Abstract
Warfarin is the only approved anticoagulant after mechanical valve replacement, but it is a well described risk factor for calciphylaxis among patients with end-stage kidney disease. Our patient with end-stage kidney disease rapidly developed calciphylaxis after dual mechanical valve replacement in association with warfarin initiation, posing significant challenges in clinical management and a fatal outcome. (Level of Difficulty: Intermediate.)
Key Words: anticoagulation, disorders of calcium disease, thrombosis, valve replacement
Abbreviations and Acronyms: DOAC, direct oral anticoagulant; ESKD, end-stage kidney disease
Central Illustration
History of Presentation
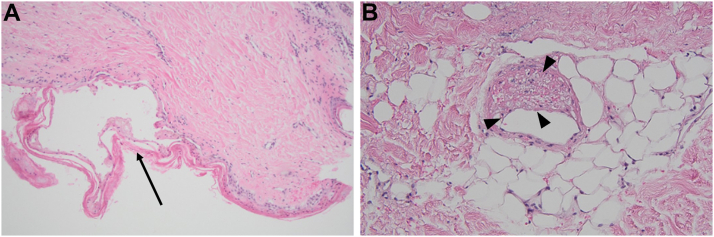
A 54-year-old man with end-stage kidney disease (ESKD) was hospitalized for elective dual valve replacement for severe symptomatic aortic and mitral valve stenosis. On presentation, vital signs were in normal ranges and physical examination revealed a late peaking ejection systolic murmur in the aortic area. The etiology of the dual valvular disease was thought to be accelerated calcification in the context of a milieu of secondary hyperparathyroidism (Figure 1). After a thorough discussion between cardiology and cardiothoracic surgery, and by shared decision making with the patient, dual mechanical (On-X) prostheses were placed in the aortic and mitral positions. His intraoperative course was smooth, and he was able to come off cardiopulmonary bypass uneventfully. The immediate postoperative course was unremarkable, unfractionated heparin infusion was started to bridge to warfarin, and he was transferred to the general floor from the intensive care unit. Ten days after the surgery and 3 days after starting warfarin at 2 mg daily while bridging with unfractionated heparin, he developed painful necrotic ulcers on bilateral lower extremities. His examination was notable for retiform purpura on the right hip and thigh with several necrotic eschars without hemorrhagic bullae on bilateral lower extremities (Figure 2).
Learning Objectives
-
•
To recognize calciphylaxis as a deadly complication in patients with ESKD.
-
•
To recognize and consider the role of warfarin in the development and progression of calciphylaxis among ESKD patients undergoing mechanical valve replacement.
-
•
To recognize the complex decision making inherent in the choice of valvular prosthesis among patients with ESKD.
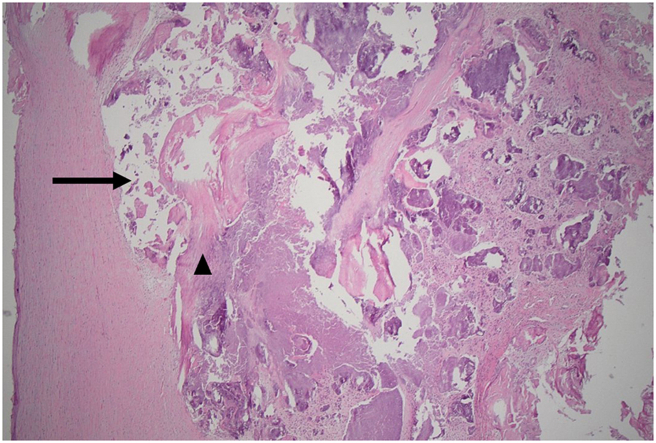
Figure 1.
Aortic Valve Tissue Histopathology
Valvular calcification (arrow) and myxoid degeneration (arrowhead).
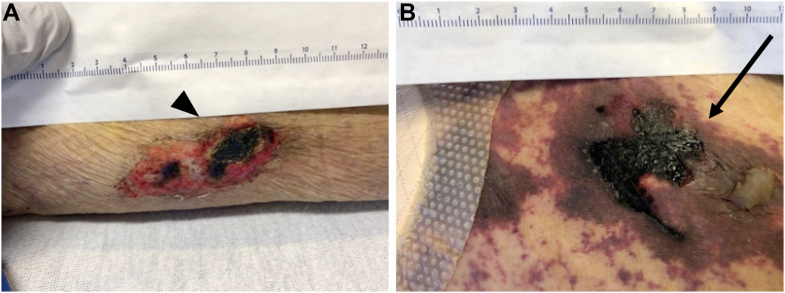
Figure 2.
Clinical Images of Ulcers
(A) Left lateral thigh necrotic ulcer (arrowhead). (B) Right anterior thigh with necrotic ulcer covered by black eschar (arrow).
Past Medical History
The patient had a history of ESKD from focal segmental glomerulosclerosis and had been receiving renal replacement therapy for 8 years, presenting on peritoneal dialysis. He also had a history of esophageal cancer in remission, severe calcific aortic valve stenosis and severe mitral valve stenosis with annular calcification. He had no history of protein C or S deficiency.
