Abstract
Sequence analysis of the hemin uptake locus (hmu) of Yersinia pestis revealed five genes, hmuRSTUV, required for use of hemin and hemoproteins as iron sources. The translated gene products have homologies with proteins of the hemin transport genes of several gram-negative bacteria. Promoters were identified upstream of hmuP′R (p1) and upstream of hmuS (p2); p1, which contains a Fur box, is regulated by iron and Fur, while p2 exhibits weak, but constitutive, activity. HmuR, which has homology with TonB-dependent outer membrane (OM) receptors, is localized to the OM of Y. pestis and is required for utilizing hemin and all hemoproteins under iron-depleted conditions. The proposed ABC transporter, HmuTUV, is necessary for use of hemin, hemin-albumin, and myoglobin, but not hemoglobin, hemoglobin-haptoglobin, or heme-hemopexin, as iron sources. In the absence of HmuTUV, HmuS, a cytoplasmic protein, is involved in use of hemoglobin and heme-hemopexin. In mice, the 50% lethal doses of Y. pestis ΔhmuP′RSTUV mutants injected subcutaneously or retro-orbitally did not differ from that of the Hmu+ parent strain. Thus, the hmu system is not essential for infection in mice via these routes. Growth studies showed that a ΔhmuP′RSTUV mutant could grow in iron-depleted medium containing high concentrations of hemoglobin, suggesting that an Hmu-independent, lower-affinity hemoglobin uptake system may exist.
Pathogenic bacteria are capable of scavaging iron, an essential nutrient for bacterial growth, from a number of mammalian host iron-sequestering proteins including transferrin, lactoferrin, ferritin, and/or hemoproteins by one or more defined energy-dependent, iron-regulated uptake systems (7, 26, 34). Iron or heme from the various iron/heme-containing proteins can be captured either by bacterial secreted products including siderophores or hemophores, respectively, or directly by outer membrane (OM) receptors specific for these substrates (7).
The plague bacillus, Yersinia pestis, contains at least three iron transport systems that may be important in various niches of its mammalian and/or insect hosts for the establishment and progression of bubonic or pneumonic plague. Two of these are inorganic iron transport systems important to the pathogenesis of plague: (i) the yersiniabactin-iron transport system (Ybt), a siderophore-dependent system (4, 16, 18, 44), and (ii) an iron and manganese uptake system, Yfe (5, 6).
Previously we described a third transport system in Y. pestis, the Hmu (for “hemin utilization”) transport system, which acquires heme from a variety of hemoproteins and is independent of the nonnutritional hemin storage system (Hms) of Y. pestis (24, 30, 44, 45). Heme is an abundant iron-containing compound found within mammals; it is the prosthetic group of a class of proteins referred to as hemoproteins and is the cofactor in reactions involved in various cellular functions including oxygen transport and electron transfer (26). The majority of heme is found intracellularly in the form of hemoglobin, and any free heme or hemoglobin released extracellularly is rapidly sequestered by the serum heme carrier proteins, hemopexin and albumin, or the serum hemoglobin carrier protein, haptoglobin (39). Y. pestis can grow in iron-deficient medium supplemented with free hemin or various hemoproteins including hemoglobin, hemoglobin-haptoglobin, hemin-albumin, heme-hemopexin, and myoglobin (47, 55, 58). We identified an 8.6-kb locus (designated hmu) from the Y. pestis chromosome that encodes at least five proteins and is involved in utilizing not only free hemin but also the various hemoproteins as iron sources (24). Introduction of the hmu locus into an Escherichia coli strain containing mutations in heme and enterobactin biosynthesis (hemA aroB) allows this strain to use hemin as iron and porphyrin sources but only under iron-poor conditions, providing evidence that the entire hemin moiety is transported into the cell and that the Hmu system may be tightly iron regulated (24).
In this study, we have characterized further the Hmu transport system of Y. pestis KIM6+. We determined the sequence of the hmu locus and identified eight open reading frames (ORFs), orfXY and hmuP′RSTUV. Database searches show that the deduced proteins of the hmu locus have strong homologies with proteins of the hemin transport systems of Yersinia enterocolitica and Shigella dysenteriae (38, 59, 60, 71). Although hemin transport proteins from other gram-negative bacteria have been reported to be important in hemin utilization, the roles of these proteins in utilization of host hemoproteins have not been studied. In this study, we have characterized the roles that the various Hmu proteins play in both hemin and host hemoprotein utilization by Y. pestis and E. coli. Additionally, we have determined the effect of a ΔhmuP′RSTUV deletion upon virulence in mice.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions.
All bacterial strains and plasmids used in this study are listed in Table 1. All strains were stored at −20°C in phosphate-buffered glycerol. Y. pestis cells were grown routinely at 30°C on Congo red agar (63) from glycerol stocks and then grown in heart infusion broth or on tryptose-blood agar base (TBA). Unless otherwise noted, iron-deficient Y. pestis cells used in various experiments were obtained by growth from TBA slants through two transfers (∼6 to 8 generations) aerobically at 37°C in the deferrated defined medium PMH (58). Iron-replete strains were grown as above with 10 μM ferric chloride (FeCl3) added. For plate assays, iron-deficient Y. pestis cells were grown on PMH–100 μM EDDA (ethylenediamine-N,N′-diacetic acid) solidified with 1% agarose (PMHA-EDDA). All glassware used in iron-restricted studies were cleaned in a potassium dichromate solution and rinsed copiously with deionized water. All E. coli strains were grown routinely at 37°C in Luria-Bertani broth (LB) or LB solidified with 1.2% Bacto Agar (Difco). For plate assays with the various E. coli 1017 isogenic strains, cells were grown on Tris-glucose-thymidine (excluding FeCl3) (57) containing 100 μM EDDA and solidified with 1% agarose (TGA-EDDA). Where appropriate, antibiotics were included in the medium at the following concentrations: ampicillin (Ap), 100 μg/ml; chloramphenicol (Cm), 30 or 20 μg/ml (for Y. pestis KIM6-2063.1+); kanamycin (Km), 50 μg/ml; spectinomycin (Spc), 25 μg/ml (on Congo red agar) or 100 μg/ml (on TBA slants or in broth cultures); tetracycline (Tc), 12.5 μg/ml.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Relevant characteristic(s)a | Source or reference(s) |
|---|---|---|
| E. coli strains | ||
| DH5α | Cloning host | 3 |
| HB101 1017 | ent::Tn5, Kmr | 11 |
| M15(pREP4) | pREP4 (lacI, Kmr); host for inducing protein expression from pQE30-derived clones | 69; Qiagen, Inc. |
| SY327 (λpir) | Host for propagating pCVD442-derived clones | 36 |
| Y. pestis strainsb | ||
| KIM5-2044.11 | Pgm− Hum− (ΔhmuP′RSTUV-2044.1) Lcr+ YopJ− [pCD1::Mu dI1734-73 (yopJ::Mu dI1734)], Kmr; derived from KIM6-2044.1 | This study |
| KIM5-2044.21+ | Pgm+ Psa− (Δpsa2053.1) Hmu− (ΔhmuP′RSTUV-2044.1) Lcr+ YopJ− [pCD1::Mu dI1734-73 (yopJMu dI1734)], Kmr; derived from KIM6-2044.2+ | This study |
| KIM5-2060.21+ | Pgm+ Psa− (Δpsa2053.1) Hmu− (ΔhmuP′RSTUV-2060.1) Lcr+ YopJ− [pCD1::Mu dI1734-73 (yopJ::Mu dI1734)], Kmr; derived from KIM6-2060.2+ | This study |
| KIM5-3173 | Pgm− Hmu+ Lcr+ YopJ− [pCD1::Mu dI1734-73 (yopJ::Mu dI1734)], Kmr | 48 |
| KIM6+ | Pgm+ Lcr− Psa+ Hmu+ | 45, 55, 56 |
| KIM6 | Pgm− (Δpgm [Δhms Δybf+) Lcr− Psa+ Hmu+ | 44, 46, 55, 56 |
| KIM6-(pRT240)+ | Pgm+ Lcr− Psa+ Hmu+ pRT240 [LacZ(Con), Apr] | 58 |
| KIM6-2030+ | Pgm+ Lcr− Psa+ Fur− (fur9::kan) Hmu+ Kmr | 58 |
| KIM6-2044.1+ | Pgm+ Lcr− Psa+ Hmu− (Δorf-XYhmuP′RSTUV-2044.1) | 24 |
| KIM6-2044.1 | Pgm− Lcr− Psa+ Hmu− (Δorf-XYhmuP′RSTUV-2044.1); Δpgm mutant from KIM6-2044.1+ | This study |
| KIM6-2044.2+ | Pgm+ Lcr− Psa− (Δpsa2053.1) Hmu− (ΔhmuP′RSTUV-2044.1); derived from KIM6-2044.1(pCVDPSA1)+ | This study |
| KIM6-2053.1+ | Pgm+ Lcr− Psa− (Δpsa2053.1) Hmu+ | 4 |
| KIM6-2060.1+ | Pgm+ Lcr− Psa+ Hmu− (ΔhmuP′RSTUV-2060.1); derived from KIM6(pHMU61)+ | This study |
| KIM6-2060.2+ | Pgm+ Lcr− Psa− (Δpsa2053.1) Hmu− (ΔhmuP′RSTUV-2060.1); derived from KIM6-2053.1(pHMU61)+ | This study |
| KIM6-2061.1+ | Pgm+ Lcr− Psa+ Hmu− (in-frame ΔhmuR-2061.1); derived from KIM6(pHMU47)+ | This study |
| KIM6-2062.1+ | Pgm+ Lcr− Psa+ Hmu− (in-frame ΔhmuS-2062.1); derived from KIM6(pHMU64)+) | This study |
| KIM6-2063.1+ | Pgm+ Lcr− Psa+ Hmu− (hmuT::cat-2063.1); derived from KIM6(pHMU48)+ | This study |
| Plasmids | ||
| pACYC184 | 4.2-kb cloning vector; Cmr Tcr | 3 |
| pBluescriptII KS+ | 3.0-kb cloning vector; Apr | Stratagene |
| pBR322 | 4.4-kb cloning vector; Apr Tcr | 3 |
| pCD1::Mu dl1734-73 | Modified Lcr plasmid pCD1 (yopJ::Mu dI1734); Lcr+ YopJ−, Kmr | 62 |
| pCVD442 | 6.2-kb suicide vector, pir-dependent replication; Apr, SacB+ | 13 |
| pCVDPSA1 | Δpsa suicide plasmid construct; Apr | 4 |
| pEU730 | 15.2-kb cloning vector, promoterless lacZ; Spcr | 17 |
| pKRP10 | 3.7-kb plasmid carrying Cmr cassette; Apr Cmr | 49 |
| pQE30 | 3.5-kb His-tagged protein expression cloning vector; Apr | Qiagen, Inc. |
| pHMU4 | 10.7-kb EcoRI-BglII fragment cloned into pBR322; Apr, Hmu+ (orfXY+ hmuP′RSTUV+) | 24 |
| pHMU6 | 6.2-kb EcoRI-SalI fragment cloned into pBR322; Apr, Hmu− (orfXY+ hmuP′RST+ hmuU′) | 24 |
| pHMU7 | 8.6-kb EcoRI-PstI fragment cloned into pBR322; Tcr, Hmu+ (orfXY+ hmuP′RSTUV+) | 24 |
| pHMU23 | Mu dI1734 insertion into pHMU4; Apr Kmr, Hmu− (hmuR::Mu dI1734) | 24 |
| pHMU28 | 2.1-kb EcoRI-EcoRV fragment from pHMU6 cloned into pBluescriptII KS+; Apr | This study |
| pHMU30 | 2.6-kb AseI fragment from pHMU6 cloned into pACYC184; Cmr Tcr, hmuP′R+ | This study |
| pHMU35 | 7.8-kb PvuI-PstI fragment from pHMU4 cloned into pBR322 lacking bla promoter; Tcr, hmuP′RSTUV+ | This study |
| pHMU37 | 5.4-kb EcoRI-NruI fragment from pHMU6 cloned into pBR322; Tcr, hmuP′RS+ hmuT′ | This study |
| pHMU39 | 0.7-kb ClaI-EcoRV fragment from pHMU30 and 1.0-kb DraI-BamHI fragment from pHMU30 cloned sequentially into pBluescriptII KS+; Apr, in-frame ΔhmuR-2061.1 | This study |
| pHMU43 | ∼1.9-kb fragment containing hmuR (minus signal sequence region) cloned into pQE30; Apr, His-tagged HmuR | This study |
| pHMU44 | 123-bp AseI-NdeI fragment from pHMU28, blunt ended and cloned into pEU730; Spcr, p1::lacZ reporter fusion | This study |
| pHMU46 | 1.7-kb PvuII-SalI fragment from pHMU6 cloned into pBluescriptII KS+ and 0.8-kb SmaI fragment of pKRP10 cloned within NruI site of construct; Apr Cmr, hmuT::cat-2063.1 | This study |
| pHMU47 | 1.8-kb SalI-SacI fragment from pHMU39 cloned into pCVD442; Apr, in-frame ΔhmuR-2061.1 | This study |
| pHMU48 | 2.5-kb XhoI-SacI fragment from pHMU46 cloned into pCVD442; Apr Cmr, hmuT::cat-2063.1 | This study |
| pHMU51 | 2.1-kb DraI-HpaI fragment from pHMU6 cloned into pBR322; Apr, hmuS+ hmuT′ | This study |
| pHMU52 | 1.0-kb PCR fragment of hmuS from pHMU6 cloned into pEQ30; Apr, His-tagged HmuS | This study |
| pHMU53 | 1.23-kb BstBI-EcoRV fragment from pHMU51 replaced with 0.26-kb BstBI-EcoRV PCR fragment generated with gene splicing by overlap extension technology, as reported by Ho et al. (23); Apr, in-frame ΔhmuS | This study |
| pHMU55 | 130-bp PCR product generated from end of hmuR to start of hmuS with pHMU6 and cloned into pEU730; Spcr, p2::lacZ reporter fusion | This study |
| pHMU60 | 5.9-kb deletion consisting of 1.1-kb SspI fragment and 4.8-kb SspI-SwaI fragment from pHMU7; Tcr; ΔhmuP′RSTUV-2060.1 | This study |
| pHMU61 | 1.8-kb NdeI-SspI fragment from pHMU60 cloned into pCVD442; Apr, ΔhmuP′RSTUV-2060.1 | This study |
| pHMU62 | 3.7-kb SspI-SalI fragment from pHMU6 cloned into pBR322; Apr, hmuST+ hmuU′ | This study |
| pHMU63 | 1.23-kb BstBI-EcoRV fragment from pHMU62 replaced with 0.26-kb BstBI-EcoRV fragment from pHMU53; Apr, in-frame ΔhmuS-2062.1 | This study |
| pHMU64 | 2.7-kb SspI-NheI fragment from pHMU63 cloned into pCVD442; Apr, in-frame ΔhmuS-2062.1 | This study |
| pHMU66 | 2.1-kb PmlI-Bsu36I fragment from pHMU4 replaced with ∼1.1-kb PmlI-Bsu36I fragment from pHMU63; Apr, in-frame ΔhmuS hmuP′RSTUV+ | This study |
Pgm, pigmentation (102-kb chromosomal locus containing genes of hemin storage system [hms] and siderophore biosynthetic and transport system [ybt/psn] [44]); Lcr, low Ca2+ response (associated with pCD1); psa, gene encoding pH 6 antigen; Psa, pH 6 antigen, Hmu, hemin utilization; LacZ(Con), constitutive expression of β-galactosidase; ent, enterobactin gene.
