Abstract
Background:
Granulosa cells (GCs) play key roles in oocyte maturation by providing required estradiol (E2). Since the presence of immature oocytes has been reported in cases with polycystic ovary syndrome (PCOS), in this study, the levels of mitochondrial membrane transporter proteins involved in E2 synthesis were determined. E2 concentration and parameters of oxidative status were also measured in follicular fluids of PCOS women.
Methods:
Forty-three women with PCOS and 43 healthy women who were candidates for IVF procedure due to their husbands' infertility were enrolled in this case-control study. The gene expression and protein levels of mitochondrial translocator protein (TSPO) and voltage-dependent anion channel 1 (VDAC1) were determined in GCs using RT-qPCR and immunocytochemistry assay, respectively. E2 level was measured with electrochemiluminescence, whereas total cholesterol, total antioxidant capacity (TAC), total oxidant status (TOS), and malondialdehyde (MDA) were determined using colorimetric methods in follicular fluids. Data were analyzed using unpaired t-test or Mann-Whitney U test, and Spearman’s correlation coefficient.
Results:
VDAC1 and TSPO were significantly lower in mRNA (p<0.05) and protein levels (p<0.001) of PCOS patients. PCOS patients had lower cholesterol, estradiol, and TAC levels, and higher TOS and MDA contents. E2 level had direct correlation with VDAC1, TSPO, and TAC while it was negatively correlated with TOS, oxidative stress index (OSI), and MDA (p<0.001). Higher E2 levels were associated with higher numbers of high-quality oocytes and conceived embryos (p<0.001).
Conclusion:
Decreased E2 levels and increased oxidative stress in the follicular fluid may be the cause of immature oocytes in PCOS cases.
Keywords: Estradiol, Granulosa cells, Oxidative stress, Polycystic ovary syndrome, TSPO protein, Voltage-dependent anion channel 1
Introduction
Infertility is the third serious disorder worldwide and World Health Organization (WHO) estimates that about 9% of couples are struggling with infertility across the world (1, 2). Polycystic ovary syndrome (PCOS) is introduced as the most prevalent endocrine disorder and its prevalence is 5–20% among females of reproductive age based on different diagnostic criteria (3). PCOS is approximately found in 80% of women with ovulatory infertility (4) and the presence of poor-quality oocytes decreases the chance of successful in vitro fertilization (IVF) in these patients.
Granulosa cells (GCs) are responsible for production and secretion of sex steroids into follicular fluid during follicle maturation. The hormonal level of follicular fluid is involved in processes such as embryo development, implantation, and fertilization (5–7).
Association of estradiol level of follicular fluid with follicular diameter and maturity has previously been shown (8); however, earlier reports for estradiol levels in PCOS are in progress and factors involved in the synthesis and secretion of steroid hormones by GCs are yet to be carefully revealed (5, 7). Mitochondrion is the main site of steroidogenesis in the adrenal glands, gonads, and placenta cells. Mitochondrial translocator protein (TSPO), voltage-dependent anion channel 1 (VDAC-1), and steroidogenic acute regulatory (StAR) protein are key players in steroidogenesis (9, 10). TSPO monomer binds to cholesterol, undergoes polymerization, and then associates with VDAC1 to form TSPO-VDAC1 which plays the main role in translocation of cholesterol into mitochondrial matrix where synthesis of steroid hormones occurs (11).
In addition, TSPO appears to cooperate with VDAC1 to regulate production of reactive oxygen species (ROS) (12) whereby high TSPO expression modulates ROS production through VDAC-dependent pathway (13). Since elevated total oxidant status (TOS) and decreased total antioxidant capacity (TAC) level in the follicular fluid of PCOS women have already been reported and the effect of imbalance in oxide-redox status on the formation of high-quality embryo has also been observed (14–16), it is assumed that TSPO and VDAC1 may also indirectly impact fertilization process through regulating cellular redox status and consequently affecting follicular maturation.
Additionally, since TSPO and VDAC1 are involved in transportation of cholesterol, as the main substrate for steroidogenesis, it is hypothesized that reduced concentration of estradiol (E2) in follicular fluid of PCOS patients is possibly due to the altered level of TSPO and VDAC1 proteins in GCs. In other words, reduced level of mitochondrial TSPO and VDAC1 proteins in GCs may lead to lower estradiol level in follicular fluid and promote development and presence of immature follicles in PCOS women. Moreover, the imbalance in redox status which is induced by altered TSPO and VDAC1 levels may foster formation of immature follicles. Thus, for the first time, in the present study, the correlation of mitochondrial protein (TSPO and VDAC1) levels with estradiol concentration and oxide-redox status was investigated in follicular fluid of PCOS patients in comparison with the control group.
Methods
Study population and study design: Eighty-six women aged 25–38 years who were candidates for IVF procedure in Fertility Centre of Fatemieh Hospital and Omid Infertility Centre of Hamadan, Iran were enrolled in this case-control study. Forty-three women were diagnosed with PCOS, based on Rotterdam criteria (17). The equal number of healthy women with normal ovulation cycle and no signs of hyperandrogenism who were candidates for IVF due to their husbands' infertility were considered as control subjects. Since the age is a confounder variable for fertility, PCOS patients and healthy women from control group were matched for their age. In addition, congenital adrenal hyperplasia, adrenal tumors, taking insulin-sensitizing drugs, Cushing syndrome, and presence of prolactinoma were considered as exclusion criteria.
All experiments were conducted in accordance with the ethical guidelines of the Declaration of Helsinki (1967; version 2013). Formal written consents were received from all participants and Research Ethics Committee of Hamadan University of Medical Sciences (IR.UMSHA.REC.1398.008) approved the study.
