Abstract
The two most generally diagnosed Neurodegenerative diseases are the Alzheimer and Parkinson diseases. So this paper presents a fully automated early screening system based on the Capsule network for the classification of these two Neurodegenerative diseases. In this study, we hypothesized that the Neurodegenerative diseases-Caps system based on the Capsule network architecture accurately performs the multiclass i.e. three class classification into either the Alzheimer class or Parkinson class or Healthy control and delivers better results in comparison other deep transfer learning models. The real motivation behind choosing the capsule network architecture is its more resilient nature towards the affine transformations as well as rotational & translational invariance, which commonly persists in the medical image datasets. Apart from this, the capsule networks overcomes the pooling layers related deficiencies from which conventional CNNs are mostly affected and unable to delivers accurate results especially in the tasks related to image classification. The various Computer aided systems based on machine learning for the classification of brain tumors and other types of cancers are already available. Whereas for the classification of Neurodegenerative diseases, the amount of research done is very limited and the number of persons suffering from this type of diseases are increasing especially in developing countries like India, China etc. So there is a need to develop an early screening system for the correct multiclass classification into Alzheimer’s, Parkinson’s and Normal or Healthy control cases. The Alzheimer disease and Parkinson progression (ADPP) dataset is used in this research study for the training of the proposed Neurodegenerative diseases-Caps system. This ADPP dataset is developed with the aid of both the Parkinson's Progression Markers Initiative (PPMI) and Alzheimer’s disease Neuroimaging Initiative (ADNI) databases. There is no such early screening system exist yet, which can perform the accurate classification of these two Neurodegenerative diseases. For the sake of genuine comparison, other popular deep transfer learning models like VGG19, VGG16, ResNet50 and InceptionV3 are implemented and also trained over the same ADPP dataset. The proposed Neurodegenerative diseases-Caps system deliver accuracies of 97.81, 98, 96.81% for the Alzheimer, Parkinson and Healthy control or Normal cases with 70/30 (training/validation split) and performs way better as compare to the other popular Deep transfer learning models.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11571-022-09787-1.
Keywords: Neurodegenerative diseases, Parkinson, Alzheimer, Capsule network, ResNet50, VGG 16, VGG 19, InceptionV3 etc.
Introduction
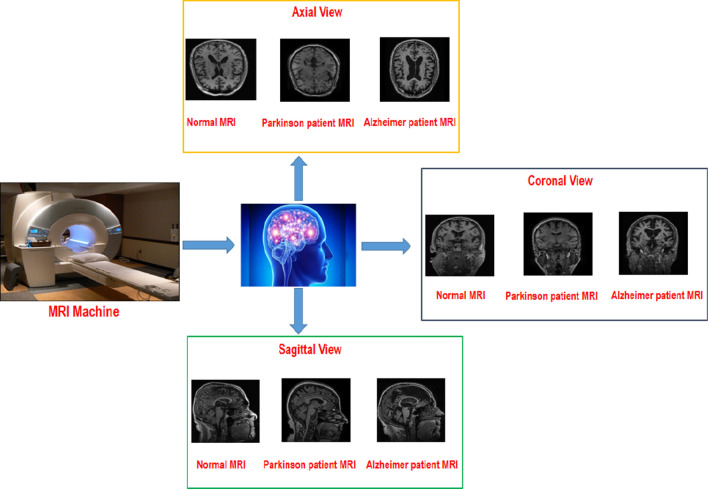
The Parkinson disease (PD) and Alzheimer's disease (AD) are the two most common diagnosed Neurodegenerative diseases, which generally diagnosed in old age people (Alzheimer's Association 2017). The common symptoms of a person suffering from Alzheimer are memory loss, difficulty in completing normal tasks, challenges in planning etc. The lessening of certain specific nerve cells alongside plaques (neuritic) just outside the neuron and accumulation of neurofibrillary tangles in the inner side of neuron (McKhann et al. 1984) is the major cause for the Alzheimer disease and disturbing the normal functionality of brain cells. The early diagnosis of AD will be very helpful for the proper care of patients as this AD is incurable. The most effective way of diagnosing the AD is the use of various medical imaging modalities like Positron emission tomography (PET), Computed tomography (CT), Magnetic resonance imaging (MRI) etc. to carry out the patient’s brain scans. Since the James Parkinson was the first one to explain this Parkinson’s disease so it is known as the Parkinson’s disease. This PD is also commonly diagnosed in old age people. The slow movements, face with no expressions, difficulty in standing from the chair, muscle rigidity, tremors, shaky handwriting, very low speech etc. are its major symptoms (Borek et al. 2006). The deficiency of Dopamine (acts as a neurotransmitter) in the human brain is the major cause of PD, which occurs due to the depletion of neurons (Ferreri et al. 2006). The PD just like AD is also highly progressive in nature and at the same time incurable too. So its diagnosis in the early stages will be very helpful in terms of providing proper healthcare to patients. The MRIs scans are mostly preferred by the physicians as compare to other medical imaging modalities like CT, PET etc. for the diagnosis of PD because the accurate Neuro-anatomic biomarkers are exhibited by the MRIs scans. Even the radiologists find it very tough to examine and perceive the inherent details of subcortical structures taken in these MRI scans with the help of bare eye because of the three dimensional nature. So there is an imperative need of an early screening system which can assist the radiologist and perform the analysis at the same time easily handle the minute intrinsic details of these three dimensional brain structures (Bakator and Radosav 2018; Lundervold and Lundervold 2019). The general set up for acquiring the MRI images of patients suffering from Alzheimer and Parkinson is presented with the aid of Fig. 1 below.
Fig. 1.
General Procedure to acquire the MRI sequences from the patients suffering from Alzheimer and Parkinson diseases
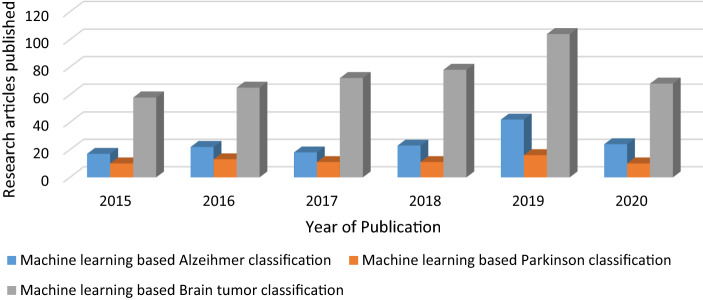
The application of machine and deep learning in the context of classification of various brain structural disorders is proved to be very useful and research oriented especially in the last five years. The CAD systems based on machine learning utilizing the medical imaging data and electronic medical records are proved to be very accurate in terms of accurate classification as well as prediction of various brain disorders. Apart from this substantial amount of research is previously been carried out in order to propose numerous CAD systems for automating the task of diagnosis of liver cancer, breast cancer, lung cancers etc. utilizing the Structural MRI scans, fMRI, PET, CT etc. But still the magnitude of research done in the domain of Neurodegenerative disease classification is far less as compare to Glioma and other types of brain tumor detection. The Fig. 2 below simply presents a statistical comparison among the number of research articles and studies published in the domain of Brain tumor (Glioma) classification and Neurodegenerative diseases i.e. Alzheimer, Parkinson based on machine and deep learning.
Fig. 2.