Differential diagnosis
The likely differential diagnosis after development of ulcers included warfarin necrosis, vasculitis, endarteritis obliterans, and calciphylaxis.
Investigations
Transthoracic echocardiography of both aortic and mitral valves revealed severe stenosis before the surgery (Figure 3). The presurgical admission corrected calcium was 7.5 mg/dL (reference: 8.6-10.0 mg/dL) and phosphorus 7.5 mg/dL (reference: 2.5-4.5 mg/dL). Parathyroid hormone (PTH) 2 months earlier was measured at 3,134 pg/mL (reference: 16-65 pg/mL). On presentation, serum calcium and phosphorus were in target ranges, 9.0 mg/dL and 5.1 mg/dL respectively, and PTH was elevated at 609 pg/mL. Skin punch biopsies of the ulcers revealed epidermal necrosis and microthrombi within small vessels in the subcutis with no endothelial cell damage and no red cell extravasation (Figure 4). The same skin tissue was evaluated for antibodies and there was no evidence of immunoreactivity.
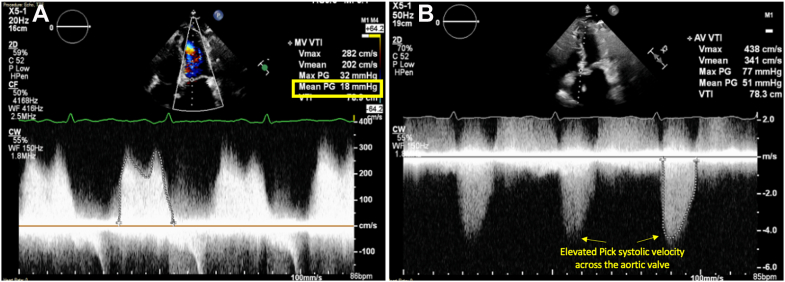
Figure 3.
Transthoracic Echocardiograms of Both Mitral and Aortic Valves Before Surgery
(A) Markedly elevated transmitral gradient of 18 mm Hg at heart rate of 87 beats/min (yellow rectangle), consistent with severe mitral stenosis. (B) Aortic valve Doppler signal consistent with severe aortic stenosis (yellow arrows).
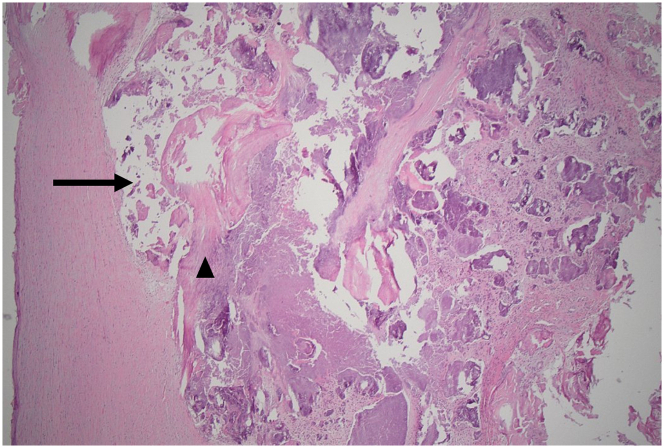
Figure 4.
Right Anterior Thigh Ulcer Punch Biopsies
(A) Epidermal necrosis (arrow) with (B) microthrombi within rare small vessels in the superficial subcutis (arrowheads).
Management
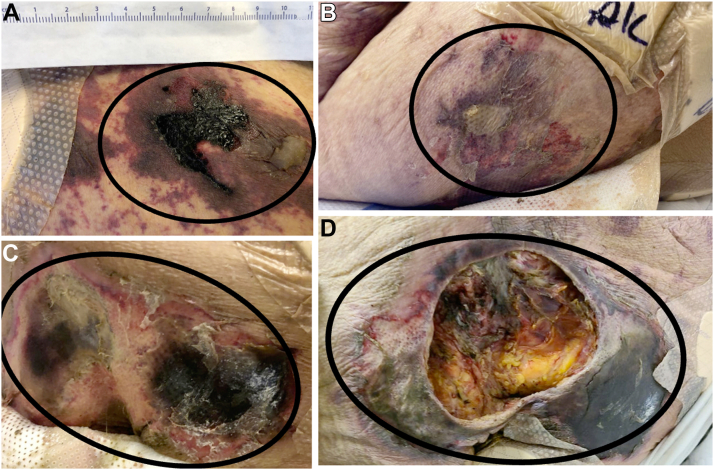
A clinical diagnosis of calciphylaxis was determined. Therapy with sodium thiosulfate, cinacalcet, and sevelamer was initiated, in addition to surgical debridement of eschars and wound care. Calcitriol, cholecalciferol, and calcium-based binders were discontinued. Despite discontinuation of warfarin and continuing unfractionated heparin infusions, eschars worsened, and wound infection rapidly progressed, leading to septicemia despite antibiotic therapy (Figure 5). The decision on a viable oral anticoagulant alternative to warfarin presented a significant management dilemma in the setting of a mechanical prosthesis. An interdisciplinary team, in consultation with the patient, recommended off-label use of apixaban as a long-term anticoagulation option as unfractionated heparin was not a long-term viable option and low-molecular-weight heparin was contraindicated owing to ESKD. However, the patient opted to move to comfort-based care.
