Abstract
Multiple studies and extensive clinical experience have shown that COVID-19 can impact the hepatobiliary system, with most reports describing primarily hepatocellular injury with elevations of aspartate aminotransferase and alanine aminotransferase. In addition to hepatocellular injury, recent literature has described a pattern of severe biliary tract injury resulting in patients with COVID-19. This novel syndrome, termed COVID-19 cholangiopathy, may have severe consequences for affected patients. This article will examine the literature describing this novel entity, its relationship to secondary sclerosing cholangitis, clinical outcomes, and proposed mechanisms underlying this form of biliary injury.
Keywords: COVID-19, bile duct injury, cholangiopathy, liver, sclerosing cholangitis
COVID-19 has been known to involve both the gastrointestinal tract and the liver. Enteric symptoms have been noted in 20% or more of patients with COVID-19 and may emerge prior to development of respiratory symptoms.1 These enteric symptoms consist primarily of anorexia, nausea, vomiting, and diarrhea,2,3 and are hypothesized to be related to the gut-lung axis, which is the relationship between respiratory tract flora and gut flora through immune regulation.4 The most common effect of COVID-19 on the liver has been hepatocellular injury, with elevations of serum aspartate aminotransferase and alanine aminotransferase up to twice the upper limit of normal (ULN) and occasionally more prominent elevations (>2 × ULN).3,4 These laboratory abnormalities were observed during the initial hospitalization of patients with COVID-19. COVID-19 cholangiopathy shows a different pattern of injury, with marked elevations of serum alkaline phosphatase (ALP) often accompanied by hyperbilirubinemia and radiographic evidence of biliary duct injury similar to that in primary sclerosing cholangitis (PSC) or the rarer secondary sclerosing cholangitis (SSC) after critical illness. The cholangiopathy in patients with COVID-19 has been observed several weeks to months after initial diagnosis of SARS-CoV-2 infection and entails potentially serious or life-threatening prognostic implications.
Another recent worldwide epidemic, AIDS, was also caused by a virus (HIV) that came to be recognized as a cause of biliary tract injury similar to PSC. AIDS cholangiopathy, first described by Margulis and colleagues,5 was associated with advanced AIDS and contributed to the poor prognosis of patients in the era before antiviral therapy, with worsening sclerosing cholangitis, and even some case reports describing progression to cholangiocarcinoma.6-9 Although HIV was demonstrated to directly infect hepatocytes, Kupffer cells, and endothelial cells,10 the cholangiopathy was thought to be caused by opportunistic infection of the biliary tract with Cryptosporidium parvum, cytomegalovirus, microsporidia such as Enterocytozoon bieneusi, or disseminated Mycobacterium avium complex.11 Treatment of AIDS cholangiopathy involves eradicating the underlying opportunistic pathogens, or initiation of highly active antiretroviral therapy to refurbish the immune system.
Clinical Presentation
Unlike for AIDS cholangiopathy, however, opportunistic biliary tract infection has not been invoked as a potential mechanism underlying COVID-19 cholangiopathy. As described by Faruqui and colleagues in November 2020 at the American Association for the Study of Liver Diseases Annual Meeting, COVID-19 cholangiopathy was identified by biochemical signs of severe cholestasis in patients who had developed severe illness from COVID19 and had required lengthy intensive care unit (ICU) admissions.12 The diagnosis of biliary tract disease was usually made weeks or even several months after initial admission for COVID-19. Laboratory features included markedly elevated ALP levels and often hyperbilirubinemia accompanied by substantial alanine aminotransferase elevations. Contemporaneously, a similar report appeared from the United Kingdom.13 Additionally, in the first fully published case series of COVID-19 cholangiopathy, Roth and colleagues characterized COVID-19 cholangiopathy in 2 men and 1 woman, ranging from 25 to 40 years of age, recovering from prolonged courses of critical illness.14 Liver biopsies in the 3 patients showed predominant cholangiocyte injury with microvascular changes, as well as evidence of bridging fibrosis, which led the authors to hypothesize the likely long-lasting negative effects of COVID-19 cholangiopathy on all patients. The Table details characteristics of patients diagnosed with COVID-19 cholangiopathy as described in the literature. Elaborating upon their initial report, Faruqui and colleagues further described 12 patients, mostly male (11/12), with a mean age of 58 years.12,15 The mean time of COVID-19 diagnosis to diagnosis of cholangiopathy by magnetic resonance cholangiopancreatography (MRCP) was 118 days. Total bilirubin was markedly elevated, with a median value of 13 mg/dL, as was ALP, with a median value of 1945 U/L. Inflammatory markers were also markedly elevated, including C-reactive protein and ferritin, and 6 patients had peak D-dimer levels greater than 10,000 ng/mL. All 12 patients required mechanical ventilation, with 3 (25%) patients receiving extracorporeal membrane oxygenation. Eight of the 12 patients (67%) experienced a thromboembolic event and required therapeutic anticoagulation treatment. All 12 patients had abnormal MRCP findings: beaded appearance of intrahepatic ducts (11 patients), peribiliary diffusion high signal (10 patients), and thickening and hyperenhancement of the bile duct wall (7 patients) (Figures 1-3). Liver biopsy findings in 4 patients showed acute and/or chronic large duct obstruction without definitive bile duct loss, as well as mild fibrosis of some portal tracts. There was prominent staining of hepatocytes in immunostaining for keratin 7, which is typical of cholestatic liver disease. These findings are also seen in PSC.
