Abstract
Background:
The purpose of the current study was to assess pooled prevalence (PP) of SARS-CoV-2 in semen and pooled estimates including weighted mean difference (WMD) and risk ratio (RR) of semen characteristics in infected cases as compared with healthy controls.
Methods:
Major databases were searched by two authors. SARS-CoV-2-positive cases were assigned to the exposed arm (group A), whereas the controls to the unex-posed (group B). Risk of bias was assessed with Newcastle-Ottawa Scale and PRISMA guidelines were followed. Random-effects model was employed for analyzing the heterogeneity and fixed-effects model for homogeneity of studies.
Results:
Of 170 studies, 14 studies were eligible involving 507 subjects (316 in group A, 191 in group B). The risk of bias was the highest for “comparability” domain. SARS-CoV-2 RNA was found in only two studies among 7 subjects (PP= 2.10%, 95%CI 0.58–4.42). There was a significant decrease in sperm concentration (WMD= −15.29, 95%CI −24.70 – −5.88) and total sperm in ejaculate (WMD= −47.58, 95%CI −86.40 – −8.75) in group A. The effect of COVID-19 upon progressive motility, ejaculate volume, and leukocyte presence in semen was not significant.
Conclusion:
Prevalence of SARS-CoV-2 in semen among the infected cases is low. Sexual transmission through semen is improbable and of little concern for public health. Sperm concentration and total sperm in ejaculate are significantly reduced as compared with controls. Due to limited information of the current research, longer follow-up is needed to identify delayed or progressive impact.
Keywords: Coronavirus, COVID-19, Meta-analysis, Pandemics, SARS-CoV-2, Semen
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in December 2019 in Wuhan City (Hubei Province, People’s Republic of China) and its spread rapidly escalated leading to global pandemic. Until 19th December, 2020, more than 75 million infected cases were reported and over 1.6 million people died worldwide. The virus causes respiratory tract disease, officially called corona-virus disease 2019 (COVID-19), and it mainly spreads through respiratory droplets during face-to-face contact with an infected person. The important issue is to establish efficient strategies for possible contagion to prevent further expansion of the pandemic (1). Using real-time reverse transcription polymerase chain reaction (rRT-PCR), viral nucleic acid was found in nasopharyngeal, nasal, and pharyngeal smear, samples of saliva, blood, stool, urine, and tears (2, 3). The virus enters the target cell through the viral spike (S) protein and angiotensin-converting enzyme 2 (ACE2) interaction (4). The presence of this enzyme in testes could justify viral appearance in semen. That could be an additional route of disease transmission. So far, the reports related to presence of SARS-CoV-2 in semen specimens are contradictory. Moreover, it is still unknown whether COVID-19 disease decreases semen quality which is used as a surrogate marker of male fertility. Although many narrative reviews have emerged, none of the researchers has attempted statistical analysis.
Thus, the aim of this study was to perform the first meta-analysis of retrospective observational studies, summarizing the current literature and providing an answer to the question of whether SARS-CoV-2 can be detectable in human semen; if this can be confirmed, the semen might be a clinically important route of COVID-19 transmission. The additional goal was to determine the possible effects that the disease will have upon semen characteristics (volume, sperm concentration, total sperm per ejaculate, sperm motility, and leukocyte presence).
Methods
Study design and literature search: It was a meta-analysis of observational studies reporting analysis of semen in subjects who have suffered COVID-19. Two researchers independently performed literature search on the 18th May, 2021. PubMed MEDLINE, Scopus, SciELO, Embase, and Web of Science were searched for the combination of the following terms:
COVID-19, SARS-CoV-2, novel coronavirus, semen, sperm, and their corresponding synonyms. No temporal restrictions were imposed and all languages were acceptable. The study has not been registered in PROSPERO. References of the relevant articles were reviewed in case any potentially contributory paper was missed. Full search strategy is demonstrated by Electronic Supplementary Material 1. In case of insufficient data, corresponding authors were emailed. For achieving clarity and transparency in the systematic approach, PRISMA guidelines were adopted (Electronic Supplementary Material 2). The null hypotheses are as follows:
95% confidence interval (CI) of weighted mean differences (WMDs) of continuous variables includes 0 and 95% CI of risk ratio (RR) of binary variables includes 1 suggesting that SARS-CoV-2 and COVID-19 do not affect semen quality.
