Abstract
Left ventricular outflow tract obstruction (LVOTO) can complicate percutaneous mitral valve replacement and may preclude patients considered high surgical risk from transcatheter therapies. We report a case of mitral valve-in-valve procedure in a patient at high risk for LVOTO. (Level of Difficulty: Advanced.)
Key Words: ablation, mitral valve, valve replacement
Abbreviations and Acronyms: ASA, alcohol septal ablation; CTA, computed tomography angiogram; ICE, intracardiac echocardiography; LVEF, left ventricular ejection fraction; LVOT, left ventricular outflow tract; LVOTO, left ventricular outflow tract obstruction; MAC, mitral annular calcification; MV, mitral valve; RFA, radiofrequency ablation; TMVR, transcatheter mitral valve replacement; TTE, transthoracic echocardiogram; ViV, valve-in-valve
Central Illustration
History of Presentation
An 82-year-old woman presented with worsening dyspnea on exertion for 1 month. She had been doubling her dose of furosemide without relief. Physical examination was grossly normal with no significant murmurs or peripheral edema, but right basilar rales. Blood pressure was 164/69 mm Hg with a regular pulse rate of 66 beats/min, respiratory rate of 18 breaths/min, and oxygen saturation of 98% on room air.
Learning Objectives
-
•
To be able to identify patients at excessive risk of surgical MV replacement and increased risk of LVOTO.
-
•
To understand the role of novel therapies for neoLVOT enlargement in patients deemed not to be candidates for invasive surgical strategies or other less invasive therapies for LVOT enlargement such as ASA.
Past Medical History
She had a history of mitral valve (MV) endocarditis and underwent bioprosthetic MV replacement in 2015 out of state, and the operative report was not available.
Differential Diagnosis
Differential diagnosis included congestive heart failure exacerbation, chronic obstructive pulmonary disease exacerbation, coronary artery or valvular heart disease, including bioprosthetic MV regurgitation/stenosis.
Investigations
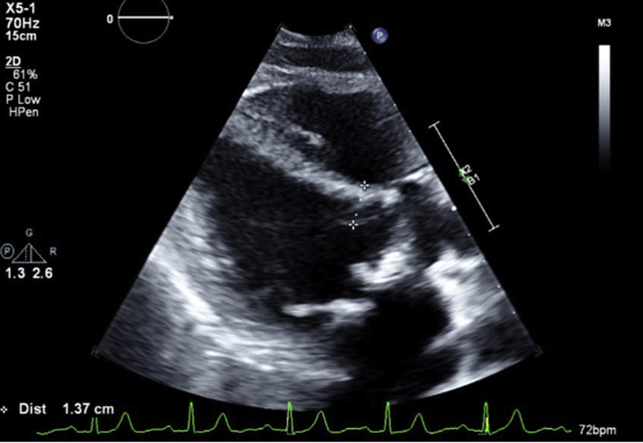
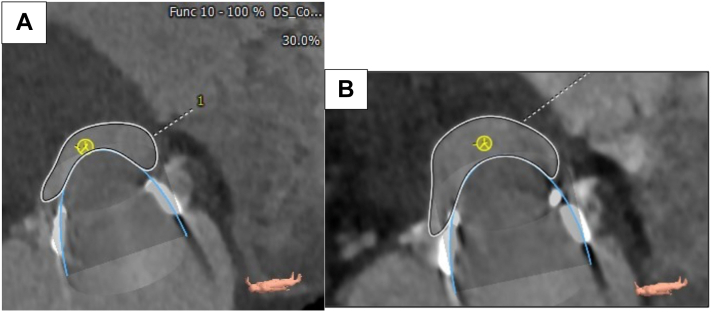
Recent pharmacologic nuclear stress test showed no ischemia. Labs revealed a high-sensitivity troponin T 22 ng/L (≤14 ng/L) and Pro B-type natriuretic peptide 1,043 pg/mL. Transthoracic echocardiogram (TTE) showed normal left ventricular ejection fraction (LVEF) and severe bioprosthetic MV stenosis with a mean gradient of 12 mm Hg at a pulse rate of 74 beats/min and septal thickness 1.37 cm (Figure 1, Video 1). Transesophageal echocardiogram showed patchy calcification of the leaflets with partial fusion, trace regurgitation, and severe bioprosthetic MV stenosis. Gated cardiac computed tomography angiogram (CTA) revealed high-risk features for left ventricular outflow tract obstruction (LVOTO) with percutaneous mitral valve-in-valve (ViV) procedure with estimated neo-left ventricular outflow tract (LVOT) using a 26-mm valve of 149 mm2 (Figure 2A), short prosthesis to septal distance, and relatively unfavorable aorto-mitral angle. Based on the fluoroscopy and preprocedural CT measurements, the valve appeared to be a 27-mm Magna Ease bioprosthetic valve.
