Abstract
Background:
A trial with trifluridine/tipiracil (FTD/TPI) versus placebo in patients with heavily pretreated metastatic gastric cancer showed that FTD/TPI is effective with manageable toxicity in these patients. However, real-world data on the effects of FTD/TPI in patients with advanced gastric cancer (AGC) are limited.
Methods:
We retrospectively collected and analyzed the clinicopathological data of patients with AGC who received FTD/TPI monotherapy at our institutions (Kobe City Medical Center General Hospital, Osaka Red Cross Hospital, Himeji Red Cross Hospital, and Kansai Medical University Hospital) between September 2019 and July 2021. Tumor responses were evaluated based on the Response Evaluation Criteria in Solid Tumors, version 1.1. Overall survival (OS) and progression-free survival were estimated using the Kaplan-Meier method.
Results:
A total of 53 patients were included in the study. The median age was 70 (range, 37-85) years; 39 patients (74%) were men; the numbers of patients with Eastern Cooperative Oncology Group performance status scores of 0, 1, and 2 were 10 (19%), 39 (74%), and 4 (8%), respectively; and 27 patients (51%) had diffuse-type histology. A total of 29 patients (56%) had ascites. Prior nivolumab therapy was administered to 49 patients (92%). The response rate and disease control rate (DCR) were 2% and 35%, respectively. The median progression-free survival was 2.4 months, and OS was 5.8 months. Patients with ascites exhibited significantly shorter OS (8.6 vs 4.7 months, P = .0291) than those without ascites, and DCR (54% vs 18%, P = .0055) was significantly worse in patients with ascites. There was no significant difference in the frequency of adverse events of grade 3 or higher between patients with and without ascites.
Conclusion:
In a real-world setting, FTD/TPI has similar effectiveness as late-line chemotherapy for patients with AGC, including those who previously had received nivolumab.
Keywords: Gastric cancer, trifluridine, chemotherapy, real-world data
Introduction
Gastric cancer is the third leading cause of cancer-related death and the fifth most common malignancy diagnosed worldwide,1 and systemic chemotherapy is the standard treatment for unresectable metastatic gastric cancer. The results of the nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with human epidermal growth factor receptor 2-negative, untreated, unresectable advanced or recurrent gastric or gastroesophageal junction cancer (ATTRACTION-4) and first-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastroesophageal junction, and esophageal adenocarcinoma (CheckMate 649) trials led to the development of a combination of chemotherapy and nivolumab as the new standard regimen for first-line treatment of advanced gastric cancer (AGC).2,3 In cases where third-line treatment is possible, trifluridine/tipiracil (FTD/TPI)- or irinotecan (CPT-11)-based regimens may be considered. FTD/TPI is an oral therapy comprising the thymidine analogs trifluridine and tipiracil, the latter preventing trifluridine degradation.4 The FTD/TPI versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS) trial, which was a phase 3 study involving patients with AGC treated with two or more regimens, showed that compared with placebo, FTD/TPI resulted in a significant increase in progression-free survival (PFS) and overall survival (OS).5 Currently, FTD/TPI is approved in the United States, Europe, and Japan for patients previously treated for AGC.
However, in actual clinical practice, many patients with AGC exhibit severe conditions, such as ascites and poor general condition, which are rare among those registered in clinical trials. While the TAGS study comprises data on patients with peritoneal dissemination, information on those with ascites or performance status (PS) score 2 is absent. Furthermore, only 6% of patients received nivolumab before FTD/TPI administration. Therefore, we retrospectively evaluated the effectiveness and safety of FTD/TPI therapy in patients with AGC in a real-world setting, including those who had previously received nivolumab.
Methods
Patients
We reviewed the medical records of consecutive patients with AGC who were treated with FTD/TPI between January 2019 and July 2021 at Kobe City Medical Center General Hospital, Kansai Medical University Hospital, Himeji Red Cross Hospital, and Osaka Red Cross Hospital, Japan. The inclusion criteria were as follows: (1) unresectable gastric cancer, (2) histologically proven gastric adenocarcinoma, (3) refractory or intolerant to at least two prior regimens, and (4) at least one evaluable lesion.
Treatment
The patients received oral FTD/TPI 35 mg/m² twice daily on days 1 to 5 and days 8 to 12 of each 28-day treatment cycle until the disease progressed or until the patient developed an intolerance to the treatment.