Ovarian stimulation: A basic transvaginal ultra-sound test was performed on the second day of menstrual cycle and no cysts or ovarian follicles above 10 mm in diameter were observed in participants. Recombinant FSH and Cinnal-F® (CinnaGen Co., Iran) were prescribed by specialist for all subjects. To evaluate follicles size, serial ultra-sonography was carried out and when the follicle diameter reached 14 mm, 0.25 μg of Cetrotide antagonist (Merck Serono Co., Germany) was administered daily. Then, the recombinant OvitrellehCG (Merck Serono Co., Germany) was administered when at least three follicles with 18 mm diameter were observed in the ovaries. Finally, after 36 hr, the mature follicles were collected via transvaginal ultrasound-guided puncture, oocytes were separated, and the follicular fluids containing GCs were obtained.
Isolation of follicular fluid and granulosa cells: Follicular fluids were collected from participants during follicular puncture and after removing oocytes. Samples were transferred into sterile tubes and centrifuged at 1000×g for 4 min at 21°C. The supernatant (follicular fluid) was separated from cell debris and stored at −80°C for further analysis. The pellet containing GCs was washed and centrifuged with 20 ml red blood cell lysing buffer (RLB) containing 2 M ammonium chloride, 1 M NaHCO3, and EDTA. The washing/centrifugation step was repeated 3 times. Then, DMEM F12 medium was added to the granulosa cells and the mixture was centrifuged at 500×g for 2 min at 21°C. Finally, GCs were suspended in phosphate buffered saline (PBS) and centrifuged for 1 min at 300×g.
Quantitative RT-PCR for gene expression assay: VDAC1 and TSPO gene expression in GCs was determined by qRT-PCR using RealQ Plus 2x Master Mix Green (Ampliqon, Denmark) on LightCycler® 96 System (Roche Life Science, Germany). Briefly, the RNA was obtained by phenol-chloroform extraction. NanoDrop (Thermo Fisher Scientific, USA) was used to quantify the extracted RNA and 1% agarose gel electrophoresis was used to confirm the quality and integrity of total RNA samples. In the next step, cDNA was synthesized using 500 ng of total RNA and HyperScript Reverse Transcriptase cDNA Kit (GeneAll Biotechnology Co., Korea).
Allele ID® software vs. 7.7 (Premier Biosoft, USA) was used for primer designing and online primer-BLAST software (https://www.ncbi.nlm.nih.gov) was applied to reconfirm the specificity of primers. Forward 5'GGACAGCAGGAAACA GTAACA-3' and reverse 5'-CCTGGCTTTAGAG TCTGAGTG-3' primers were used to detect VDA C1 gene expression while 5′-TACCTGGTCTGG AAAGAGCTG-3′ and 5′-TTGTCGGGCACCAA AGAAGAT-3′ primers were used as forward and reverse primers, respectively to determine TSPO expression. In addition, a primer set of forward 5′-CAC CAA CTG GGA CGA CAT-3′and reverse 5′-ACA GCC TGG ATA GCA ACG-3′ was applied to assess expression of β-actin as housekeeping gene. Finally, the relative mRNA expression levels were calculated using 2−ΔCt method.
Immunocytochemistry for VDAC1 and TSPO protein assay: The cell suspensions were placed on sterile gelatin slides for 24 hr, rinsed with PBS, and refrigerated for 4 min with 4% paraformalde-hyde. The lamellae were washed with PBS in 2N HCL and incubated at ambient temperature for 20 min. The lamellae were then exposed to 0.3% Triton for 30 min to make cell membrane permeable to antibodies. Next, 10% goat serum was added and the cells were incubated overnight with primary (Santa Cruz Biotechnology, USA, sc-390996) and secondary (Abcam, USA, ab6785) antibodies against VDAC1 and primary (Boster Bio, USA, m01153) and secondary (Abcam, USA, ab191866) antibodies against TSPO, and stored overnight at 4°C in a humidity chamber. Cells were then washed twice with PBS and exposed to diluted secondary antibodies at a ratio of 1:200 in PBS for 1 hr. After washing with PBS, 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) was used to stain the nuclei, samples were examined under a fluorescence microscope (Olympus, Japan), and data were quantified by ImageJ software v1.52 (https://imagej.nih.gov/ij/).
Electrochemiluminescence assessment of estradiol (E2) in follicular fluid: Estradiol (E2) content of follicular fluids was determined using Elecsys Estradiol III assay (Roche Diagnostics GmbH, Germany) and cobas e 411 Analyzer (Roche Diagnostics GmbH, Germany). Briefly, based on a competitive principle using two monoclonal antibodies specifically directed against 17ß-estradiol, the released endogenous estradiol from the sample by mesterolone competes with the added estradiol derivative for the binding sites on the biotinylated antibody. Serial dilutions of follicular fluids were prepared (1:100, 1:200, 1:300, and 1:400). The lower and upper limits of detection were 5 and 3,000 pg/ml, respectively. Results were determined using an instrument which specifically generated 2-point calibration curve, expressed as pg/ml.
Measurement of total cholesterol in follicular fluid: Total cholesterol levels in follicular fluids of PCOS patients and control group were determined to investigate whether the difference in the concentration of estradiol (E2) in follicular fluids is due to the disability of GCs in translocation of cholesterol into mitochondrial matrix (as primary substrate for cholesterol biosynthesis pathway) or is a result of different available amounts of cholesterol in follicular fluid. Measurement of cholesterol was carried out using a commercial colorimetric cholesterol kit (Delta Darman Part, Iran) and the results were expressed as mg/dl.
Determination of total antioxidant capacity (TAC) in follicular fluid: A ready-to-use NaxiferTM Kit (Navand Salamat Co., Iran) was used to determine TAC level in follicular fluids. Briefly, based on Ferric Reducing Antioxidant Power Assay (FRAP method), antioxidant potential of samples to reduce ferric iron (Fe3+) to ferrous iron (Fe2+) was assessed. Changes in color resulting from the reaction was evaluated by a microplate reader at 593 nm. Finally, the quantity of antioxidant capacity was determined against the standard curve (Fe2+ solution) and expressed as mmol Fe2+/L.