Table.
Characteristics of Pts With COVID-19 Cholangiopathy
| Reference | Age (yrs) and Sex of Pt(s) | Clinical Findings | COVID-19 Diagnosis to COVID-19 Cholangiopathy Diagnosis Duration | Medications Used During Initial Hospitalization | Clinical Follow-Up |
|---|---|---|---|---|---|
| Sanders et al38 | 57, male | Pt with cholangitis requiring ERCP and acalculous cholecystitis; upon repeat ERCP for stent removal 2 weeks later, significant intrahepatic ductal dilation found; diagnosed with SSC | ~3 months | NA | NA |
| Weaver et al39 | 63, male | Pt underwent ERCP for elevated LFTs and sludge in gallbladder on ultrasound; irregular and beaded appearance of intrahepatic ducts | 48 days | Hydroxychloroquine, azith- romycin, tocilizumab, plasma exchange, fluconazole | NA |
| Faruqui et al15 | Pt 1: 73, male Pt 2: 39, male Pt 3: 77, male Pt 4: 77, male Pt 5: 46, male Pt 6: 72, male Pt 7: 38, male Pt 8: 60, male Pt 9: 42, male Pt 10: 57, male Pt 11: 68, male Pt 12: 62, female |
Pts with bile duct injury noted with serum ALP >3 × ULN and with MRCP findings of beading of intrahepatic ducts (11/12), peribiliary diffusion high signal (10/12), and all showing progressive biliary tract injury (12/12); on biopsy, 4 pts had acute or chronic large duct obstruction without clear bile duct loss | Pt 1: 238 days Pt 2: 175 days Pt 3: 319 days Pt 4: 358 days Pt 5: 302 days Pt 6: 229 days Pt 7: 240 days Pt 8: 314 days Pt 9: 138 days Pt 10: 138 days Pt 11: 308 days Pt 12: 189 days |
Pt 1: azithromycin, apixaban Pt 2: tocilizumab, azithromycin, enoxaparin Pt 3: hydroxychloroquine, azithromycin, enoxaparin Pt 4: hydroxychloroquine, azithromycin, remdesivir, apixaban Pt 5: hydroxychloroquine, tocilizumab, azithromycin, enoxaparin Pt 6: hydroxychloroquine, azithromycin, heparin Pt 7: hydroxychloroquine, azithromycin, heparin Pt 8: hydroxychloroquine, azithromycin, enoxaparin Pt 9: remdesivir, valacyclovir, foscarnet, heparin, bivalirudin Pt 10: azithromycin, heparin Pt 11: hydroxychloroquine, heparin Pt 12: azithromycin, heparin |
Pt 1: alive, declined as liver transplant candidate Pt 2: alive, no liver transplant Pt 3: alive, had living donor transplant Pt 4: alive, no liver transplant, on ursodiol Pt 5: alive, no liver transplant, laparoscopic cholecystectomy, off ursodiol since Pt 6: deceased Pt 7: deceased Pt 8: alive, listed for liver transplant Pt 9: deceased Pt 10: deceased Pt 11: alive, declined liver transplant evaluation Pt 12: alive, no liver transplant |
| Roth et al14 | Pt 1: 38, male Pt 2: 25, male Pt 3: 40, female |
Pts had marked cholestasis during recovery from severe COVID-19; MRCP in 2 pts showed irregularity of intrahepatic biliary tree with intervening areas of beading; liver biopsies showed at least moderate portal/periportal fibrosis and extensive degenerative cholangiocyte injury with prominent cholangiocyte vacuolization | Pt 1: 158 days Pt 2: 88 days Pt 3: 178 days |
Pt 1: hydroxychloroquine, azithromycin, tocilizumab Pt 2: hydroxychloroquine, azithromycin, ivermectin, corticosteroids, tocilizumab, anakinra, convalescent plasma, remdesivir, meropenem, piperacillin-tazobactam, vancomycin, caspofungin, fluconazole Pt 3: hydroxychloroquine, azithromycin, corticosteroids, anakinra, aztreonam, cefepime, ertapenem, meropenem, nitrofurantoin, piperacillin-tazobactam, vancomycin, fluconazole |
NA |
| Durazo et al16 | 47, male | Pt with elevated LFTs, including ALP of 2730 U/L, total bilirubin of 19 mg/dL; liver biopsy showed mechanical bile duct obstruction; ERCP showed diffuse intrahepatic biliary strictures; MRCP had similar findings of intrahepatic biliary ductal dilation with multifocal strictures/beading | 73 days | Hydroxychloroquine, azithromycin, high-dose vitamin C | Underwent liver-kidney OLT on day 108 from initial presentation; at time of publication, pt was 7 months posttransplant and doing well in recovery |
| Lee et al17 | 64, male | Pt, while in inpatient rehabilitation, had rising total bilirubin to 6.8 mg/dL and jaundice; ERCP showed relative ductopenia and ductal bleeding; MRCP showed mild intrahepatic biliary ductal dilation and mild patchy T2 hyperintensity in right liver | 51 days | Hydroxychloroquine, azithromycin, tocilizumab, convalescent plasma | Underwent OLT 259 days after initial COVID-19 diagnosis, with normal allograft function at 8 months posttransplant |
