Abstract
The implementation of biologic therapy has improved the treatment and clinical course of patients with inflammatory bowel disease since the initial approval of infliximab for Crohn’s disease in 1998. However, the efficacy and safety profiles of currently available therapies are still less than optimal in several ways, highlighting the need for novel therapeutic targets. Several new drug classes (Janus kinase inhibitors, anti-integrins, sphingosine-1-phosphate receptor modulators, anti–interleukin-23 antibodies, and stem cell therapies) are currently being studied in Crohn’s disease and ulcerative colitis with promising results. This article reviews the current literature and provides an updated overview of the emerging therapies.
Keywords: Novel therapies, inflammatory bowel disease, Janus kinase inhibitors, sphingosine-1-phosphate receptor modulators, anti-integrins, anti–interleukin-23 antibodies, stem cell therapies
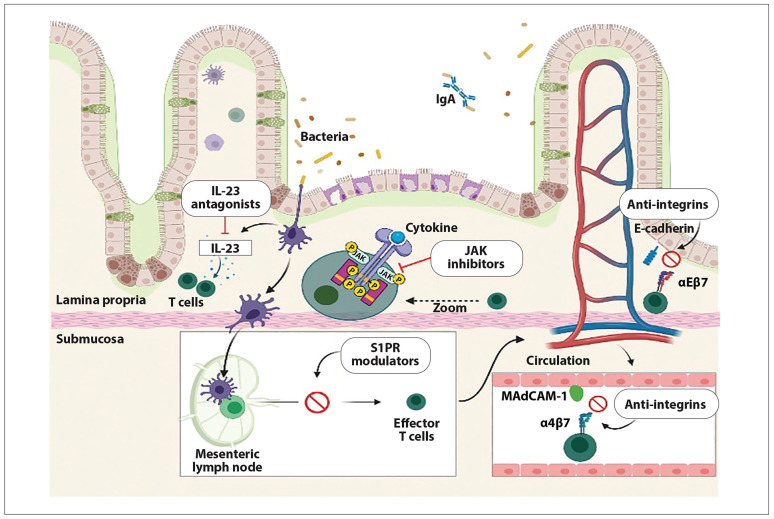
The treatment of inflammatory bowel disease (IBD) has significantly changed in the past 2 decades. The biologic era has introduced the concept of treat-to-target strategies and has shifted the emphasis to treatments that can control inflammation and modify the course of IBD. The multifactorial pathogenesis of IBD, including genetic susceptibility and environmental exposure leading to a complex inflammatory cascade, offers a vast territory for multiple immunologic and genetic targets (Figure 1).
Figure 1.
Representation of mucosal inflammation in inflammatory bowel disease and inflammatory targets for multiple novel therapies. Therapies are grouped by categories according to their main mechanism of action. Figure created with BioRender.com.
Ig, immunoglobulin; IL, interleukin; JAK, Janus kinase; MAdCAM-1, mucosal vascular addressin cell adhesion molecule 1; S1PR, sphingosine-1-phosphate receptor.
Tumor necrosis factor (TNF) inhibitors are effective for the treatment of IBD and represent well-established therapies for induction and maintenance of remission in patients with ulcerative colitis (UC) and Crohn’s disease (CD).1-6 Vedolizumab (Entyvio, Takeda) and ustekinumab (Stelara, Janssen) also represent well-studied biologic agents that have been approved for patients with IBD.7-10 Nevertheless, up to one-third of patients treated with anti-TNF agents are primary nonresponders and 23% to 46% develop secondary loss of response.11-13 In addition, biologics may predispose patients to opportunistic infections, immunosuppression side effects, and increased malignancy risk. Therefore, there is an unmet need for the development of highly effective and safer medications that can advance the management field in IBD and provide better clinical, endoscopic, and histologic outcomes.
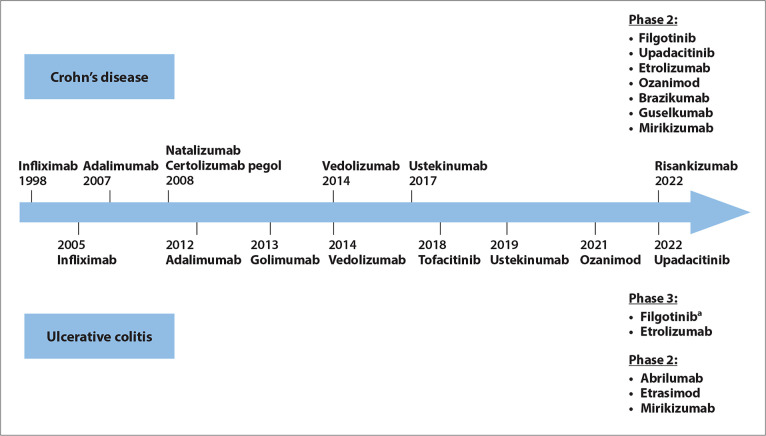
With recent advances in molecular biology and understanding of immunologic pathways in IBD, novel pharmacologic treatments have emerged. These therapies include new biologics such as anti-integrins and anti–interleukin (IL)-23 antibodies, small molecules such as Janus kinase (JAK) inhibitors and sphingosine-1-phosphate receptor (S1PR) modulators, and stem cell therapies. This article aims to provide an overview of recent clinical trials involving these novel drugs for the management of UC and CD (Figure 2).
Figure 2.
Timeline of drugs approved by the US Food and Drug Administration for the treatment of Crohn’s disease and ulcerative colitis, and published manuscripts or abstracts for positive phase 2 and 3 trials.
aFilgotinib was approved for ulcerative colitis by the European Medicines Agency for use in the European Union in 2021.
New Janus Kinase Inhibitors
JAKs are intracellular signaling proteins that belong to the family of tyrosine kinases and include the JAK1 to 3 proteins and tyrosine kinase 2 (TYK2).14,15 JAKs phosphorylate activated cytokine receptors on the cell surface and subsequently allow signal transducer and activator of transcription (STAT) proteins to bind the receptors and enter a phosphorylated state. Once phosphorylated, STATs dissociate from the receptor and form dimers, which translocate to the cell nucleus to induce transcription of target genes.16 The JAK-STAT pathway mediates the signaling of multiple proinflammatory cytokines. This pathway has become the target of several new therapies that inhibit JAK signaling (Figure 3). In addition, small molecule inhibitors exhibit the advantages of oral administration and lack of immunogenicity when compared with monoclonal antibodies.17,18
Figure 3.
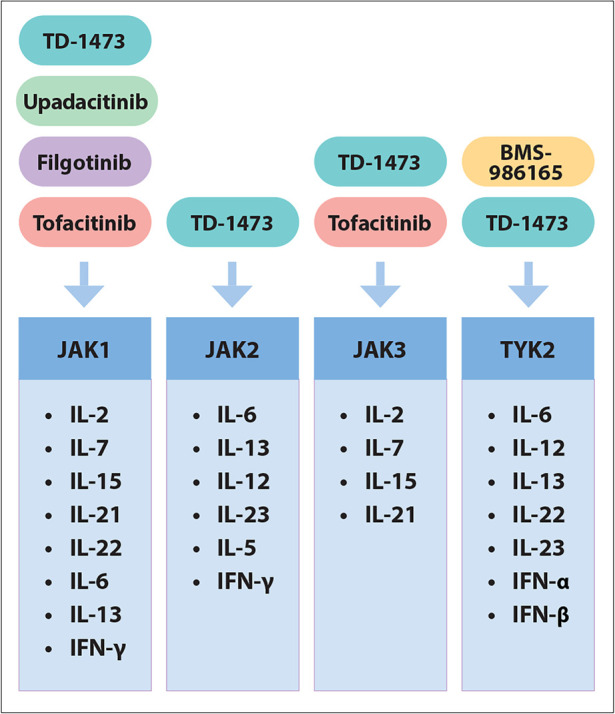
Summary of JAK receptors with downstream targets and new JAK inhibitor drugs.
IFN, interferon; IL, interleukin; JAK, Janus kinase; TYK, tyrosine kinase.
Tofacitinib
Tofacitinib (Xeljanz, Pfizer) is an oral JAK1, JAK3, and, to a lesser degree, JAK2 inhibitor that blocks the effect of several cytokines, including IL-2, IL-3, IL-4, IL-5, IL-6, IL-12, IL-15, IL-21, and interferon (IFN)-γ.19-21 Tofacitinib was well studied in the OCTAVE trials and was the first JAK inhibitor approved by the US Food and Drug Administration (FDA) for the treatment of patients with IBD, specifically for moderate-to-severe UC.22,23 In contrast to its efficacy for UC, tofacitinib has failed to demonstrate significant benefit in CD, as 2 phase 2 clinical trials for induction and maintenance of remission in CD did not meet their primary endpoints.24,25 More recently, tofacitinib was evaluated for efficacy and safety with dose escalation and de-escalation in the open-label, long-term extension OCTAVE Open study.26 With tofacitinib de-escalation in patients in remission with tofacitinib 10 mg twice daily (BID), 84.1% and 74.6% of patients maintained clinical response (defined as a decrease from induction study baseline total Mayo score of ≥3 points and ≥30%, plus a decrease in rectal bleeding subscore of ≥1 point or an absolute rectal bleeding subscore of 0 or 1) and remission, respectively, at 12 months. Furthermore, of patients who were induction responders and had treatment failure with tofacitinib 5 mg BID as maintenance, 49.1% were able to achieve remission at 12 months. Final analysis from OCTAVE Open demonstrated consistent long-term safety and tolerability of tofacitinib 5 mg or 10 mg for up to 7.0 years.27 The most frequent serious adverse event (AE) was worsening UC (4.4%). Incidence rates (per 100 patient-years) for other AEs were: death, 0.25; major adverse cardiovascular event, 0.16; pulmonary embolism, 0.21; and malignancy (excluding nonmelanoma skin cancer), 1.03. This study also showed that efficacy of tofacitinib was maintained for up to 36 months, with 66.9% and 40.3% clinical response rates in the groups receiving tofacitinib 5 mg and 10 mg BID, respectively.