KIM5 strains contain a form of pCD1 (Lcr plasmid), while KIM6 strains have been cured of the Lcr plasmid. A “+” after strain name designates a Pgm+ phenotype.
Recombinant DNA techniques.
Plasmid extractions, plasmid transformations, and Southern blot hybridizations were described previously (24). Standard cloning and ligation methods (52) were used to construct the various plasmids in Table 1 including the p1::lacZ promoter fusion in pEU730 (pHMU44). To construct the p2::lacZ promoter fusion (pHMU55), restriction endonuclease sites (KpnI and AscI) were introduced onto the ends of the p2 promoter region (extending from the end of hmuR to the start of hmuS) by PCR for directional cloning into pEU730. To construct the hmuR and hmuS expression clones (pHMU43 and pHMU52, respectively), we cloned a modified hmuR gene (lacking the nucleotide sequence encoding the signal peptide) and an hmuS gene (in which BamHI and HindIII restriction sites were introduced at the ends of the hmuS gene by PCR) into pQE30 (Qiagen, Chatsworth, Calif.). This places a His6 affinity tag at the amino-terminal end of the expressed proteins. Oligonucleotide primers were purchased from Integrated DNA Technologies, Inc.
Construction of Y. pestis chromosomal mutations in hmu genes.
To construct a deletion encompassing all of the hmu genes, a 5.9-kb fragment, consisting of a 1.1-kb SspI fragment and a 4.8-kb SspI-SwaI fragment, was deleted from pHMU7 to generate pHMU60 (Table 1 and Fig. 1 [ΔhmuP′RSTUV-2060.1]). An in-frame mutation within hmuR was constructed by ligating two fragments from pHMU30, generating a deletion of the 880-bp EcoRV-DraI fragment (pHMU39) (Table 1 and Fig. 1 [ΔhmuR-2061.1]). An in-frame mutation within hmuS was generated by overlap extension, as described by Ho et al. (23). Two sets of primers were constructed to delete ∼1 kb of DNA from hmuS, and the primers used in this experiment were as follows. Set 1 consists of SP1 (5′-CCCCCCTTCGAAAAAGAGTATTACACGCCACAAG-3′) and SP2 (5′-CTGGTGCTGCTGGGGCTGTGATGCGTTCATAATG-3′), and set 2 consists of SP3 (5′-CAGCCCCAGCAGCACCAGCCAGAACAAAACCAAT-3′) and SP4 (5′-AACCACAATCTCATCACCTGCACCGAGTGCATAG-3′); the underlined sequences show the overlapping region designed to keep the transcriptional product in frame. Two different PCRs, using primers SP1 and SP2 or SP3 and SP4, were set up to amplify DNA from pHMU6. The resulting PCR products were mixed together, the overlapping regions were allowed to anneal, and the annealed products were amplified with primers SP1 and SP4. The final PCR product containing an in-frame ΔhmuS was digested with BstBI and EcoRV and used to replace the wild-type hmuS gene in pHMU62, generating pHMU63 (Table 1 and Fig. 1 [ΔhmuS-2062.1]). To construct a polar mutation in hmuT which should disrupt transcription of hmuTUV, we introduced a chloramphenicol cassette (cat) into the NruI site within the hmuT nucleotide sequence, generating pHMU46 (Table 1 and Fig. 1 [hmuT::cat-2063.1]). The deletions in pHMU60 (ΔhmuP′RSTUV-2060.1), pHMU39 (in-frame ΔhmuR-2061.1), and pHMU63 (in-frame ΔhmuS-2062.1) and the insertion within pHMU46 (hmuT::cat-2063.1) were confirmed by sequence analysis. The mutated fragments from each of the plasmids listed above were then ligated separately into the suicide vector pCVD442 and transformed into E. coli SY327 (λpir), as described previously (24). Each of the resulting recombinant plasmids (Table 1)—pHMU61 (ΔhmuP′RSTUV-2060.1), pHMU47 (in-frame ΔhmuR-2061.1), pHMU64 (in-frame ΔhmuS-2062.1), and pHMU48 (hmuT::cat-2063.1)—were transformed separately into Y. pestis KIM6+, and double recombinants were selected as described previously (24). For each resulting mutant (Table 1 and Fig. 1)—KIM6-2060.1+ (ΔhmuP′RSTUV-2060.1), KIM6-2061.1+ (in-frame ΔhmuR-2061.1), KIM6-2062.1+ (in frame ΔhmuS-2062.1), and KIM6-2063.1+ (hmuT::cat-2063.1)—PCR or Southern blot hybridization analysis was used to confirm allelic exchange of the mutated locus for the wild-type locus.
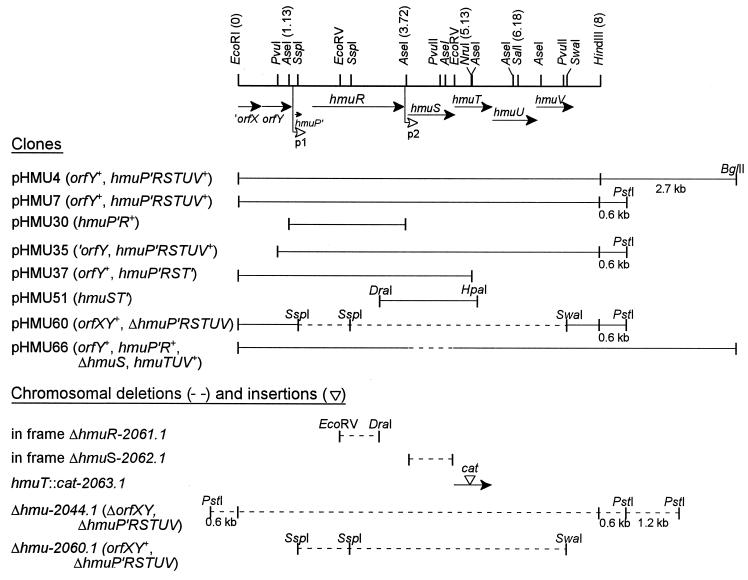
FIG. 1.
Partial restriction map of the hemin uptake locus (hmu) of Y. pestis KIM6+ illustrating the locations and directions of transcription of ORFs designated hmuP′RSTUV and upstream ORFs designated orfXY. The sequence for the ′orfX gene does not extend to a predicted 5′ start site. The sequence of this region (7,623 bp) from the EcoRI site to beyond the end of hmuV has been deposited in GenBank under accession no. U60647. The location of the primary promoter region (p1) is shown upstream of hmuP′; a second promoter region (p2) is proposed upstream of hmuS (open arrowheads). Numbers next to the restriction enzymes refer to the locations (in kilobases) of key restriction sites. Clones generated from the hmu locus as well as deletions and insertions generated in the Y. pestis KIM6+ chromosome that were used in this study are illustrated below the genetic map. The in-frame ΔhmuS mutation generated within pHMU66 is identical to that generated within the chromosome (ΔhmuS-2062.1).
DNA sequence determination and analysis.
The DNA sequence of the previously cloned hmu locus (24) was determined by the dideoxynucleotide chain termination method (53) with Sequenase, version 2.0, and [35S]dATP (Amersham Corp.). Either single-stranded or denatured double-stranded DNA templates were used in the sequencing reactions. Sequencing products were separated on 6% polyacrylamide gels containing 8.3 M urea, as described by Sambrook et al. (52). Extension of the nucleotide sequence on either DNA strand was obtained by using oligonucleotide primers (Integrated DNA Technologies, Inc.) or M13 forward/reverse primers on various subclones of pHMU4 or subclones generated from unidirectional nested deletions with the Erase-a-Base system, as described by Promega Corp. (Madison, Wis.). To sequence directly the hmuP′ gene from the chromosome, we amplified a 274-bp region from Y. pestis KIM6+ or KIM6 chromosome by using primers 5′-TGAGCCAGGATTAGCCCGTAAG-3′ and 5′-GCATTCGCCCTGATGGACGATA-3′, treated the PCR-generated products with Klenow fragment, and cloned the products into pBluescriptII KS+. The DNA sequence of the hmu locus was analyzed by the IntelliGenetics (IG) suite (Mountain View, Calif.) or the Genetics Computer Group (GCG) package (12). The properties of the deduced amino acid sequences were determined with programs in the IG Suite and PSORT (40) or SignalP (41) programs in ExPASy Proteomic Tools. Searches of protein databases for sequences homologous to the Hmu deduced amino acid sequences were performed with the BLAST program (1, 2). Protein sequence alignments were performed with the Bestfit or Gap programs in the GCG package (12) or the multiple sequence alignment program of CLUSTAL W (66).
The 7,623-bp nucleotide sequence of the hmu locus, containing ′orfX, orfY, and hmuP′RSTUV, was determined. The designation ′orfX refers to an incomplete sequence lacking a 5′ region. orfXY, designated originally orfAB, were renamed to indicate the homologies between these gene products and those of S. dysenteriae shuXY (71). Recent corrections in the nucleotide sequence lengthened the orfX (formerly orfA) reading frame in comparison to that illustrated by Wyckoff et al. (71).
RNA isolation and primer extension analysis.
