Abstract
Immunization with a plasmid DNA containing the gene encoding the catalytic domain of trans-sialidase (TS) elicits protective immune responses against experimental Trypanosoma cruzi infection. As several studies provided strong evidence that during infection CD4 Th1 and CD8 T cytotoxic type 1 (Tc1) cells are important factors in host resistance, the present study was designed to evaluate which T-cell types were activated in DNA-vaccinated BALB/c mice. We found that bulk cells from DNA-immunized mice had CD4 and CD8 T cells that produced gamma interferon (IFN-γ) but not interleukin-4 (IL-4) or IL-10. To characterize the TS-specific T cells at the clonal level, we generated CD4 and CD8 clones. We obtained cytotoxic CD4 clones of the Th1 type that secreted large amounts of IFN-γ but not IL-4 or IL-10. Unexpectedly, we obtained other CD4 clones with a Th2 phenotype, secreting IL-4 and IL-10 but not IFN-γ. All CD8 clones were cytotoxic and produced IFN-γ. IL-4 and IL-10 were not secreted by these cells. Using synthetic peptides, we determined a CD8 epitope recognized by several clones as being represented by amino acids IYNVGQVSI. The antiparasitic activity of a CD4 Th1 and a CD8 Tc1 clone was assessed in vitro. CD4 or CD8 T cells significantly inhibited T. cruzi development in infected macrophages or fibroblasts, respectively. We concluded that DNA vaccine efficiently generates potentially protective CD4 Th1 and CD8 Tc1 cells specific for a T. cruzi antigen, therefore reinforcing the possibility of using this strategy for developing a preventive or therapeutic vaccine against Chagas’ disease.
Despite the significant reduction in transmission observed in the last 20 years, Chagas’ disease (American trypanosomiasis) is still a major problem for most Latin American countries, afflicting between 16 and 18 million individuals. After contact with trypomastigotes of Trypanosoma cruzi, humans develop the acute phase of Chagas’ disease characterized by a patent parasitemia that lasts for few weeks. The chronic phase initiates when the parasitemia declines significantly becoming subclinical.
The efficacy of conventional chemotherapy with nifurtimox or benznidazole is low. In children recently infected, treatment is 55.8% efficient (9). Most of the individuals in the chronic phase of infection are resistance to treatment with conventional chemotherapy and carry the infection for life (47). Approximately one-third of them progress slowly to the symptomatic forms of the disease characterized by cardiomyopathy and/or mega-gastrointestinal syndromes. Patients who do not develop symptoms pose a major threat to blood supplies, transmitting the disease through blood transfusions. The lack of efficient drug treatment necessitates the development of new strategies to prevent or ameliorate the disease.
During T. cruzi infection in mice or humans, parasite-specific CD4 and CD8 T cells are activated. These T cells are important factors for host resistance, as genetically modified mice that do not have either CD4 or CD8 cells are significantly more susceptible to infection than wild-type animals (34, 43; reviewed in references 11 and 42). Several bodies of evidence strongly suggest that immunoprotection is mediated by CD4 Th1 and CD8 Tc1 cells (1, 27, 48). Their antiparasitic effect is mediated in part by their ability to secrete gamma interferon (IFN-γ), a cytokine that inhibits the development of T. cruzi.
A number of studies have focused on the role of IFN-γ in resistance to T. cruzi infection. Initial reports indicated that the administration of recombinant IFN-γ to mice reduced the parasitemia and prevented death due to infection (29). IFN-γ participates in naturally acquired immunity, as treatment with neutralizing monoclonal antibodies (MAbs) significantly reduced the resistance to acute infection (40). IFN-γ increases host resistance by contributing to the production of nitric oxide (NO), a potent inhibitor of intracellular development of T. cruzi (26, 39). The critical role for IFN-γ in the induction of NO production during experimental infection was confirmed in studies using genetically modified mice that lack the IFN-γ receptor. This mouse strain fails to produce NO during the course of the disease and was extremely susceptible to infection (17).
Recently, we and others reported that immunization with plasmids containing genes encoding for antigens expressed on the surface of infective trypomastigotes of T. cruzi induced protective immunity against experimental Chagas’ disease (6, 49). We used the gene encoding for the catalytic domain of an enzyme called trans-sialidase (TS) (7, 38, 44). Immunization of BALB/c mice with the TS gene elicited immune responses, as measured by antibody production and T-cell activation. Most relevant, these mice had a significant reduction in peak parasitemia and survived lethal T. cruzi infection (6). In view of data suggesting an important role of IFN-γ-producing T cells in resistance to experimental infection, we considered it important to evaluate whether DNA vaccination with the TS gene could efficiently generate these potentially protective CD4 Th1 and CD8 T cytotoxic type 1 (Tc1) cells. For this purpose, we performed a detailed analysis of the type of T-cell immune response in animals immunized with the TS gene.
MATERIALS AND METHODS
Parasites and animals.
Trypomastigotes of the Y strain were obtained from tissue culture as described in reference 38. Female 5- to 8-week-old BALB/c mice used in this study were purchased from University of São Paulo.
Plasmid generation, purification, and immunization.
Plasmid 154/13 was created as described by Costa et al. (6). It contains the nucleotide sequence coding for amino acids 1 to 678 of TS inserted into the commercially available plasmid pcDNA3 (Invitrogen, San Diego, Calif.). As control, we used plasmid pcDNA3 alone. Plasmids were produced in Escherichia coli DH5α and purified on cesium chloride density gradients according to standard protocols (35). DNA concentration was estimated at 260 nm. DNA was diluted in saline at a concentration of 1 mg/ml, and 50 μl was injected per leg. BALB/c mice were immunized according to the protocol described by Costa et al. (6). Each mouse received intramuscularly four doses of 100 μg of plasmid DNA at 0, 3, 5, and 7 weeks. Two to six weeks after the last dose, lymph node and spleen cells were collected.
The recombinant TS was produced in E. coli transformed with plasmid pTS-cat7 (30). The purity of recombinant TS was determined by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis. A single band of 70 kDa was visualized in the gel. Protein concentration was estimated by the Bradford procedure (Bio-Rad, Hercules, Calif.).
Cell culture.
Cells were cultured in RPMI medium (Life Technologies, GIBCO-BRL, Gaithersburg, Md.) supplemented with 10 mM HEPES and 2 mM l-glutamine. For T-cell proliferation, we added 5 × 10−5 M 2- mercaptoethanol, 1 mM sodium pyruvate, 1% (vol/vol) nonessential amino acids solution, 1% (vol/vol) vitamin (all purchased from Life Technologies), penicillin and streptomycin (100 U/ml; Sigma, St. Louis, Mo.), and 2% (vol/vol) normal human serum. For stimulation of spleen cells and maintenance of T-cell clones, we added to the medium 2-mercaptoethanol, sodium pyruvate, nonessential amino acid solutions, vitamin, 10% fetal calf serum (FCS; Hyclone, Logan, Utah), and 30 U of recombinant human interleukin-2 (IL-2; kindly provided by Hoffmann-LaRoche) per ml. The cultures were maintained at 37°C in an atmosphere containing 5% CO2.
T-cell proliferative response.