Multiple side effects noted in rheumatoid arthritis (RA) studies have raised concern regarding the long-term safety of tofacitinib, however. Drug safety communications from the FDA have warned about higher rates of serious heart-related events, blood clots, cancer, and death in RA patients treated with tofacitinib compared with TNF inhibitors.28,29 However, the RA patient population was considerably older and with more medical comorbidities than the typical UC population. The ORAL Surveillance study included RA patients with active disease who were 50 years of age or older and had at least 1 additional cardiovascular risk factor.30 In addition, 48.2% of patients had ever smoked. All of these factors likely contributed to an overall increased risk of cardiovascular events and cancer. In contrast to the RA findings, no increased rates of cancer or major cardiovascular events have been reported in the UC trials and real-world experience.
Filgotinib
Filgotinib is a JAK1-selective small molecule inhibitor with a 28-fold selectivity for JAK1 over JAK2.31 The FITZROY study was a phase 2, double-blind, randomized controlled trial that studied the efficacy and safety of filgotinib in 174 patients with moderately to severely active CD.17 At week 10, 47% of 128 patients treated with once-daily filgotinib 200 mg achieved clinical remission (primary endpoint defined as Clinical Disease Activity Index [CDAI] <150 in this study), vs 23% of 44 patients treated with placebo (P=.0077). Among patients naive to anti-TNF agents, this difference was even higher: 60% of 57 patients in the filgotinib group achieved clinical remission vs 13% of 16 patients in the placebo group. Nevertheless, no significant difference was found between filgotinib and placebo for secondary endpoints such as endoscopic remission (14% vs 7%; P=.31), mucosal healing (4% vs 2%; P=.82), and deep remission (8% vs 2%; P=.31). The authors discussed that the 10-week endpoint may have limited this interpretation, as endoscopic and deep remission are often delayed compared with clinical response. Although filgotinib demonstrated an acceptable safety profile, the frequency of serious AEs was higher with filgotinib compared with placebo (9% vs 4%), including a higher rate of serious infections (3% vs 0%). Similarly, the OCTAVE trials of tofacitinib in UC have reported serious infections in up to 5% of patients.23,32 In addition, a recent systematic review and meta-analysis assessed herpes zoster infection in 44 studies and 48,093 patients with immune-mediated diseases exposed to JAK inhibitors (tofacitinib, upadacitinib [Rinvoq, AbbVie], filgotinib, and baricitinib [Olumiant, Eli Lilly]) and reported an incidence rate of 2.11 per 100 patient-years.33 A pooled analysis including only controlled studies with a total of 9572 patients demonstrated a significantly higher relative risk of herpes zoster infection among patients who were treated with JAK inhibitors (relative risk, 1.57; 95% CI, 1.04-2.37).
A phase 3 trial evaluating the efficacy and safety of filgotinib in the induction and maintenance of remission in patients with moderately to severely active CD is currently in progress (DIVERSITY1; ClinicalTrials.gov identifier: NCT02914561), in addition to a long-term extension study of its safety for up to 432 weeks (DIVERSITYLTE; NCT02914600). Furthermore, phase 2 trials of filgotinib in small-bowel CD (DIVERGENCE 1; NCT03046056) and fistulizing CD (DIVERGENCE 2; NCT03077412) are underway.
Findings from SELECTION, a phase 2b/3 trial of filgotinib for moderate-to-severe UC, were recently published.34 This multicenter trial was the first to evaluate filgotinib for UC and included 2 induction studies (A, biologic-naive patients; B, biologic-experienced patients), and 1 maintenance study. Patients were randomized 2:2:1 for oral daily filgotinib 200 mg, 100 mg, or placebo for 11 weeks, with a primary endpoint of clinical remission by Mayo endoscopic, rectal bleeding, and stool frequency subscores at weeks 10 and 58. A total of 659 patients were enrolled in induction study A and 689 patients in induction study B, and 664 patients were able to enter the maintenance study. Patients on filgotinib 200 mg daily had significantly higher clinical remission rates compared with placebo at week 10 (study A: 26.1% vs 15.3%; P=.0157; study B: 11.5% vs 4.2%; P=.0103) and at week 58 (37.2% vs 11.2%; P<.0001). Although clinical remission at week 10 for filgotinib 100 mg was not significant, it achieved significance by week 58. In addition, filgotinib was well tolerated, demonstrating a similar incidence of serious AEs as placebo. Filgotinib is a promising new IBD therapy, and data from ongoing clinical trials will help determine its applicability in clinical practice. It was approved for moderately to severely active UC by the European Commission on November 15, 2021 in the European Union.35 Nevertheless, filgotinib is undergoing assessment by the FDA given concerns of possible impact on sperm parameters. MANTA (NCT03201445) and MANTA-RAy (NCT03926195) are 2 ongoing trials designed to investigate filgotinib’s effects on sperm concentration, motility, count, volume, and morphology.36,37
Upadacitinib
Upadacitinib is another novel oral selective JAK1 inhibitor, first approved by the FDA for the treatment of RA in August 2019.38,39 Upadacitinib has also been approved by the FDA for psoriatic arthritis and most recently for atopic dermatitis.40,41 Upadacitinib was shown to block the effects of several proinflammatory cytokines, including IL-2, IL-4, IL-6, IL-7, IL-9, IL-15, IL-21, and IFN-γ, which are important mediators in CD.42 As a result, upadacitinib was recently evaluated in the CELEST study, a double-blind, phase 2 trial of patients with moderately to severely active CD with inadequate response or intolerance to immunosuppressants or TNF antagonists, and showed promising results, particularly at a dosage of 24 mg BID.43 CELEST was a dose-ranging, multicenter study that evaluated 220 patients for a 16-week placebo-controlled induction period, followed by a 36-week maintenance period (P<.1 defined as significant). At week 16, clinical remission was achieved in 13%, 27% (P<.1), 11%, 22%, and 14% of patients on upadacitinib 3 mg, 6 mg, 12 mg, and 24 mg twice daily, and 24 mg once daily, respectively, compared with 11% of patients in the placebo group. Furthermore, endoscopic remission occurred in 10% (P<.1), 8%, 8% (P<.1), 22% (P<.01), and 14% (P<.05) of patients receiving upadacitinib, respectively, compared with 0% of patients receiving placebo. Notably, upadacitinib 24 mg twice daily was the dosage consistently associated with multiple important clinical and endoscopic endpoints during the induction phase. Overall, patients who responded to the induction regimen demonstrated continued clinical and endoscopic responses during the 36-week maintenance period. The serious AE rate ranged from 5% to 27.8% across the induction arms, with the highest rate (27.8%) in the 12 mg twice-daily arm, vs 5.4% in the placebo group. The safety profile of upadacitinib was comparable to previous trials of JAK inhibitors.24,25 In addition, health-related outcomes from the CELEST study were recently published. Improvements in quality of life and work productivity with upadacitinib were statistically significant at 8 weeks and 16 weeks, respectively, with sustained effect for up to 52 weeks.44 Analysis of exposure-response relationships of upadacitinib induction dosages from CELEST determined that 18 mg to 24 mg twice daily (immediate release) or 45 mg to 60 mg daily (extended release) provide the greatest efficacy, which will inform further phase 3 trials.45
In UC, upadacitinib has shown promising results as an induction therapy in U-ACHIEVE, a phase 2b trial of 250 patients.46 Patients were randomly assigned to groups of extended-release upadacitinib 7.5 mg, 15 mg, 30 mg, or 45 mg once daily or placebo for 8 weeks. The primary endpoint of clinical remission was achieved in 8.5%, 14.3%, 13.5%, and 19.6% of patients receiving upadacitinib, respectively, and in no patient receiving placebo (P=.052, P=.013, P=.011, and P=.002, respectively). Significant endoscopic improvement was also reported across all upadacitinib groups (14.9%, 30.6%, 26.9%, and 35.7%, respectively), compared with 2.2% in the placebo group. A more recent evaluation of the U-ACHIEVE trial demonstrated that upadacitinib also improved bowel urgency and abdominal pain, as 46% of patients receiving upadacitinib 45 mg reported no bowel urgency (vs 9% on placebo; P≤.001) and 38% reported no abdominal pain (vs 13% on placebo; P=.015).47
Two phase 3 induction trials of upadacitinib in adults with moderate-to-severe UC were recently presented at the 16th Congress of the European Crohn’s and Colitis Organisation and demonstrated that upadacitinib met the primary endpoint of clinical remission (per adapted Mayo Score) at week 8 and all ranked secondary endpoints, including endoscopic and histologic outcomes.48,49 A total of 474 patients were enrolled in phase 3 of U-ACHIEVE and randomized 2:1 to upadacitinib 45 mg daily or placebo for 8 weeks.48 Clinical remission (per adapted Mayo score, defined as stool frequency subscore ≤1 and not greater than baseline, rectal bleeding subscore of 0, and Mayo endoscopic subscore of ≤1 at week 8) was achieved in 26.1% of patients receiving upadacitinib vs 4.8% in the placebo group (P<.001), and clinical response (decrease in adapted Mayo score ≥2 points and ≥30% from baseline and a decrease in rectal bleeding subscore ≥1 or an absolute rectal bleeding score ≤1) was detected as early as week 2 (60.1% vs 27.3%; P<.001). The other induction study, U-ACCOMPLISH, enrolled 522 patients using a similar randomization ratio and endpoints and showed that 33.5% of patients in the upadacitinib group vs 4.1% in the placebo group achieved the primary endpoint (P<.001).49 In addition, a significantly higher proportion of patients receiving upadacitinib vs placebo achieved endoscopic improvement and remission (44.0% vs 8.3%, and 18.2% vs 1.7%, respectively; all P<.001). No new safety signals were demonstrated in either trial. Clinical responders of both the U-ACHIEVE and U-ACCOMPLISH induction studies were rerandomized to upadacitinib 15 mg, upadacitinib 30 mg, or placebo for an additional 52 weeks in the phase 3 maintenance trial.50 Clinical remission was significantly higher in the upadacitinib 15 mg and 30 mg groups compared with placebo (42% and 52% vs 12%; P<.001). All secondary endpoints were met, including endoscopic improvement, corticosteroid-free clinical remission, and histologicendoscopic mucosal improvement. Results from these 3 trials were recently published in full manuscript format.51 Upadacitinib was approved by the FDA in March 2022 for the treatment of adults with moderately to severely active UC who had an inadequate response or intolerance to 1 or more TNF blockers.52 The recommended induction dosage for this indication is 45 mg once daily for 8 weeks, and the recommended maintenance dosage is 15 mg once daily with a consideration for 30 mg once daily if refractory or with severe or extensive UC. Upadacitinib was approved with the boxed warnings for the JAK inhibitor class, including increased risk of serious cardiovascular events, malignancies, thrombosis, and death. Use of upadacitinib also requires an effective contraception method during treatment and for 4 weeks after final dose given potential embryo-fetal toxicity in animal studies.