Total RNA was isolated from iron-deficient Y. pestis KIM6+, KIM6-2044.1+, or KIM6-2044.1(pHMU4)+ cells harvested during exponential growth by the acid-sodium dodecyl sulfate (SDS) lysis/hot phenol method described by Von Gabain et al. (70) and treated with DNase I. Primers 5′-ATATTCGGCAGCAGGTTCGTCATTTAACATT-3′ and 5′-CATATTTGCCAGGGTGCTCTGCTTTAGCCTGTA-3′, which are complementary to the 5′ ends of the coding regions of hmuP′ and hmuS, respectively, were used in primer extension analysis to determine the transcriptional start sites within the p1 and p2 promoter regions, respectively. The primer extension reactions were carried out with 32P-labeled primers and 15, 30, or 100 μg of total RNA, as described by Ausubel et al. (3). The extension products were analyzed on a 6% polyacrylamide–8.3 M urea gel simultaneously with the noncoding sequence ladder of the corresponding p1 or p2 region generated by dideoxynucleotide sequencing with the same oligonucleotide primers.
β-Galactosidase assays.
Y. pestis KIM6+, KIM6-2044.1+, and KIM6-2030+ cells containing either pHMU44 (p1::lacZ) or pHMU55 (p2::lacZ) were harvested during exponential growth from second transfer cultures in PMH broth containing either no added iron source, 10 μM FeCl3, 10 μM hemin, or 2.5 μM hemoglobin. The siderophore desferrioxamine mesylate (Sigma, St. Louis, Mo.), which is not used by Y. pestis (32), was added to the hemin and hemoglobin solutions at a concentration of 20 μM to chelate any contaminating inorganic iron. β-Galactosidase activities from whole-cell lysates of these cultures were measured as previously described (15, 35). Since Y. pestis is naturally β-galactosidase negative in this assay (58), the activity obtained from strains carrying either reporter plasmid correlates directly with promoter activity.
Cellular fractionation of Y. pestis and Western blot analysis.
Cellular fractions of KIM6(pRT240)+ were separated according to a method described by Lucier et al. (32). Isopycnic sucrose density gradient centrifugation separates OMs from inner membranes (IMs) and a mixture (termed mixed membranes) that contains IM and OM components not separated by this procedure. The periplasmic fraction was concentrated 30- to 60-fold with Centricon 10 filtration units (Millipore, Bedford, Mass.). Protein concentrations of the cellular fractions were determined by BCA protein assays (Pierce, Rockford, Ill.).
For Western blot analysis, equal protein concentrations of whole-cell extracts (12.5 μg) of iron-deficient or iron-replete Y. pestis KIM6+ isogenic strains (obtained from exponentially growing cells in second transfer cultures) or cellular fractions (∼25 μg) of Y. pestis KIM6(pRT240)+ were separated on SDS–9% or –12% polyacrylamide gels and immunoblotted to polyvinylidene difluoride membranes (Immobilon P; Millipore). For immunodetection of the blots, we used the procedure of Towbin et al. (68). The blocked membranes were treated with anti-HmuR or anti-HmuS antiserum (diluted 1:1,000 and 1:5,000, respectively) and secondary antibody (anti-rabbit immunoglobulin G [IgG]-alkaline phosphatase conjugate [Sigma] diluted 1:15,000) and detected with 5-bromo-4-chloro-3-indolylphosphate–nitro blue tetrazolium (Sigmafast tablets; Sigma). Similarly, blots were treated with mouse anti-β-Galactosidase IgG1 monoclonal antibody (Life Technologies, Gaithersburg, Md.) or rabbit anti-β-lactamase polyclonal antibodies (5 Prime→3 Prime, Inc., Boulder, Colo.) at 1:500 dilutions, and the secondary antibody used for detecting anti-β-galactosidase antibody was anti-mouse IgG-alkaline phosphatase conjugate (Sigma). We found very little contamination of the cytoplasmic fraction with periplasmic protein, and some cross-reacting bands were observed with anti-β-galactosidase antibody, especially in the cytoplasmic fraction. We found only minor contamination of the periplasmic and membrane fractions with cytoplasmic proteins.
Antiserum preparation.
His-tagged HmuR and His-tagged HmuS were expressed from E. coli M15(pREP4) containing either pHMU43 (the cloned hmuR gene lacking the nucleotide sequence encoding the signal peptide) or pHMU52 (the cloned hmuS gene), respectively, and isolated by nickel chromatography columns, as described by Qiagen, Inc. The 71-kDa His-tagged HmuR or 39-kDa His-tagged HmuS protein band was excised from an SDS–12% polyacrylamide gel, homogenized and emulsified with Freund’s complete adjuvant, and injected into New Zealand female rabbits. The rabbits were immunized with two booster injections (in Freund’s incomplete adjuvant) at 3 to 4 weeks after each injection. Antiserum was collected 1 week after each booster injection.
Hemin and hemoprotein utilization studies.
Growth conditions for Y. pestis strains for hemin and hemoprotein utilization studies in broth or on solid medium have been described by Hornung et al. (24). Y. pestis strains were grown through two transfers (∼6 to 8 generations) in iron-deficient PMH and transferred either into PMH or PMH-EDDA or onto PMH-EDDA plates. Twenty microliters of 500 μM hemin, 10 μM human hemoglobin, 500 μM horse myoglobin, 100 μM hemoglobin-human haptoglobin, 200 μM hemin-bovine albumin, or 100 μM heme-rabbit hemopexin was added to wells cut into the solidified medium. The concentrations of hemoglobin-haptoglobin (50% saturated), hemin-albumin (50% saturated), and heme-hemopexin (95% saturated) refer to the concentrations of the respective carrier proteins within the mixture. Hemin and hemoprotein solutions were prepared as described previously (24). Growth around the wells was monitored daily for 7 days at 37°C. Zones of growth around utilized substrates were approximately equivalent to those reported previously (24). Use of hemin by E. coli 1017 isogenic strains was determined on TGA-EDDA plates at 37°C, as described previously (24). Growth was monitored daily for 5 days.
Virulence testing in mice.
The 50% lethal doses (LD50s) for HmuP′RSTUV− mutants KIM5-2044.21+ and KIM5-2060.21+, injected subcutaneously into NIH/Swiss Webster mice (Harlan Sprague-Dawley, Inc., Indianapolis, Ind.), were determined with a protocol described by Bearden et al. (4). Cells were grown at 26°C in PMH containing 50 μM hemin to potentially mimic the flea environment. For determinations of the LD50s of strains lacking the Ybt iron transport system, cells were grown at 37°C in deferrated PMH. Cells of KIM5-3173 (Ybt− Hmu+) and KIM5-2044.11+ (Ybt− Hmu−) were injected retro-orbitally (62) into BALB/c mice (Harlan Sprague-Dawley, Inc.). Groups of five mice were used for each dosage, and the bacterial doses used in each experiment ranged from ∼10 to 105 CFU (increasing in 10-fold increments) per 0.1-ml injection. The mice were monitored daily for 14 to 21 days. LD50s were calculated by a method described by Reed and Muench (50).
Nucleotide sequence accession number.
The 7,623-bp nucleotide sequence of the hmu locus has been deposited in the GenBank database under accession no. U60647.
RESULTS
Genetic characterization of the hmu locus.
Analysis of the DNA sequence of the hmu locus revealed eight ORFs, orfXY and hmuP′RSTUV, with the genetic organization shown in Fig. 1. The ORF for orfX is not complete, and analysis of the homologous sequence (∼99% identical at the DNA level) from the unfinished Y. pestis CO92 genomic sequence database of The Sanger Centre (65) shows a possible start site for orfX ∼57 bp upstream of the EcoRI restriction site. Only 33 nucleotides (nt) separate orfX from orfY, suggesting that these genes may be cotranscribed; however, 85 nt separate orfY from hmuP′.
The predicted amino acid sequences for OrfXY are homologous to ShuXY (Table 2), the predicted proteins from the S. dysenteriae hemin utilization gene cluster, shuTWXY, whose functions are unknown (71). A region spanning 22 amino acids within the carboxy-terminal end of OrfX (SVQFFNQQGEVMFKVYVGRDED) has 63% similarity and 50% identity with a 22-amino-acid region in the middle of Y. pestis HmuS (125-SIQFFDHQGDALHKVYVTEQTD-146) and similar homology with the HmuS homologs Y. enterocolitica HemS and S. dysenteriae ShuS and may represent a possible signature motif for these proteins.
TABLE 2.
Properties and homologies of proteins of the hmu locus and upstream genes
| Protein | Mass (kDa)a | pIa | Locationb (putative function) | Homology (% similarity/% identity)c |
|---|---|---|---|---|
| OrfXd | ? | ? | ? (unknown) | ShuX (67/57) |
| OrfY | 23.2 | 7.2 | IM (unknown) | ShuY (64/58) |
| HmuP′ | 4.5 | 4.7 | CP (unknown) | Truncated homolog of HemP (68/64) |
| HmuR | 74.2/71.2 | 4.8/4.7 | OMe (TonB-dependent receptor) | HemR (88/86), ShuA (75/69), ChuA (75/69), HutA (30/23), PhuR |
| HmuS | 39.1 | 5.4 | CPe (possibly involved in iron release from heme) | HemS (92/89), ShuS (73/66), PhuS (50/43) |
| HmuT | 29.6/26.8 | 74./5.9 | PP (periplasm-binding protein) | HemT (91/91), ShuT (73/66), PhuT (41/33), HutB (47/35) |
| HmuU | 35.5 | 11.6 | IM (permease) | HemU (96/93), ShuU (74/68), PhuU (54/58), HutC (51/41) |
| HmuV | 29.6 | 6.6 | IMf (ATP-binding protein) | HemV (91/88), ShuV (66/59), PhuV (51/45), HutD (51/42) |
Calculated masses and pIs were determined for each protein with the IG suite software package. The values for HmuR and HmuT are for unprocessed/processed proteins. Signal cleavage sites for HmuR and HmuT were predicted between amino acids 28 and 29 and between amino acids 25 and 26, respectively, by using the SignalP program (41).
Predicted protein locations were determined with the PSORT program (40). CP, cytoplasmic; PP, periplasmic.
Percent homologies were determined by using the Gap program of the GCG software package. A Gap comparison between HmuR and PhuR showed no significant homology; however, a Bestfit alignment of these two proteins showed 44% similarity of PhuR with HmuR over a stretch of 185 amino acids. Hem proteins are from Y. enterocolitica (accession no. X68147 and X77867 [59, 60]), Shu proteins are from S. dysenteriae (accession no. U64516 [38, 71]), ChuA is from E. coli (accession no. U67920 [67]), Phu proteins are from P. aeruginosa (accession no. AF055999 [43]), and Hut proteins are from V. cholerae (accession no. AF016580 [20, 42]).
The sequence for the gene encoding the deduced OrfX protein is incomplete and lacks a 5′ start site.
Protein locations of HmuR and HmuS were determined by Western blot analysis of cellular fractions of Y. pestis with HmuR- or HmuS-specific polyclonal antibodies.
HmuV, an ATP-binding protein, is most likely associated with HmuU, the IM permease.
The genetic organization of the Y. pestis hmuP′RSTUV genes is identical to that of the Y. enterocolitica hemin uptake genes, hemPRSTUV (59, 60), and the nomenclature for the hmu genes was derived from the highly homologous hem genes. Intergenic regions of 187 and 116 nt were observed between hmuP′ and hmuR and between hmuR and hmuS, respectively. However, the translational start codons for hmuS, hmuT, hmuU, and hmuV all overlap, suggesting that these four genes are cotranscribed. Sequence analysis of the hmu region and comparison with the Y. enterocolitica hem sequences (59, 60) identified putative promoters upstream of hmuP′ within the 85 nt separating orfY and hmuP′ (p1) and in the intergenic region between hmuR and hmuS (p2) (Fig. 1). Using the IG dyad program, we identified inverted repeats downstream of hmuR and hmuV with ΔGs of −13.2 and −30.5 kcal/mol, respectively, indicating possible sites for transcriptional termination.