Cells were obtained from draining lymph nodes (popliteal and inguinal) or from the spleens of mice immunized four times with plasmid 154/13 or pcDNA3. These cells were washed three times in RPMI medium and resuspended in complete medium to a concentration of 2.5 × 106 viable cells per ml. The assay was performed in 96-well flat-bottom plates (Corning); 0.2 ml of cell suspension was pipetted into each well and the antigen was added at the desired concentration. Each determination was done in triplicate; results are reported as average ± standard deviation (SD). Recombinant TS or concanavalin A (Sigma) were added at a final concentration of 10 or 2.5 μg/ml, respectively. Anti-CD4 and anti-CD8 MAbs (see description below) were added at a final concentration of 1 μg/ml. After 4 days, we collected supernatants for cytokine determination and 0.5 μCi of [methyl-3H]thymidine ([3H]TdR; Amersham, Little Chalfont, England) was added to each well. At the end of incubation period (18 to 20 h later), lymphocytes were collected with the aid of a semiautomatic cell harvester.
Cloning of CD4 T cells, proliferation, and cytokine production.
CD4 T-cell clones were derived from nonadherent lymph node cells of mice immunized with plasmid 154/13. T cells (3 × 106/ml) were cultured for 7 days in the presence of irradiated normal spleen cells (feeder cells; 5 × 106/ml) and recombinant TS (10 μg/ml). Cells were cultured in 24-well plates (Costar) in a final volume of 2 ml per well. Viable cells were collected through a Ficoll-Paque gradient (Pharmacia, Uppsala, Sweden), and 3 × 106/ml T cells were maintained in culture for 3 days. After that period, feeder cells (5 × 106/ml) and antigen (10 μg/ml) were added, and the plates were incubated for 8 days. T cells were then cloned by limiting dilution in 96-well plates in the presence of feeder cells (5 × 105/well), recombinant TS (10 μg/ml), and human recombinant IL-2 (30 U/ml). T-cell clones were maintained by biweekly stimulation of T cells (0.5 × 106/ml) in the presence of feeder cells (2.5 × 106/ml), recombinant TS (10 μg/ml), and human recombinant IL-2 (30 U/ml). For cytokine production, T cells (105/well) and feeder cells (5 × 105/well) were cultured in the presence or absence of recombinant TS (10 μg/ml). Cultures were performed in 96-well plates in a final volume of 0.2 ml. The supernatants were collected 4 days later, and IFN-γ, IL-4, and IL-10 levels were estimated as described below.
Transfection of A20J cells with the TS gene.
A20J cells expressing class I and II major histocompatibility complex molecules were kindly provided by Moriya Tsuji, New York University School of Medicine. Transfections were performed with the aid of Lipofectin (Life Technologies) according to the protocol provided by the manufacturer. Cells were selected in the presence of Geneticin (800 μg/ml; Life Technologies). Geneticin-resistant cells transfected with plasmid pcDNA3 (A20J-pcDNA3 cells) or 154/13 (A20J-TS cells) were cloned by limiting dilution. One clone was selected and grown in medium containing Geneticin (800 μg/ml). The supernatant of these cells was collected, filtered, and used for determination of TS enzymatic activity as previously described (30).
Spleen cells cultures and cytokine production.
Spleen cells of DNA-immunized mice, obtained 3 to 6 weeks after the last immunization, were stimulated (4 × 107 cells/10 ml) in vitro in the presence of 4 × 106 irradiated A20J-TS cells. After 6 to 7 days in culture, cells were washed, counted, and incubated at different concentration with 105 irradiated A20J-TS or A20J-pcDNA3 cells in a flat-bottom 96-well plate with a final volume of 0.2 ml. Depletion of T-cell subpopulations was obtained by treating 107 cells with 100 μg of anti-CD4 (MAb GK1.5) or anti-CD8 (MAb 2.43) or both. After 45 min on ice, cells were centrifuged, resuspended in medium containing 10% rabbit low-toxicity complement (Cedarlane, Hornby, Ontario, Canada), and incubated for an additional 45 min at 37°C. These cells were washed twice and counted, and 105 cells were incubated with the same amount of A20J-TS or A20J-pcDNA3 cells. After 18 h in culture, the supernatants were collected and IFN-γ, IL-4, and IL-10 levels were estimated as described below.
Cloning and maintenance of CD8 T cells.
Anti-CD4-treated spleen cells that had been stimulated in vitro with A20J-TS cells were cloned by limiting dilution exactly as described by Rodrigues et al. (33). T-cell clones were also established and maintained as described previously (33). Briefly, cytotoxic T-lymphocyte clones (0.125 × 106 cells/ml) were restimulated weekly with irradiated feeder cells (2.5 × 106/ml) and irradiated A20J-TS cells (3.75 × 105/ml). Four days later, and after the addition of an equal volume of medium, the cultures were divided and grown under the same conditions for 2 to 3 more days. T cells were collected, washed, and resuspended in RPMI medium containing 10% FCS immediately before use.
Determination of IFN-γ, IL-4, IL-10, nitrite, and BLT-esterase in T-cell supernatants.
A20J-TS or A20J-pcDNA3 cells (105/well) were cultured with 105 CD8 T cells in flat-bottom 96-well plates. The final volume was 0.2 ml. After 15 h, the plates were centrifuged; culture supernatants were collected and used to estimate IFN-γ, IL-4, IL-10 and N-benzyloyxcarbonyl l-lysine thiobenzyl ester (BLT)-esterase. The concentration of cytokines was estimated by capture enzyme-linked immunosorbent assay (ELISA) using antibodies and recombinant cytokines purchased from Pharmingen (San Diego, Calif.). The capture MAbs for IFN-γ, IL-4, and IL-10 were R46A2, BVD4-1D11, and JES-2A5, respectively; detection was done with biotinylated MAbs XMG1.2, BVD6-24G2, and SXC-1, respectively. Briefly, high-binding microtiter plates (Costar) were coated with 0.05 ml of capture MAb (5 μg/ml) and incubated overnight at 4°C. After washes with phosphate-buffered saline (PBS)–Tween 20 (0.05%, vol/vol), wells were blocked for 2 h with PBS containing 2.5% (wt/vol) bovine serum albumin (PBS-BSA). After removing the blocking solution, 0.05 ml of the T-cell supernatants were added per well. In many cases, the supernatants were diluted twice or up to 100 times in order to estimate precisely the cytokine concentration. Each determination was performed in triplicate. After overnight incubation at 4°C, plates were washed and the biotinylated MAbs were added at a final concentration of 5 μg/ml in PBS-BSA. After washes, 0.05 ml of avidin-peroxidase (Kiekegaard & Perry Laboratories, Gaithersburg, Md.) diluted in PBS-BSA was added to each well at a final concentration of 2 μg/ml. After a 1-h incubation at room temperature, excess-labeled avidin was removed during washing, and the reaction was developed with o-phenylenediamine (Sigma). Plates were read at 492 nm on an ELISA reader (Labsystems Multiskan MS). The concentration of cytokine in each sample was determined from standard curves executed in parallel with known concentration of recombinant IFN-γ, IL-4, or IL-10. The detection limit of the assays was 0.2 ng/ml.
Nitrite production production by cultured cells was assessed by incubation of 50 μl of each supernatant with 50 μl of Griess solution (1% sulfanilamide, 0.1% naphthylene diamine dihydrochloride, 2% H3PO4; all purchased from Sigma). The absorbance was measured at 540 nm in a microplate ELISA reader. Sodium nitrite diluted in culture medium was used as a standard.