Three additional phase 3 clinical trials are registered for CD (NCT03345836, NCT03345823, and NCT03345849). A press release in December 2021 announced positive results from U-EXCEED, an induction study that evaluated the efficacy of upadacitinib 45 mg at week 12.53 A significantly higher proportion of patients treated with upadacitinib achieved the coprimary endpoints of clinical remission (CDAI <150: 39% vs 21%; P<.0001) and endoscopic response (35% vs 4%; P<.0001) compared with placebo. Another press release in February 2022 reported that the other phase 3 induction study, U-EXCEL, demonstrated positive results.54
Izencitinib
A novel gut-selective pan-JAK inhibitor, TD-1473 (izencitinib), was evaluated in a preclinical and clinical translational program that included a phase 1b study in patients with moderate-to-severe UC.55 TD-1473 inhibits JAK1, JAK2, JAK3, and TYK2 and has been shown to reduce JAK-mediated signaling in colonic tissue from mice and from patients with IBD. In the phase 1b study, 40 patients with UC were enrolled to receive once-daily oral TD-1473 20 mg, 80 mg, or 270 mg, or placebo for 28 days. Results were not powered for efficacy analyses. However, descriptive analyses showed increased rates of clinical and endoscopic response in patients receiving all dosages of TD-1473 vs placebo. A phase 2b induction study of TD-1473 in UC did not meet the primary endpoint of a change in Mayo Score or secondary endpoint of clinical remission at week 8.56 Results are pending from the 16-week extended induction and 44-week maintenance portions of this study (NCT03758443). A phase 2 trial is underway for TD-1473 in CD, with results expected in 2022 (NCT03635112).
Deucravacitinib
Another therapeutic target is TYK2, a member of the JAK family that has limited downstream effects on IL-12, IL-13, IFN-α, and IFN-β, and therefore may lead to fewer AEs when inhibited.57 TYK2 inhibitors and other JAK inhibitors both can promote robust clinical efficacy, but TYK2 inhibitors may have a better safety profile. In contrast to the other JAK inhibitors that bind to the adenosine triphosphate site of the catalytic domain (JH1 domain) of the JAK protein, BMS-986165 (deucravacitinib) is a selective TYK2 inhibitor that binds to the pseudokinase (JH2) domain of TYK2 and inhibits its effects by an allosteric mechanism. BMS-986165 is currently being investigated for IBD. Despite the high efficacy of BMS-986165 in psoriasis trials, the phase 2 LATTICE-UC study failed to meet the primary and secondary endpoints (NCT03934216).58 Another phase 2 study in UC includes a higher dosage of BMS986165 (NCT04613518), and a phase 2 study for CD (NCT03599622) is underway.
Overall, the JAK-STAT pathway offers promising therapeutic targets for further development of drugs inhibiting JAK signaling.
Inhibition of Lymphocyte Adhesion: New Anti-Integrins
Manipulating lymphocyte adhesion with subsequent inhibition of immune cell trafficking is an established therapeutic intervention for IBD. α4β7 integrin and its subunits have been a target of multiple therapies, as these molecules are specifically expressed on activated lymphocytes in the gut lymphatics.59 Vedolizumab is an FDA-approved monoclonal antibody against α4β7 integrin that is given via intravenous (IV) administration and has improved the care of patients with IBD for nearly 10 years now.7,8
Abrilumab
Similarly, abrilumab is an anti-α4β7 integrin antibody with a subcutaneous (SC) route of administration, which may be a preferred route for patients. A phase 2b study of 354 patients evaluated the efficacy of abrilumab in patients with moderate-to-severe UC.60 After 8 weeks of treatment, remission rates for abrilumab 70 mg, 210 mg, and placebo were 13.3%, 12.7%, and 4.3%, respectively (P<.05 for 70 mg and 210 mg vs placebo). Abrilumab also demonstrated significant clinical response and mucosal healing with these dosages.
Etrolizumab
Etrolizumab is an anti-β7 integrin antibody that blocks both α4β7 and αEβ7 integrins and therefore reduces β7-positive lymphocyte trafficking and retention in the inflamed gut mucosa by inhibiting interaction with E-cadherin.61 In addition to controlling αEβ7-expressing inflammatory cells in the gut lining, the inhibition of αEβ7 integrin is important to control αEβ7-expressing cells that will continue to migrate to the mucosa by the α4β1/vascular cell adhesion molecule 1 pathway, which is not inhibited by α4β7 antagonists such as vedolizumab.62 Etrolizumab has been evaluated for induction of remission in UC, with beneficial therapeutic outcomes.63 In a phase 2 study of 124 patients with moderate-to-severe UC, etrolizumab induced remission at week 10 in 21% of patients in the etrolizumab 100 mg group (P=.0040), 10% in the etrolizumab 300 mg group (P=.048), and none in the placebo group.64 Similarly, interim results from substudy 1 of the phase 3 BERGAMOT induction cohort for CD showed that CDAI remission at week 14 was greater with etrolizumab 105 mg and 210 mg than with placebo (23.3% and 28.9% vs 16.9%, respectively) (NCT02394028).65 More patients also achieved endoscopic remission with etrolizumab and the drug was well tolerated. The full results of the trial are pending.
A comprehensive phase 3 clinical program of etrolizumab in IBD has evaluated the efficacy and safety of etrolizumab in several UC trials, and the program is in development for CD trials, including head-to-head comparisons against established anti-TNF agents.62 The results of the UC trials were mixed. GARDENIA was a phase 3, randomized, head-to-head study designed as a superiority trial of etrolizumab vs infliximab for moderately to severely active UC in patients who were naive to anti-TNF agents.66 The primary endpoint was both clinical response at week 10 and clinical remission at week 54, which was met by 18.6% and 19.7% of patients receiving etrolizumab and infliximab, respectively (P=.81), not proving superiority of etrolizumab. HICKORY was a placebo-controlled UC study in patients previously exposed to TNF inhibitors.67 At week 14, etrolizumab was significantly more effective at induction of remission compared with placebo (18.5% vs 6.3%; P=.0033). However, statistical significance was not achieved for the primary maintenance endpoint of remission at week 66 among patients with a clinical response at week 14. Maintenance therapy with etrolizumab was well tolerated. HIBISCUS I and HIBISCUS II were identically designed, placebo-controlled, head-to-head studies comparing etrolizumab with placebo and adalimumab in patients with UC who were naive to TNF inhibitors.68 A significantly higher proportion of patients receiving etrolizumab achieved remission at week 10 than with placebo in HIBISCUS I (19.4% vs 6.9%; P=.017); however, etrolizumab was not superior to placebo in HIBISCUS II (18.2% vs 11.1%; P=.17). In addition, no significant differences in efficacy were detected in the prespecified pooled analysis of etrolizumab vs adalimumab. In LAUREL, the phase 3 maintenance study of etrolizumab vs placebo in TNF inhibitor–naive patients with UC, patients received a 10-week openlabel induction with etrolizumab and proceeded to the double-blind maintenance phase until week 62 if they achieved clinical response at week 10.69 No significant difference was found between etrolizumab vs placebo for the primary endpoint of remission at week 62 (29.6% vs 20.6%; P=.19). The hierarchical study design precluded formal statistical testing of secondary endpoints; however, a numerical benefit for etrolizumab was seen in several secondary endpoints, including endoscopic improvement and remission, and histologic remission.