SARS-CoV-2 RNA in human semen is not detectable or its prevalence is marginal.
Eligibility: For an article to be eligible, it had to (1) evaluate semen in COVID-19 patients (either in acute phase, in recovery phase, or both), (2) address only sexually mature subjects (arbitrarily set at 15 years of age), and (3) contain 5 or more subjects for analysis. On the other hand, studies were excluded in case of encountering any of the following:
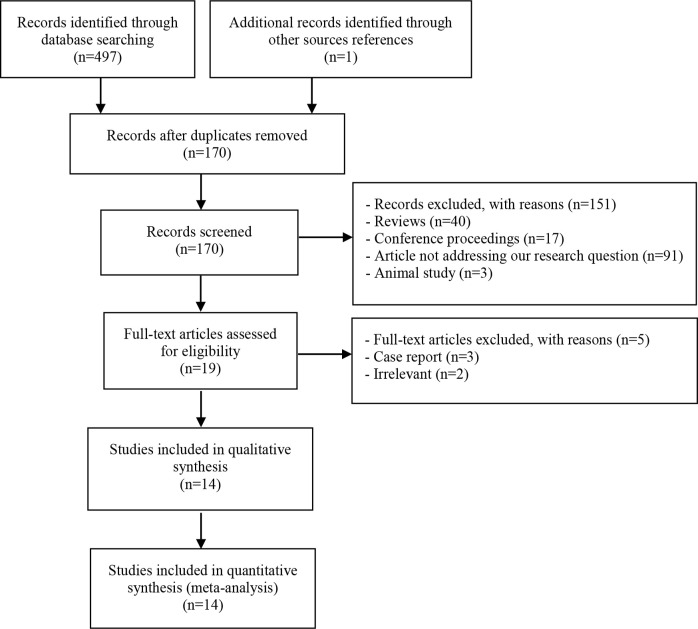
(1) reviews, (2) commentaries, (3) conference proceedings, (4) number of subjects less than five, (5) chapters, (6) posters, (7) surveys, (8) errata, (9) published material in non-peer-reviewed supplements, (10) high index of suspicion for the overlapping data, and (11) insufficient data in spite of an attempt to communicate with corresponding authors. All inclusion procedures are shown in figure 1.
Figure 1.
Study selection process
Data extraction and curation: The purpose of the current paper was to extract the following items: (1) type of the study, (2) total number of subjects, (3) mean age, (4) mean/median follow-up, (5) number of patients in acute (active) phase, (6) number of patients in recovery phase, (7) number of controls, (8) geographical origin of the study, (9) number of patients with SARS-CoV-2 in semen at each phase of the disease (active or recovery), (10) laboratory method for SARS-CoV-2 confirmation, (11) number of patients requiring hospitalization, (12) number of patients with fever, (13) duration of symptoms, (14) time between symptoms appearance and semen collection, (15) number of patients with testicular pain or discomfort during infection, and (16) semen characteristics (volume, sperm concentration, motility, leukocyte count, and bacterial presence). Data was disaggregated into its components, then reaggre-gated for the statistical analysis. Missing means or standard deviations were calculated using the dis-aggregated data. In case of parameters that could not be aggregated into the mean of the whole sample due to insufficient data, the latest parameters were chosen as indicative of the recovery phase. The data was curated in Microsoft Excel 2016 and subjects were divided into group A consisting of SARS-CoV-2-positive patients and group B comprising healthy controls.