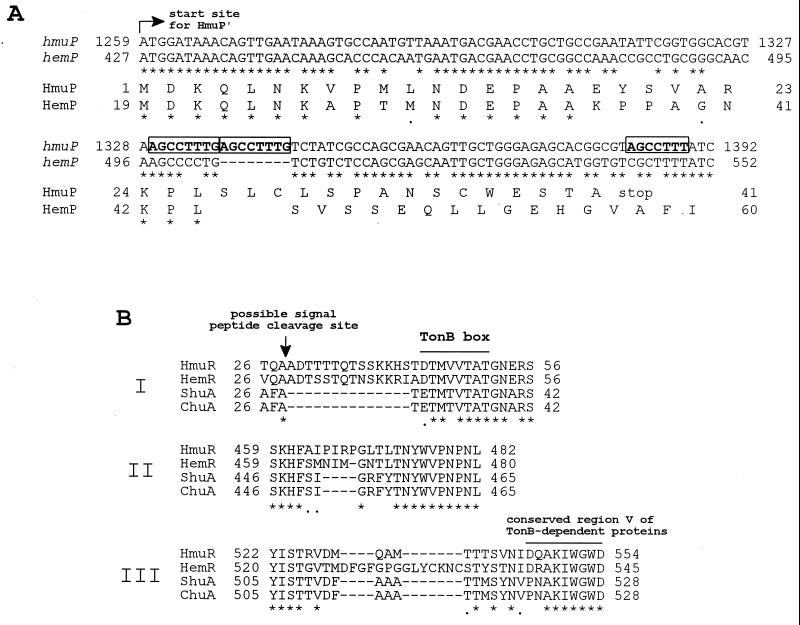
The predicted sizes, pIs, protein locations, and putative functions for HmuP′RSTUV, as well as percent homologies of these deduced amino acid sequences with similar sequences in the protein databases, are shown in Table 2. Analysis of the deduced amino acid sequence of HmuP′ showed that it is homologous with HemP over a stretch of 26 amino acids (Fig. 2A). The potential translational start site for hmuP′ is located 54 nt downstream of that designated for hemP. In addition, we found an 8-bp repeat (AGCCTTTG [Fig. 2A]) within the hmuP′ sequence that causes a shift in the ORF and a similar repeat sequence (AGCCTTT [Fig. 2A]) 39 bp downstream of the first repeat introducing a premature stop codon (TAG). To confirm that the mutations were not introduced during cloning or subsequent growth of strains containing these clones, we sequenced PCR-generated clones of the hmuP′ region from the KIM6+ and KIM6 chromosomes and found the repeated sequences within each chromosomal fragment. Thus, the resulting 41-amino-acid gene product, HmuP′, would have a different carboxy-terminal sequence and would be truncated in comparison to HemP (81 amino acids).
FIG. 2.
CLUSTAL W alignments of hmuP′ and HmuP′ sequences and selected HmuR sequences from Y. pestis with other homologous sequences. (A) Nucleotide and amino acid sequence comparisons of hmuP′ and the predicted translated gene product (HmuP′) of Y. pestis with hemP and HemP of Y. enterocolitica. Only the coding strands of hmuP′ and hemP are shown, and the translational start site for HmuP′ is indicated by the arrow. Repeated nucleotide sequences within hmuP′ are indicated by boldface letters in boxes. Conserved nucleotides or amino acids are indicated by asterisks. (B) Three regions (I, II, and III) of variability in hemin-uptake OM receptors from Y. pestis (HmuR), Y. enterocolitica (HemR), S. dysenteriae (ShuA), and E. coli O157:H7 (ChuA). The predicted signal peptide cleavage site for HmuR is indicated by the vertical arrow. The TonB box region and another conserved region (V) found to be associated with all TonB-dependent OM proteins are shown by overlines. Conserved amino acids are indicated by asterisks.
While HmuR has high homologies with HemR, ShuA, and ChuA (Table 2), there are three regions of notable variability in the primary structures between these OM proteins that lie outside of the conserved regions of TonB-dependent receptors. The first difference is in the sequence between the putative signal peptide cleavage site and the TonB box (Fig. 2B). While this region appears to be conserved within HmuR and HemR, it is absent from ShuA and ChuA. The second difference lies in the amino acid sequence just before conserved region IV of TonB-dependent proteins (Fig. 2B). Again, this region is conserved within HmuR and HemR; however, there are deletions within this area in ShuA and ChuA (Fig. 2B). Finally, the third difference was found in the sequence between conserved regions IV and V (region V shown on Fig. 2B) of TonB-dependent proteins. HmuR, ShuA, and ChuA all appear to contain a deletion within this area in comparison to HemR.
The deduced amino acid sequences of HmuTUV have homologies with members of binding protein-dependent transporters, a family of ABC transporters, involved in iron, hemin, hemoprotein, and vitamin B12 uptake (Table 2 and data not shown). A region within the amino-terminal portion of the 279-amino-acid HmuT protein (72-TLNAEGILAMKPTMLL-87) has similarities with the signature sequence of the cluster 8 siderophore-binding periplasmic proteins, including two of three conserved residues, a glutamate residue at position 5 and a proline residue at position 12 (64). The HmuU deduced amino acid sequence contains an EAA motif (223-EAAHYLGVNVRQAKLRLLLL-242) common to IM permeases (EAA(X3) G (X9) IXLP [54, 60]). The predicted HmuV amino acid sequence has a Walker A motif (44-GPNGAGKS-51) and a Walker B motif (169-LFLDE-173) similar to those of other ATP-binding proteins (22, 60).
Characterization of promoter activity from p1 and p2.
Stoljiljkovic and Hantke (59, 60) provided sequence data suggesting two separate operons, hemPR and hemSTUV, within the Y. enterocolitica hem cluster. Sequence analysis of the homologous Y. pestis hmu gene cluster revealed two potential promoters in the intergenic regions upstream of hmuP′ (designated p1) and upstream of hmuS (designated p2). We identified putative −35 regions, −10 regions, and Fur-binding sequences (FBS) for each promoter (Fig. 3). The FBS regions of p1 and p2 are 68 and 79% homologous to the E. coli consensus sequence (GATAATGATAATCATTATC), respectively. Although, the putative FBS within the p2 promoter region is 5 bp longer than the E. coli consensus FBS, it still exhibits dyad symmetry.
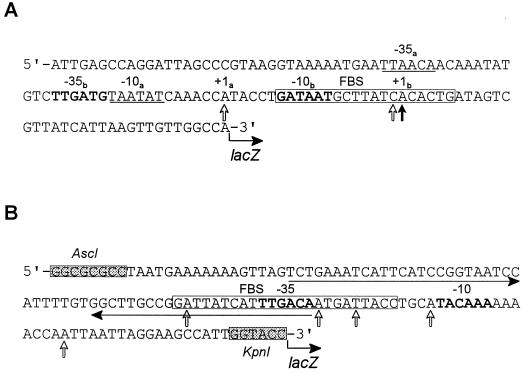
FIG. 3.
Nucleotide sequences of the p1 (A) and p2 (B) promoter regions. (A) The 123-bp fragment containing the p1 promoter (upstream of hmuP′) was cloned into a single-copy-number lacZ reporter plasmid, pEU730, generating pHMU44. (B) The 130-bp fragment of the intergenic sequence between hmuR and hmuS containing the p2 promoter region was cloned into pEU730, generating pHMU55. The shaded boxes shown in the p2 sequence are AscI and KpnI restriction sites that were designed into the oligonucleotide primers used in PCR. Potential FBS (in open boxes) were identified within p1 and p2; the p2 FBS is 5 bp longer than the E. coli consensus sequence (GATAATGATAATCATTATC). The solid vertical arrow in the p1 promoter region indicates the probable transcriptional start site of the major primer extension product of RNA isolated from Y. pestis KIM6+ or KIM6-2044.1(pHMU4)+. Open vertical arrows in both the p1 and p2 sequences show potential transcriptional start sites of the minor primer extension products of RNA isolated from KIM6+ (p1 only) and KIM6-2044.1(pHMU4)+. Boldface letters or underlined sequences indicate potential −35 and −10 regions. Within the p1 sequence, the −35b and −10b regions correspond to the major transcriptional start site (+1b), and the −35a and −10a regions may correspond to a weaker transcriptional start site (+1a). The horizontal arrows show imperfect inverted repeats within the p2 promoter region.
Primer extension analysis of the p1 region revealed one major product and two minor products from cellular RNA isolated from iron-depleted cells of Y. pestis KIM6+ and KIM6-2044.1(pHMU4)+ (data shown schematically in Fig. 3A). Two −35 and −10 regions that may correspond to these primary and secondary transcriptional start sites were identified by sequence analysis (Fig. 3A). No primer extension products were observed from RNA preparations from the ΔhmuP′RSTUV-2044.1 strain, KIM6-2044.1+, grown in iron-deficient medium or KIM6+ grown in iron-replete medium (data not shown). These results suggest that the FBS region in p1 is functional and regulated by iron and Fur at the transcriptional level. Mapping of the p2 region did not provide enough data to determine conclusively a possible transcriptional start site. Five minor products were observed within the p2 region by primer extension analysis of cellular RNA isolated from KIM6+ (at 100-μg RNA concentrations) and KIM6-2044.1(pHMU4)+ (at 30-μg RNA concentrations) grown in iron-deficient medium (data shown schematically in Fig. 3B), suggesting that the p2 promoter exhibits weak activity. No primer extension products were observed from KIM6-2044.1+ (ΔhmuP′RSTUV-2044.1) RNA preparations (data not shown). Potential −35 and −10 regions within this putative promoter sequence that overlap the putative transcriptional termination sequence at the end of hmuR were identified (Fig. 3B).
We generated promoter (p1 or p2) fusions to lacZ in pEU730, a low-copy-number reporter plasmid (17), and used the resulting constructs, pHMU44 (p1::lacZ) and pHMU55 (p2::lacZ), to examine iron and Fur regulation of transcription from p1 and p2 in Y. pestis KIM6+ (parent strain), KIM6-2044.1+ (ΔhmuP′RSTUV-2044.1), and KIM6-2030+ (fur9::kan). The β-galactosidase activities from the various strains carrying one or the other reporter plasmid are shown in Table 3. High β-galactosidase activities (∼27,000 Miller units) from strains containing the p1::lacZ fusion were observed, whereas low β-galactosidase activities (∼1,600 Miller Units) from strains containing the p2::lacZ fusion were observed (Table 3). These results correlate with RNA primer extension results, suggesting that p1 exhibits much stronger promoter activity than p2.
TABLE 3.
β-Galactosidase activities of Y. pestis KIM6+ derivatives containing p1::lacZ or p2::lacZ reporter plasmid
| Strain | β-Galactosidase activity of cells grown in deferrated PMH witha:
|
β-Galactosidase ratiosa
|
|||||
|---|---|---|---|---|---|---|---|
| No added iron | 10 μM FeCl3 | 10 μM Heminb | 2.5 μM Hemoglobinb | −Fe/+Fe | −Fe/+Hemin | −Fe/ +Hemoglobin | |
| With pHMU44 (p1::lacZ) | |||||||
| KIM6+ | 26,946 (±4,802) | 1,458 (±551) | 23,606 (±3,430) | 12,312 (±1,528) | 20.9 (±9.2) | 1.1 (±0.1) | 2.2 (±0.4) |
| KIM6-2044.1+ (ΔhmuP′RSTUV-2044.1) | 27,544 (±3,679) | 962 (±150) | 24,798 (±3,386) | 19,764 (±4,009) | 29.1 (±4.9) | 1.2 (±0.1) | 1.6 (±0.2) |
| KIM6-2030+ (fur::kan-9) | 27,960 (±3,444) | 26,300 (±3,653) | NTc | NT | 1.0 (±0.2) | NT | NT |
| With pHMU55 (p2::lacZ) | |||||||
| KIM6+ | 1,604 (±113) | 1,222 (±188) | 1,484 (±81) | 955 (±131) | 1.3 (±0.3) | 1.1 (±0.1) | 1.7 (±0.4) |
| KIM6-2044.1+ (ΔhmuP′RSTUV-2044.1) | 911 (±155) | 567 (±49) | 1,211 (±76) | 596 (±43) | 1.6 (±0.2) | 0.7 (±0.1) | 1.5 (±0.2) |
| KIM6-2030+ (fur::kan-9) | 1,098 (±161) | 828 (±73) | NT | NT | 1.3 (±0.3) | NT | NT |
Cells were grown approximately six generations in deferrated PMH at 37°C and harvested during mid-log-phase growth. β-Galactosidase activity is expressed in Miller units (35), and the values represent an average of individual reactions from whole-cell lysates. Mean β-galactosidase ratios were obtained from averaged β-galactosidase activities from three or four experiments. Standard deviations are shown in parentheses.