The yearly rise in statistics related to the amount of research studies associated with the use of artificial intelligence for the classification of Alzheimer, Parkinson and Brain tumor as per IEEE Xplore and PubMed databases from 2015 to 2020
The conventional neural networks and deep transfer learning models are very popular and widely used by the researchers because of their high performance and efficient nature. As in these neural networks and deep transfer learning models, there is no need of manual segmentation and feature extraction unlike conventional machine learning models, so these deep learning models are more popular and hence completely automate the task of either binary or multi class classification (Russakovsky et al. 2015). But these conventional neural networks also tends to suffered from some deficiencies especially related to pooling layers, which affects their performance especially in the image classification task. So in order to overcome the deficiencies of existing neural networks and deep transfer learning models, the Capsule networks are used especially for the small size, medium size and augmented image datasets. The use of Capsule network offers advantages like robustness towards data rotations and affine transformation, also requires small size training datasets etc. (Sabour et al. 2017). Another important attribute of Capsule network is the use of routing by agreement or dynamic routing in order to overcome the issues related to pooling layers (Sara et al. 2017; Worrall et al. 2017). The application of the Capsule network based models are already being tried out especially in the field of Covid-19 classification utilizing the Chest X ray and CT scans images (Afshar et al. 2020; Tiwari and Jain 2021; Heidarian et al. 2021) and has delivers very encouraging results. The Capsule network based models has also delivered very good results in the domain of breast cancer classification (Wang et al. 2021), Lung Cancer classification (Ali and Ali 2021; Adu et al. 2021a, b),Brain tumor classification (Adu et al. 2021a, b) and other cancer type classification. The Capsule network performs better for the image classification tasks as it tends to learn the good weights for feature extraction and image classification along with how to infer spatial pose parameters from the image (Jiménez-Sánchez et al. 2018). All these attributes makes the Capsule network an ideal alternative for the classification of multiclass Neurodegenerative disease classification. The major contributions of this research study are as follows:
A Capsule network based framework was proposed for the Multiclass classification of Neurodegenerative diseases for the first time.
In order to showcase the robustness, efficiency and applicability of this Neurodegenerative diseases-Caps system in real time, large size ADPP dataset is used for the training and testing purpose.
To study the behavior and performance of our proposed Neurodegenerative diseases-Caps system in comparison with other popular deep transfer learning models. The VGG-16, VGG-19, ResNet 50 and InceptionV3 are also implemented and trained over the same ADPP dataset.
Related work
The magnitude of research done in the domain of Alzheimer and Parkinson disease classification using Machine and Deep learning is very limited. Initially the Alzheimer and Parkinson classification approaches based on Machine learning uses the classifiers like Support vector machine (SVM), Radial based function SVM (RBFS), least absolute shrinkage and selection operator (LASSO) etc. (Li et al. 2014a, b; Dyrba et al. 2015; Garali et al. 2018; Peng et al. 2017; Amoroso et al. 2018). These classifiers are trained with the help of Diffusion tensor images (DTI) features, Graph theoretical measures, Entropy features, shape based and volume based features etc., which are extracted from the segmented region of interest. These machine learning based approaches in turn tends to perform either binary classification or multiclass classification categorizing the Alzheimer or Parkinson cases into their corresponding stages.
With the advent of Convolutional neural networks and Deep learning, the fully automated approaches for the binary as well as multiclass classification of Alzheimer and Parkinson diseases into their corresponding stages are proposed. Generally the Deep Convolutional Neural Network, Max Pooling based CNN, 3D neural network, enhanced probabilistic neural network framework (EPNN) etc. models are used in those approaches (Fulton et al. 2019; Talo et al. 2019; Yagis et al. 2019; Ramzan et al. 2020; Naz et al. 2021; Mozhdehfarahbakhsh et al. 2021). Majority of these Alzheimer and Parkinson classification approaches are utilizing the Global datasets like ADNI, PPMI, MIRIAD and OASIS etc. for the training and testing purpose. These datasets are having the Axial and Sagittal view MRI sequences. A brief comparison among some of the state of the art Alzheimer and Parkinson classification approaches based on the Machine as well as Deep learning are presented with the help of Tables 1 and 2 below.
Table 1.
A comparison among some of the state of the art Alzheimer disease classification approaches based on the Machine and Deep learning
| Author name and year | Machine learning classifier or Deep learning model | Modalities used | Result (Accuracy) AD vs. healthy control (%) | Datasets used |
|---|---|---|---|---|
| Li et al. (2014a) | LBP and SVM classifier | MRI sequence | 82.8 | ADNI database |
| Li et al. (2014b) | SVM and Diffusion tensor images (DTI) features | T1 sequence MRI | 94.3 | Local dataset |
| Dyrba et al. (2015) | SVM | sMRI, dMRI, Resting state-fMRI | 85 | Local dataset |
| Farzan et al. (2015) | RBFS | MRI sequence | 91.7 | ADNI |
| Ni et al. (2016) | SVM and multiple kernel learning (MKL) | Resting state-fMRI | 76 | ADNI |
| Doan et al. (2017) | LASSO | T1 sequence MRI | 81 | Local dataset |
| Glozman et al. (2017) | SVM | PET and MRI | 88.13 | ADNI |
| Guo et al. (2017) | Multi-kernel SVM | Resting state-fMRI | 91.60 | Local dataset |
| Garali et al. (2018) | SVM | FDG-PET | 97.88 | Local dataset |
| Wang et al. (2018) | The leaky rectified linear unit and max pooling based CNN model | T1-weighted Gradient-Echo sequence and Magnetization-Prepared Rapid Gradient-Echo (MP-RAGE) | 97.7 | Local dataset |
| Choi and Jin (2018) | Deep convolutional neural network | PET images | 96 | ADNI |
| Goceri (2019) | 3D neural network | MRI images | 98.06 | ADNI |
| Fulton et al. (2019) | Resnet-50 | MRI images | 98 | OASIS |
| Talo et al. 2019 | Resnet-34 | MRI images | 99 | Local dataset |
| El-Sappagh et al. (2020) | Stacked convolutional neural network (CNN) and Bidirectional long short-term memory (BiLSTM) | MRI images | 92 | ADNI |
| Feng et al. (2020) | 3D-CNN-SVM | MRI images | 92 | ADNI |
| Ramzan et al. (2020) | ResNet18 | rs-fMRI | 97 | ADNI |
| Naz et al. (2021) | VGGNet, AlexNet, GoogLeNet, ResNet, DenseNet, Inceptionv3, InceptionResNet | MRI images | 97 delivered by VGGNet | ADNI |
Table 2.
A comparison among some of the state of the art Parkinson disease classification approaches based on the Machine and Deep learning
| Author name and year | Machine learning classifier or deep learning model | Modalities used | Result (accuracy) PD vs. healthy control (%) | Datasets used |
|---|---|---|---|---|
| Adeli et al. (2016) | Least squares formulation of linear discriminant analysis (LS-LDA) classifier | T1 sequence MRI | 82 | PPMI |
| Peng et al. (2017) | Multi-kernel support vector machine (SVM) | T1 weighted MRI | 86 | PPMI |
| Amoroso et al. (2018) | Random forest and SVM classifiers | T1 weighted MRI | 93 | PPMI |
| Oliveira et al. (2017) | SVM | The single-photon emission computed tomography scans (FP-CIT SPECT) | 98 | PPMI |
| Yagis et al. (2019) | VGG 16 and ResNet 50 | T1 weighted MRI | 82 | PPMI |
| Chakraborty et al. (2020) | 3D convolutional neural network (CNN) architecture | T1 weighted MRI | 95 | PPMI |
| Vyas et al. (2021) | 2D and 3D CNN | T1 weighted MRI | 88.9 | PPMI |
| Mozhdehfarahbakhsh et al. (2021) | Customize CNN | T1 weighted MRI | 94 | PPMI |
The following points can be concluded from the brief literature review done above, which are as follows:
There is no unified system proposed yet either based on the Machine learning or deep learning for early screening of both the neurodegenerative diseases like Alzheimer and Parkinson. There is a need of a unified automated system in order to classify both the Alzheimer and Parkinson disease as both are neurodegenerative diseases in real time.
