: 10.2307/1468026. [DOI] [Google Scholar]
- 40.Stevenson RJ. Scale-dependent determinants and consequences of benthic algal heterogeneity. J. North Am. Benthol. Soc. 1997;16:248–262. doi: 10.2307/1468255. [DOI] [Google Scholar]
- 41.U.S. Geological Survey. NLCD 2011 land cover. Multi-Resolution Land Characteristics.https://www.mrlc.gov/data/nlcd-2011-land-cover-conus (2011).
- 42.Adamski JC. Geochemistry of the Springfield Plateau Aquifer of the Ozark Plateaus Province in Arkansas, Kansas, Missouri and Oklahoma, USA. Hydrol. Process. 2000;14:849–866. doi: 10.1002/(SICI)1099-1085(20000415)14:5<849::AID-HYP973>3.0.CO;2-7. [DOI] [Google Scholar]
- 43.Woods AJ, et al. Ecoregions of Oklahoma (Color Poster with Map, Descriptive Text, Summary Tables, and Photographs) U.S. Geological Survey; 2005. [Google Scholar]
- 44.Unklesbay AG, Vineyard JD. Missouri Geology: Three Billion Years of Volcanoes, Seas, Sediments, and Erosion. University of Missouri Press; 1992. [Google Scholar]
- 45.Eigenmann, C. H. A new blind fish. In Proceedings of the Indiana Academy of Science 1897 (ed Waldo, C. A.) 231 (1898).
- 46.Graening GO, Fenolio DB, Niemiller ML, Brown AV, Beard JB. The 30-year recovery effort for the Ozark cavefish (Amblyopsis rosae): Analysis of current distribution, population trends, and conservation status of this threatened species. Environ. Biol. Fish. 2010;87:55–88. doi: 10.1007/s10641-009-9568-2. [DOI] [Google Scholar]
- 47.Niemiller ML, Near TJ, Fitzpatrick BM. Delimiting species using multilocus data: Diagnosing cryptic diversity in the southern cavefish, Typhlichthys subterraneus (Teleostei: Amblyopsidae) Evolution. 2012;66:846–866. doi: 10.1111/j.1558-5646.2011.01480.x. [DOI] [PubMed] [Google Scholar]
- 48.Hobbs HH, Jr, Brown AV. A new troglobitic crayfish from northwestern Arkansas (Decapoda: Cambaridae) Proc. Biol. Soc. Wash. 1987;100:1040–1048. [Google Scholar]
- 49.Graening GO, Slay ME, Brown AV, Koppelman JB. Status and distribution of the endangered Benton cave crayfish, Cambarus aculabrum (Decapoda: Cambaridae) Southwest. Nat. 2006;51:376–381. doi: 10.1894/0038-4909(2006)51[376:SADOTE]2.0.CO;2. [DOI] [Google Scholar]
- 50.Faxon W. Cave animals from southwestern Missouri. Bull. Mus. Comp. Zool. 1889;17:225–240. [Google Scholar]
- 51.Graening GO, Hobbs HH, III, Slay ME, Elliott WR, Brown AV. Status update for bristly cave crayfish, Cambarus setosus (Decapoda: Cambaridae), and range extension into Arkansas. Southwest. Nat. 2006;51:382–392. doi: 10.1894/0038-4909(2006)51[382:SUFBCC]2.0.CO;2. [DOI] [Google Scholar]
- 52.Hobbs HH., III Cambarus (Jugicambarus) subterraneus, a new cave crayfish (Decapoda: Cambaridae) from northeastern Oklahoma, with a key to the troglobitic members of the subgenus Jugicambarus. Proc. Biol. Soc. Wash. 1993;106:719–727. [Google Scholar]
- 53.Graening GO, Fenolio DB. Status update of the Delaware County cave crayfish, Cambarus subterraneus (Decapoda: Cambaridae) Proc. Okla. Acad. Sci. 2005;85:85–89. [Google Scholar]
- 54.Hobbs HH, Jr, Cooper MR. A new troglobitic crayfish from Oklahoma (Decapoda: Astacidae) Proc. Biol. Soc. Wash. 1972;85:49–56. [Google Scholar]