Sphingosine-1-Phosphate Receptor Modulators
S1PR modulators offer another unique therapeutic approach in IBD. This class of drugs acts by trapping and sequestering lymphocytes within lymphoid organs, in addition to regulating several inflammatory pathways through 5 S1PRs (S1PR1-5) with different downstream effects.59
Ozanimod
Ozanimod (Zeposia, Bristol Myers Squibb) is an oral agent that selectively targets S1PR1 and S1PR5. The large phase 3 study True North evaluated ozanimod 1 mg daily as induction and maintenance in UC, with 1012 patients enrolled.70 In the double-blind first cohort, at week 10, 18.4% and 6.0% of patients in the ozanimod and placebo groups, respectively, achieved the primary endpoint of clinical remission (P<.001). In addition, secondary endpoints were all statistically significant for ozanimod vs placebo: clinical response was achieved in 47.8% vs 25.9%, endoscopic improvement in 27.3% vs 11.6%, and mucosal healing in 12.6% vs 3.7% (all P<.001), respectively. A second cohort of an additional 367 patients received open-label ozanimod at the same dosage for induction, with a clinical remission rate of 21.0%. A total of 457 patients with clinical response to ozanimod during induction at 10 weeks were rerandomized to maintenance therapy with ozanimod or placebo and evaluated for up to 52 weeks. Significantly more patients achieved clinical remission with ozanimod maintenance therapy vs placebo (37.0% vs 18.5%; P<.001). Serious infections occurred in less than 2% in each group. Liver aminotransferase level elevations were more common with ozanimod; however, no patients had drug-induced liver injury or severe liver injury. Furthermore, recent data from the True North open-label extension (OLE) study were presented at Digestive Disease Week 2022. A substudy of 150 patients who did not achieve clinical response at week 10 and entered an OLE showed that 48.7% achieved symptomatic clinical response at week 10 of the OLE, with 44% achieving this response as early as week 5 of the OLE.71 In total, the True North OLE consisted of 823 patients who were followed up to week 142.72 Patients were enrolled in the OLE from the phase 3 True North study if they were nonresponders at the end of induction, lost response during maintenance, or completed maintenance treatment, or if they participated in the phase 2 Touchstone OLE and remained at study closure receiving once-daily oral ozanimod 0.92 mg. Observed case analyses showed that the rates of clinical response, clinical remission, endoscopic improvement, and corticosteroid-free remission were maintained during follow-up (week 94: 84%, 51%, 57%, and 50%, respectively). At 94 weeks, 34% of all patients and 55% of the responders maintained clinical response, with no new safety concerns. Comparably, STEPSTONE, a recent phase 2 uncontrolled trial of ozanimod in 69 patients with moderate-to-severe CD demonstrated a clinical remission rate of 39.1% and a response rate of 56.5% at week 12.73 Endoscopic response was noted in 23.2% of the patients.
Based on the data from True North, the FDA approved ozanimod for moderately to severely active UC in May 2021.74 Ozanimod is also approved by the FDA for multiple sclerosis. Because ozanimod can lead to a transient decrease in heart rate and atrioventricular conduction delays, an electrocardiogram is required at baseline. Given the potential risk of varicella zoster virus (VZV) meningitis, patients without a history of vaccination against VZV or a confirmed past history of VZV need baseline VZV antibodies testing and vaccination if antibody-negative prior to starting treatment. Vaccination with Shingrix (GlaxoSmithKline) is also recommended owing to the increased incidence of herpes zoster in ozanimod-treated patients. An increased risk of macular edema has been reported with S1PR modulators, especially in patients with diabetes mellitus or uveitis. These conditions warrant an ophthalmic evaluation prior to the initiation of ozanimod and regular follow-up.74
Etrasimod
Another oral selective S1PR modulator is etrasimod, which acts on S1PR1, S1PR4, and S1PR5 and was studied in moderately to severely active UC. In the phase 2 OASIS study, 156 patients with UC were randomized to once-daily etrasimod 1 mg, 2 mg, or placebo for 12 weeks.75 Etrasimod 2 mg resulted in significant improvement of modified Mayo Scores compared with placebo (P=.009), and 41.8% of patients receiving etrasimod 2 mg vs 17.8% receiving placebo achieved endoscopic improvement (P=.003). Results from an OLE of OASIS with etrasimod 2 mg in 112 patients for an additional 34 to 40 weeks showed that week 12 clinical response, clinical remission, or endoscopic improvement was maintained in 85%, 60%, or 69% of patients, respectively.76 The safety profile was also favorable. Overall, the new class of S1PR modulators for IBD, and especially for UC, might represent a safe and efficacious option for induction and maintenance of remission.
Selective Interleukin-23 Antagonists
The cytokines IL-12 and IL-23 play an essential role in IBD pathogenesis. Importantly, they share the subunit p40, as IL-12 is formed by p35/p40 and IL-23 is formed by p19/p40. However, IL-12 and IL-23 mediate distinct inflammatory responses, as IL-12 drives a Th1 phenotype including the TNF pathway, whereas IL-23 promotes Th17 differentiation and upregulation of IL-17, resulting in intestinal inflammation through the recruitment of neutrophils, macrophages, and dendritic cells.59,77,78 The monoclonal antibody ustekinumab targets p40, thus exhibiting downstream effects in both IL-12 and IL-23 inflammatory pathways. Ustekinumab has demonstrated its effectiveness in several clinical trials and has been approved for the treatment of both CD and UC.9,10,79
New therapeutic approaches have focused on targeting the subunit p19 for selective inactivation of IL-23 downstream effects.80,81 Monoclonal antibodies that specifically inhibit the p19 subunit include brazikumab, risankizumab (Skyrizi, AbbVie), guselkumab (Tremfya, Janssen), and mirikizumab.
Brazikumab
Brazikumab was evaluated in a phase 2a study of 119 patients with CD with failure to TNF treatment who were randomized 1:1 to receive placebo or brazikumab 700 mg IV at weeks 0 and 4.82 Induction consisted of a double-blind 12-week period, which was followed by a 100-week OLE of receiving brazikumab 210 mg SC every 4 weeks. Clinical response was reported in 49.2% of patients at week 8, compared with 26.7% in the placebo group (P=.01). IL-22 was identified as a potential biomarker for clinical response, as higher levels of IL-22 at baseline were associated with greater response to brazikumab. The 100-week OLE period showed that brazikumab was well tolerated, and the most reported treatment-emergent AEs were headache (22.1%), nasopharyngitis (22.1%), abdominal pain (18.3%), and CD (16.3%).83
Risankizumab
Risankizumab was approved for moderate-to-severe plaque psoriasis by the FDA in 2019 and for active psoriatic arthritis in January 2022.84 The efficacy of risankizumab in moderate-to-severe CD was initially evaluated in a double-blind, placebo-controlled, phase 2 study for induction therapy, followed by an OLE period for reinduction or washout, and finally a maintenance period of 26 weeks.85,86 Patients were randomized 1:1:1 to risankizumab 200 mg IV, risankizumab 600 mg IV, or placebo. The primary outcome was clinical remission with CDAI less than 150 at week 12, which was achieved by 31% of pooled risankizumab patients vs 15% of the placebo group (P=.0489), with statistical significance driven mainly by the 600-mg group.85 Patients who had not achieved combined clinical and endoscopic remission received reinduction with open-label risankizumab 600 mg IV for 12 weeks.86 Subsequently, patients in clinical remission at week 26 entered an open-label maintenance phase with risankizumab 180 mg every 8 weeks for 26 weeks. Clinical remission at week 26 was achieved by 53% (54/101) of the patients treated with 600 mg of risankizumab, demonstrating that an extended induction was effective in enhancing clinical remission rates. At week 52, 71% of patients maintained clinical remission, 81% demonstrated clinical response, 35% demonstrated endoscopic remission, and 55% demonstrated endoscopic response. Promising results were also reported for mucosal healing in 24% of patients and deep remission in 29% of patients at week 52. Overall, the drug was well tolerated, with no serious AEs or death.
The phase 3 ADVANCE study evaluated risankizumab 600 mg IV, risankizumab 1200 mg IV, or placebo in patients with CD with inadequate response or intolerance to conventional and/or biologic therapy.87 The proportion of patients achieving the coprimary endpoints of clinical remission and endoscopic response was significantly higher with risankizumab 600 mg and 1200 mg than placebo (CDAI remission: 45.2% and 41.6% vs 25.2%; endoscopic response: 40.3% and 32.2% vs 12%; P<.001 for both). Risankizumab was efficacious independent of prior biologic exposure; however, efficacy rates were higher in patients with no previous inadequate response to biologics. No new serious AE signals were demonstrated.
MOTIVATE, another phase 3 induction study of risankizumab for CD, included patients with prior failure to biologics (1 vs >1 biologic) and showed similar efficacy results.88 Risankizumab 600 mg and 1200 mg were superior to placebo for the same coprimary endpoints as the ADVANCE study (P≤.001). Regardless of the number of biologics failed, risankizumab demonstrated efficacy; however, a greater proportion of patients who failed only 1 vs more than 1 biologic achieved the endpoint.