Assessment of total oxidant status (TOS) in follicular fluid: A ready-to-use NatosTM Kit (Navand Salamat Co., Iran) was used to determine TOS level in follicular fluids. The detection was based on the oxidation reaction which occurs by large quantities of amplifying molecules present in the reaction medium. The color intensity which is directly proportional to the total amount of oxidant molecules in the specimen was evaluated by spectrophotometry. Hydrogen peroxide was used for calibration of the test and results were expressed as equivalent liquid peroxide per liter (μmol H2O2 Eq/L). Based on manufacturer’s instruction, the standard range for TOS level was 0.156–10 μmol Eq/L and the sensitivity was 0.023 μmol Eq/L. In addition, oxidative stress index (OSI), calculated as the ratio of TOS to TAC, was evaluated in each group and expressed as μmol Eq H2O2/mmol Fe2+.
Determination of lipid peroxidation level in follicular fluid: To determine the level of lipid peroxidation in follicular fluids, malondialdehyde (MDA), a reactive aldehyde formed by oxidative degradation of lipids, was measured using commercial NalondiTM Kit (Navand Salamat Co., Iran). Briefly, the MDA produced by peroxidation of lipids in follicular fluid reacted with thiobarbituric acid (TBA) at high temperature to generate a pink MDA-TBA adduct, which was quantified colorimetrically at 550 nm against standard curve. Finally, lipid peroxidation level was expressed as nmol/ml.
Statistical analysis: Data analysis was done by SPSS software vs. 22.0 (IBM., USA) and Graph-Pad Prism software vs. 8.0 (GraphPad Software Inc., USA). Mann-Whitney U test, Kolmogorov-Smirnov test, Chi square test and student’s t-test were applied for analysis of data. Spearman’s correlation coefficients were used for evaluating relationship between the variables. Data was expressed as mean±SEM and p value less than 0.05 was considered as statistically significant.
Results
Demographic characteristics and IVF results: Demographic characteristics and IVF outcome for all participants are shown in table 1. Data analysis showed no significant difference between participants in maternal age. PCOS subjects had higher body mass index (BMI) than controls. Menstrual status in women with PCOS was significantly irregular (72.1%) compared to the control group (14%). PCOS patients also had significantly higher number of retrieved oocytes (14.27±1.11) versus control women (7.71±0.99). Similarly, the number of metaphase I-oocytes and germinal vesicles, which were obtained from PCOS subjects, was markedly higher compared with those of control women (Table 1).
Table 1.
Demographic characteristics, hormonal status, and outcome of IVF procedures in participants (Mean±SEM)
| Variables | Control | PCOS | p-value |
|---|---|---|---|
| Age (year) | 30.88±0.55 | 30.93±0.47 | NS |
| BMI (kg/m 2) | 24.26±0.38 | 27.22±0.55 | <0.001 |
| Regular menstrual cycle (%) | 86% | 27.9% | <0.001 |
| Retrieved oocytes (N) | 7.71±0.99 | 14.27±1.11 | <0.001 |
| Number of oocytes in MII | 5.58±0.83 | 11.00±1.10 | <0.001 |
| Number of oocytes in MI | 0.95±0.22 | 0.30±0.11 | 0.001 |
| Number of oocytes in GV | 1.11±0.16 | 2.86±0.48 | 0.014 |
| M-oocytes/T-oocytes | 0.755±0.28 | 0.676±0.28 | 0.023 |
| IM-oocytes/T-oocytes | 0.245±0.28 | 0.323±0.28 | 0.026 |
| Embryo/M-oocytes | 0.749±0.03 | 0.593±0.04 | 0.006 |
BMI: Body Mass Index, MI and MII: Metaphase I and II, GV: Germinal Vesicle, M-oocytes: Number of Mature Oocytes Obtained in Metaphase-II, IM-oocytes: Immature Oocytes, T-oocytes: Total Number of Oocytes, NS: No Significant Difference between groups
While the ratio of the number of mature oocytes obtained in metaphase-II to the total number of oocytes (M-oocytes/T-oocytes) was significantly higher in control women (p=0.023), PCOS patients showed higher ratio of the number of immature oocytes to the total number of oocytes (p= 0.026). In addition, data analysis confirmed that Embryo/M-oocytes ratio (the ratio of number of embryos to mature oocytes obtained in meta- phase-II) was lower in PCOS patients compared with control women (0.593±0.04 vs. 0.749±0.03, respectively).
Expression of mitochondrial membrane transporter proteins in PCOS: The relative mRNA expression levels of VDAC1 and TSPO were significantly lower in women with PCOS compared with control subjects (Table 2). In addition, at the protein level, immunocytochemical analysis showed that the green fluorescence intensity which indicates cytoplasmic VDAC1-and TSPO-specific antibody complexes and the intensity ratio of green/blue fluorescence (cytoplasmic protein to nuclear fluorescence ratio) were significantly lower in PCOS patients (Figures 1 and 2).
Table 2.
The relative gene expression and protein levels of mitochondrial membrane proteins, VDAC1 and TSPO, in controls and PCOS patients
| VDAC1 gene expression | VDAC1 protein level (%) | TSPO gene expression | TSPO protein level (%) | |
|---|---|---|---|---|
| Control group | 0.189±0.07 | 38.42±1.13 | 0.074±0.03 | 45.49±3.31 |
| PCOS group | 0.152±0.06 | 11.66±0.62 | 0.060±0.03 | 15.09±1.23 |
| p value | p=0.023 | p<0.001 | p=0.044 | p<0.001 |
The relative mRNA expression levels were calculated using 2−ΔCt method while protein levels were determined using immunocytochemistry.
TSPO: Translocator Protein, VDAC1: Voltage-Dependent Anion Channel 1. Data is represented as mean±SEM
Figure 1.