BLT-esterase activity was detected by adding 20 μl of culture supernatants in 0.18 ml of Tris-HCl buffer (Tris 20 mM, EDTA 1 mM [pH 7.5]) containing 200 μM 5,5′-dithiobis-(2-nitrobenzoic acid; Sigma) to 220 μM BLT (Sigma). The samples were kept at room temperature for 30 min before reading at 405 nm.
Indirect immunofluorescence and flow cytometry.
T cells (106) were incubated on ice for 45 min with hybridoma supernatants precipitated with ammonium sulfate. After being washed twice, cell samples were incubated with fluorescein-labeled goat anti-rat immunoglobulin G (IgG; Kierkegaard & Perry Laboratories) for additional 45 min on ice, washed, and fixed in PBS containing 2% (wt/vol) paraformaldehyde.
Fluorescence was analyzed in an Optishot-2 fluorescence microscope (Nikon) or by fluorescence-activated cell sorting (FACS) in a FACScan cytometer (Becton Dickinson), gating for size by forward and sideward light scatter, both amplified on linear scales; the fluorescence signals were amplified on a logarithmic scale.
The rat IgG MAbs used for staining were anti-CD8 2.43, anti-CD4 GK1.5, anti-CD44 KM 703, and anti-VLA-4/LPAM-1 R1-2. All hybridoma cells were purchased from the American Type Culture Collection (Rockville, Md.).
DNA degradation assay.
The assay was performed as detailed described in reference 24. For this in vitro assay, we used as targets A20J-TS or A20J-pcDNA3 cells labeled overnight with 5 μCi of [3H]TdR per ml. After being washed, target cells (105/well) were cultured at the indicated T cell/target ratio in 96-well plates in a final volume of 0.2 ml. At the end of the 2-h incubation period, supernatants were collected with a semiautomatic cell harvester. The percentage of DNA degradation was calculated as follows: [(experimental − spontaneous release cpm − 1)/total − spontaneous release cpm] × 100.
Chromium release assay.
Target cells were labeled for 2 h with 100 μCi of 51Cr (ICN Biomedicals, Irvine, Calif.). After being washed, target cells were cultured at different T cell/target ratios in 96-well plates in a final volume of 0.2 ml. For the CD4 clones, we used 5 × 103 target cells and an incubation time of 18 h. For the CD8 clones, 1 × 104 to 5 × 104 target cells were added per well and the incubation period was only 5 h. At the end of the incubation period, plates were centrifuged for 2 min, 0.1 ml of each sample was collected, and the amount of 51Cr was estimated with a gamma counter. The percentage of specific lysis was calculated as follows: (experimental − spontaneous release cpm/total − spontaneous release cpm) × 100.
Synthetic peptides.
Synthetic peptides were purchased from Neosystem (Strasbourg, France). As estimated by high-pressure liquid chromatography analysis, peptide IYNVGQVSI was more than 90% pure. This peptide represents amino acids 359 to 367 encoded by TS gene 154 (44). The other two peptides, VYSLVFARL (amino acids 395 to 403) and CGPAVTTVGL (amino acids 442 to 451), were more than 70 and 80% pure, respectively. The control peptide SYVPSAEQI, kindly provided by Moriya Tsuji, is a CD8 epitope of the Plasmodium yoelii circumsporozoite protein that binds to H-2Kd (32).
In vitro inhibition of T. cruzi development.
Macrophages were adherent cells collected from peritoneal exudates of nonmanipulated BALB/c mice. After washes in PBS, peritoneal exudate cells were resuspended in RPMI medium containing 10% FCS. Aliquots of 200 μl containing 5 × 105 cells were loaded on top of sterile glass coverslips placed inside 24-well plates (Costar). After 4 h at 37°C, nonadherent cells were washed thoroughly, and the macrophages were maintained overnight in culture until infection with T. cruzi. Tissue culture trypomastigotes (5 × 106) were obtained as described in reference 38 and added to each well in a final volume of 1 ml. After 4 h, the parasites were removed by vigorous washes, and the desired activation stimulus, or medium, was added to the culture. A total of 5 × 105 CD4 Th1 cells of clone 2F1 or mouse recombinant IFN-γ (1 μg/ml; R&D Systems, Minneapolis, Minn.) were added to each well. In some experiments, we added the supernatant of T cells that had been activated with infected macrophages (see below). After 72 h of incubation, the supernatants were removed to estimate the concentration of nitrite, IFN-γ, IL-4, or IL-10 or used to test antiparasitic activity. Glass coverslips were washed with PBS and stained with Giemsa. The number of amastigotes per 100 macrophages as well as the percentage of infected cells were estimated microscopically by counting at least 200 macrophages per coverslip.
Fibroblast cell lines BALB/c 3T3 (H-2D) and NIH 3T3 cells (both purchased from the American Type Culture Collection) were cultured overnight in 24-well plates at a concentration of 0.125 × 106 per ml in a final volume of 1 ml. Tissue culture trypomastigotes (1.25 × 106) were added to each well. After overnight incubation, the parasites were removed by vigorous washes, and the desired amount of T cells was added to each well in a final volume of 2 ml. The number of trypomastigotes released was estimated at days 3, 4, 5, and 6 after infection. For that purpose, the medium was thoroughly homogenized, and an aliquot of 0.02 ml was taken. The number of parasites was estimated by counting the number motile trypomastigotes in a hematocytometer.
RESULTS
Secretion of IFN-γ by CD4 cells of mice immunized with plasmid 154/13 upon in vitro restimulation with recombinant TS.
In our earlier study, we found that lymph nodes and spleen cells of mice immunized with plasmid 154/13 proliferated in vitro upon stimulation with recombinant TS (6). We have now determined that the proliferative response was dependent on CD4 T cells, as the addition of the anti-CD4 MAb almost completely inhibited [3H]TdR uptake of lymph node cells. In contrast, addition of anti-CD8 did not modify the proliferative response (Fig. 1A). Complete inhibition [3H]TdR uptake by MAb anti-CD4 was also observed when we used spleen cells from mice immunized with plasmid 154/13 (data not shown).
FIG. 1.
Immunization of mice with plasmid 154/13 generates a CD4-dependent proliferative response and IFN-γ production by lymph node cells. BALB/c mice were immunized as described in Materials and Methods. Three weeks after the last immunization, lymph node cells pooled from three mice immunized with either plasmid 154/13 or pcDNA3 were cultured in the presence or absence of recombinant (Rec.) TS (10 μg/ml). Anti-CD4 (α CD4) or anti-CD8 (α CD8) was added to the cultures at a final concentration of 1 μg/ml (A). After 4 days, culture supernatants were collected and the levels of IFN-γ and IL-4 were estimated by capture ELISA (B). Some cultures received concanavalin A (ConA; 2.0 μg/ml). The results are expressed as the average of triplicate cultures ± SD.