The majority of approaches for the classification of Alzheimer and Parkinson diseases are utilizing only the axial MRI scans, whereas there is still experimentation is required to be done with the sagittal view MRI scans and can even generate more accurate results. As these sagittal view MRI scans are mostly used by the radiologists for the diagnosis of Neurodegenerative diseases as they present more information about the grey matter content of the human brain.
As ADNI and PPMI databases are the mostly used repositories consist of MRI images of both the neurodegenerative diseases. So these two databases are used to develop a common ADPP datasets consisting of sagittal view Alzheimer diseases and Parkinson Progression MRI scans.
As Capsule networks are the evolving fields of deep neural networks delivering better results in present scenario. So the Capsule network can be utilized for the development of the Neurodegenerative disease as no such approach exist yet.
Material and methods
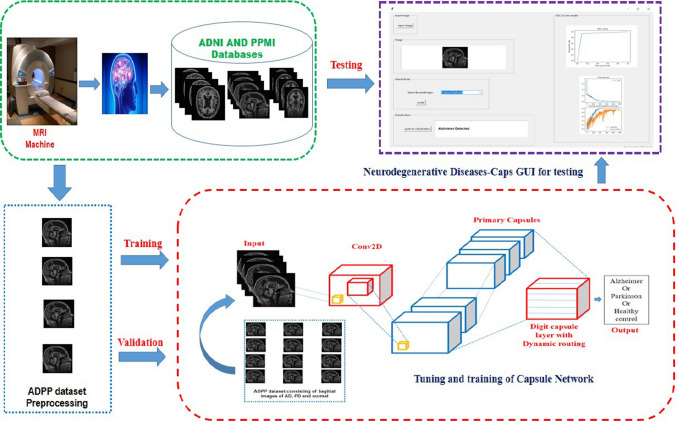
There are two major stages in the proposed Neurodegenerative diseases-Caps system. The initial stage is all about downloading the Sagittal view MRI scans of Alzheimer and Parkinson’s diseases along with the healthy cases from the ADNI and PPMI global databases. As these MRI scans are present in the DICOM (Digital Imaging and Communications in Medicine) format, so convert them into the PNG (Portable Network Graphics) format as it offers compression of lossless nature. Followed by the proper selection of informative and precise sagittal view MRI scans with the aid of the Radiologists in order to develop training and testing datasets. The fine tuning and training of the Capsule Network with the help of this multiclass ADPP dataset is done in the second stage in order to perform the multiclass classification of Neurodegenerative dieses into Alzheimer or Parkinson or Healthy cases. The overall proposed Neurodegenerative disease-Caps system is illustrated with the help of Fig. 3 given below.
Fig. 3.
The proposed neurodegenerative disease-Caps system architecture for the multiclass classification of Neurodegenerative diseases
ADPP dataset
This dataset consist four thousand and eight hundred MRI scans of patients suffering from the Alzheimer and Parkinson diseases along with the scans of healthy controls i.e. normal cases for the training and validation purpose. From the ADNI and PPMI databases, around sixteen hundred Sagittal view MRI scans each of Alzheimer patients, Parkinson’s patients and healthy controls are taken for training purpose. Almost all the researchers working in the domain of research are using these two ADNI (https://www.adni.loni.usc.edu) and PPMI (Marek et al. 2011) datasets. From the PPMI and ADNI databases around 90 healthy control cases, 80 Alzheimer and 80 Parkinson are downloaded in DICOM format. Each downloaded case will results into around 188–190 sagittal images, when converted from the DICOM format into the PNG format. Now out of those 188–190 sagittal images per case or subject around 20 sagittal images are selected with the help of Radiologist. In case of Alzheimer patient, the sagittal images that presents the best view of regions like entorhinal cortex, cerebral cortex and hippocampus are selected as these regions are precisely diagnosed by the radiologist for the correct prognosis of Alzheimer diseases. Whereas the sagittal images that best represents the Striatum, Substantia Nigra and Nigrostriatal regions of human brain are selected for the Parkinson disease cases as these regions are mostly affected in a patient suffering from the Parkinson disease. Similarly the sagittal images that best presents the above mentioned regions of human brain are selected in case of healthy control. The selected images undergoes preprocessing in order to remove the image artefacts. The images free from all type of artefacts are now used for the training and testing purpose. Apart from this a separate ADPP testing dataset is also used consist of only 200 sagittal images of Alzheimer, Parkinson and Normal cases each.
The Table 3 below simply illustrates the demographic information of the ADPP dataset used in this paper.
Table 3.
The Demographic information of the ADPP dataset
| Global dataset | Type | No. of cases taken for training | No. of cases taken for testing | Gender | Cases age group | MRI Modality view | Number of scans taken for | |
|---|---|---|---|---|---|---|---|---|
| Training | Testing | |||||||
| ADNI | AD | 80 | 14 | 24 females and 26 males | 65–90 | Sagittal | 1600 | 200 |
| HC | 50 | 8 | 35 females and 15 males | 60–93 | Sagittal | 900 | 100 | |
| PPMI | PD | 80 | 14 | 20 females and 30 males | 39–80 | Sagittal | 1600 | 200 |
| HC | 40 | 8 | 26 females and 24 males | 31–80 | Sagittal | 700 | 100 | |
Capsule network
Capsule network comprises of capsules. In capsule network each capsule is a group of neurons. Activity vectors of the neurons of a capsule represents different instantiation parameters of the concerned entity. Length of these vectors denotes the probability that a spatial entity exists (Hinton et al. 2011). In CNN, existence of pooling layers can be attributed for most deficiencies but this is not the case with capsule networks since capsule networks uses routing by agreement principle. Using this principle, the generated outputs of the first layer are communicated to parental level capsules of next layer (Sara et al. 2017).
Even though the capsules’ coupling coefficient are different, all the capsules attempts to recognize the parental capsules’ outputs. Expected conformations to the objectified outputs of the parent capsules causes an increase in the coupling-coefficient of the related capsules (Shahroudnejad et al. 2018). Therefore if output of the capsule x is , it’s exposure for the parent capsule y is calculated using the formula
where denotes the higher layer yth capsule’s expected vector of its output as calculated by the capsule x in the succeeding layers. The weighted coefficient matrix as learned in the regressive process is .
The amount of conformations of the beneath layer and parent capsules work as the foundation of the coupling coefficient can be deduced as
where denotes the probability of log, for deciding whether capsule x is connected to capsule y or not and initialized to zero at the beginning of the process. Thus the parent capsule input vector can be defined as
The subsequent as mentioned below is applied to ensure that capsules’ outputs never go past 1. Initial vector can be used to define finial outputs of each capsule according to below equation
where and denotes the capsule y’s input vector and output respectively. In such a routing process, the log updates’ probability is numerous, it means that considering the contracts between and exploiting the realities of the two supportive vectors, produces a bigger inner product. Hence, contract for updating the log probability as well as coupling coefficient can be calculated as:
A function that aims high loss values on capsules having long output instigation parameters when the existence of an entity is not recorded is called loss function and it is calculated by:
where is 0 but it is 1 whenever class k exists. The , , parameters should be established when learning procedure begins since these are important parameters. The architecture of capsule network consists of a single layer of convolutional filters and two layers of capsules as discussed in (Mukhometzianov and Carrillo 2018). Exceptionally, it needed three layers of interconnected neurons that make an effort to recreate the inputs by means of the instantiation parameters from the capsules associated to true labels.
Designing the neurodegenerative diseases-Caps
After exploring the numerous architectures of the capsule network, we decided to create a model that consist of four layers along with the Input layer. The layers of our created model is as follows:
In order to cut training time as well as for decreasing parameters, the MRI sequences are down-sized from 512 × 512 to 480 × 120.