Figure 1.
TTE Parasternal Long-Axis View Pre-Mitral ViV Procedure
Septal thickness 1.37 cm. TTE = transthoracic echocardiogram; ViV = valve-in-valve.
Figure 2.
CTA Chest for Preprocedural Planning Mitral ViV Measurements and Post-RFA for Reassessment of neoLVOT
(A) Estimated neoLVOT at 30% systolic phase, using a 26-mm valve, of 149 mm2, short prosthesis to septal distance, and unfavorable aorto-mitral angle. (B) Enlargement of neoLVOT at 40% systolic phase to 232 mm2. CTA = computed tomography angiography; LVOT = left ventricular outflow tract; RFA = radiofrequency ablation; other abbreviation as in Figure 1.
Management
The heart team deemed her excessive risk for re-do surgical MV replacement, and septal reduction intervention was recommended for anticipated percutaneous mitral ViV procedure. Coronary angiography showed no obstructive coronary disease; however, her anatomy was unsuitable for alcohol septal ablation (ASA) (Video 2). Therefore, she was referred for radiofrequency ablation (RFA) of septum to reduce the risk of LVOTO.
After obtaining informed consent, an electrophysiology study was performed with the patient under general anesthesia. Using intracardiac echocardiography (ICE), anatomic mapping of the aortic root, coronary cusps, LVOT, and the left ventricle was performed. Under the guidance of 3-dimensional electro-anatomic mapping system (CARTO 3, Biosense Webster), using the retrograde aortic approach under therapeutic anticoagulation, a 3.5-mm-tip open-irrigation catheter was used to perform a detailed anatomic and activation map of the His-Purkinje system, left anterior and posterior fascicle, septum, and LVOT. The MV apparatus-septal contact area was marked using ICE onto the anatomic map, and this region became the target for RFA. Avoiding the conduction system, ablation was initiated at 35 watts and 1-minute application time was used per lesion (Figure 3). A total of 47 minutes of energy was applied. Procedural endpoint included complete coverage of the valve-septal contact area, as well as basal septal akinesis on ICE imaging.
Figure 3.
Septal Ablation Mapping
Right anterior oblique view of electro-anatomic mapping demonstrating distance of 5.5 mm between ablation and bundle of His. Purple, red, and yellow circles represent bundle branch potentials, area of ablation, and bundle of His potentials, respectively.
Postprocedure TTE showed a small pericardial effusion with normal LVEF 65% to 70%, and she was discharged with plan for elective mitral ViV procedure in 6 to 8 weeks. However, because of severely limiting symptoms, a repeat cardiac CTA was performed in 3.5 weeks confirming enlargement of neoLVOT to 232 mm2 (Figure 2B). She subsequently underwent successful transcatheter mitral ViV procedure with 26-mm S3 Edwards Sapien valve via percutaneous transeptal approach (Videos 3 and 4).
Discussion
LVOTO can complicate percutaneous MV replacement and may preclude patients considered high surgical risk from transcatheter therapies. Acute LVOTO after transcatheter mitral valve replacement (TMVR) is associated with increased mortality. Preprocedure assessment and interventions to prevent this complication are vital. RFA is a novel technique that can be useful in patients unsuitable for other septal reduction strategies.