To determine which type of CD4 T cells (Th1 or Th2) was being activated, we collected the supernatants of these cultures and estimated the presence of selected cytokines. Lymph node cells of mice immunized with plasmid 154/13 secreted detectable levels of IFN-γ upon stimulation with recombinant TS (Fig. 2B). In contrast, IL-4 levels were below the detection limit of the assay. IL-10 was also undetectable (data not shown). IFN-γ production was dependent on the in vivo priming with plasmid 154/13, as cells from mice immunized with control plasmid pcDNA3 fail to secrete this cytokine when stimulated by recombinant TS (Fig. 1B). As positive control, lymphocytes were stimulated with concanavalin A. Upon stimulation with this mitogen, lymph node cells from mice immunized with both plasmids produced detectable amounts of IFN-γ and IL-4. In vitro secretion of IFN-γ, but not IL-4, was also observed when we used spleen cells from mice immunized with plasmid 154/13 (data not shown).
FIG. 2.
IFN-γ, IL-4, and IL-10 production by CD4 T-cell clones. CD4 clones (105/well) and irradiated feeder cells (5 × 105/well) were cultivated in the presence (hatched bars) or absence (open bars) of recombinant TS (10 μg/ml). After 4 days, culture supernatants were collected and the lymphokine concentration was estimated by capture ELISA. The results are expressed as the average of triplicate cultures ± SD.
Characterization of CD4 clones derived from lymph node cells of DNA-immunized mice.
To characterize proliferating CD4 cells at the clonal level, we derived T-cell clones. From two cloning procedures using lymph node cells that had been expanded in vitro in the presence of recombinant TS, we obtained five T-cell clones. By indirect immunofluorescence assay, we determined that these clones expressed CD4 but not CD8 surface marker (data not shown). To determine the pattern of cytokine production of these T-cell clones, we estimated the presence of IFN-γ, IL-4, and IL-10 in the cell supernatants following stimulation with recombinant TS. Three of the clones (2F1, 2F3, and 2H10) secreted IFN-γ, but not IL-4 or IL-10, whereas in supernatants of the two other clones (A1 and B9), we detected the presence of the type 2 cytokines IL-4 and IL-10 but not IFN-γ (Fig. 2).
To determine whether these Th1 clones had cytolytic activity, we used A20J-TS cells. These transfected cells secreted TS, as estimated by its enzymatic activity (data not shown). A20J-pcDNA3 cells served as a control. Th1 clones and Th2 clones were tested for the ability to lyse A20J-TS cells in a 51Cr release assay. As shown in Fig. 3, while both Th1 clones (2F1 and 2F3) were highly cytotoxic for A20J-TS, the Th2 clones (A1 and B9) fail to lyse these cells. The cytotoxic activity was dependent on the recognition of the antigen, as T cells minimally lysed control (A20J-pcDNA3) cells.
FIG. 3.
Cytotoxic activity of Th1 CD4 T-cell clones. CD4 clones were incubated at the indicated T cell/target ratio with A20J-TS or A20J-pcDNA3 cells labeled with 51Cr. After 18 h, the percentage of lysis was estimated as described in Materials and Methods. The results are expressed as average of triplicate cultures ± SD.
The antiparasitic activity of a TS-specific Th1 clone was evaluated in vitro by coculture of T cells with infected syngeneic macrophages. After 72 h of incubation, macrophages cocultured with cells of clone 2F1 had less than 2% of the number of amastigotes found in control cultures (Table 1). Similar inhibitory activity was achieved when we added to these cultures mouse recombinant IFN-γ. We then collected the supernatant of clone 2F1 cells incubated with infected macrophages and assayed for the presence of IFN-γ, IL-4, IL-10, and nitrite. Also, we added this supernatant to infected macrophages and estimated its antiparasitic activity. We found that supernatant collected from cultures containing clone 2F1 cells and infected macrophages had IFN-γ and nitrite but no IL-4 or IL-10. Most relevant, this supernatant strongly inhibited development of T. cruzi in infected macrophages (Table 1). Infected macrophages cocultured with a control Th1 clone specific for ovalbumin failed to eliminate amastigotes of T. cruzi (data not shown).
TABLE 1.
Inhibition of T. cruzi development in infected macrophages by CD4 Th1 clone 2F1
| Expt | Stimulus | Infected macrophages (%)a | Inhibition (%) | No. of amastigotes/100 macrophagesa | Inhibition (%) | IFN-γ (ng/ml)a | Nitrite (μM)b | IL-4 (ng/ml)b | IL-10 (ng/ml)b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Medium | 31.6 ± 2.4 | 247 ± 7 | >0.2 ± 0 | 0.21 | >0.2 | >0.2 | ||
| T-cell clone 2F1c | 3 ± 0 | 90.5 | 4 ± 1 | 98.4 | 9.2 ± 0.2 | 1.3 | >0.2 | >0.2 | |
| Recombinant IFN-γ (1 μg/ml) | 2.5 ± 1.5 | 92.1 | 4 ± 3 | 98.4 | NTd | 2.36 | NT | NT | |
| 2 | Medium | 31.1 ± 2.7 | 262 ± 15 | NT | 0.1 | NT | NT | ||
| Supernatant of T-cell clone 2F1e | 0.5 ± 0.5 | 93.4 | 0.5 ± 0.5 | 99.8 | NT | 2.2 | NT | NT | |
| Recombinant IFN-γ (1 μg/ml) | 1.5 ± 0.5 | 95.2 | 2.9 ± 1 | 98.9 | NT | 0.78 | NT | NT |
BALB/c macrophages (5 × 105/well) were infected with culture trypomastigotes as described in Materials and Methods. Results are expressed as the average of triplicate cultures ± SD.
Cytokines were estimated in supernatants collected after 72 h. Results are expressed as the average of duplicate cultures.
5 × 105 T cells per well.
NT, not tested.
Supernatants were collected after 72-h incubation of T-cell clone 2F1 with infected macrophages.
Secretion of IFN-γ by CD4 and CD8 cells of mice immunized with plasmid 154/13 upon in vitro restimulation with A20J-TS cells.
The presence of IFN-γ-producing splenic cells of DNA-immunized mice was estimated 6 days after in vitro expansion upon stimulation with A20J-TS cells. In the supernatants of cells that had been derived from mice immunized with plasmid 154/13 and subsequently incubated with A20J-TS cells, we observed a high concentration of IFN-γ. These IFN-γ-producing cells were induced by in vivo priming with plasmid 154/13, because negligible levels of this cytokine were detected in supernatants of spleen cells from pcDNA3-immunized mice stimulated with A20J-TS cells. The IFN-γ secretion was antigen specific, i.e., dependent on TS recognition, as very low levels of this cytokine were produced when spleen cells were cocultured with A20J-pcDNA3 cells (Fig. 4A). Supernatants were also tested for the presence of type 2 cytokines. Despite the fact that in some instances spleen cells supernatants from mice immunized with plasmid 154/13 had over 2,000 ng of IFN-γ per ml, IL-4 and IL-10 concentrations were below the detection limit of the assay (data not shown).
FIG. 4.