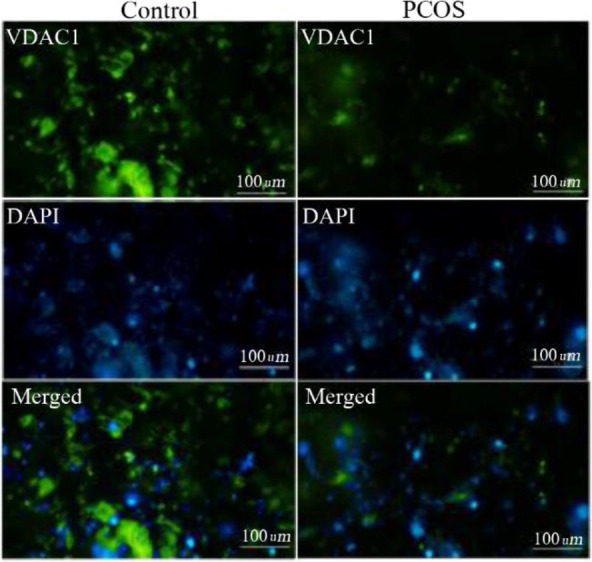
VDAC1 protein levels were obtained by immunocytochemistry where green fluorescence intensities indicated cytoplasmic VDAC1 and blue DAPI fluorescence showed nuclear counterstain. Green and blue intensities were merged to obtain intensity ratio of green/blue fluorescence corresponding to the relative cytoplasmic VDAC1 levels and quantified with ImageJ software
Figure 2.
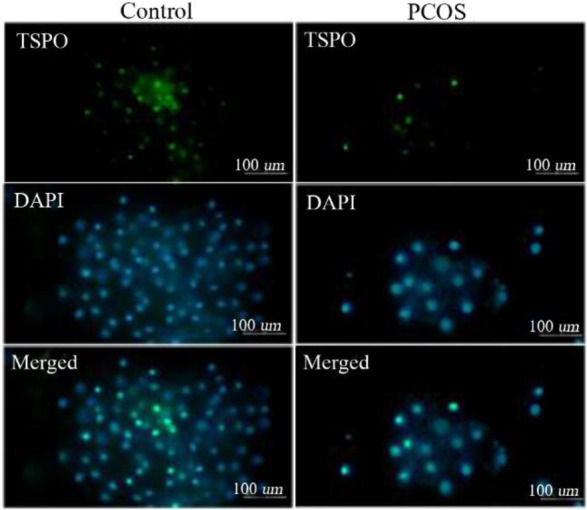
TSPO protein levels were obtained by immunocytochemistry where green fluorescence intensities indicated cytoplasmic TSPO and blue DAPI fluorescence showed nuclear counterstain. Green and blue intensities were merged to obtain intensity ratio of green/blue fluorescence corresponding to the relative cytoplasmic TSPO levels and quantified with ImageJ software
Interestingly, both VDAC1 and TSPO protein levels in GCs of PCOS patients were found nearly 65% lower than those of control subjects (Table 2). Spearman’s correlation analysis also showed a positive correlation between VDAC1 and TSPO concentrations (r=0.534, p<0.001), indicating that lower VDAC1 is accompanied with lower TSPO level in mitochondrial membrane.
Concentrations of estradiol in follicular fluid of PCOS patients: As shown in figure 3A, the mean estradiol level in follicular fluid of PCOS subjects was markedly lower than that of control group. Although a nearly 30% lower E2 level was observed in follicular fluid of PCOS patients, only a slightly (8%) lower concentration of total cholesterol was observed in their follicular fluid (Figure 3B) compared with control women (31.21± 0.86 vs. 34.21±0.53 mg/dl, respectively), indicating that slightly different amounts of available cholesterol in follicular fluid were not probably the main cause of marked difference (30%) in E2 concentration between groups.
Figure 3.
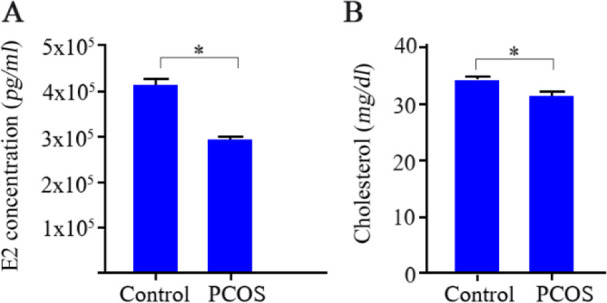
Determination of estradiol (E2) concentration and total cholesterol level in follicular fluids of 43 controls and 43 PCOS patients. A) Concentration of E2 as determined by electrochemiluminescence and, B) the mean level of total cholesterol. Data is represented as mean±SEM and asterisk (*) indicates significant difference between groups (p<0.001)
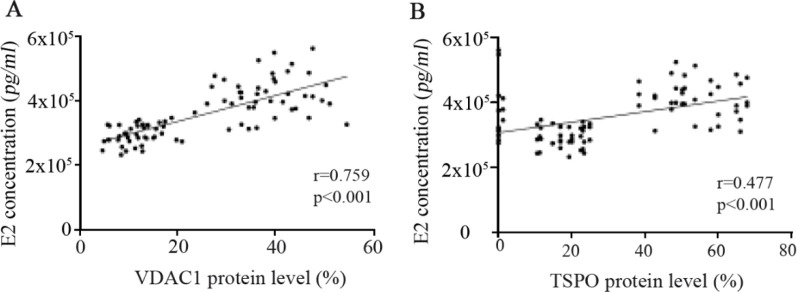
E2 concentration in follicular fluid and VDAC1 and TSPO expression: As represented in figure 4, Spearman's correlation analysis showed strongly positive associations between estradiol concentration in follicular fluid with the expression of mitochondrial membrane transporter proteins, VDAC1 (r=0.769, p<0.001) and TSPO (r=0.477, p<0.001), in granulosa cells. In other words, the greater concentration of E2 in follicular fluid was correlated with higher expression of VDAC1 and TSPO proteins in GCs.
Figure 4.