Desferrioxamine mesylate (Desferal) was added to a final concentration of 20 μM to chelate any contaminating inorganic iron in the hemin and hemoglobin solutions.
NT, not tested.
Expression of the p1::lacZ fusion in the Y. pestis parent strain or the ΔhmuP′RSTUV-2044.1 strain was inhibited strongly, >20-fold, in the presence of 10 μM FeCl3; however, expression of this promoter fusion in a Fur− strain was not affected by inorganic iron (Table 3). Thus, activity from p1 is regulated tightly by inorganic iron and the corepressor, Fur. In contrast, p1::lacZ activity did not appear to be repressed by 10 μM hemin but was repressed about twofold by 2.5 μM hemoglobin (Table 3), suggesting that hemoglobin (at an iron concentration equivalent to that of hemin) may be utilized more efficiently as an iron source than hemin. However, growth responses in liquid PMH with or without EDDA indicated that there is no preference for hemoglobin over hemin as an iron source and that these solutions contain very little inorganic iron contamination (data not shown). The expression of the p2::lacZ fusion in the various Y. pestis strains did not appear to be affected significantly by the presence of FeCl3, hemin, or hemoglobin (Table 3). Thus, activity from the p2 region does not appear to be regulated significantly by any iron source or Fur and may represent basal-level constitutive activity for this promoter. Similarities between the β-galactosidase activity ratios from KIM6-2044.1+ strains containing either pHMU44 or pHMU55 and those from their KIM6+ counterparts suggest that no genes within the deleted region of the KIM6-2044.1+ genome including the hmu genes are involved in regulation of promoter p1 or p2 (Table 3).
Analysis of HmuR and HmuS expression in Y. pestis.
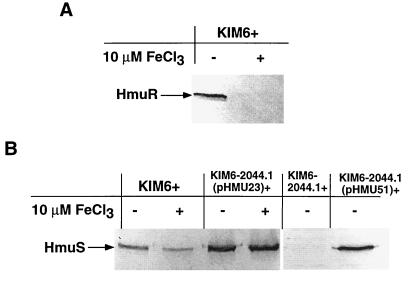
To determine whether hmu p1 and p2 promoter activity and regulation correlated with Hmu protein expression, we used Western blot analysis to detect production of HmuR and HmuS, one protein product of each putative operon (hmuP′R and hmuSTUV), by Y. pestis KIM6+ strains grown in the presence or absence of 10 μM FeCl3. HmuR production by the KIM6+ parent strain was inhibited strongly when inorganic iron was present in the medium (Fig. 4A), confirming that expression from the hmuP′R operon is tightly iron regulated. In contrast to the p2::lacZ data above, HmuS production from the parent strain was regulated slightly by iron; a decrease in the intensity of the HmuS band from KIM6+ grown with iron in comparison to that grown without iron was observed (Fig. 4B, lanes 1 and 2). We did not detect HmuS from whole-cell lysates of a ΔhmuP′RSTUV strain (KIM6-2044.1+) grown in the absence of iron (Fig. 4B, lane 5), suggesting that the HmuS antiserum is specific for HmuS and does not cross-react with any other protein encoded outside of the hmu locus.
FIG. 4.
Western blot analysis of expression of HmuR and HmuS from various Y. pestis KIM6+ isogenic strains. Iron-depleted cells of Y. pestis KIM6+ and KIM6+ derivatives were grown at 37°C in deferrated PMH medium in the presence or absence of 10 μM FeCl3. (A) Equal concentrations of proteins from whole-cell lysates of KIM6+ were separated in an SDS–9% polyacrylamide gel, and the immunoblot was reacted with anti-HmuR antiserum. (B) Equal concentrations of proteins from whole-cell lysates of KIM6+, KIM6-2044.1+ (ΔhmuP′RSTUV-2044.1), and KIM6-2044.1+ containing either pHMU23 (hmuR::Mu dI1734) or pHMU51 (hmuST′ clone) were separated in SDS–12% polyacrylamide gels, and the immunoblots were reacted with anti-HmuS antiserum.
Next, we examined whether HmuS production by Y. pestis is independent of HmuR production. The hmuS gene was cloned into pBR322, generating pHMU51 (Fig. 1); in vitro transcription-translation of pHMU51 showed a product ∼35 kDa in size, which corresponds to the predicted size for HmuS (data not shown). The hmuS clone was introduced into KIM6-2044.1+, and Western blot analysis confirmed that HmuS was expressed from pHMU51 (Fig. 4B, lane 6). Furthermore, we examined whether HmuS was produced by a KIM6-2044.1+ isogenic strain containing a plasmid (pHMU23) with a Mu dI1734 transcriptional fusion in hmuR that should exhibit a polar effect on transcription of downstream genes, hmuSTUV, if no other promoters are active except p1. While no HmuR was produced by this strain (data not shown), similar amounts of HmuS appeared to be produced by this strain grown with or without FeCl3 (Fig. 4B, lanes 3 and 4), suggesting that a promoter upstream of hmuS (probably within the p2 region) is functional but not iron regulated. The increase in the intensity of the bands in comparison to those from the parent strain, KIM6+, may be due to the moderate copy number of pHMU23 containing hmuS. Therefore, HmuS can be produced independently of HmuR probably as the result of weak activity from the p2 promoter.
Localization of HmuR and HmuS in Y. pestis cellular fractions.
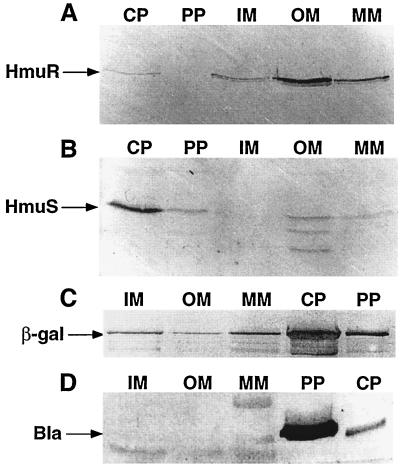
Mills and Payne (37) found that S. dysenteriae ShuA, an HmuR homolog, was localized to the OM; however, the cellular locations of other Hmu homologs have not been determined empirically. Computer analysis predicted HmuR and HmuS to be OM and cytoplasmic proteins, respectively. Western blot analysis of cellular fractions from Y. pestis KIM6(pRT240)+ confirmed that HmuR was found predominantly within the OM (Fig. 5A) and that HmuS was found predominantly within the cytoplasm (Fig. 5B). The presence of HmuR and HmuS in other cellular fractions (Fig. 5A and B) may be the result of incomplete separation of these proteins from the various cellular fractions. We could predict cross-contamination of proteins within cellular fractions during the separation procedure by Western blot analysis using antisera to proteins expressed from pRT240, β-lactamase (a periplasmic protein), and β-galactosidase (a cytoplasmic protein). As expected, our results showed that β-lactamase and β-galactosidase were found predominantly in the periplasmic and cytoplasmic fractions, respectively; however, these proteins were found in other cellular fractions as well (Fig. 5C and D), confirming minor cross-contamination of proteins between the various cellular fractions.
FIG. 5.
Localization of HmuR and HmuS in Y. pestis cellular fractions by Western blot analysis. Iron-depleted Y. pestis KIM6+(pRT240) cells were grown in deferrated PMH broth at 30°C. Periplasm (PP), cytoplasm (CP), IM, OM, and mixed-membrane (MM) fractions were isolated, and equal concentrations of proteins in each cell fraction were separated in an SDS–12% polyacrylamide gel. The immunoblot was reacted with anti-HmuR antiserum (A), anti-HmuS antiserum (B), anti-β-galactosidase (β-gal) IgG1 antibody (C), or anti-β-lactamase (Bla) polyclonal antibody (D).
Roles of hmu genes in hemoprotein utilization.
To examine the importance of individual hmu genes in the utilization of hemin and various hemoproteins, we determined the ability of KIM6-2061.1+ (in-frame ΔhmuR-2061.1), KIM6-2062.1+ (in-frame ΔhmuS-2062.1), KIM6-2063.1+ (hmuT::cat-2063.1), and KIM6-2044.1+ (ΔhmuP′RSTUV-2044.1) strains containing various cloned hmu genes to use hemin and hemoproteins (Table 4). KIM6-2044.1+, the Δhmu2044.1 mutant, could not grow around wells containing hemin or any hemoprotein; however, introduction of pHMU4, a clone containing the entire hmu locus, into this strain restored its ability to use hemin and the various hemoproteins as iron sources (24) (Table 4). Since pHMU4 contains an incomplete copy of orfX, the data suggest that orfXY are probably not involved in hemoprotein utilization. This was confirmed with pHMU35, which lacks orfX and the 5′ region of orfY but still contains hmuP′RSTUV; complementation of the ΔhmuP′RSTUV-2044.1 strain with this plasmid restored its ability to use hemin and hemoglobin (other hemoproteins were not tested) as sources of iron (Table 4).
TABLE 4.
Analysis of hemoprotein utilization by various Y. pestis hmu mutants
| Y. pestis strain | Relevant hmu characteristics
|
Hemoprotein utilization on PMHA-EDDA plates after 3 days at 37°Ca
|
||
|---|---|---|---|---|
| Chromosome | Plasmid | Growth | No growth | |
| KIM6+ | orfXY+ hmuP′RSTUV+ | None | He, Hb, Mb, Hb-Hp, He-alb, Hx | None |
| KIM6-2044.1+ | ΔorfXY ΔhmuP′RSTUV-2044.1 | None | None | He, Hb, Mb, Hb-Hp, He-alb, Hx |
| KIM6-2044.1(pHMU4)+ | ΔorfXY ΔhmuP′RSTUV-2044.1 | orfY+ hmuP′RSTUV+ | He, Hb, Mb, Hb-Hp, He-alb, Hx | None |
| KIM6-2044.1(pHMU35)+ | ΔorfXY ΔhmuP′RSTUV-2044.1 | ′orfY hmuP′RSTUV+ | He, Hb | NT |
| KIM6-2044.1(pHMU37)+ | ΔorfXY ΔhmuP′RSTUV-2044.1 | orfY+ hmuP′RS+ hmuT′ | Hb, Hb-Hp, Hx | He, Mb, He-alb |
| KIM6-2044.1(pHMU30)+ | ΔorfXY ΔhmuP′RSTUV-2044.1 | hmuP′R+ | None | He, Hb, Mb, Hb-Hp, He-alb, Hx |
| KIM6-2044.1(pHMU51)+ | ΔorfXY ΔhmuP′RSTUV-2044.1 | hmuS+ hmuT′ | None | He, Hb, Mb, Hb-Hp, He-alb, Hx |
| KIM6-2044.1(pHMU30 + pHMU51)+ | ΔorfXY ΔhmuP′RSTUV-2044.1 | hmuP′R+ hmuS+ humT′ | Hb | He |
| KIM6-2044.1(pHMU66)+ | ΔorfXY ΔhmuP′RSTUV-2044.1 | orfY+ hmuP′R+ ΔhmuS hmuTUV+ | He, Hb, Mb, Hb-Hp, He-alb, Hx | None |
| KIM6-2061.1+ | In-frame ΔhmuR-2061.1 | None | None | He, Hb, Mb, Hb-Hp, He-alb, Hx |
| KIM6-2061.1(pHMU30)+ | In-frame ΔhmuR-2061.1 | hmuP′R+ | He, Hb, Mb, Hb-Hp, He-alb, Hx | None |
| KIM6-2062.1+ | In-frame ΔhmuS-2062.1 | None | He, Hb, Mb, Hb-Hp, He-alb, Hx(+/−) | None |
| KIM6-2063.1+ | hmuT::cat-2063.1 | None | Hb, Hx | He, Mb, Hb-Hp(?), He-alb |
Approximately 107 cells were overlaid onto deferrated PMH–100 μM EDDA agarose (PMHA-EDDA) plates. Twenty microliters of 500 μM hemin (He), 10 μM hemoglobin (Hb), 500 μM myoglobin (Mb), 100 μM hemoglobin-haptoglobin (Hb-Hp), 200 μM hemin-albumin (He-alb), or 100 μM heme-hemopexin (Hx) was added to 0.3-cm wells cut into the agarose, and growth around the wells was recorded after 3 days at 37°C. The concentrations of Hb-Hp (50% saturated), He-alb (50% saturated), and Hx (95% saturated) refer to the concentrations of the respective carrier proteins within the mixture. These results are based on at least three experiments. Hx(+/−), growth in two of three experiments; Hb-Hp(?), no growth in five of nine experiments; NT, no hemoproteins tested other than hemin and hemoglobin.