In our model, 2nd layer is the main capsule layer comprising of 32 channels for convolution where every capsule contains 9 × 9 kernel and stride value is 2 (Sara et al. 2017).
There are 3-capsules in the final year corresponding to 3-class i.e. AD, PD and Healthy control. These two classes actually represents the major Neurodegenerative diseases, we have considered in our study. These capsules has a dimension of 16.
Three connected layers consisting of 512, 1024, 4096 neurons form the decoder segment. To reduce the re-building loss neurons in the input layer is equal to the quantity of image pixels in the output layer.
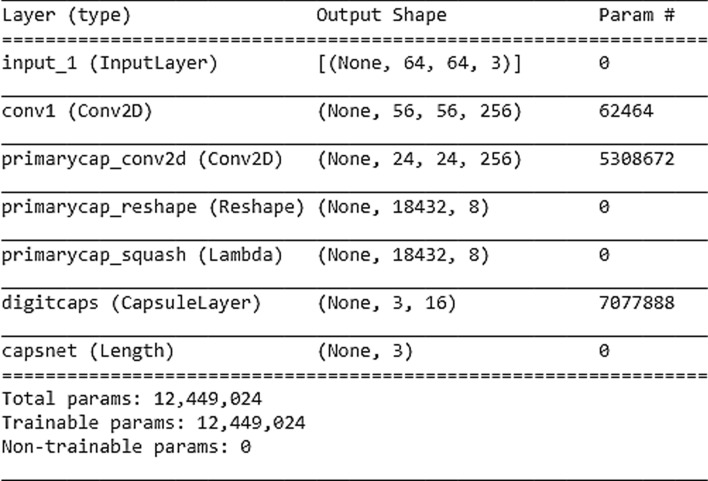
Although considering the risk of over-fitting and under-fitting, an early stopping algorithm (Goodfellow et al. 2016) is used but our network achieved actually well on the large size ADPP dataset. Accordingly, the training process will stop when the validation accuracy starts declining at every epoch’s end for the period of model training. The model summary of the Neurodegenerative diseases-Caps is presented with the help of Fig. 4 below.
Fig. 4.
The proposed neurodegenerative diseases-Caps model summary
Comparison approach
The four popular deep transfer learning models like VGG16 (Simonyan and Zisserman 2015), VGG19 (Liu and Wang 2019), ResNet50 (He et al. 2016) and InceptionV3 (Szegedy et al. 2014) are used for the sake of comparison and evaluation on the ADPP dataset. These four DTL models are initially fine-tuned and trained using the augmented ADPP dataset. The ultimate object of this comparison is to illustrate that how these commonly used DTL models performs on the ADPP dataset and their performance can be compared with the Capsule network based Neurodegenerative diseases-Caps model. The Algorithm 2 for the comparison approach is given as: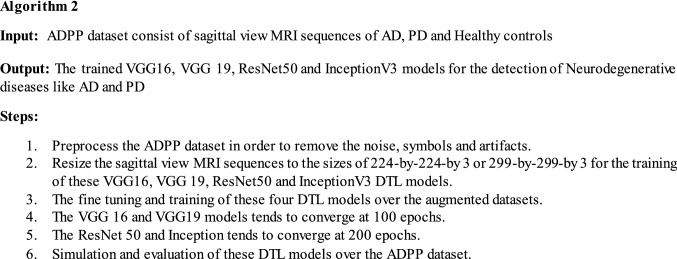
All the four deep transfer learning models are trained and evaluated with a learning rate equal to 0.00001, mini-batch size of 16 and Adam (Kingma 2015) is opted as an optimizer technique for the weights adjustment. Whereas the size of input image and number of epochs required to converge varies from model to model. The dropout method (Srivastava et al. 2014) is utilized in order to avoid the problem of over fitting. The Table 4 below illustrates the configuration parameters of VGG16, VGG19, InceptionV3 and ResNet50 DTL models.
Table 4.
The configuration parameters of VGG16, VGG19, InceptionV3 and ResNet50 DTL models
| DTL models parameters | VGG16 | VGG19 | InceptionV3 | ResNet50 |
|---|---|---|---|---|
| Input image size | 224*224 | 224*224 | 299*299 | 224*224 |
| Number of layers | 16 | 19 | 48 | 50 |
| Learning rate | 0.00001 | 0.00001 | 0.00001 | 0.00001 |
| Batch size | 16 | 16 | 16 | 16 |
| Number of epochs to converge | 100 | 100 | 200 | 200 |
| Momentum | 0.9 | 0.9 | 0.9 | 0.9 |
| Optimizer | Adam | Adam | Adam | Adam |
Results and simulation
The Google Colaboratory (colab) platform powered by the NVidia Tesla T4 GPU along with Python 3.6 is used for the implementation and experimentation. The ADPP dataset is used in the 70/30 split, which means 70% of ADPP dataset is used for training and remaining 30% for validation. For testing additional 200 AD, PD and normal cases MRI sequence are taken as the ADPP testing dataset. The Multiclass confusion matrix of the proposed Capsule network based system over the ADPP testing dataset is presented with the help of Table 5 below. Whereas the Multiclass confusion matrices of the four deep transfer learning models over the ADPP testing dataset used for the comparison are illustrated with the help of Table 6 below. The numbers highlighted in bold in the table 5 and 6 are the correctly classified MRI sequences of AD, PD and normal cases in the ADPP test dataset by the proposed Neurodegenerative diseases-Caps system and four deep transfer learning based systems used for comparison. From these Multiclass confusion matrices, the values of True positive (TP), True Negative (TN), False Positive (FP) and False Negative (FN) are derived for the Alzheimer class, Parkinson class and Normal or Healthy class. The step by step procedure for determining the values of these TP, TN, FP and FN parameters for each class is somewhat different in Multiclass i.e. three class classification as compare to the conventional binary class classification. Although this step by step procedure for determining the TP, TN, FP and FN values for the Alzheimer class, Parkinson class and Normal class is clearly illustrated with the help of Appendix 1. These derived TP, TN, FP and FN values for each class i.e. Alzheimer, Parkinson and Healthy control are finally used in the formulas of various statistical parameters or classification rates in order to determine the performance evaluation of this proposed system. The derived TP, TN, FP and FN values for the Alzheimer class, Parkinson class and Healthy control class of the proposed Neurodegenerative diseases—Caps system over the ADPP testing dataset are presented with the help of Table 7.
Table 5.
Multi class confusion matrix of the proposed Neurodegenerative diseases-Caps system over the ADPP test dataset for the multiclass i.e. three class classification
| 200/200/200 | Alzeihmer | Parkinson | Healthy control |
|---|---|---|---|
| Alzeihmer | 193 | 4 | 3 |
| Parkinson | 1 | 198 | 1 |
| Healthy control | 7 | 8 | 187 |
Table 6.
Confusion matrices of the (a) VGG 16, (b) VGG19, (c) ResNet50 and (d) Inceptionv3 based systems used for comparison over the ADPP test dataset
| 200/200/200 | Alzeihmer | Parkinson | Healthy control |
|---|---|---|---|
| (a) | |||
| Alzeihmer | 171 | 15 | 14 |
| Parkinson | 10 | 178 | 12 |
| Healthy control | 10 | 18 | 172 |
| (b) | |||
| Alzeihmer | 185 | 8 | 7 |
| Parkinson | 6 | 189 | 5 |
| Healthy Control | 12 | 14 | 174 |
| (c) | |||
| Alzeihmer | 168 | 14 | 18 |
| Parkinson | 15 | 171 | 14 |
| Healthy control | 14 | 16 | 170 |
| (d) | |||
| Alzeihmer | 164 | 16 | 20 |
| Parkinson | 16 | 168 | 16 |
| Healthy control | 18 | 17 | 165 |
Table 7.