Lastly, FORTIFY, a phase 3 maintenance trial in CD, was conducted to evaluate risankizumab SC maintenance therapy vs withdrawal after clinical response to the induction phases from the ADVANCE and MOTIVATE studies.89 Patients were rerandomized 1:1:1 to risankizumab 360 mg SC, risankizumab 180 mg SC, or placebo every 8 weeks for 52 weeks. Clinical remission rates were significantly higher for risankizumab groups when compared with placebo at week 52 (per CDAI: 52.2% in the 360 mg group and 55.4% in the 180 mg group vs 40.9% with placebo; P≤.01). Risankizumab 180 mg and 360 mg were both superior to placebo for endoscopic response rates (47.1% and 46.8% vs 22.0%, respectively; P≤.01). A dose-response relationship was noted for the endpoints of endoscopic remission, deep remission, and inflammatory biomarkers such as fecal calprotectin, C-reactive protein, and IL-22. Serious AE rates were similar among groups, and maintenance treatment was well tolerated. Complete results from the ADVANCE and MOTIVATE induction trials and from the FORTIFY maintenance trial have been fully published.90,91
Guselkumab
Guselkumab is another monoclonal antibody that targets the subunit p19 of IL-23. It is approved by the FDA for the treatment of moderate-to-severe plaque psoriasis and recently for active psoriatic arthritis.92,93 Guselkumab is currently being investigated for use in moderate-tosevere CD. GALAXI, a large, randomized, phase 2/3 trial program, is underway, comparing different IV and SC dosages of guselkumab to placebo and ustekinumab (NCT03466411). The program includes 3 separate studies: a 48-week, phase 2 dose-ranging study (GALAXI 1) and two 48-week, phase 3 confirmatory studies (GALAXI 2 and GALAXI 3, both enrolling). Results from GALAXI 1 were published in abstract form for early clinical outcomes during induction with guselkumab vs placebo.94 Rates for clinical remission, clinical response, and clinical biomarker response were higher in the combined guselkumab group (200 mg, 600 mg, and 1200 mg IV) vs placebo at weeks 4, 8, and 12 (54.0% vs 15.7% at week 12; P<.001). The proportion of patients achieving these early outcomes continued to increase until week 12, and a similar trend was also observed in subgroups of patients who failed either biologic or conventional therapy. A recent press release for the GALAXI 1 study confirmed that clinical remission rates with guselkumab increased at week 48 from those previously reported at week 12, with up to 65% of patients achieving clinical remission.95 Another multicenter trial of guselkumab is being conducted in Japan as a phase 3, open-label study for CD (NCT04397263).
Mirikizumab
Mirikizumab also targets IL-23 subunit p19 and was studied in a phase 2 randomized trial for UC with treatment groups receiving mirikizumab 50 mg IV, mirikizumab 200 mg IV, mirikizumab 600 mg IV, or placebo.96 Clinical remission rates at week 12 were 15.9% (P=.066), 22.6% (P=.004), and 11.5% (P=.142) in the mirikizumab 50 mg, 200 mg, and 600 mg groups, respectively, compared with 4.8% in the placebo group. Even though the primary endpoint was not significant, clinical response was significant in all respective groups: 41.3% (P=.014), 59.7% (P<.001), and 49.2% (P=.001) vs 20.6% for placebo. Clinical responders to mirikizumab at week 12 were randomized to maintenance with mirikizumab 200 mg SC every 4 weeks or every 12 weeks, with 46.8% and 37.0%, respectively, sustaining clinical remission at week 52. A continuation of this study was conducted as an open-label extended induction treatment in patients who did not initially respond to mirikizumab.97 Those patients were offered an additional 12-week induction period with mirikizumab 600 mg or 1000 mg IV. If clinical response was achieved at week 24, patients entered a maintenance phase with mirikizumab 200 mg SC for up to 52 weeks. This extended induction period offered an important clinical benefit: up to 50.0% of those patients achieved a clinical response by week 24. In addition, encouraging results for sustained response were demonstrated, with 65.8% of the responders to extended induction doses maintaining clinical response at week 52. The safety profile was good and consistent with results from studies of other IL-23 antagonists. A press release for mirikizumab has recently confirmed that this is the first anti–IL-23p19 to demonstrate efficacy for maintenance of remission for UC in a phase 3 study (LUCENT-2; NCT03524092).98 Patients with UC who had responded to 12-week induction with mirikizumab in LUCENT-1 (NCT03518086) were rerandomized to maintenance treatment with mirikizumab or placebo.99 Mirikizumab was superior to placebo for the primary endpoint of clinical remission at 1 year (49.9% vs 25.1%, respectively; P<.001). Moreover, secondary endpoints were also achieved with mirikizumab (P<.001), including endoscopic remission, resolution or near-resolution of bowel urgency, and improvement in endoscopic histologic intestinal inflammation. Serious AEs were numerically lower in the mirikizumab group than with placebo.
Mirikizumab was recently evaluated in SERENITY, a phase 2 dose-ranging study of patients with CD (NCT02891226), with a primary endpoint of endoscopic response at week 12.100 This endpoint was met in each of the 3 mirikizumab groups, demonstrating superiority to placebo (mirikizumab 200 mg: 25.8%, P=.079; mirikizumab 600 mg: 37.5%, P=.003; mirikizumab 1000 mg: 43.8%, P<.001; placebo: 10.9%). In addition, CDAI response rates at week 12 for mirikizumab 200 mg, 600 mg, and 1000 mg were 48.4% (P<.05), 56.3% (P<.01), and 42.2% (P<.05), respectively, vs 23.4% for placebo. Mirikizumab also demonstrated durability of its effects during the maintenance period up to week 52, as endoscopic and clinical response or remission rates were similar to or numerically higher than those at week 12. A large phase 3 study aiming to enroll 1150 patients with CD is also underway (VIVID-1; NCT03926130), with a subsequent OLE study (VIVID-2; NCT04232553).
Overall, targeting the p19 subunit of IL-23 is a promising strategy in the treatment of IBD and can possibly become an effective and safe alternative to standard biologics.
Stem Cell Therapy
Mesenchymal stem cells (MSCs) have emerged as an innovative and effective therapy for fistulizing CD, given their anti-inflammatory and regenerative properties.59 A phase 3 placebo-controlled trial showed that injection of allogeneic MSCs (Cx601) into a fistula tract for refractory perianal CD fistulas was safe and led to a combined remission rate of 50% in treated patients vs 34% in patients receiving placebo (P=.024).101 The high placebo response rate may have been related to aggressive fistula curettage at baseline, as both groups underwent a fistula preparation visit with examination under anesthesia, curettage, and seton placement if clinically indicated. In the phase 1 STOMP trial, 12 patients with CD with perianal fistulas received concentrated autologous MSCs attached to a bioabsorbable matrix, and 83% of the patients achieved complete clinical healing and radiographic response on magnetic resonance imaging at 6 months.102 An autologous MSC-coated fistula plug was also studied in 15 patients with CD with transsphincteric cryptoglandular fistulas and resulted in healing in most patients (3 had complete clinical healing, 8 had partial healing, and 11 had radiographic improvement) with no serious AEs.103 Finally, a small phase 1 clinical trial for rectovaginal fistulas showed that treatment with autologous MSCs on a plug was feasible and safe, and led to a reduction in the size and drainage of fistulas in the 5 enrolled patients.104
Autologous hematopoietic stem cell transplantation (aHSCT) has been widely used in the treatment of hematologic malignancies, and its use has extended to patients with the most severe refractory CD.105 To date, only small case series and the randomized ASTIC trial were conducted on aHSCT for CD. The ASTIC trial’s intervention involved all patients undergoing stem cell mobilization prior to randomization to immunoablation and aHSCT (n=23) or control (aHSCT deferred for 1 year; n=22).106 This was a negative trial, as there were no significant differences in sustained disease remission rates (aHSCT 8.7% vs control 4.5%), CDAI less than 150, or freedom from active disease between groups. Despite some benefits in controlling disease activity, there was an alarming incidence of 76 serious AEs in 19 patients in the aHSCT group vs 38 serious AEs in 15 controls, and 1 death in the aHSCT group. Overall, aHSCT was associated with significant toxicity, especially from infections related to pancytopenia. A more recent study evaluated the efficacy and safety of aHSCT in 82 patients with CD in Europe and demonstrated that 68% had clinical improvement or remission at latest follow-up and 27% required no further medical therapy post-aHSCT.107 Further, 57% (24/42) of patients who required reinitiation of medical therapies achieved clinical remission or improvement with therapies to which they were previously nonresponders. Lastly, the ASTIClite trial in the United Kingdom will assess for possible benefits of aHSCT with a regimen less intense than for ASTIC.108 Further studies are needed to explore the role of aHSCT in refractory CD.
Conclusion
Current biologics offer an overall effective profile for the treatment of patients with moderate-to-severe IBD. However, challenges remain with those therapies, including safety concerns with immunosuppression, along with difficulties faced with primary nonresponse and secondary loss of response. Therefore, new promising targets for the treatment of IBD have emerged, with novel drugs developed to modulate several inflammatory and molecular pathways. Encouraging results from trials of selective JAK inhibitors suggest that these oral small molecules are effective alternatives with no associated immunogenicity. Systemic toxicity from JAK inhibitors could be potentially avoided with gut-selective options such as TD-1473. Etrolizumab may emerge as a new anti-integrin option for UC and CD, with a phase 3 program underway, including multiple trials and head-tohead comparisons with standard anti-TNF agents. S1PR modulators offer a unique mechanism of action of lymphocyte trapping within lymphoid organs and are likely effective for IBD, especially UC. The class of selective IL-23p19 monoclonal antibodies has been investigated primarily in CD, along with mirikizumab in UC, and has shown very promising results with a good safety profile. In conclusion, the future of IBD looks promising, with a multitude of emerging therapies that can potentially address the current therapeutic challenges.