KIM6-2044.1+ containing hmuP′RST′ on pHMU37 could utilize hemoglobin, hemoglobin-haptoglobin, and heme- hemopexin, but not hemin, myoglobin, or hemin-albumin, for growth, while hmuP′R(pHMU30) or hmuST′(pHMU51) alone did not allow KIM6-2044.1+ to use hemin or any hemoproteins for growth (Table 4). However, the presence of both plasmids in KIM6-2044.1+ restored growth on hemoglobin but not hemin (Table 4). KIM6-2063.1+ (hmuT::cat-2063.1) containing a polar chromosomal mutation that is genetically similar to KIM6-2044.1(pHMU37)+ in lacking functional hmuTUV genes utilized hemoglobin and heme-hemopexin but not hemin, myoglobin, or hemin-albumin (Table 4). In contrast to KIM6-2044.1(pHMU37)+, the hmuT::cat-2063.1 mutant did not grow around the hemoglobin-haptoglobin well in five of nine experiments and grew only poorly around this hemoprotein complex when we did observe growth (Table 4). An in-frame deletion within hmuR on the chromosome generated a mutant (KIM6-2061.1+) that was unable to use hemin or any hemoprotein for growth; however, introduction of the hmuP′R clone into this mutant restored its ability to use hemin and all hemoproteins for growth (Table 4). Finally, mutants containing an in-frame deletion of hmuS either on a plasmid (KIM6-2044.1[pHMU66]+) or on the chromosome (KIM6-2061.1+) were capable of using hemin and all hemoproteins for growth on PMHA-EDDA plates (Table 4).
Since the deletion in the KIM6-2044.1+ chromosome encompasses ∼2 kb more DNA on either side of the hmu genes, we constructed a second ΔhmuP′RSTUV mutant strain, KIM6-2060.1+, which contains a deletion encompassing only hmuP′RSTUV (Fig. 1). The patterns of heme compounds used by both mutants with and without various complementing plasmids were the same (data not shown), indicating that genes adjacent to hmuP′RSTUV are not required for hemoprotein utilization.
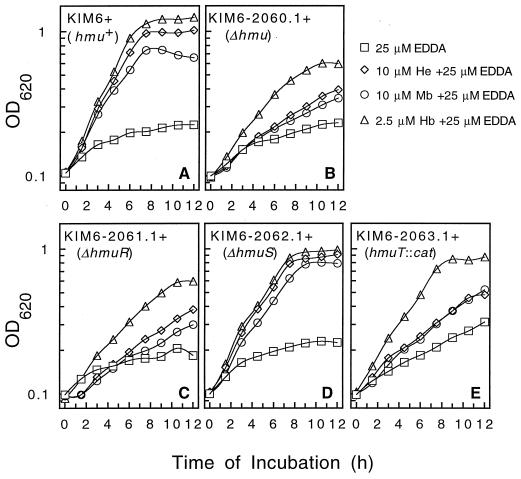
Furthermore, we examined the growth of KIM6+ (parent strain), KIM6-2060.1+ (ΔhmuP′RSTUV-2060.1), and the various chromosomal mutants—KIM6-2061.1+ (in-frame ΔhmuR-2061.1), KIM6-2062.1+ (in-frame ΔhmuS-2062.1), and KIM6-2063.1+ (hmuT::cat-2063.1)—in broth cultures (PMH–25 μM EDDA) containing 10 μM hemin, 10 μM myoglobin, or 2.5 μM hemoglobin to determine growth responses over a shorter period of time (Fig. 6). While the parent strain and the ΔhmuS-2062.1 mutant grew well with all three iron sources (Fig. 6A and D), the ΔhmuP′RSTUV-2060.1 and ΔhmuR-2061.1 mutants grew poorly with all three iron sources; however, the growth of both of the latter mutants was stimulated moderately in the presence of hemoglobin (Fig. 6B and C). The hmuT::cat-2063.1 insertional mutant responded poorly to hemin and myoglobin, but it attained near-wild-type growth levels with hemoglobin (Fig. 6E), indicating that the hmuTUV gene products are not essential for use of hemoglobin.
FIG. 6.
Growth of Y. pestis KIM6+ derivatives in PMH-EDDA containing various heme compounds. Iron-depleted cells (described in Materials and Methods) were transferred to PMH–25 μM EDDA medium alone or medium supplemented with 10 μM hemin (He), 10 μM myoglobin (Mb), or 2.5 μM hemoglobin (Hb). The Y. pestis strains used in these experiments include KIM6+ (Hmu+ parent strain) (A), KIM6-2060.1+ (ΔhmuP′RSTUV-2060.1) (B), KIM6-2061.1+ (in-frame ΔhmuR-2061.1) (C), KIM6-2062.1+ (in-frame ΔhmuS-2062.1) (D), and KIM6-2063.1 (hmuT::cat-2063.1) (E). The cells were grown at 37°C, and growth was monitored by measuring the optical density of each culture at 620 nm (OD620). These graphs depict one of two separate experiments.
Mills and Payne (37, 38) found that S. dysenteriae shuA, an hmuR homolog, allowed hemin utilization by E. coli 1017, an HB101-derived siderophore synthesis mutant incapable of transporting hemin. To determine whether hmuR would allow hemin uptake by E. coli 1017, we introduced pHMU4 (hmuP′RSTUV clone), pHMU30 (hmuP′R), pHMU37 (hmuP′RST′), and pHMU66 (hmuP′RTUV, in-frame ΔhmuS) into this E. coli strain and examined growth of the various constructed strains around a well containing ∼3 or 0.5 mM hemin on TGA-EDDA plates. After 3 to 5 days at 37°C, no growth was observed for 1017 alone or 1017(pHMU30) around any hemin well; however we did observe growth of 1017(pHMU4), 1017(pHMU37), and 1017(pHMU66) around the hemin wells (data not shown). Thus, hmuP′R in combination with hmuS or hmuTUV are required to allow hemin usage by E. coli 1017.
Evidence for a secondary hemoglobin uptake system.
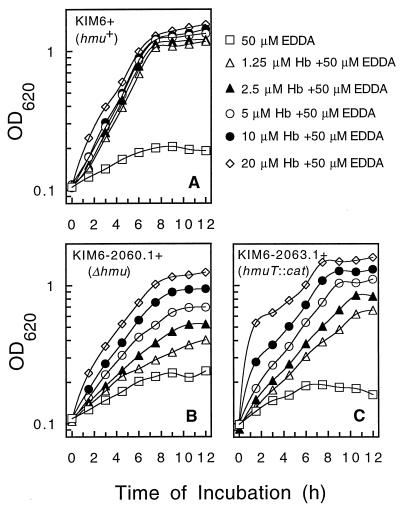
The results from Fig. 6B suggested that KIM6-2060.1+ (ΔhmuP′RSTUV-2060.1 mutant) could utilize hemoglobin via a secondary or lower-affinity hemoglobin transport system. Therefore, we examined the growth responses of KIM6+, KIM6-2060.1+ (ΔhmuP′RSTUV-2060.1), and KIM6-2063.1+ (hmuT::cat-2063.1) cells in PMH–50 μM EDDA to increasing concentrations of hemoglobin (1.25, 2.5, 5, 10, and 20 μM) (Fig. 7). KIM6+ exhibited maximum growth with as little as 1.25 μM hemoglobin (Fig. 7A). However, the growth rates of both mutants increased with increasing concentrations of hemoglobin (Fig. 7B and C); similar results were observed with the ΔhmuP′RSTUV-2044.1 mutant, KIM6-2044.1+ (data not shown). These results suggest that a lower-affinity hemoglobin uptake system may be present within Y. pestis.
FIG. 7.
Growth of Y. pestis KIM6+ derivatives in PMH-EDDA with increasing concentrations of hemoglobin. Iron-depleted cells (described in Materials and Methods) were transferred to PMH–50 μM EDDA containing 1.25, 2.5, 5, 10, or 20 μM hemoglobin (Hb). The Y. pestis strains used in these experiments include KIM6+ (Hmu+ parent strain) (A), KIM6-2060.1+ (ΔhmuP′RSTUV-2060.1) (B), and KIM6-2063.1+ (hmuT::cat-2063.1) (C). The cells were grown at 37°C, and growth was monitored by measuring the optical density of each culture at 620 nm (OD620). These graphs depict one of two separate experiments.
To determine whether the response of the Δhmu mutants was specific for hemoglobin and not due to release of hemin, we grew KIM6+ and KIM6-2060.1 in PMH–50 μM EDDA broth with increasing concentrations of hemin 5, 10, 20, 40, and 80 μM) corresponding to hemin-equivalent concentrations of hemoglobin used in the previous experiment. The growth response of KIM6+ was similar to that shown in Fig. 7A for KIM6+ grown with hemoglobin (data not shown). The growth response for KIM6-2060.1+ at all concentrations of hemin was poor and never exceeded that observed for KIM6-2060.1+ grown in 5 μM hemoglobin (data not shown). Also, we examined growth of KIM6-2060.1+ in PMH–50 μM EDDA containing 5 μM hemoglobin and 10 μM bovine serum albumin (to bind any free hemin) and found no significant difference in the growth of this mutant with or without serum albumin (data not shown). These results suggest that the growth response to hemoglobin is specific and not due to degradation of hemoglobin.
LD50 studies in mice infected subcutaneously or intravenously with HmuP′RSTUV− strains.
The effect of an HmuP′RSTUV− phenotype on infections within mice was examined by comparing the LD50 of the ΔhmuP′RSTUV-2044.1 or ΔhmuP′RSTUV-2060.1 deletion mutant with that of parent strains via subcutaneous or retro-orbital (intravenous) routes of infection. The LD50 for BALB/c mice injected retro-orbitally with the Y. pestis parent strain KIM5-3173 (Δpgm yopJ::Mu dI1734) or the isogenic strain KIM5-2044.11 (ΔhmuP′RSTUV-2044.1 Δpgm yopJ::Mu dI1734) was 73 and 91 CFU, respectively. As well, the LD50 for NIH/Swiss Webster mice injected subcutaneously with either KIM5-2044.21+ (ΔhmuP′RSTUV-2044.1 Δpsa2053.1 yopJ::Mu dI1734) or KIM5-2060.21+ (ΔhmuP′RSTUV-2060.1 Δpsa2053.1 yopJ::Mu dI1734) was 88 and 42 CFU, respectively. In comparison, the LD50 for NIH/Swiss Webster mice injected subcutaneously with the parent strain, KIM2053.11+, was reported previously as 130 CFU by Bearden et al. (4). These results indicate that the ΔhmuP′RSTUV-2044.1 and ΔhmuP′RSTUV-2060.1 mutations had no significant effect on virulence in mammals whether infected subcutaneously or intravenously.