Correlation between concentration of estradiol (E2) in follicular fluid with the expression of (A) voltage-dependent anion channel 1 (VDAC1) and (B) translocator protein (TSPO) in GCs of 43 PCOS patients and 43 controls, as determined by Spearman’s correlation analysis
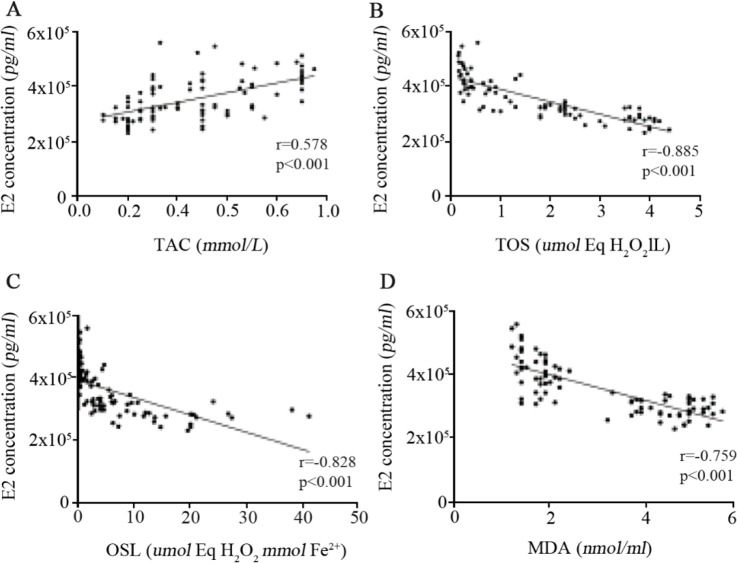
The level of oxidative stress and its association with E2 concentration in follicular fluid: Follicular fluid of PCOS patients had significantly lower total antioxidant capacity whereas an almost six-fold greater total oxidant status level together with higher oxidative stress index and greater lipid peroxidation marker were observed in PCOS patients (Table 3), confirming the presence of considerably substantial oxidants in follicular fluids of PCOS subjects compared with control women. Moreover, as shown in figure 5, Spearman's correlation analysis confirmed the presence of a direct correlation between E2 concentration with TAC level (r=0.578, p<0.001) whereas a strong negative correlation was found between E2 concentration with TOS (r=−0.885, p<0.001), OSI (r= −0.828, p<0.001), and MDA (r= −0.759, p<0.001). Interestingly, direct correlations were also observed between the expression of both VDAC1 and TSPO proteins with TAC level (Table 4) while the expression of these mitochondrial membrane transporter proteins were negatively correlated with TOS, OSI, and MDA in follicular fluids.
Table 3.
Associations of oxidative stress markers of follicular fluids with TSPO and VDAC1 gene and protein levels in all subjects
| Variables | TSPO gene | TSPO protein | VDAC1 gene | VDAC1 protein | Embryo (N) |
|---|---|---|---|---|---|
| E2 | 0.177 | 0.477 | 0.104 | 0.769 | 0.216 |
| 0.103 | <0.001 | 0.339 | <0.001 | 0.047 | |
| TOS | −0.112 | −0.573 | −0.176 | −0.778 | −0.231 |
| 0.303 | 0.001 | 0.105 | <0.001 | 0.033 | |
| TAC | 0.096 | 0.333 | 0.032 | 0.340 | 0.457 |
| 0.380 | 0.002 | 0.769 | 0.001 | <0.001 | |
| OSI | −0.112 | −0.526 | −0.154 | −0.696 | −0.286 |
| 0.306 | <0.001 | 0.158 | <0.001 | 0.008 | |
| MDA | −0.167 | −0.503 | −0.258 | −0.763 | 0.057 |
| 0.125 | <0.001 | 0.016 | <0.001 | 0.606 |
TAC: Total Antioxidant Capacity, TOS: Total Oxidant Status, OSI: Oxidative Stress Index, MDA: Malondialdehyde. Data is represented as mean±SEM and in each column, p value represents significant difference between groups
Figure 5.
Correlations between concentrations of E2 in follicular fluid and total antioxidant capacity (A), total oxidant status (B), oxidative stress index (C), and the level of malondialdehyde (D) in follicular fluid of 43 PCOS patients and 43 controls, as determined by Spearman’s correlation analysis
Table 4.
Correlation of E2, TOS, TAC, OSI, and MDA with the gene and protein expression of mitochondrial membrane transporters
| TAC (nmol/L) | TOS (μmol Eq H2O2/L ) | OSI (μmol Eq H2O2/mmol Fe 2+ ) | MDA (nmol/ml) | |
|---|---|---|---|---|
| Control group | 0.57±0.03 | 0.52±0.05 | 1.52±0.26 | 1.82±0.04 |
| PCOS group | 0.36±0.02 | 3.00±0.13 | 11.99±1.42 | 4.66±0.09 |
| p-value | p<0.001 | p<0.001 | p<0.001 | p<0.001 |
In each cell, the upper number represents correlation (r) of the variables and the lower number indicates p value. E2: Estradiol, MDA: Malondialdehyde, N: Number, OSI: Oxidative Stress Index, TAC: Total Antioxidant Capacity, TOS: Total Oxidant Status, TSPO: Translocator Protein, VDAC1: Voltage-Dependent Anion channel 1
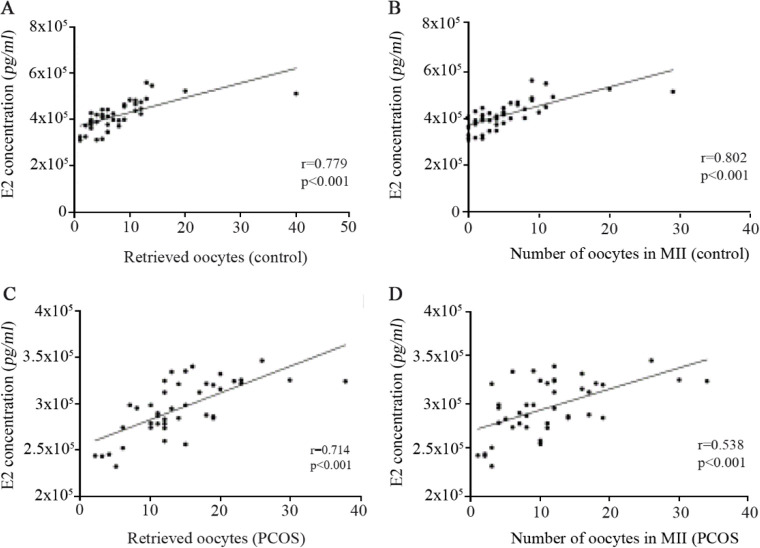
The concentration of E2 in follicular fluid and its correlation with oocyte quality: The number of obtained oocytes and the number of retrieved oocytes in metaphase-II were associated with the concentration of E2 in follicular fluid in both healthy women (Figures 6A and B) and PCOS patients (Figures 6C and D). Therefore, greater number of both retrieved oocytes and oocytes with higher quality (oocytes in metaphase-II) was found in the higher concentration of E2, regardless of the presence or absence of the disease. E2 concentration in follicular fluid showed positive correlation with the number of conceived embryos in all participants (Table 4), indicating that higher number of embryos was observed in the greater concentrations of E2.
