Abstract
Patients with lymphoproliferative diseases are at an increased risk of an incomplete immune response following vaccination or SARS‐CoV‐2 infection and might develop persistent viral infection and severe COVID‐19 disease. We present a case of successful treatment of persistent and mechanical‐ventilation‐requiring SARS‐CoV‐2 infection in a del17+ CLL patient using exogenous antibodies.
Keywords: Casirivimab and Imdevimab, COVID‐19, haemato‐oncological patients, immunity
Short abstract
Patients with chronic lymphocytic leukemia (CLL) might have an incomplete immune response following vaccination or SARS‐CoV‐2 infection. There is therapeutic potential of monoclonal antibodies in persistent and severe COVID‐19, mirrored in a CLL patient.
1. INTRODUCTION
Chronic lymphocytic leukemia (CLL) is a lymphoproliferative neoplasm characterized by the expansion and accumulation of CD5+ clonal B‐cell population. Dysregulation of innate and adaptive immune responses leading to immunosuppression is a main feature of this disease and infections are a major cause of morbidity and mortality. 1 , 2 Furthermore, patients with del17+ (TP53 mutation) and unmutated immunoglobulin heavy‐chain variable region gene (IGHV) show worse prognosis. 3 Chemoimmunotherapy agents can further impair the immune response to common pathogens, rendering patients more susceptible to infections. 1 , 2
Patients with hematological malignancies showed an increased susceptibility to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection and a higher risk of hospitalization and death. 4 , 5 A recent study showed that 77% of CLL patients diagnosed with COVID‐19 were hospitalized, 28% of which died from COVID‐19 and its complications. 6 CLL patients are also at an increased risk of an incomplete immune response following vaccination. About 60% of patients are unable to develop SARS‐CoV‐2 antibodies after two doses of mRNA vaccination. This figure purportedly higher in those who have already received directed CLL‐therapy, thus preventing these patients to effectively eradicate the virus. 7 , 8
Ronapreve™ comprises two monoclonal antibodies, Casirivimab and Imdevimab, approved for the treatment of early SARS‐CoV‐2 infection or post‐exposure prophylaxis in patients at high risk of progression to severe COVID‐19. 9 , 10 These recombinant human monoclonal antibodies are directed to nonrelated epitopes of the receptor‐binding domain of the spike protein of the virus. Its attachment prevents the SARS‐CoV‐2 virus from entering human cells through the angiotensin converting enzyme 2 (ACE2) receptors. 11 Due to the role of these antibodies in preventing viral infection of human cells, they are recommended for administration in early stages of SARS‐CoV‐2 infection and preferably before oxygen supplementation is implemented. 9 , 10 Ronapreve™ has been shown to reduce SARS‐CoV‐2 viral load and the risk of COVID‐19‐related hospitalization or death from any cause, 11 however, no cases of CLL patients under Ronapreve™ treatment have been described.
We present a case of persistent SARS‐CoV‐2 infection in a del17+ CLL patient, successfully treated with Ronapreve™ in later disease stages.
2. CASE HISTORY
The patient is a 69‐year‐old male with a history of asthma, pulmonary aspergillosis, tuberculosis, and insulin‐dependent type 2 diabetes. He was also diagnosed with CLL with 17p deletion in 2010 and was previously submitted to multiple therapeutic lines: 6 cycles of Rituximab, Fludarabine, and Cyclophosphamide (Rituximab was only administered in the first cycle due to intolerance) as first‐line therapy; second line with methylprednisolone for 6 months; third‐line with Alemtuzumab for 12 weeks, and fourth‐line with Ibrutinib, having achieved partial response (although transfusion dependent). The patient has been in complete remission under a fifth therapeutic line with Venetoclax for the past four years, administered as a 400 mg daily dose. While previously vaccinated with two doses of the mRNA SARS‐CoV‐2 vaccine, the patient failed to produce antibodies and antispike (AS) and antinucleocapsid (AN) were negative at admission.
The patient presented with mild respiratory symptoms on September 10, 2021 (D0) and had a positive antigen test for SARS‐CoV‐2 on D2. On D4, he was admitted to the hospital with complaints of dyspnea and had a PaO2/FiO2 (P/F) ratio of 322.
A SARS‐CoV‐2 polymerase chain reaction (PCR) test confirmed the infection, with an initial cycle threshold (Ct) of 18.2. Antibody tests showed a nonseroconversion pattern with both AS and AN antibodies negative. The COVID‐19 disease severity markers were leukopenia (3100 cells/μl) and elevated serum C‐reactive protein level (15.72 mg/dl), serum ferritin (4167 ng/ml), and D‐dimer (2233 ng/ml).