DISCUSSION
The ability of pathogenic bacteria to acquire iron from free heme and host hemoproteins has been studied by many laboratories (reviewed in references 7 and 26). In various Vibrio spp., Haemophilus spp., and Neisseria spp., one or more TonB-dependent OM receptors that bind specifically to free hemin and/or one or more hemoproteins have been isolated (9, 14, 27–29, 31, 33, 51, 61). Unique heme carrier uptake systems in Serratia marcescens (Has system) and Haemophilus influenzae (Hxu system), consisting of a secreted protein that acquires heme from hemoglobin and heme-hemopexin, respectively, and a heme carrier-specific OM receptor, have been described (8, 9, 19). Homologous hemin transport systems in Y. enterocolitica and S. dysenteriae that are involved in transporting the entire heme moiety into the cytoplasm have been described (37, 38, 59, 60, 71). These systems consist primarily of a TonB energy-dependent OM receptor (HemR, ShuA), a transport system homologous to a family of ATP-binding cassette (ABC) transporters (HemTUV, ShuTUV), and a putative heme-degrading protein (HemS, ShuS). Several pathogenic E. coli strains contain a gene that hybridizes to shuA of S. dysenteriae (71), and a shuA homolog, chuA, was cloned from E. coli O157:H7 (67). Components of a hemin transport system discovered in Vibrio cholerae have been shown to have homologies with those of Y. enterocolitica and S. dysenteriae (20, 42). Previously, we determined that Y. pestis contains a hemin/hemoprotein utilization system which we designated Hmu, since only modest hybridization to Y. enterocolitica DNA was detected (24). In this study, we identified eight genes (orfXY, hmuP′RSTUV) within an 8.6-kb chromosomal fragment whose deduced amino acid sequences have high degrees of identity and similarity to those of the Hem hemin transport system of Y. enterocolitica. In addition, the genetic organization of the hmuP′RSTUV genes is identical to that of the hemPRSTUV genes (59, 60). The amino acid sequences and signature motifs of HmuT (periplasmic hemin-binding protein), HmuU (IM permease), and HmuV (ATP-binding protein) are nearly identical to their Y. enterocolitica counterparts, HemTUV, and have been discussed in detail by Stojiljkovic and Hantke (60). Significant similarities to the S. dysenteriae Shu, V. cholerae Hut, and Pseudomonas aeruginosa Phu hemin transport systems were apparent (Table 2). Southern blot analysis with a Y. pestis DNA probe containing the hmu locus showed modest hybridization to genomic DNA from Y. enterocolitica, E. coli, S. dysenteriae, Salmonella enteritidis, Klebsiella pneumoniae, and Proteus vulgaris, suggesting that the latter three bacteria may possess related hemin transport systems. Yersinia pseudotuberculosis DNA exhibited strong hybridization to the hmu fragment, indicating that it likely contains a homologous Hmu/Hem hemin transport system (24).
In contrast to the deduced amino acid sequences of HmuSTUV, HmuP′ and HmuR exhibited differences in deduced amino acid sequences from those of HemP (protein with unknown function) and HemR (TonB-dependent OM protein). HmuP′ is truncated in comparison to HemP due to an 8-bp repeated sequence within the nucleotide sequence that may be indicative of excision of a transposable element. Stoljiljkovic and Hantke (60) provided evidence that HemP may not be essential for utilization of hemin, at least by E. coli; therefore, disruption of the hmuP′ ORF by the direct repeat may not affect hemin and hemoprotein utilization by Y. pestis. The differences in the primary amino acid sequences of the OM receptors HmuR and HemR were within one region in the carboxy-terminal end of the proteins between conserved regions IV and V of TonB-dependent receptors described by Kadner (25). Differences in the primary amino acid sequences of the HmuR/HemR-homologous proteins, S. dysenteriae ShuA and E. coli ChuA, were also apparent within this region, as well as within two other regions located at the amino-terminal ends of these proteins and before conserved region IV of TonB-dependent receptors. Either Y. enterocolitica hemR or S. dysenteriae shuA was the only gene necessary to allow hemin utilization by E. coli HB101-derived strains that are naturally incapable of transporting hemin (37, 60). However, either hmuS or hmuTUV were needed in addition to hmuR for hemin utilization by E. coli HB101 derivatives. These differences in hemin utilization by E. coli strains containing shuA, hemR, or hmuP′R and the differences in the primary amino acid sequences of the gene products might indicate structural or functional differences in these OM receptors in interacting either with the hemin moiety or with the cytoplasmic membrane transport system.
Many iron and heme transport systems are repressed by iron and Fur under iron-rich conditions (10, 26). Expression of Y. enterocolitica hemR and S. dysenteriae shuA was found to be repressed by iron in a Fur-dependent manner (38, 59). As well, Mills and Payne (38) found that shuA was also repressed by hemin, but to a lesser extent than that found for iron. Stoljiljkovic and Hantke (59, 60) identified two possible promoters within the hem locus of Y. enterocolitica as elements of two operons, one upstream of hemPR that contained an FBS and one upstream of hemSTUV. We identified similar promoter regions upstream of hmuP′R (p1) and hmuSTUV (p2). Studies with a p1::lacZ reporter fusion showed that p1 was a strong promoter that was regulated by iron in a Fur-dependent manner, and Western blot analysis of whole-cell extracts with anti-HmuR antiserum confirmed that HmuR was absent under iron-rich conditions. Studies with a p2::lacZ reporter fusion showed that p2 promoter activity is much weaker than that of p1 and does not appear to be iron or Fur regulated, suggesting that the putative FBS, which is 5 nt longer than the FBS consensus sequence, may not be recognized by the Fur-Fe2+ complex. In contrast, Western blot analysis shows that expression of HmuS by Y. pestis cells exhibits modest iron regulation when the entire hmu system is intact. However, expression of HmuS from Y. pestis ΔhmuP′RSTUV-2044.1 containing either an hmuS clone or an hmuP′RSTUV clone with a polar mutation in hmuR did not appear to be regulated by iron. The most likely explanation of modest iron regulation of HmuS expression is that p1, a strong iron/Fur-regulated promoter, may produce an hmuP′RSTUV transcript and p2, a weak constitutive promoter, may produce an hmuSTUV transcript. Further experiments examining the number and sizes of transcripts from the hmu locus will be necessary to confirm this hypothesis.
Mutations in various hemin transport genes from Y. enterocolitica (HemPRSTUV), S. dysenteriae (ShuA, ShuS, ShuT, ShuUV), and V. cholerae (HutA, HutBCD) have been shown to affect hemin uptake to various degrees (20, 37, 38, 42, 59, 60, 71). However, the roles of these genes in hemoprotein utilization have not been studied. The hmu locus from Y. pestis allows it to use hemin as well as host hemoproteins including hemoglobin, myoglobin, hemin-albumin, heme-hemopexin, and hemoglobin-haptoglobin as sources of iron (24). OrfX and OrfY, which are homologous to S. dysenteriae ShuX and ShuY, do not appear to have functional roles in hemin/hemoprotein transport by Y. pestis.
Surprisingly, mutations altering production of HmuR, HmuS, or HmuTUV affected hemin and hemoprotein utilization by Y. pestis to various degrees. HmuR plays an important role in the utilization of hemin and all hemoproteins as iron sources. An in-frame ΔhmuR-2061.1 mutation on the Y. pestis chromosome prevents growth on iron-deficient medium containing hemin or any hemoprotein at low concentrations. Western blot analysis confirmed that HmuR is an OM protein, so the ability of this protein to bind to hemin or the various hemoproteins suggests that it recognizes a common structural component or the heme moiety for each of these compounds. In contrast, Y. pestis hmuTUV-negative cells did not use hemin, myoglobin, or hemin-albumin but still utilized hemoglobin and heme-hemopexin. As well, disruption of various genes encoding homologous ABC transport systems of Y. enterocolitica, S. dysenteriae, and V. cholerae inhibits uptake of hemin (21, 42, 60, 71), and the cloned Hmu, Hem, Shu, and Hut systems are sufficient for uptake of the entire heme moiety into E. coli (21, 24, 37, 59). This suggests that while the Hmu ABC transporter may translocate the entire hemin moiety into the cytoplasm, it is not exclusively involved in uptake of iron or heme from hemoglobin and heme-hemopexin through the cytoplasmic membrane.
Mutants carrying an in-frame deletion within hmuS on the chromosome or on a moderate-copy-number plasmid could still utilize hemin and hemoproteins for growth. Stoljiljkovic and Hantke (60) suggested that Y. enterocolitica HemS was required to reduce heme toxicity in E. coli when hemPR was present on a high-copy-number plasmid. However, the increased copy number of the hmuP′R and hmuTUV genes on the moderate-copy-number plasmid containing an in-frame ΔhmuS did not appear to produce any deleterious effects in Y. pestis. Wyckoff et al. (71) showed that disruption of S. dysenteriae shuS did affect the colony size of Salmonella typhimurium on iron-chelated, hemin agar plates. While our study provides evidence that HmuS plays a role in the utilization of some hemoproteins, its role is still enigmatic. Genetic constructs that are hmuP′RS+ and hmuTUV-negative use hemoglobin and heme-hemopexin, but not hemin, myoglobin, or hemin-albumin, while hmuP′R+ hmuSTUV-negative cells cannot use hemin or any hemoprotein at low concentrations. Increased expression of HmuR and HmuS from a moderate-copy-number plasmid may allow use of hemoglobin-haptoglobin by the ΔhmuP′RSTUV-2044.1 mutant, which lacks the HmuTUV transport system. While HmuP′RS appear to be involved in hemoglobin and heme-hemopexin utilization, uptake of either heme or hemoprotein fragments from these compounds through the cytoplasmic membrane in the absence of HmuTUV may be mediated by a lower-affinity system.
It is not uncommon for pathogenic bacteria to possess more than one transport system for iron or heme/hemoprotein uptake (7, 26). The growth rate of a ΔhmuP′RSTUV-2060.1 mutant in broth cultures containing increasing concentrations of hemoglobin was only slightly lower than that observed for the hmuT::cat-2063.1 mutant, and yet this growth response was specific for hemoglobin and not a hemin degradation product. This observation suggests that an Hmu-independent lower-affinity uptake system for hemoglobin may exist in Y. pestis. From our analysis of the roles of hmu genes in hemin and hemoprotein utilization, we hypothesize that at least three hemin/hemoprotein uptake mechanisms may be present in Y. pestis: (i) HmuR and HmuTUV constitute a system involved in uptake of the heme moiety from host hemoproteins used by Y. pestis; (ii) HmuR, HmuS, and an unidentified lower-affinity cytoplasmic transport system may be components of a system in the use of hemoglobin and heme-hemopexin; and (iii) a lower-affinity Hmu-independent uptake system may be involved in hemoglobin utilization. The hypothesis that an Hmu-independent system functions in Y. pestis KIM6+ is strengthened by our identification of has-like sequences in the unfinished Y. pestis CO92 genomic sequence database of The Sanger Centre (65).
Acquisition of iron is critical for pathogenic bacteria to grow and survive during the progression of an infection; therefore, the ability of the pathogen to scavenge iron in various microenvironments within its host is essential for survival (34). Y. pestis strains containing deletions in various components of the Ybt system or a deletion of the pgm locus (Δpgm) encompassing both the hemin storage system and the Ybt system are essentially avirulent via the subcutaneous route of infection in mice; however, a Δpgm mutant remains virulent via the intravenous route of infection in mice by utilizing the Yfe iron and manganese transport system (4, 5, 44). We found that Y. pestis strains lacking hmuP′RSTUV are as virulent as their corresponding parent strains when injected subcutaneously or retro-orbitally (an intravenous route of infection). Thus, the HmuP′RSTUV hemin transport system does not appear to be essential by these routes of infection in mice. Further experiments will be required to determine if this hemin uptake system is required in other animal models, in the flea, or in other routes or stages of infection such as a pneumonic route of infection or an intracellular stage of infection. Alternatively, the putative secondary hemoglobin and heme-hemopexin utilization systems or the various inorganic transport systems may serve as alternative systems that can acquire sufficient iron to counteract the loss of the Y. pestis Hmu system. If the has genes detected in Y. pestis CO92 (65) are present and functional in KIM strains, it may be necessary to mutate both the hmu and has systems to observe in vivo effects on heme-iron acquisition.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI33481 from the National Institutes of Health.