| Meersseman et al40 | Pt 1: 67, male Pt 2: 68, male Pt 3: 66, male Pt 4: 45, male |
Pts developed progressive cholestatic liver injury that persisted after ARDS resolved; MRCP showed diffuse beading of intrahepatic biliary system, focal strictures in hepatic ducts, and diminished arborization of intrahepatic biliary tree | NA | Pt 1: ketamine, corticosteroids Pt 2: ketamine, corticosteroids Pt 3: ketamine, corticosteroids, antiviral medication Pt 4: ketamine, corticosteroids |
Pt 1: underwent OLT at 167 days after initial admission; at time of publication, alive at last follow-up of 267 days Pt 2: underwent liver-kidney transplant at 132 days; deceased6 weeks posttransplant from multiresistant Pseudomonas pneumonia and multifactorial acute kidney injury Pt 3: no liver transplant; deceased on day 169 of hemorrhagic shock Pt 4: no liver transplant; at time of publication, alive at 258 days with resolved cholestasis |
| Tafreshi et al19 | 38, male | Pt with severe cholestasis and jaundice after discharge had markedly elevated ALP to 3665 U/L; MRCP showed marked beading and irregularity of intrahepatic bile ducts and diffuse periductal enhancement; liver biopsy showed cholestatic hepatitis with cholangiocyte injury, and focal bridging fibrosis | NA | NA | At time of publication, pt was being evaluated for liver transplant |
| Klindt et al20 | 47, male | Pt admitted with mildly elevated ALP that rose; ultrasound showed multiple changes in diameter of small and medium-sized intrahepatic ducts and increased sonographic reflexes of biliary ducts (seen in sclerosing cholangitis); MRCP consistent with these findings; liver biopsy showed enlarged portal tracts with mixed inflammatory infiltrate, degenerative changes of bile duct epithelium, and ductular reaction | 20 days | Lopinavir-ritonavir, remdesivir, piperacillin-tazobactam, clarithromycin, meropenem | Underwent OLT; no further details given |
| Edwards et al13 | 57, male | During ICU course, pt had elevated LFTs; MRCP demonstrated intrahepatic biliary duct dilation and beading; ERCP showed uncommon appearance of common bile duct with large column of sludge | NA | Levofloxacin, vancomycin, co-trimoxazole, caspofungin | At time of publication, waiting for liver biopsy to evaluate degree of hepatocyte and sinusoidal injury; had undergone 2 ERCPs and on ursodiol |
| Bütikofer et al21 | 4 pts, median age of 61.5, 75% male | Pts with cholestasis and jaundice while in ICU; MRCP showed irregularities or bile ducts with stricturing | NA | NA | 1 pt being evaluated for OLT; 2 pts deceased after ICU discharge; 1 pt with persistently elevated ALP and γ-glutamyltransferase |
| Rojas et al26 | 29, female | Pt, during initial ICU course, developed jaundice, markedly elevated bilirubin (>15 mg/dL) and ALP (>6000 U/L); liver biopsy showed periportal inflammatory infiltrate with severe obstructive cholestatic pattern | ~3 months | Piperacillin-tazobactam, vancomycin, nystatin, meropenem, vancomycin, linezolid, cefepime, clarithromycin, enoxaparin, aspirin, colchicine | At time of publication, pt with continued laboratory abnormalities and other sequelae of long-haul COVID-19 (memory loss, dizziness, headaches); on ursodiol and cholestyramine |
| Linneweber et al25 | Pt 1: 64, male Pt 2: 72, male |
Pt 1: During early rehabilitation, presented with elevated LFTs; ERCP showed inflammation, stricture formation, and rarefication of peripheral bile duct system, and was performed 4 times with continued bile duct dilation Pt 2: After transfer from ICU, had elevated LFTs with peak γ-glutamyltransferase of 2848 U/L and MRCP with dilation of common bile duct but no intrahepatic cholestasis; subsequent MRCP showed bile duct irregularities; ERCP showed subtotal destruction of intrahepatic bile ducts |
NA | NA | Pt 1: with continued elevated bilirubin and LFTs Pt 2: deceased from septic shock from cholangitis 8 months after initial COVID-19 diagnosis |
ALP, alkaline phosphatase; ARDS, acute respiratory distress syndrome; ERCP, endoscopic retrograde cholangiopancreatography; ICU, intensive care unit; LFT, liver function test; MRCP, magnetic resonance cholangiopancreatography; NA, not available; OLT, orthotopic liver transplant; pt, patient; SSC, secondary sclerosing cholangitis; ULN, upper limit of normal.
Figure 1.