Figure 6.
Correlations between concentrations of E2 in follicular fluid and the number of retrieved oocytes and the number of oocytes obtained in MII from 43 PCOS patients and 43 controls, as determined by Spearman’s correlation analysis. Correlation of E2 with (A) the number of retrieved oocytes and (B) the number of oocytes in MII in controls. Correlation of E2 with (C) the number of retrieved oocytes and (D) the number of oocytes in MII in PCOS patients
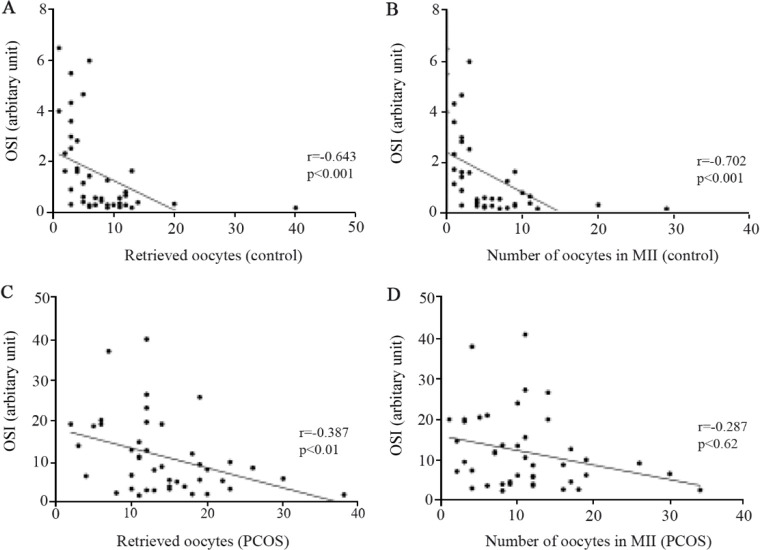
Oxidative stress in follicular fluid and its correlation with oocyte quality: Data analysis showed that the higher levels of oxidative stress index (OSI) in follicular fluids were associated with the lower number of retrieved oocytes in both control women and PCOS patients (Figure 7). Spearman’s correlation analysis also confirmed that higher OSI levels in follicular fluid were associated with the presence of only a few number of oocytes in metaphase-II (usually considered as higher quality oocytes) where the number of oocytes increased by decreasing oxidative stress in both control women and PCOS patients (Figure 7). Interestingly, the number of conceived embryos showed positive correlation with TAC whereas negative correlation was observed with TOS and OSI, as indicated in table 4.
Figure 7.
Correlations between oxidative stress index (OSI) of follicular fluid and the number of retrieved oocytes and the number of oocytes obtained in MII from 43 PCOS patients and 43 controls, as determined by Spearman’s correlation analysis. Correlation of E2 with (A) the number of retrieved oocytes and (B) the number of oocytes in MII in controls. Correlation of E2 with (C) the number of retrieved oocytes and (D) the number of oocytes in MII in PCOS patients
Discussion
According to World Health Organization (WHO) report, it is estimated that about 9% of couples are entangled with infertility worldwide (1, 2). PCOS is a common endocrine disease and the main cause of infertility in women of reproductive age and despite production of a large number of oocytes in these patients, the produced oocytes have low quality which leads to infertility (18).
Oocytes closely interact with GCs and follicular fluids. In granulosa cells, mitochondria are directly involved in a compartmentalized process, synthesizing estradiol and secreting E2 into follicular fluid where E2 governs maturation of follicles and improvement of oocyte quality (19, 20). It is now clear that in PCOS cases, inadequate synthesis or disruption in E2 production in GCs is the main contributor to impairment of oocyte maturation. E2 is synthesized in mitochondrial matrix using cholesterol as substrate. Translocation of cholesterol from cytosol into mitochondria is mediated by mitochondrial membrane integrated proteins, TSPO and VDAC1, in collaboration with steroidogenic acute regulatory protein (StAR). Trans-location of cholesterol across mitochondrial membrane is postulated as a rate-limiting step in the synthesis of E2 by granulosa cells (12, 13, 21). Therefore, in the present study, the correlation of expression level in mitochondrial membrane proteins (TSPO and VDAC1) with estradiol concentration and oxide-redox status in follicular fluids of PCOS patients was investigated.