Evaluation of ascites
We assessed the extent of ascites using computed tomographic scans, based on which patients were categorized into the following groups: massive (extending throughout the abdominal cavity), moderate (neither mild nor massive), mild (localized at the pelvic cavity or liver surface), or no ascites (ascites not detected). Moderate and massive ascites were defined as high ascites burden (HAB), whereas mild and no ascites were categorized as low ascites burden (LAB), based on previous reports.6,7
Response evaluation and statistical analysis
Tumor response was evaluated based on the Response Evaluation Criteria in Solid Tumors, version 1.1. OS was defined as the period from the date of initiation of FTD/TPI treatment to death. Patients who were alive or whose data were missing at the cutoff point were censored. PFS was defined as the duration between the date of initiation of FTD/TPI treatment and disease progression or death from any cause. Patients for whom information regarding tumor progression was missing were censored. OS and PFS were estimated using the Kaplan-Meier method. Cox proportional hazards models were used to identify the risk factors associated with OS. Statistical analyses were performed using the JMP software version 12 (SAS Institute Inc, Cary, NC, USA). Toxicity was assessed using the Common Terminology Criteria for Adverse Events, version 4.1.
Results
Between January 2019 and July 2021, 53 patients received FTD/TPI after failures of at least two prior regimens. The patients’ characteristics are shown in Table 1. The median age was 70 (range, 37-85) years, and most patients were men (74%). The numbers of patients with Eastern Cooperative Oncology Group PS scores of 0, 1, and 2 were 10 (19%), 39 (74%), and 4 (8%), respectively. While 38 patients (72%) had peritoneal dissemination, 29 (56%) had ascites, of whom 14 (26%) were considered to have HAB. Most patients (94%-98%) had received prior regimens containing 5-fluorouracil, platinum drugs, taxanes, and ramucirumab. While 49 patients (92%) had previously received nivolumab, 19 (36%) had undergone treatment with a regimen of CPT-11.
Table 1.
Characteristics of the study participants.
| All (n = 53) | With ascites (n = 29) | Without ascites (n = 24) | P | |
|---|---|---|---|---|
| Sex (male) | 39 (74%) | 20 (69%) | 19 (79%) | .5350 |
| Age (years), median (range) | 70 (37-85) | 67 (37-85) | 71 (52-84) | .0248 |
| PS score | .0577 | |||
| 0 | 10 (19%) | 4 (14%) | 6 (25%) | |
| 1 | 39 (74%) | 21 (72%) | 18 (75%) | |
| 2 | 4 (8%) | 4 (14%) | 0 | |
| Histology (diffuse type) | 27 (51%) | 21 (72%) | 6 (33%) | .0009 |
| HER2 status (positive) | 12 (23%) | 3 (10%) | 9 (38%) | .0245 |
| MSI status (high) | 0 | 0 | 0 | |
| Prior gastrectomy | 23 (43%) | 14 (48%) | 9 (38%) | .5787 |
| Metastases to more than 1 organ | 42 (79%) | 21 (72%) | 21 (88%) | .3078 |
| Liver metastasis | 18 (34%) | 8 (28%) | 10 (42%) | .3841 |
| Peritoneum dissemination | 38 (72%) | 27 (93%) | 11 (46%) | .0002 |
| Ascites | 29 (55%) | 29 (100%) | 0 | - |
| High ascites burden | 14 (26%) | 14 (48%) | 0 | - |
| Number of prior regimens | .8110 | |||
| 2 | 4 (8%) | 3 (10%) | 1 (4%) | |
| 3 | 27 (51%) | 15 (52%) | 12 (50%) | |
| >3 | 22 (42%) | 11 (38%) | 11 (46%) | |
| Prior regimens | ||||
| 5-FU | 52 (98%) | 28 (97%) | 24 (100%) | >.999 |
| Platinum | 50 (94%) | 26 (90%) | 24 (100%) | .2424 |
| Taxane | 51 (96%) | 28 (97%) | 23 (96%) | >.999 |
| CPT-11 | 19 (36%) | 10 (34%) | 9 (38%) | >.999 |
| Ramucirumab | 50 (94%) | 27 (93%) | 23 (96%) | >.999 |
| Nivolumab | 49 (92%) | 26 (90%) | 23 (96%) | .6173 |
Abbreviations: CPT-11, irinotecan; 5-FU, 5-fluorouracil; HER2, human epidermal growth factor receptor 2; MSI, microsatellite instability; PS, performance status.