References
- 1.Hanauer SB, Feagan BG, Lichtenstein GR et al. ACCENT I Study Group. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359(9317):1541–1549. doi: 10.1016/S0140-6736(02)08512-4. [DOI] [PubMed] [Google Scholar]
- 2.Rutgeerts P, Sandborn WJ, Feagan BG et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462–2476. doi: 10.1056/NEJMoa050516. [DOI] [PubMed] [Google Scholar]
- 3.Colombel JF, Sandborn WJ, Rutgeerts P et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132(1):52–65. doi: 10.1053/j.gastro.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 4.Schreiber S, Khaliq-Kareemi M, Lawrance IC et al. PRECISE 2 Study Investigators. Maintenance therapy with certolizumab pegol for Crohn’s disease. N Engl J Med. 2007;357(3):239–250. doi: 10.1056/NEJMoa062897. [DOI] [PubMed] [Google Scholar]
- 5.Reinisch W, Sandborn WJ, Hommes DW et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780–787. doi: 10.1136/gut.2010.221127. [DOI] [PubMed] [Google Scholar]
- 6.Sandborn WJ, Feagan BG, Marano C et al. PURSUIT-Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014;146(1):96–109.e1.. doi: 10.1053/j.gastro.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Feagan BG, Rutgeerts P, Sands BE et al. GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi: 10.1056/NEJMoa1215734. [DOI] [PubMed] [Google Scholar]
- 8.Sandborn WJ, Feagan BG, Rutgeerts P et al. GEMINI 2 Study Group. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2013;369(8):711–721. doi: 10.1056/NEJMoa1215739. [DOI] [PubMed] [Google Scholar]
- 9.Feagan BG, Sandborn WJ, Gasink C et al. UNITI–IM-UNITI Study Group. Ustekinumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2016;375(20):1946–1960. doi: 10.1056/NEJMoa1602773. [DOI] [PubMed] [Google Scholar]
- 10.Sands BE, Sandborn WJ, Panaccione R et al. UNIFI Study Group. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201–1214. doi: 10.1056/NEJMoa1900750. [DOI] [PubMed] [Google Scholar]
- 11.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33(9):987–995. doi: 10.1111/j.1365-2036.2011.04612.x. [DOI] [PubMed] [Google Scholar]
- 12.Roda G, Jharap B, Neeraj N, Colombel JF. Loss of response to anti-TNFs: definition, epidemiology, and management. Clin Transl Gastroenterol. 2016;7(1):e135. doi: 10.1038/ctg.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 14.Danese S, Argollo M, Le Berre C, Peyrin-Biroulet L. JAK selectivity for inflammatory bowel disease treatment: does it clinically matter? Gut. 2019;68(10):1893–1899. doi: 10.1136/gutjnl-2019-318448. [DOI] [PubMed] [Google Scholar]
- 15.Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285(1-2):1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- 16.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J Biol Chem. 2007;282(28):20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 17.Vermeire S, Schreiber S, Petryka R et al. Clinical remission in patients with moderate-to-severe Crohn’s disease treated with filgotinib (the FITZROY study): results from a phase 2, double-blind, randomised, placebo-controlled trial. Lancet. 2017;389(10066):266–275. doi: 10.1016/S0140-6736(16)32537-5. [DOI] [PubMed] [Google Scholar]
- 18.White JR, Phillips F, Monaghan T et al. Review article: novel oral-targeted therapies in inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47(12):1610–1622. doi: 10.1111/apt.14669. [DOI] [PubMed] [Google Scholar]
- 19.Coskun M, Salem M, Pedersen J, Nielsen OH. Involvement of JAK/STAT signaling in the pathogenesis of inflammatory bowel disease. Pharmacol Res. 2013;76:1–8. doi: 10.1016/j.phrs.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Coskun M, Vermeire S, Nielsen OH. Novel targeted therapies for inflammatory bowel disease. Trends Pharmacol Sci. 2017;38(2):127–142. doi: 10.1016/j.tips.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 21.Danese S, Grisham M, Hodge J, Telliez J-B. JAK inhibition using tofacitinib for inflammatory bowel disease treatment: a hub for multiple inflammatory cytokines. Am J Physiol Gastrointest Liver Physiol. 2016;310(3):G155–G162. doi: 10.1152/ajpgi.00311.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.New York, NY: Pfizer, Inc; Xeljanz [prescribing information]. December 2021. [Google Scholar]
- 23.Sandborn WJ, Su C, Sands BE et al. OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 24.Sandborn WJ, Ghosh S, Panes J, Vranic I, Wang W, Niezychowski W. A phase 2 study of tofacitinib, an oral Janus kinase inhibitor, in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(9):1485–1493.e2.. doi: 10.1016/j.cgh.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 25.Panés J, Sandborn WJ, Schreiber S et al. Tofacitinib for induction and maintenance therapy of Crohn’s disease: results of two phase IIb randomised placebo-controlled trials. Gut. 2017;66(6):1049–1059. doi: 10.1136/gutjnl-2016-312735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sands BE, Armuzzi A, Marshall JK et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020;51(2):271–280. doi: 10.1111/apt.15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sandborn WJ, Lawendy N, Danese S et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE Open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55(4):464–478. doi: 10.1111/apt.16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.https://www.fda.gov/drugs/drug-safety-and-availability/initial-safety-trial-results-find-increased-risk-seriousheart-related-problems-and-cancer-arthritis US Food and Drug Administration. Initial safety trial results find increased risk of serious heart-related problems and cancer with arthritis and ulcerative colitis medicine Xeljanz, Xeljanz XR (tofacitinib). Published February 4, 2021.
- 29.https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-seriousheart-related-events-cancer-blood-clots-and-death US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. Published September 1, 2021.
- 30.Ytterberg SR, Bhatt DL, Mikuls TR et al. ORAL Surveillance Investigators. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 31.Namour F, Diderichsen PM, Cox E et al. Pharmacokinetics and pharmacokinetic/ pharmacodynamic modeling of filgotinib (GLPG0634), a selective JAK1 inhibitor, in support of phase IIB dose selection. Clin Pharmacokinet. 2015;54(8):859–874. doi: 10.1007/s40262-015-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panés J, Vermeire S, Lindsay JO et al. Tofacitinib in patients with ulcerative colitis: health-related quality of life in phase 3 randomised controlled induction and maintenance studies. J Crohns Colitis. 2018;12(2):145–156. doi: 10.1093/ecco-jcc/jjx133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554–1573.e12.. doi: 10.1053/j.gastro.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Feagan BG, Danese S, Loftus EV, Jr et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397(10292):2372–2384. doi: 10.1016/S0140-6736(21)00666-8. [DOI] [PubMed] [Google Scholar]
- 35.https://www.globenewswire.com/en/news-release/2021/11/15/2334506/0/en/Jyseleca-filgotinib-approved-in-the-European-Union-for-the-treatment-of-ulcerative-colitis.html GlobeNewswire. Jyseleca® (filgotinib) approved in the European Union for the treatment of ulcerative colitis [press release]. Published November 15, 2021.
- 36.https://www.gilead.com/news-and-press/press-room/press-releases/2020/8/gilead-receives-complete-response-letter-for-filgotinib-for-the-treatment-of-moderately-to-severelyactive-rheumatoid-arthritis Gilead. Gilead receives complete response letter for filgotinib for the treatment of moderately to severely active rheumatoid arthritis [press release]. Published August 18, 2020.
- 37.Hellstrom WJG, Dolhain RJEM, Ritter TE et al. MANTA and MANTA-RAy: rationale and design of trials evaluating effects of filgotinib on semen parameters in patients with inflammatory diseases. Adv Ther. 2022;39(7):3403–3422. doi: 10.1007/s12325-022-02168-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burmester GR, Kremer JM, Van den Bosch F et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECTNEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2018;391(10139):2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]
- 39.North Chicago, IL: AbbVie Inc; Rinvoq [prescribing information]. January 2022. [Google Scholar]
- 40.https://news.abbvie.com/news/press-releases/rinvoq-upadacitinib-receives-us-fda-approval-for-active-psoriatic-arthritis.htm#:~:text=14%2C%202021%20%2FPRNewswire%2F%20%2D%2D,or%20more%20tumor%20necrosis%20factor%20 AbbVie. RINVOQ® (upadacitinib) receives U.S. FDA approval for active psoriatic arthritis [press release]. Published December 14, 2021.
- 41.https://news.abbvie.com/news/press-releases/us-fda-approves-rinvoq-upadacitinib-to-treat-adults-and-children-12-years-and-older-with-refractory-moderate-to-severe-atopic-dermatitis.htm AbbVie. U.S. FDA approves RINVOQ® (upadacitinib) to treat adults and children 12 years and older with refractory, moderate to severe atopic dermatitis [press release]. Published January 14, 2022.