It was shown that despite a slight difference (8%) in cholesterol levels in follicular fluids of patients and controls, E2 concentration markedly differed between groups being almost 30% lower in PCOS patients. Concurrently, PCOS women had also remarkably lower expression level of TSPO and VDAC1 proteins which, to some extent, explains disruption in synthesis of E2 among these subjects. Significant direct correlations were also observed between E2 concentration in follicular fluid and VDAC1 and TSPO proteins in this study. Considering the role of TSPO and VDAC1 in steroidogenesis, these findings convincingly accentuated the importance of TSPO/VDAC1 in oocyte maturation and fertility. These observations are similar to previous reports confirming the role of TSPO protein in cholesterol transport and steroidogenesis where reduced TSPO protein level is associated with decreased synthesis of sex steroids (9, 22, 23). Taken together, it can be concluded that insufficient estradiol levels in follicular fluids of PCOS patients compared with control women is plausibly due to the down-regulation of TSPO and VDAC1 in these subjects.
Polycystic ovary syndrome is believed to be associated with mitochondrial dysfunction in regulating oxide-redox status in follicular environment (24). Mitochondria play fundamental roles in cellular fate by metabolizing nutrients and releasing reactive oxygen species (ROS) as by-products (24). Although at proper levels, ROS may favorably impact the biological functions, increased oxidative stress is portrayed as a villain in the network of metabolic pathways which induces deleterious effects on the cell. In physiological conditions, cellular delicate antioxidant system potentially counteracts oxidative stress to mitigate harmful effects of oxidizing components; however, it seems that in PCOS cases, persistent oxidative stress further aggravates the unpleasant environment for follicular maturation (25–27). In fact, internalized cholesterol may also become oxidized under oxidative stress condition (which is accompanied with PCOS) and consequently further enhances oxidative stress in these patients (28). In line with these reports, the important role of oxidative stress in women's infertility has previously been shown (29, 30) and increased oxidative stress in follicular fluid of PCOS individuals has been documented (5, 15). In addition, the effect of imbalanced oxide-redox status on the quality of embryo has been reported in different studies (18, 20). Likewise, our results showed reduced antioxidant capacity and increased TOS, OSI, and MDA level in follicular fluids of PCOS patients. Interestingly, our data showed that increased oxidative stress in follicular fluid was accompanied with decreased E2 level which is a necessary steroid hormone for oocyte maturation. The ratio of mature-oocytes/total-oocytes was higher in control women whereas PCOS patients had significantly higher ratio of immature-oocytes to total number of oocytes. In addition, the ratio of conceived embryo to the mature-oocytes was lower in PCOS patients compared with control women. Number of retrieved oocytes and number of high-quality oocytes obtained in metaphase-II directly increased by E2 level increment in all participants and they decreased by enhancing oxidative stress, as determined by OSI. Consequently, positive association of conceived embryos with E2 concentration and TAC level and negative correlations with TOS and OSI were observed in the present study confirming that lower E2 concentration and higher oxidative status are important contributors to lower number of embryos in PCOS women. Our observations were in line with previous reports that showed lower TAC content (5, 16) and higher lipid peroxidation level (16, 31) in follicular fluid of PCOS women; moreover, association of increased ROS with poor oocyte quality and IVF-outcome in PCOS patients was another finding of the current research (32).
A prominent biochemical feature of VDAC1 and TSPO proteins is their cooperation in ROS signaling and their role in cellular oxide-redox state (12). Joo et al. have reported that overexpression of TSPO in endothelial cells leads to the reduction of ROS level via VDAC1 dependent pathway (21). Thus, it is postulated that down-regulation of TSPO and VDAC1 proteins in PCOS (as observed in the present study) might also be a potential causative factor in instantaneous increase of oxidative stress in these patients.
Due to the limitations of our study, it seems that (1) determination of granola cells and ROS levels in follicular fluid, (ii) measurement of E2 in both serum and follicular fluid, and (iii) follow-up of embryo quality and fertilization rate may strengthen the results of this study. Moreover, an outstanding question that should be addressed is whether evoking E2 production in GCs can be used as a therapeutic strategy to obtain mature high-quality oocytes.
Conclusion
In conclusion, it was shown that lower expression of mitochondrial membrane proteins (TSPO and VDAC1) is involved in the pathogenesis of PCOS. In addition, an important implication of the present findings is that increasing E2 level and reducing oxidative stress in follicular fluid may be envisioned as therapeutic strategies in PCOS women.
Acknowledgement
Authors would like to acknowledge the financial support of Hamadan University of Medical Sciences. This manuscript was prepared based on a part of PhD thesis project (Project No: 98090565-44).
Footnotes
Conflict of Interest
The authors declare that they have no competing interests.