He started oxygen supplementation on admission to hospital, which was increased according to need. On D6 an angio‐computerized tomography (angio‐CT) was performed revealing SARS‐CoV‐2 pneumonia, with ground glass opacity and reticulation affecting over 50% of both lungs (Figure 1). He was admitted to our intensive care unit (ICU) on that day.
FIGURE 1.
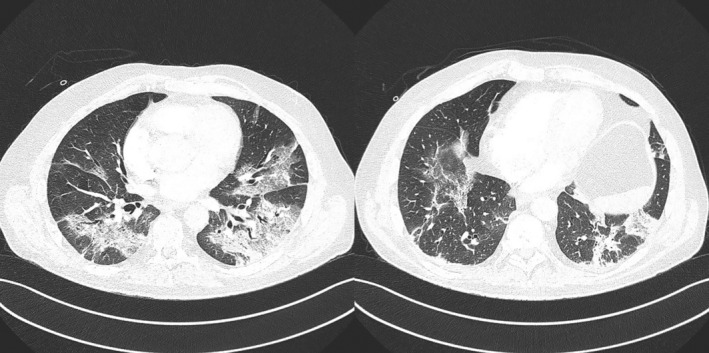
CT scans showing SARS‐CoV‐2 pneumonia, with ground glass opacity and reticulation. This reticulation was extended to every lobe and affected >50% of both lungs.
To address the COVID‐19 pneumonia, he was initially treated with remdesivir for 10 days (100 mg daily) and methylprednisolone 1 mg/kg/day. Considering the patient's CLL and immunosuppression, antimicrobial prophylaxis with atovaquone, acyclovir, and voriconazole were prescribed. The patient was monitored for cytomegalovirus (CMV) reactivation and serum galactomannan levels. None of those infections were identified.
After discussion with the patient's hematologist, Venetoclax was discontinued due to the severity of COVID‐19 pneumonia and a lymphopenia of 620 cells/μl. Hypogammaglobulinemia motivated a total of three administrations of polyclonal intravenous immunoglobulin during his ICU stay.
Due to past history and unfavorable prognosis, alternative therapies were discussed. Convalescent plasma was considered but, given the uncertainty associated with the high antibody titre and the risk of subsequent resistant infection, the use of Ronapreve™ was favored. 12 At that time, Ronapreve™ had not yet been approved for hospitalized patients, therefore approval was sought from both the Hospital's pharmacy and the European Medical Agency (EMA).
The patient developed a slow disease progression and was under noninvasive ventilation and intermittent high flow oxygen for 36 days (D7‐D43).
A follow‐up CT‐scan on D17 showed worsening of the pulmonary infiltrates, patchy consolidations, and subpleural sparing, suggesting progression to organizing pneumonia (Figure 2). By that time, antifibrotic therapy with pirfenidone was started with a gradual increment to a full dose of 2.4 g/day.
FIGURE 2.
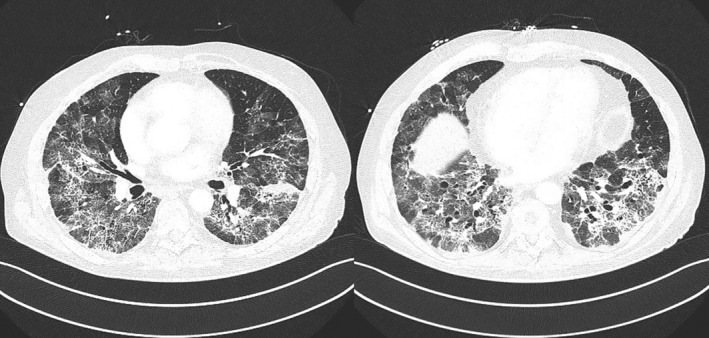
CT scans showing worsening of the pulmonary infiltrates, patchy consolidations and subpleural sparing, suggesting progression to organizing pneumonia.
Due to respiratory distress and deterioration of P/F ratio, mechanical ventilation was initiated on D43.