- 42.Aguilar D, Planell N, Panes J et al. P843 Upadacitinib-induced endoscopic improvement is associated with modulation of pathways involved in Crohn’s disease pathogenesis. J Crohns Colitis. 2018;12(supplement_1):S542–S543. [Google Scholar]
- 43.Sandborn WJ, Feagan BG, Loftus EV, Jr et al. Efficacy and safety of upadacitinib in a randomized trial of patients with Crohn’s disease. Gastroenterology. 2020;158(8):2123–2138.e8.. doi: 10.1053/j.gastro.2020.01.047. [DOI] [PubMed] [Google Scholar]
- 44.Peyrin-Biroulet L, Louis E, Loftus EV, Jr et al. Quality of life and work productivity improvements with upadacitinib: phase 2b evidence from patients with moderate to severe Crohn’s disease. Adv Ther. 2021;38(5):2339–2352. doi: 10.1007/s12325-021-01660-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed MF, Klünder B, Lacerda AP, Othman AA. Exposure-response analyses for upadacitinib efficacy and safety in the Crohn’s disease CELEST study and bridging to the extended-release formulation. Clin Pharmacol Ther. 2020;107(3):639–649. doi: 10.1002/cpt.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandborn WJ, Ghosh S, Panes J et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158(8):2139–2149.e14.. doi: 10.1053/j.gastro.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 47.Ghosh S, Sanchez Gonzalez Y, Zhou W et al. Upadacitinib treatment improves symptoms of bowel urgency and abdominal pain, and correlates with quality of life improvements in patients with moderate to severe ulcerative colitis. J Crohns Colitis. 2021;15(12):2022–2030. doi: 10.1093/ecco-jcc/jjab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Danese S, Vermeire S, Zhou W et al. OP24 Efficacy and safety of upadacitinib induction therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 U-ACHIEVE study. J Crohns Colitis. 2021;15(suppl 1):S022–S024. [Google Scholar]
- 49.Vermeire S, Danese S, Zhou W et al. OP23 Efficacy and safety of upadacitinib as induction therapy in patients with moderately to severely active ulcerative colitis: results from phase 3 U-ACCOMPLISH study. J Crohns Colitis. 2021;15(suppl 1):S021–S022. [Google Scholar]
- 50.Panaccione R, Hebuterne X, Lindsay J et al. Efficacy and safety of upadacitinib maintenance therapy in patients with moderately to severely active ulcerative colitis: results from a randomized phase 3 study. United European Gastroenterol J. 2021;9:1207–1209. [Google Scholar]
- 51.Danese S, Vermeire S, Zhou W et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113–2128. doi: 10.1016/S0140-6736(22)00581-5. [DOI] [PubMed] [Google Scholar]
- 52.https://news.abbvie.com/news/press-releases/rinvoq-upadacitinib-receives-fda-approval-for-treatment-adults-with-moderately-to-severely-active-ulcerative-colitis.htm AbbVie. RINVOQ® (upadacitinib) receives FDA approval for the treatment of adults with moderately to severely active ulcerative colitis [press release]. Published March 16, 2022.
- 53.https://news.abbvie.com/news/press-releases/upadacitinib-rinvoq-achieved-primary-and-key-secondary-endpoints-in-first-phase-3-induction-study-in-patients-withcrohns-disease.htm AbbVie. Upadacitinib (RINVOQ®) achieved primary and key secondary endpoints in first phase 3 induction study in patients with Crohn’s disease [press release]. Published December 6, 2021.
- 54.https://news.abbvie.com/news/press-releases/news-type/rd-news/second-phase-3-induction-study-confirms-upadacitinib-rinvoq-improvedclinical-and-endoscopic-outcomes-in-patients-with-crohns-disease.htm AbbVie. Second phase 3 induction study confirms upadacitinib (RINVOQ®) improved clinical and endoscopic outcomes in patients with Crohn’s disease [press release]. Published February 24, 2022.
- 55.Sandborn WJ, Nguyen DD, Beattie DT et al. Development of gut-selective pan-Janus kinase inhibitor TD-1473 for ulcerative colitis: a translational medicine programme. J Crohns Colitis. 2020;14(9):1202–1213. doi: 10.1093/ecco-jcc/jjaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.https://investor.theravance.com/news-releases/newsrelease-details/theravance-biopharma-inc-announces-top-line-results-phase-2b Theravance Biopharma. Theravance Biopharma, Inc. announces top-line results from phase 2b dose-finding induction study of izencitinib in patients with ulcerative colitis [press release]. Published August 23, 2021.
- 57.Wrobleski ST, Moslin R, Lin S et al. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J Med Chem. 2019;62(20):8973–8995. doi: 10.1021/acs.jmedchem.9b00444. [DOI] [PubMed] [Google Scholar]
- 58.https://news.bms.com/news/details/2021/Bristol-Myers-Squibb-Provides-Update-on-Phase-2-Study-of-Deucravacitinib-in-Patients-With-Moderate-to-Severe-Ulcerative-Colitis/default.aspx Bristol Myers Squibb. Bristol Myers Squibb provides update on phase 2 study of deucravacitinib in patients with moderate to severe ulcerative colitis [press release]. Published October 7, 2021.
- 59.Misselwitz B, Juillerat P, Sulz MC, Siegmund B, Brand S. Swiss IBDnet, an official working group of the Swiss Society of Gastroenterology. Emerging treatment options in inflammatory bowel disease: Janus kinases, stem cells, and more. Digestion. 2020;101(suppl 1):69–82. doi: 10.1159/000507782. [DOI] [PubMed] [Google Scholar]
- 60.Sandborn WJ, Cyrille M, Hansen MB et al. Efficacy and safety of abrilumab in a randomized, placebo-controlled trial for moderate-to-severe ulcerative colitis. Gastroenterology. 2019;156(4):946–957.e18.. doi: 10.1053/j.gastro.2018.11.035. [DOI] [PubMed] [Google Scholar]
- 61.Lichnog C, Klabunde S, Becker E et al. Cellular mechanisms of etrolizumab treatment in inflammatory bowel disease. Front Pharmacol. 2019;10:39. doi: 10.3389/fphar.2019.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandborn WJ, Vermeire S, Tyrrell H et al. Etrolizumab Global Steering Committee. Etrolizumab for the treatment of ulcerative colitis and Crohn’s disease: an overview of the phase 3 clinical program. Adv Ther. 2020;37(7):3417–3431. doi: 10.1007/s12325-020-01366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rosenfeld G, Parker CE, MacDonald JK, Bressler B. Etrolizumab for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2015;12:CD011661. doi: 10.1002/14651858.CD011661.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vermeire S, O’Byrne S, Keir M et al. Etrolizumab as induction therapy for ulcerative colitis: a randomised, controlled, phase 2 trial. Lancet. 2014;384(9940):309–318. doi: 10.1016/S0140-6736(14)60661-9. [DOI] [PubMed] [Google Scholar]
- 65.Selinger C, Sandborn W, Panes J et al. OTU-003 Etrolizumab as induction therapy in moderate to severe Crohn’s disease: results from Bergamot cohort 1. Gut. 2018;67(suppl 1):A53. [Google Scholar]
- 66.Danese S, Colombel JF, Lukas M et al. Etrolizumab versus infliximab for the treatment of moderately to severely active ulcerative colitis (GARDENIA): a randomised, double-blind, double-dummy, phase 3 study. Lancet Gastroenterol Hepatol. 2022;7(2):118–127. doi: 10.1016/S2468-1253(21)00294-6. [DOI] [PubMed] [Google Scholar]
- 67.Peyrin-Biroulet L, Hart A, Bossuyt P et al. Etrolizumab as induction and maintenance therapy for ulcerative colitis in patients previously treated with tumour necrosis factor inhibitors (HICKORY): a phase 3, randomised, controlled trial. Lancet Gastroenterol Hepatol. 2022;7(2):128–140. doi: 10.1016/S2468-1253(21)00298-3. [DOI] [PubMed] [Google Scholar]
- 68.Rubin DT, Dotan I, DuVall A et al. HIBISCUS Study Group. Etrolizumab versus adalimumab or placebo as induction therapy for moderately to severely active ulcerative colitis (HIBISCUS): two phase 3 randomised, controlled trials. Lancet Gastroenterol Hepatol. 2022;7(1):17–27. doi: 10.1016/S2468-1253(21)00338-1. [DOI] [PubMed] [Google Scholar]
- 69.Vermeire S, Lakatos PL, Ritter T et al. LAUREL Study Group. Etrolizumab for maintenance therapy in patients with moderately to severely active ulcerative colitis (LAUREL): a randomised, placebo-controlled, double-blind, phase 3 study. Lancet Gastroenterol Hepatol. 2022;7(1):28–37. doi: 10.1016/S2468-1253(21)00295-8. [DOI] [PubMed] [Google Scholar]
- 70.Sandborn WJ, Feagan BG, D’Haens G et al. True North Study Group. Ozanimod as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2021;385(14):1280–1291. doi: 10.1056/NEJMoa2033617. [DOI] [PubMed] [Google Scholar]
- 71.Panaccione R, Afzali A, Hudesman D . San Diego: CA; 2022. Tu1450 Extended therapy with ozanimod for delayed responders to ozanimod in moderately to severely active ulcerative colitis: data from the True North open-label study. Presented at DDW. May 21-24, 2022. [Google Scholar]
- 72.Wolf D, Colombel JF, Ponich T . Presented at DDW. San Diego: CA; 2022. 2022. Tu1458 Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis. May 21-24. [Google Scholar]
- 73.Feagan BG, Sandborn WJ, Danese S et al. Ozanimod induction therapy for patients with moderate to severe Crohn’s disease: a single-arm, phase 2, prospective observer-blinded endpoint study. Lancet Gastroenterol Hepatol. 2020;5(9):819–828. doi: 10.1016/S2468-1253(20)30188-6. [DOI] [PubMed] [Google Scholar]
- 74.Summit, NJ: Celgene; Zeposia [prescribing information]. December 2021. [Google Scholar]