References
- 1.Fainberg J, Kashanian JA. Recent advances in understanding and managing male infertility. F1000-Res. 2019;8:F1000 Faculty Rev-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarhan D, El Mazny A, Taha T, Aziz A, Azmy O, Fakhry D, et al. Estradiol and luteinizing hormone concentrations in the follicular aspirate during ovum pickup as predictors of in vitro fertilization (IVF) outcome. Middle East Fertil Soci J. 2017;22(1):27–32. [Google Scholar]
- 3.Crespo RP, Bachega TA, Mendonça BB, Gomes LG. An update of genetic basis of PCOS pathogenesis. Arch Endocrinol Metab. 2018;62(3):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687–708. [DOI] [PubMed] [Google Scholar]
- 5.Masjedi F, Keshtgar S, Agah F, Karbalaei N. Association between sex steroids and oxidative status with vitamin D levels in follicular fluid of non-obese PCOS and healthy women. J Reprod Infertil. 2019;20(3):132–42. [PMC free article] [PubMed] [Google Scholar]
- 6.Li J, Ma XJ, Wu X, Si SJ, Li C, Yang PK, et al. Adiponectin modulates steroid hormone secretion, granulosa cell proliferation and apoptosis via binding its receptors during hens’ high laying period. Poult Sci. 2021;100(7):101197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu L, Liu M, Wang Z, Liu T, Liu S, Wang B, et al. Correlation between steroid levels in follicular fluid and hormone synthesis related substances in its exosomes and embryo quality in patients with polycystic ovary syndrome. Reprod Biol Endocrinol. 2021;19(1):74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wen X, Li D, Tozer AJ, Docherty SM, Iles RK. Estradiol, progesterone, testosterone profiles in human follicular fluid and cultured granulosa cells from luteinized pre-ovulatory follicles. Reprod Biol Endocrinol. 2010;8:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller WL. Steroid hormone synthesis in mitochondria. Mol Cell Endocrinol. 2013;379(1–2):62–73. [DOI] [PubMed] [Google Scholar]
- 10.Sreerangaraja Urs DB, Wu WH, Komrskova K, Postlerova P, Lin YF, Tzeng CR, et al. Mitochondrial function in modulating human granulosa cell steroidogenesis and female fertility. Int J Mol Sci. 2020;21(10):3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rone MB, Midzak AS, Issop L, Rammouz G, Jagannathan S, Fan J, et al. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol Endocrinol. 2012;26(11): 1868–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Veenman L, Shandalov Y, Gavish M. VDAC activation by the 18 kDa translocator protein (TSPO), implications for apoptosis. J Bioenerg Biomembr. 2008;40(3):199–205. [DOI] [PubMed] [Google Scholar]
- 13.Gatliff J, Campanella M. TSPO is a REDOX regulator of cell mitophagy. Biochem Soc Trans. 2015; 43(4):543–52. [DOI] [PubMed] [Google Scholar]
- 14.Yilmaz N, Inal HA, Gorkem U, Sargin Oruc A, Yilmaz S, Turkkani A. Follicular fluid total anti-oxidant capacity levels in PCOS. J Obstet Gynaecol. 2016;36(5):654–7. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Yu Z, Zhao S, Cheng L, Man Y, Gao X, et al. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J Assist Reprod Genet. 2021;38(2):471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artimani T, Karimi J, Mehdizadeh M, Yavangi M, Khanlarzadeh E, Ghorbani M, et al. Evaluation of pro-oxidant-antioxidant balance (PAB) and its association with inflammatory cytokines in polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2018;34(2):148–52. [DOI] [PubMed] [Google Scholar]
- 17.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group . Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 18.Tan IF, Lim AJ, Indran IR, Kramer MS, Yong EL. Reproductive outcomes of women with polycystic ovarian syndrome following in-Vitro fertilization—a meta-analysis and systematic review. Fertil Reprod. 2019;1(4):193–201. [Google Scholar]
- 19.Pellicer A, Valbuena D, Bauset C, Albert C, Bonilla-Musoles F, Remohı J, et al. The follicular endocrine environment in stimulated cycles of women with endometriosis: steroid levels and embryo quality. Fertil Steril. 1998;69(6):1135–41. [DOI] [PubMed] [Google Scholar]
- 20.Masjedi F, Keshtgar S, Zal F, Talaei-Khozani T, Sameti S, Fallahi S, et al. Effects of vitamin D on steroidogenesis, reactive oxygen species production, and enzymatic antioxidant defense in human granulosa cells of normal and polycystic ovaries. J Steroid Biochem Mol Biol. 2020;197:105521. [DOI] [PubMed] [Google Scholar]
- 21.Joo HK, Lee YR, Lim SY, Lee EJ, Choi S, Cho EJ, et al. Peripheral benzodiazepine receptor regulates vascular endothelial activations via suppression of the voltage-dependent anion channel-1. FEBS Lett. 2012;586(9):1349–55. [DOI] [PubMed] [Google Scholar]
- 22.Fan J, Wang K, Zirkin B, Papadopoulos V. CRISPR/Cas9‒mediated Tspo gene mutations lead to reduced mitochondrial membrane potential and steroid formation in MA-10 mouse tumor Leydig cells. Endocrinology. 2018;159(2):1130–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen DR, Fan J, Campioli E, Venugopal S, Midzak A, Daly E, et al. TSPO mutations in rats and a human polymorphism impair the rate of steroid synthesis. Biochem J. 2017;474(23):3985–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Bao Y, Zhou X, Zheng L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol. 2019;17(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sulaiman MA, Al-Farsi YM, Al-Khaduri MM, Saleh J, Waly MI. Polycystic ovarian syndrome is linked to increased oxidative stress in Omani women. Int J Womens Health. 2018;10:763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moti M, Amini L, Ardakani SSM, Kamalzadeh S, Masoomikarimi M. Oxidative stress and anti-oxidant defense system in Iranian women with polycystic ovary syndrome. Iran J Reprod Med. 2015; 13(6):373–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Mazloomi S, Sheikh N, Sanoee Farimani M, Pilehvari S. Association of Prx4, total Oxidant status, and inflammatory factors with insulin resistance in polycystic ovary syndrome. Int J Endocrinol. 2021; 2021:9949753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilkan Z, Akar FG. The mitochondrial translocator protein and the emerging link between oxidative stress and arrhythmias in the diabetic heart. Front Physiol. 2018;9:1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamilian M, Modarres SZ, Siavashani MA, Karimi M, Mafi A, Ostadmohammadi V, et al. The influences of chromium supplementation on glycemic control, markers of cardio-metabolic risk, and oxidative stress in infertile polycystic ovary syndrome women candidate for in vitro fertilization: a randomized, double-blind, placebo-controlled trial. Biol Trace Element Res. 2018;185(1): 48–55. [DOI] [PubMed] [Google Scholar]
- 30.Adeoye O, Olawumi J, Opeyemi A, Christiania O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist Reprod. 2018;22 (1):61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Zolfaghari Z, et al. Abdominal obesity can induce both systemic and follicular fluid oxidative stress independent from polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2015;184:112–6. [DOI] [PubMed] [Google Scholar]
- 32.Lai Q, Xiang W, Li Q, Zhang H, Li Y, Zhu G, et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front Med. 2018;12(5):518–24. [DOI] [PubMed] [Google Scholar]