Abstract
Persistent infection with Pseudomonas aeruginosa increases interleukin-8 (IL-8) levels and causes dense neutrophil infiltrations in the airways of patients with chronic airway diseases. Recently, we have reported that nitrite reductase from P. aeruginosa induces the production of IL-8 in respiratory cells, including bronchial epithelial cells. To determine the molecular mechanism(s) of nitrite reductase-induced IL-8 expression in respiratory cells, A549 epithelial cells were transfected with plasmids containing serial deletions of the 5′-flanking region of the IL-8 gene and then exposed to nitrite reductase. Nitrite reductase significantly enhanced IL-8 gene promoter-driven reporter activity. This increased IL-8 gene expression was inhibited by mutating the nuclear factor-κB (NF-κB) binding element. Nitrite reductase enhanced nuclear localization of the NF-κB binding complex. Furthermore, nitrite reductase induced the degradation of IκBα, the major cytoplasmic inhibitor of NF-κB, and the expression of IκBα mRNA. These data support the critical role of the activation of NF-κB in nitrite reductase-induced IL-8 gene expression in airway epithelium.
The importance of Pseudomonas aeruginosa as an opportunistic pathogen in the lower respiratory tract is well established (6, 31). Mucoid strains of P. aeruginosa often appear as a chronic infection in the late stages of chronic airway disease (CAD), such as cystic fibrosis and diffuse panbronchiolitis, and worsen the prognosis of these diseases (7, 37).
Inflammation in the airways of patients with CAD is characterized by dense neutrophil infiltrations (9, 19). Levels of cytokines, including interleukin-8 (IL-8), a potent neutrophil chemoattractant factor, have been reported to be elevated in bronchoalveolar lavage fluids from patients with such inflammatory diseases and to be decreased after low-dose, long-term erythromycin therapy, suggesting that these cytokines are important in airway inflammatory processes (9, 19, 28). We previously reported that persistent P. aeruginosa infection increased the levels of IL-8 and induced dense neutrophil infiltrations in the airways of patients with CAD (28). These observations confirmed that a perpetual cycle of IL-8 production and neutrophil accumulation caused by persistent P. aeruginosa infection plays an important role in the pathogenesis of CAD. IL-8 is known to be released by monocytes (41), macrophages (4), and fibroblasts (32), and recent data showed that airway epithelial cells are an important source of this chemokine (8, 25, 28, 36). Consistent with the in vivo observations, several products of P. aeruginosa stimulate bronchial epithelial cells to produce IL-8 in vitro (5, 16). Recently, our studies indicated that a heat-stable protein purified from the supernatants of sonicated P. aeruginosa stimulates human bronchial epithelial cells to produce IL-8 (27). We purified the protein to homogeneity and determined its N-terminal amino acid sequence. It completely matched a sequence at the N-terminal part of the mature protein form of Pseudomonas nitrite reductase (PNR) (27). Therefore, PNR is an IL-8 inducer in human respiratory cells.
However, the mechanism(s) by which PNR activates IL-8 gene expression in human epithelial cells has not yet been clarified. The IL-8 gene is regulated at both the transcriptional and the posttranscriptional levels. The former is primarily mediated by multiple cis elements, including a CCAAT box, a steroid-responsive element, an HNF-1 element, two IRF-1 elements, an activating protein 1 (AP-1) sequence, an AP-3 site, a C/EBP sequence, and a nuclear factor-κB (NF-κB)–NF-IL-6 overlapping sequence (10, 18, 23, 30). Activation of NF-κB is the most crucial step for IL-8 gene transcription in most cells, but NF-IL-6 and AP-1 binding sites are also required for IL-8 transcriptional activation by IL-1 or tumor necrosis factor alpha (TNF-α) (22). Synergistic interaction between NF-κB and NF-IL-6 may play an important role in the transcription of the IL-8 gene (11, 18). Depending on the cell line, cooperation between NF-κB and either NF-IL-6 or AP-1 is sufficient for IL-8 gene activation (22). In resting cells, NF-κB is present in the cytoplasm bound to its inhibitor, IκBα (2). Subsequent to cellular activation and through proteolytic degradation of IκBα, NF-κB is released and translocated to the nucleus, where it transactivates several genes. As several microbial, environmental, and inflammatory stimuli can activate NF-κB (3, 34), the mutual regulation of NF-κB and IκBα is critical to ensuring a transient and limited host response. In this study, we investigated the molecular mechanisms responsible for the induction of the IL-8 gene by PNR. We show that the NF-κB element is essential for activation of IL-8 gene expression by PNR. On exposure to PNR, human epithelial cells exhibit increased NF-κB activity and increased turnover of IκBα.
MATERIALS AND METHODS
Purification of PNR.
A serum-sensitive strain with a mucoid phenotype, P. aeruginosa 5276, was isolated from a patient with diffuse panbronchiolitis (28). This strain was grown overnight in Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.). Bacteria in the post-log phase were harvested in sterile normal saline. Harvested bacterial cells were sonicated 10 times with an ultrasonifier (Cell Disruptor 185; Branson Ultrasonics Co., Danbury, Conn.) with 1-min intervals. The sonicated supernatant of P. aeruginosa was obtained following ultracentrifugation at 18,000 × g for 60 min at 4°C and filtration through a 0.45-μm-pore-size filter. The PNR was purified as previously described (27). The purified PNR was stored at −80°C until use.
Cell culture and transfection.
A549 cells (Riken Cell Bank, Tsukuba Science City, Japan), a tumor cell line with the properties of alveolar epithelial cells (14), were cultured at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated fetal calf serum (GIBCO-BRL, Grand Island, N.Y.), penicillin (50 U/ml), and streptomycin (50 μg/ml). The cells were subcultured twice weekly.
DNA constructs.