- 75.Sandborn WJ, Peyrin-Biroulet L, Zhang J et al. Efficacy and safety of etrasimod in a phase 2 randomized trial of patients with ulcerative colitis. Gastroenterology. 2020;158(3):550–561. doi: 10.1053/j.gastro.2019.10.035. [DOI] [PubMed] [Google Scholar]
- 76.Vermeire S, Chiorean M, Panés J et al. Long-term safety and efficacy of etrasimod for ulcerative colitis: results from the open-label extension of the OASIS study. J Crohns Colitis. 2021;15(6):950–959. doi: 10.1093/ecco-jcc/jjab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abraham C, Dulai PS, Vermeire S, Sandborn WJ. Lessons learned from trials targeting cytokine pathways in patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):374–388.e4.. doi: 10.1053/j.gastro.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aggeletopoulou I, Assimakopoulos SF, Konstantakis C, Triantos C. Interleukin 12/interleukin 23 pathway: biological basis and therapeutic effect in patients with Crohn’s disease. World J Gastroenterol. 2018;24(36):4093–4103. doi: 10.3748/wjg.v24.i36.4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sandborn WJ, Rebuck R, Wang Y et al. Five-year efficacy and safety of ustekinumab treatment in Crohn’s disease: the IM-UNITI trial. Clin Gastroenterol Hepatol. 2022;20(3):578–590.e4.. doi: 10.1016/j.cgh.2021.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma C, Jairath V, Khanna R, Feagan BG. Investigational drugs in phase I and phase II clinical trials targeting interleukin 23 (IL23) for the treatment of Crohn’s disease. Expert Opin Investig Drugs. 2018;27(8):649–660. doi: 10.1080/13543784.2018.1506764. [DOI] [PubMed] [Google Scholar]
- 81.Ma C, Panaccione R, Khanna R, Feagan BG, Jairath V. IL12/23 or selective IL23 inhibition for the management of moderate-to-severe Crohn’s disease? Best Pract Res Clin Gastroenterol. 2019. pp. 38–39.pp. 101604 [DOI] [PubMed]
- 82.Sands BE, Chen J, Feagan BG et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology. 2017;153(1):77–86.e6.. doi: 10.1053/j.gastro.2017.03.049. [DOI] [PubMed] [Google Scholar]
- 83.Sands BE, Gasser Jr RA, Gommoll C, Danese S. Long-term safety in the open-label period of a phase 2a study of brazikumab, an antibody against interleukin-23: 590. Am J Gastroenterol. 2018;113:S339. [Google Scholar]
- 84.North Chicago, IL: AbbVie; Skyrizi [prescribing information]. January 2022. [Google Scholar]
- 85.Feagan BG, Sandborn WJ, D’Haens G et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-tosevere Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389(10080):1699–1709. doi: 10.1016/S0140-6736(17)30570-6. [DOI] [PubMed] [Google Scholar]
- 86.Feagan BG, Panés J, Ferrante M et al. Risankizumab in patients with moderate to severe Crohn’s disease: an open-label extension study. Lancet Gastroenterol Hepatol. 2018;3(10):671–680. doi: 10.1016/S2468-1253(18)30233-4. [DOI] [PubMed] [Google Scholar]
- 87.D’Haens GR, Colombel JF, Bossuyt P et al. 775a Risankizumab induction therapy in patients with moderate-to-severe Crohn’s disease with intolerance or inadequate response to conventional and/or biologic therapy: results from the phase 3 ADVANCE study. Gastroenterology. 2021;161(2):e28. [Google Scholar]
- 88.Panaccione R, Schreiber S, Peyrin-Biroulet L et al. S754 Risankizumab as induction therapy in patients with moderately to severely active Crohn’s disease who failed 1 vs >1 prior biologic treatment: results from the MOTIVATE study. Am J Gastroenterol. 2021;116:S348. [Google Scholar]
- 89.Ferrante MPR, Feagan BG, Sandborn WJ et al. Efficacy and safety of risankizumab as maintenance therapy in patients with Crohn’s disease: 52 week results from the phase 3 FORTIFY study. United European Gastroenterol J. 2021;9(10):1210–1213. [Google Scholar]
- 90.D’Haens G, Panaccione R, Baert F et al. Risankizumab as induction therapy for Crohn’s disease: results from the phase 3 ADVANCE and MOTIVATE induction trials. Lancet. 2022;399(10340):2015–2030. doi: 10.1016/S0140-6736(22)00467-6. [DOI] [PubMed] [Google Scholar]
- 91.Ferrante M, Panaccione R, Baert F et al. Risankizumab as maintenance therapy for moderately to severely active Crohn’s disease: results from the multicentre, randomised, double-blind, placebo-controlled, withdrawal phase 3 FORTIFY maintenance trial. Lancet. 2022;399(10340):2031–2046. doi: 10.1016/S0140-6736(22)00466-4. [DOI] [PubMed] [Google Scholar]
- 92.Boehncke WH, Brembilla NC, Nissen MJ. Guselkumab: the first selective IL-23 inhibitor for active psoriatic arthritis in adults. Expert Rev Clin Immunol. 2021;17(1):5–13. doi: 10.1080/1744666X.2020.1857733. [DOI] [PubMed] [Google Scholar]
- 93.Horsham, PA: Janssen Biotech; Tremfya [prescribing information] July 2020. [Google Scholar]
- 94.Danese S, Sandborn WJ, Feagan BG et al. OP28 The effect of guselkumab induction therapy on early clinical outcome measures in patients with moderately to severely active Crohn’s disease: results from the phase 2 GALAXI 1 study. J Crohns Colitis. 2021;15(suppl 1):S027–S028. [Google Scholar]
- 95.https://www.jnj.com/janssen-reports-positive-topline-week-48-phase-2-results-for-tremfya-guselkumab-in-adults-with-moderately-to-severely-active-crohns-disease Johnson & Johnson. Janssen reports positive topline week 48 phase 2 results for TREMFYA® (guselkumab) in adults with moderately to severely active Crohn’s disease [press release]. Published November 17, 2021.
- 96.Sandborn WJ, Ferrante M, Bhandari BR et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with ulcerative colitis. Gastroenterology. 2020;158(3):537–549.e10.. doi: 10.1053/j.gastro.2019.08.043. [DOI] [PubMed] [Google Scholar]
- 97.Sandborn WJ, Ferrante M, Bhandari BR et al. Efficacy and safety of continued treatment with mirikizumab in a phase 2 trial of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2022;20(1):105–115.e14.. doi: 10.1016/j.cgh.2020.09.028. [DOI] [PubMed] [Google Scholar]
- 98.https://investor.lilly.com/news-releases/news-release-details/mirikizumabdemonstrates-superiority-over-placebo-phase-3 Lilly. Mirikizumab demonstrates superiority over placebo in phase 3 maintenance study in ulcerative colitis, supporting regulatory submissions in 2022 [press release]. Published December 14, 2021.
- 99.Dubinsky M, Irving PM, Li X . San Diego: CA; 2022. 2022. 867e: Efficacy and safety of mirikizumab as maintenance therapy in patients with moderately to severely active ulcerative colitis: results from the phase 3 LUCENT-2 study. Presented at DDW. May 21-24. [PMC free article] [PubMed] [Google Scholar]
- 100.0.Sands BE, Peyrin-Biroulet L, Kierkus J. et al. Efficacy and safety of mirikizumab in a randomized phase 2 study of patients with Crohn’s disease. Gastroenterology. 2022. 162 2 495 508 [DOI] [PubMed] [Google Scholar]
- 101.1.Panés J, García-Olmo D, Van Assche G. et al. ADMIRE CD Study Group Collaborators. Expanded allogeneic adipose-derived mesenchymal stem cells (Cx601) for complex perianal fistulas in Crohn’s disease: a phase 3 randomised, double-blind controlled trial. Lancet. 2016. 388 10051 1281 1290 [DOI] [PubMed] [Google Scholar]
- 102.2.Dietz AB, Dozois EJ, Fletcher JG. et al. Autologous mesenchymal stem cells, applied in a bioabsorbable matrix, for treatment of perianal fistulas in patients with Crohn’s disease. Gastroenterology. 2017. 153 1 59 62.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.3.Dozois EJ, Lightner AL, Mathis KL. et al. Early results of a phase I trial using an adipose-derived mesenchymal stem cell-coated fistula plug for the treatment of transsphincteric cryptoglandular fistulas. Dis Colon Rectum. 2019. 62 5 615 622 [DOI] [PubMed] [Google Scholar]
- 104.4.Lightner AL, Dozois EJ, Dietz AB. et al. Matrix-delivered autologous mesenchymal stem cell therapy for refractory rectovaginal Crohn’s fistulas. Inflamm Bowel Dis. 2020. 26 5 670 677 [DOI] [PubMed] [Google Scholar]
- 105.5.Snowden JA, Sharrack B, Akil M. et al. Autologous haematopoietic stem cell transplantation (aHSCT) for severe resistant autoimmune and inflammatory diseases— a guide for the generalist. Clin Med (Lond). 2018. 18 4 329 334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.6.Hawkey CJ, Allez M, Clark MM. et al. Autologous hematopoetic stem cell transplantation for refractory Crohn disease: a randomized clinical trial. JAMA. 2015. 314 23 2524 2534 [DOI] [PubMed] [Google Scholar]
- 107.7.Brierley CK, Castilla-Llorente C, Labopin M. et al. European Society for Blood and Marrow Transplantation [EBMT] Autoimmune Diseases Working Party [ADWP]. Autologous haematopoietic stem cell transplantation for Crohn’s disease: a retrospective survey of long-term outcomes from the European Society for Blood and Marrow Transplantation. J Crohns Colitis. 2018. 12 9 1097 1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.8.Snowden JA, Hawkey C, Hind D. et al. Autologous Stem Cell Transplantation in Refractory CD -Low Intensity Therapy Evaluation Study Investigators; European Society for Blood and Marrow Transplantation (EBMT) Autoimmune Diseases Working Party (ADWP). Autologous stem cell transplantation in refractory Crohn’s disease -low intensity therapy evaluation (ASTIClite): study protocols for a multicentre, randomised controlled trial and observational follow up study. BMC Gastroenterol. 2019. 19 1 82 [DOI] [PMC free article] [PubMed] [Google Scholar]