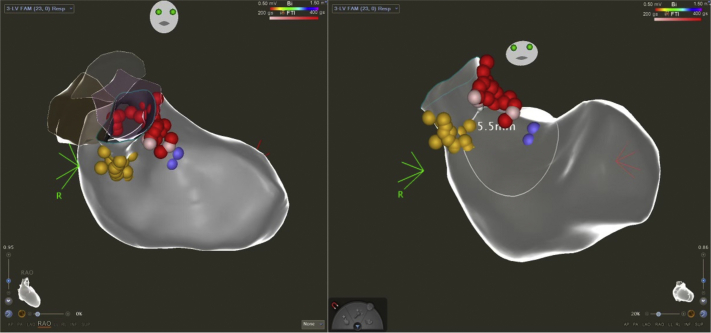
Repeat operation following MV replacement or repair can be required in a significant proportion of patients,1 and with the emergence of patients with TMVR who may otherwise be considered high risk for surgical MV repair or replacement are now presented with a new treatment modality. Preprocedural evaluation of LVOT is critical because of the complex anatomy of the MV, and risk of LVOTO with TMVR. LVOTO may have a low incidence, approximately 7% to 9%,2 and with higher rates after valve in mitral annular calcification (MAC)3 or valve-in-ring procedures,4 but can be associated with significant periprocedural morbidity and mortality.4 We measured neoLVOT from multiplanar images using a proprietary software (3Mensio, Pie Medical) as recommended by the consensus document.5 Briefly, using multiplanar reconstruction, the segmentation of the basal ring of the mitral prosthesis was performed in mid-late systole. A virtual 26-mm valve was then modeled and offset at the level of surgical valve post and planimetry of the neoLVOT area was performed (Figures 2 and 4).
Figure 4.
Multiplanar Image Assessment of neoLVOT
Virtual Sapien 3 26-mm valve modeled and offset at the level of surgical valve post. Abbreviations as in Figure 2.
LVOTO following TMVR in a ViV procedure is caused by pushing the bioprosthetic leaflet into the LVOT covering the open cells of the balloon expandable valve. The size of the neoLVOT and the aorto-mitral angle are associated with increased risk of LVOTO after TMVR.6 ASA has been previously used in patients undergoing TMVR at high risk for LVOTO with success7; however, not all patients have suitable anatomy to undergo ASA with associated risk of need for permanent pacing and large myocardial infarction. In our patient, significant tortuosity of the left anterior descending artery and the absence of a single suitable proximal septal that supplied the area of interest, made her not a good candidate for ASA. She would have likely required injection of multiple septal branches and would have been at very high risk of pacemaker placement. RFA is a novel technique that has been previously used in patients unsuitable for LVOT enlargement with open procedures, such as surgical myomectomy or laceration of the anterior leaflet (LAMPOON) who require TMVR for native MV disease associated with MAC.8 To our knowledge, our case represents the first report of successful use of RFA for neoLVOT enlargement in anticipated TMVR for a mitral ViV procedure.
Follow-Up
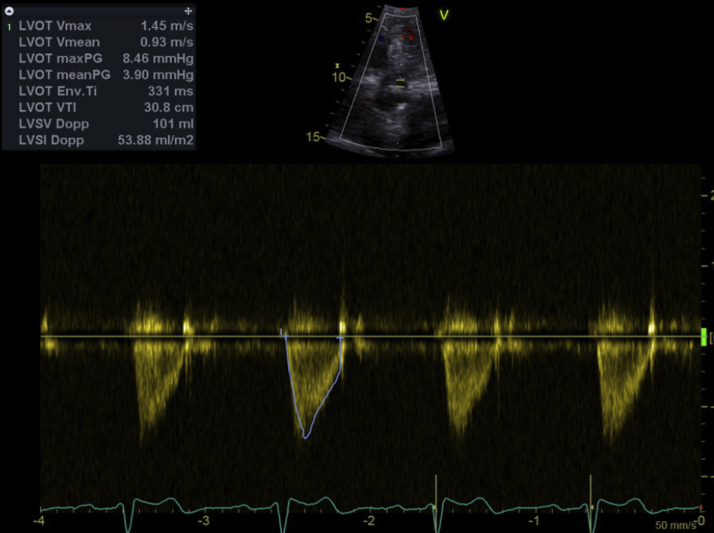
After the ViV procedure, postprocedural transesophageal echocardiogram and TTE showed normal LVEF and a mitral bioprosthesis with a mean gradient across the valve of 2 mm Hg with a pulse rate of 77 beats/min and peak LVOT velocity of 1.4 m/s (Figures 5 and 6). She had an uncomplicated postprocedural course and was discharged home the next day. At 4 months, she has had no recurrent hospitalizations and continues to report significant improvement in her ability to perform activities of daily living and overall quality of life.