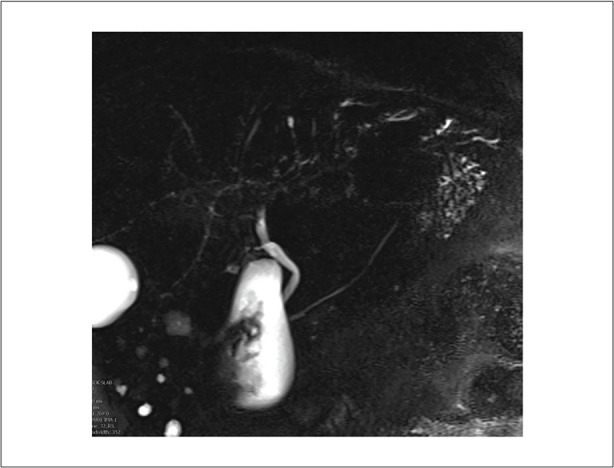
Coronal magnetic resonance cholangiopancreatography image of a 60-year-old man demonstrating multifocal mild diffuse intrahepatic biliary dilatation with beaded appearance.
Figure 3.
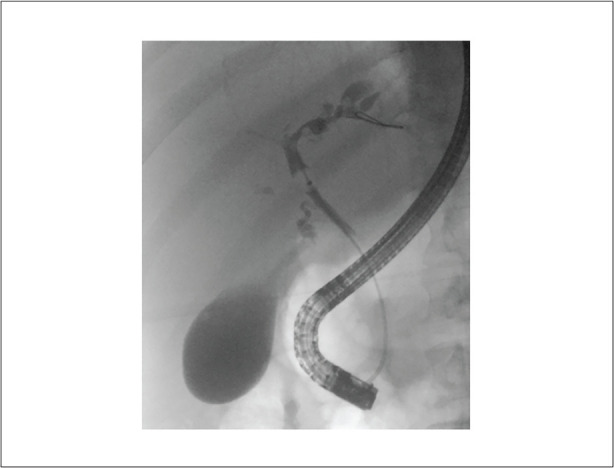
Coronal endoscopic retrograde cholangiopancreatography image demonstrating multifocal intrahepatic biliary dilation with stricturing, predominantly involving the left lobe.
Figure 2.
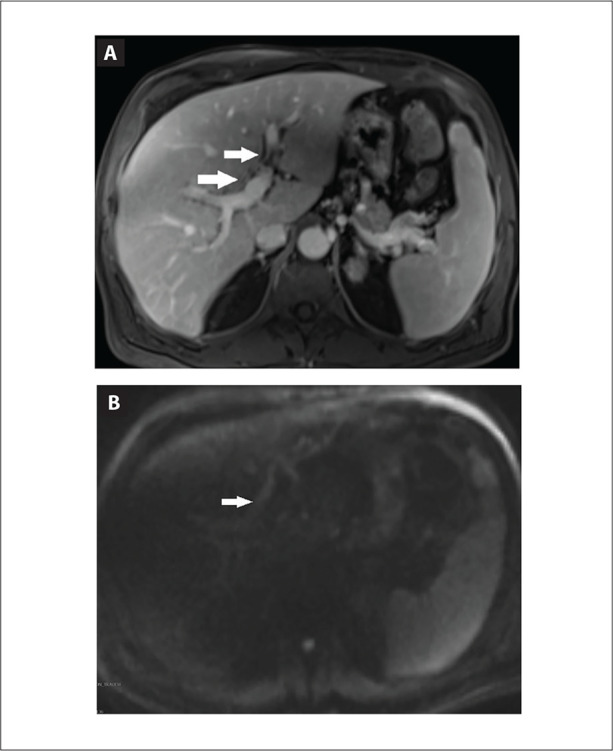
Axial contrast-enhanced T1-weighted magnetic resonance image (A) and diffusion-weighted image (B) demonstrating peribiliary enhancement and diffusion restriction (arrows) compatible with active inflammation.
Long-Term Implications and Role of Liver Transplantation
Not much is known about the long-term implications of COVID-19 cholangiopathy. Faruqui and colleagues observed no complete resolution of signs of biliary injury in any patient examined.15 Eleven of the 12 patients studied were treated with ursodiol, without clear improvement in clinical status or liver function tests. Five patients were considered for liver transplant, and 1 patient received a living donor liver transplant. MRCP showed worsening of biliary stricturing disease. An additional patient in the series, after remaining stable but with severe cholangiopathy for 1 year, developed decompensated cirrhosis with liver and kidney failure despite treatment with ursodiol throughout.
At least 4 additional patients with COVID-19 cholangiopathy undergoing liver transplant have been reported. Durazo and colleagues16 described a 47-yearold man, severely obese with a body mass index of 51, who presented to their institution with SARS-CoV-2 infection. The patient had a lengthy hospital admission that included prolonged mechanical ventilation (29 days) and continuous venovenous hemofiltration. On day 73 of hospitalization, the patient had markedly elevated liver tests, with total bilirubin of 10.9 mg/dL and ALP of 970 U/L, and underwent endoscopic retrograde cholangiopancreatography (ERCP) with findings of diffuse intrahepatic biliary strictures. The patient’s liver tests continued to rise, with a total bilirubin of 19 mg/dL and ALP of 2730 U/L, and MRCP and ERCP showed continued stricturing and beading of intrahepatic ducts. Because of worsening disease and concern for progression to liver failure, the patient underwent orthotopic liver transplant (OLT) on day 108 from initial presentation. The native liver weighed 4000 grams, with histologic findings of severe sclerosing cholangitis and hepatic abscesses. Seven months after OLT, the patient’s hepatic allograft function was normal, and the patient was doing well in recovery.