The TP, TN, FP and FN values obtained from the Multiclass confusion matrix of the proposed Neurodegenerative diseases-Caps system
| Values/class | Alzheimer class | Parkinson class | Healthy control class |
|---|---|---|---|
| True positive | 193 | 198 | 185 |
| True negative | 392 | 389 | 396 |
| False positive | 7 | 2 | 15 |
| False negative | 8 | 12 | 4 |
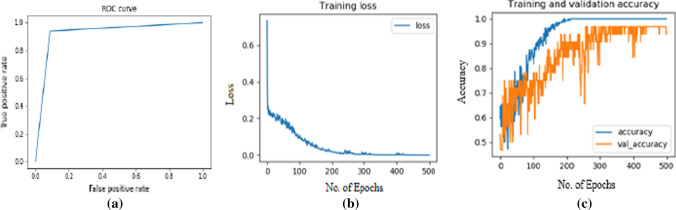
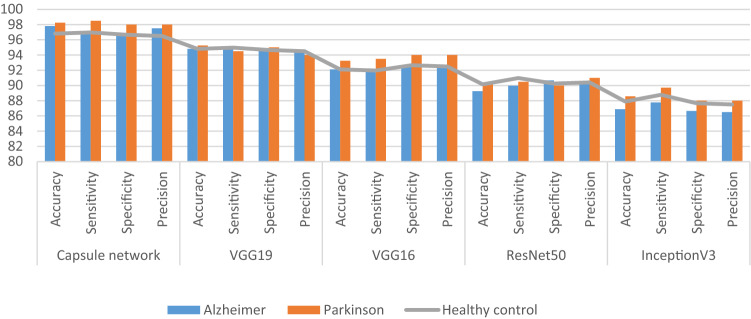
The statistical parameters or classification rates like Accuracy, Sensitivity, Specificity and F1-score etc., which are used for the performance evaluation are well defined in the Table 8 below. The Tables 8, 9 and 10 below simply illustrates the performance of the proposed system as well as the VGG16, VGG19, InceptionV3 and ResNet50 DTL models over the ADPP dataset for the multiclass classification of Neurodegenerative diseases. The Fig. 5 below simply illustrates the ROC curve, training loss and training & validation accuracy graphs of the proposed Neurodegenerative-Caps system. The Fig. 6 below simply showcase the performance comparison graph for comparing the performance of Capsule network based proposed system with the VGG19, VGG16, ResNet50 and InceptionV3 based system.
Table 8.
Performance of the proposed Neurodegenerative diseases-Caps system on the ADPP dataset
| Classification rates | Their formulas | Multiclass classification into | ||
|---|---|---|---|---|
| Alzheimer | Parkinson | Healthy control | ||
| Accuracy | (TP + TN)/(TP + TN + FP + FN) | 97.81 | 98 | 96.81 |
| Sensitivity | TP/(TP + FN) | 96.02 | 94.2 | 97.8 |
| Specificity | TN/(FP + TN) | 98.25 | 99 | 96.66 |
| Precision | TP/(TP + FP) | 96.5 | 99 | 92.5 |
| Negative predictive value | TN/(TN + FN) | 97.89 | 97 | 99 |
| False positive rate | FP/(FP + TN) | 0.0175 | 0.005 | 0.0332 |
| False discovery rate | FP/(FP + TP) | 0.035 | 0.01 | 0.0750 |
| False negative rate | FN/(FN + TP) | 2.03 | 0.57 | 0.021 |
| F1 score | 2TP/(2TP + FP + FN) | 96.26 | 96.24 | 95.1 |
TP True positive, TN true negative, FP false positive, FN false negative
Table 9.
The performance of the VGG 19 and VGG16 based systems on the ADPP dataset
| Classification rates | Multiclass classifications into | |||||
|---|---|---|---|---|---|---|
| Alzheimer | Parkinson | Healthy control | ||||
| VGG19 | VGG16 | VGG19 | VGG16 | VGG19 | VGG16 | |
| Accuracy | 94.6 | 92 | 95 | 91 | 94 | 91 |
| Sensitivity | 91.13 | 90 | 90 | 85 | 94 | 87 |
| Specificity | 96.22 | 92.9 | 97 | 94 | 94 | 93.03 |
| Precision | 92.5 | 86 | 95 | 89 | 87 | 86 |
| Negative predictive value | 95.5 | 95 | 95.5 | 92 | 97 | 93.5 |
| False positive rate | 0.037 | 0.070 | 0.028 | 0.056 | 0.0628 | 0.069 |
| False discovery rate | 0.075 | 0.145 | 0.055 | 0.11 | 0.13 | 0.14 |
| False negative rate | 0.088 | 0.10 | 0.104 | 0.156 | 0.064 | 0.13 |
| F1 score | 91.8 | 87.4 | 92 | 86.6 | 90.16 | 86.4 |
Table 10.
The performance of ResNet50 and InceptionV3 based systems on the ADPP dataset
| Classification rates | Multiclass classifications into | |||||
|---|---|---|---|---|---|---|
| Alzheimer | Parkinson | Healthy control | ||||
| ResNet50 | InceptionV3 | ResNet50 | InceptionV3 | ResNet50 | InceptionV3 | |
| Accuracy | 89.8 | 88 | 90.17 | 89 | 89.6 | 88 |
| Sensitivity | 85.2 | 83 | 85.07 | 83.5 | 84.14 | 82.09 |
| Specificity | 92 | 91 | 92 | 91.9 | 92.46 | 91.2 |
| Precision | 84 | 82 | 85.5 | 84 | 85 | 82.5 |
| Negative predictive value | 92 | 91.5 | 92.5 | 91.7 | 91.89 | 91 |
| False positive rate | 0.079 | 0.089 | 0.072 | 0.0802 | 0.075 | 0.087 |
| False discovery rate | 0.16 | 0.18 | 0.145 | 0.16 | 0.15 | 0.175 |
| False negative rate | 0.147 | 0.1717 | 0.149 | 0.16 | 0.158 | 0.179 |
| F1 score | 84.6 | 82.4 | 85.2 | 83.7 | 84.58 | 82.2 |
Fig. 5.
The ROC curve (a), training loss curve (b) and training and validation accuracy graph (c) of the proposed Neurodegenerative-Caps system
Fig. 6.
The performance comparison graph for comparing the performance of Capsule network based proposed system with the VGG19, VGG16, ResNet50 and InceptionV3 based system
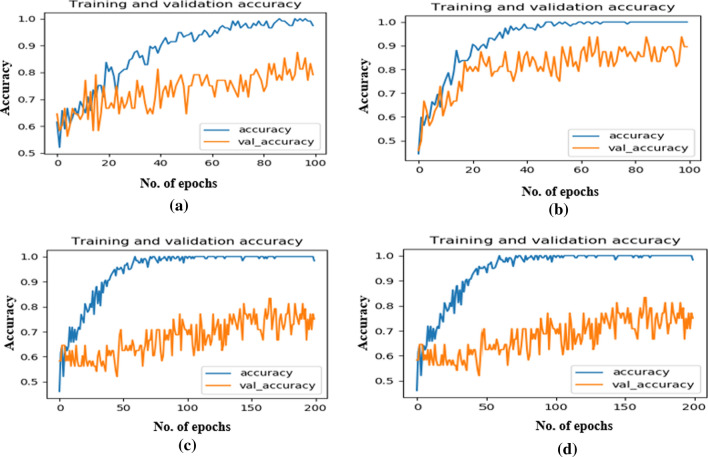
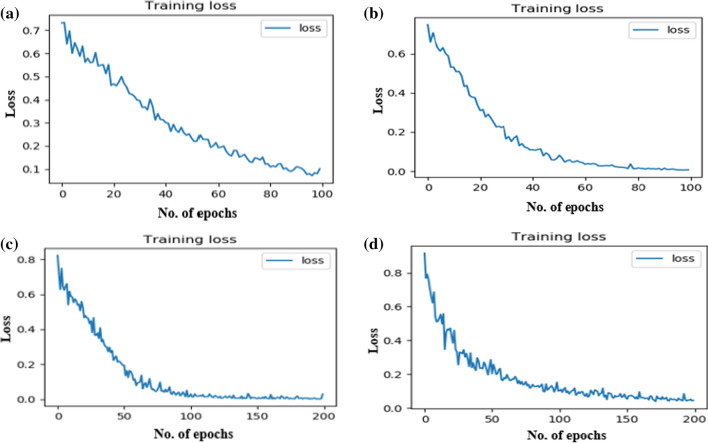
Whereas the training and validation graph of four DTL models on ADPP dataset is presented with the help of Fig. 7 above. The training loss curve of all the four DTL models used for the comparison are presented with the help of Fig. 8 above. The proposed Capsule network based system is also compared with some of the state of the art approaches proposed in the recent years for either performing the Alzheimer classification or Parkinson classification on the ADNI or PPMI datasets. Their comparison is presented with the help of Tables 11 and 12. The classification rates of the proposed Neurodegenerative diseases-Caps system on the ADNI and PPMI datasets are highlighted in bold in table 11 and 12.