Ronapreve™ was approved and delivered to the hospital within 3 days of mechanical ventilation (D46). Despite the indication that Ronapreve™ antibodies should be administered to patients not on mechanical‐ventilation support, preferably in an out‐patient scenario, we considered that the patient's endogenous immune response had not been initiated since his serologic antibodies remained negative and the SARS‐CoV‐2 PCR tests remained positive, with a Ct of 24.3. Therefore, Ronapreve™ was administered in a single dose of 8000 mg, diluted in 250 ml of sodium chloride 0.9%. The intravenous infusion administered in an hour period was not associated with adverse effects.
During his long stay at the ICU (a total of 70 days), he developed infectious complications and received antibiotic treatment, including a 4‐day course of Piperacillin‐tazobactam directed to a complicated urinary infection caused by Proteus mirabilis, escalated to Meropenem considering clinical deterioration; a 21‐day course of Trimethoprim‐sulfamethoxazole due to ventilator‐associated pneumonia to Stenotrophomonas maltophilia, and, finally, 15 days of Ceftazidime‐Avibactam plus 9 days of Amikacin following Bacteremia with carbapenem‐resistant Klebsiella pneumoniae.
3. OUTCOME AND FOLLOW‐UP
Following treatment with Ronapreve™, and termination of Venetoclax, the patient had a favorable clinical outcome and ventilatory support was progressively reduced. Tracheostomy was performed on D57, 14 days after mechanical ventilation, to avoid complications of prolonged endotracheal intubation.
The patient showed clinical improvement and was successfully weaned off the ventilator at D65. Methylprednisolone was maintained on the initial dose during 27 days, followed by slow dose taper, until substitution for oral prednisolone.
After Ronapreve™ administration, SARS‐CoV‐2 PCR tests were performed weekly, with progressive increase of Ct values, reaching values above 36 after 3 weeks. By that time, AS antibodies were also present (174.6 AU/ml), while AN were negative, indicating that the patient did not develop natural immunity to the virus but acquired exogenous antibodies. To assess the efficacy of these Ronapreve™ induced antibodies, a neutralization assay was performed, which confirmed patient immunity. Patient was discharged from the ICU 70 days after admission, to continue rehabilitation on an Internal Medicine ward.
The patient was soon decannulated and oxygen supplementation was ceased. After three months of respiratory and physical therapy in a rehabilitation center, he is currently at home. In March 2022, his hemogram showed an increase of lymphocytes, and the immunophenotypic analysis was compatible with CLL progression, motivating Venetoclax reinstitution.
4. DISCUSSION
Due to disease‐inherent and treatment‐induced reduced immune response, characterized by hypogammaglobulinemia, B‐ and T‐cells defects, decreased levels of CD4+ T‐cells, neutropenia and innate immunity malfunction, CLL patients are at a higher risk of developing severe forms of COVID‐19 disease. They become unable to seroconvert after complete mRNA vaccination or to effectively eradicate SARS‐CoV‐2 after infection. 7 , 8 , 13 Clinical evidence on SARS‐CoV‐2 immune responses following mRNA vaccination in hemato‐oncological patients, and the impact of the available therapeutic agents on the immune response to vaccinations, is extremely scarce. However, even among patients with hematological malignancies, CLL patients appear to be at increased risk of poor outcomes after SARS‐CoV‐2 infection. 13
Efforts have been made to clarify the impact of CLL‐directed therapy during COVID‐19 disease and to decide whether or not it should be discontinued. A general recommendation was made that CLL patients with controlled disease under antileukemic treatments should not discontinue therapy, unless it produces significant immunosuppression. 14 Recent publications also suggest that Bruton tyrosine kinase inhibitors (BTKi) should be continued, not only due to its ability to control CLL progression but also to decrease the hyperinflammatory status. As for Venetoclax, no standard recommendation is available but patients under its treatment had increased rates of SARS‐CoV‐2 infection and consequent hospitalization. 15
One of the aspects that increases the risk of SARS‐CoV‐2 infection in these patients is that the levels of directed antibodies induced by vaccines or SARS‐CoV‐2 infection itself, when present, have a slower increase, lower peak and more transient profile compared to healthy individuals. 13 Those physiology aspects promote a more aggressive infection and a higher risk of recurrence of COVID‐19 in these patients. Regarding that, therapies that help neutralize the virus load, diminishing cell infection and viral proliferation, could reduce both disease installation and progression.
One of the available therapeutic options is the convalescent plasma, rich in neutralizing antibodies from patients recently recovered from the SARS‐CoV‐2 infection. Initially related to shorter hospital stay and lower mortality, 16 it was later associated with limitations, including the number of available donors, low titer of neutralizing antibodies, and infusion‐related reactions or infections. 12 Ronapreve™ is an alternative therapeutic approach that might overcome those limitations and has proven to be safe and effective.