Effectiveness
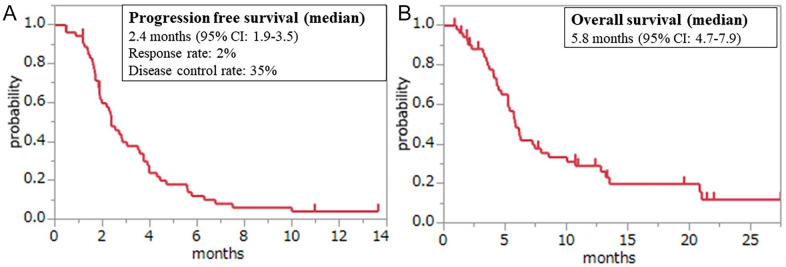
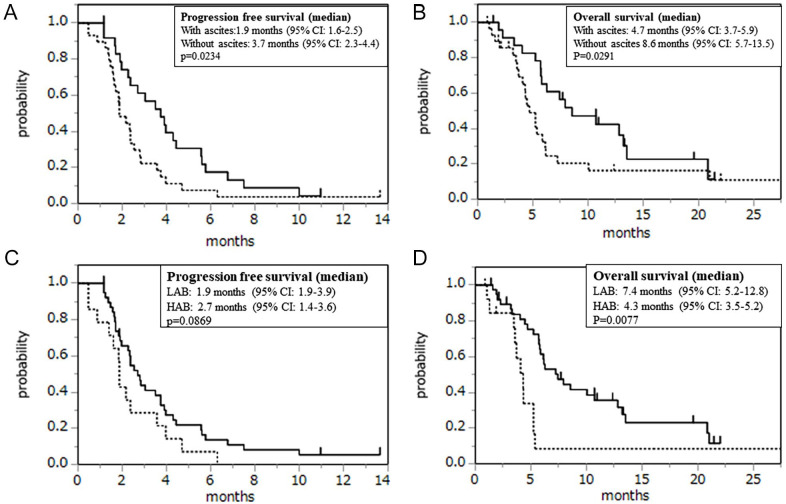
Measurable lesions were found in 52 patients. A partial response was observed in 2% of patients, and 33% showed stable disease, resulting in a response rate (RR) of 2% and a disease control rate (DCR) of 35%. The median follow-up time was 5.4 (range, 0.87-27.5) months for the censored patients. The median PFS was 2.4 months (95% confidence interval [CI], 1.9-3.5), and the median OS was 5.8 months (95% CI, 4.7-7.9; Figure 1). The efficacy of treatment in patients with ascites (RR, 0%; DCR, 18%; median PFS, 1.9 months [95% CI, 1.6-2.5]; and OS, 4.7 months [95% CI, 3.7-6.1]) and that of those without ascites (RR, 4%; DCR, 54%; median PFS, 3.7 months [95% CI, 2.3-4.4]; and OS, 8.6 months [95% CI, 5.7-13.5]) are depicted in Figure 2A and B. There were significant differences in PFS (P = .0092), OS (P = .0149), and DCR (P = .0088) between patients with and without ascites. Furthermore, OS (4.3 vs 7.4 months, P = .0077) was significantly worse in the HAB group than in the LAB group. PFS (1.9 vs 2.7 months, P = .0869; Figure 2C and D) and DCR (21% vs 39%, P = .3286) were poorer in the HAB group than in the LAB group, although the differences were not statistically significant (Table 2). Only one patient each with ascites (5%) and HAB (7%) demonstrated reduced ascites.
Figure 1.
Kaplan-Meier plots of (A) progression-free survival and (B) overall survival among study participants.
Figure 2.
Kaplan-Meier plots of (A) progression-free survival and (B) overall survival among study participants (the dotted line indicates the patient group with ascites; the normal line indicates the patient group without ascites). Kaplan-Meier plots of (C) progression-free survival and (D) overall survival among study participants (the dotted line indicates the patient group with HAB; the normal line indicates the patient group with LAB). HAB indicates high ascites burden; LAB, low ascites burden.
Table 2.