Rabbit hemopexin was kindly provided by M. Pendrak. Shelley Payne kindly provided E. coli 1017. We thank Vinnie Bertolino for participation in sequencing part of the hmu locus and constructing some of the clones used in analyzing promoter activity and developing antibodies to various Hmu proteins. We also thank Jackie Fetherston and Scott Bearden for participation in the animal work required for developing antibodies to HmuR and HmuS and for testing an hmu mutant for virulence.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1987. [Google Scholar]
- 4.Bearden S W, Fetherston J D, Perry R D. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect Immun. 1997;65:1659–1668. doi: 10.1128/iai.65.5.1659-1668.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bearden S W, Perry R D. The Yfe system of Yersinia pestis transports iron and manganese and is required for full virulence of plague. Mol Microbiol. 1999;32:403–414. doi: 10.1046/j.1365-2958.1999.01360.x. [DOI] [PubMed] [Google Scholar]
- 6.Bearden S W, Staggs T M, Perry R D. An ABC transporter system of Yersinia pestis allows utilization of chelated iron by Escherichia coli SAB11. J Bacteriol. 1998;180:1135–1147. doi: 10.1128/jb.180.5.1135-1147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun V, Hantke K, Köster W. Bacterial iron transport: mechanisms, genetics, and regulation. Met Ions Biol Syst. 1998;35:67–145. [PubMed] [Google Scholar]
- 8.Cope L D, Thomas S E, Hrkal Z, Hansen E J. Binding of heme-hemopexin complexes by soluble HxuA protein allows utilization of this complexed heme by Haemophilus influenzae. Infect Immun. 1998;66:4511–4516. doi: 10.1128/iai.66.9.4511-4516.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cope L D, Yogev R, Müller-Eberhard U, Hansen E J. A gene cluster involved in the utilization of both free heme and heme:hemopexin by Haemophilus influenzae type b. J Bacteriol. 1995;177:2644–2653. doi: 10.1128/jb.177.10.2644-2653.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crosa J H. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol Mol Biol Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daskaleros P A, Stoebner J A, Payne S M. Iron uptake in Plesiomonas shigelloides: cloning of the genes for the heme-iron uptake system. Infect Immun. 1991;59:2706–2711. doi: 10.1128/iai.59.8.2706-2711.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins C, Totten P A, Olsen B, Thomas C E. Role of the Haemophilus ducreyi Ton system in internalization of heme from hemoglobin. Infect Immun. 1998;66:151–160. doi: 10.1128/iai.66.1.151-160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fetherston J D, Bearden S W, Perry R D. YbtA, an AraC-type regulator of the Yersinia pestis pesticin/yersiniabactin receptor. Mol Microbiol. 1996;22:315–325. doi: 10.1046/j.1365-2958.1996.00118.x. [DOI] [PubMed] [Google Scholar]
- 16.Fetherston J D, Lillard J W, Jr, Perry R D. Analysis of the pesticin receptor from Yersinia pestis: role in iron-deficient growth and possible regulation by its siderophore. J Bacteriol. 1995;177:1824–1833. doi: 10.1128/jb.177.7.1824-1833.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froehlich B, Husmann L, Caron J, Scott J R. Regulation of rns, a positive regulatory factor for pili of enterotoxigenic Escherichia coli. J Bacteriol. 1994;176:5385–5392. doi: 10.1128/jb.176.17.5385-5392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gehring A M, DeMoll E, Fetherston J D, Mori I, Mayhew G F, Blattner F R, Walsh C T, Perry R D. Iron acquisition in plague: modular logic in enzymatic biogenesis of yersiniabactin by Yersinia pestis. Chem Biol. 1998;5:555–568. doi: 10.1016/s1074-5521(98)90115-6. [DOI] [PubMed] [Google Scholar]
- 19.Ghigo J-M, Létoffé S, Wandersman C. A new type of hemophore-dependent heme acquisition system of Serratia marcescens reconstituted in Escherichia coli. J Bacteriol. 1997;179:3572–3579. doi: 10.1128/jb.179.11.3572-3579.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson D P, Payne S M. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB-dependent proteins. J Bacteriol. 1994;176:3269–3277. doi: 10.1128/jb.176.11.3269-3277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson D P, Payne S M. Cloning and characterization of the Vibrio cholerae genes encoding the utilization of the iron from haemin and haemoglobin. Mol Microbiol. 1993;7:461–469. doi: 10.1111/j.1365-2958.1993.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 22.Higgins C F. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 23.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 24.Hornung J M, Jones H A, Perry R D. The hmu locus of Yersinia pestis is essential for utilization of free haemin and haem-protein complexes as iron sources. Mol Microbiol. 1996;20:725–739. doi: 10.1111/j.1365-2958.1996.tb02512.x. [DOI] [PubMed] [Google Scholar]
- 25.Kadner R J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990;4:2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee B C. Quelling the red menace: haem capture by bacteria. Mol Microbiol. 1995;18:383–390. doi: 10.1111/j.1365-2958.1995.mmi_18030383.x. [DOI] [PubMed] [Google Scholar]
- 27.Lee B C, Levesque S. A monoclonal antibody directed against the 97-kilodalton gonococcal hemin-binding protein inhibits hemin utilization by Neisseria gonorrhoeae. Infect Immun. 1997;65:2970–2974. doi: 10.1128/iai.65.7.2970-2974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lewis L A, Dyer D W. Identification of an iron-regulated outer membrane protein of Neisseria meningitidis involved in the utilization of hemoglobin complexed to haptoglobin. J Bacteriol. 1995;177:1299–1306. doi: 10.1128/jb.177.5.1299-1306.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis L A, Gray E, Wang Y-P, Roe B A, Dyer D W. Molecular characterization of hpuAB, the haemoglobulin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol. 1997;23:737–749. doi: 10.1046/j.1365-2958.1997.2501619.x. [DOI] [PubMed] [Google Scholar]
- 30.Lillard J W, Jr, Bearden S W, Fetherston J D, Perry R D. The haemin storage (Hms+) phenotype of Yersinia pestis is not essential for the pathogenesis of bubonic plague in mammals. Microbiology. 1999;145:197–209. doi: 10.1099/13500872-145-1-197. [DOI] [PubMed] [Google Scholar]
- 31.Litwin C M, Byrne B L. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect Immun. 1998;66:3134–3141. doi: 10.1128/iai.66.7.3134-3141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucier T S, Fetherston J D, Brubaker R R, Perry R D. Iron uptake and iron-repressible polypeptides in Yersinia pestis. Infect Immun. 1996;64:3023–3031. doi: 10.1128/iai.64.8.3023-3031.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maciver I, Latimer J L, Liem H H, Muller-Eberhard U, Hrkal Z, Hansen E J. Identification of an outer membrane protein involved in utilization of hemoglobin-haptoglobin complexes by nontypeable Haemophilus influenzae. Infect Immun. 1996;64:3703–3712. doi: 10.1128/iai.64.9.3703-3712.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mietzner T A, Morse S A. The role of iron-binding proteins in the survival of pathogenic bacteria. Annu Rev Nutr. 1994;14:471–493. doi: 10.1146/annurev.nu.14.070194.002351. [DOI] [PubMed] [Google Scholar]
- 35.Miller J H. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 36.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mills M, Payne S M. Genetics and regulation of heme iron transport in Shigella dysenteriae and detection of an analogous system in Escherichia coli O157:H7. J Bacteriol. 1995;177:3004–3009. doi: 10.1128/jb.177.11.3004-3009.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills M, Payne S M. Identification of shuA, the gene encoding the heme receptor of Shigella dysenteriae, and analysis of invasion and intracellular multiplication of a shuA mutant. Infect Immun. 1997;65:5358–5363. doi: 10.1128/iai.65.12.5358-5363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muller-Eberhard U, Nikkilä H. Transport of tetrapyrroles by proteins. Semin Hematol. 1989;26:86–104. [PubMed] [Google Scholar]
- 40.Nakai K, Kanehisa M. Expert system for predicting protein localization sites in Gram-negative bacteria. Proteins. 1991;11:95–110. doi: 10.1002/prot.340110203. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Occhino D A, Wyckoff E E, Henderson D P, Wrona T J, Payne S M. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol Microbiol. 1998;29:1493–1507. doi: 10.1046/j.1365-2958.1998.01034.x. [DOI] [PubMed] [Google Scholar]
- 43.Ochsner U A, Johnson Z, Vasil Z, Vasil M L. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. DNA sequence and expression of a Pseudomonas aeruginosa receptor and ABC transporter locus homologous to haemin iron uptake systems of bacterial pathogens, abstr. B258; p. 98. [Google Scholar]
- 44.Perry R D, Fetherston J D. Yersinia pestis—etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perry R D, Lucier T S, Sikkema D J, Brubaker R R. Storage reservoirs of hemin and inorganic iron in Yersinia pestis. Infect Immun. 1993;61:32–39. doi: 10.1128/iai.61.1.32-39.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry R D, Pendrak M L, Schuetze P. Identification and cloning of a hemin storage locus involved in the pigmentation phenotype of Yersinia pestis. J Bacteriol. 1990;172:5929–5937. doi: 10.1128/jb.172.10.5929-5937.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perry R D, Brubaker R R. Accumulation of iron by yersiniae. J Bacteriol. 1979;137:1290–1298. doi: 10.1128/jb.137.3.1290-1298.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price S B, Cowen C, Perry R D, Straley S C. The Yersinia pestis V antigen is a regulatory protein necessary for Ca2+-dependent growth and maximal expression of low-Ca2+ response virulence genes. J Bacteriol. 1991;173:2649–2657. doi: 10.1128/jb.173.8.2649-2657.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reece K S, Phillips G J. New plasmids carrying antibiotic-resistance cassettes. Gene. 1995;165:141–142. doi: 10.1016/0378-1119(95)00529-f. [DOI] [PubMed] [Google Scholar]
- 50.Reed L J, Muench H. A simple method for estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 51.Ren Z, Jin H, Morton D J, Stull T L. hgpB, a gene encoding a second Haemophilus influenzae hemoglobin- and hemoglobin-haptoglobin-binding protein. Infect Immun. 1998;66:4733–4741. doi: 10.1128/iai.66.10.4733-4741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 53.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saurin W, Köster W, Dassa E. Bacterial binding protein-dependent permeases: characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol Microbiol. 1994;12:993–1004. doi: 10.1111/j.1365-2958.1994.tb01087.x. [DOI] [PubMed] [Google Scholar]
- 55.Sikkema D J, Brubaker R R. Outer membrane peptides of Yersinia pestis mediating siderophore-independent assimilation of iron. Biol Met. 1989;2:174–184. doi: 10.1007/BF01142557. [DOI] [PubMed] [Google Scholar]
- 56.Sikkema D J, Brubaker R R. Resistance to pesticin, storage of iron, and invasion of HeLa cells by yersiniae. Infect Immun. 1987;55:572–578. doi: 10.1128/iai.55.3.572-578.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon E H, Tessman I. Thymidine-requiring mutants of phage T4. Proc Natl Acad Sci USA. 1963;50:526–532. doi: 10.1073/pnas.50.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Staggs T M, Perry R D. Identification and cloning of a fur regulatory gene in Yersinia pestis. J Bacteriol. 1991;173:417–425. doi: 10.1128/jb.173.2.417-425.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stojiljkovic I, Hantke K. Hemin uptake system of Yersinia enterocolitica: similarities with other TonB-dependent systems in Gram-negative bacteria. EMBO J. 1992;11:4359–4367. doi: 10.1002/j.1460-2075.1992.tb05535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stojiljkovic I, Hantke K. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol Microbiol. 1994;13:719–732. doi: 10.1111/j.1365-2958.1994.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 61.Stojiljkovic I, Hwa V, de Saint Martin L, O’Gaora P, Nassif X, Heffron F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 62.Straley S C, Bowmer W S. Virulence genes regulated at the transcriptional level by Ca2+ in Yersinia pestis include structural genes for outer membrane proteins. Infect Immun. 1986;51:445–454. doi: 10.1128/iai.51.2.445-454.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Surgalla M J, Beesley E D. Congo red-agar plating medium for detecting pigmentation in Pasteurella pestis. Appl Microbiol. 1969;18:834–837. doi: 10.1128/am.18.5.834-837.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tam R, Saier M H., Jr Structural, functional, and evolutionary relationships among extracellular solute-binding receptors of bacteria. Microbiol Rev. 1993;57:320–346. doi: 10.1128/mr.57.2.320-346.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.The Sanger Centre Yersinia pestis Genomic Project. 27 July 1998, revision date (sequence updated weekly). [Online.] Yersinia pestis CO92 genomic sequence database. The Sanger Centre, Hinxton, Cambridge, United Kingdom. ftp://ftp.sanger.ac.uk/pub/pathogens/yp/YP.dbs.
- 66.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Torres A G, Payne S M. Haem iron-transport system in enterohaemorrhagic Escherichia coli O157:H7. Mol Microbiol. 1997;23:825–833. doi: 10.1046/j.1365-2958.1997.2641628.x. [DOI] [PubMed] [Google Scholar]
- 68.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villarejo M R, Zabin I. β-Galactosidase from termination and deletion mutant strains. J Bacteriol. 1974;120:466–474. doi: 10.1128/jb.120.1.466-474.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.von Gabain A, Belasco J G, Schottel J L, Chang A C, Cohen S N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci USA. 1983;80:653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wyckoff E E, Duncan D, Torres A G, Mills M, Maase K, Payne S M. Structure of the Shigella dysenteriae haem transport locus and its phylogenetic distribution in enteric bacteria. Mol Microbiol. 1998;28:1139–1152. doi: 10.1046/j.1365-2958.1998.00873.x. [DOI] [PubMed] [Google Scholar]