Assessing quality and risk of bias: Risk of bias was assessed both qualitatively and quantitatively. Qualitative assessment was based upon the Newcastle-Ottawa Scale (NOS), whereas for quantitative interpretation, a funnel plot was created. For the NOS, the exposure was defined as SARS-CoV-2 infection with or without symptoms. The endpoints of interest were defined as presence of the viral RNA in semen and/or semen quality as compared to World Health Organization reference values (5th centile was adopted as the lower cutoff value) (5). Each paper was reviewed for three domains including selection, comparability, and outcome (endpoints). A total number of stars that could have been given for each domain was as follows:
for “selection” four stars, for “comparability” two stars, and for “outcome” three stars. Lack of any stars in a given domain showed the risk of bias was high. At least one star meant the risk of bias was moderate. A maximum number of stars demonstrated the risk of bias was low. If the study does not present any controls at all, then for the entire “comparability” domain as well as for “selection of the non-exposed cohort”, it is given zero stars. The most important control factor was age. Additional control factors were smoking and mean body mass index (BMI). Since it was suggested that SARS-CoV-2 might affect semen even in the acute phase (6), adequacy of minimal follow-up of cohorts (time from diagnosis to semen collection) was set at 0 days (the day of diagnosis).
Statistical analysis: The meta-analysis was performed using MetaXL 5.3 (Epigear International Pty Ltd, Australia) and Comprehensive Meta-Analysis V3 (Biostat Inc., USA). Relative risk (risk ratio; RR) and weighted mean difference (WMD) were calculated with corresponding confidence interval of 95%. Heterogeneity was assessed based on I2 and chi2 values. Interpretation of I2 value was made as commonly accepted: 0 to 40% as not important, 30 to 60% as moderate heterogeneity, 50 to 90% as substantial heterogeneity, and 75 to 100% as considerable heterogeneity. Level of significance for Cochran’s Q was arbitrarily set to <10% (<0.10), whereas p-value of comparative tests was universally set to <5% (<0.05). Based on principles of good research practice, in case of I2 ≥40% (heterogeneity), random effects model would be employed, whereas for I2 <40% (homogeneity), fixed effects model would qualify for analysis.
Sensitivity analysis: For sensitivity analysis, WMDs were approached again after excluding the study of Temiz et al. (7) where a considerable part of the sample had a negative throat swab test yet still were included solely based on symptoms.
Results
Study characteristics: Initial search of all databases yielded 497 results. One paper was additionally found during reference check. After de-duplication, 170 records were obtained. Screening of the titles and abstracts led to exclusion of 149 studies (Figure 1) and 19 full-text articles were evaluated. Ultimately, 14 papers were eligible for analysis, including the single paper that had been collected in the reference check (6–19). In general, 9 (64.3%) studies were conducted in Asia (including two Turkish studies) and 2 (14.3%) in Europe, and three (21.4%) were done in North America. The studies involved 507 subjects that were grouped into two categories: 316 in group A (62.3%; an exposed arm of infected individuals) and 191 (37.7%) healthy controls in group B (Table 1).
Table 1.
Characteristics of studies
| Study name | Study type | Global region | Method of systemic COVID-19 testing | No. of COVID-19 subjects (n) | No. of subjects with SARS-CoV-2 in semen |
|---|---|---|---|---|---|
| Guo et al. (2020) | Case series | Asia | RT-PCR | 23 | 0 |
| Holtmann et al. (2020) | Cohort study | Europe | RT-PCR or serum IgA, IgG | 20 | 0 |
| Kayaaslan et al. (2020) | Case series | Asia | RT-PCR | 16 | 0 |
| Li, Jin, et al. (2020) | Case series | Asia | RT-PCR | 38 | 6 |
| Li, Xiao, et al. (2020) | Cohort study | Asia | RT-PCR | 23 | 0 |
| Ma et al. (2020) | Case series | Asia | RT-PCR or serum IgM, IgG | 12 | 0 |
| Pan et al. (2020) | Case series | Asia | qRT-PCR | 34 | 0 |
| Pavone et al. (2020) | Case series | Europe | RT-PCR | 9 | 0 |
| Rawlings et al. (2020) | Case series | North America | dd-PCR | 6 | 0 |
| Ruan et al. (2020) | Cohort study | Asia | RT-PCR | 70 | 0 |
| Song et al. (2020) | Case series | Asia | RT-PCR or serum IgM, IgG | 12 | 0 |
| Temiz et al. (2020) | Cohort study | Asia | RT-PCR | 17* | 0 |
| Machado et al. (2021) | Case series | North America | RT-PCR | 15 | 1 |
| Burke et al. (2021) | Case series | North America | RT-PCR | 18 | 0 |
20 subjects but in 3, the swab test was negative. They were regarded by Temiz et al. as SARS-CoV-2-positive.