- 55.Graening GO, et al. Range extension and status update for the Oklahoma cave crayfish, Cambarus tartarus (Decapoda: Cambaridae) Southwest. Nat. 2006;51:94–99. doi: 10.1894/0038-4909(2006)51[94:REASUF]2.0.CO;2. [DOI] [Google Scholar]
- 56.Hobbs HH., III A new cave crayfish of the genus Orconectes, subgenus Orconectes, from southcentral Missouri, USA, with a key to the stygobitic species of the genus (Decapoda, Cambaridae) Crustaceana. 2001;74:635–646. doi: 10.1163/156854001750377911. [DOI] [Google Scholar]
- 57.Miller BV. The Hydrology of the Carroll Cave-Toronto Springs System: Identifying and Examining Source Mixing Through Dye Tracing, Geochemical Monitoring, Seepage Runs, and Statistical Methods. Western Kentucky University; 2010. [Google Scholar]
- 58.Mouser JB, Brewer SK, Niemiller ML, Mollenhauer R, Van Den Bussche R. Comparing visual and environmental DNA surveys for detection of stygobionts. Subterr. Biol. 2021;39:79–105. doi: 10.3897/subtbiol.39.64279. [DOI] [Google Scholar]
- 59.Longmire JL, Maltbie M, Baker RJ. Use of “Lysis Buffer” in DNA Isolation and Its Implication for Museum Collections. Museum of Texas Tech University; 1997. [Google Scholar]
- 60.Mouser JB, Mollenhauer R, Brewer SK. Relationships between landscape constraints and a crayfish assemblage with consideration of competitor presence. Divers. Distrib. 2019;25:61–73. doi: 10.1111/ddi.12840. [DOI] [Google Scholar]
- 61.U.S. Geological Survey. 1 Arc-second digital elevation models (DEMs)—USGS national map 3DEP downloadable data collection. https://data.usgs.gov/datacatalog/data/USGS:35f9c4d4-b113-4c8d-8691-47c428c29a5b (U.S. Geological Survey, 2017).
- 62.Oak Ridge National Laboratory Distributed Active Archive Center. 2018. MODIS and VIIRS land products global subsetting and visualization tool. Oak Ridge National Laboratory Distributed Active Archive Center. [DOI]
- 63.Horton JD, San Juan CA, Stoeser DB. The state geologic map compilation (SGMC) geodatabase of the conterminous United States. U.S. Geol. Surv. 2017 doi: 10.3133/ds1052. [DOI] [Google Scholar]
- 64.MacKenzie DI, et al. Estimating site occupancy rates when detection probabilities are less than one. Ecology. 2002;83:2248–2255. doi: 10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2. [DOI] [Google Scholar]
- 65.Tyre AJ, et al. Improving precision and reducing bias in biological surveys: Estimating false negative error rates. Ecol. Appl. 2003;13:1790–1801. doi: 10.1890/02-5078. [DOI] [Google Scholar]
- 66.Gelman A, Hill J. Data Analysis Using Regression and Multilevel Hierarchical Models. Cambridge University Press; 2007. [Google Scholar]
- 67.Kéry M, Royle JA. Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness in R and BUGS. Academic Press; 2016. [Google Scholar]
- 68.Plummer M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. In: Hornik K, Leisch F, Zeileis A, editors. Proceedings of the 3rd International Workshop on Distributed Statistical Computing. Austrian Science Foundation; 2003. pp. 1–10. [Google Scholar]
- 69.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2020. [Google Scholar]
- 70.Kellner, J. jagsUI: A wrapper around 'rjags' to streamline 'JAGS' analyses. https://CRAN.R-project.org/package=jagsUI (R-project, 2019).