Fig. 7.
The training and validation graph of four DTL models on ADPP dataset a VGG16, b VGG19, c ResNet50, d InceptionV3
Fig. 8.
The training loss graph of four DTL models on ADPP dataset a VGG16, b VGG19, c ResNet50, d InceptionV3
Table 11.
Performance comparison of the proposed Neurodegenerative disease-Caps system with the existing approaches for the Alzheimer classification on the ADNI dataset
| Author and year | Machine learning classifier or deep learning model used | Accuracy (%) | Precision (%) | Sensitivity (%) | F1 score (%) |
|---|---|---|---|---|---|
| Li et al. (2014a, b) | Support vector machine | 83 | 82.8 | 80.4 | 81 |
| Glozman et al. (2017) | Support vector machine | 80.54 | 81 | 70.59 | 76 |
| Talo et al. 2019 | ResNet34 | 81.3 | 80.5 | 79.9 | 79 |
| Proposed one | Capsule network | 97.81 | 96.5 | 96.02 | 96.26 |
Table 12.
Performance comparison of the proposed Neurodegenerative disease-Caps system with the existing approaches for the Parkinson’s disease classification on the PPMI dataset
| Author and year | Machine learning classifier or deep learning model used | Accuracy (%) | Precision (%) | Sensitivity (%) | F1 score (%) |
|---|---|---|---|---|---|
| Peng et al. (2017) | Multi-kernel support vector machine (SVM) | 85.78 | 85 | 87.64 | 86.2 |
| Sivaranjini et al. (2019) | Alexnet | 88.9 | 87.5 | 89.3 | 88.2 |
| Yagis et al. (2019) | VGG16 and ResNet 50 | 82 | 81.2 | 80.1 | 81.9 |
| Proposed one | Capsule network | 98 | 99 | 94.2 | 96.24 |
Discussion
The proposed Neurodegenerative disease-Caps system based on the Capsule network is proved to be very accurate especially in terms of performing the multiclass classification as compare to other deep transfer learning models. The major reason of employing the Capsule network is its ability to deal with affine transformations and invariance (rotational and translational), which very commonly persists in the MRI modalities datasets. This type of automated systems can be very effectively used for doing the early screening of patients suffering from the Neurodegenerative disease. The need of such systems is even more felt or realized in the developing and densely populated countries, where patient’s to doctor ratio is drastically low. Such automated system can enhance the diagnostic capabilities as well as efficiency of the radiologists. As the radiologists are more inclined towards using the sagittal view MRI sequence for the correct diagnosis of these two Neurodegenerative diseases as compare to the axial and coronal view. So we have decided to utilize this sagittal view MRI sequence for performing the experimentation in this research study. Another important reason of utilizing this view of MRI sequence only is the availability of high quality MRI sequences, which are selected with the aid of radiologist and tends to deliver better results. There are already approaches based on machine and deep learning utilizing the axial view of MRI sequence for performing the classification of either Alzheimer into sub stages or Parkinson but the experimentation is still required to be done with the sagittal view. The sagittal view MRI sequence of human brain offers more information related to the specific Alzheimer as well Parkinson affected brain regions like rostral hippocampus, rostral hippocampus, Medial amygdala, Medial amygdala, caudal area etc. in 2D as compare to the axial and coronal view. The sagittal view MRI sequence thus proved to be more efficient and reliable for the correct classification of these Neurodegenerative diseases and this fact is very proved in the result section.
An experimentation that involves feeding of the proposed Neurodegenerative diseases—Caps system with input MRI sequences with different sizes are done. This experimentation is done in order to reduce the training time quotient of the proposed model. The different size MRI sequences are tried as input in order to find the best input size that offers the least training time while offering the best accuracy quotient. As the proposed Capsule network based model is offering the highest training time with input as 512*512 size MRI sequence. Whereas the proposed model offered the best accuracy quotient with least training time with input of 480*120 size MRI sequence, so this input size is used in the research study. The other input sizes that are used in the experimentation are 512*480, 480*480, 480*240, 480*60 etc. This experimentation with different MRI input sizes is also done in order to observe or determine that how the proposed capsule network based model responds to the low resolution input images in the training phase. This experimentation has given answers to questions like:
Whether the proposed model can deliver good results with low resolution images?
Whether the proposed model is able to better optimize itself whenever trained with low resolution input images etc.?
So, yes the proposed model is delivering best accuracy with least training time with 480*120 size MRI sequence. Another very important observation of this experimentation is that the proposed model is able to better optimize itself during the training phase, whenever trained with input MRI sequence with resolution of 480*120 in order to classify the test images correctly later in the testing phase. The parameters associated with the Capsule network for the proper tuning are decided after performing a number of experiments. The Capsule network tends to converges at 500 epochs during training and after that its validation accuracy is not at all improving.
The performance comparison along with the more complex deep transfer learning models in the result section simply proves that the Capsule network based system is more robust and accurate. Whereas the other four DTL models which tends to converge much earlier during training at either 100 or 200 epochs. Apart from the fact, that the Capsule network are taking more time to converge and requires lot of hardware resources, there are no other drawback, which can be associated with this proposed system. The major reasons behind the better performance of the proposed Neurodegenerative disease-Caps system in comparison to the deep transfer learning models over the ADPP dataset are as follows:
The proposed Neurodegenerative disease-Caps system is based on the routing by agreement principle, so it is generalizing the invariance and affine transformation infected input test samples correctly. Whereas the four deep transfer learning models are completely fail to generalize such test samples as these DTL models are based on the pooling layers concept.
The deep transfer learning models performs well on the very large size training datasets, which is not the case here as the size of the ADPP dataset is not very large.
The Capsule networks allows the experimentation to be done with the input size of training samples. This type of experimentation will helps in identifying the correct input size samples, which proved to be accurate and training time efficient. Whereas the four deep transfer learning models are rigid in terms of size of input samples and provides no scope for experimentation.
The proposed Capsule network based system is far less complex as it consist of small number of layers i.e. (4 convolution layers + primary Input capsule layer). This less complex nature of the proposed system makes the hyper parameter tuning part easy and hence result in more accurate model. Unlike deep transfer learning models, which consist of large number of layers and making the task of hyper parameter tuning a difficult one.