Additionally, Lee and colleagues described a patient with COVID-19 cholangiopathy who underwent OLT successfully.17 The patient was a 64-year-old man who required ICU care for severe COVID-19. On day 51 after his diagnosis of COVID-19, the patient’s total bilirubin significantly increased from 1.6 mg/dL to 6.8 mg/dL, and on day 52, the patient underwent ERCP with findings of cholangiopathy. The patient was listed for OLT on day 224 after his initial diagnosis of COVID-19, and underwent OLT on day 259. The native liver was noted to be cholestatic and firm in appearance, with pathology showing bridging fibrosis, diffuse hepatic injury, and evidence of intrahepatocellular cholestasis and scattered loss of bile ducts in various portal tracts. At the time of publication, 8 months after transplant, the patient was doing well and had returned to work. Further, Blondeel and colleagues described 2 patients who underwent liver transplant for refractory cholangitis.18
Tafreshi and colleagues19 described a patient who underwent evaluation for liver transplant as a result of what the authors initially dubbed SSC owing to COVID-19. This 38-year-old patient was treated for COVID-19– related pneumonia in March 2020, requiring mechanical ventilation and extracorporeal membrane oxygenation. The patient had normal liver tests on admission; however, a few months later, during the same admission, the patient developed severe cholestasis with jaundice (peak ALP of 3665 U/L). Computed tomography of the abdomen/pelvis with contrast showed mild intrahepatic biliary ductal dilatation, periportal edema, and a mildly dilated, hyperenhancing common bile duct. On a later hospitalization in August 2020, the patient underwent MRCP, which showed marked beading and irregularity of intrahepatic bile ducts as well as diffuse periductal enhancement. Liver biopsy showed cholestatic hepatitis with cholangiocyte injury, as well as focal bridging fibrosis.
COVID-19 Cholangiopathy and Secondary Sclerosing Cholangitis
Although the etiology of biliary injury remains unclear, COVID-19 cholangiopathy presents similarly to SSC after critical illness. Several additional case studies have described the presence of SSC in patients with severe COVID-19, which at least descriptively reflects COVID-19 cholangiopathy, raising the possibility of a shared pathophysiology.13,19-21 First reported in 2001 by Scheppach and colleagues, SSC is an ischemic form of cholangiopathy observed in patients with lengthy ICU admissions.22,23 Patients were diagnosed with SSC by ERCP and/or liver histology. A case series by Laurent and colleagues examined 13 patients with endoscopic findings of biliary casts with impairment of biliary flow and subsequent cholangitis, and liver biopsy showed confirmed cholangitis and hemorrhagic exudates in bile ducts.24 MRCP findings showed endoluminal defects of bile ducts corresponding to biliary casts, strictures, and thickening of intrahepatic bile ducts. Compared with studies of COVID-19 cholangiopathy wherein values increased after weeks to months, ALP values increased after a median time of 11 days, peaking on day 15. All patients also received 10 mg/kg to 15 mg/kg of ursodeoxycholic acid, as did most of the patients reported by Faruqui and colleagues.15 Two patients in the study by Laurent and colleagues died from liver failure, 1 patient had worsening stricturing of intrahepatic ducts (with no further complication), and 9 patients had persistent minor stricturing and no significant complications.24 One patient had normal liver tests, whereas no patient reported in the literature thus far with COVID-19 cholangiopathy has demonstrated complete spontaneous recovery.
Klindt and colleagues described a case of SSC in a 47-year-old man admitted for SARS-CoV-2–mediated acute respiratory distress syndrome.20 Upon admission, the patient had mildly elevated markers of biliary injury (ALP of 1.56 × ULN and normal total bilirubin). Inflammatory makers were strikingly elevated (C-reactive protein of 66.8 × ULN). The patient’s ALP continually increased throughout admission, reaching 1097 U/L on day 20 of admission. Liver ultrasound on day 39 showed changes in the diameter of the small and medium-sized intrahepatic biliary ducts, as well as increased sonographic reflexes of biliary ducts (seen in sclerosing cholangitis). MRCP findings were consistent with ultrasound findings.
Although significant biliary tract injury was present earlier than in other cases of COVID-19 cholangiopathy, the patient’s ALP remained elevated at 1538 U/L, and total bilirubin rose to 18 mg/dL on day 110 of admission. The patient ultimately underwent liver transplant because of continued worsening of liver function.
Edwards and colleagues,13 Bütikofer and colleagues,21 Linneweber and colleagues,25 and Rojas and colleagues26 also described SSC in patients with severe COVID-19. Edwards and colleagues described a 57-year-old patient who was critically ill owing to the virus and went into multiorgan failure.13 During the ICU admission, the patient had elevations of liver tests first attributed to the patient’s critically ill state. However, elevations persisted and MRCP and ERCP also showed characteristic beading appearance.