RT-PCR: Real-Time Polymerase Chain Reaction, dd-PCR: droplet digital Polymerase Chain Reaction
Mean age in group A was 33.5 years and mean age in group B was 39.9 years. Also, mean BMI in group A was 25.74 kg/m2. Semen specimens were collected at a mean time of 23 days from the disease onset (range of 0–109 days). At the time of semen collection, only a minority of subjects were in acute or active phase of COVID-19 (n=73; 23.1%), whereas most were in recovery period (n=237; 75%). Data regarding stage of the disease of six subjects was unavailable. Moreover, it was reported that 121 participants (38.3%) had symptomatic course of COVID-19.
Risk of bias assessment: Summary of the qualitative risk of bias assessment using the Newcastle-Ottawa Scale are show in table 2. The highest risk of bias was found in “comparability” domain. Only one study provided controls who were age-matched, BMI-matched, and smoking status- matched. The lowest risk of bias was identified in “outcome” domain. Additional risk of bias was found in the study of Temiz et al. as they had included three subjects whose throat swab tests were negative. Therefore, a sensitivity analysis was conducted omitting this study from analysis (see below).
Table 2.
Qualitative assessment of risk of bias using Newcastle-Ottawa Scale
| Study name | Selection | Comparability | Outcome |
|---|---|---|---|
| Guo et al. (2020) | ** | *** | |
| Holtmann et al. (2020) | *** | * | ** |
| Kayaaslan et al. (2020) | ** | *** | |
| Li, Jin, et al. (2020) | * | ** | |
| Li, Xiao, et al. (2020) | *** | * | *** |
| Ma et al. (2020) | ** | *** | |
| Pan et al. (2020) | ** | *** | |
| Pavone et al. (2020) | ** | *** | |
| Rawlings et al. (2020) | ** | *** | |
| Ruan et al. (2020) | *** | * | *** |
| Song et al. (2020) | ** | *** | |
| Temiz et al. (2020) | *** | ** | *** |
| Machado et al. (2021) | ** | *** | |
| Burke et al. (2021) | ** | *** |
A total number of stars that could have been given for each domain was as follows: for “selection” four stars, for “comparability” two stars, and for “outcome” three stars
Pooled effects: Pooled prevalence of SARS-CoV-2 RNA in semen was estimated at 2.10% (95% CI 0.58–4.42; I2=21%, Cochran’s Q=16.49, p=0.22, fixed-effects model). Pooled mean of semen volume for group A was 2.61 ml versus 3.17 ml for group B. The weighted mean difference (WMD) for semen volume was −0.35 (95% CI −0.70 −0.00; I2=0%, Cochran’s Q=0.1, p=0.951, fixed-effects model). Pooled prevalence of leukocytes in semen for group A was 50.57% and for group B was 40.06% with insignificant risk ratio (RR) of 1.69 (95% CI 0.49–5.88; I2=78%, Cochran’s Q=9.25, p=0.01, random-effects model). Mean sperm concentration in group A and group B were 53.30 [106/ml] and 70.60 [106/ml], respectively with statistically significant WMD of −15.29 (95% CI −24.70–5.88; I2=15%, Cochran’s Q=3.51, p=0.32, fixed-effects model). Mean semen volume in group A and group B were 2.61 ml and 3.17 ml, respectively with insignificant WMD of −0.35 (95%CI −0.70–0.00; I2=0%, Cochran’s Q=0.1, p=0.95, fixed-effects model). Mean of total sperm in ejaculate was 126.51 [106] and 239.24 [106] in group A and group B, respectively with statistically significant WMD of −47.58 (95% CI −86.40 – −8.75; I2=23%, Cochran’s Q=2.59, p=0.27, fixed-effects model). Mean of progressive motility in group A and group B was 41.38% (95% CI 38.58–44.18) and 39.97% (95%CI 38.27–41.16), respectively with insignificant WMD of 2.26 (95%CI −1.07–5.58; I2=0%, Cochran’s Q=1.22, p=0.54, fixed-effects model). The tabular summary of findings with comments are provided in table 3.