Immunization of mice with plasmid 154/13 generates splenic IFN-γ-producing CD4 and CD8 cells. BALB/c mice were immunized as described in Materials and Methods. Three weeks after the last immunization, pooled spleen cells obtained from two mice immunized with plasmid 154/13 (closed symbols) or pcDNA3 (open symbols) were expanded for 6 days in the presence of irradiated A20J-TS cells. (A) The indicated numbers of splenic cells were incubated with irradiated A20J-TS cells (squares) or A20J-pcDNA3 cells (circles). (B) Cells from mice immunized with plasmid 154/13 were expanded in the presence irradiated A20J-TS. After 6 days, the cells were treated with anti-CD4 (α-CD4) or anti-CD8 (α-CD8) or both antibodies and then incubated with rabbit low-toxicity complement (C′). Cells used as control were treated with complement alone. These cells (105) were cultivated in the presence of irradiated A20J-TS cells (hatched bars) or A20J-pcDNA3 cells (open bars). The supernatants were collected after 18 h, and the concentration of IFN-γ was estimated by capture ELISA. The results are expressed as the average of triplicate cultures ± SD.
The phenotype of the TS-specific IFN-γ-producing cells was established by using a selective in vitro depletion approach. Spleen cells that had been expanded in vitro for 6 days in the presence of A20J-TS cells were treated with either anti-CD4 or anti-CD8 MAb or both MAbs or were left untreated. In several experiments, we found that depletion of CD4 cells reduced IFN-γ production by 60 to 65%. On other hand, depletion of CD8 cells decreased the production of this cytokine by 30 to 35%. Most relevant was the fact that the depletion of CD4 and CD8 cells almost completely abolished IFN-γ production (Fig. 4B). We concluded that spleen cells of mice immunized with plasmid 154/13 contained antigen-specific CD4 and CD8 cells that secreted IFN-γ.
Characterization of CD8 clones derived from spleen cells of DNA-immunized mice.
From a single cloning procedure using in vitro-expanded spleen cells that had been depleted of CD4 cells, we obtained 18 T-cell clones. Ten of these clones were chosen for subsequent in vitro characterization. By indirect immunofluorescence, we determined that all clones expressed CD8 but not CD4 surface marker (data not shown). In five clones (A4, A8, A10, A11 and A12), expression of surface markers was determined by FACS analysis. As exemplified in Fig. 5, T-cells clones expressed CD8 but not CD4 on their surface. In addition, they expressed high levels of CD44 and detectable levels of VLA-4 (CD49d), two surface molecules that have been implicated in the antiparasitic activity of CD8 T cells (32).
FIG. 5.
Expression of CD8, CD44, and VLA-4 surface molecules in clones A4 and A10. T-cell clones were stained with MAbs specific for the indicated surface markers, followed by fluorescein-conjugated goat anti-rat antibody (secondary), and analyzed by FACS. The relative mean fluorescence obtained with each MAb is indicated at the right in each histogram.
The cytotoxic activity of these clones was established initially by using the DNA degradation assay (24). As shown in Fig. 6A, cells from clone A10 induced DNA degradation of A20J-TS cells in a dose-dependent manner. The DNA degradation event was specific, as these cells failed to degrade the DNA of A20J-pcDNA3 cells. Although some minor variations were observed among them, all 10 clones displayed significant cytotoxic activity in vitro (Fig. 6B). We also performed conventional 51Cr release assays with four CD8 clones (A4, A10, A11, and A12). All of them lysed A20J-TS cells. The lysis was specific, as control cells (A20J-pcDNA3) were minimally lysed by these T-cell clones (data not shown).
FIG. 6.
DNA degradation induced by CD8 T-cell clones. (A) Clone A10 was incubated at different T cell/target ratios with A20J-TS or A20J-pcDNA3 cells; (B) T-cell clones were incubated with either A20J-TS or pcDNA3 cells at a T cell/target ratio of 2.5:1. The results are expressed as the average of triplicate cultures ± SD.
Next, we estimated the amounts of the cytokines and a granule-associated protein secreted by these clones following stimulation with A20J-TS or A20J-pcDNA3 cells. As shown in Table 2, upon stimulation with A20J-TS cells, all clones produced IFN-γ and the enzyme BLT-esterase. In contrast, we detected no type 2 cytokines (IL-4 and IL-10) in the supernatant of activated T cells. This finding indicates that all CD8 clones that we analyzed were Tc1 cells.
TABLE 2.
CD8 T-cell clones secrete IFN-γ and BLT-esterase but not IL4 or IL-10a
| T-cell clone | IFN-γ (ng/ml)
|
IL-4 (ng/ml)
|
IL-10 (ng/ml)
|
BLT-esterase (OD/ml)
|
||||
|---|---|---|---|---|---|---|---|---|
| A20J-pcDNA3 | A20J-TS | A20J-pcDNA3 | A20J-TS | A20J-pcDNA3 | A20J-TS | A20J-pcDNA3 | A20J-TS | |
| A1 | 35 ± 11 | 301 ± 20 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.003 | 0.265 |
| A3 | 85 ± 5 | 770 ± 31 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.029 | 0.197 |
| A4 | <0.2 ± 0 | 245 ± 5 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.034 | 0.147 |
| A6 | 73 ± 5 | 620 ± 20 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.021 | 0.096 |
| A8 | 12 ± 1 | 352 ± 11 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.014 | 0.281 |
| A9 | <0.2 ± 0 | 420 ± 20 | <0.2 ± 0 | <0.5 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.001 | 0.130 |
| A10 | <0.2 ± 0 | 342 ± 18 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.037 | 0.165 |
| A11 | <0.2 ± 0 | 320 ± 10 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.024 | 0.196 |
| A12 | <0.2 ± 0 | 365 ± 5 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.009 | 0.178 |
| A13 | <0.2 ± 0 | 545 ± 35 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | <0.2 ± 0 | 0.000 | 0.176 |
Supernatants were collected 18 h after incubation of T cells and target cells. The results for IFN-γ, IL4, and IL-10 are expressed as the average of triplicate cultures ± SD; results for BLT-esterase are expressed as the optical density (OD) reading average of duplicate cultures.
To determine the epitope recognized by some CD8 T cells, we tested the three synthetic peptides based on the TS sequence. Peptides were selected by the presence of sites for binding to H-2kd (underlined; IYNVGQVSI and VYSLVFARL [12]) or H-2Dd (CGPAVTTVGL). No sequences were found that matched the binding motif for H-2Ld. Three CD8 clones were tested for the ability to lyse target A20J cells coated with these peptides or a control CD8 epitope derived from a malaria parasite (32). T-cell clones A4, A10, and A11 specifically lysed target cells in the presence of 10−10 M peptide IYNVGQVSI (Table 3). At that same concentration, the two other TS peptides were not recognized by any of these T-cell clones. In dose-response experiments, we observed that peptide IYNVGQVSI led to ∼50% maximal lysis in a concentration of ∼10−12 M (data not shown). In contrast, target cells were not lysed in the presence of a much higher concentration (10−8 M) of the three other peptides (data not shown).
TABLE 3.