Plasmids containing serial deletions of the 5′-flanking region of the IL-8 gene linked to luciferase expression vectors were constructed from a firefly luciferase expression vector (29). Site-directed mutagenesis of the IL-8 AP-1, NF-IL-6, and NF-κB sites in the −133-luc plasmid was introduced, converting the AP-1 site TGACTCA (−126 to −120 bp) to TatCTCA, the NF-IL-6 site CAGTTGCAAATCGT (−94 to −81 bp) to agcTTGCAAATCGT, and the κB site GGAATTTCCT (−80 to −71 bp) to taAcTTTCCT (sites of mutation are indicated in lowercase). These constructs were designated as AP-1 site-mutated, NF-IL-6 site-mutated, and κB site-mutated plasmids, respectively. Two copies of the IL-8 AP-1 binding site (TGACTCA), three copies of the NF-IL-6 site (CAGTTGCAAATCGTG), or three copies of the IL-8 κB site (GGAATTTCCT) were inserted upstream of the IL-8 enhancerless core promoter (−50 to +44 bp) linked to the firefly luciferase gene (−50-luc plasmid).
Transient-transfection and luciferase assay.
A549 cells were plated at a density of 3 × 105 cells/60-mm tissue culture plate 16 h before transfection. Transfection of plasmid DNA into A549 cells was done by the calcium phosphate coprecipitation method (21). In brief, each luciferase reporter construct (2 μg) and a cytomegalovirus promoter-Renilla luciferase expression plasmid (0.1 μg; pRL-CMV, as an internal control) were placed in a sterile microcentrifuge tube, and 62 μl of 2 M CaCl2 was slowly added by drops. Then, 2× HEBS solution (50 mM HEPES [pH 7.05], 1.5 mM Na2HPO4, 10 mM KCl, 280 mM NaCl, and 12 mM glucose) was added. The mixture was kept still at room temperature for 20 min. One milliliter of the above solution was added per plate, and cells were incubated at 37°C. After a 4-h incubation, the cells were exposed to a 15% glycerol–1× HEBS solution for 3 min and then washed twice with serum-free DMEM. Fresh medium was applied, and cells were incubated at 37°C. After 24 h, the transfected A549 cells were incubated in serum-free DMEM in the absence or the presence of PNR (5 μg/ml) and incubated at 37°C. To measure reporter gene expression, cells were harvested 6 h later and resuspended in 250 μl of lysis buffer. The cells were then lysed by freeze-thaw and centrifuged, and 20 μl of supernatant was evaluated for luciferase activity with a Lumat model LB9505C luminometer (Berthold, Bad Wildbad, Germany). Levels of luciferase expression were normalized to Renilla luciferase activity. Each experiment was performed three times.
Nuclear extract preparation.
Nuclear extracts from A549 cells were prepared as previously described (1). After the treatment indicated, cells were scraped and washed in phosphate-buffered saline and then resuspended in 200 μl of cold buffer A (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM dithiothreitol [DTT], and 2 mM aminoethyl benzenesulfonyl fluoride). After the cells were allowed to swell at 4°C for 10 min, they were lysed in the presence of Nonidet P-40 (final concentration, 0.8%). The homogenate was centrifuged for 5 min at 400 × g, and the nuclear pellet was resuspended in 75 μl of cold buffer C (20 mM HEPES [pH 7.9], 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 2 mM aminoethyl benzenesulfonyl fluoride, 33 μg of aprotinin per ml, 10 μg of leupeptin per ml, 10 μg of pepstatin per ml, and 10 μg of E-64 per ml). This suspension was agitated at 4°C for 30 min, followed by microcentrifugation for 15 min at 4°C. The resulting supernatant was stored in small aliquots at −80°C. Protein concentrations were determined by the Bradford method (Bio-Rad protein assay) with bovine serum albumin as a standard.
Electrophoretic mobility shift assay (EMSA).
EMSAs were performed as previously described (20). Binding sites for double-stranded oligonucleotides, κB (5′-CGTGGAATTTCCTCTG-3′, −83 to −68 bp) and AP-1 (5′-GTGATGACTCAGGTT-3′, −130 to −116 bp), from the IL-8 promoter were end labeled with [α-32P]dCTP and [α-32P]dATP with the large-fragment DNA polymerase (Klenow) (GIBCO-BRL) and were used as probes. The binding reactions were performed at room temperature, and the reaction mixtures contained 20 μl of binding buffer (10 mM Tris-HCl [pH 7.5], 50 mM NaCl, 1 mM EDTA, 5% glycerol, and 1 mM DTT), 5 μg of nuclear proteins, and 1 μg of poly(dI-dC) (Pharmacia, Piscataway, N.J.). After 15 min at room temperature, 50,000 cpm of 32P-labeled DNA probe was added to the reaction mixture for 15 min at room temperature. The DNA-protein complexes were separated on native 4% polyacrylamide gels (prerun at 150 V for 30 min) in 0.25× TBE buffer (22.3 mM Tris-HCl [pH 7.5], 22.2 mM boric acid, and 0.5 mM EDTA) at 150 V for 2 h at 4°C. After electrophoresis was performed, the gels were dried and exposed for autoradiography. In competition studies, a 25-fold excess of unlabeled wild-type oligonucleotides, oligonucleotides corresponding to the κB element from IL-2 receptor α (5′-CGGCAGGGGAATCTCCCTCTC-3′), oligonucleotides containing the consensus sequence for the AP-1 DNA binding site (5′-CGCTTGATGAGTCAGCCGGAA-3′), or oligonucleotides containing mutant NF-κB (5′-CGTtaAcTTTCCTCTG-3′) or AP-1 (5′-GTGATatCTCAGGTT-3′) binding sites (sites of mutation are indicated in lowercase) was included in the reaction mixture along with the radiolabeled probe. For supershift experiments, affinity-purified rabbit antibodies (2 μg/reaction) to p50, p65, c-Rel, p52, c-Fos, FosB, Fra-1, Fra-2, c-Jun, JunB, or JunD (Santa Cruz Biotechnology, Inc., Santa Cruz, Calif.) were incubated in the standard reaction mixture and incubated at room temperature for 45 min before the labeled oligonucleotides were added.
Western blot analysis.