Table 3.
Summary of findings
| Endpoint | Graphic illustration | Pooled effect size (95% CI) | No. of participants (studies) | Comment |
|---|---|---|---|---|
| Leukocytes in semen | Figure 2A | RR 1.69 (0.49–5.88) | 107 (3) | Insignificant effect on leukocytes in semen |
| Sperm concentration | Figure 2B | WMD-15.29 (−24.70 – −5.88) | 307 (4) | Significantly reduced sperm concentration [106/ml] |
| Semen volume | Figure 2C | WMD-0.35 (−0.70–0.00) | 262 (3) | Insignificant trend towards reduced volume (CI includes 0) |
| Total sperm per ejaculate | Figure 2D | WMD-47.58 (−86.40 – −8.75) | 262 (3) | Significantly reduced total sperm per ejaculate [106] |
| Progressive motility | Figure 2E | WMD 2.26 (−1.07–5.58) | 262 (3) | Insignificant effect on motility |
| SARS-CoV-2 RNA in semen | Figure 2F | Prevalence 2.10% (0.58–4.42) | 313 (14) | Only two studies showed presence (see “discussion”) |
CI: Confidence Interval, RR: Risk Ratio, WMD: Weighted Mean Difference, No: Number
Figure 2.

Forest plots of pooled effect size with corresponding 95% confidence intervals. A) RR for leukocytes in semen, B) WMD for sperm concentration, C) WMD for sperm volume, D) WMD for total sperm in ejaculate E) WMD for progressive motility, F) Pooled prevalence of SARS-CoV-2 RNA in semen. RR: Risk Ratio, WMD: Weighted Mean Difference
Sensitivity analysis: For sensitivity analysis, the study of Temiz et al. (7) was excluded from semen quality analysis due to involvement of three subjects in group A (15% of group A sample, 10% of their study population) who had negative throat swab test but still were included solely based on symptoms. Thus, weighted mean differences and risk ratios were recalculated and compared with primary results:
(1) RR of 1.51 (95% CI 0.37–6.17) for presences of leukocytes in semen remained insignificant, (2) WMD of −17.82 (95%CI −27.83 – −7.82) for sperm concentration remained significant, (3) WMD of −0.347 (95%CI −0.71–0.015) for semen volume remained insignificant, (4) WMD of −61.64 (95% CI −104.22 – −19.07) for total sperm per ejaculate remained significant, and (5) WMD of 2.96 (95% CI −0.63–6.54) for progressive motility remained insignificant.