Figure 5.
Progression and Worsening of Calciphylaxis Lesions, Days After Warfarin Initiation
(A) Day 3 of lesions, (B) day 10 of lesions, (C) day 12 of lesions, (D) day 22 of lesions (black circles).
Discussion
Warfarin is the recommended anticoagulant for antithrombotic therapy for mechanical prosthetic valves.1 It is also an established independent risk factor for calciphylaxis in patients with ESKD, a condition that is associated with increased risk of mortality.2 We have described a unique case of rapidly progressive calciphylaxis following initiation of warfarin after dual mechanical valve replacement in a patient with ESKD, conferring a considerable conundrum for antithrombotic therapy.
Calciphylaxis is associated with high mortality rates, particularly in patients with underlying cardiovascular disease and in association with the use of warfarin.2 Common risk factors associated with calciphylaxis include ESKD, hyperparathyroidism, mineral bone disease, and dialysis.3 Calciphylaxis has been associated with warfarin use, but is typically described in association with long-term use rather than a rapidly developing and catastrophic course as experienced by our patient.4 The development of this feared complication in association with the use of warfarin in the present case does not by any means determine causality, but there was certainly a strong temporal association with its use.
Warfarin is the only proven long-term oral anticoagulant in the context of mechanical prosthetic cardiac valves. There are currently no data to support the use of direct oral anticoagulants (DOACs) in this context. Dabigatran is the only DOAC studied in a randomized controlled trial among patients with a mechanical prosthesis and showed an increased risk of thromboembolic and bleeding complications compared with warfarin, without any offsetting benefits.5 Apixaban is currently being studied in the PROACT Xa (A Trial to Determine if Participants With an On-X Aortic Valve Can be Maintained Safely on Apixaban; NCT04142658) and has been successfully used in a reported case of a patient with double mechanical valves.6,7
This case exemplifies the clinical conundrum and dilemma inherent in the exceedingly difficult clinical choice of prosthetic valves in patients with ESKD.8 Owing to abnormalities of mineral metabolism associated with ESKD, bioprosthetic valves have the risk of rapid degeneration and structural failure in ESKD. On the other hand, mechanical valves necessitate the use of warfarin, and are associated with an increased risk of bleeding complications as well as the risk of calciphylaxis.
Follow-Up
The patient was transferred to home hospice, where he died 3 days later.
Conclusions
This case reveals some crucial clinical lessons. The choice of bioprosthetic vs mechanical valve in ESKD patients is extremely nuanced and necessitates careful consideration of risks vs benefits by the extended heart-kidney team in conjunction with the patient with the use of a shared decision-making format.8 Clinicians should be aware of the association of calciphylaxis with use of warfarin, which necessitates immediate discontinuation of warfarin given the associated high mortality risk. The medical community eagerly awaits the results of the PROACT Xa trial as well as future trials evaluating DOACs in patients with mechanical valves, particularly those with ESKD.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
This paper was presented as a poster at the American College of Cardiology's Annual Scientific Session & Expo 2022, April 2, 2022.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Otto C.M., Nishimura R.A., Bonow R.O., et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2021;77:e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 2.Santos P.W., He J., Tuffaha A., Wetmore J.B. Clinical characteristics and risk factors associated with mortality in calcific uremic arteriolopathy. Int Urol Nephrol. 2017;49(12):2247–2256. doi: 10.1007/s11255-017-1721-9. [DOI] [PubMed] [Google Scholar]
- 3.Nigwekar S.U., Zhao S., Wenger J., et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27(11):3421–3429. doi: 10.1681/ASN.2015091065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudwaleit M., Schwarz A., Trautmann C., Offermann G., Distler A. Severe calciphylaxis in a renal patient on long-term oral anticoagulant therapy. Am J Nephrol. 1996;16(4):344–348. doi: 10.1159/000169021. [DOI] [PubMed] [Google Scholar]
- 5.Eikelboom J.W., Connolly S.J., Brueckmann M., et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369(13):1206–1214. doi: 10.1056/NEJMoa1300615. [DOI] [PubMed] [Google Scholar]
- 6.Eom J.Y., Shin J.K., Kwon C.H. Apixaban use in an atrial fibrillation patient with double mechanical heart valves: a case report. Eur Heart J Case Rep. 2021;5(7):ytab285. doi: 10.1093/ehjcr/ytab285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jawitz O.K., Wang T.Y., Lopes R.D., et al. Rationale and design of PROACT Xa: a randomized, multicenter, open-label, clinical trial to evaluate the efficacy and safety of apixaban versus warfarin in patients with a mechanical On-X Aortic Heart Valve. Am Heart J. 2020;227:91–99. doi: 10.1016/j.ahj.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shroff G.R., Bangalore S., Bhave N.M., et al. Evaluation and management of aortic stenosis in chronic kidney disease: a scientific statement from the American Heart Association. Circulation. 2021;143(25):e1088–e1114. doi: 10.1161/CIR.0000000000000979. [DOI] [PubMed] [Google Scholar]