The antibody used in these experiments was a rabbit polyclonal antibody to IκBα, C-21 (Santa Cruz Biotechnology, Inc.). Cells were retrieved by scraping, washed, and lysed by incubation in radioimmunoprecipitation assay buffer (0.5% sodium deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate, 66 μg of aprotinin per ml, 100 μg of phenylmethylsulfonyl fluoride per ml, and 1 mM sodium orthovanadate) for 30 min at 4°C. Equal amounts (50 μg) of protein from cell lysates were electrophoresed on sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis gels and transferred to polyvinylidene difluoride membranes. The membranes were washed with Tris-buffered saline–Tween (0.05%) with 3% nonfat dried milk overnight at 4°C and then blotted for 45 min with the antibody. The membranes were washed with Tris-buffered saline–Tween and incubated with a 1:5,000 dilution of horseradish peroxidase-conjugated anti-rabbit immunoglobulin (Amersham, Arlington Heights, Ill.). Thereafter, membranes were developed with enhanced chemiluminescence reagents (Amersham) and exposed to film.
RNA extraction and reverse transcriptase PCR.
Total RNA was isolated with Trizol reagent (GIBCO-BRL) according to the manufacturer’s recommendations and converted to single-strand cDNA with an oligo(dT) primer and reverse transcriptase under reaction conditions as described previously (27). PCR was performed on the cDNA after the addition of Taq DNA polymerase, deoxynucleoside triphosphate, and each primer with a DNA thermal cycler (Perkin-Elmer, Branchburg, N.J.) as previously described (27). An aliquot of each reaction mixture was then analyzed by electrophoresis on a 1% agarose gel, and the amplified DNA band was visualized by ethidium bromide staining. The amplification products of human IL-8 and human glyceraldehyde triphosphate dehydrogenase (GAPDH) were 983 and 750 bp, respectively.
Northern blot analysis.
Equal amounts of RNA (20 μg per lane) were loaded and run on a 1% agarose–formaldehyde gel. RNA was then transferred to a nylon membrane and hybridized to a cDNA probe encoding human IκBα, a kind gift of Dean W. Ballard (Vanderbilt University School of Medicine, Nashville, Tenn.). A cDNA probe for human GAPDH was used to confirm equal loading of RNA in all the wells. All probes were labeled with [α-32P]dCTP with a random primer labeling kit (Amersham).
Statistical analysis.
Statistical analysis of results was performed with Student’s t test.
RESULTS
Effect of PNR on IL-8 mRNA.
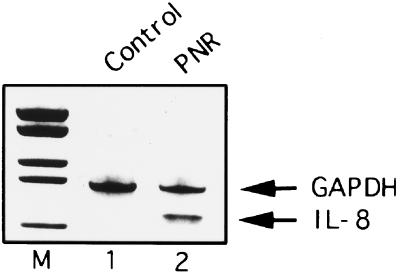
Initial studies were performed to examine IL-8 transcription following PNR exposure. Reverse transcriptase PCR analyses demonstrated that A549 cells did not express IL-8 mRNA transcripts constitutively but expressed them markedly after exposure to PNR (Fig. 1). Control GAPDH mRNA transcripts were observed in similar amounts in both unstimulated cells and cells exposed to PNR.
FIG. 1.
The effect of PNR on IL-8 gene expression in A549 bronchial epithelial cells. The IL-8 mRNA expression induced by PNR (5 μg/ml) for 3 h is shown. M, molecular mass markers.
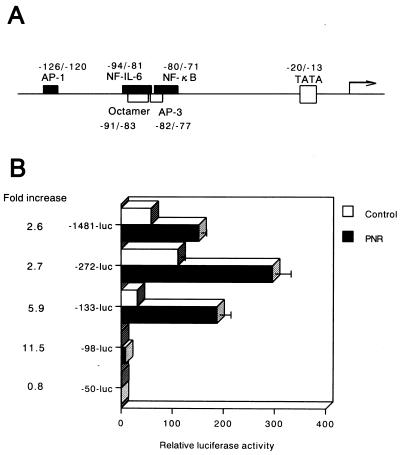
A sequence spanning positions −98 to −50 of the IL-8 gene promoter is sufficient to mediate activation by PNR.
To delineate the mechanism by which PNR induces IL-8 gene transcription, PNR-responsive promoter elements in the IL-8 promoter were identified in this study. This was done by transfecting A549 cells with plasmid constructs containing the luciferase reporter gene driven by the IL-8 promoter (Fig. 2A). Twenty-four hours posttransfection, cells were stimulated with PNR. The full-length promoter was reproducibly activated by PNR. These results indicate that PNR induces IL-8 expression in A549 cells at the level of transcription. Deletion of sequences upstream of position −98 decreased the constitutive promoter activity but had little effect on PNR inducibility (Fig. 2B). In contrast, deletion of sequences upstream of −50 abolished inducibility by PNR. This maps the region from −98 to −50 bp as a PNR-responsive region which is likely to contain individual PNR-responsive regulatory elements.
FIG. 2.
Sequences within −98 to −50 bp of the IL-8 promoter are necessary for activation by PNR. (A) Schematic representation of the 5′-flanking region of the IL-8 gene, demonstrating locations of several known binding sites for transcription factors. (B) Effect of PNR on IL-8 gene expression in A549 epithelium. A549 cells were transfected with plasmids containing serial deletions in the 5′-flanking region of the IL-8 gene. After 24 h, cells were incubated for 6 h with either medium alone (control) or PNR (5 μg/ml). Relative luciferase activity was calculated from the luciferase activity of each reporter plasmid relative to the value of −50-luc, incubated with medium alone and assigned a value of 1. Data are also expressed as fold increases in luciferase activity in PNR-exposed cells over that in control cells. These results represent the averages of three independent experiments. Error bars indicate standard errors. The mean fold increase relative to the untreated medium control was significantly lower in −50-luc than in the other reporter plasmids (P < 0.01).