Conclusion
To the best of our knowledge, there is no system proposed yet for the multiclass classification of major Neurodegenerative diseases like Alzheimer and Parkinson. There are deep learning based automated approaches for the classification of either Alzheimer or Parkinson diseases alone. So the proposed Neurodegenerative disease-Caps system is a unified framework based on Capsule network for the multiclass classification of these Neurodegenerative diseases. This systems delivers an encouraging accuracy of above 97, 98 and 96% for the correct classification of Alzheimer, Parkinson and Normal cases. Whereas the VGG19, VGG16, ResNet50 and InceptionV3 DTL models delivers average accuracies of 92, 90, 89 and 87% respectively. The result section moreover proves that the proposed Neurodegenerative disease-Caps system based on Capsule Network outperforms the VGG19, VGG16, ResNet50 and InceptionV3 models in terms of accuracy and performance. In future, quotient of multiclass classification of this system can be further enhanced in order to perform the classification of other brain structural disorders like brain tumors, Schizophrenia, dementia etc.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We like to thank Dr. Pushraj Bhatele (Chief Radiologist) of MP MRI and CT scan center at MP MRI and CT scan center, Netaji Subhash Chandra Bose Medical College, Jabalpur, India for providing his valuable support in terms of medical knowledge and validate this proposed work. We also like to extend our gratitude to the Sanya Hospital and Diagnostics Pvt. Ltd. Delhi for supporting this research study.
Funding
This research study is supported by the Sanya Hospital and Diagnostics Pvt. Ltd. Delhi.
Availability of data and materials
The dataset used in this research is publically available.
Code availability
The code will be provided on request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All the authors have given their consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kirti Raj Bhatele, Email: kirtirajbhatele8@gmail.com.
Anand Jha, Email: mail2anandjha@yahoo.co.in.
Kavish Kapoor, Email: kavishkapoor97@gmail.com.
Devanshu Tiwari, Email: devanshu.tiwari28@gmail.com.
References
- Adeli E, Shi F, An L, Wee CY, Wu G, Wang T, et al. Joint feature-sample selection and robust diagnosis of Parkinson’s disease from MRI data. J Neuroimaging. 2016;141:206–219. doi: 10.1016/j.neuroimage.2016.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu K, Yu Y, Cai J, Owusu-Agyemang K, Twumasi BA, Wang X. DHS-CapsNet: dual horizontal squash capsule networks for lung and colon cancer classification from whole slide histopathological images. Int J Imaging Syst Technol. 2021;31(4):2075–2092. doi: 10.1002/ima.22569. [DOI] [Google Scholar]
- Adu K, Yu Y, Cai J, Mensah PK, Owusu-Agyemang K. MLAF-CapsNet: multi-lane atrous feature fusion capsule network with contrast limited adaptive histogram equalization for brain tumor classification from MRI images. J Intell Fuzzy Syst. 2021;41(2):3933–3950. doi: 10.3233/JIFS-202261. [DOI] [Google Scholar]
- Afshar P, Heidarian S, Naderkhani F, Oikonomou A, Plataniotis KN, Mohammadi A. COVID-CAPS: a capsule network-based framework for identification of COVID-19 cases from X-ray images. Pattern Recogn Lett. 2020;138:638–643. doi: 10.1016/j.patrec.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali M, Ali R. Multi-input dual-stream capsule network for improved lung and colon cancer classification. Diagnostics. 2021;11(8):1485. doi: 10.3390/diagnostics11081485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Association A. Alzheimer’s disease facts and figures. Alzheimer's Dementia. 2017;13(4):325–373. [Google Scholar]
- Amoroso N, La Rocca M, Monaco A, Bellotti R, Tangaro S. Complex networks reveal early MRI markers of Parkinson’s disease. Med Image Anal. 2018;48:12–24. doi: 10.1016/j.media.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Bakator M, Radosav D. Deep learning and medical diagnosis: a review of literature. Multimodal Technol Interact. 2018;2(3):47. doi: 10.3390/mti2030047. [DOI] [Google Scholar]
- Borek LL, Amick MM, Friedman JH. Non-motor aspects of Parkinson’s disease. CNS Spectrum. 2006;11(7):541–554. doi: 10.1017/s1092852900013560. [DOI] [PubMed] [Google Scholar]
- Chakraborty S, Aich S, Kim HC. Detection of Parkinson’s disease from 3T T1 Weighted MRI scans using 3D convolutional neural network. Diagnostics. 2020;10(6):402–419. doi: 10.3390/diagnostics10060402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H, Jin KH. Alzheimer’s disease neuroimaging predicting cognitive decline with deep learning of brain metabolism and amyloid imaging. Behavior Brain Resonance. 2018;344:103–109. doi: 10.1016/j.bbr.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Doan NT, Engvig A, Zaske K, Persson K, Lund MJ, Kaufmann T, et al. Distinguishing early and late brain aging from the Alzheimer’s disease spectrum: consistent morphological patterns across independent samples. Neuroimaging. 2017;158:282–295. doi: 10.1016/j.neuroimage.2017.06.070. [DOI] [PubMed] [Google Scholar]
- Dyrba M, Grothe M, Kirste T, Teipel SJ. Multimodal analysis of functional and structural disconnection in Alzheimer’s disease using multiple kernels SVM. Hum Brain Mapp. 2015;36(6):2118–2131. doi: 10.1002/hbm.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sappagh S, Abuhmed T, Riazul Islam SM, Kwak KS. Multimodal multitask deep learning model for Alzheimer’s disease progression detection based on time series data. Neurocomputing. 2020;418:197–215. doi: 10.1016/j.neucom.2020.05.087. [DOI] [Google Scholar]
- Farzan A, Mashohor S, Ramli AR, Mahmud R. Boosting diagnosis accuracy of Alzheimer’s disease using high dimensional recognition of longitudinal brain atrophy patterns. Behav Brain Res. 2015;290:124–130. doi: 10.1016/j.bbr.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Feng W, Halm-Lutterodt NV, Tang H, Mecum A, Mesregah MK, Ma Y, Guo X. Automated MRI-based deep learning model for detection of Alzheimer’s disease process. Int J Neural Syst. 2020;30(6):2050032. doi: 10.1142/S012906572050032X. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Agbokou C, Gauthier S. Recognition and management of neuropsychiatric complications in Parkinson’s disease. J L'assoc Med Can. 2006;175(12):1545–1552. doi: 10.1503/cmaj.060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton LV, Dolezel D, Harrop J, Yan Y, Fulton CP. Classification of Alzheimer’s disease with and without Imagery using gradient boosted machines and ResNet-50. Brain Sci. 2019;9(9):212. doi: 10.3390/brainsci9090212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garali I, Adel M, Bourennane S, Guedj E. Histogram-based features selection and volume of interest ranking for brain PET image classification. IEEE J Transl Eng Health Med. 2018;6:2100212. doi: 10.1109/JTEHM.2018.2796600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow IJ, Bengio Y, Courville A. Deep learning. Cambridge MIT Press. 2016 doi: 10.4258/hir.2016.22.4.351. [DOI] [Google Scholar]
- Glozman T, Solomon J, Pestilli F, Guibas L. Alzheimer’s disease neuroimaging: shape-attributes of brain structures as biomarkers for Alzheimer’s disease. J Alzheimer’s Dis. 2017;56(1):287–295. doi: 10.3233/JAD-160900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goceri E. Diagnosis of Alzheimer's disease with Sobolev gradient-based optimization and 3D convolutional neural network. Int J Numer Methods Biomed Eng. 2019;35:e3225. doi: 10.1002/cnm.3225. [DOI] [PubMed] [Google Scholar]