Bütikofer and colleagues conducted a retrospective, single-center cohort study of patients admitted to the ICU at the University Hospital of Zurich between March and June 2020.21 The study included 34 patients admitted with influenza A pneumonia and 34 patients admitted with COVID-19 pneumonia. In both groups, patients were in acute hypoxemic respiratory failure. In the COVID-19 cohort, 4 patients (12%) had persistently elevated cholestasis and abnormal MRCP (with characteristic findings of irregularities of bile ducts with stricturing). In the influenza A group, 2 patients (6%) had severe cholestasis during admission that resolved spontaneously on follow-up. This study showed that COVID-19 is more likely to cause bile duct injury than influenza A, another virus commonly known to cause acute respiratory distress syndrome.
Linneweber and colleagues described 2 patients in Germany who developed biliary injury during early rehabilitation.25 The patients were a 64-year-old man and a 72-year-old man, both with lengthy courses in the ICU. ERCP performed on the 64-year-old patient showed stricture formation consistent with SSC, and the patient underwent a total of 4 ERCPs for biliary ductal dilation and stent placement. The 72-year-old patient developed elevated liver tests, with peak γ-glutamyltransferase of 2848 U/L. MRCP showed findings consistent with SSC, and the patient underwent ERCP with dilation and metal stent placement. On repeat ERCP, a cholangiogram showed almost complete destruction of intrahepatic bile ducts, and no further intervention was completed. The patient died 8 months after his initial diagnosis.
Rojas and colleagues described a 29-year-old woman who showed evidence of COVID-19 cholangiopathy.26 The patient had a difficult ICU course, requiring mechanical ventilation and eventually tracheostomy. On month 3 of her hospitalization, the patient developed jaundice, elevated transaminases, and markedly elevated bilirubin and ALP, with a total bilirubin greater than 15 mg/dL and ALP greater than 6000 U/L. Although stricturing and other features typical of COVID-19 cholangiopathy were not seen on imaging or ERCP, the patient underwent liver biopsy with findings of periportal inflammatory infiltrate with a severe obstructive cholestatic pattern. This biopsy led the authors to believe that this patient may have COVID-19 cholangiopathy. At the time of publication, the patient still had significant laboratory abnormalities as well as other sequelae of long-haul COVID-19, including memory loss, dizziness, and headaches.
Pathophysiology of COVID-19 Cholangiopathy
Although the etiology of the injury to the biliary system is unclear, there have been several hypotheses of the cause, most commonly that it is similar in pathology to SSC, with bile duct ischemia leading to cholangiocyte necrosis and cast formation.27 The intrahepatic biliary epithelium is more susceptible to ischemia, as it has a single blood supply via the hepatic arteries (unlike the common bile duct and hepatocytes, which have a dual blood supply from the portal vein and hepatic artery).28,29 Further, in an autopsy series by Chai and colleagues, patients with COVID-19 showed mild hepatic steatosis with little inflammation and numerous platelet-fibrin microthrombi in hepatic sinusoids.29 Another autopsy series, by Lagana and colleagues, showed sinusoidal microthrombi in 15% of 40 patients.30 In several of the studies discussed herein, there was no significant thrombotic injury observed upon liver biopsy or in autopsy specimens.15,16,21 Bütikofer and colleagues described transmural necrosis of perihilar bile ducts but noted no thrombi in small peribiliary vessels, whereas Faruqui and colleagues identified widespread platelets or exosomes in small sinusoidal aggregates but no intravascular thrombi. However, the absence of intravascular thrombi does not preclude significant vascular injury and thrombosis elsewhere in the liver as sinusoidal microthrombi.30
Another hypothesis is that biliary tract injury is thought to be mediated by inflammation. Both SSC related to critical illness and COVID-19 cholangiopathy include cytokine release syndrome,27,31 which is known to release proinflammatory cytokines and inflammatory mediators that may contribute to cholestatic liver injury and fibrosis.31 Ponnuraj and Hayward found that, in animal models, TNF-α–Tnfsrf1 signaling was implicated in the development of sclerosing cholangitis in a chronic infection with C parvum.32
Other hypotheses include direct virus-mediated damage to the biliary epithelium and drug-induced liver injury. SARS-CoV-2 enters the host through the angiotensin-converting enzyme 2 (ACE2) receptor in the respiratory epithelium, which is also expressed in cholangiocytes. Research has found that ACE2 is expressed more in cholangiocyte clusters (59.7%) than in hepatocytes (2.6%).29 The ACE2 expression in cholangiocytes has been thought to be comparable to ACE2 expression in lung alveolar type II cells. Drug-induced liver injury, particularly with combined amoxicillin and clavulanate, can cause cholestatic injury.33 Patients with COVID-19 receive a variety of both novel and known medications, with remdesivir being implicated in liver injury, but predominantly in the form of alanine aminotransferase elevations.34
Additionally, ketamine-associated cholangiopathy has been reported in the literature. One case series examined ketamine-associated cholangiopathy in 5 patients with severe COVID-19, and Knooihuizen and colleagues described a case in a 54-year-old woman with severe COVID-19.35,36 Ketamine, a nonbarbiturate general anesthetic drug, is a second-line medication for maintenance sedation of patients with acute respiratory distress syndrome and is metabolized by the liver. Research has shown that norketamine is present in human bile after ketamine poisoning,37 and Knooihuizen and colleagues’ series found that these patients, while under ketamine, developed cholestatic liver injury and MRCP findings of sclerosing cholangitis, similar to patients with COVID19 cholangiopathy. However, ketamine exposure has not been noted in other reports of this syndrome. Although patients with COVID-19 cholangiopathy studied in case series and reports were not known to be administered any medications known to cause cholestatic liver injury, there are large numbers of medications that were studied in COVID-19, particularly early in the epidemic.