We described a case of a 69‐year‐old male patient with CLL, treated with Venetoclax and vaccinated with 2 doses of the mRNA SARS‐CoV‐2 vaccine that did not develop specific humoral immunity and ended up being hospitalized in the ICU with a COVID‐19 pneumonia and, posteriorly, mechanically ventilated. The failure of all therapeutic options allowed infection to persist 43 days with active accumulation of the SARS‐CoV‐2 virus. As a rescue therapy, an isolated infusion of Ronapreve™ antibodies was performed 46 days from initial diagnosis, promoting the appearance of serum AS antibodies, effective against SARS‐CoV‐2 infection and resulting in a favorable clinical, analytical, and radiological outcome for the patient.
Our results provide, to our knowledge, the first evidence of the therapeutic potential of this antibody combination in an immunosuppressed hospitalized COVID‐19 patient, previously vaccinated without seroconversion and under ventilatory support. However, it is imperative to design studies to assess their efficacy in a wider range of patients with lymphoid malignancies, including CLL, under different antileukemic treatments, so that specific guidelines can be established to decrease patients' morbidity and mortality and to improve their long‐term outcomes.
5. PATIENT'S PERSPECTIVE (DECEMBER 2021)
“I first had mild respiratory symptoms and mild fatigue only. My wife, however, had more severe symptoms and we started suspecting they could be attributed to the SARS‐CoV‐2 virus, so we had an antigen test that came back positive. We immediately called the national health line that gave us the instructions to go to the hospital's emergency department, where the PCR test confirmed the infection.
Being a retired Pneumologist myself makes me more aware of not only the possible severity and consequences of the Covid‐19 disease but also the possibility of not developing antibodies against this virus because of my disease, CLL, so I started to be very worried. Throughout my pre‐intubation days in the ICU, I felt everyone from the ICU medical team, my Haematologist and my family tried to give me the best possible care. Since I continued not to develop directed antibodies against the virus, the team started to consider Ronapreve™ antibodies as a potential therapeutic option. At the time I felt that could be my chance of recovery, the solution to this problem.
I started to feel more and more fatigued over time, a fatigue that was palpable when trying to perform simple motor tasks. The medical and nursing team, that would give me feedback from my progression, needed to gradually increment the oxygen supplementation and I knew I was clinically getting worse. There are a lot of things that people, including some of my family members, tell me I said or they have told me during the days prior to the intubation that I don't really remember. I only recall waking up feeling very distressed because I knew that was a considerable possibility, I wouldn't develop the antibodies to the virus and I wouldn't get out of that bed.
Throughout the rest of my stay in the ICU, I had a big fluctuation of feelings – some of the times I would feel better and in others I would feel apprehensive and, psychologically, very confused. It was a shock, initially, when I woke up and couldn't move my legs, however, after the physical therapy I was under in the ICU and the one I'm doing now, I feel better from a motor as well as respiratory point of view. The otolaryngology team has been evaluating me and I've already been decannulated. I'm currently in a swallowing rehabilitation training with the speech‐language therapists.
I still feel psychologically tired (although I don't tend to look back and relive the period from my ICU stay) and I know it's going to be a long rehabilitation process, but I do feel Ronapreve™ antibodies made a difference and I feel hopeful about the recovery that lies ahead.”
AUTHOR CONTRIBUTIONS
Fernanda Braga Seganfredo: Conceptualization; writing – original draft; writing – review and editing. Ana Raquel Dias: Conceptualization; writing – original draft; writing – review and editing. Pedro Ribeiro Santos: Writing – original draft; writing – review and editing. Marta Rebelo: Conceptualization; writing – original draft; writing – review and editing. Cristina João: Supervision; writing – review and editing. Dina Mendes: Writing – original draft; writing – review and editing. Maria Eduarda Carmo: Supervision; writing – review and editing.
FUNDING INFORMATION
This research did not receive any specific grant from funding agencies in the public, commercial, or non‐for‐profit sectors.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
CONSENT
Patient consent was obtained and patient's identifiable data were omitted. Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
The case complexity, inherent to the patient's preconditions, required a multidisciplinary approach, namely intensivists, the patient's hematologist, clinical pharmacists, therapists, otolaryngologists, and nurses to achieve the best quality of care. We would like to extend our gratitude to all of them.