Discussion
SARS-CoV-2 in semen: This meta-analysis shows that SARS-CoV-2 RNA in semen is an exceptional finding, which has been reported only twice. Therefore, the main question is whether the finding of Li, Jin, et al. (6) and Machado et al. (10) were incidental or their specimens were contaminated. Information regarding the methodology in the former study was scarce as it lacked description on the procedure of semen collection and processing. Many other studies, which refuted the presence of virus in semen, furnished detailed methodology, depicting careful washing of hands and penis with soap and water and the ways for drying them with paper towels, besides showing sterile containers, sterile transport system, as well as complex laboratory processing materials and methods (8, 9, 18). Therefore, since studies of higher quality disprove the results of Li, Jin, et al.'s findings, the computed prevalence of semen viremia (2.10% [95% CI 0.58–4.42]) is possibly overestimated and falls closer to the lower end of the confidence interval. On the other hand, it has been suggested that PCR can be affected by many inhibitors (20). In terms of semen collection and processing, two conditions are theoretically possible:
(1) urea contamination by urine, and (2) contamination by powder from gloves (20, 21). This might be a potential cause of false negative results, leading to underestimation of the real incidence of virus presence in semen. Nevertheless, as for now, there appears to be a low risk of sexual transmission of the novel coronavirus.
Spermatogenesis deregulation: In spite of the absence in semen, febrile SARS-CoV-2 infection might still affect male fertility. As we showed in this meta-analysis, both sperm concentration and total sperm in ejaculate were significantly reduced. Statistical significance here might not be tantamount to clinical significance since, although reduced, these two surrogate markers of fertility are still within normal range according to World Health Organization reference values (5). One hypothesis that could explain the decrease in the quality parameters of semen is elevated body temperature. Fever of any cause is a known factor impairing spermatogenesis and disfiguring the results of semen testing (22). However, Holtmann et al. (19) suggested that deregulation of spermatogenesis in COVID-19 is independent of fever as they did find reduced sperm concentration and motility, which did not differ in subgroup analysis between the febrile and the afebrile cases. This, however, might be highly biased due to the small sample size in their groups (n=10 for febrile and n=8 for afebrile).
This meta-analysis has several limitations. Firstly, most of the current literature presents evidence no stronger than class III, thereby weakening the outcomes. Moreover, definitions of acute and recovery phase or description of PCR methodology differ among the authors precluding solid bias-free subgroup analysis. This is a common issue encountered when comparing different studies. Therefore, the subgroup analysis in acute and recovery phase was not performed. Additionally, as indicated in table 2, many studies exhibited low quality, which may further weaken the outcomes. The study of Temiz et al. included three subjects (15%) who, despite being symptomatic, turned out to have negative swab test results and this limitation was addressed in sensitivity analysis. Nevertheless, it is the only meta-analysis on the topic, systematically and statistically approaching the ongoing debate whether SARS-CoV-2 infection affects human semen or not.
In relation to standard semen parameters, not only the stage of disease and the general status of the patients but also selection of control subjects is of great importance. This was addressed in assessment of the quality of each study.
Conclusion
Current literature shows that SARS-CoV-2 is usually undetectable in human semen. This indirectly indicates that sexual transmission through semen is unlikely. As only two of fourteen studies have confirmed presence of the virus in semen, it should be currently regarded as an exceptional finding which, if repeated, needs to be reported. Sperm concentration and total sperm in ejaculate are significantly decreased as compared with controls, yet they are still within normal range according to WHO reference values. Progressive motility, ejaculate volume, and leukocyte presence in semen are not affected by COVID-19.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Cipriano M, Giacalone A, Ruberti E. Sexual behaviors during COVID-19: the potential risk of transmission. Arch Sex Behav. 