Conclusion
Several studies have shown that patients who are critically ill from COVID-19 may develop severe cholestatic injury, now known as COVID-19 cholangiopathy. This has been found to be similar to SSC after critical illness, although the mechanism of biliary tract injury remains uncertain. Given its severe prognostic implications, with persistent cholangiopathy, cases of progressive liver failure, and even the need for liver transplant having been reported, COVID-19 cholangiopathy requires further study, including trends in incidence in the current era of vaccination and refinements in treatment.
References
- 1.Guan WJ, Ni ZY, Hu Y et al. China Medical Treatment Expert Group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Q, Wang B, Zhang T, Xu J, Shang S. The mechanism and treatment of gastrointestinal symptoms in patients with COVID-19. Am J Physiol Gastrointest Liver Physiol. 2020;319(2):G245–G252. doi: 10.1152/ajpgi.00148.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan L, Mu M, Yang P et al. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am J Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Budden KF, Gellatly SL, Wood DLA et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol. 2017;15(1):55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]
- 5.Margulis SJ, Honig CL, Soave R, Govoni AF, Mouradian JA, Jacobson IM. Biliary tract obstruction in the acquired immunodeficiency syndrome. Ann Intern Med. 1986;105(2):207–210. doi: 10.7326/0003-4819-105-2-207. [DOI] [PubMed] [Google Scholar]
- 6.Naseer M, Dailey FE, Juboori AA, Samiullah S, Tahan V. Epidemiology, determinants, and management of AIDS cholangiopathy: a review. World J Gastroenterol. 2018;24(7):767–774. doi: 10.3748/wjg.v24.i7.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangeya N, Mafukidze AT, Pascoe M et al. Cholangiocarcinoma presenting in an adolescent with vertically acquired HIV infection. Int J STD AIDS. 2008;19(10):717–718. doi: 10.1258/ijsa.2008.008078. [DOI] [PubMed] [Google Scholar]
- 8.Charlier C, Lecuit M, Furco A et al. Cholangiocarcinoma in HIV-infected patients with a history of cholangitis. J Acquir Immune Defic Syndr. 2005;39(2):253–255. [PubMed] [Google Scholar]
- 9.Datta J, Shafi BM, Drebin JA. Extrahepatic cholangiocarcinoma developing in the setting of AIDS cholangiopathy. Am Surg. 2013;79(3):321–322. [PubMed] [Google Scholar]
- 10.Puri P, Kumar S. Liver involvement in human immunodeficiency virus infection. Indian J Gastroenterol. 2016;35(4):260–273. doi: 10.1007/s12664-016-0666-8. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox CM, Mönkemüller KE. Hepatobiliary diseases in patients with AIDS: focus on AIDS cholangiopathy and gallbladder disease. Dig Dis. 1998;16(4):205–213. doi: 10.1159/000016868. [DOI] [PubMed] [Google Scholar]
- 12.Faruqui S, Okoli F, Olsen S Bile duct injury and severe cholestasis in patients recovering from severe COVID-19: a novel entity of COVID-associated cholangiopathy [abstract 24542]. 2020. Presented at the Liver Meeting Digital Experience; November 13-16.