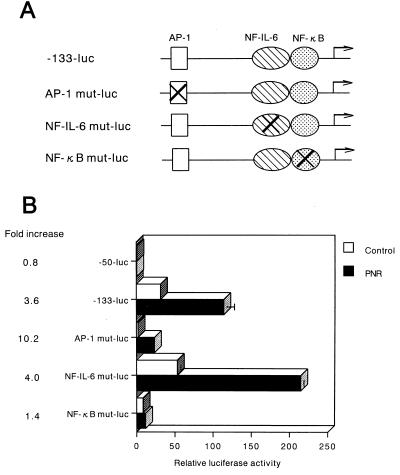
Previous studies have shown that the AP-1 or the NF-IL-6 site acts cooperatively with NF-κB to induce IL-8 gene transcription (11, 18, 22). To identify the cis-acting elements in the −133 to −50 bp region of the IL-8 promoter which served as a PNR-responsive regulatory element, site-directed mutant constructs were prepared and tested (Fig. 3A). The results shown in Fig. 3B indicate that mutation in the NF-κB site (NF-κB mut-luc) resulted in a significant reduction of IL-8 inducibility by PNR. Mutation of the AP-1 site (AP-1 mut-luc) decreased basal activity but had no effect on PNR-induced luciferase activity. However, mutation of the NF-IL-6 site (NF-IL-6 mut-luc) resulted in no decrease in either the basal activity or the activity inducible by PNR. These results indicate that activation of the IL-8 promoter in A549 epithelial cells in response to PNR stimulation requires an intact binding site for the NF-κB element.
FIG. 3.
The relative importance of the AP-1, NF-IL-6, and NF-κB binding sites in the IL-8 promoter. (A) The constructs used are diagrammed. The AP-1 site, NF-IL-6 site, or κB site in the IL-8 promoter (−133 to +44 bp), linked to the luciferase gene, was mutated. (B) The wild-type and mutated plasmids were transfected into A549 cells. The transfected cells were incubated without PNR (open bars) or with PNR (5 μg/ml) (solid bars). After a 6-h incubation, the luciferase activity was determined. Relative luciferase activity was calculated from the luciferase activity of each reporter plasmid relative to the value of −50-luc, incubated with medium alone and assigned a value of 1. Mean fold increase relative to the untreated medium control was significantly lower in NF-κB mut-luc than in −133-luc (P < 0.01).
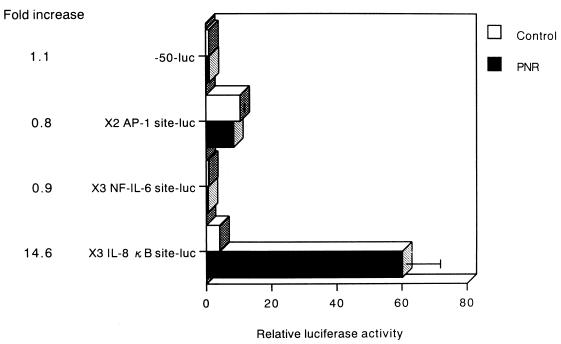
The NF-κB site of the IL-8 gene is sufficient to mediate PNR-induced gene activation.
The IL-8 gene fragment spanning positions −133 to −50 bp contains three prominent DNA-protein interaction sites for the transcription factors AP-1, NF-IL-6, and NF-κB. To further determine which sites are sufficient for activation by PNR, several constructs were used (Fig. 4). Each construct contained three tandemly repeated copies of one of the following elements: the NF-κB or NF-IL-6 site from the IL-8 gene or two copies of the AP-1 binding site from the IL-8 gene. Each was linked to the minimal IL-8 promoter spanning −50 to +44 bp and to the luciferase reporter gene.
FIG. 4.
PNR-induced IL-8 gene expression is specific for the κB region of the IL-8 gene. PNR induces luciferase expression in A549 cells transfected with ×3 IL-8 κB site-luc. Relative luciferase activity was calculated from the luciferase activity of each reporter plasmid relative to the value of −50-luc, incubated with medium alone and assigned a value of 1. Mean fold increase relative to the untreated medium control was significantly higher in ×3 IL-8 κB site-luc than in −50-luc (P < 0.01).
The construct ×3 IL-8 κB site-luc, which contains three tandem repeats of the NF-κB site from the IL-8 gene, generated a 14.6-fold stimulation by PNR. In contrast, constructs ×2 AP-1 site-luc and ×3 NF-IL-6 site-luc did not exhibit PNR inducibility in A549 cells (Fig. 4). These results demonstrate that the NF-κB site of the IL-8 gene is important for activation of PNR-induced gene expression. In contrast, the AP-1 and NF-IL-6 sites, essential for IL-8 induction by various stimuli in other cell types, are not involved in this form of PNR responsiveness.
Stimulation with PNR induces a single predominant κB binding complex, while AP-1 is constitutively expressed in A549 cells.
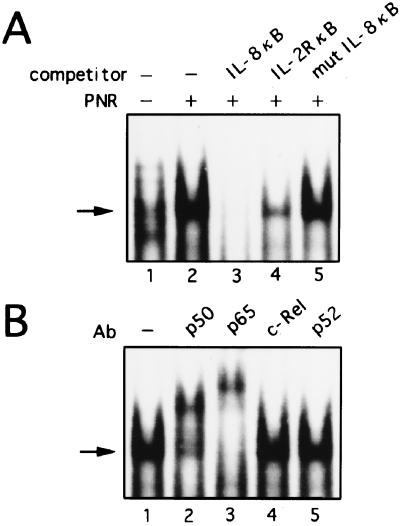
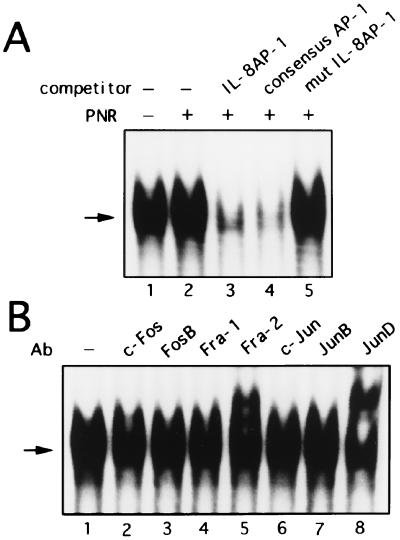
To investigate the trans-acting factors that are involved in the activation of IL-8 by PNR, we performed EMSA with a probe spanning positions −83 to −68 bp of the IL-8 promoter, which includes the NF-κB binding site at −80 to −71 bp (Fig. 5A). PNR treatment for 3 h induced a single predominant κB binding complex (Fig. 5A, lanes 1 and 2). This binding was specifically competed by an excess of wild-type unlabeled NF-κB from the IL-8 gene or the IL-2 receptor α gene (Fig. 5A, lanes 3 and 4) but not by an oligonucleotide containing point mutations which abolished NF-κB binding (Fig. 5A, lane 5). To further characterize the κB complex, supershift experiments using specific antisera were performed. An antibody against NF-κB p50 or p65 specifically shifted and diminished the formation of complex (Fig. 5B, lanes 2 and 3), while an antibody against c-Rel or p52 had no effect (Fig. 5B, lanes 4 and 5). These data suggest that this DNA-protein complex represents a p50-p65 heterodimer product. In contrast, AP-1 binding was constitutively present in nuclei from unstimulated cultures, appearing as a single band on EMSA (Fig. 6A, lane 1). Nuclear extracts from PNR-treated A549 cells incubated with the AP-1 probe showed no increase in binding over unstimulated extracts (Fig. 6A, lane 2). The complex was specifically competed by the IL-8 AP-1 or consensus AP-1 probe but not by the mutant AP-1 fragment (Fig. 6A, lanes 3 to 5). To determine if the complex contains AP-1, antibodies were used in a supershift assay. Anti-Fra-2 and anti-JunD antibodies reduced the complex and resulted in supershifted bands (Fig. 6B, lanes 5 and 8). Taken together, the cold competition and supershift assays indicate that PNR-induced proteins bind the NF-κB site but not the AP-1 site, which is in agreement with the functional data shown in Fig. 2 to 4.