Figure 5.
TEE Post Mitral ViV
Peak transmitral velocity 1.4 m/s and mean gradient across valve 2 mm Hg. TEE = transesophageal echocardiography; other abbreviation as in Figure 1.
Figure 6.
TTE Post Mitral ViV
Abbreviations as in Figure 1.
Conclusions
Acute LVOTO after transcatheter mitral intervention is associated with increased mortality. Preprocedure assessment and interventions to prevent this complication are vital. RFA is a novel technique that can be useful in patients unsuitable for other septal reduction strategies.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental videos, please see the online version of this paper.
Appendix
Preprocedure TTE Parasternal Long-Axis View
Coronary angiography, Right Anterior Oblique Cranial View
Intraprocedural Fluoroscopy, Percutaneous Mitral Valve-in-Valve Procedure
Postprocedure TEE, Mid-Esophageal Long-Axis View
References
- 1.Bourguignon T., Bouquiaux-Stablo A.L., Loardi C., et al. Very late outcomes for mitral valve replacement with the Carpentier-Edwards peri- cardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg. 2014;148:2004–2011.e1. doi: 10.1016/j.jtcvs.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 2.Regueiro A., Granada J.F., Dagenais F., Rodés-Cabau J. Transcatheter mitral valve replacement: insights from early clinical experience and future challenges. J Am Coll Cardiol. 2017;69(17):2175–2192. doi: 10.1016/j.jacc.2017.02.045. [DOI] [PubMed] [Google Scholar]
- 3.Guerrero M., Dvir D., Himbert D., et al. Transcatheter mitral valve replacement in native mitral valve disease with severe mitral annular calcification: results from the first multicenter global registry. J Am Coll Cardiol Intv. 2016;9(13):1361–1371. doi: 10.1016/j.jcin.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 4.Dvir D., Webb J. Mitral valve-in-valve and valve-in-ring: technical aspects and procedural outcomes. EuroIntervention. 2016;12(Y):Y93–Y96. doi: 10.4244/EIJV12SYA25. [DOI] [PubMed] [Google Scholar]
- 5.Reid A., Ben Zekry S., Turaga M., et al. Neo-LVOT and transcatheter mitral valve replacement: expert recommendations. J Am Coll Cardiol Img. 2021;14(4):854–866. doi: 10.1016/j.jcmg.2020.09.027. [DOI] [PubMed] [Google Scholar]
- 6.Murphy D.J., Ge Y., Don C.W., et al. Use of cardiac computerized tomography to predict neo-left ventricular outflow tract obstruction before transcatheter mitral valve replacement. J Am Heart Assoc. 2017;6(11) doi: 10.1161/JAHA.117.007353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D.D., Guerrero M., Eng M.H., et al. Alcohol septal ablation to prevent left ventricular outflow tract obstruction during transcatheter mitral valve replacement: first-in-man study. J Am Coll Cardiol Intv. 2019;12(13):1268–1279. doi: 10.1016/j.jcin.2019.02.034. [DOI] [PubMed] [Google Scholar]
- 8.Killu A.M., Guerrero M., Siontis K.C., et al. A novel technique—prophylactic septal radiofrequency ablation to prevent left ventricular outflow tract obstruction with transcatheter mitral valve replacement (RADIO-TMVR) J Cardiovasc Electrophysiol. 2020;31(11):3048–3055. doi: 10.1111/jce.14720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Preprocedure TTE Parasternal Long-Axis View
Coronary angiography, Right Anterior Oblique Cranial View
Intraprocedural Fluoroscopy, Percutaneous Mitral Valve-in-Valve Procedure
Postprocedure TEE, Mid-Esophageal Long-Axis View