Specificity of CD8 T-cell clones A4, A10, and A11
| T-cell clone | Specific lysis in presence of indicated peptidea (%)
|
||||
|---|---|---|---|---|---|
| None | IYNVGQVSI | VYSLVFARL | CGPAVTTVGL | SYVPSAEQI | |
| None | 2 ± 2 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| A4 | 2 ± 2 | 80 ± 4 | 5 ± 4 | 9 ± 2 | 5 ± 4 |
| A10 | 13 ± 3 | 79 ± 6 | 20 ± 3 | 12 ± 5 | 12 ± 3 |
| A11 | 0 ± 0 | 82 ± 7 | 12 ± 5 | 14 ± 7 | 10 ± 3 |
A20J cells labeled with 51Cr were incubated with each peptide at a final concentration of 10−10 M. The assay was performed as described in Materials and Methods, using a T cell/target ratio of 5:1. Peptide IYNVGQVSI represents amino acids 359 to 367 encoded by TS gene 154 (44); VYSLVFARL and CGPAVTTVGL represent, respectively, amino acids 395 to 403 and 442 to 451 encoded by the same gene. The control peptide SYVPSAEQI is a CD8 epitope of the P. yoelii circumsporozoite protein that binds to H-2Kd (32). Results are expressed as the average of triplicate cultures ± SD.
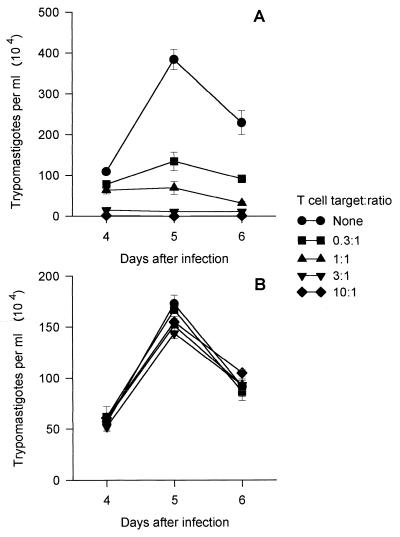
Finally, we found that coculture of CD8 A11 cells with infected BALB/c 3T3 cells significantly reduced the number of trypomastigotes released at days 4, 5, and 6 after infection (Fig. 7A). The inhibitory activity was dependent on the amount of cells added per well, and a ∼50% reduction of parasite development was achieved with a T cell/target ratio of 0.3:1. The inhibitory activity was dependent on the major histocompatibility complex haplotype of the infected target cells, as in parallel cultures, CD8 T cells were unable to reduce trypomastigote development in infected NIH 3T3 cells, which are not H-2D (Fig. 7B). Very similar results of inhibition of T. cruzi development in BALB/c 3T3 cells were observed with T-cell clone A4 (data not shown).
FIG. 7.
In vitro inhibition of trypomastigote development by CD8 clone A11. Fibroblast cell lines BALB/c 3T3 (A) and NIH 3T3 (B) were infected with tissue culture trypomastigotes as described in Materials and Methods. After overnight interaction, the parasites were removed and cells of CD8 clone A11 were added at the indicated T cell/target ratio. The number of trypomastigotes released in the medium was estimated at days 4, 5, and 6 after infection. The results are expressed as the average of triplicate cultures ± SD.
DISCUSSION
Several bodies of evidence indicate that T. cruzi activates specific CD4 and CD8 cells which participate in resistance to experimental infection (34, 43). Th1 and Tc1 are thought to be the cell types that mediate protective immunity, in part, by their ability to secrete IFN-γ. In mice, this cytokine is the main activator of NO production, a potent inhibitor of T. cruzi development (17).
In the present study, we performed a detailed analysis of the cellular immune response of mice vaccinated with a T. cruzi gene and shown to be protected against a lethal challenge with infective forms of the parasite (6). Our data indicate that DNA vaccination efficiently induces IFN-γ-producing CD4 and CD8 T cells specific for TS. Up to now, to our knowledge, only active infection with T. cruzi had been shown to effectively generate specific CD4 and CD8 cells that secrete IFN-γ (1, 48).
At the bulk cell level, IFN-γ was the predominant type of cytokine. Type 2 cytokines, such as IL-4 and IL-10, could not be detected. Our results confirm and extend several studies that reports a predominant role for Th1 cells after immunization of mice with DNA vaccines (10, 14, 15, 18, 23, 25, 28, 37, 46, 50). Nevertheless, the vast majority of reports fail to extend their characterization to the clonal level. By analyzing T-cell clones derived from these DNA-vaccinated mice, we confirmed the presence of CD4 Th1 cells that secreted large amounts of IFN-γ but not IL-4 or IL-10. In addition to secreting IFN-γ, these clones were highly cytotoxic in vitro and strongly inhibited T. cruzi development in infected macrophages. Whether the cytotoxic activity of these cells plays any role in inhibiting T. cruzi development in vitro and in vivo remains to be determined.
In addition to Th1 cells, we isolated clones that had a clear Th2 type secreting IL-4 and IL-10 but not IFN-γ. The presence of Th2 cells may explain our earlier observation that sera of DNA-vaccinated mice contained TS-specific antibodies of the IgG1 subclass (6). The precise reason why these cells cannot be detected in bulk cultures is not known but is most likely attributable to a low frequency of precursors. We concluded that although Th1 cells were predominant, the TS gene induced mixed Th1 and Th2 responses. The coexistence of these two cell populations had been also noticed in mice vaccinated with some genes (3, 4, 25).
The results obtained with the TS gene are compatible with recent reports showing that DNA produces a better Th1 type response than adjuvants such as alum or incomplete Freund’s adjuvant (22, 37). The reason for such a bias toward a Th1-type response is attributed to the immunomodulatory capacity of bacterial DNA. Bacterial plasmids contain CpG oligonucleotides that activate macrophages in vitro to produce IL-12 (19–21), a cytokine that has a pivotal role in the development of a Th1-type response (13, 41). Direct evidence of the immunomodulatory properties of DNA was provided by the in vivo coadministration of bacterial plasmid DNA or CpG-containing oligonucleotides with soluble antigens. This type of immunization generated a Th1-dominated response, confirming that they can induce Th1 responses (5, 8, 19, 22, 37).
Detailed analysis of CD8 clones revealed that immunization with the TS gene led to a uniform response mediated by Tc1 cells, which secreted large amounts of IFN-γ but not IL-4 or IL-10. These clones were highly cytotoxic in vitro, mediating lysis and DNA degradation of target cells. Also, in vitro these cells strongly inhibited T. cruzi development in nonphagocytic syngeneic cells. Similar findings had been reported for cytotoxic CD8 cells specific for a trypomastigote surface antigen (48, 49). In preliminary experiments, we observed that the adoptive transfer of CD8 clones A4 and A10 to naive mice significantly reduced the peak parasitemia and mortality after challenge with trypomastigotes of T. cruzi (33a). The exact mechanism(s) used by CD8 cells to inhibit T. cruzi development in vitro and in vivo has yet to be determined. Altogether, these observations confirm and extend earlier studies demonstrating that DNA vaccination was effective at inducing effector CD8 cells (reviewed in reference 45).
Using these clones, we could determine a CD8 epitope. This epitope can be useful to test whether DNA immunization with a plasmid containing only a minigene encoding the CD8 epitope will be effective in producing IFN-γ-secreting cells and protective immunity against T. cruzi infection.