FIG. 5.
Induction of specific NF-κB complex formation by exposure of A549 cells to PNR. (A) Characterization of nuclear proteins bound to the NF-κB binding site of the IL-8 gene. Nuclear proteins were extracted from A549 cells cultured for 3 h without (lane 1) or with (lanes 2 to 5) PNR. EMSA was performed on nuclear extracts preincubated with no reagents (lanes 1 and 2) or the indicated competitors (lanes 3 to 5), and the NF-κB binding site of the IL-8 gene was used as a labeled probe. (B) Further characterization of nuclear proteins bound to the κB binding site of the IL-8 gene. Nuclear proteins were extracted from A549 cells that were cultured with PNR for 3 h. EMSA was performed on nuclear proteins preincubated with no reagents (lane 1) or the indicated antisera (lanes 2 to 5), and the κB binding site of the IL-8 gene was used as a labeled probe. Ab, antibody. Arrows indicate the specific complex.
FIG. 6.
The AP-1 binding complex is constitutively expressed in A549 cells. (A) Nuclear extracts containing equal protein concentrations from A549 cells unstimulated or stimulated with PNR were analyzed for AP-1 binding activity by EMSA. The IL-8 AP-1 probe was incubated with nuclear extracts from unstimulated cells (lane 1) or PNR-stimulated A549 cells alone (lanes 2 to 5), without (lane 2) or with (lanes 3 to 5) a 25-fold excess of the indicated competitors. (B) Anti-Fra-2 and anti-JunD sera recognize AP-1 binding complex in nuclear extracts from A549 cells. Nuclear extracts from A549 cells that were unstimulated were analyzed for AP-1 binding by EMSA. The AP-1 probe was incubated with nuclear extracts from A549 cells. The nuclear extracts were preincubated for 45 min with the indicated antibodies (Ab) (lanes 2 to 8). Arrows indicate the specific complex.
Activation of IκBα by PNR.
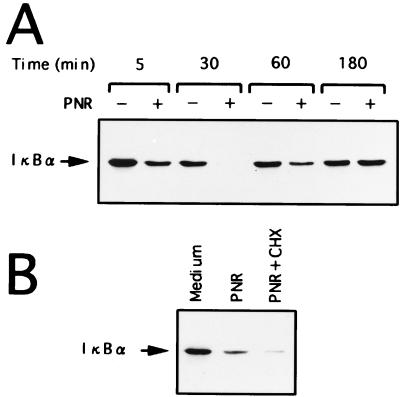
Since signal-induced proteolytic degradation of IκBα precedes the appearance of NF-κB DNA binding activity, we examined the conditions for activation of IκBα in A549 cells that were stimulated by PNR. Kinetic analysis of PNR-induced degradation of IκBα in A549 cells revealed gradual replacement of IκBα levels, complete restoration of IκBα levels to those in unstimulated A549 cells being achieved after 3 h of culture (Fig. 7A). Immunoblots of whole-cell extracts of A549 cells that were cultured with PNR or medium for 1 h were prepared (Fig. 7B). Cycloheximide (50 μg/ml) was added to some dishes along with PNR. In PNR-stimulated A549 cells, loss of IκBα reactivity was observed at 1 h. Cycloheximide inhibited the partial recovery of IκBα levels in stimulated A549 cells, suggesting that accumulation of IκBα in A549 cells after activation depends on new protein synthesis.
FIG. 7.
Induction of IκBα in PNR-stimulated A549 cells. (A) Kinetic analysis of IκBα activity in A549 cells cultured without (−) or with (+) (5 μg/ml) PNR for the indicated time. Degradation of IκBα is seen as early as 5 min after stimulation of A549 cells with PNR and is completed by 30 min. Restoration of IκBα levels in PNR-stimulated A549 cells to that in unstimulated A549 cells is seen after 3 h of culture. (B) A549 cells were cultured with PNR (5 μg/ml) for 1 h in the presence or the absence of cycloheximide (CHX; 50 μg/ml).
Induction of IκBα mRNA expression in A549 cells by PNR.