- 71.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J. Comput. Graph. Stat. 1998;7:434–455. doi: 10.1080/10618600.1998.10474787. [DOI] [Google Scholar]
- 72.Kruschke JK. Doing Bayesian Data Analysis: A Tutorial with R, JAGS, and Stan. Academic Press; 2015. [Google Scholar]
- 73.Hobbs NT, Hooten MB. Bayesian Models. Princeton University Press; 2015. [Google Scholar]
- 74.Conn PB, Johnson DS, Williams PJ, Melin SR, Hooten MB. A guide to Bayesian model checking for ecologists. Ecol. Monogr. 2018;88:526–542. doi: 10.1002/ecm.1314. [DOI] [Google Scholar]
- 75.Allan JD. Landscapes and riverscapes: The influence of land use on stream ecosystems. Annu. Rev. Ecol. Evol. Syst. 2004;35:257–284. doi: 10.1146/annurev.ecolsys.35.120202.110122. [DOI] [Google Scholar]
- 76.Paul MJ, Meyer JL. Streams in the urban landscape. Annu. Rev. Ecol. Evol. Syst. 2001;32:333–365. doi: 10.1146/annurev.ecolsys.32.081501.114040. [DOI] [Google Scholar]
- 77.Wicks C, Kelley C, Peterson E. Estrogen in a karstic aquifer. Groundwater. 2004;42:384–389. doi: 10.1111/j.1745-6584.2004.tb02686.x. [DOI] [PubMed] [Google Scholar]
- 78.Buřič M, Kouba A, Máchová J, Mahovská I, Kozák P. Toxicity of the organophosphate pesticide diazinon to crayfish of differing age. Int. J. Environ. Sci. Technol. 2013;10:607–610. doi: 10.1007/s13762-013-0185-4. [DOI] [Google Scholar]
- 79.Sohn L, Brodie RJ, Couldwell G, Demmons E, Sturve J. Exposure to a nicotinoid pesticide reduces defensive behaviors in a non-target organism, the rusty crayfish Orconectes rusticus. Ecotoxicology. 2018;27:900–907. doi: 10.1007/s10646-018-1950-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Noltie DB, Wicks CM. How hydrogeology has shaped the ecology of Missouri’s Ozark cavefish, Amblyopsis rosae, and southern cavefish, Typhlichthys subterraneus: Insights on the sightless from understanding the underground. Environ. Biol. Fish. 2001;62:171–194. doi: 10.1023/A:1011815806589. [DOI] [Google Scholar]
- 81.Kuhajda BR, Mayden RL. Status of the federally endangered Alabama cavefish, Speoplatyrhinus poulsoni (Amblyopsidae), in Key Cave and surrounding caves, Alabama. Environ. Biol. Fish. 2001;62:215–222. doi: 10.1023/A:1011817023749. [DOI] [Google Scholar]
- 82.Hutchins BT. The conservation status of Texas groundwater invertebrates. Biodivers. Conserv. 2018;27:475–501. doi: 10.1007/s10531-017-1447-0. [DOI] [Google Scholar]
- 83.Niemiller ML, et al. Discovery of a new population of the federally endangered Alabama cave shrimp, Palaemonias alabamae Smalley, 1961, in northern Alabama. Subterr. Biol. 2019;32:43–59. doi: 10.3897/subtbiol.32.38280. [DOI] [Google Scholar]
- 84.Abell R, Allan JD, Lehner B. Unlocking the potential of protected areas for freshwaters. Biol. Conserv. 2007;134:48–63. doi: 10.1016/j.biocon.2006.08.017. [DOI] [Google Scholar]
- 85.Liu Y, et al. A review on effectiveness of best management practices in improving hydrology and water quality: Needs and opportunities. Sci. Total Environ. 2017;601–602:580–593. doi: 10.1016/j.scitotenv.2017.05.212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Oak Ridge National Laboratory Distributed Active Archive Center. 2018. MODIS and VIIRS land products global subsetting and visualization tool. Oak Ridge National Laboratory Distributed Active Archive Center. [DOI]
Data Availability Statement
The dataset generated and analyzed during the current study is available in the SHAREOK repository [10.22488/okstate.22.000004].