Responses among patients with measurable lesions.
| All (n = 53) | With ascites (n = 28) | Without ascites (n = 24) | HAB (n = 14) | LAB (n = 38) | |
|---|---|---|---|---|---|
| CR | 0 | 0 | 0 | 0 | 0 |
| PR | 1 | 0 | 1 | 0 | 1 |
| SD | 17 | 5 | 12 | 3 | 14 |
| PD | 34 | 23 | 6 | 11 | 23 |
| RR (%) | 2% | 0% | 4% | 0% | 3% |
| OR: NE (95% CI: NE) P = .4615 |
OR: NE (95% CI: NE) P > .999 |
||||
| DCR (%) | 41% | 18% | 50% | 21% | 39% |
| OR: 5.43 (95% CI:
1.55-19.11) P = .0088 |
OR: 2.39 (95% CI:
0.57-10.02) P = .3286 |
||||
Abbreviations: CI, confidence interval; CR, complete response; DCR, disease control rate (CR + PR + SD); HAB, high ascites burden; LAB, low ascites burden; NE, not evaluated; OR, odds ratio; PD, progressive disease; PR, partial response; RR, response rate; SD, stable disease.
We performed univariate and multivariate analyses to investigate prognostic factors. Poor PS and ascites were found to be statistically significant factors for poor prognosis in multivariate analyses (Table 3).
Table 3.
Univariate and multivariate analyses of overall survival.
| Factor | Univariate | Multivariate | |||
|---|---|---|---|---|---|
| RR (95% CI) | P | RR (95% CI) | P | ||
| PS score | 1 or 2 vs 0 | 5.28 (2.01-18.08) | .0003 | 5.70 (2.07-20.40) | .0003 |
| Ascites | Yes vs no | 1.84 (0.97-3.57) | .0621 | 2.21 (1.01-4.89) | .0462 |
| Liver metastasis | Yes vs no | 0.86 (0.44-1.73) | .661 | 0.90 (0.43-1.81) | .7694 |
| Histology | Diffuse vs intestinal type | 1.07 (0.57-2.02) | .8368 | 0.59 (0.27-1.29) | .1862 |
Abbreviations: RR, risk ratio; CI, confidence interval; PS, performance status.
Safety
Adverse events that occurred among the study participants are shown in Table 4. Major adverse events were neutropenia (45%), anemia (25%), infection (11%), decreased platelet count (6%), fatigue (6%), and anorexia (4%). The frequency of adverse events of grade 3 or higher was not significantly different between patients with and without ascites (P > .999). Similarly, no significant difference was observed in this frequency between the HAB and LAB groups (P = .3623).
Table 4.
Frequency of occurrence of adverse events among study participants.
| All (n = 53) | With ascites (n = 29) | Without ascites (n = 24) | ||||
|---|---|---|---|---|---|---|
| All | Grade 3/4 | All | Grade 3/4 | All | Grade 3/4 | |
| Fatigue | 22 (42%) | 3 (6%) | 11 (38%) | 2 (7%) | 11 (53%) | 1 (5%) |
| Anorexia | 29 (55%) | 2 (4%) | 17 (59%) | 2 (7%) | 12 (50%) | |
| Nausea | 13 (25%) | 9 (31%) | 4 (17%) | |||
| Vomiting | 6 (11%) | 4 (14%) | 2 (8%) | |||
| Diarrhea | 7 (13%) | 3 (10%) | 4 (17%) | |||
| Infection | 7 (13%) | 5 (11%) | 3 (10%) | 2 (7%) | 4 (17%) | 3 (13%) |
| Dysgeusia | 1 (2%) | 1 (4%) | ||||
| Mucositis | 5 (9%) | 2 (7%) | 3 (13%) | |||
| Brain infarction | 1 (2%) | 1 (2%) | 1 (3%) | 1 (3%) | ||
| Febrile neutropenia | 1 (2%) | 1 (2%) | 1 (3%) | 1 (3%) | ||
| Hyperkalemia | 1 (2%) | 1 (2%) | 1 (3%) | 1 (3%) | ||
| Neutropenia | 28 (53%) | 24 (45%) | 10 (36%) | 8 (28%) | 18 (75%) | 16 (67%) |
| Anemia | 31 (58%) | 13 (25%) | 17 (64%) | 6 (24%) | 14 (58%) | 7 (29%) |
| Decreased platelet count | 9 (17%) | 3 (6%) | 4 (14%) | 1 (3%) | 5 (21%) | 2 (8%) |
Dose modification was warranted in 66% of patients (dose reduction, 32%; dose delay, 60%). There was no significant difference in the frequency of dose reduction between patients with and without ascites (31% vs 33%, P > .999) or between the HAB and LAB groups (29% vs 33%, P > .999).