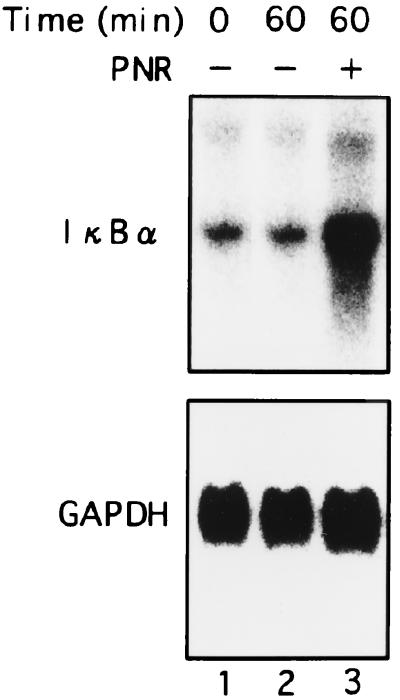
It is possible that PNR suppressed the transcription of the IκBα gene. To examine this possibility, we carried out Northern blot analysis of IκBα mRNA expression in A549 cells stimulated with PNR. A549 cells were cultured with PNR or medium, and total RNA was extracted at 1 h of culture. Induction of IκBα mRNA by PNR was observed (Fig. 8). It was reported previously that transcription of the IκBα gene is regulated by the NF-κB binding site in the promoter and is activated by NF-κB p65 or c-Rel (12). These results clearly indicate that the decrease of IκBα protein induced by PNR did not result from suppression of transcription but from modulation of posttranscriptional events, probably at the protein level. Cumulatively, these results indicate that PNR activates A549 cells, as reflected by a loss of IκBα protein and up-regulation of its mRNA.
FIG. 8.
Induction of IκBα mRNA in A549 cells by PNR. A549 cells were cultured with PNR (5 μg/ml, lane 3) or medium alone (lane 2) for 1 h. Total RNA was assessed for IκBα expression. Equal loading was assessed by hybridization of a stripped blot with a probe for GAPDH.
DISCUSSION
P. aeruginosa affects neutrophil recruitment into the airways by direct and indirect mechanisms. Among the chemotactic factors for neutrophils produced by the airways, IL-8 is one of the most potent. Because epithelial cells are sources of IL-8 in the airways, we and others hypothesized that P. aeruginosa may affect neutrophil migration by stimulating the production of IL-8 in airway epithelial cells. This hypothesis provides an important model to elucidate the mechanism by which P. aeruginosa infection increases IL-8 levels and causes dense neutrophil infiltration in the airways of patients with CAD. Previous studies have shown that a nonprotein factor of less than 1 kDa in culture supernatant of P. aeruginosa stimulates IL-8 expression and production by bronchial epithelial cells (16). Pilin-mediated adherence of P. aeruginosa as well as Pseudomonas autoinducer was reported to be a potent stimulus for IL-8 production by bronchial epithelial cells (5). Through the analysis of an inducer among Pseudomonas products for IL-8 production in bronchial epithelial cells, we have recently reported the nitrite reductase from P. aeruginosa to be a potent IL-8 inducer in respiratory cells (27). The PNR, with a molecular mass of 60,204 Da, is recognized as a periplasmic component active in energy generation (35). This enzyme oxidizes reduced Pseudomonas cytochrome c551 under aerobic conditions and also reduces nitrite to nitric oxide by using reduced Pseudomonas cytochrome c551 under anaerobic conditions (38, 39). It seems that the purified PNR did not stimulate epithelial cells to produce IL-8 by released nitric oxide through its enzymatic reaction, because in vitro cultures were under aerobic conditions without electron donors such as Pseudomonas cytochrome c551 (38). Our previous studies with reduced Pseudomonas cytochrome c551 under aerobic conditions showed loss of potent cytochrome oxidase activity in the purified PNR (27). Indeed, the purified PNR did not stimulate nitric oxide production under the culture conditions (data not shown). Therefore, the enzymatic activity of PNR is not essential for the IL-8-inducing activity of PNR, and a direct stimulation of bronchial epithelial cells by the PNR is a possible mechanism for IL-8 gene induction (27).
We have recently reported that PNR could be released from P. aeruginosa after exposure to antimicrobial agents or complement and that released PNR is active in inducing IL-8 production by bronchial epithelial cells (33). Moreover, we found increased levels of immunoglobulin G and immunoglobulin A antibodies against PNR in sera from patients with CAD and P. aeruginosa infections. However, hyperimmune sera from these patients did not neutralize PNR-mediated IL-8 induction in bronchial epithelial cells (26).
This study was done to define the sites in the IL-8 promoter that are required for PNR induction of IL-8 gene expression in airway epithelium. Optimal PNR-induced IL-8 gene activation required an NF-κB binding site in the 5′-flanking region of the gene. In addition, PNR incubation directly results in activation of the transcription factor that binds to this site, NF-κB.
We initially demonstrated, by use of luciferase constructs with serial deletions in the IL-8 promoter, that the region between −1481 and −98 bp in the 5′-flanking region of the IL-8 gene is not essential for PNR-induced IL-8 gene expression. Further deletions up to −50 bp completely abolished the response of the promoter to PNR. This suggests that the region between −98 and −50 bp contains elements that are essential for PNR induction of IL-8 gene activation. Within this segment of the 5′-flanking region of the IL-8 gene, there are several potential binding sites for transcription factors, including NF-κB and NF-IL-6 (Fig. 2A).
Several prior studies, using various stimuli and cell lines, demonstrated that NF-κB and NF-IL-6 play a vital role in the regulation of IL-8 gene expression. The studies also suggested that each factor may possess a variable degree of importance, depending on the cell line and the stimulus used (22), and that induction of the IL-8 gene by TNF-α, IL-1, phorbol myristate acetate, and hepatitis B virus protein X requires the cooperative effect of NF-κB and NF-IL-6 (15, 23). On the other hand, IL-8 gene activation by TNF-α, gamma interferon, cytomegalovirus, and paclitaxel appears to be mediated by the AP-1 site in conjunction with the NF-κB site in a human gastric cancer cell line (40), a monocytic cell line (24), and a human ovarian carcinoma cell line (13). In contrast to previous reports, we now report that PNR induction of IL-8 is independent of intact NF-IL-6 and AP-1 sites. These results suggest that different sets of nuclear transcription factors may be responsible for the regulation of IL-8 gene transcription in a cell type- and stimulus-specific manner. Interestingly, AP-1 was the preferred transcription factor (over NF-IL-6) for cooperative interaction with NF-κB for IL-8 gene expression in respiratory syncytial virus-infected A549 cells (17). However, our present results demonstrate that the activation of NF-κB, but not that of NF-IL-6 and AP-1, was indispensable for PNR-induced IL-8 gene transcription, suggesting that the molecular mechanism involved in IL-8 gene transcription differed, even within the same cell, depending on the employed stimuli.