T. cruzi is a parasite that persists for long periods of time, causing a chronic inflammatory disease. The current therapeutic treatment to eliminate the parasite and terminate the disease has not been effective in the vast majority of patients in the chronic phase of infection. On the other hand, the cellular immune response plays a role in host resistance during chronic human infection (31, 36). During that stage, the parasite-specific immune response is mediated mainly by IFN-γ-producing T cells (2). A portion of the cells that secrete IFN-γ are specific for epitopes present in TS. Upon stimulation with a recombinant protein representing the catalytic domain of the enzyme, T cells of 88% of patients with Chagas’ disease secreted IFN-γ (29a). The possibility of vaccinating patients with chronic infection is hindered by a concern that an exacerbated immune response may cause an increase in tissue pathology or autoimmunity (16). However, a recent report showed that adoptive transfer of large amounts of IFN-γ-producing T cells specific for a parasite epitope significantly reduced infection and promoted survival of mice given a lethal infection with T. cruzi (48). These results indicate that IFN-γ-producing cells did not aggravate inflammatory reactions or autoimmunity but instead promoted elimination of parasites.
The fact that DNA vaccination with a T. cruzi gene is effective in generating IFN-γ-producing CD4 and CD8 cells and protective immunity against infection suggests that DNA vaccines may provide one more strategy for treatment or prevention of Chagas’ disease.
ACKNOWLEDGMENTS
We thank Zuleica Caulada, ICB-USP, for helping with the FACS analysis, Moriya Tsuji for helping with the CD4 clones, and Mariano Levin and Sergio Schenkman for comments on the manuscript.
This work was supported by grants from FAPESP, PADCT, CNPq, PRONEX, FINEP (Brazil), and INSERM réseau NORD-SUD (contract 4N002C) (France).
REFERENCES
- 1.Abrahamsohn I A, Coffman R L. Cytokine and nitric oxide regulation of immunosuppression in Trypanosoma cruzi infection. J Immunol. 1995;155:3955–3963. [PubMed] [Google Scholar]
- 2.Arnholdt A C V, Piuvezam M R, Russo D M, Lima A P C, Pedrosa R C, Reed S G, Scharfstein J. Analysis and partial epitope mapping of human T cell responses to Trypanosoma cruzi cysteinyl proteinase. J Immunol. 1993;151:3171–3179. [PubMed] [Google Scholar]
- 3.Bonato V L D, Lima V M F, Tascon R E, Lowrie D B, Silva C L. Identification and characterization of protective T cells in hsp65 DNA-vaccinated and Mycobacterium tuberculosis-infected mice. Infect Immun. 1998;66:169–175. doi: 10.1128/iai.66.1.169-175.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chow Y-H, Chiang B-L, Lee Y-L, Chi W-K, Liu W-C, Chen Y-T, Tao M-H. Development of Th1 and Th2 Populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 5.Chu R S, Targoni O S, Krieg A M, Lehmann P V, Harding C V. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J Exp Med. 1997;186:1623–1631. doi: 10.1084/jem.186.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa F, Franchin G, Pereira-Chioccola V L, Ribeirão M, Schenkman S, Rodrigues M M. Immunization with a plasmid DNA containing the gene of trans-sialidase reduces Trypanosoma cruzi infection in mice. Vaccine. 1998;16:768–774. doi: 10.1016/s0264-410x(97)00277-6. [DOI] [PubMed] [Google Scholar]
- 7.Cross G A M, Tackle G B. The surface trans-sialidase family of Trypanosoma cruzi. Annu Rev Microbiol. 1993;46:383–411. doi: 10.1146/annurev.mi.47.100193.002125. [DOI] [PubMed] [Google Scholar]
- 8.Davis H L, Weerantsa R, Waldschmidt T J, Tygrett L, Schorr J, Krieg A M. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J Immunol. 1998;160:870–876. [PubMed] [Google Scholar]
- 9.de Andrade A L, Zicker F, de Oliveira R M, Almeida Silva S, Luquetti A, Travassos L R, Almeida I C, de Andrade S S, de Andrade J G, Martelli C M. Randomised trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet. 1996;348:1407–1413. doi: 10.1016/s0140-6736(96)04128-1. [DOI] [PubMed] [Google Scholar]
- 10.Denis O, Tanghe A, Palfifliet K, Jurion F, van der Berg T-P, Vanonckelen A, Ooms J, Saman E, Ulmer J B, Content J, Huygen K. Vaccination with plasmid encoding mycobacterial antigen 85A stimulates CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect Immun. 1998;66:1527–1533. doi: 10.1128/iai.66.4.1527-1533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dos Reis G A. Cell-mediated immunity in experimental Trypanosoma cruzi infection. Parasitol Today. 1997;13:335–342. doi: 10.1016/s0169-4758(97)01073-9. [DOI] [PubMed] [Google Scholar]
- 12.Falk K, Rötzschke O, Dres K, Metzger J, Jung G, Rammensee H-G. Identification of naturally processed viral nonapeptide allows their quantification in infected cells and suggests an allele-specific T cell epitope forecast. J Exp Med. 1991;174:425–434. doi: 10.1084/jem.174.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fearon D T, Locksley R M. The instructive role of innate immunity in acquired immune response. Science. 1996;272:50–53. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 14.Feltquate D M, Heaney S, Webster R G, Robinson H L. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol. 1997;158:2278–2284. [PubMed] [Google Scholar]
- 15.Grunathan S, Sacks D L, Brown D R, Reiner S L, Charest H, Glaichenhaus N, Seder R A. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J Exp Med. 1997;186:1137–1147. doi: 10.1084/jem.186.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higuchi M, de Brito T, Reis M, Barbosa A, Belloti G, Pereira-Barreto A, Pileggi F. Correlation between Trypanosoma cruzi parasitism and myocardial inflammatory infiltrate in human chronic Chagas’ disease and chagasic myocarditis: light microscopy and immunohistochemical findings. Cardiovasc Pathol. 1993;2:101–104. doi: 10.1016/1054-8807(93)90021-S. [DOI] [PubMed] [Google Scholar]
- 17.Hölscher C, Kölher G, Müller U, Mossman H, Schaub G A, Brombacher F. Defective nitric oxide effector function lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect Immun. 1998;66:1208–1215. doi: 10.1128/iai.66.3.1208-1215.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, Dewitt C M, Orme I M, Baldwin S, D’Souza C, Drowart A, Lozes E, Vandenbussche P, van Vooren J P, Liu M A, Ulmer J B. Immunogenicity and protective efficacy of tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 19.Jakob T, Walker P S, Krieg A M, Udey M C, Vogel J C. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J Immunol. 1998;161:3042–3049. [PubMed] [Google Scholar]
- 20.Klinman D M, Yi A-K, Beacauge S L, Conover J, Krieg A M. CpG motifs present in bacterial DNA rapidly induce lymphocytes to secrete interleukin-6, interleukin-12, and interferon-γ. Proc Nat Acad Sci USA. 1996;93:2879–2883. doi: 10.1073/pnas.93.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinman D M, Yamshchikov G, Ishigaatsubo Y. Contribution of CpG motif to the immunogenicity of DNA vaccines. J Immunol. 1997;158:3635–3639. [PubMed] [Google Scholar]
- 22.Leclerc C, Deriaud E, Rojas M, Whalen R G. The preferential induction of a Th1 immune response by DNA-based immunization is mediated by immunostimulatory effect of plasmid DNA. Cell Immunol. 1997;179:97–106. doi: 10.1006/cimm.1997.1161. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Sambhara S, Li C X, Ewasyshyn M, Parrington M, Caterini J, James O, Cates G, Du R-P, Klein M. Protection against respiratory syncitial virus infection by DNA immunization. J Exp Med. 1998;158:681–688. doi: 10.1084/jem.188.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzinger P. The JAM test. A simple assay for DNA degradation and cell death. J Immunol Methods. 1991;15:185–192. doi: 10.1016/0022-1759(91)90325-a. [DOI] [PubMed] [Google Scholar]
- 25.Mor G, Klinman D M, Shapiro S, Hagiwara E, Sedegah M, Norman J A, Hoffman S L, Steinberg A D. Complexity of the cytokine and antibody response elicited by immunization mice with Plasmodium yoelii circumsporoite protein plasmid DNA. J Immunol. 1995;155:2039–2046. [PubMed] [Google Scholar]
- 26.Munoz-Fernandez M A, Fernandez M A, Fresno M. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur J Immunol. 1992;22:301–307. doi: 10.1002/eji.1830220203. [DOI] [PubMed] [Google Scholar]
- 27.Nickell S P, Keane M, So M. Further characterization of protective Trypanosoma cruzi-specific CD4+ T-cell clones: T helper type 1-like phenotype and reactivity with shed trypomastigote antigens. Infect Immun. 1993;61:3250–3258. doi: 10.1128/iai.61.8.3250-3258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raz E, Tighe H, Corr M, Dudler J A, Roman M, Swain S L, Spielgeberg H L, Carson D A. Preferential induction of Th1 immune response and inhibition of specific IgE antibody formation by plasmid immunization. Proc Natl Acad Sci USA. 1996;93:5141–5145. doi: 10.1073/pnas.93.10.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed S G. In vivo administration of IFN-γ induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infection. J Immunol. 1988;140:4342–4347. [PubMed] [Google Scholar]
- 29a.Ribeirao, M. Unpublished observations.