2020;49(5):1431–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karia R, Gupta I, Khandait H, Yadav A, Yadav A. COVID-19 and its Modes of Transmission. SN Compr Clin Med. 2020:1–4. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li W, Moore MJ, Vasllieva N, Sui J, Wong SK, Berne MA, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HWG, Behre HM, et al. World health organization reference values for human semen characteristics. Hum Reprod Update. 2010;16(3):231–45. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Jin M, Bao P, Zhao W, Zhang S. Clinical characteristics and results of semen tests among men with Coronavirus disease 2019. JAMA Netw Open. 2020;3(5):e208292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Temiz MZ, Dincer MM, Hacibey I, Yazar RO, Celik C, Kucuk SH, et al. Investigation of SARS-CoV-2 in semen samples and the effects of COVID-19 on male sexual health by using semen analysis and serum male hormone profile: a cross-sectional, pilot study. Andrologia. 2021;53(2):e13912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo L, Zhao S, Li W, Wang Y, Li L, Jiang S, et al. Absence of SARS-CoV-2 in semen of a COVID-19 patient cohort. Andrology. 2021;9(1):42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song C, Wang Y, Li W, Hu B, Chen G, Xia P, et al. Absence of 2019 novel coronavirus in semen and testes of COVID-19 patients. Biol Reprod. 2020;103 (1):4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado B, Barra GB, Scherzer N, Massey J, dos Santos Luz H, Jacomo RH, et al. Presence of SARS-CoV-2 RNA in semen-cohort study in the United States COVID-19 positive patients. Infect Dis Rep. 2021;13(1):96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke CA, Skytte A, Kasiri S, Howell D, Patel ZP, Trolice MP, et al. A cohort study of men infected with COVID-19 for presence of SARS-CoV-2 virus in their semen. J Assist Reprod Genet. 2021; 38(4):785–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Xiao X, Zhang J, Zafar MI, Wu C, Long Y, et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine. 2020;28:100604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rawlings SA, Ignacio C, Porrachia M, Du P, Smith DM, Chaillon A. No evidence of SARS-CoV-2 seminal shedding despite SARS-CoV-2 persistence in the upper respiratory tract. Open Forum Infect Dis. 2020;7(8):ofaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavone C, Giammanco GM, Baiamonte D, Pinelli M, Bonura C, Montalbano M, et al. Italian males recovering from mild COVID-19 show no evidence of SARS-CoV-2 in semen despite prolonged nasopharyngeal swab positivity. Int J Impot Res. 2020;32(5):560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Y, Hu B, Liu Z, Liu K, Jiang H, Li H, et al. No detection of SARS-CoV-2 from urine, expressed prostatic secretions, and semen in 74 recovered COVID-19 male patients: a perspective and urogenital evaluation. Andrology. 2021;9(1): 99–106. [DOI] [PubMed] [Google Scholar]
- 16.Pan F, Xiao X, Guo J, Song Y, Li H, Patel DP, et al. No evidence of severe acute respiratory syndrome–coronavirus 2 in semen of males recovering from coronavirus disease 2019. Fertil Steril. 2020; 113(6):1135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Xie W, Li D, Shi L, Ye G, Mao Y, et al. Evaluation of sex-related hormones and semen characteristics in reproductive-aged male COVID-19 patients. J Med Virol 2021;93(1):456–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kayaaslan B, Korukluoglu G, Hasanoglu I, Kalem AK, Eser F, Akinci E, et al. Investigation of SARS-CoV-2 in semen of patients in the acute stage of COVID-19 infection. Urol Int. 2020;104 (9–10):678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtmann N, Edimiris P, Andree M, Doehmen C, Baston-Buest D, Adams O, et al. Assessment of SARS-CoV-2 in human semen-a cohort study. Fertil Steril. 2020;114(2):233–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrader C, Schielke A, Ellerbroek L, Johne R. PCR inhibitors-occurrence, properties and removal. J Appl Microbiol. 2012;113(5):1014–26. [DOI] [PubMed] [Google Scholar]
- 21.Tobe SS, Swaran YC, Dennany L, Sibbing U, Schulze Johann K, Welch L, et al. A proof of principal study on the use of direct PCR of semen and spermatozoa and development of a differential isolation protocol for use in cases of alleged sexual assault. Int J Legal Med. 2017;131(1):87–94. [DOI] [PubMed] [Google Scholar]
- 22.Carlsen E, Andersson AM, Petersen JH, Skakkebæk NE. History of febrile illness and variation in semen quality. Hum Reprod 2003;18(10):2089–92. [DOI] [PubMed] [Google Scholar]