To complement these observations, we also determined if PNR could induce the degradation of IκBα in A549 epithelial cells. Similar to other transient stimuli, PNR induced the degradation of IκBα in A549 cells. Degradation of IκBα in PNR-induced A549 cells was associated with translocation of NF-κB and increased steady-state transcription of IκBα mRNA. Further, the levels of IκBα protein in PNR-induced A549 cells were restored to a level similar to that of unstimulated A549 cells after 3 h of incubation. Restoration of IκBα in PNR-stimulated A549 cells was sensitive to the effect of cycloheximide, indicating a dependency on new protein synthesis.
Infections with P. aeruginosa trigger dense neutrophil infiltrations in the airways of patients with CAD (28). PNR-induced NF-κB activation and the subsequent up-regulation of IL-8 could contribute to inflammatory cell recruitment. Because of its pivotal role in inflammation, NF-κB could be an obvious target for new types of anti-inflammatory treatments for CAD. Blocking the activation of NF-κB may prevent the early events in the inflammatory cascade, decreasing P. aeruginosa-induced inflammation.
In summary, we have shown that PNR regulates IL-8 gene expression at the transcriptional level, acting through the 5′-flanking region of the IL-8 gene. The transcriptional factor NF-κB is necessary for PNR-induced IL-8 up-regulation, indicating one molecular mechanism of P. aeruginosa-induced airway inflammation.
ACKNOWLEDGMENTS
We thank Dean W. Ballard for providing the cDNA probe encoding human IκBα. We gratefully acknowledge Mika Yamamoto and Masako Sasaki for excellent technical assistance and Bent W. Nielsen for his helpful comments during the preparation of the manuscript.
This work was supported by a Grant-in-Aid for Encouragement of Young Scientists from the Ministry of Education, Science, Sports and Culture of Japan.
REFERENCES
- 1.Antalis T M, Godbolt D. Isolation nuclei from hematopoietic cell types. Nucleic Acids Res. 1991;19:4301. doi: 10.1093/nar/19.15.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle P A, Baltimore D. IκB: a specific inhibitor of the NF-κB transcriptional factor. Science. 1988;242:540–546. doi: 10.1126/science.3140380. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle P A, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 4.Carre P C, Mortenson R L, King T E, Jr, Noble P W, Sable C L, Riches D W H. Increased expression of the interleukin-8 gene by alveolar macrophages in idiopathic pulmonary fibrosis: a potential mechanism for the recruitment and activation of neutrophils in lung fibrosis. J Clin Investig. 1991;88:1802–1810. doi: 10.1172/JCI115501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiMango E, Zar H J, Bryan R, Prince A. Diverse Pseudomonas aeruginosa gene products stimulate respiratory epithelial cells to produce interleukin-8. J Clin Investig. 1995;96:2204–2210. doi: 10.1172/JCI118275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fick R B., Jr Pathogenesis of the Pseudomonas lung infection in cystic fibrosis. Clinical implication of basic research. Chest. 1989;96:158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- 7.Hoiby N. Pseudomonas aeruginosa infection in cystic fibrosis: diagnostic and prognostic significance of Pseudomonas aeruginosa precipitins determined by means of crossed immunoelectrophoresis. Acta Pathol Microbiol Scand. 1977;262:1–96. [PubMed] [Google Scholar]
- 8.Inoue H, Massion P P, Ueki I F, Grattan K M, Hara M, Dohrman A F, Chan B, Lausier J A, Golden J A, Nadel J A. Pseudomonas stimulates interleukin-8 mRNA expression selectively in airway epithelium, in gland ducts, and in recruited neutrophils. Am J Respir Cell Mol Biol. 1994;11:651–653. doi: 10.1165/ajrcmb.11.6.7946394. [DOI] [PubMed] [Google Scholar]
- 9.Kadota J, Sakito O, Khono S, Sawa H, Mukae H, Oda H, Kawakami K, Fukushima K, Hiratani K, Hara K. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1993;147:153–159. doi: 10.1164/ajrccm/147.1.153. [DOI] [PubMed] [Google Scholar]
- 10.Kunsch C, Rosen C A. NF-κB subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunsch C, Lang R K, Rosen C A, Shannon M F. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- 12.Le Bail O, Schmidt-Ullrich R, Israel A. Promoter analysis of the gene encoding the IκB-α/MAD3 inhibitor of NF-κB: positive regulation by members of the rel/NF-κB family. EMBO J. 1993;12:5043–5049. doi: 10.1002/j.1460-2075.1993.tb06197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee L-F, Haskill J S, Mukaida N, Matsushima K, Ting J P-Y. Identification of tumor-specific paclitaxel (Taxol)-responsive regulatory elements in the interleukin-8 promoter. Mol Cell Biol. 1997;17:5097–5105. doi: 10.1128/mcb.17.9.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G. A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer. 1976;17:62–70. doi: 10.1002/ijc.2910170110. [DOI] [PubMed] [Google Scholar]
- 15.Mahe Y, Mukaida N, Kuno K, Akiyama M, Ikeda N, Matsushima K, Murakami S. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor κB and CCAAT/enhancer-binding protein-like cis-elements. J Biol Chem. 1991;266:13759–13763. [PubMed] [Google Scholar]
- 16.Massion P P, Inoue H, Richman-Eisenstat J, Grunberger D, Jorens P G, Housset B, Pittet J-F, Wiener-Kronish J P, Nadel J A. Novel Pseudomonas product stimulates interleukin-8 production in airway epithelial cells in vitro. J Clin Investig. 1994;93:26–32. doi: 10.1172/JCI116954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mastronarde J G, Monick M M, Mukaida N, Matsushima K, Hunninghake G W. Activator protein-1 is the preferred transcription factor for cooperative interaction with nuclear factor-κB in respiratory syncytial virus-induced interleukin-8 gene expression in airway epithelium. J Infect Dis. 1998;177:1275–1281. doi: 10.1086/515279. [DOI] [PubMed] [Google Scholar]
- 18.Matsusaka T, Fujikawa K, Nishio Y, Mukaida N, Matsushima K, Kishimoto T, Akira S. Transcription factors NF-IL6 and NF-κB synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc Natl Acad Sci USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElvaney N G, Nakamura H, Birrer P, Hebert C A, Wong W L, Alphonso M, Baker J B, Catalano M A, Crystal R G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Investig. 1992;90:1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori N, Prager D. Transactivation of the interleukin-1α promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood. 1995;87:3410–3417. [PubMed] [Google Scholar]
- 21.Mori N, Murakami S, Oda S, Prager D, Eto S. Production of interleukin 8 in adult T-cell leukemia cells: possible transactivation of the interleukin 8 gene by human T-cell leukemia virus type I tax. Cancer Res. 1995;55:3592–3597. [PubMed] [Google Scholar]
- 22.Mukaida N, Okamoto S, Ishikawa Y, Matsushima K. Molecular mechanism of interleukin-8 gene expression. J Leukoc Biol. 1994;56:554–558. [PubMed] [Google Scholar]
- 23.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor-κB- and cis-regulatory enhancer binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990;265:21128–21132. [PubMed] [Google Scholar]
- 24.Murayama T, Ohara Y, Obuchi M, Khabar K S A, Higashi H, Mukaida N, Matsushima K. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1 and NF-κB-binding sites of the interleukin-8 gene. J Virol. 1997;71:5692–5695. doi: 10.1128/jvi.71.7.5692-5695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakamura H, Yoshimura K, Jaffe H A, Crystal R G. Interleukin-8 gene expression in human bronchial epithelial cells. J Biol Chem. 1991;266:19611–19617. [PubMed] [Google Scholar]
- 26.Oishi, K. Unpublished data.