Treatment was terminated because of progressive disease in 52 patients, of whom 16 (31%) received subsequent therapy. Of these, 7 patients (13%) received a CPT-11-based regimen, 3 (6%) nivolumab, 3 (6%) trastuzumab deruxtecan, 3 (6%) a paclitaxel-based regimen, 2 (4%) capecitabine plus trastuzumab, 1 (2%) capecitabine plus oxaliplatin plus trastuzumab, and 1 (2%) S-1 plus oxaliplatin. There was no significant difference in the treatment transition rate between patients with and without ascites (24% vs 39%, P = .3649), although it was significantly lower in the HAB group than in the LAB group (7% vs 39%, P = .0399).
Discussion
In this retrospective study, we used real-world data of patients with AGC treated with FTD/TPI. We observed levels of efficacy and safety that were similar to those demonstrated in the TAGS trial, namely an RR of 4%, PFS of 2 months, and OS of 5.7 months among patients undergoing third-line or later chemotherapy. However, there were some differences between the results of our study and those of the TAGS trial. In the TAGS trial, 63% of the patients received three or more prior regimens, and 34% received ramucirumab. In our study, 92% of the patients received three or more prior regimens, and 94% received ramucirumab. In addition, even though 92% of patients previously received nivolumab, 31% of patients received subsequent systemic therapy after FTD/TPI failure. In the TAGS trial, 25% of patients received subsequent systemic therapy. Our study included a higher number of older patients, patients with poorer PS, and patients who underwent more pretreatments; all these factors reflect the situation in clinical practice. Our study findings suggest that FTD/TPI is an important regimen for the treatment of patients with AGC who have received ramucirumab and nivolumab.
Immune checkpoint inhibitors (ICIs), such as nivolumab, are among the most important drugs used to treat AGC. The ATTRACTION-2 trial showed a significant clinical benefit in patients with AGC who had previously received two or more prior chemotherapy regimens.8 In addition, nivolumab plus chemotherapy significantly improved OS compared with chemotherapy alone in the CheckMate 649 trial and has become the standard first-line treatment.2 Although FTD/TPI in third-line or later chemotherapy becomes more important when nivolumab is used as the first-line treatment, the efficacy and safety of FTD/TPI after administration of ICIs were unclear. In the TAGS trial, only 7% of the patients received ICIs before administration of FTD/TPI, whereas in our study, 92% of the patients received ICIs before FTD/TPI administration. The efficacy and safety exhibited in our study were similar to those in the TAGS trial, which suggests that FTD/TPI is one of the key drugs for the treatment of AGC after the administration of ICIs.
A previous study reported that prior ICI administration enhances the effectiveness of cytotoxic drugs in non-small cell lung cancer.9 Few studies reported the efficacy of cytotoxic chemotherapy in patients with AGC who previously had received ICIs. In Japan, a large prospective observational study (the REVIVE study, clinical trial registration UMIN000032182 [umin.ac.jp]) has been conducted.10 This study includes patients with FTD/TPI administration and is awaiting publication.
In our study, 60% of the patients experienced adverse events of grade 3 or 4. This finding was similar compared with that of the TAGS trial (56%). There was no significant difference in the frequency of such adverse events between patients with ascites and those without ascites or between the LAB and HAB groups. Dose reduction and dose delay were required in 32% and 60% of patients, respectively, which were higher frequencies than those in the TAGS trial (11% and 47%, respectively). Our study showed almost the same safety profile as the TAGS trial, but the dose modification tended to be higher. This result suggests that FTD/TPI is tolerable in clinical practice; however, attention should be paid to dose modification.
Considering the results of a phase 3 trial of FTD/TPI in patients with colorectal cancer (CRC),11,12 dose modification of FTD/TPI was observed more frequently in patients with AGC than in patients with CRC. In addition, compared with a Japanese multicenter observational study of patients with CRC, our study showed that severe adverse events of FTD/TPI tended to be more frequent in patients with AGC.13 Thus, compared with patients with CRC, those with AGC treated using FTD/TPI require careful management. It has been suggested that biweekly administration of FTD/TPI may reduce toxicity in patients with CRC compared with normal dosages of FTD/TPI14; a similar investigation related to AGC is awaited.