Seganfredo FB, Dias AR, Santos PR, et al. Successful treatment of persistent and severe SARS‐CoV‐2 infection in a high‐risk chronic lymphocytic leukemia patient using Ronapreve™ antibodies. Clin Case Rep. 2022;10:e06548. doi: 10.1002/ccr3.6548
Fernanda Braga Seganfredo and Ana Raquel Dias should be considered joint first authors.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Arruga F, Gyau BB, Iannello A, Vitale N, Vaisitti T, Deaglio S. Immune response dysfunction in chronic lymphocytic leukemia: dissecting molecular mechanisms and microenvironmental conditions. Int J Mol Sci. 2020;21(5):1825. doi: 10.3390/ijms21051825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Forconi F, Moss P. Perturbation of the normal immune system in patients with CLL. Blood. 2015;126(5):573‐581. doi: 10.1182/blood-2015-03-567388 [DOI] [PubMed] [Google Scholar]
- 3. Parikh SA, Shanafelt TD. Prognostic factors and risk stratification in chronic lymphocytic leukemia. Semin Oncol. 2016;43(2):233‐240. doi: 10.1053/j.seminoncol.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 4. Chatzikonstantinou T, Kapetanakis A, Scarfò L, et al. COVID‐19 severity and mortality in patients with CLL: an update of the international ERIC and campus CLL study. Leukemia. 2021;35:3444‐3454. doi: 10.1038/s41375-021-01450-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mato AR, Roeker LE, Lamanna N, et al. Outcomes of COVID‐19 in patients with CLL: a multicenter international experience. Blood. 2020;136(10):1134‐1143. doi: 10.1182/blood.2020006965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blixt L, Bogdanovic G, Buggert M, et al. Covid‐19 in patients with chronic lymphocytic leukemia: clinical outcome and B‐ and T‐cell immunity during 13 months in consecutive patients. Leukemia. 2022;36:476‐481. doi: 10.1038/s41375-021-01424-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Roeker LE, Knorr DA, Thompson MC, et al. COVID‐19 vaccine efficacy in patients with chronic lymphocytic leukemia. Leukemia. 2021;35:2703‐2705. doi: 10.1038/s41375-021-01270-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Herishanu Y, Avivi I, Aharon A, et al. Efficacy of the BNT162b2 mRNA COVID‐19 vaccine in patients with chronic lymphocytic leukemia. Blood. 2021;137(23):3165‐3173. doi: 10.1182/blood.2021011568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sidebottom DB, Gill D. Ronapreve for prophylaxis and treatment of covid‐19. BMJ. 2021;374:n2136. doi: 10.1136/bmj.n2136 [DOI] [PubMed] [Google Scholar]
- 10. Roche . Ronapreve approved by European Commission to treat non‐hospitalised COVID‐19 patients and for prophylaxis of the disease. 2021. Accessed November 22, 2021. https://www.roche.com/investors/updates/inv‐update‐2021‐11‐12.htm
- 11. Weinreich DM, Sivapalasingam S, Norton T, et al. REGN‐COV2, a neutralizing antibody cocktail in outpatient with Covid‐19. N Engl J Med. 2021;384:238‐251. doi: 10.1056/NEJMoa2035002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nagoba B, Gavkare A, Jamadar N, Mumbre S, Selkar S. Positive aspects, negative aspects and limitations of plasma therapy with special reference to COVID‐19. J Infect Public Health. 2020;13(12):1818‐1822. doi: 10.1016/j.jiph.2020.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Agha M, Blake M, Chilleo C, Wells A, Haidar G. Suboptimal response to COVID‐19 mRNA vaccines in hematologic malignancies patients. MedRxiv [Preprint]. 2021. doi: 10.1101/2021.04.06.21254949 [DOI] [PMC free article] [PubMed]
- 14. Von Lilienfeld‐Toal M, Vehreschild JJ, Cornely O, et al. Frequently asked questions regarding SARS‐CoV‐2 in cancer patients‐recommendations for clinicians caring for patients with malignant diseases. Leukemia. 2020;34:1487‐1494. doi: 10.1038/s41375-020-0832-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mihaila RG. Management of patients with chronic lymphocytic leukemia during the SARS‐CoV‐2 pandemic. Oncol Lett. 2021;22(2):636. doi: 10.3892/ol.2021.12897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen L, Xiong J, Bao L, Shi Y. Convalescent plasma as a potential therapy for COVID‐19. Lancet Infect Dis. 2020;20(4):398‐400. doi: 10.1016/S1473-3099(20)30141-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