- 27.Oishi K, Sar B, Wada A, Hidaka Y, Matsumoto S, Amano H, Sonoda F, Kobayashi S, Hirayama T, Nagatake T, Matsushima K. Nitrite reductase from Pseudomonas aeruginosa induces inflammatory cytokines in cultured respiratory cells. Infect Immun. 1997;65:2648–2655. doi: 10.1128/iai.65.7.2648-2655.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oishi K, Sonoda F, Kobayashi S, Iwagaki A, Nagatake T, Matsushima K, Matsumoto K. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect Immun. 1994;62:4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okamoto S, Mukaida N, Yasumoto K, Rice N, Ishikawa Y, Horiguchi H, Murakami S, Matsushima K. The interleukin-8 AP-1 and κB-like sites are genetic end targets of FK506-sensitive pathway accompanied by calcium mobilization. J Biol Chem. 1994;269:8582–8589. [PubMed] [Google Scholar]
- 30.Oliveira I C, Mukaida N, Matsushima K, Vilcek J. Transcriptional inhibition of the interleukin-8 gene by interferon is mediated by the NF-κB site. Mol Cell Biol. 1994;14:5300–5308. doi: 10.1128/mcb.14.8.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pennington J E, Reynolds H Y, Carbone P P. Pseudomonas pneumonia: a retrospective study of 36 cases. Am J Med. 1973;55:155–160. doi: 10.1016/0002-9343(73)90163-0. [DOI] [PubMed] [Google Scholar]
- 32.Rolfe M W, Kunkel S L, Standiford T J, Chensue S W, Allen R M, Evanoff H L, Phan S H, Strieter R M. Pulmonary fibroblast expression of interleukin-8: a model for alveolar macrophage-derived cytokine networking. Am J Respir Cell Mol Biol. 1991;5:493–501. doi: 10.1165/ajrcmb/5.5.493. [DOI] [PubMed] [Google Scholar]
- 33.Sar B, Oishi K, Wada A, Hirayama T, Matsushima K, Nagatake T. Nitrite reductase from Pseudomonas aeruginosa released by antimicrobial agents and complement induces interleukin-8 production in bronchial epithelial cells. Antimicrob Agents Chemother. 1999;43:794–801. doi: 10.1128/aac.43.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 35.Silvestrini M C, Galeotti C L, Gervais M, Schinina E, Barra D, Bossa F, Brunori M. Nitrite reductase from Pseudomonas aeruginosa: sequence of the gene and the protein. FEBS Lett. 1989;254:33–38. doi: 10.1016/0014-5793(89)81004-x. [DOI] [PubMed] [Google Scholar]
- 36.Takizawa H, Ohtoshi T, Kikutani T, Okazaki H, Akiyama N, Sato M, Shoji S, Ito K. Histamine activates bronchial epithelial cells to release inflammatory cytokines in vitro. Int Arch Allergy Immunol. 1995;108:260–267. doi: 10.1159/000237162. [DOI] [PubMed] [Google Scholar]
- 37.Tanimoto H. A review of the recent progress in treatment of patients with diffuse panbronchiolitis associated with Pseudomonas aeruginosa infection in Japan. Antibiot Chemother (Basel) 1991;44:94–98. doi: 10.1159/000420303. [DOI] [PubMed] [Google Scholar]
- 38.Yamanaka T, Okunuki K. Crystalline Pseudomonas cytochrome oxidase. I. Enzymatic properties with special reference to the biological specificity. Biochim Biophys Acta. 1963;67:379–393. doi: 10.1016/0006-3002(63)91844-4. [DOI] [PubMed] [Google Scholar]
- 39.Yamanaka T, Kijimoto S, Okunuki K. Biological significance of Pseudomonas cytochrome oxidase in Pseudomonas aeruginosa. J Biochem. 1963;53:416–421. doi: 10.1093/oxfordjournals.jbchem.a127716. [DOI] [PubMed] [Google Scholar]
- 40.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor α and interferon γ synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-κB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–22511. [PubMed] [Google Scholar]
- 41.Yoshimura T, Matsushima K, Oppenheim J J, Leonard E J. Neutrophil chemotactic factor produced by lipopolysaccharide (LPS)-stimulated human blood mononuclear leukocytes: partial characterization and separation from interleukin 1 (IL 1) J Immunol. 1987;139:788–793. [PubMed] [Google Scholar]