Peritoneal dissemination is common in patients with AGC, approximately 40% of whom exhibit ascites as a clinical symptom.15,16 To the best of our knowledge, this study is the first to investigate the effectiveness of FTD/TPI in AGC patients with ascites. We found significant differences in OS and DCR between patients with and without ascites. In addition, OS was significantly shorter in patients with HAB. There was no significant difference in the frequency of adverse events of grade 3 or higher between patients with and without ascites. Therefore, FTD/TPI may be well tolerated, although its effect is inadequate in patients with AGC and ascites.
Kawazoe et al17 reported that in patients with AGC previously treated with second- to fourth-line chemotherapy, FTD/TPI plus ramucirumab showed promising efficacy (RR, 16%; DCR, 77%; PFS, 5.3 months). Increased levels of vascular endothelial growth factor receptor 2 ligand in patients with ascites may be associated with poor prognosis, and FTD/TPI plus ramucirumab may be effective in treating AGC patients with ascites.18 However, data related to the efficacy and safety of this treatment are not available. Kawazoe et al also reported that the prior use of ICIs enhanced the efficacy of FTD/TPI plus ramucirumab. There are no data on the correlation between the effectiveness of FTD/TPI and prior use of ICIs. In our study, 92% of patients received prior nivolumab, and it was difficult to determine whether prior nivolumab use affected the efficacy of FTD/TPI treatment.
To the best of our knowledge, this is the first study to evaluate the effectiveness and safety of FTD/TPI therapy in patients with AGC in a real-world setting. However, this study has certain limitations. This was a retrospective analysis with small sample size. Therefore, a prospective multicenter study involving more patients with AGC should be conducted to clarify the effectiveness and safety of FTD/TPI in this population.
Conclusions
Our results indicate that FTD/TPI has modest effectiveness and tolerable toxicity in patients with AGC in a real-world setting. Although 92% of patients had received nivolumab as a prior treatment, our study results suggest that FTD/TPI is as effective and tolerable as in the TAGS study. However, considering that the effectiveness of this treatment is inadequate, further research focusing on improving its efficacy, such as combination therapy, is needed.
Acknowledgments
The authors thank the study participants and their families for their cooperation.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Toshihiko Matsumoro received research funding from Ono Pharmaceutical Co, Ltd and Sanofi Co, Ltd; honoraria from Bayer Co, Ltd, Bristol-Myers Squibb Co, Ltd, Chugai Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Eli Lilly Japan Co, Ltd, Merck Bio Pharma Co, Ltd, MSD Co, Ltd, Ono Pharmaceutical Co, Ltd, Sanofi Co, Ltd, Taiho Pharmaceutical Co, Ltd, Takeda Co, Ltd, Teijin Pharmaceutical Co, Ltd and Yakult Honsha Co, Ltd. Hironaga Satake received research funding from Ono Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Taiho Pharmaceutical Co, Ltd, Takeda Pharmaceutical Co, Ltd, and Sanofi Co, Ltd; honoraria from Bayer Co, Ltd, Bristol-Myers Squibb Co, Ltd, Chugai Pharmaceutical Co, Ltd, Daiichi Sankyo Co, Ltd, Eli Lilly Japan Co, Ltd, Merck Bio Pharma Co, Ltd, MSD Co, Ltd, Ono Pharmaceutical Co, Ltd, Sanofi Co, Ltd, Taiho Pharmaceutical Co, Ltd, Takeda Co, Ltd, and Yakult Honsha Co, Ltd. Hisateru Yasui received honoralia from Taiho Pharmaceutical Co, Ltd. All the remaining authors declare that they have no competing interests.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Author Contributions: TM, SY, YK, TY, SB, NS, HN, TK, TT, YM, MT, HY, and HS participated in the literature search, data acquisition, data analysis, and data interpretation. TM conceived and designed the study, critically revised the manuscript, performed the research, wrote the first draft, and collected and analyzed the data. TM, SY, YK, TY, SB, HN, TK, TT, YM, MT, HY, and HS wrote and revised the manuscript. All authors have read and approved the manuscript.