- 30.Ribeirão M, Pereira-Chioccola V L, Eichinger D, Rodrigues M M, Schenkman S. Temperature differences for trans-glycosylation and hydrolysis reaction reveal an acceptor binding site in the catalytic mechanism of Trypanosoma cruzi trans-sialidase. Glycobiology. 1997;7:1237–1246. doi: 10.1093/glycob/7.8.1237. [DOI] [PubMed] [Google Scholar]
- 31.Rocha A, de Menezes A C, da Silva A M, Ferreira M S, Nishioka S A, Burgarelli M K, Almeida E, Turcato Jr G, Metze K, Lopes E R. Pathology of patients with Chagas’ disease and acquired immunodeficiency syndrome. Am J Trop Med Hyg. 1994;50:261–268. doi: 10.4269/ajtmh.1994.50.261. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues M, Nussenzweig R S, Romero P, Zavala F. In vivo cytotoxic activity of CD8+ T cell clones correlates with their level of expression of adhesion molecules. J Exp Med. 1992;175:895–905. doi: 10.1084/jem.175.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodrigues M M, Cordey A S, Arreaza G, Romero P, Corradin G, Maryanski J L, Nussenzweig R, Zavala F. Cytotoxic T cell clones derived against the circumsporozoite protein of Plasmodium yoelii protects against malaria. Int Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 33a.Rodrigues, M. M., and V. L. Pereira-Chioccola. Unpublished observations.
- 34.Rottenberg M E, Bakhiet M, Olsson T, Kristensson K, Mak T, Wigzell H, Orn A. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect Immun. 1993;61:5129–5133. doi: 10.1128/iai.61.12.5129-5133.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 36.Sartori A M, Shikanai-Yasuda M A, Neto V A, Lopes M H. Follow-up of 18 patients with human immunodeficiency virus infection and chronic Chagas’ disease, with reactivation of Chagas’ disease in three patients. Clin Infect Dis. 1998;26:177–179. doi: 10.1086/516257. [DOI] [PubMed] [Google Scholar]
- 37.Sato Y, Roman M, Tighe H, Lee D, Corr M, Nguyen M, Silverman G J, Lotz M, Carson D A, Raz E. Immunostimulatory DNA sequences necessary for effective intradermal gene immunization. Science. 1996;273:352–354. doi: 10.1126/science.273.5273.352. [DOI] [PubMed] [Google Scholar]
- 38.Schenkman S, Man-Shiow J, Hart G W, Nussenzweig V. A novel cell surface trans-sialidase of Trypanosoma cruzi generates a stage-specific epitope required for invasion of mammalian cells. Cell. 1991;65:1117–1125. doi: 10.1016/0092-8674(91)90008-m. [DOI] [PubMed] [Google Scholar]
- 39.Silva J S, Vespa G N R, Cardoso M A G, Aliberti J C S, Cunha F Q. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect Immun. 1995;63:4862–4867. doi: 10.1128/iai.63.12.4862-4867.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. Interleukin 10 and IFN-gamma regulation of experimental Trypanosoma cruzi infection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tarleton R L. The role of T cells in Trypanosoma cruzi infections. Parasitol Today. 1995;11:7–12. doi: 10.1016/0169-4758(95)80095-6. [DOI] [PubMed] [Google Scholar]
- 42.Tarleton R L, Sun J, Zhang L, Postan M, Glimcher L. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int Immunol. 1996;8:13–22. doi: 10.1093/intimm/8.1.13. [DOI] [PubMed] [Google Scholar]
- 43.Trinchieri G. IL-12: a cytokine produced by antigen-presenting cells with immunoregulatory function in the generation of T-helper cell type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 44.Uemura H, Schenkman S, Nussenzweig V, Eichinger D. Only some members of a gene family in Trypanosoma cruzi encode proteins that express both trans-sialidase and neuraminidase activities. EMBO J. 1992;11:3837–3844. doi: 10.1002/j.1460-2075.1992.tb05476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ulmer J B, Sadoff J C, Liu M A. DNA vaccines. Curr Opin Immunol. 1996;8:531–536. doi: 10.1016/s0952-7915(96)80042-2. [DOI] [PubMed] [Google Scholar]
- 46.Ulmer J B, Fu T-M, Deck R R, Friedman A, Guan L, DeWitt C, Liu X, Wang S, Liu M A, Donnelly J J, Caulfield M J. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72:5648–5653. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Viotti R, Vigliano C, Armenti H, Segura E. Treatment of chronic Chagas’ disease with benznidazole: clinical and serologic evolution of patients with long-term follow up. Am Heart J. 1994;127:151–162. doi: 10.1016/0002-8703(94)90521-5. [DOI] [PubMed] [Google Scholar]
- 48.Wizel B, Nunes M, Tarleton R. Identification of Trypanosoma cruzi trans-sialidase family members as targets of protective CD8+ TC1 responses. J Immunol. 1997;159:6120–6130. [PubMed] [Google Scholar]
- 49.Wizel B, Garg N, Tarleton R. Vaccination with trypomastigote surface antigen 1-encoding plasmid DNA confers protection against lethal Trypanosoma cruzi infection. Infect Immun. 1998;66:5073–5081. doi: 10.1128/iai.66.11.5073-5081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu X, Venkaprasad N, Thangaraj H S, Hill M, Singh M, Ivanyi J, Vordermeier H M. Functions and specificity of T cells following nucleic acid vaccination of mice against Mycobacterium tuberculosis infection. J Immunol. 1997;158:5921–5926. [PubMed] [Google Scholar]