- Guo H, Zhang F, Chen J, Xu Y, Xiang J. Machine learning classification combining multiple features of a hyper-network of fMRI data in Alzheimer’s disease. Front Neurosci. 2017;11:615. doi: 10.3389/fnins.2017.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. CVPR, pp 770–778. arXiv:1512.03385
- Heidarian S, Afshar P, Enshaei N, et al. COVID-FACT: a fully-automated capsule network-based framework for identification of COVID-19 cases from chest CT scans. Front Artif Intell. 2021;4:598932. doi: 10.3389/frai.2021.598932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton GE, Krizhevsky A, Wang SD (2011) Transforming auto-encoders. In: Lecture notes in computer science. Springer, Berlin, Heidelberg, pp 44–51. 10.1007/978-3-642-21735-7_6
- Jiménez-Sánchez A, Albarqouni S, Mateus D (2018) Capsule networks against medical imaging data challenges. In: Intravascular imaging and computer assisted stenting and large-scale annotation of biomedical data and expert label synthesis. Springer, Cham, pp 150–60. 10.1007/978-3-030-01364-6_17
- Kingma DP (2015) ADAM: a method for stochastic optimization. arXiv:1412.6980
- Li M, Qin Y, Gao F, Zhu W, He X. Discriminative analysis of multivariate features from structural MRI and diffusion tensor images. Magn Reson Imaging. 2014;32(8):1043–1051. doi: 10.1016/j.mri.2014.05.008. [DOI] [PubMed] [Google Scholar]
- Li M, Oishi K, He X, Qin Y, Gao F, Mori S, et al. An efficient approach for differentiating Alzheimer’s disease from normal elderly based on multicentre MRI using gray-level invariant features. J PLoS One. 2014;9(8):e105563. doi: 10.1371/journal.pone.0105563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Wang Z. HiCNN: a very deep convolutional neural network to better enhance the resolution of Hi-C data. Bioinformatics. 2019;35(21):4222–4228. doi: 10.1093/bioinformatics/btz251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundervold AS, Lundervold A. An overview of deep learning in medical imaging focusing on MRI. Z Med Phys. 2019;29:102–127. doi: 10.1016/j.zemedi.2018.11.002. [DOI] [PubMed] [Google Scholar]
- Marek K, Jennings D, Lasch S, Siderowf A, Tanner C, Simuni T, Coffey C, Kieburtz K, et al. The Parkinson progression marker initiative (PPMI) Prog Neurobiol. 2011;95:629–635. doi: 10.1016/j.pneurobio.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the nincds-adrda work group* under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mozhdehfarahbakhsh A, Chitsazian S, Chakrabarti P, Chakrabarti T, Kateb B, Nami M (2021) An MRI-based deep learning model to predict Parkinson’s disease stages. medRxiv. 10.1101/2021.02.19.21252081
- Mukhometzianov R, Carrillo J (2018) CapsNet comparative performance evaluation for image classification. arXiv:1805.11195
- Naz S, Ashraf A, Zaib A. Transfer learning using freeze features for Alzheimer neurological disorder detection using ADNI dataset. Multimedia Syst. 2021 doi: 10.1007/s00530-021-00797-3. [DOI] [Google Scholar]
- Ni H, Zhou L, Ning X, Wang L. Alzheimer’s disease neuroimaging: exploring multi fractal-based features for mild Alzheimer’s disease classification. Magn Reson Med. 2016;76(1):259–269. doi: 10.1002/mrm.25853. [DOI] [PubMed] [Google Scholar]
- Oliveira FPM, Faria DB, Costa DC, Castelo-Branco M, Tavares JMRS. Extraction, selection and comparison of features for an effective automated computer-aided diagnosis of Parkinson’s disease based on FP-CIT SPECT images. Eur J Nucl Med Mol Imaging. 2017;45(6):1052–1062. doi: 10.1007/s00259-017-3918-7. [DOI] [PubMed] [Google Scholar]
- Peng B, Wang S, Zhou Z, Liu Y, Tong B, Zhang T, et al. A multilevel-ROI-features-based machine learning method for detection of morph metric biomarkers in Parkinson’s disease. Neurosci Lett Elsevier. 2017;651:88–94. doi: 10.1016/j.neulet.2017.04.034. [DOI] [PubMed] [Google Scholar]
- Ramzan F, Khan MUG, Rehmat A, Iqbal S, Saba T, Rehman A, Mehmood Z. A deep learning approach for automated diagnosis and multi-class classification of Alzheimer’s disease stages using resting-state fMRI and residual neural networks. J Med Syst. 2020;44:37. doi: 10.1007/s10916-019-1475-2. [DOI] [PubMed] [Google Scholar]
- Russakovsky O, Deng J, Su H, Krause J, Satheesh S, Ma S, Huang Z, Karpathy A, Khosla A, Bernstein M, Berg AC, Fei-Fei L. ImageNet large scale visual recognition challenge. Int J Comput vis. 2015;115(3):211–252. doi: 10.1007/s11263-015-0816-y. [DOI] [Google Scholar]
- Sabour S, Frosst N, Hinton GE (2017) Dynamic routing between capsules. In: 31st conference on neural information processing systems, Long Beach, CA, USA
- Sara S, Nicholas F, Hinton GE (2017) Dynamic routing between capsules. In: Proceedings of the 31st international conference on neural information processing systems, pp 3859–3869
- Shahroudnejad A, Mohammadi A, Plataniotis KN (2018) Improved explainability of capsule networks: relevance path by agreement. arXiv:1802.10204v1
- Simonyan K, Zisserman A (2015) Very deep convolutional networks for large-scale image recognition. arXiv:1409.1556
- Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R. Dropout: a simple way to prevent neural networks from over fitting. J Mach Learn Res. 2014;15(56):1929–1958. [Google Scholar]
- Szegedy C, Liu W, Jia Y, Sermanet P, Reed S, Anguelov D, Erhan D, Vanhoucke V, Rabinovich A (2014) Going deeper with convolutions. Technical report, pp 1–25. arXiv:1409.4842
- Talo M, Baloglu UB, Yıldırım Ö, Acharya R. Application of deep transfer learning for automated brain abnormality classification using MR images. Cogn Syst Res. 2019;54:176–188. doi: 10.1016/j.cogsys.2018.12.007. [DOI] [Google Scholar]
- Tiwari S, Jain A. Convolutional capsule network for COVID-19 detection using radiography images. Int J Imaging Syst Technol. 2021 doi: 10.1002/ima.22566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas T, Yadav R, Solanki C, Darji R, Desai S, Tanwar S. Deep learning-based scheme to diagnose Parkinson's disease. Expert Syst. 2021 doi: 10.1111/exsy.12739. [DOI] [Google Scholar]
- Wang SH, Phillips P, Sui Y, Liu B, Yang M, Cheng H. Classification of Alzheimer’s disease based on eight-layer convolutional neural network with leaky rectified linear unit and max pooling. J Med Syst. 2018;42(5):85. doi: 10.1007/s10916-018-0932-7. [DOI] [PubMed] [Google Scholar]
- Wang P, Wang J, Li Y, Li P, Li L, Jiang M Automatic classification of breast cancer histopathological images based on deep feature fusion and enhanced routing. Biomed Signal Process Control. 2021;65:102341. doi: 10.1016/j.bspc.2020.102341. [DOI] [Google Scholar]
- Worrall DE, Garbin SJ, Turmukhambetov D, Brostow GJ (2017) Harmonic networks: deep translation and rotation equivariance. In: IEEE conference on computer vision and pattern recognition IEEE computer society, pp 7168–7177. 10.1109/CVPR.2017.758
- Yagis E, De Herrera AGS, Citi L (2019) Generalization performance of deep learning models in neurodegenerative disease classification. In: IEEE international conference on bioinformatics and biomedicine (BIBM), San Diego, USA, pp 1692–1698. 10.1109/BIBM47256.2019.8983088
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used in this research is publically available.
The code will be provided on request.