- 13.Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS-CoV-2 infection. BMJ Case Rep. 2020;13(11):e237984. doi: 10.1136/bcr-2020-237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roth NC, Kim A, Vitkovski T et al. Post-COVID-19 cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116(5):1077–1082. doi: 10.14309/ajg.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 15.Faruqui S, Okoli FC, Olsen SK et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol. 2021;116(7):1414–1425. doi: 10.14309/ajg.0000000000001264. [DOI] [PubMed] [Google Scholar]
- 16.Durazo FA, Nicholas AA, Mahaffey JJ et al. Post-Covid-19 cholangiopathy— a new indication for liver transplantation: a case report. Transplant Proc. 2021;53(4):1132–1137. doi: 10.1016/j.transproceed.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee A, Wein AN, Doyle MBM, Chapman WC. Liver transplantation for post-COVID-19 sclerosing cholangitis. BMJ Case Rep. 2021;14(8):e244168. doi: 10.1136/bcr-2021-244168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blondeel J, Meersseman P, Gilbo N et al. Liver transplantation for COVID-19-associated cholangiopathy [abstract LB 24]. Am J Transplant. 2021;21(suppl 3) [Google Scholar]
- 19.Tafreshi S, Whiteside I, Levine I, D’Agostino C. A case of secondary sclerosing cholangitis due to COVID-19. Clin Imaging. 2021;80:239–242. doi: 10.1016/j.clinimag.2021.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klindt C, Jensen BE, Brandenburger T et al. Secondary sclerosing cholangitis as a complication of severe COVID-19: a case report and review of the literature. Clin Case Rep. 2021;9(5):e04068. doi: 10.1002/ccr3.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bütikofer S, Lenggenhager D, Wendel Garcia PD et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID-19. Liver Int. 2021;41(10):2404–2417. doi: 10.1111/liv.14971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheppach W, Druge G, Wittenberg G et al. Sclerosing cholangitis and liver cirrhosis after extrabiliary infections: report on three cases. Crit Care Med. 2001;29(2):438–441. doi: 10.1097/00003246-200102000-00042. [DOI] [PubMed] [Google Scholar]
- 23.Gelbmann CM, Rümmele P, Wimmer M et al. Ischemic-like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102(6):1221–1229. doi: 10.1111/j.1572-0241.2007.01118.x. [DOI] [PubMed] [Google Scholar]
- 24.Laurent L, Lemaitre C, Minello A et al. Cholangiopathy in critically ill patients surviving beyond the intensive care period: a multicentre survey in liver units. Aliment Pharmacol Ther. 2017;46(11-12):1070–1076. doi: 10.1111/apt.14367. [DOI] [PubMed] [Google Scholar]
- 25.Linneweber L, Mann AB, Denk G, Kraft E, Weber S. Cholangiopathy in early rehabilitation after intensive care treatment of patients with COVID-19. Am J Gastroenterol. 2022;117(1):197–198. doi: 10.14309/ajg.0000000000001511. [DOI] [PubMed] [Google Scholar]
- 26.Rojas M, Rodríguez Y, Zapata E, Hernández JC, Anaya J-M. Cholangiopathy as part of post-COVID syndrome. J Transl Autoimmun. 2021;4:100116. doi: 10.1016/j.jtauto.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonhardt S, Veltzke-Schlieker W, Adler A et al. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care. 2015;19(1):131. doi: 10.1186/s13054-015-0861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonhardt S, Veltzke-Schlieker W, Adler A et al. Secondary sclerosing cholangitis in critically ill patients: clinical presentation, cholangiographic features, natural history, and outcome: a series of 16 cases. Medicine (Baltimore). 2015;94(49):e2188. doi: 10.1097/MD.0000000000002188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chai X, Hu L, Zhang Y Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. Preprint posted online February 4, 2020. bioRxiv. doi: https://doi.org/10.1101/2020.02.03.931766.
- 30.Lagana SM, Kudose S, Iuga AC et al. Hepatic pathology in patients dying of COVID-19: a series of 40 cases including clinical, histologic, and virologic data. Mod Pathol. 2020;33(11):2147–2155. doi: 10.1038/s41379-020-00649-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006;44(5):1063–1074. doi: 10.1002/hep.21405. [DOI] [PubMed] [Google Scholar]
- 32.Ponnuraj EM, Hayward AR. Requirement for TNF-Tnfrsf1 signalling for sclerosing cholangitis in mice chronically infected by Cryptosporidium parvum. Clin Exp Immunol. 2002;128(3):416–420. doi: 10.1046/j.1365-2249.2002.01861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sundaram V, Björnsson ES. Drug-induced cholestasis. Hepatol Commun. 2017;1(8):726–735. doi: 10.1002/hep4.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olry A, Meunier L, Délire B, Larrey D, Horsmans Y, Le Louët H. Drug-induced liver injury and COVID-19 infection: the rules remain the same. Drug Saf. 2020;43(7):615–617. doi: 10.1007/s40264-020-00954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Intravenous ketamine and progressive cholangiopathy in COVID-19 patients. J Hepatol. 2021;74(5):1243–1244. doi: 10.1016/j.jhep.2021.02.007. Keta-Cov research group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knooihuizen SAI, Aday A, Lee WM. Ketamine-induced sclerosing cholangitis (KISC) in a critically ill patient with COVID-19. Hepatology. 2021;74(1):519–521. doi: 10.1002/hep.31650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Licata M, Pierini G, Popoli G. A fatal ketamine poisoning. J Forensic Sci. 1994;39(5):1314–1320. [PubMed] [Google Scholar]
- 38.Sanders D, Bomman S, Irani S. COVID-19-induced bile duct casts and cholangitis: a case report. Cureus. 2021;13(4):e14560. doi: 10.7759/cureus.14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weaver M, McHenry S, Das KK. COVID-19 and jaundice. Gastroenterology. 2021;160(7):e1–e3. doi: 10.1053/j.gastro.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meersseman P, Blondeel J, De Vlieger G, van der Merwe S, Monbaliu D. Collaborators Leuven Liver Transplant program. Secondary sclerosing cholangitis: an emerging complication in critically ill COVID-19 patients. Intensive Care Med. 2021;47(9):1037–1040. doi: 10.1007/s00134-021-06445-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