Ethics Approval and Consent to Participate: All procedures were performed in accordance with institutional and national standards on human experimentation, as confirmed by the ethics committee of all institutions, and with the Helsinki Declaration of 1964 and its later amendments. This study was approved by the institutional review boards of Kobe City Medical Center General Hospital (examination number: zn211018), Himeji Red Cross Hospital, Kansai Medical University Hospital, and Osaka Red Cross Hospital. The requirement for informed consent was waived because this was an observational study. However, we followed the opt-out consent approach, which was approved by the ethics committees of Kobe City Medical Center General Hospital and Himeji Red Cross Hospital. All administrative permissions to access the data used in this study were acquired.
Consent for Publication: Not applicable.
Availability of Data and Materials: All data and materials supporting the conclusions are included in the main article. The data sets used in this study are available from the corresponding author upon reasonable request.
ORCID iDs: Toshihiko Matsumoto  https://orcid.org/0000-0002-7397-1006
https://orcid.org/0000-0002-7397-1006
Nobuhiro Shibata  https://orcid.org/0000-0001-8647-5098
https://orcid.org/0000-0001-8647-5098
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [DOI] [PubMed] [Google Scholar]
- 2. Janjigian YY, Shitara K, Moehler M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet. 2021;398:27-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kang YK, Chen LT, Ryu MH, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastrooesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2022;23:234-247. [DOI] [PubMed] [Google Scholar]
- 4. Emura T, Murakami Y, Nakagawa F, Fukushima M, Kitazato K. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. Int J Mol Med. 2004;13:545-549. [PubMed] [Google Scholar]
- 5. Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:1437-1448. [DOI] [PubMed] [Google Scholar]
- 6. Nakajima TE, Yamaguchi K, Boku N, et al. Randomized phase II/III study of 5-fluorouracil/l-leucovorin versus 5-fluorouracil/l-leucovorin plus paclitaxel administered to patients with severe peritoneal metastases of gastric cancer (JCOG1108/WJOG7312G). Gastric Cancer. 2020;23:677-688. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki H, Yamada T, Sugaya A, et al. Retrospective analysis for the efficacy and safety of nivolumab in advanced gastric cancer patients according to ascites burden. Int J Clin Oncol. 2021;26:370-377. [DOI] [PubMed] [Google Scholar]
- 8. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390: 2461-2471. [DOI] [PubMed] [Google Scholar]
- 9. Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90-95. [DOI] [PubMed] [Google Scholar]
- 10. Narita Y, Shoji H, Kawai S, et al. REVIVE study: a prospective observational study in chemotherapy after nivolumab therapy for advanced gastric cancer. Future Oncol. 2021;17:869-875. [DOI] [PubMed] [Google Scholar]
- 11. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372:1909-1919. [DOI] [PubMed] [Google Scholar]
- 12. Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol. 2018;36:350-358. [DOI] [PubMed] [Google Scholar]
- 13. Moriwaki T, Fukuoka S, Taniguchi H, et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese society for cancer of the colon and rectum multicenter observational study. Oncologist. 2018;23:7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Satake H, Kato T, Oba K, et al. Phase Ib/II study of biweekly TAS-102 in combination with bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (BiTS study). Oncologist. 2020;25:e1855-e1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitayama J, Ishigami H, Kaisaki S, et al. Weekly intravenous and intraperitoneal paclitaxel combined with S-1 for malignant ascites due to advanced gastric cancer. Oncology. 2010;78:40-46. [DOI] [PubMed] [Google Scholar]
- 16. Tahara M, Ohtsu A, Boku N, et al. Sequential methotrexate and 5-fluorouracil therapy for gastric cancer patients with peritoneal dissemination: a retrospective study. Gastric Cancer. 2001;4:212-218. [DOI] [PubMed] [Google Scholar]
- 17. Kawazoe A, Ando T, Hosaka H, et al. Safety and activity of trifluridine/tipiracil and ramucirumab in previously treated advanced gastric cancer: an open-label, single-arm, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6:209-217. [DOI] [PubMed] [Google Scholar]
- 18. Fushida S, Oyama K, Kinoshita J, et al. VEGF is a target molecule for peritoneal metastasis and malignant ascites in gastric cancer: prognostic significance of VEGF in ascites and efficacy of anti-VEGF monoclonal antibody. Onco Targets Ther. 2013;6:1445-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]